Abstract
An extracellular α-glucuronidase was purified and characterized from a commercial Aspergillus preparation and from culture filtrate of Aspergillus tubingensis. The enzyme has a molecular mass of 107 kDa as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and 112 kDa as determined by mass spectrometry, has a determined pI just below 5.2, and is stable at pH 6.0 for prolonged times. The pH optimum for the enzyme is between 4.5 and 6.0, and the temperature optimum is 70°C. The α-glucuronidase is active mainly on small substituted xylo-oligomers but is also able to release a small amount of 4-O-methylglucuronic acid from birchwood xylan. The enzyme acts synergistically with endoxylanases and β-xylosidase in the hydrolysis of xylan. The enzyme is N glycosylated and contains 14 putative N-glycosylation sites. The gene encoding this α-glucuronidase (aguA) was cloned from A. tubingensis. It consists of an open reading frame of 2,523 bp and contains no introns. The gene codes for a protein of 841 amino acids, containing a eukaryotic signal sequence of 20 amino acids. The mature protein has a predicted molecular mass of 91,790 Da and a calculated pI of 5.13. Multiple copies of the gene were introduced in A. tubingensis, and expression was studied in a highly overproducing transformant. The aguA gene was expressed on xylose, xylobiose, and xylan, similarly to genes encoding endoxylanases, suggesting a coordinate regulation of expression of xylanases and α-glucuronidase. Glucuronic acid did not induce the expression of aguA and also did not modulate the expression on xylose. Addition of glucose prevented expression of aguA on xylan but only reduced the expression on xylose.
Xylan is the most abundant hemicellulose structure present in plant cell walls. It consists of a β-1,4-linked backbone of xylose residues which can be replaced with a number of different functions such as acetyl, arabinosyl, ferulic acid, and 4-O-methyl-α-glucuronic acid residues. To ensure cell wall rigidity, xylan is linked to other cell wall polymers, such as pectin and lignin. Two residues attached to xylan are involved in these linkages. Ferulic acid, connected to the xylan backbone through arabinose, can form a covalent linkage with other phenolic acid residues present in pectin or lignin (7, 9, 17). The other residue so far identified to be involved in cross-linking cell wall polymers is 4-O-methylglucuronic acid. Indications for an ester linkage between lignin and glucuronoxylan through 4-O-methylglucuronic acid have been found in beech wood (22). Calculations indicated that approximately one-third of the glucuronic acid residues attached to the xylan backbone are involved in this linkage.
Many bacteria and fungi are capable of degrading polymeric structures from plant cell walls by producing a large number of enzymes which specifically cleave certain linkages in these polymers. Endoxylanases (EC 3.2.1.8) cleave the xylan backbone, whereas β-xylosidase (EC 3.2.1.37) cleaves off xylose monomers from the nonreducing end of xylo-oligomers. To remove the side groups from the xylose backbone, arabinofuranosidases (EC 3.2.1.55), acetylxylan esterases (EC 3.1.1.72), ferulic acid esterases, and α-glucuronidases (EC 3.2.1.139) are needed. A complex synergy exists between these enzymes, resulting in an efficient degradation of the xylan polymer. α-Glucuronidase releases 4-O-methyl-α-glucuronic acid from xylan. Although many organisms have been reported to produce extracellular α-glucuronidases (3, 8, 10, 16, 20, 21), for Aspergillus only two intracellular α-glucuronidases have been described (23). These intracellular α-glucuronidases have slightly different properties than extracellular α-glucuronidases from other fungi (3, 10, 20, 21), which all have molecular masses between 90 and 130 kDa and a slightly acidic pI and which are active mainly on small xylo-oligomers. So far, the molecular structure of α-glucuronidase-encoding genes has been described for only two organisms. An activity screening of Trichoderma reesei cDNA clones resulted in the isolation of a clone which contained the α-glucuronidase-encoding gene (13). A gene encoding α-glucuronidase was also isolated from the hyperthermophilic bacterium Thermotoga maritima (18).
We have purified an extracellular α-glucuronidase from Aspergillus tubingensis and, using reverse genetics, cloned the corresponding gene.
MATERIALS AND METHODS
Strains, libraries, and plasmids.
The A. tubingensis strains used were NW756 and NW241 (pyrA2 fwnA1). Escherichia coli DH5αF′ (BRL, Life Technologies Inc., Gaithersburg, Md.) was used for routine plasmid propagation. E. coli LE392 was used as a host for phage λ. pBluescript was used for subcloning. The genomic library from A. tubingensis was previously described (5).
Media and culture conditions.
Minimal medium (MM) contained the following (per liter): 6.0 g of NaNO3, 1.5 g of KH2PO4, 0.5 g of KCl, 0.5 g of MgSO4, and trace elements (25) and 1% (wt/vol) glucose as a carbon source unless otherwise indicated. For complete medium (CM), MM was supplemented with 2% (wt/vol) tryptone, 1% (wt/vol) yeast extract, 1% (wt/vol) Casamino Acids, and 0.5% (wt/vol) yeast ribonucleic acids. Liquid cultures were inoculated with 106 spores/ml and incubated at 30°C in an orbital shaker at 250 rpm. Agar at 1.5% (wt/vol) was added for solid medium. For the growth of strains with auxotrophic mutations, the necessary supplements were added to the medium.
Chemicals.
d-Glucuronic acid was obtained from Fluka (Buchs, Switzerland). p-Nitrophenol-β-d-xylopyranoside, d-xylose, d-glucose, l-arabinose 3,5-dimethoxy-4-hydroxycinnamic acid, and birchwood xylan were obtained from Sigma (St. Louis, Mo.). Aldotriouronic acid, xylo-oligosaccharides, and Xylazyme tablets were obtained from Megazyme International (Dublin, Ireland). Endoproteinase Lys-C and bovine serum albumin were from Boehringer (Mannheim, Germany). N-Glycosidase F was from Oxford GlycoSystems (Oxon, United Kingdom). Taq polymerase, Q-Sepharose FF, Phenyl Sepharose FF, Superdex 200 PG, Butyl Sepharose FF, protein molecular weight markers, and fast protein liquid chromatography Mono Q HR 5/5 and Superose 6 HR 10/30 columns were purchased from Pharmacia (Uppsala, Sweden). Poros 10 HQ medium was obtained from PerSeptive Biosystems (Cambridge, Mass.). Sumizyme AC was obtained from Sumitomo (Osaka, Japan). A PA 100 column was obtained from Dionex Corp. (Sunnyvale, Calif.).
α-Glucuronidase assay.
The incubation mixture for the α-glucuronidase assay (total volume, 0.2 ml) contained 0.16 ml of substrate (2 mg of aldotriouronic acid-aldobiuronic acid [80:20] in 0.05 M sodium acetate buffer [pH 5.0]) and 0.04 ml of enzyme solution to be assayed. The incubation was started by addition of the enzyme. After 30 min of incubation at 40°C, the reaction was stopped by boiling the samples for 4 min. Precipitates were removed by centrifugation (10,000 × g), after which the supernatant was transferred to a new tube. To each tube, 0.6 ml of copper reagent prepared as described by Milner and Avigad (14) was added, and then the sample was boiled for 10 min and cooled on ice. Subsequently, 0.4 ml of arsenomolybdate reagent prepared as described by Nelson (15) was added. The samples were mixed gently, 0.8 ml of H2O was added, and the absorbance at 600 nm was measured against H2O. Controls were prepared by boiling a complete assay mixture at time zero, before incubation at 40°C. A substrate control was made by adding water instead of enzyme solution. A standard curve was prepared by using d-glucuronic acid. One α-glucuronidase unit is the amount of enzyme liberating 1 μmol of glucuronic or 4-O-methylglucuronic acid per min under standard assay conditions.
β-Xylosidase assay.
The β-xylosidase assay mixture contained 600 μl of substrate (5.5 mg of p-nitrophenyl-β-d-xylopyranoside in 6 ml of 50 mM sodium acetate [pH 4.2]) and 100 μl of purified β-xylosidase. The assay mixture was incubated at 40°C. At 0, 7, 15, and 22 min, a 100-μl sample was removed and added to 600 μl of stop reagent (0.13 M Na2CO3), after which the absorbance at 405 nm was measured. A substrate blank was prepared by adding water instead of enzyme solution. One β-xylosidase unit is the amount of enzyme which liberates 1 μmol of xylose per min at 40°C.
Endoxylanase assay.
Xylanase activity was determined by the amount of blue color liberated from azurine-dyed cross-linked birchwood xylan (Xylazyme tablets) under conditions recommended by the manufacturer.
HPLC analysis of monomeric and oligomeric residues from xylan.
A 2.5-ml solution of birchwood xylan (0.5%) in 50 mM sodium acetate (pH 5.0) was incubated with 0.45 U of purified α-glucuronidase, 7.0 mU of purified xylanase A from A. tubingensis, 5.8 mU of xylanase complex (Sumizyme AC), and 0.48 U of purified β-xylosidase. The four enzymes were incubated alone and in all possible combinations in a total volume of 3.25 ml for 3 h at 45°C. The incubation was stopped by boiling the samples for 3 min. The samples were analyzed on a Dionex high-pressure liquid chromatography (HPLC) system equipped with a Dionex PA 100 column and a pulsed electrochemical detector with a pH reference electrode. Elution was carried out with a 12-min linear gradient from 0.02 to 0.05 M followed by a 33-min linear gradient from 0.05 to 0.12 mM sodium acetate in 0.1 M NaOH at a flow rate of 1 ml/min.
Protein determination.
During the α-glucuronidase purification, the protein concentrations were determined by measuring the absorbance at 280 nm. Protein concentrations in the pooled samples were determined in microtiter plates by a sensitive method (2) performed according to instructions given by Bio-Rad (1a). Bovine serum albumin was used as a standard.
PAGE and Western analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), native gel electrophoresis, and isoelectric focusing were carried out by using the Novex (San Diego, Calif.) system with precast gels. Both electrophoresis and silver staining of the gels were done according to the instructions of the manufacturer. Western analysis of supernatant samples from A. tubingensis cultures was performed with polyclonal antibodies raised in mice against purified α-glucuronidase A (AGUA) from A. tubingensis.
Purification.
α-Glucuronidase was isolated from a commercial enzyme preparation, Pektinase 146 (Danisco Ingredients, Brabrand, Denmark), derived from Aspergillus niger and from culture filtrate of A. tubingensis NW241::pIM3212.8. All procedures were performed at room temperature.
(NH4)2SO4 was added to 200 ml of Pektinase 146 or 20 ml of A. tubingensis culture filtrate to 45% saturation. After 30 min of stirring, the precipitated protein was recovered by centrifugation for 20 min at 11,000 × g. The pellet was solubilized in 120 ml of Phenyl Sepharose buffer consisting of 20 mM sodium acetate (pH 5.0) and 1.5 M (NH4)2SO4. The sample was applied to a 155-ml Phenyl Sepharose FF column equilibrated in Phenyl Sepharose buffer. α-Glucuronidase was eluted with a 1,320-ml linear gradient from 1.5 to 0 M (NH4)2SO4 in 20 mM sodium acetate (pH 5.0) with a flow rate of 4 ml/min during which 12-ml fractions were collected. Fractions 60 to 101 (500 ml) were pooled, concentrated, and desalted in Q-Sepharose buffer (20 mM triethanolamine, pH 7.3) by ultrafiltration in an Amicon 8400 unit equipped with a 10-kDa membrane. The resulting sample was applied to a 106-ml Q-Sepharose FF column equilibrated with Q-Sepharose buffer. After the column was washed with 240 ml of Q-Sepharose buffer, the α-glucuronidase was eluted with a 420-ml linear gradient from 0 to 0.4 M sodium chloride in Q-Sepharose buffer at a flow rate of 3 ml/min, during which 7.5-ml fractions were collected. Fractions 23 to 36 (105 ml) were pooled and concentrated by ultrafiltration. The concentrated sample (7 ml) was loaded onto a Superdex 200 PG column (180 ml) equilibrated in 20 mM sodium acetate (pH 5.0)–0.1 M sodium chloride. α-Glucuronidase was eluted from the column with a flow rate of 1 ml/min, during which fractions of 2 ml were collected. Fractions 22 to 41 were pooled (18 ml), concentrated, and desalted. This sample was separated on a Mono Q HR 5/5 column in six runs with 20 mM triethanolamine buffer (pH 7.3). The column was washed with an 18-ml sodium chloride gradient from 0 to 0.1 M, and then α-glucuronidase was eluted at a constant concentration of 0.1 M sodium chloride in the same buffer at a flow rate of 1.5 ml/min, during which fractions of 0.75 ml were collected. The α-glucuronidase-containing fractions were pooled (27 ml), and (NH4)2SO4 was added to a final concentration of 1.5 M. This sample was loaded on a 30-ml Butyl Sepharose FF column equilibrated with Phenyl Sepharose buffer. After the column was washed with 50 ml of this buffer, the α-glucuronidase was eluted with a 160-ml linear gradient from 1.5 to 0 M (NH4)2SO4 in Phenyl Sepharose buffer at a flow rate of 2 ml/min, during which 4-ml fractions were collected. Fractions 22 to 26 (20 ml) were pooled, concentrated, and desalted as described above. A final purification was achieved by loading the sample on a 4-ml Poros 10 HQ column equilibrated in Q-Sepharose buffer; 5 ml was loaded per run. Elution was performed with a 22-ml linear gradient of sodium chloride from 0 to 0.3 M in Q-Sepharose buffer at a flow rate of 2 ml/min. Fractions of 1 ml were collected and screened for α-glucuronidase activity.
Determination of N-terminal and internal peptide sequences of AGUA.
The purified freeze-dried enzyme (100 μg) was dissolved in 50 μl of a solution containing 8 M urea and 0.4 M NH4HCO3 (pH 8.4). After the solution was flushed with N2, 5 μl of 45 mM dithiothreitol was added, and the protein was denatured and reduced for 15 min at 50°C under N2. After the solution had cooled to room temperature, 5 μl of 100 mM iodoacetamide was added for the cysteines to be derivatized for 15 min at room temperature in the dark under N2. Subsequently, 135 μl of water and 5 μg of endoproteinase Lys-C in 5 μl of water were added, and the sample was incubated at 37°C under N2 for 24 h. The resulting peptides were separated by reverse-phase HPLC on a VYDAC C18 column (0.46 by 15 cm; particle size, 10 μm; The Separation Group, Hesparia, Calif.) using solvent A (0.1% trifluoroacetic acid [TFA] in water) and solvent B (0.1% TFA in acetonitrile). Selected peptides were rechromatographed on a Develosil C18 column (0.46 by 10 cm; Novo Nordisk, Bagsværd, Denmark) with the same solvent system, prior to N-terminal sequencing. Sequencing was done on a 476A sequencer using pulsed-liquid fast cycles according to the instructions of the manufacturer (Applied Biosystems, Foster City, Calif.). For direct N-terminal sequencing, the purified protein was passed through a Brownlee C2 Aquapore column (0.46 by 3 cm; particle size, 7 μm; Applied Biosystems) with the same solvent system as above. N-terminal sequencing was performed as described above.
Deglycosylation.
Deglycosylation of the pure α-glucuronidase was performed with N-glycosidase F (Oxford GlycoSystems) according to the procedure recommended by the manufacturer, with denaturation of the protein before addition of the N-glycosidase F.
Characterization of the α-glucuronidase.
The molecular masses of the native and the recombinant α-glucuronidases were determined by gel permeation chromatography on a Superose 6 column at a flow rate of 0.5 ml/min with 20 mM triethanolamine (pH 7.3) as the eluent and RNase A (13.7 kDa), ovalbumin (43 kDa), aldolase (158 kDa), and catalase (232 kDa) as size standards.
The optimum temperature was determined by the assay described above, with incubation for 10 min in 0.05 M sodium acetate buffer (pH 5.0) at different temperatures. The optimum pH was determined by using 0.1 M sodium acetate in a pH range from 3.5 to 6.7; pH values were determined for the assay tubes at room temperature. Temperature stability was determined by incubating 200 μl of purified α-glucuronidase in 50 mM sodium acetate buffer (pH 5.0) at different temperatures for 20 h, after which the α-glucuronidase activity was determined as described above. pH stability was determined by incubating 150 μl of purified α-glucuronidase in 500 μl of 0.2 M sodium acetate (pH 4.0)–0.2 M bis-Tris (pH 6.0) or 0.2 M Tris (pH 8.0) for 3, 10, 13, 28, and 62 days at room temperature. Residual activities were measured as described above.
Determination of the molecular mass by mass spectrometry.
Samples containing 10 μl of native and recombinant α-glucuronidase were mixed with 1 μl of 10% acetonitrile and desalted for 2 h at room temperature by use of VSWP013 filters (Millipore). MALDI/TOF mass spectrometry was performed with a Voyager Biospectrometry Work Station (PerSeptive Biosystems). Samples were prepared by mixing 1 μl of desalted proteins and 2 μl of matrix solution (saturated solution of 3,5-dimethoxy-4-hydroxycinnamic acid in 60% acetonitrile with 0.1% TFA). A 1-μl sample of the mixture was spotted into a well of the MALDI sample plate and allowed to air dry prior to introduction into the mass spectrometer. Data for 100 3-ns laser pulses were averaged for each spectrum, and linear, positive-ion TOF detection was performed with an accelerating voltage of 20,000 V. Spectra were smoothed with a 19-point Savitzky-Golay filter.
PCR cloning of a specific fragment of the aguA gene.
Several degenerate oligonucleotides were designed and synthesized on an Applied Biosystems 392 DNA synthesizer. PCRs were performed with a Biometra Personal Cycler using these oligonucleotides at 55°C and chromosomal DNA from A. tubingensis NW756. A PCR using oligonucleotides 5 and 9 (5′-GGNCCNATHGAYTTYCARGT-3′ and 5′-ARRTCRTARTTNACNCC-3′ with H, Y, and R representing A/C/T, C/T, and A/G, respectively) resulted in a fragment of 1,142 bp which was cloned into the pGEM-T vector system (Promega). Sequence analysis was performed as described below.
Isolation, cloning, and characterization of the aguA gene.
Plaque hybridization using nylon replicas was performed as described by Benton and Davies (1). Hybridizations were performed overnight at 65°C with the PCR fragment used as a probe. The filters were washed with SSC and SDS (final concentrations, 0.2× and 0.5%) (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate [pH 7.6]). Positive plaques, identified on duplicate replicas after autoradiography, were recovered from the original plates and purified by rescreening at a low plaque density. Standard methods were used for other DNA manipulations, such as Southern and Northern analysis, subcloning, DNA digestions, and λ phage and plasmid DNA isolations (19). Chromosomal DNA was isolated as previously described (4). Sequence analysis was performed on both strands of DNA with a Sequenase DNA sequencing kit (United States Biochemical Corporation, Cleveland, Ohio) and a T7Sequencing Kit (Pharmacia LKB), using additional oligonucleotides. Nucleotide and amino acid sequences were analyzed with the computer programs of Devereux et al. (6). Aspergillus transformations were performed as described by Kusters-van Someren et al. (12).
Nucleotide sequence accession number.
The EMBL accession number for aguA from A. tubingensis is Y15405.
RESULTS
Purification of α-glucuronidase.
α-Glucuronidase was purified as described in Materials and Methods. A summary of the purification from the Pektinase 146 preparation is shown in Table 1. Throughout the purification, α-glucuronidase always eluted as a single peak. The enzyme was purified 371-fold, with a yield of 5.8%. SDS-PAGE patterns showing the different steps of the purification are given in Fig. 1. The purified α-glucuronidase had no β-xylosidase activity or endoxylanase activity.
TABLE 1.
Purification of the A. niger α-glucuronidase
| Fraction | Protein (mg) | Total activity (μmol/min) | Sp act (μmol/min/mg of protein) | Purification factor | Yield (%) |
|---|---|---|---|---|---|
| Pektinase 146 | 2,740 | 380 | 0.14 | 1 | 100 |
| 45% (NH4)2SO4 | 1,899 | 300 | 0.16 | 1.1 | 79 |
| Phenyl Sepharose | 619 | 195 | 0.31 | 2.2 | 51 |
| Q-Sepharose | 88 | 162 | 1.83 | 13.1 | 42 |
| Superdex 200 PG | 57 | 102 | 1.80 | 12.9 | 27 |
| Mono Q | 11 | 69 | 6.20 | 44.3 | 18 |
| Butyl Sepharose | 2.2 | 54 | 24.3 | 175 | 14.2 |
| Poros 10 HQ | 0.42 | 22 | 52.3 | 371 | 5.8 |
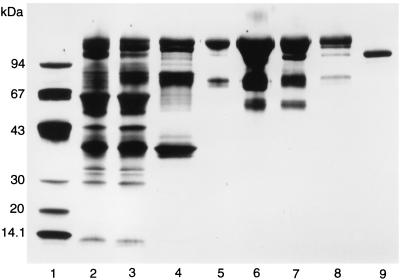
FIG. 1.
SDS-PAGE of the different steps in the purification of α-glucuronidase. Lane 1, low-molecular-mass standard proteins; lane 2, starting material; lane 3, 45% ammonium sulfate precipitate; lane 4, Phenyl Sepharose FF pool; lane 5, Q-Sepharose pool; lane 6, Superdex 200 PG pool; lane 7, Mono Q pool; lane 8, Butyl Sepharose pool; lane 9, Poros 10 HQ pool.
Enzyme properties.
The apparent molecular mass of α-glucuronidase was 107,000 Da as determined by SDS-PAGE (Fig. 1) and 100,000 Da as determined by gel filtration, indicating that the native enzyme consists of a single peptide chain. For the recombinant enzyme, an apparent molecular mass of 115 kDa was determined by SDS-PAGE. After N deglycosylation, a molecular mass of 95,000 Da was observed for both the native and the recombinant enzyme by SDS-PAGE. The molecular mass was also determined by mass spectrometry, resulting in values of 112,079 Da for the native enzyme and 116,488 for the recombinant enzyme.
The isoelectric point for the α-glucuronidase was just below 5.2. The pH and temperature optima were 4.5 to 6 and 70°C, respectively. At pH 6.0, the α-glucuronidase was completely stable for at least 62 days at room temperature. A loss of 15% of the activity was observed after 13 days, at pH 4, but even after 62 days, 68% of the activity was recovered. pH 8 was the least favorable of the tested pH values. After 13 days, 82% of the activity was recovered; after 62 days, this value had dropped to 45%. At pH 5, the α-glucuronidase was 100% stable at 10°C for 20 h. At the same pH at 45, 50, 55, and 60°C, the recoveries after 20 h were 88, 70, 52, and 10%, respectively. Loss in activity during freezing was not observed. With aldotriouronic acid-aldobiuronic acid as a substrate, the Km for α-glucuronidase was determined to be 0.14 ± 0.03 mg/ml (mean ± standard deviation). For the recombinant enzyme, similar results were obtained.
Cloning and overexpression of aguA from A. tubingensis.
Amino acid sequences were obtained for AGUA as described in Materials and Methods. In total, seven fragments containing 201 amino acids were sequenced (with six uncertainties). On the basis of these amino acid sequences, nine degenerate oligonucleotides were designed and used in PCRs with A. tubingensis chromosomal DNA. Although several DNA fragments were generated, only one combination (primers 5 and 9, based on peptides 5 and 4, respectively; see Materials and Methods) resulted in a fragment in which both the amino acid sequences on which the primers were based could be identified. The size of this fragment is 1,142 bp.
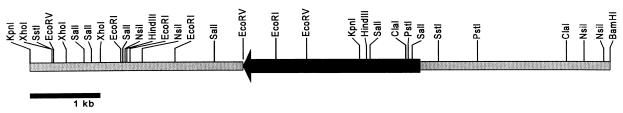
A genomic library of A. tubingensis was screened by using this fragment as a probe, and four hybridizing phage λ clones were isolated and purified. From one of these phages, a 7-kb XhoI/BamHI fragment and a 4-kb KpnI fragment containing part of the aguA gene and some flanking regions were cloned (pIM3210 and pIM3211). These fragments were combined, resulting in plasmid pIM3212 (Fig. 2), which was used to generate A. tubingensis multicopy transformants. Transformation of this construct resulted in a number of transformants with elevated levels of AGUA as determined by Western analysis (data not shown). Transformant NW241::pIM3212.8 had the highest level of α-glucuronidase activity (10 times the wild-type activity) and was selected for further experiments. Slot blot analysis indicated the presence of 11 copies of the aguA gene in this transformant.
FIG. 2.
Restriction map of the insert containing the aguA gene (arrow) which is present in the functional construct pIM3212.
Analysis of the nucleotide sequence of aguA and the derived amino acid sequence of the enzyme.
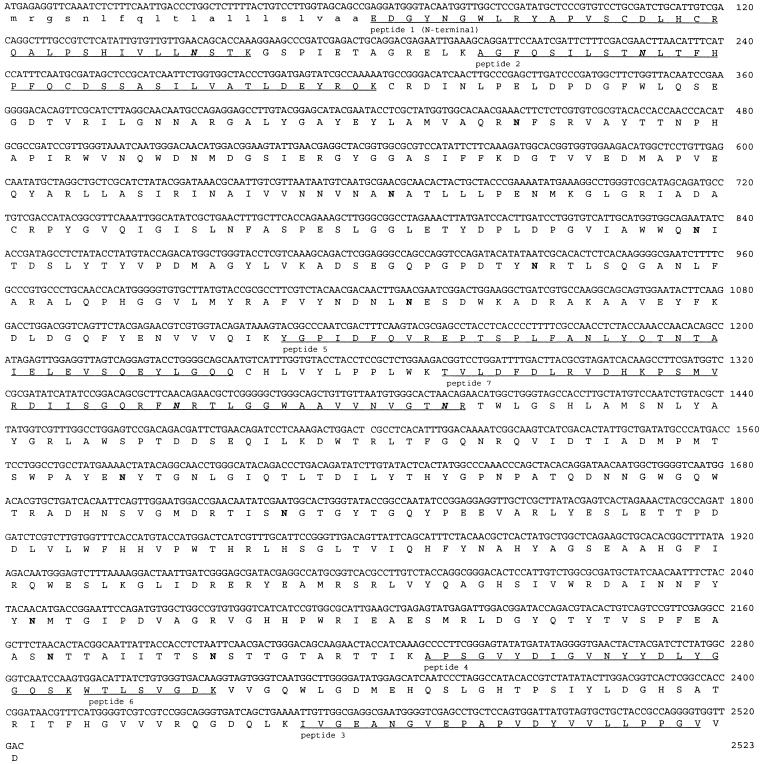
Subclones were made from pIM3210 and pIM3211 and sequenced. Additional sequence data were obtained by using specific oligonucleotides. The aguA gene consists of an open reading frame of 2,523 bp which contains no introns and codes for a protein of 841 amino acids (Fig. 3). Analysis of the derived amino acid sequence indicated a putative eukaryotic signal sequence of 20 amino acids, which was confirmed by the N-terminal amino acid sequence of the mature protein starting at position 21. The mature protein contains 14 putative N-glycosylation sites, of which 4 were confirmed by the presence of an unidentifiable amino acid residue in the sequenced peptides. The enzyme has a calculated pI of 5.18, which is identical to the measured value. The calculated molecular mass of the mature protein is 93,904 Da, which is similar to the value determined by mass spectrometry. In the promoter region of the gene, several boxes possibly involved in transcription and regulation were identified. A TATA box was found 65 bp upstream from the ATG, and CAAT boxes were found at positions −106, −161, and −313. Putative binding sites for the CREA protein (11), involved in carbon catabolite repression, were found at positions −100, −123, −247, and −440. Only the first site is present in the upper strand; the others are in the complementary strand.
FIG. 3.
Nucleotide sequence of aguA and derived amino acid sequence. The signal peptide (lowercase letters), putative (boldface roman letters) and confirmed (boldface italics) N-glycosylation sites, and the determined amino acid sequences (underlined) are indicated.
Alignment of the amino acid sequences of AGUA and two other α-glucuronidases.
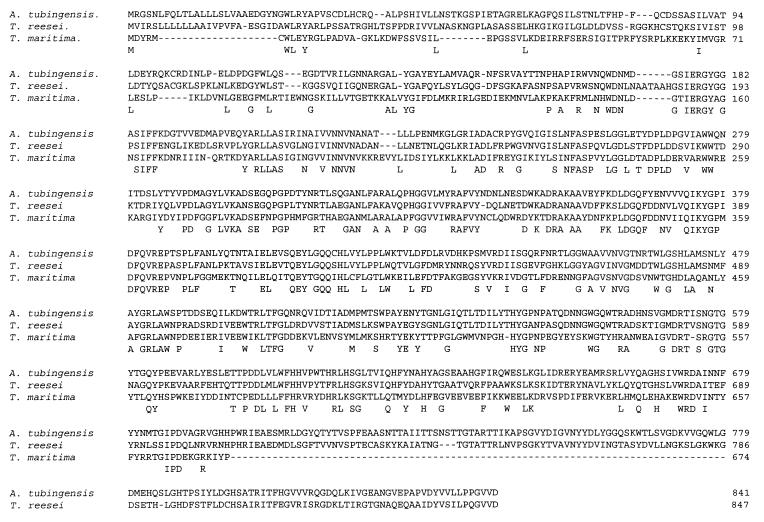
The deduced amino acid sequence of AGUA from A. tubingensis was aligned with the deduced amino acid sequences of AGUA from T. maritima (18) and GLRI from T. reesei (13) as shown in Fig. 4. AGUA was 59.3% identical to GLRI from T. reesei and 39.3% identical to AGUA from T. maritima. No clear highly identical boxes could be identified, although the level of identity is highest in the middle region of the enzymes.
FIG. 4.
Alignment of the amino acid sequences of α-glucuronidase from A. tubingensis, T. reesei, and T. maritima. Identical amino acids are depicted below the amino acid sequences.
The aguA gene is induced by xylan and xylose but not by glucuronic acid.
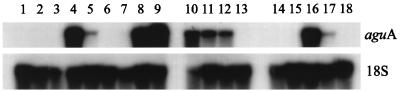
The induction of aguA was studied by a transfer experiment. Transformant NW241::pIM3212.8 was grown for 16 h in CM (3% fructose). The mycelium was harvested, washed with MM, and transferred to MM with different carbon sources. After 6 h, the mycelium was harvested and stored at −70°C. A Northern analysis was performed using RNA isolated from the mycelium samples. Induction of aguA was observed on xylose, arabinose, xylobiose, and birchwood xylan alone but not on glucose, fructose, glycerol, or glucuronic acid (Fig. 5). The presence of glucose completely inhibited the expression on birchwood xylan but only reduced the expression on xylose. Addition of glucuronic acid to the monomeric carbon sources did not result in an increase in the expression of aguA.
FIG. 5.
Northern analysis of the induction of aguA on different carbon sources. The top panel was probed with the internal 2-kb SalI fragment of aguA, and the bottom panel was probed with a 700-bp EcoRI fragment from the A. niger 18S ribosomal DNA and served as a loading control. Lane 1, mycelium from the preculture on fructose; other lanes, mycelium transferred to the following carbon sources: lane 2, 1% glucose; lane 3, 1% fructose; lane 4, 1% xylose; lane 5, 1% arabinose; lane 6, 1% glycerol; lane 7, 1% glucuronic acid; lane 8, 0.2% xylobiose; lane 9, 0.5% birchwood xylan; lane 10, 1% xylose–0.2% glucose; lane 11, 1% xylose–1% glucose; lane 12, 1% xylose–2% glucose; lane 13, 0.5% birchwood xylan–1% glucose; lane 14, 1% glucose–1% glucuronic acid; lane 15, 1% fructose–1% glucuronic acid; lane 16, 1% xylose–1% glucuronic acid; lane 17, 1% arabinose–1% glucuronic acid; and lane 18, 1% glycerol–1% glucuronic acid.
The presence of endoxylanase or β-xylosidase enhances the activity of α-glucuronidase on xylan.
The purified native α-glucuronidase was able to liberate minor amounts of 4-O-methylglucuronic acid from birchwood xylan and wheat bran (data not shown) but had a much higher activity on xylan-derived oligomers. The influence of endoxylanase and β-xylosidase on α-glucuronidase activity was studied by incubating combinations of these enzymes with birchwood xylan as described in Materials and Methods. Addition of xylanase A, xylanase complex, and β-xylosidase increased the amount of 4-O-methylglucuronic acid liberated (Table 2). The amount of small oligomers (xylobiose and xylotriose) is larger when a combination of α-glucuronidase and endoxylanase is used than when endoxylanase is used alone, indicating a positive effect of α-glucuronidase on the activity of endoxylanase on xylan. The most efficient degradation of birchwood xylan was achieved when a combination of α-glucuronidase, endoxylanase, and β-xylosidase was used.
TABLE 2.
Synergistic effects of α-glucuronidase, endoxylanase, and β-xylosidase activity on release of 4-O-methylglucuronic acid, xylose, and xylo-oligomers from birchwood xylan
| Enzyme(s) used in reaction mixtures | Amt released (μg/ml)a
|
|||
|---|---|---|---|---|
| 4-O-Methylglucuronic acid | Xylose | Xylobiose | Xylotriose | |
| None (control) | 63 | 0 | 0 | 0 |
| α-Glucuronidase | 62 | 0 | 0 | 0 |
| Xylanase complex | 127 | 1,260 | 440 | 129 |
| Xylanase A | 95 | 1,300 | <50 | <50 |
| β-Xylosidase | 63 | 270 | 0 | 0 |
| α-Glucuronidase plus: | ||||
| Xylanase complex | 379 | 1,210 | 680 | 210 |
| Xylanase A | 206 | 1,360 | 60 | 70 |
| β-Xylosidase | 187 | 1,060 | <50 | <50 |
| Xylanase A + β-xylosidase | 107 | 1,320 | <50 | <50 |
| Xylanase complex + β-xylosidase | 146 | 1,410 | <50 | <50 |
| α-Glucuronidase + xylanase A + β-xylosidase | 398 | 1,500 | <50 | <50 |
The amount of 4-O-methylglucuronic acid was determined by the colorimetric method. The amounts of the other compounds were determined by HPLC analysis.
DISCUSSION
Purification of the α-glucuronidase required a complex procedure. The ammonium sulfate precipitation applied as the first step did not give much purification with respect to the increase in specific activity but was important because it removed much of the colored contaminants (possibly phenolic compounds), which would otherwise have interfered with later column chromatography steps. Gel filtration always gave a large loss in activity but could not be omitted in the procedure. The most essential step was the Poros 10 HQ column, which had a high selectivity for the α-glucuronidase. However, the separation capacity of Poros 10 HQ was poor compared to that of Mono Q, and it was not possible to eliminate the Mono Q column.
The molecular mass determined by SDS-PAGE after treatment with N-glycosidase F was still slightly higher than the calculated molecular mass from the amino acid sequence, due to either O glycosylation or running effects in the SDS-PAGE gel. Since the difference in molecular mass is small, the amount of O glycosylation, if any, will be small. The large difference in molecular masses observed between the mature and the deglycosylated enzyme (107 and 95 kDa, respectively, for the native enzyme) suggests that most of the 14 putative N-glycosylation sites are actually involved in glycosylation. The difference in molecular masses for the native and the recombinant enzyme is probably due to differences in the degree of glycosylation.
The molecular mass is similar to those of some other fungal α-glucuronidases (8, 21) but is lower than those of Agaricus bisporus α-glucuronidase (16), which has a molecular mass of 160,000 Da as determined by SDS-PAGE, and the two internal α-glucuronidases from A. niger (23), which have molecular masses of 130 and 150 kDa, respectively. The pI of AGUA is similar to the pI of the A. niger α-glucuronidases.
The amino acid sequence of AGUA had a high level of identity to the amino acid sequences of the α-glucuronidases from T. reesei and T. maritima (13, 18), as shown in Fig. 4. The homology was present throughout the sequence until the end of the T. maritima amino acid sequence. The additional amino acid sequence from the two fungal α-glucuronidases (starting at the end of the T. maritima sequence) also showed a high level of identity, indicating that this region might be specific for fungal α-glucuronidases. Screening of the databases did not detect any other enzymes which had a significant level of identity with the α-glucuronidase from A. tubingensis.
The aguA gene was induced on xylose, xylobiose, and xylan, which resembles the induction of xlnA, encoding an A. tubingensis endoxylanase (5), and xlnD from A. niger, encoding a β-xylosidase (24). These data suggest that aguA is regulated by a xylan- or xylose-specific system, which induces genes coding for xylan-degrading enzymes in the presence of xylose or xylan. The low level of expression on arabinose has also been observed for other xylanolytic genes (unpublished data). Since a minor amount of xylose is present in the arabinose purchased from Sigma, this could explain the low level of expression, rather than a possible inducing effect of l-arabinose itself. Glucose repressed the expression of aguA completely in the presence of xylan but only partly in the presence of xylose. The presence of four putative CREA binding sites in the region directly upstream from the structural part of aguA suggests that glucose repression occurs through this regulator protein. The reason for the leaky repression in the presence of xylose is not clear and requires further study.
The hydrolysis of birchwood xylan by xylanases was enhanced in the presence of α-glucuronidase, but complete hydrolysis to xylose was not observed. Although the enzymes tested in this study clearly have a synergistic effect, other enzymes are needed as well to completely degrade xylan. A synergistic effect of the addition of (endo)xylanase to an α-glucuronidase incubation mixture was found. The activity of α-glucuronidases on polymeric substrates is very low compared to the activity on small-oligomer substrates, indicating that the presence of a xylanase is essential for efficient α-glucuronidase activity in vivo. Siika-aho et al. (21) found that α-glucuronidase seemed to act exclusively on bonds between the terminal xylose at the nonreducing end and 4-O-methylglucuronic acid attached to it. From this data, a synergistic effect of addition of β-xylosidase to an incubation mixture with α-glucuronidase (and xylanase) was expected, which was confirmed by the experiments described in this paper. Although the xylanase complex already contains β-xylosidase, addition of this enzyme resulted in an increase in the amount of liberated 4-O-methylglucuronic acid and a further degradation of xylobiose and xylotriose to xylose.
In this investigation, we have studied the hydrolysis of only hardwood xylan. A comparison with softwood xylan or deacetylated xylan could elucidate whether the acetylation present in hardwood is of any influence on the hydrolysis by α-glucuronidase. Hardwood xylan is a linear xylan, while wheat bran xylan is highly branched. The influence of branching requires further investigation in relation to the activity of α-glucuronidase on wheat bran xylan and applications thereof.
ACKNOWLEDGMENTS
We thank Masoud R. Zargahi for excellent technical assistance during purification and characterization of the enzyme, Clive Phipps Walter for performance of the amino acid sequencing, and Yvonne Thomassen and Tine Suhr for assistance during cloning and characterization of the gene.
REFERENCES
- 1.Benton W D, Davies R W. Screening λgt recombinant clones by hybridization to single plaques in situ. Science. 1977;196:180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- 1a.Bio-Rad Laboratories. Bio-Rad bulletin 1177 EG. Richmond, Calif: Bio-Rad Laboratories; 1984. [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Bronnenmeier K, Meissner H, Stocker S, Staudenbauer W L. α-d-Glucuronidases from the xylanolytic thermophiles Clostridium stercorarium and Thermoanaerobacterium saccharolyticum. Microbiology. 1995;141:2033–2040. doi: 10.1099/13500872-141-9-2033. [DOI] [PubMed] [Google Scholar]
- 4.de Graaff L H, van den Broeck H W J, Visser J. Isolation and transformation of the pyruvate kinase gene of Aspergillus nidulans. Curr Genet. 1988;13:315–321. doi: 10.1007/BF00424425. [DOI] [PubMed] [Google Scholar]
- 5.de Graaff L H, van den Broeck H C, van Ooijen A J J, Visser J. Regulation of the xylanase-encoding xlnA gene of Aspergillus tubingensis. Mol Microbiol. 1994;12:479–490. doi: 10.1111/j.1365-2958.1994.tb01036.x. [DOI] [PubMed] [Google Scholar]
- 6.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iiyama K, Bach-Tuyet Lam T, Stone B A. Covalent cross-links in the cell wall. Plant Physiol. 1994;104:315–320. doi: 10.1104/pp.104.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishihara M, Inagaki S, Hayashi N, Shimizu K. 4-O-methyl-d-Glucuronic acid residue liberating enzyme in the enzymatic hydrolysis of hardwood xylan. Bull For For Prod Res Inst. 1990;359:141–157. [Google Scholar]
- 9.Ishii T. Isolation and characterization of a diferuloyl arabinoxylan hexasaccharide from bamboo shoot cell-walls. Carbohydr Res. 1991;219:15–22. doi: 10.1016/0008-6215(91)89039-i. [DOI] [PubMed] [Google Scholar]
- 10.Khandke K M, Vithayathil P J, Murthy S K. Purification and characterization of an α-d-glucuronidase from a thermophilic fungus, Thermoascus aurantiacus. Arch Biochem Biophys. 1989;274:511–517. doi: 10.1016/0003-9861(89)90464-5. [DOI] [PubMed] [Google Scholar]
- 11.Kulmburg P, Mathieu M, Dowzer C, Kelly J M, Felenbok B. Specific binding sites in the alcR and alcA promoters of the ethanol regulon for the CREA repressor mediating carbon catabolite repression in Aspergillus nidulans. Mol Microbiol. 1993;7:847–857. doi: 10.1111/j.1365-2958.1993.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 12.Kusters-van Someren M A, Harmsen J A M, Kester H C M, Visser J. The structure of the Aspergillus niger pelA gene and its expression in Aspergillus niger and Aspergillus nidulans. Curr Genet. 1991;20:293–299. doi: 10.1007/BF00318518. [DOI] [PubMed] [Google Scholar]
- 13.Margolles-Clark E, Saloheimo M, Siika-aho M, Penttilä M. The α-glucuronidase-encoding gene of Trichoderma reesei. Gene. 1996;172:171–172. doi: 10.1016/0378-1119(96)00167-9. [DOI] [PubMed] [Google Scholar]
- 14.Milner Y, Avigad G. A copper reagent for the determination of hexuronic acid and certain ketohexoses. Carbohydr Res. 1967;4:359–361. [Google Scholar]
- 15.Nelson N. A photometric adaptation of the Somogyi method for determination of glucose. J Biol Chem. 1944;153:375–380. [Google Scholar]
- 16.Puls J, Schmidt O, Granzow C. α-Glucuronidase in two microbial xylanolytic systems. Enzyme Microb Technol. 1987;9:83–88. [Google Scholar]
- 17.Ralph J, Grabber J H, Hatfield R D. Lignin-ferulate crosslinks in grasses: active incorporation of ferulate polysaccharide esters into ryegrass lignins. Carbohydr Res. 1995;275:167–178. [Google Scholar]
- 18.Ruile P, Winterhalter C, Liebi W. Isolation and characterization of a gene encoding α-glucuronidase, an enzyme with a novel primary structure involved in the breakdown of xylan. Mol Microbiol. 1997;23:267–279. doi: 10.1046/j.1365-2958.1997.2011568.x. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 20.Shao W, Obi S K C, Puls J, Wiegel J. Purification and characterization of the α-glucuronidase from Thermoanaerobacterium sp. strain JW/SL-YS485, an important enzyme for the utilization of substituted xylans. Appl Environ Microbiol. 1995;61:1077–1081. doi: 10.1128/aem.61.3.1077-1081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siika-aho M, Tenkanen M, Buchert J, Puls J, Viikari L. An α-glucuronidase from Trichoderma reesei RUT C-30. Enzyme Microb Technol. 1994;16:813–819. [Google Scholar]
- 22.Takahashi N, Koshijima T. Ester linkages between lignin and glucuronoxylan in a lignin-carbohydrate complex from beech (Fagus crenata) wood. Wood Sci Technol. 1988;22:231–241. [Google Scholar]
- 23.Uchida H, Nanri T, Kawabata Y, Kusakabe I, Murakami K. Purification and characterization of intracellular α-glucuronidase from Aspergillus niger 5-16. Biosci Biotechnol Biochem. 1992;56:1608–1615. [Google Scholar]
- 24.van Peij N N M E, Brinkmann J, Vršanska M, Visser J, de Graaff L H. β-Xylosidase activity, encoded by xlnD, is essential for complete hydrolysis of xylan by Aspergillus niger but not for induction of the xylanolytic spectrum. Eur J Biochem. 1997;245:164–173. doi: 10.1111/j.1432-1033.1997.00164.x. [DOI] [PubMed] [Google Scholar]
- 25.Vishniac W, Santer M. The thiobacilli. Bacteriol Rev. 1957;21:195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]