Abstract
Background:
Diroximel fumarate (DRF) is approved for adults with relapsing–remitting multiple sclerosis (RRMS) in Europe and for relapsing forms of MS in the United States. DRF and dimethyl fumarate (DMF) yield bioequivalent exposure of the active metabolite monomethyl fumarate. Prior studies indicated fewer gastrointestinal (GI)-related adverse events (AEs) with DRF compared with DMF.
Objective:
To report final outcomes from EVOLVE-MS-1.
Methods:
EVOLVE-MS-1 was an open-label, 96-week, phase 3 study assessing DRF safety, tolerability, and efficacy in patients with RRMS. The primary endpoint was safety and tolerability; efficacy endpoints were exploratory.
Results:
Overall, 75.7% (800/1057) of patients completed the study; median exposure was 1.8 (range: 0.0–2.0) years. AEs occurred in 938 (88.7%) patients, mostly of mild (28.9%) or moderate (50.3%) severity. DRF was discontinued due to AEs in 85 (8.0%) patients, with < 2% discontinuing due to GI or flushing/flushing-related AEs. At Week 96, mean number of gadolinium-enhancing lesions was significantly reduced from baseline (72.7%; p < 0.0001); adjusted annualized relapse rate was 0.13 (95% confidence interval: 0.11–0.15).
Conclusion:
DRF was generally well tolerated over 2 years, with few discontinuations due to AEs; radiological measures indicated decreased disease activity from baseline. These outcomes support DRF as a treatment option in patients with RRMS.
Keywords: Diroximel fumarate, relapsing–remitting multiple sclerosis, multiple sclerosis, clinical trial, disease-modifying therapy, safety, tolerability, efficacy
Introduction
Diroximel fumarate (DRF) is an oral fumarate approved in Europe for adult patients with relapsing–remitting multiple sclerosis (RRMS) and in the United States for adult patients with relapsing forms of MS.1,2 Oral administration of DRF leads to rapid conversion via esterase cleavage in the small intestine to monomethyl fumarate (MMF), the same pharmacologically active metabolite as dimethyl fumarate (DMF). 3 DRF 462 mg and DMF 240 mg produce bioequivalent exposures of MMF, 4 and therefore efficacy and safety profiles for DRF and DMF are expected to be comparable at these doses. DMF has demonstrated significant and clinically meaningful efficacy in clinical trials and real-world studies of patients with MS.5–10 However, gastrointestinal (GI) adverse events (AEs) are commonly reported in patients with MS receiving DMF treatment, including in up to 40% of participants in clinical trials.6,9
DRF has demonstrated improved GI tolerability compared with DMF in a prior study, possibly because of DRF’s different chemical structure. This results in lower production of methanol as a metabolite, which might contribute to a reduced level of irritation within the GI tract than occurs with DMF treatment. 11 DRF’s physicochemical properties may also cause fewer off-target interactions than DMF, subsequently provoking less GI irritation. 11 DRF demonstrated improved GI tolerability compared with DMF in the phase 3, randomized, head-to-head, double-blind, 5-week EVOLVE-MS-2 study of patients with RRMS. 12 As of 31 December 2022, approximately 33,989 patients had been treated with DRF, representing 35,420 patient-years of exposure. Of these, 1477 patients (1718 patient-years) were from clinical trials (Biogen. Data on file).
EVOLVE-MS-1 was an open-label, phase 3 study to evaluate the long-term safety and tolerability of DRF in adults with RRMS who had either previously participated in EVOLVE-MS-2 or were newly initiated onto DRF. Here, we report the final safety, tolerability, and exploratory efficacy outcomes from the EVOLVE-MS-1 study.
Methods
Study design
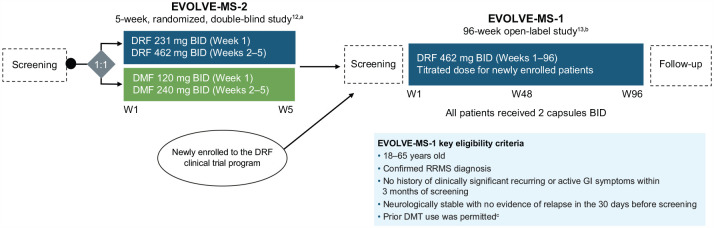
EVOLVE-MS-1 (NCT02634307) was an open-label, single-arm, phase 3 study assessing the long-term safety, tolerability, and efficacy of DRF 462 mg twice daily over 96 weeks in patients with RRMS (Figure 1). The study population included patients who were newly enrolled in the DRF clinical trial program and patients who were eligible to enter having completed the 5-week, randomized, double-blind, phase 3 EVOLVE-MS-2 (NCT03093324) study of DRF and DMF.
Figure 1.
EVOLVE-MS-1 study design.
BID: twice daily; DMF: dimethyl fumarate; DMT: disease-modifying therapy; DRF: diroximel fumarate; GI: gastrointestinal; RRMS: relapsing–remitting multiple sclerosis; W: week.
EVOLVE-MS-1 was conducted from 10 December 2015 to 11 November 2021.
aAdapted from Naismith RT, et al. CNS Drugs. 2020;34(2):185–196; http://creativecommons.org/licenses/by-nc/4.0/.
bAdapted from Naismith RT, et al. Mult Scler. 2020;26(13):1729–1739; http://creativecommons.org/licenses/by-nc/4.0/.
cExclusion criteria for newly enrolled patients included the use of teriflunomide within 2 years of Visit 2 (Week 1); natalizumab within 2 months of Visit 2; alemtuzumab; fingolimod within 90 days of Visit 2; daclizumab within 6 months of Visit 2; or B-cell therapies within 12 months of screening.
Patients
Eligible patients were aged 18–65 years, had a confirmed diagnosis of RRMS, and were neurologically stable with no evidence of relapse in the 30 days before screening. Patients who transitioned from the EVOLVE-MS-2 study were required to have completed the full EVOLVE-MS-2 treatment period within 7 days of EVOLVE-MS-1 study Visit 2. Prior disease-modifying therapy (DMT), including DMF, was permitted. Further details regarding inclusion and exclusion criteria for the EVOLVE-MS-2 and EVOLVE-MS-1 studies have been reported previously.12,13
The study was approved by local and central ethics committees and conducted in accordance with the International Council on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent. A full list of study sites is provided in Supplementary Table 1.
Study endpoints
The primary endpoint was DRF safety and tolerability. Exploratory efficacy endpoints included radiological (gadolinium-enhancing (Gd+), new/newly enlarging T2, and new T1 hypointense lesion counts), brain volume, clinical outcomes (annualized relapse rate (ARR), MS relapse, confirmed disability progression (CDP), Expanded Disability Status Scale (EDSS) score, no evidence of disease activity-3 (NEDA-3), timed 25-foot walk (T25-FW) score), and patient-reported outcomes (PROs; EQ-5D-5 L and the 12-Item Short Form Health Survey (SF-12)).
Assessments and analysis of populations
Final safety and exploratory efficacy (clinical and radiological) outcomes were evaluated in the overall EVOLVE-MS-1 study population. The overall population consisted of subgroups entering from EVOLVE-MS-2 (having received either DRF or DMF) and those who had not previously received DRF (de novo group). Exploratory efficacy outcomes were also reported for newly diagnosed patients, defined as patients from the overall population who were diagnosed with MS for <1 year and were treatment-naive with respect to DMTs.
Safety evaluations included treatment-emergent adverse events (TEAEs) and laboratory parameters (chemistry, hematology, and urinalysis). Absolute lymphocyte count (ALC) less than the lower limit of normal (LLN) was defined as <0.91 × 109/L. DRF was temporarily withheld if ALC reached a confirmed level of <0.5 × 109/L and then permanently discontinued if levels remained <0.5 × 109/L for ⩾4 weeks after discontinuation. Patients who permanently discontinued the study with a last measured ALC of <0.8 × 109/L were followed for 6 months afterward for lymphocyte monitoring. Moderate and severe prolonged lymphopenia were defined as ALC ⩾0.5 × 109/L to <0.8 × 109/L and <0.5 × 109/L, respectively, sustained for >6 months.
Tolerability-related AEs were classified using the MedDRA Preferred Terms within the System Organ Class for GI disorders and for flushing/flushing-related AEs. Per the study protocol, MS relapses were recorded as AEs. AEs of special interest included anaphylaxis and serious angioedema, lymphopenia, liver injury, renal injury, cardiac disorders, GI tolerability AEs (serious or leading to discontinuation), abuse potential, pancreatitis, opportunistic infections, and all serious infections, malignancies, and pre-malignant conditions. AEs were followed until resolution, or deemed stable by the investigator, or until the patient was deemed lost to follow-up by the investigator.
Protocol-defined MS relapse was defined as new or recurrent neurologic symptoms (not associated with fever/infection) lasting ⩾24 hours and accompanied by at least one of the following: new objective neurological findings and increase of ⩾0.5 in EDSS score since the previous visit; an increase of ⩾2 in one functional system score; or an increase of ⩾1 in two functional systems (apart from bladder or cognitive changes). ARR values on study reflect protocol-defined MS relapses. Baseline ARR values reflect relapses in the 12 months before the study started that were historically obtained and not confirmed by protocol-defined MS relapse criteria. NEDA-3 was defined as no relapses, no CDP sustained for 12 weeks per EDSS, and no new/newly enlarging T2 hyperintense or Gd+ lesions. Written documentation was required to confirm a patient’s willingness to continue the study in the instance of MS relapse, disability progression measured by EDSS, or total Gd+ lesion count ⩾5 assessed by the central magnetic resonance imaging (MRI) facility. The Kaplan–Meier product limit method was used to estimate the proportions of patients who were free from CDP, were relapse-free, and had NEDA-3.
Statistical analyses
Summary statistics were provided for all parameters. Safety analyses were based on the safety population, defined as all enrolled patients who received ⩾1 dose of DRF. Safety assessments were summarized using descriptive statistics. Exploratory efficacy endpoints were based on the full analysis set, defined as all enrolled patients who received ⩾1 dose of DRF and had ⩾1 post-baseline efficacy assessment. Adjusted ARR was based on a Poisson regression model. MRI and clinical endpoints were summarized using descriptive statistics. For de novo participants, newly enrolled in EVOLVE-MS-1, baseline was defined as Visit 2 (Week 1). For prior DMF and prior DRF participants who rolled over from the EVOLVE-MS-2 study, baseline disease characteristics were obtained from the baseline visit in EVOLVE-MS-2.
Results
Patients
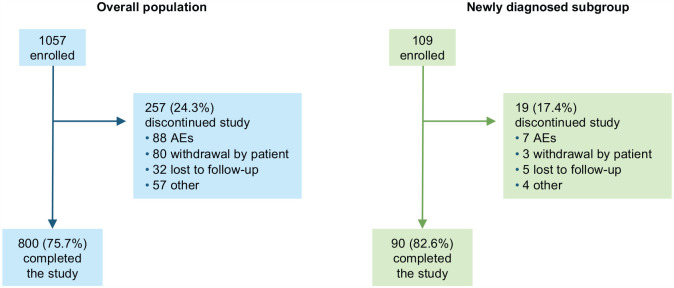
Of 1057 patients enrolled in EVOLVE-MS-1 who received at least one dose of DRF (Figure 2), 593 were newly enrolled (de novo group) and 464 had transitioned after completing EVOLVE-MS-2 (n = 239 received DRF; n = 225 received DMF); 109 patients had been newly diagnosed with RRMS. In the overall population, the mean (standard deviation (SD)) age was 42.5 (10.8) years, and 762 (72.1%) patients were female (Table 1). Most patients (681/1057; 64.4%) had received prior DMTs, of which the most common were interferon (398/1057; 37.7%) and glatiramer acetate (267/1057; 25.3%). Overall, 453 (42.9%) patients were enrolled at US sites and 604 (57.1%) at sites in Canada or Europe.
Figure 2.
EVOLVE-MS-1 patient disposition.
AE: adverse event.
Table 1.
Baseline demographics and disease characteristics in EVOLVE-MS-1.
| Characteristics | Overall population n = 1057 |
Newly diagnosed subgroup n = 109 |
De novo enrollment n = 593 |
EVOLVE-MS-2 rollover | |
|---|---|---|---|---|---|
| Prior DMF n = 225 |
Prior DRF n = 239 |
||||
| Age, years, mean (SD) | 42.5 (10.8) | 36.0 (10.8) | 41.5 (11.0) | 43.7 (9.8) | 44.0 (11.0) |
| Female, n (%) | 762 (72.1) | 78 (71.6) | 427 (72.0) | 170 (75.6) | 165 (69.0) |
| Race, n (%) | |||||
| White | 972 (92.0) | 104 (95.4) | 547 (92.2) | 205 (91.1) | 220 (92.1) |
| Black or African American | 72 (6.8) | 5 (4.6) | 37 (6.2) | 16 (7.1) | 19 (7.9) |
| Other | 13 (1.2) | 0 | 9 (1.5) | 4 (1.8) | 0 |
| BMI, kg/m2, mean (SD) | 26.6 (6.1) | 25.4 (6.2) | 26.2 (6.1) | 27.6 (6.2) | 27.0 (5.9) |
| Region, n (%) | |||||
| Non-US | 604 (57.1) | 78 (71.6) | 385 (64.9) | 104 (46.2) | 115 (48.1) |
| US | 453 (42.9) | 31 (28.4) | 208 (35.1) | 121 (53.8) | 124 (51.9) |
| Prior DMT a , n (%) | 681 (64.4) | 0 | 379 (63.9) | 143 (63.6) | 159 (66.5) |
| Interferon | 398 (37.7) | 0 | 232 (39.1) | 81 (36.0) | 85 (35.6) |
| Glatiramer acetate | 267 (25.3) | 0 | 145 (24.5) | 61 (27.1) | 61 (25.5) |
| Time since diagnosis, years, mean (SD) | 7.6 (7.3) b | 0.4 (0.5) | 7.6 (7.1) c | 7.8 (7.5) | 7.4 (7.8) |
| No. of relapses in the previous year, mean (SD) | 0.7 (0.8) | 1.2 (0.7) | 0.8 (0.8) | 0.6 (0.7) | 0.6 (0.7) |
| EDSS score, mean (SD) | 2.7 (1.5) | 2.0 (1.1) | 2.7 (1.5) | 2.7 (1.4) | 2.6 (1.5) |
| No. of Gd+ lesions, mean (SD) | 1.1 (3.5) d | 1.9 (5.1) | 1.3 (4.2) | 0.9 (2.6) | 0.8 (2.2) e |
| Gd+ lesion free, n (%) | 741 (70.1) | 61 (56.0) | 406 (68.5) | 159 (70.7) | 176 (73.6) |
| GI comorbidity, n (%) | 132 (12.5) | 17 (15.6) | 89 (15.0) | 26 (11.6) | 17 (7.1) |
BMI: body mass index; DMF: dimethyl fumarate; DRF: diroximel fumarate; DMT: disease-modifying therapy; EDSS: Expanded Disability Status Scale; Gd+: gadolinium-enhancing; GI: gastrointestinal.
Prior DMT includes immunomodulatory and immunosuppressant (investigational or approved).
n =1056.
n =592.
n =1053.
n =235.
Overall, 800/1057 (75.7%) patients completed the study and 257/1057 (24.3%) discontinued; the most common reasons provided for discontinuation were AEs (88/1057; 8.3%) and withdrawal by the patient (80/1057; 7.6%). The median (range) duration of DRF exposure was 1.8 (0.0–2.0) years.
Safety
Summary of safety
TEAEs were reported in 938/1057 (88.7%) patients (Table 2); for most patients, these were mild (306/1057; 28.9%) or moderate (532/1057; 50.3%) in severity. The most common individual TEAE was flushing, which occurred in 288/1057 (27.2%) patients. Serious TEAEs were reported in 123/1057 (11.6%) patients. Four deaths occurred, of which the causes were reported as bacterial pneumonia (n = 1), fall (n = 1), hypertensive heart disease (n = 1), and cardiac arrest (n = 1); none were considered related to study treatment. No cases of progressive multifocal leukoencephalopathy were reported. The types and frequencies of TEAEs were similar across the de novo, prior DMF, and prior DRF patient groups.
Table 2.
Safety summary in the overall population and by prior enrollment status.
| AE, n (%) | Overall population n = 1057 |
De novo enrollment n = 593 |
EVOLVE-MS-2 rollover | |
|---|---|---|---|---|
| Prior DMF n = 225 |
Prior DRF n = 239 |
|||
| Any AE | 938 (88.7) | 519 (87.5) | 207 (92.0) | 212 (88.7) |
| Mild | 306 (28.9) | 170 (28.7) | 70 (31.1) | 66 (27.6) |
| Moderate | 532 (50.3) | 297 (50.1) | 112 (49.8) | 123 (51.5) |
| Severe | 100 (9.5) | 52 (8.8) | 25 (11.1) | 23 (9.6) |
| AEs leading to treatment discontinuation | 85 (8.0) | 49 (8.3) | 13 (5.8) | 23 (9.6) |
| Most common AEs leading to treatment discontinuation (occurring in ⩾0.5% of patients) | ||||
| Lymphopenia | 14 (1.3) | 6 (1.0) | 3 (1.3) | 5 (2.1) |
| MS relapse | 11 (1.0) | 7 (1.2) | 0 | 4 (1.7) |
| GI disorders a | 7 (0.7) | 5 (0.8) | 1 (0.4) | 1 (0.4) |
| Lymphocyte count decreased | 7 (0.7) | 1 (0.2) | 1 (0.4) | 5 (2.1) |
| Flushing | 5 (0.5) | 2 (0.3) | 1 (0.4) | 2 (0.8) |
| Any SAE | 123 (11.6) | 69 (11.6) | 25 (11.1) | 29 (12.1) |
| Most common SAEs (occurring in ⩾0.5% of patients) | ||||
| Nervous system disorders b | 68 (6.4) | 35 (5.9) | 13 (5.8) | 20 (8.4) |
| MS relapse c | 61 (5.8) | 32 (5.4) | 10 (4.4) | 19 (7.9) |
| GI disorder b | 10 (0.9) | 5 (0.8) | 3 (1.3) | 2 (0.8) |
| Injury, poisoning, and procedural complications b | 10 (0.9) | 7 (1.2) | 3 (1.3) | 0 |
| Infections and infestations b | 9 (0.9) | 4 (0.7) | 3 (1.3) | 2 (0.8) |
| Reproductive system and breast disorders b | 7 (0.7) | 5 (0.8) | 1 (0.4) | 1 (0.4) |
| Cardiac disorders b | 6 (0.6) | 3 (0.5) | 1 (0.4) | 2 (0.8) |
| Death d | 4 (0.4) | 3 (0.5) | 0 | 1 (0.4) |
| Most common AEs (occurring in ⩾10% of patients cumulatively) | ||||
| Flushing | 288 (27.2) | 226 (38.1) | 29 (12.9) | 33 (13.8) |
| MS relapse | 206 (19.5) | 113 (19.1) | 45 (20.0) | 48 (20.1) |
| Upper respiratory tract infection | 153 (14.5) | 76 (12.8) | 35 (15.6) | 42 (17.6) |
| Nasopharyngitis | 137 (13.0) | 86 (14.5) | 27 (12.0) | 24 (10.0) |
| Lymphopenia | 124 (11.7) | 51 (8.6) | 38 (16.9) | 35 (14.6) |
| Diarrhea | 109 (10.3) | 66 (11.1) | 25 (11.1) | 18 (7.5) |
| AEs of special interest (category) e | ||||
| Lymphopenia (SMQ hematopoietic leukopenia) | 187 (17.7) | 82 (13.8) | 56 (24.9) | 49 (20.5) |
| Lymphopenia (lymphocyte relevant) | 163 (15.4) | 68 (11.5) | 48 (21.3) | 47 (19.7) |
| Cardiac disorders f | 145 (13.7) | 70 (11.8) | 38 (16.9) | 37 (15.5) |
| Liver injury | 79 (7.5) | 39 (6.6) | 19 (8.4) | 21 (8.8) |
| Renal injury | 37 (3.5) | 13 (2.2) | 10 (4.4) | 14 (5.9) |
| Infections g | 16 (1.5) | 7 (1.2) | 4 (1.7) | 5 (2.1) |
| Malignancies | 5 (0.5) | 1 (0.2) | 1 (0.4) | 3 (1.3) |
AE: adverse event; DMF: dimethyl fumarate; DRF: diroximel fumarate; MS: multiple sclerosis; GI: gastrointestinal; SAE: serious adverse event; SMQ: standardized MedDRA query.
Diarrhea (n = 3); anal incontinence, dyspepsia, irritable bowel syndrome, and peptic ulcer (all n = 1).
System organ class.
Preferred term.
Accidental fall, bacterial pneumonia, hypertensive heart disease, and cardiac arrest; none of the deaths were considered related to the study drug by the investigator.
AEs of special interest included anaphylaxis and angioedema (serious), cardiac disorders, lymphopenia (SMQ hematopoietic leukopenia) and lymphopenia (lymphocyte relevant), liver injury, renal injury, GI tolerability AEs (serious or leading to discontinuation), abuse potential, and pancreatitis.
Within the cardiac disorders category, the most common AE was dizziness (57/1057; 5.4%).
Includes opportunistic infections (AEs and SAEs) and all serious infections (including serious opportunistic infections).
Tolerability
AEs affecting the GI system occurred in 337/1057 (31.9%) patients; for most patients, the greatest severity was mild (205/337; 60.8%) or moderate (113/337; 33.5%). Severe GI AEs occurred in 19/1057 patients (1.8%); of these, 10 were receiving concomitant medication for treating GI AEs, and two discontinued DRF. Of patients with GI AEs, 155/337 (46.0%) received concomitant therapy for treating GI AEs. GI AEs resolved in 309/337 (91.7%) patients; the median (10th–90th percentile) duration was 10 (1–135) days. Patients with unresolved GI AEs were followed until deemed stable by investigators at the last follow-up visit. Among patients with treatment-emergent GI AEs for whom complete start dates were recorded (n = 336), 159 (47.3%) reported that their first GI AE occurred within the first month of treatment.
Flushing/flushing-related AEs were reported in 394/1057 (37.3%) patients; in addition to flushing, the most common were pruritus (70/1057; 6.6%), erythema (51/1057; 4.8%), and rash (29/1057; 2.7%). For flushing/flushing-related AEs, the number and percentage of patients by severity were as follows: mild (300/394; 76.1%), moderate (84/394; 21.3%), and severe (10/394; 2.5%). Of patients with flushing/flushing-related AEs, 67/394 (17.0%) received concomitant therapy for treating flushing/flushing-related AEs. In most patients (317/394; 80.5%), flushing/flushing-related AEs resolved. Patients with unresolved flushing/flushing-related AEs were followed until deemed stable by investigators at the last follow-up visit. In patients with complete start and end dates recorded (n = 328), the median (10th–90th percentile) duration of flushing/flushing-related AEs was 13 (1–364) days. Of patients with flushing/flushing-related AEs with complete start and end dates recorded, most patients (279/388; 71.9%) reported the first occurrence within the first month of treatment.
There were 85 (8.0%) AEs leading to discontinuations; 7/1057 (0.7%) patients discontinued due to GI AEs, and 8/1057 (0.8%) due to flushing/flushing-related AEs. Overall, 19/1057 (1.8%) patients discontinued due to serious AEs, the most common of which was MS relapse (n = 4); all serious AEs leading to discontinuation are listed in Supplementary Table 2. Of 132/1057 (12.5%) patients who had GI comorbidities at baseline, 38 (28.8%) discontinued.
AEs of special interest
Overall, 145/1057 (13.7%) patients experienced an AE within the “cardiac disorders” category; these AEs were mild (98/145; 67.6%), moderate (41/145; 28.3%), or severe (6/145; 4.1%), and the most common AE was dizziness (57/1057; 5.4%). AEs within the “liver injury” category were reported in 79/1057 (7.5%) patients, including 59/1057 (5.6%) with alanine aminotransferase (ALT) increase and 32/1057 (3.0%) with aspartate aminotransferase (AST) increase; in 29/1057 (2.7%) patients, both AEs of ALT and AST increase were reported. AEs in the liver injury category were assessed as mild (55/79; 69.6%), moderate (21/79; 26.6%), or severe (3/79; 3.8%). No cases of liver injury met laboratory criteria for Hy’s law (total bilirubin ⩾2 × upper limit of normal (ULN) and ALT or AST ⩾3× ULN). AEs within the “renal injury” category were reported in 37/1057 (3.5%) patients; all cases were mild (33/37; 89.2%) or moderate (4/37; 10.8%).
Serious infections were reported in 10/1057 (0.9%) patients: appendicitis (n = 2), bacterial pneumonia (n = 1), cellulitis (n = 1), chronic gastritis (n = 1), pneumonia (n = 1), pharyngeal abscess (n = 1), pharyngitis (n = 1), sepsis (n = 1), and urinary tract infection (n = 1). All AEs of serious infection were moderate (4/10; 40%) or severe (6/10; 60%). Malignancies were reported in 5/1057 (0.5%) patients: invasive ductal breast carcinoma (n = 1), basal cell carcinoma (n = 1), malignant melanoma (n = 1), Bowen’s disease (n = 1), and diffuse large B-cell lymphoma (n = 1). All AEs of malignancy were moderate in severity (5/5; 100%).
Lymphocyte counts
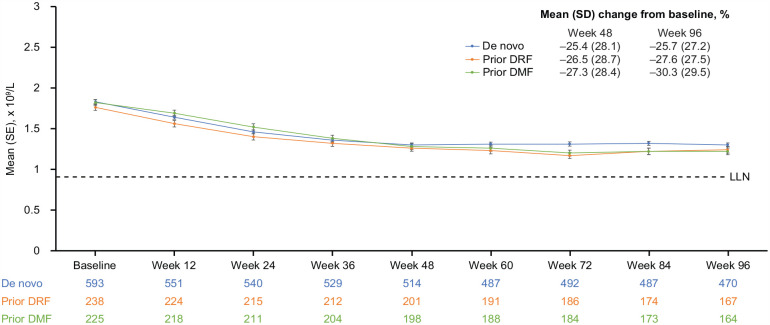
Mean (SD) ALCs for the de novo (n = 593), prior DRF (n = 239), and prior DMF groups (n = 225) declined from baseline to Week 48 by −25.4% (28.1%), −26.5% (28.7%), and −27.3% (28.4%), respectively, and then plateaued (Figure 3). For 56.7% (593/1045) of patients, ALCs remained above the LLN for the duration of treatment. Prolonged moderate lymphopenia was reported in 147/1045 (14.1%) patients, including 80/582 (13.7%) de novo patients, 28/225 (12.4%) prior DMF patients, and 39/238 (16.4%) prior DRF patients. No cases of prolonged severe lymphopenia were seen due to a stop rule in the study protocol. DRF was discontinued in 14 (1.3%) patients due to AEs of lymphopenia.
Figure 3.
Mean (standard error (SE)) absolute lymphocyte counts (ALCs) declined from baseline to Week 48 and plateaued to Week 96.
DMF: dimethyl fumarate; DRF: diroximel fumarate; LLN: lower limit of normal.
LLN was defined as < 0.91×109/L. The prior DMF group includes patients who rolled over from receiving DMF in EVOLVE-MS-2. The prior DRF group includes patients who rolled over from receiving DRF in EVOLVE-MS-2. De novo group includes patients who had not previously received DRF and were newly enrolled in the DRF clinical trial program.
Efficacy
Clinical outcomes
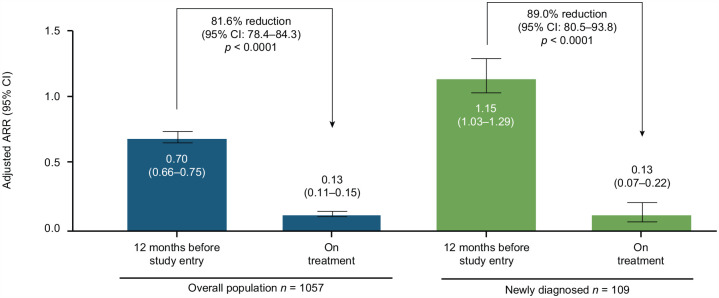
Overall adjusted ARR on DRF was 0.13 (95% confidence interval (CI): 0.11–0.15) compared with 0.70 (95% CI: 0.66–0.75) reported for the 12 months before study entry, representing a significant reduction on treatment (81.6% reduction (95% CI: 78.4–84.3); p < 0.0001; Figure 4). In newly diagnosed patients, adjusted ARR on DRF was 0.13 (95% CI: 0.07–0.22) compared with 1.15 (95% CI: 1.03–1.29) in the 12 months before study entry, representing a reduction of 89.0% (95% CI: 80.5–93.8; p < 0.0001). Adjusted ARR on DRF was 0.13 (95% CI: 0.11–0.17) for de novo patients, 0.13 (95% CI: 0.09–0.18) for prior DMF patients, and 0.12 (95% CI: 0.09–0.16) for prior DRF patients.
Figure 4.
Adjusted annualized relapse rate (ARR)a on treatment compared with 12 months before study entry.
aCalculation of ARR was based on patient-reported relapses in the 12 months before study entry compared with protocol-defined relapses during the study period.
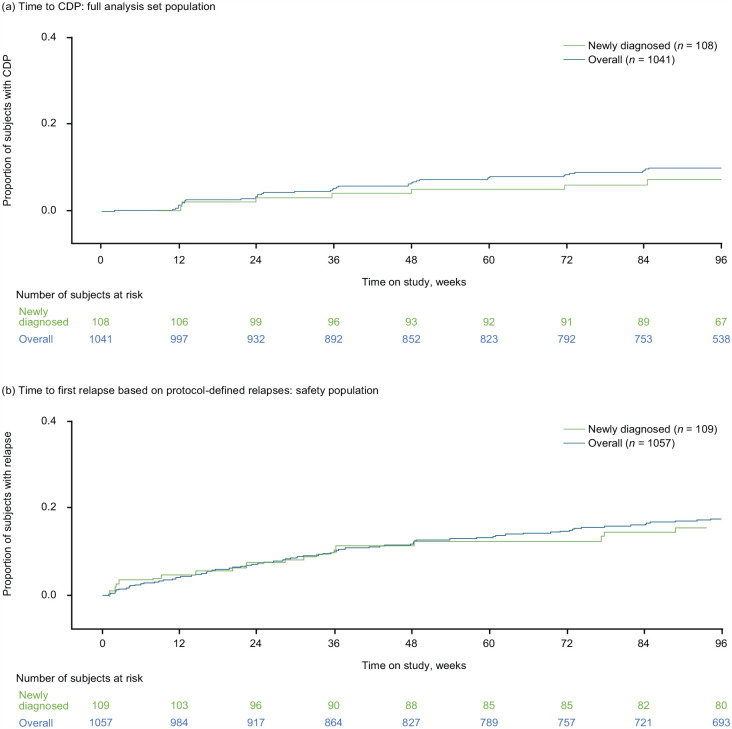
Overall in this study, the estimated proportions of patients who were free from CDP were 93.4% at Week 48% and 90.2% at Week 96; in newly diagnosed patients, the corresponding proportions were 95.1% and 93.0%, respectively (Figure 5(a)). At Weeks 48 and 96, respectively, estimated proportions who were free from CDP were 94.5% and 91.1% in the de novo group, 90.4% and 88.7% in the prior DMF group, and 93.6% and 89.3% in the prior DRF group.
Figure 5.
Time to (a) confirmed disability progression (CDP) and (b) first relapse.
Estimated proportions of patients who were relapse-free were 87.7% (Week 48) and 82.4% (Week 96), overall, and 88.6% (Week 48) and 84.5% (Week 96) in the newly diagnosed population (Figure 5(b)). When separated based on prior enrollment, estimated proportions of patients who were relapse-free at Weeks 48 and 96, respectively, were 87.1% and 82.3% (de novo), 88.0% and 81.7% (prior DMF), and 89.0% and 83.3% (prior DRF).
Estimates of patients with NEDA-3 were 65.4% at Week 48 and 41.1% at Week 96. Median (interquartile range) EDSS scores were 2.5 (1.5–3.5) at baseline (n = 1041) and 2.5 (1.5–3.5) at Week 96 (n = 816). In newly diagnosed patients, estimates were 56.1% at Week 48 and 34.4% at Week 96. At Weeks 48 and 96, respectively, estimates of patients with NEDA-3 were 61.1% and 35.7% in the de novo group, 69.8% and 46.1% in the prior DMF group, and 72.0% and 50.0% in the prior DRF group.
Radiological endpoints
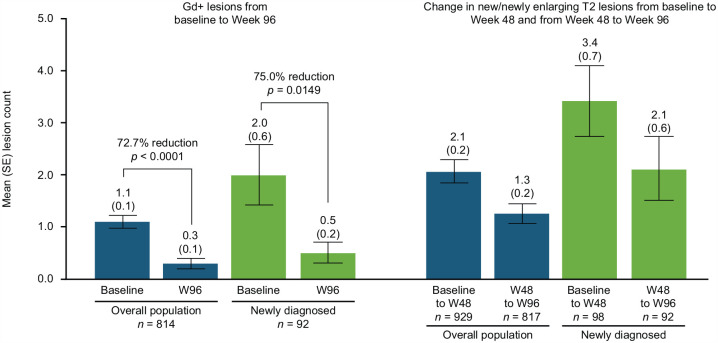
Compared with baseline, Gd + lesion count at Week 96 was reduced by 72.7% in the overall population (mean (standard error (SE)) number of lesions: 1.1 (0.1) vs. 0.3 (0.1); p < 0.0001)) and by 75.0% in the newly diagnosed subgroup (2.0 (0.6) vs. 0.5 (0.2); p = 0.0149) (Figure 6). In the overall population, the mean (SE) number of new/newly enlarging T2 lesions was 2.1 (0.2) from baseline to Week 48 and 1.3 (0.2) from Week 48 to Week 96. In the newly diagnosed patient group, the mean (SE) number of new/newly enlarging T2 lesions was 3.4 (0.7) from baseline to Week 48 and 2.1 (0.6) from Week 48 to Week 96. Based on prior enrollment, from baseline to Week 48 and from Week 48 to Week 96, respectively, the mean (SE) number of new/newly enlarging T2 lesions was 2.7 (0.3) and 1.6 (0.3) in the de novo group, 1.1 (0.2) and 0.6 (0.2) in the prior DMF group, and 1.4 (0.4) and 0.9 (0.3) in the prior DRF group.
Figure 6.
Change in number of gadolinium-enhancing (Gd+) lesions, baseline to Week 96, and in new/newly enlarging T2 lesions, baseline to Week 48 and Weeks 48–96.
W: Week.
Baseline MRI was performed any time between screening and Visit 2 (Day 1) for patients newly enrolled in the DRF clinical trial program (de novo group) and within 1 week after Visit 2 (Day 1) for patients who had transitioned after completing EVOLVE-MS-2.
Mean (SD) brain volume change from baseline to Week 48 was −0.4% (0.6%), and from baseline to Week 96 was −0.74% (0.72%), in the overall population; corresponding changes in the newly diagnosed patient group were −0.38% (0.63%) and −0.76% (0.79%), respectively. In patient groups separated by prior enrollment, mean (SD) brain volume changes from baseline to Weeks 48 and 96, respectively, were −0.38% (0.63%) and −0.71% (0.76%) in the de novo group, −0.39% (0.57%) and −0.77% (0.69%) in the prior DMF group, and −0.44% (0.54%) and −0.8% (0.65%) in the prior DRF group.
T25-FW and PROs
In the overall population, T25-FW and PROs remained stable over the study period. Median (Q1, Q3) T25-FW was 5.65 (4.7, 7.25) seconds at baseline (n = 1041) and 5.59 (4.65, 7.05) seconds at Week 96 (n = 810). Mean (SD) EQ-5D-5 L visual analog scale scores were 76.6 (16.9) at baseline (n = 1034) and 75.5 (18.7) at Week 96 (n = 824) (higher scores indicating perception of better health based on patient’s own assessment). Mean (SD) SF-12 physical scores were 43.8 (10.7) at baseline (n = 1037) and 44.0 (10.6) at Week 96 (n = 820).
Discussion
Final results reported for the full duration of the EVOLVE-MS-1 study with patients receiving DRF for up to 96 weeks indicate favorable safety, tolerability, and efficacy outcomes, consistent with those reported earlier for the interim analysis of EVOLVE-MS-1. 13
Overall, 938 (88.7%) patients experienced AEs, most of which were mild or moderate. AEs reported on DRF were consistent with those reported previously for DMF; the most common were flushing (27.2%) and MS relapse (19.5%). No new safety signals were observed. A total of 85 (8.0%) patients discontinued DRF due to AEs; 800 (75.7%) patients completed the study. Tolerability is a notable factor in terms of adherence to therapy and is therefore a key consideration for the long-term treatment of chronic diseases such as MS. These data from EVOLVE-MS-1 support that long-term treatment (up to 96 weeks) with DRF was generally well tolerated.
With DMF treatment, GI AEs and flushing are among the most commonly experienced AEs. 14 Since improved GI tolerability compared with DMF was part of the rationale behind the development of DRF, GI AEs are of significant interest in EVOLVE-MS-1. The favorable GI tolerability of DRF compared with DMF has previously been demonstrated in an analysis of the EVOLVE-MS-2 study. 12 In addition, based on outcomes from EVOLVE-MS-2, DRF’s GI tolerability has been linked to quality-of-life benefits compared with DMF. 15 In this final report of EVOLVE-MS-1, 31.9% of patients experienced GI AEs. Most of these events were mild-to-moderate in severity and 47.3% occurred within the first month on DRF. Discontinuation of DRF due to GI symptoms was uncommon, occurring in 7 (0.7%) patients.
Although EVOLVE-MS-1 was not designed to evaluate the clinical efficacy of DRF, outcomes were favorable in both the overall population and the subgroup of newly diagnosed patients during treatment with DRF and were consistent with efficacy outcomes previously reported for fumarates in patients with MS.6,9 ARR was reduced by 81.6% and 89.0% compared with the 12 months prior to DRF treatment initiation in the overall population and the newly diagnosed subgroup, respectively. Estimates for adjusted ARRs across de novo, prior DMF, and prior DRF patient groups were consistent with that for the overall study population. Furthermore, at Week 96, an estimated 90.2% of the overall population and 93.0% of newly diagnosed patients were free from CDP. A decrease from baseline in disease activity was seen with DRF treatment, based on radiological measures; Gd + lesion count was reduced by 72.7% overall and new/newly enlarging T2 lesions were lower for Weeks 48–96 compared with baseline to Week 48. Brain volume change was comparable with ranges observed in healthy adults.16,17 Similar clinical and radiological outcomes on DRF were observed in the subgroup of newly diagnosed patients. In the overall population, T25-FW scores and PROs remained stable throughout treatment. Together, these outcomes support that DRF is an effective treatment option for patients with RRMS, including those with newly diagnosed disease.
Limitations associated with the EVOLVE-MS-1 study, inherent to the open-label, single-arm study design, include the lack of blinding and the absence of a comparator arm. Outcomes for relapses were compared with data for relapses in the 12 months prior to the study started that were historically obtained and not confirmed by protocol-defined MS relapse criteria; therefore, these limitations should be considered when reviewing the data, as a formal efficacy evaluation would require a comparator. In addition, the assessment of on-study relapses was more stringent than for those reported before the study; therefore, the true effect on ARR might be smaller than the estimates of ARR as per the protocol. Regression to the mean could have also influenced outcomes, although the inclusion criteria did not require a minimum number of relapses for entry into the study. Discontinuation rates due to GI AEs might have been higher if more patients had been naive to treatment with fumarates, with 464 patients having already completed a 5-week course of DMF or DRF in EVOLVE-MS-2 before enrolling in EVOLVE-MS-1. However, since the study was primarily aimed at assessing the long-term safety and tolerability of DRF in patients with RRMS, these limitations do not lessen the value of the study outcomes.
Conclusion
In the phase 3 EVOLVE-MS-1 study, treatment with DRF was associated with a favorable safety and tolerability profile, as well as favorable clinical and radiological outcomes over the 96-week treatment duration, providing further support that DRF is a valuable option for the treatment of patients with RRMS.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585231205708 for Diroximel fumarate in patients with relapsing–remitting multiple sclerosis: Final safety and efficacy results from the phase 3 EVOLVE-MS-1 study by Barry A Singer, Douglas L Arnold, Jelena Drulovic, Mark S Freedman, Ralf Gold, Mark Gudesblatt, Elzbieta Jasinska, Christopher C LaGanke, Robert T Naismith, Donald Negroski, Jiwon Oh, Miguel Angel Hernandez Perez, Krzysztof Selmaj, Florian Then Bergh, Annette Wundes, Tjalf Ziemssen, Wanda Castro-Borrero, Hailu Chen, Seth Levin, Matthew Scaramozza, Sai L Shankar, Ting Wang and Sibyl Wray in Multiple Sclerosis Journal
Supplemental material, sj-pdf-2-msj-10.1177_13524585231205708 for Diroximel fumarate in patients with relapsing–remitting multiple sclerosis: Final safety and efficacy results from the phase 3 EVOLVE-MS-1 study by Barry A Singer, Douglas L Arnold, Jelena Drulovic, Mark S Freedman, Ralf Gold, Mark Gudesblatt, Elzbieta Jasinska, Christopher C LaGanke, Robert T Naismith, Donald Negroski, Jiwon Oh, Miguel Angel Hernandez Perez, Krzysztof Selmaj, Florian Then Bergh, Annette Wundes, Tjalf Ziemssen, Wanda Castro-Borrero, Hailu Chen, Seth Levin, Matthew Scaramozza, Sai L Shankar, Ting Wang and Sibyl Wray in Multiple Sclerosis Journal
Acknowledgments
The authors wish to acknowledge the contributions of Dr Dragana Obradovic, of the Military Medical Academy, Belgrade, Serbia, who sadly passed away in July 2021. Dr Obradovic was a Professor of Neurology, an MS researcher, and an advocate not only for patients in Serbia but for patients globally. Dr Obradovic was an investigator in the EVOLVE-MS-1 study and had contributed as an author in previous EVOLVE-MS-1 publications.
The authors thank the EVOLVE-MS-1 study patients, investigators, and staff. Biogen provided funding for medical writing support in the development of this manuscript. Katherine Ayling-Rouse, MSc, and David Pertab, PhD, both from Excel Scientific Solutions, wrote the first draft of the manuscript based on input from the authors. The authors had full editorial control of the manuscript and provided their final approval of all content.
Some of these data have been previously presented at the 38th Congress of the European Committee for Treatment & Research in Multiple Sclerosis (ECTRIMS), Amsterdam, Netherlands, 26–28 October 2022.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BAS: research grant support from AbbVie, Biogen, Bristol Myers Squibb, Greenwich Biosciences, Novartis, and Sanofi and consulting and/or speaking fees from Alexion, Biogen, Bristol Myers Squibb, Cigna, Cycle, EMD Serono, Genentech, Horizon, Janssen, Novartis, Octave Bioscience, Roche, Sanofi, and TG Therapeutics.
DLA: consulting fees from Albert Charitable Trust, Alexion Pharma, Biogen, Celgene, Frequency Therapeutics, Genentech, Med-Ex Learning, Merck, Novartis, Population Council, Receptos, Roche, and Sanofi-Aventis; grants from Biogen, Immunotec, and Novartis; and equity interest in NeuroRx.
JD: advisory boards for Amicus, Biogen, Janssen, Medis, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva; speaker bureaus for Biogen, Bayer, Hemofarm, Janssen, Medis, Medtronic, Merck, Novartis, Roche, Sanofi-Genzyme, Teva, and Zentiva; and research grant from Roche.
MSF: research/educational grants from Sanofi-Genzyme; honoraria/consultation fees from Alexion/AstraZeneca, BMS (Celgene), EMD Serono, Hoffman La-Roche, Actelion/Janssen (J&J), Novartis, Quanterix, Sanofi-Genzyme, and Teva Canada Innovation; advisory boards/boards of directors for Alexion/AstraZeneca, Atara Biotherapeutics, Bayer Healthcare, Celestra Health, Hoffman La-Roche, Actelion/Janssen (J&J), EMD Serono/Merck Serono, Novartis, and Sanofi-Genzyme; and participated in speakers bureau for Sanofi-Genzyme and EMD Serono.
RG: research support and speaker’s honoraria from Bayer-Schering, Biogen Idec, BMS, Chugai, Eisai, Genesis, Janssen, Merck Serono, Nikkiso Pharma, Novartis, Roche, Sanofi-Genzyme, Sandoz, and Teva; consulting honoraria from ZLB Behring, Baxter, Roche, and Talecris; and personal stock options in Bayer, Merck, and Roche.
MG: consulting fees from Biogen, EMD Serono, Novartis, and Sanofi-Genzyme; research support from Alkermes; and speaker bureaus for Biogen, EMD Serono, Genentech-Roche, and Sanofi-Genzyme.
EJ: advisory boards for Biogen and speaker fees from Biogen, Novartis, Roche, and Sanofi.
CLG: consultant/advisory boards/speaker bureaus for Biogen, Bristol Myers Squibb, EMD Serono, Genentech, Janssen, Novartis, Sanofi-Genzyme, and TG Therapeutics.
RTN: consultant for Abata Therapeutics, Banner Life Sciences, BeiGene, Biogen, Bristol Myers Squibb, Genentech, Genzyme, GW Therapeutics, Janssen, Horizon Therapeutics, Lundbeck, NervGen, and TG Therapeutics.
DN: research support from and consultant/advisory boards/speaker bureaus for Adamas, Alkermes, Alexion, Bayer, Biogen, Celgene/BMS, EMD Serono, Janssen, Novartis, Roche-Genentech, and Sanofi-Genzyme.
JO: research support from Biogen Idec, EMD Serono, and Roche and personal compensation for consulting/speaking from Biogen Idec, BMS, EMD Serono, Eli Lilly, Roche, Sanofi-Genzyme, and Novartis.
MAHP: consultant/advisory boards/speaker bureaus for Biogen, Bristol Myers Squibb, EMD Serono, Genentech, Janssen, Novartis, Sanofi-Genzyme, and TG Therapeutics.
KS: research support from Merck; advisory boards for Biogen, Celgene/BMS, Merck, Novartis, Roche, Sanofi, and TG Therapeutics.
FTB: research support and travel grants, through his institution, from the German Science Fund (DFG), German Federal Ministry of Education and Science (BMBF), Bayer-Schering, Merck, Novartis, Pfizer, Roche, Sanofi, and Teva and speaker fees from and advisory boards for Actelion, Alexion, Bayer, Biogen, CSL Behring, Fresenius, Horizon, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva.
AW: adviser fees from AbbVie and research support from AbbVie, Alkermes, and Biogen.
TZ: personal compensation for consulting services and speaker honoraria from Bayer, Biogen Idec, Novartis, Sanofi, Synthon, and Teva and financial support for research activities from Bayer, Biogen Idec, Novartis, Sanofi-Aventis, and Teva.
WC-B, HC, SL, MS, SLS, and TW: employees of and hold stock/stock options in Biogen.
SW: consulting fees from and advisory boards for Biogen, Celgene, and EMD Serono; speaker bureaus for Biogen, Celgene, EMD Serono, Roche-Genentech, and Sanofi-Genzyme; and research support from Biogen, Celgene, EMD Serono, Novartis, Receptos, Roche-Genentech, Sanofi-Genzyme, and TG.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The EVOLVE-MS-1 study was sponsored by Biogen (Cambridge, MA, USA).
ORCID iDs: Douglas L Arnold  https://orcid.org/0000-0003-4266-0106
https://orcid.org/0000-0003-4266-0106
Jelena Drulovic  https://orcid.org/0000-0002-4516-3458
https://orcid.org/0000-0002-4516-3458
Elzbieta Jasinska  https://orcid.org/0000-0003-0016-9896
https://orcid.org/0000-0003-0016-9896
Robert T Naismith  https://orcid.org/0000-0003-0520-4283
https://orcid.org/0000-0003-0520-4283
Jiwon Oh  https://orcid.org/0000-0001-5519-6088
https://orcid.org/0000-0001-5519-6088
Tjalf Ziemssen  https://orcid.org/0000-0001-8799-8202
https://orcid.org/0000-0001-8799-8202
Wanda Castro-Borrero  https://orcid.org/0009-0002-9018-4263
https://orcid.org/0009-0002-9018-4263
Data Sharing Statement: EVOLVE-MS-1 was registered with ClinicalTrials.gov (NCT02634307). Study data will be shared in accordance with applicable regulations and laws.
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Barry A Singer, The MS Center for Innovations in Care, Missouri Baptist Medical Center, St Louis, MO, USA.
Douglas L Arnold, Montreal Neurological Institute, McGill University, Montreal, QC, Canada; NeuroRx Research Inc., Montreal, QC, Canada.
Jelena Drulovic, Clinic of Neurology, University of Belgrade, Belgrade, Serbia.
Mark S Freedman, University of Ottawa and Ottawa Hospital Research Institute, Ottawa, ON, Canada.
Ralf Gold, Department of Neurology, Ruhr University Bochum, Bochum, Germany.
Mark Gudesblatt, NYU Langone South Shore Neurologic Associates, Patchogue, NY, USA.
Elzbieta Jasinska, Collegium Medicum UJK, and Clinical Center, RESMEDICA, Kielce, Poland.
Christopher C LaGanke, North Central Neurology Associates, Cullman, AL, USA.
Robert T Naismith, Washington University School of Medicine, St Louis, MO, USA.
Donald Negroski, MS Center of Sarasota, Sarasota, FL, USA.
Jiwon Oh, Division of Neurology, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada.
Miguel Angel Hernandez Perez, Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz de Tenerife, Spain.
Krzysztof Selmaj, Center of Neurology, Lodz, Poland; Department of Neurology, University of Warmia and Mazury, Olsztyn, Poland.
Florian Then Bergh, Department of Neurology, University of Leipzig, Leipzig, Germany.
Annette Wundes, Department of Neurology, University of Washington Medical Center, Seattle, WA, USA.
Tjalf Ziemssen, Center of Clinical Neuroscience, Carl Gustav Carus University Hospital, Dresden, Germany.
Wanda Castro-Borrero, Biogen, Cambridge, MA, USA.
Hailu Chen, Biogen, Cambridge, MA, USA.
Seth Levin, Biogen, Cambridge, MA, USA.
Matthew Scaramozza, Biogen, Cambridge, MA, USA.
Sai L Shankar, Biogen, Cambridge, MA, USA.
Ting Wang, Biogen, Cambridge, MA, USA.
Sibyl Wray, Hope Neurology MS Center, Knoxville, TN, USA.
References
- 1. Vumerity. Summary of product characteristics. Badhoevedorp: Biogen, 2022. [Google Scholar]
- 2. Vumerity. Prescribing information. Cambridge, MA: Biogen, 2023. [Google Scholar]
- 3. Tecfidera. Prescribing information. Cambridge, MA: Biogen;, 2023. [Google Scholar]
- 4. Wehr A. Relative bioavailability of monomethyl fumarate after administration of ALKS 8700 and dimethyl fumarate in healthy subjects. Neurology 2018; 90: P1403. [Google Scholar]
- 5. Berger T, Brochet B, Brambilla L, et al. Effectiveness of delayed-release dimethyl fumarate on patient-reported outcomes and clinical measures in patients with relapsing-remitting multiple sclerosis in a real-world clinical setting: PROTEC. Mult Scler J Exp Transl Clin 2019; 5: 2055217319887191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 7. Gold R, Arnold DL, Bar-Or A, et al. Safety and efficacy of delayed-release dimethyl fumarate in patients with relapsing-remitting multiple sclerosis: 9 years’ follow-up of DEFINE, CONFIRM, and ENDORSE. Ther Adv Neurol Disord 2020; 13: 1756286420915005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gold R, Arnold DL, Bar-Or A, et al. Long-term safety and efficacy of dimethyl fumarate for up to 13 years in patients with relapsing-remitting multiple sclerosis: Final ENDORSE study results. Mult Scler 2022; 28: 801–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 10. Kresa-Reahl K, Repovic P, Robertson D, et al. Effectiveness of delayed-release dimethyl fumarate on clinical and patient-reported outcomes in patients with relapsing multiple sclerosis switching from glatiramer acetate: RESPOND, a prospective observational study. Clin Ther 2018; 40: 2077–2087. [DOI] [PubMed] [Google Scholar]
- 11. Palte MJ, Wehr A, Tawa M, et al. Improving the gastrointestinal tolerability of fumaric acid esters: Early findings on gastrointestinal events with diroximel fumarate in patients with relapsing-remitting multiple sclerosis from the phase 3, open-label EVOLVE-MS-1 study. Adv Ther 2019; 36: 3154–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naismith RT, Wundes A, Ziemssen T, et al. Diroximel fumarate demonstrates an improved gastrointestinal tolerability profile compared with dimethyl fumarate in patients with relapsing-remitting multiple sclerosis: Results from the randomized, double-blind, phase III EVOLVE-MS-2 study. CNS Drugs 2020; 34: 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Naismith RT, Wolinsky JS, Wundes A, et al. Diroximel fumarate (DRF) in patients with relapsing-remitting multiple sclerosis: Interim safety and efficacy results from the phase 3 EVOLVE-MS-1 study. Mult Scler 2020; 26: 1729–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Phillips JT, Selmaj K, Gold R, et al. Clinical significance of gastrointestinal and flushing events in patients with multiple sclerosis treated with delayed-release dimethyl fumarate. Int J MS Care 2015; 17: 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wundes A, Wray S, Gold R, et al. Improved gastrointestinal profile with diroximel fumarate is associated with a positive impact on quality of life compared with dimethyl fumarate: Results from the randomized, double-blind, phase III EVOLVE-MS-2 study. Ther Adv Neurol Disord 2021; 14: 1756286421993999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakamura K, Mokliatchouk O, Arnold DL, et al. Effects of dimethyl fumarate on brain atrophy in relapsing-remitting multiple sclerosis: Pooled analysis phase 3 DEFINE and CONFIRM studies. Front Neurol 2022; 13: 809273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Stefano N, Stromillo ML, Giorgio A, et al. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry 2016; 87: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585231205708 for Diroximel fumarate in patients with relapsing–remitting multiple sclerosis: Final safety and efficacy results from the phase 3 EVOLVE-MS-1 study by Barry A Singer, Douglas L Arnold, Jelena Drulovic, Mark S Freedman, Ralf Gold, Mark Gudesblatt, Elzbieta Jasinska, Christopher C LaGanke, Robert T Naismith, Donald Negroski, Jiwon Oh, Miguel Angel Hernandez Perez, Krzysztof Selmaj, Florian Then Bergh, Annette Wundes, Tjalf Ziemssen, Wanda Castro-Borrero, Hailu Chen, Seth Levin, Matthew Scaramozza, Sai L Shankar, Ting Wang and Sibyl Wray in Multiple Sclerosis Journal
Supplemental material, sj-pdf-2-msj-10.1177_13524585231205708 for Diroximel fumarate in patients with relapsing–remitting multiple sclerosis: Final safety and efficacy results from the phase 3 EVOLVE-MS-1 study by Barry A Singer, Douglas L Arnold, Jelena Drulovic, Mark S Freedman, Ralf Gold, Mark Gudesblatt, Elzbieta Jasinska, Christopher C LaGanke, Robert T Naismith, Donald Negroski, Jiwon Oh, Miguel Angel Hernandez Perez, Krzysztof Selmaj, Florian Then Bergh, Annette Wundes, Tjalf Ziemssen, Wanda Castro-Borrero, Hailu Chen, Seth Levin, Matthew Scaramozza, Sai L Shankar, Ting Wang and Sibyl Wray in Multiple Sclerosis Journal