Abstract
Background
Mosquito research in Europe has a long history, primarily focused on malaria vectors. In recent years, invasive mosquito species like the Asian tiger mosquito (Aedes albopictus) and the spread of arboviruses like dengue virus, chikungunya virus or bluetongue virus have led to an intensification of research and monitoring in Europe. The risk of further dissemination of exotic species and mosquito-borne pathogens is expected to increase with ongoing globalization, human mobility, transport geography, and climate warming. Researchers have conducted various studies to understand the ecology, biology, and effective control strategies of mosquitoes and associated pathogens.
Main body
Three invasive mosquito species are established in Europe: Asian tiger mosquito (Aedes albopictus), Japanese bush mosquito (Ae. japonicus), and Korean bush mosquito (Aedes koreicus). Ae. albopictus is the most invasive species and has been established in Europe since 1990. Over the past two decades, there has been an increasing number of outbreaks of infections by mosquito-borne viruses in particular chikungunya virus, dengue virus or Zika virus in Europe primary driven by Ae. albopictus. At the same time, climate change with rising temperatures results in increasing threat of invasive mosquito-borne viruses, in particular Usutu virus and West Nile virus transmitted by native Culex mosquito species. Effective mosquito control programs require a high level of community participation, going along with comprehensive information campaigns, to ensure source reduction and successful control. Control strategies for container breeding mosquitoes like Ae. albopictus or Culex species involve community participation, door-to-door control activities in private areas. Further measures can involve integration of sterile insect techniques, applying indigenous copepods, Wolbachia sp. bacteria, or genetically modified mosquitoes, which is very unlike to be practiced as standard method in the near future.
Conclusions
Climate change and globalization resulting in the increased establishment of invasive mosquitoes in particular of the Asian tiger mosquito Ae. albopictus in Europe within the last 30 years and increasing outbreaks of infections by mosquito-borne viruses warrants intensification of research and monitoring. Further, effective future mosquito control programs require increase in intense community and private participation, applying physical, chemical, biological, and genetical control activities.
Keywords: Invasive mosquito, Spread, Outbreak, Mosquito-borne virus, Asian tiger mosquito, Arbovirus, Control strategy, Globalization Europe
Background
Mosquitoes as hematophagous ectoparasites play an important role as pest species and vectors of pathogens [1]. Therefore, research on mosquitoes has a long history worldwide. This is especially true for tropical regions with a high burden of vector-borne diseases. Malaria parasites and the dengue virus (DENV) are the most important pathogens at the global scale with a total of more than 500,000 human deaths per year [2]. Europe also has a long history of research on the ecology of mosquitoes and their associated pathogens [3]. However, most research until the twenty-first century was driven by the interest in the ecology and spatial–temporal distribution of the malaria vectors as several countries had ongoing circulation of different Plasmodium species.
Intensive studies were conducted at the beginning of the twentieth century especially in European areas with a burden of malaria cases. However, the disease disappeared by vector control, drainage measurements, drugs and general improvement of the hygienic status. Thus, the interest in mosquitoes as an object of research significantly had declined in most European countries [4].
Over the last decades research on mosquitoes in Europe and systematic mosquito control programs were predominantly restricted to areas with a high burden of mosquito plagues. This situation changed by two important events: the spread of invasive mosquito species since 1990 especially of the Asian tiger mosquito Aedes albopictus and the emergence of vector-borne pathogens, e.g. the arthropod-borne (arbo) viruses chikungunya virus (CHIKV), transmitted by the Ae. albopictus and of bluetongue virus, transmitted by biting midges (Culicoides spp.) [5]. Before the outbreaks of bluetongue virus in Central Europe the risk for the emergence of arboviruses north of the Alps was very low. Especially because the main vectors for arboviruses were regarded to be missing in Germany, e.g., Culicoides imicola for bluetongue virus or Ae. albopictus for CHIKV. However, the outbreak of bluetongue virus in North-West Europe in 2006 [6] and later followed by Schmallenberg virus in 2011 [7] demonstrated the potential of arbovirus transmission in Central Europe by native hematophagous insects. At the same time the first eggs of the Asian tiger mosquito were detected at a highway station in South-West Germany [8]. The mosquito species is known to be a highly invasive species with a high vector capacity for several important pathogens, as DENV or CHIKV. Both, the bluetongue virus outbreaks and detection of Ae. albopictus eggs, demonstrated the huge lack of knowledge regarding native and potentially invasive arthropod vector species in Central Europe including the lack of entomological trained professionals.
The outbreak of bluetongue virus and detection of exotic mosquito species resulted in the intensification of the research on mosquito-borne diseases North of the Alps, collecting baseline information on the ecology of mosquitoes and spatial–temporal distribution of vectors and associated pathogens. Intensified monitoring led to the detection of the mentioned exotic mosquitoes and the ongoing circulation of several arboviruses and parasites like Dirofilaria [9–12]. The risk for the further spread of exotic species and ongoing circulation of mosquito-borne pathogens have been expected in face of ongoing globalization and climate warming.
Current status of invasive mosquito species in Europe
Different exotic mosquito species are regularly introduced to Europe [13]. However, only three different exotic mosquito species are established in Europe: the Asian tiger mosquito (Ae. albopictus), the Japanese bush mosquito (Ae. japonicus) and the Korean bush mosquito (Ae. koreicus) [14]. The high invasive capacity of these Aedes species is driven by several ecological traits, but most importantly drought-resistant eggs and the ability to lay hibernating eggs. Thereby, the dispersal of the exotic mosquito species on different scales, i.e., continental, national, regional, is driven by different modes of human mobility dynamics. Spread between continents is predominantly the result of the transport of eggs attached to different goods with plants (e.g. lucky bamboo) and tires by freights on ships with the highest relevance [15]. Aircrafts play a relevant role in the spread [13]. On the national and regional scale, however, transport via cars and trains are the most important modes of dispersal [8, 14]. Due to the relatively small flight range of invasive species [16], active dispersal of the mosquito species probably only plays an underneath role on the local scale.
Aedes albopictus is one of the most invasive species in the world. Originating from the Asian region, the species spread all over the world. In Europe, the species was first established in Italy in 1990 and since then spreading widely around the Mediterranean Sea and towards Central Europe [5, 14, 17]. First detected north of the Alps in Germany in 2007 [5], subsequent monitoring activities confirmed regular introductions via human-mediated transport of adult mosquitoes along the highways and several established populations especially in southern Germany. At least since 2016 the species must be considered established with overwintering populations north of the Alps [18] with the recently established most northern population for Europe in Berlin, Germany (personal observation).
The second exotic species is Ae. japonicus. The species is considered established in Belgium since 2002 and is especially widely distributed in Central Europe [19, 20]. Populations of the Japanese bush mosquito have already been detected in more than 10 countries [21]. In 2008, the first invasive spread of this species in Europe was reported in Switzerland. Since then, it has even become there a dominant mosquito species. In order to prevent further spread within the country, the Netherlands and Belgium conducted systematic control measurements [22–24]. Shortly after its introduction in Switzerland, populations were established at several sites in southern Germany [23]. Since the first detection, the species spread over wide parts of Germany [14, 21] from South-West Germany up to the coast (personal observation).
The third exotic species is the Korean bush mosquito (Ae. koreicus). The species is established with relatively small populations in several countries in Europe with a main focus in Central Europe, e.g. Italy, Belgium, Slovenia, Hungary or the Netherlands [25–28]. In Germany there is currently one stable, small population known in Wiesbaden, southern Germany [29].
Mosquito-borne viruses in Europe
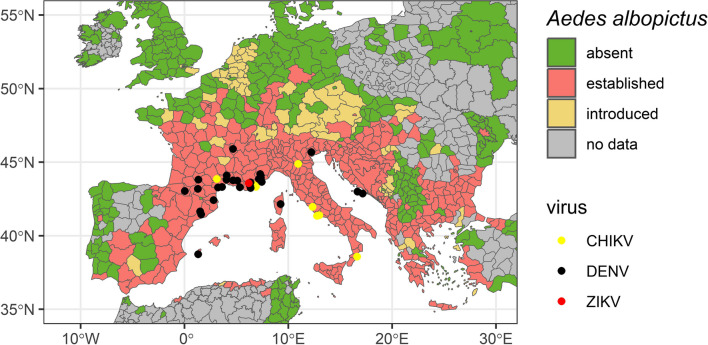
Over the last two decades of vector invasion, we observed increasing numbers of outbreaks of mosquito-borne viruses in Europe. Thereby we can distinguish two different drivers. Firstly, the establishment of Ae. albopictus in wide parts of Southern Europe allow the circulation of exotic viruses like DENV [24, 25], Zika virus (ZIKV) [26] or chikungunya virus (CHIKV) (Fig. 1). This process must be separated from the increasing activity of mosquito-borne viruses transmitted by our native Culex mosquito species, especially Usutu virus (USUV) and West Nile virus (WNV). It is highly likely that the circulation of DENV, ZIKV and CHIKV only became possible due to the establishment of Ae. albopictus. Native mosquito taxa probably only have low or no vector capacity for these viruses. In contrast, USUV and WNV are transmitted by our native Culex species. The three exotic mosquito species Ae. albopictus, Ae. japonicus and Ae. koreicus probably are no good vectors for both viruses [30–32].
Fig. 1.
Invasion status for Aedes albopictus in Europe and local outbreaks of chikungunya virus (CHIKV), dengue virus (DENV) and Zika virus (ZIKV) (data source: https://www.ecdc.europa.eu/en)
The global spread of Ae. albopictus is a significant public health concern due to its aggressive daytime-biting behaviour and its ability to feed on both humans and animals, making it a potential bridge vector for zoonotic pathogens [33, 34] (Fig. 1). This mosquito species is a known vector of various emerging arboviruses, such as DENV, CHIKV, and ZIKV [33, 35, 36]. The spread of Ae. albopictus in Europe has led to the repeated occurrence of autochthonous CHIKV and DENV infections. For instance, in 2007, a CHIKV epidemic occurred in Italy due to a traveller from India, resulting in over two hundred clinical cases [37]. Since then, small-scale circulation has been observed in France in 2010, 2014, and 2017, and a major CHIKV outbreak occurred in Italy in 2017 [38–40]. Additionally, several autochthonous DENV infections have been reported in recent years in France and Croatia [41–45]. Furthermore, the first observation of autochthonous ZIKV infections in France was reported [46, 47]. Laboratory studies using Ae. albopictus populations from Germany and Italy have demonstrated their vector competence for ZIKV and CHIKV [48–51].
The Asian bush mosquito (Ae. japonicus) is widespread in Central Europe and can have very high population densities. The species is well adapted to temperature climatic conditions with a long seasonal activity also at lower temperatures compared to Ae. albopictus [52]. The species probably has a broad host-feeding patterns with a preference for humans, but also non-human mammals or birds [53, 54]. This makes the species a potential vector of zoonotic arboviruses. Vector competence studies proved Ae. japonicus as a potential vector for different viruses, including the Japanese Encephalitis virus, WNV, and Saint Louis encephalitis virus [30, 55, 56], but potentially also for the La Crosse virus and CHIKV [57, 58].
The closely related mosquito species Ae. koreicus is considered to have a similar ecology as Ae. japonicus. Experimental studies prove a vector competence for CHIKV, ZIKV and also the filarial parasite Dirofilaria immitis [32, 59, 60]. However, the species relevance as a vector in the field is unknown but is considered to play a role as a vector of the Japanese encephalitis virus in its area of origin [61].
In summary, vector competence studies with European populations of Ae. japonicus and Ae. koreicus have demonstrated that the species probably only have a very low vector competence for currently circulating pathogens in Europe or only at high temperatures [30–32]. The populations Ae. albopictus are probably too small, spatially restricted and the temperatures are still too low to allow transmission of exotic viruses north of the Alps.
In contrast to the future risk of arbovirus transmission by invasive mosquito species, there is already ongoing circulation of several arboviruses through native species. Three viruses can be highlighted here: Sindbis virus, USUV and WNV. Large outbreaks with many human cases are only observed in northern Europe [62]. In contrast, USUV and WNV circulate in South, South-Eastern and Central Europe [63, 64]. All three pathogens have similar transmission cycles including an enzootic cycle between Culex mosquitoes and birds.
Since the start of intensified monitoring of mosquito-borne pathogens in 2009, different arboviruses and Dirofilaria spp. parasites have been detected in native mosquito species in Germany. Sindbis virus is regularly detected in mosquitoes and birds [65–68]. Several mosquito species in Germany have a very high vector competence for Sindbis virus [69]. However, compared to Finland or Sweden for unknown reasons huge outbreaks with clinically sick humans are not observed. The Batai virus is regularly observed in vertebrates in Germany with high seroprevalence in sheep, goats, and cattle, but clinical signs of Batai virus infections are rarely detected [70, 71]. The same applies to Dirofilaria repens and D. immitis, which probably also circulates continuously in Germany [9–11]. However, so far only few autochthonous human cases were detected in eastern Germany [9].
USUV and WNV show a continuous, yearly circulation after the respective first detection in 2018 [63, 64]. Both viruses share a similar transmission cycle with birds and amplification hosts and different Culex taxa as main vector species. The viruses can overwinter in the female adult mosquitoes [72, personal observation], which probably is the main factor allowing long-term local establishment although not every year outbreaks of the virus are observed. Both viruses are pathogenic for humans. However, USUV only rarely causes severe disease [73], while WNV is a serious risk for humans and equines [63].
USUV was first virus discovered in Europe in 1996, after causing deaths among Eurasian blackbirds (Turdus merula) in Italy [74]. Since then, it has spread to other countries, including Austria, Belgium, Czech Republic, France, Germany, Hungary, Spain, Switzerland, and the Netherlands [75–79]. The virus has caused outbreaks among wild and/or captive birds, often resulting in a significant die-off of blackbirds and captive great grey owls (Strix nebulosa) [76]. The emergence of USUV has posed a significant challenge for wildlife management authorities in affected regions. As a result, there has been a growing effort to monitor and study the virus, with the aim of developing effective prevention and control strategies. USUV was first detected in a mosquito pool in 2010 in Southwest Germany [80]. Only one year later, the first bird die-off was observed in 2011/12 [78]. Over the following years, USUV spread over large areas of Germany causing massive die-offs of birds and since 2018 have to be considered to spread all over Germany [64, 77, 81, 82]. The virus is known to result in significant decline of the population in European blackbirds [81]. USUV regularly infects humans, but the infections probably do not cause significant health risk for humans except for immunosuppressed patients [73].
WNV is a widely distributed mosquito-borne virus in Europe, with particular focus on South-Eastern Europe and Italy [46]. However, low levels of WNV activity have also been reported in neighbouring countries such as Germany, France, Austria, and the Czech Republic. In light of this, numerous monitoring programs have been implemented in Germany over the past ten years to screen for WNV RNA and antibodies against WNV in birds, horses, mosquitoes, and chicken eggs [67, 82–85]. The first epizootic emergence of WNV was only observed in Germany in 2018 [86]. The virus shows significant circulation especially in Central-Eastern Germany. Since 2018, yearly circulation is observed with infections in birds and horses but also infection of humans occurred every year and one fatal case was reported in 2020 [63]. WNV is also transmitted by our native mosquito species [87]. We know that different Culex species are important vectors, while Cx. torrentium must be considered as one of the most important vector species with very high transmission rates [88].
Increasing temperatures in the course of climate warming will significantly change the risk of transmission for vector-borne pathogens in Europe [89]. Increasing temperatures will result in a further spread of the thermophilic Asian tiger mosquito [90]. Over the last years we have seen a tremendous increase in the detection of different mosquito populations at different sites north of the Alps [18]. The highest risk for the future must be expected for outbreaks with CHIKV. The virus can also be transmitted at relatively low temperatures, thus, the spatial risk in Europe currently is probably predominantly restricted by the further spread and increasing population size of Ae. albopictus [50]. However, local circulation of other exotic pathogens like DENV or ZIKV, which are already observed in Mediterranean areas can be expected in the course of the spread of the Ae. albopictus in combination with increasing temperatures.
Nevertheless, the prediction of the impact of climate warming on these pathogens is a complex issue that requires more research. We know that the risk of transmission is strongly affected by the extrinsic incubation period (EIP), which represents the time period between the uptake of a pathogen through a vector and the re-transmission after development and replication in the vector. For most pathogens, the EIP is directly temperature-dependent, with increase of the temperatures the risk of transmission also increases. This has been experimentally demonstrated for the most important pathogens circulating in Europe, such as ZIKV [49] or WNV [88]. There are, however, exceptions to this rule, such as CHIKV, which does not seem to show a clear correlation between EIP and temperature [30].
The unambiguous temperature dependence of the transmission of WNV by Culex mosquitoes connotes that warmer temperatures are required for approximately two weeks with significant temperatures of over 20 ℃ [88]. This explains the main distribution of WNV in South-Eastern Europe and the first observed larger outbreak of West Nile fever in Germany during the heat summer in 2018 [63]. This definitive temperature dependence is also observed for the small outbreaks of ZIKV and DENV in the Mediterranean region [41–47]. Thus, both viruses require relative high temperature for successful transmission [49]. However, while we have a good understanding of the impact of climate warming on the development of pathogens in mosquitoes [31, 49, 88] and the ecology of exotic mosquito species like Ae. albopictus [90], there is still a great lack of knowledge regarding the impact of climate warming on native mosquito species. This is due in part to the still low research focus on these species, resulting in a lack of systematic time series data. Further research is needed to better understand the impact of climate warming on these species and the potential consequences for public health.
Control of mosquitoes with special regard to invasive mosquitoes
With the disappearance of malaria on the European continent in the 1950s the country-wide activities were strongly reduced. Laws which regulated mosquito control on a state level were based on medical aspects only. These laws were cancelled in many states as Germany since mosquitoes were no longer considered as a medical threat to humans.
The reasons for the decline of malaria in Europe are multifold:
First, the appearance of quinine—extracted from the bark of the cinchona tree—as a treatment for malaria was introduced by Pierre Pelletier in 1820 and the consistent treatment of malaria patients led to a continual reduction in the numbers of infected people. This in turn led to a drastic decrease in the probability of a mosquito taking up the parasite as Plasmodium spp. through human blood meals and becomes infected.
Ground water levels have steadily become lower through the canalization of rivers reducing the development of a large mosquito population. A reduction in malaria infection was achieved in Europe through hydraulic engineering measures before the developmental cycle of the disease-causing agent was known.
Lifestyle changes in humans have resulted in reduced contact with Anopheles mosquitoes. For example, stables and living quarters, once together in one complex, are now separate. The female An. maculipennis s.l. are mainly zoophilic, meaning they prefer large mammals as hosts for their blood meals, such as cows and horses, thereby acting as vectors.
Central Europe was climatically borderline for malaria parasites. This is one basic reason why eradication was possible. Today, all the malaria vector-competent mosquitoes are still present in Germany, however, indigenous malaria has disappeared in Central Europe, excluding single conjectural cases. This is also the case in Southern Europe, even with its more favourable climate [89]. Nevertheless, in recent years again autochthonous cases are reported from Southern Europe [46].
The high living and medical standards in Europe will avoid an epidemic re-emergence of malaria. New cases as they occur regularly in Southern Europe will be immediately treated and thus the Plasmodium-mosquito contact will be eliminated [89].
In general, the biology of the target mosquito, its importance as nuisance and vector species decides which control strategy must be chosen. In Europe we can differ in general between.
the control of mosquitoes in wetlands which hatch in masses after flooding and have a great ability for dispersal after emerging such as floodwater mosquitoes (Ae. vexans, Ae. sticticus or Ae. caspius).
the control of container-breeding mosquitoes and human-made habitats in urban and suburban areas. These species usually have a limited flight range like Ae. albopictus, Cx. pipiens or An. plumbeus.
As a principle, it is advisable to control immature stages because they are as populations usually defined and concentrated in their breeding sites, whereas adults spread from their breeding grounds several hundred meters (e.g., mosquitoes breeding in urban areas like Cx. pipiens or Ae. albopictus) up to several kilometres like the floodwater mosquitoes Aedes vexans or Ae. sticticus [16, 91]. However, when transmission of pathogens takes place, adults as a source for infections have to be controlled at least 100 m around the infection sites in the urban areas. The establishment of new populations of Ae. albopictus is mainly based on the introduction of a sufficient number of viable eggs or gravid females as founders of new populations.
In general, the Integrated Mosquito Management represents the strategic approach for the control of mosquitoes and should be implemented by specialists [92–94]. It should be adopted according to the local conditions as well as to the national/regional regulations and should comprise all appropriate available surveillance and control tools [95]. It comprises usually the following elements:
Physical control: (i) environmental management (e.g., breeding site reduction) and sanitation (modification of breeding sites e.g., by covering of the containers); (ii) surface layers to chemical and biological obstruct pupal and late larval instars; (iii) reduction of human vector contact e.g., by mosquito windows or bed nets.
Chemical control: spraying (i) adulticide mainly based on pyrethroids and (ii) larvicides mainly based on biorational chemicals, i.e., with few environmental side effects, like insect growth regulators.
Biological control: (i) Beside fish and invertebrates (e.g., copepods), microbial control agents, e.g., products based on Bacillus thuringiensis israelensis (Bti) and Lysinibacillus sphaericus are the main biological control tools applied in Europe. (ii) additional products based on Saccharopolyspora spinosa or fungi e.g., Metarhizium anisopliae and Beauveria bassiana as well as the bacterial endosymbiont Wolbachia spp. can be used after authority acceptance and registration.
Genetical control: (i) e.g., the irradiation-based sterile insect techniques (SIT) against tiger mosquito are already successfully used and (ii) genetic engineering can play an important role in future.
The design of the mosquito control strategy has to consider the biology of mosquitoes and their impact on humans. Therefore, e.g., the control strategy for floodwater mosquitoes has to be different from the strategy against urban mosquitoes. With the foundation of the European Mosquito Control Association in 2000 a forum was created for intensive exchange of knowledge in the field of sound mosquito control to solve mosquito problems in Europe.
Monitoring of the target populations
All control activities should be guided by a well-organized monitoring program to assess the occurrence, abundance and dispersal of the target populations. The monitoring should be implemented by entomologists and should comprise the following techniques: (a) inspections of the breeding sites including larval sampling; (b) employing of ovitraps and adult traps such as Biogents Gravid-Aedes Traps or Biogent Sentinel traps; (c) human-landing-collections [1, 93].
Breeding sites can be inspected for mosquito developing stages either by employing a plankton net in larger water containers or pouring the water through a plankton net when small breeding sites are examined [93]. In larger water containers like rainwater barrels the net with a handle should be drawn through the water in a figure of “8” pattern to sample the larvae. By aid of a touch the mosquito developing stages can also recognized in the water column. Nonetheless, in areas with a minor infestation it is difficult to find larvae, different to areas with a dense population where larvae can be easily detected. For transportation to the laboratory, a plastic container with a close-fitting cap filled with 3/4 of water from the site should be used which is carefully marked with the date and location of sampling for determination of the container indices. Third and fourth instar larvae can be identified to species by using appropriate keys [1]. Earlier larval instars should be reared to the 4th instar or even to the adult stage in a mosquito breeder for precise species determination.
Usually ovitraps are the main tool to monitor the presence, phenology and abundance of Ae. albopictus and to assess the effect of the control activities (quality control) as well as to estimate the population density based on the number of deposited eggs [96, 97]. The ovitrap usually consist of a dark plastic container with a total volume of about 1.5 L. They are usually filled up to an overflow hole (2/3 of the pot volume) with tap water or hay infusion. A wooden board is added to support oviposition.
In order to prevent the potential development of larvae to imagines a larvicide e.g. granule or tablet formulations based on Bti like Vectobac G (activity: 200 international toxic units/mg, Valent BioSciences, Libertyville, USA or Culinex Tab plus, Culinex, Germany) has to be added to the water [92].
Preferable the ovitraps are positioned on shaded places on the ground or hang in a height of max. 1.5 m at places which cannot be easily assessed by animals. The first positioning should be done by a skilled entomologist and the exact position should be registered in an appropriate database with geographical coordinates (e.g., Open Source Geographic Information System) and description of the sampling site for easy assessment. Each ovitrap should have a unique code on the black plastic container and should be always employed on a fixed place during the season.
The density of ovitraps has to be adjusted to the goal of the study. Usually one ovitrap is positioned per two hectares in the infested and in the surrounding areas to determine the spread of the species. The number of ovitraps can also be calculated using the Taylor equation [93].
The wooden boards are usually replaced at a bi-weekly (sometimes weekly or three weekly) interval and the water in the ovitraps has to be refilled. Before refilling the inner walls of the plastic container must be thoroughly cleaned with water and a soft sponge to remove eggs which could be attached to the inner wall of the pot [93]. The collected wooden boards have to be clearly marked at the dry end with a permanent marker to refer to the place of the ovitrap and date of collection. They should be wrapped with paper foil and stored in a plastic bag at room temperature until they are checked by means of a binocular for the presence of eggs.
A skilled person is able to distinguish between eggs of the indigenous species e.g., Ae. geniculatus and the exotic species Ae. albopictus, Ae. koreicus and Ae. japonicus. The results should be validated by hatching some of the eggs and rearing the larvae to the fourth instar for morphological determination or by validation using PCR or MALDI-TOF mass spectrometry [1].
In areas where the sterile insect technique (see below pillar 3) is practiced the sterility of the eggs on wooden boards can be checked by bleaching the exochorion of the eggs by a 10% hydrogen peroxide solution for 48 h and/or disrupting the egg-shell by means of a fine needle to prove existing embryos or unsegmented whitish egg masses.
For quick assessment of the presence of invasive day-time biting Aedes species a person can also expose either the whole body or only a part of the body (leg or arm) for a certain time period (some minutes) to collect the approaching mosquitoes by means of an aspirator from the clothes or skin [93]. The species can be determined in the laboratory and the number of biting females per time period can be assessed.
Control operations
Many control programs employing integrated vector management are based on several pillars, for example pillar (1) community participation; pillar (2) door-to-door activities [92] including the treatment of all breeding sites by trained people with larvicides e.g. Bti at appropriate intervals according to the long-lasting effect of the larvicide or a monolayer like aquataine [98]; pillar (3) the integration of the sterile insect technique to possible wipe out remaining Ae. albopictus populations. The sterile males are “helpers on wings” by mating with females deriving from cryptic and/or non-accessible breeding sites [92, 93, 95]. The fourth pillar can also include the use of indigenous copepods, Wolbachia sp. or genetically modified organisms like OX5034 Ae. aegypti females.
In the following the four main pillars of the control strategy against Ae. albopictus are described, which mostly also apply to the control of other container-breeding mosquitoes including Cx. pipiens. In addition to these four pillars, further measures are described as “Wolbachia—a potential biocontrol agent” and “Epidemiological and resistance risk assessment” are described.
Pillar I—community participation
Urban areas provide a wide range of water bodies, ranging from flower vases at cemeteries, water barrels, buckets, cans, saucers, water catch basins, bird baths and many more artificial and natural water bodies like tree holes. Mosquito control is therefore particularly successful when people are involved in the frame of community participation [99]. Community participation means that the people are becoming “actors” instead of being “spectators”. It focuses on the increase in public awareness to prevent mosquito breeding as well as to record and report the occurrence of Ae. albopictus in the frame of “passive monitoring” as an “early warning system” for the occurrence of invasive species. The programme must enable people to contribute to the solution of their mosquito problem related to their own settlement. “Help through self-help” can be achieved by a comprehensive information campaign. It includes detailed information provided to the public via distribution of leaflets/flyers, press releases, television airtime, web pages and information events, e.g. at schools, in city halls or meetings of gardener associations [93]. Through these activities usually detailed information is given on the characteristics, distribution, and the biology of the Asian tiger mosquito. In addition, easy applying measures should be simple communicated to prevent the proliferation of the mosquito. This includes the elimination of breeding sites, environmental sanitation, e.g., depositing breeding sites like buckets up-site-down that rainwater cannot be collected or the use of totally fitting lids to water containers. Additionally, in heavily infested areas, mosquito nets can be distributed to thoroughly cover water containers as mass breeding sites preventing access for female mosquitoes. Before covering the water barrels larvicides such as Bti tablets must be applied to kill the existing larval populations. Thus “help through self-help” facilitates source reduction which has a permanent effect and is highly advantageous from the cost–benefit view. Breeding sites which cannot be removed or sanitated must be treated with larvicides. It is important to keep citizen’s level of motivation high over a long period and to achieve at least 95% of the public. Unfortunately, experiences document that a certain percentage of inhabitants is ignorant and thus limit the success of community participation [92, 93]. Another positive aspect of community participation is that people report suspected mosquitoes in the frame of passive monitoring to the institution/agency/company responsible for the control and thus, immediate surveillance can be organized to prevent further proliferation of the mosquitoes.
Pillar II—door-to-door control and larviciding in private areas
Due to the lack of professional know-how and the reservation of some people, community participation alone is usually not enough to reach the goal of strong reduction or even elimination of the Asian tiger mosquito populations [92, 100]. Therefore, the implementation of door-to-door activities by trained staff along with the application of long-lasting larvicides e.g. Bti treatments in high dosages is highly recommended. Door-to-door is the most powerful tool when control is performed in all properties in regular intervals during the mosquito breeding season (minimum access threshold should be more than 95% of the properties). The staff should be easily identified by letters from the authorities and wearing unique uniforms to support the accessibility of the properties and to destroy concerns of the public. Prior to the actions the public must be informed via the local press that trained staff will visit their properties in the infested area and treat all potential breeding sites with environmentally friendly larvicides which are effective for at least two to three weeks. The necessary time intervals for re-treatments should be assessed in field studies according to the environmental and climate conditions. Door-to-door actions can also be used to distribute flyers to the citizens with thorough information for breeding site elimination and modification. The flyers should contain also contact addresses, telephone numbers, and refer to website pages where people can get additional information and assistance. Additional to flyers mosquito nets for covering mass breeding sites or larvicides for self-help like Bti tablets can be distributed that people are able to treat breeding sites e.g., water drums or water catch basins on their private premises. The staff should record all activities including information on the time of treatment, number of permanent breeding sites on a hand-hold computer (mobiles) equipped with a geographic information program. The data base can be programmed that the colour of the treated premises in the display turns to green and change the colour over time till it turns to red (mainly after four weeks). The operator is than aware that he has to re-visit these properties in a certain time. The absence of the owner should also be indicated. It is advisable that during the first round of door-to-door activities all permanent breeding sites/property are mapped as well as such properties which doesn’t contain any breeding sites. The data should be stored in a data base which allow a straightforward planning and targeted control of the properties. Heat maps can be organized which indicate the critical properties and the results of the ovitrap monitoring can also be used to identify critical areas which have to considered for more frequent treatments. The above-mentioned procedure is the optimum way to control Ae. albopictus in the private sectors, however, it has been shown that these activities are costly and communities can hardly carry the costs for a long time [97]. Therefore, an alternative is the search for an increased community cooperation by trained inhabitants who are responsible as e.g. “Tiger-mosquito inspectors” for the control in their districts in question. This requires intensive training of the public helpers and guidance by experts.
In the public area all control methods employed in the private sector can be applied but special attention should be taken to water catch basins because they are usually very productive for container-breeding mosquitoes. If sanitation is not possible they have to be treated with larvicides like formulations based on Bti. Frequently granule formulations are based on Bti or combined products of Bti and Lysinibacillus sphaericus like VectoMax G. At higher dosages these formulations can also provide a killing effect for at least three weeks.
Beside microbial control agents, insect growth regulators like pyriproxyfen and diflubenzuron as chemicals which cause physiological alterations during the development of insects can be used due to their low acute mammalian toxicology and relative safety to non-target organisms [101]. Insect growth regulators are available as liquid, granular and tablet formulations and registered products are suited to be used in water catch basins. Because of the enormous productivity the treatment of the water catch basins has to carefully conducted in regular intervals by trained staff and checked in the frame of the quality control of the operations [93].
Pillar III—the sterile insect technique
The final goal of an integrated control program is a significant reduction or ideally the eradication of Ae. albopictus populations. In some areas this goal is hardly to achieve where breeding sites are out of reach of community participation and door-to-door control. This applies especially to property owners refusing access as well as to areas with many cryptic breeding sites. In these areas sterile insect technique can be added as the third pillar using e.g., gamma-irradiated sterilizied males. Aedes albopictus is ideal for employing sterile insect technique, as the Asian tiger mosquito can easily be mass-reared, has a limited flight range, does not reproduce in enormous numbers within a very short time as the floodwater mosquitoes does, and the breeding sites are well-defined [102]. Preceding the release of sterile males, the natural Ae. albopictus population has to be strongly reduced by community participation and door-to-door control [92]. The mass-rearing should be conducted with eggs deriving from the native Aedes population of an area where the sterile males will be released to avoid the distribution of another genotype [103–105]. Based on the sexual dimorphism the smaller male pupae can be sieved or sorted out from the female pupae by Fay Morlan glass sorters [106, 107]. Precise sexing is important that the male pupae are not contaminated with female pupae more than an acceptable level of less than 1%. Following the sexing the sorted pupae are sterilized by gamma-radiation at a rate of 1.9 Gy/min for 19 min. resulting in a dosage of 35 Gy which damage the reproductive cell-lines but have no harmful or a limited negative effect to somatic cells to sustain the viability and fitness of the males for flying and mating [108]. The sterile males have to outcompete their wild counterparts resulting in a large majority of wild females laying sterile eggs [108]. In practice the release of about 2000 sterile males/ha on a weekly basis can result in an egg-sterility of more than 80% compared to a natural sterility of less than 5% [92]. The routine release in bi-weekly intervals can result in a reduced sterility of about 60%.
The employment of ovitraps and the final assessment of the sterility by e.g. bleaching the eggs with 10% hydrogen peroxide for 24 up to 48 h allows to assess the effect of the sterile insect technique [92]. The transparency of the egg shell makes it possible to recognize the embryonic structures such as the dark hatching teeth and eyes of the embryos [92] and thus prove the development or non-development of embryos. Taking the operational perspective, over the last ten years the sterile insect technique was tested in different field trials in Montenegro, Germany, Albania, Greece and France [109]. In a recent highly efficient prospective study of a large-scale deployment of the sterile insect technology in Brazil, a > 98% suppression of Ae. aegypti live progeny and a 97% reduced incidence of DENV was shown [110]. Thereby, the general effectiveness of the sterile insect technique to reduce the population size of Ae. albopictus have be demonstrated. However, there are especially two challenges preventing the usage in routine monitoring. Firstly, regulatory pathways for the release of sterilized mosquitoes are unclear and differ between the countries, and secondly there is a lack of regional factories to produce such mosquitoes.
Pillar IV—the use of copepods
The application of larvicides results usually only in a sufficient killing effect for a limited number of weeks and therefore a continuous and repeated application of larvicides is required during the mosquito breeding season which is laborious and cost-intensive [92]. Therefore, the search for alternative strategies providing a sustainable long-term control has high priority. Examples of sustainable control is the elimination or sanitation of breeding sites or the use of mosquito nets to cover mass breeding sites like water drums to avoid egg-laying mosquito females. The disadvantage of the majority of larvicides providing only a limited effect can potentially be compensated by the simultaneous inoculation of natural predators to the breeding sites, to feed upon newly hatched larvae as the impact of larvicides ceases. These predators should therefore maintain stable populations within bodies of water, creating a sustainable, long-term vector control [1, 111, 112]. In this context, copepods are considered to be the most efficient invertebrate predators of mosquito larvae and are a promising tool in the control of container-breeding mosquitoes [111]. The use of copepods against Aedes mosquitoes was primarily described 1981 by Riviere and Thirel [113]. Most effective are copepods of the largest cyclopoid genera such as Mega- or Macrocyclops (Cyclopoida: Cyclopidae) which show a positive correlation between their body size and predatory efficiency [114]. These copepods mainly prey on first instar larvae, and to a lesser extent on second instar larvae, as the further developed larval stages exceed the maximum size of potential copepod prey [111, 115]. Furthermore, they show significant differences in their preferences towards different mosquito species [116, 117]. In general, copepod species proved to prey more efficiently upon Aedes than Culex larvae, indicating their varying prey preferences [111, 118, 119]. It is mandatory that only indigenous copepod species should be considered, since they do not pose any threat towards the local ecosystem and fauna [118, 120].
Promising results using copepods as predators of Aedes were achieved in the United States, Asia and South America [119]. However, only a few studies address the use of copepods against Aedes species in the UK [114] and Italy [120], while German native copepod species have only been evaluated for their efficacy against the invasive mosquito Ae. japonicus in 2019 [121]. In recent laboratory and semi-field test the results reveal a high predation efficiency of Megacyclops viridis against first instars of Ae. albopictus resulting in a reduction rate of 92.0 ± 12.6% [111]. The copepods did not prey upon stages further developed than the first instars and, in comparison to Ae. albopictus, the predation rates on the larvae of Cx. pipiens s.l. were significantly lower. The integration of copepods as a promising biocontrol agent to the vector control strategy is therefore highly recommended, especially because of the excellent compatibility of copepods with the use of larvicides like Bti. However, the mass rearing of suitable copepods has to be guaranteed. Recently, the copepods as predators of mosquito larvae have been combined with a newly explored mosquitocidal technique applying functionalized nanoparticles representing an emerging tool against virus-transmitting mosquitoes [122–125]. Biologically synthesized toxic silver nanoparticles induce reactive oxygen radicals in the mosquito which target the DNA metabolism and mitochondrial activity of the mosquito larvae. The silver nanoparticles are generated by the endolichenic fungus Talaromyces funiculosus. The biomolecule-based nanoparticles in combination with the predatory copepod Mesocyclops aspericornis are highly mosquitocidal [123]. Thus, fungal bio-insecticides together with bio-predation by copepods represent a promising green pathway for effective mosquito management processes in future.
Wolbachia—a potential biocontrol agent
The Gram-negative bacterium Wolbachia infects as endosymbiont a wide range of arthropods including a high proportion of insects and nematodes. Wolbachia occurs primarily in the sexual organs and manipulates the reproduction of the infected organism in a way that only infected females are able to reproduce [1]. A mechanism which is not fully understood so far but guarantees in the evolutionary process the maternal induced proliferation of the bacterium as endosymbiont. The importance of Wolbachia was evident when Yen and Barr [126] found that Wolbachia induces cytoplasmatic incompatibility in Culex mosquitoes, i.e. the failure of a sperm and egg to produce viable offspring. In case that sperm of a Wolbachia-infected Culex male fertilizes eggs of a non-infected female it leads to early embryonic death; no viable offspring are produced. Offspring is also not viable when males and females are infected with different strains of Wolbachia. Viable offsprings are only produced in a population when both sexes are either uninfected by Wolbachia or when an infected female mates with an uninfected male or with an infected male embodying the same Wolbachia strain as the female is carrying. Since Wolbachia is only transmitted by females, this mechanism favourites the spread of Wolbachia and leads to a selection pressure on uninfected females as well as for selected Wolbachia strains.
These discoveries have led to new control strategies by introducing Wolbachia-infected males into mosquito populations. However, methods of artificial infections of uninfected mosquitoes with Wolbachia have been invented, thus opening a new chapter in the control of vectors and arbovirus diseases [127–130]. The Wolbachia wMel strain can reduce the lifespan of adult Ae. aegypti and thus reduce the potential for DENV transmission, but these bacteria were not used in Europe so far [129]. The virus-infected vector mosquito doesn’t live long enough to complete the extrinsic incubation period.
The Wolbachia release program in Townsville, Australia, led to a 65% reduction in predicted DENV incidence during the release period and over 95% reduction in the 24 months that followed [131]. The release of males of Ae. albopictus with a manipulated ARwP strain Wolbachia induced egg inviability in female mosquitoes which prevents the risk of exotic arbovirus transmission. This population suppression approach is referred as incompatible insect technique [132, 133]. The combination of Wolbachia-based incompatible insect technique and radiation-based sterile insect technique can be used for population suppression of Ae. aegypti. Using proteomic methods Osario et al. [134] analyzed the seminal proteome of infected males and demonstrated that Wolbachia affect the composition of the seminal fluid proteins.
In recent time multiple mechanisms of Wolbachia-mediated antiviral activity are detected for instance in Ae. aegypti carrying different Wolbachia strains [135]. Discovered were changes in RNA processing pathways and upregulation of RNA-binding proteins in the wAu Wolbachia strain-carrying mosquito, including effects on genes with known antiviral activity. Lipid transport and metabolism proteome changes also differ between strains wAu and wMel, respective. In contrast to wMel, the strain wAu antiviral activity was not rescued by cyclodextrin treatment. These results suggest that wAu could show unique features in its inhibition of arboviruses compared to previously characterized Wolbachia strains. Further, Wolbachia was shown to interfere with Zika virus replication by hijacking cholesterol metabolism in mosquito cells [136].
However, these bacteria were not used in Europe so far. There is still a huge lack of knowledge regarding the interaction between the bacterium and mosquitoes, which currently prevent the test of field releases of Wolbachia-transinfected specimens, e.g., the lack of knowledge on the prevalence of Wolbachia on the European-level. In addition, although it can be assumed that the Wolbachia-based control approaches might be more accepted than genetically modified mosquitoes, regulatory pathways for the release are largely unexplained.
Epidemiological and resistance risk assessment
The occurrence of autochthonous transmissions of DENV, ZIKV and CHIKV by Ae. albopictus in recent years in Southern Europe is a warning signal and threat underlining the necessity for mosquito surveillance and control activities [38, 41, 43–47]. In case traveller return with proven viremia a careful travel history must be conducted and close cooperation with the health departments is mandatory. Especially in critical areas where Ae. albopictus was not yet recorded or in already infested areas, the surveillance must be intensified including the use of adult traps. In case infected mosquito females or even locally acquired infections are detected, immediate control activities must be conducted or intensified. According to the results of the surveillance, the emergency vector control operation has also to include the application of adulticides like pyrethroids when needful [93].
The onset of resistance against control agents can constitute a serious problem especially due to the limited inventory of available products. Therefore, the monitoring of the susceptibility of the target organism as applied product has to be conducted in regular intervals in bioassays e.g. according to the WHO protocols [137]. So far, the use of products based on Bti has the advantage that no resistance phenomena against Bti could be demonstrated. The rotation of insecticides with different mode of actions can avoid the onset of resistance.
Conclusions
The emergence of invasive mosquito species, such as t Ae. albopictus, and the spread of arboviruses, such as CHIKV and bluetongue virus, have led to an intensification of research and monitoring in Europe. Human mobility dynamics is the primary mode of dispersal on national and regional scales, while transport of goods with plants and tires by ships is the primary mode for continental spread. The risk of further spread of exotic species and mosquito-borne pathogens is expected to increase with ongoing globalization and climate warming. Three invasive mosquito species Asian tiger mosquito, Japanese bush mosquito, and Korean bush mosquito are already established in Europe due to their ability to lay drought-resistant and hibernating eggs. Over the past two decades, there has been an increasing number of outbreaks of mosquito-borne viruses in Europe.
The emergence of exotic mosquitoes and their spread of pathogens, furthermore a transmission contributed by native species warrant the Integrated Mosquito Management for mosquitoes in Europe. Thus, community participation and public awareness by a comprehensive information campaign, should ensure source reduction and successful control of mosquitoes in settlements. Promising control programs represent door-to-door control activities, and integration of sterile insect technique to reduce and possibly wipe out remaining populations (for Ae. albopictus) and in addition the use of indigenous copepods, bacteria or genetically modified mosquitoes.
Acknowledgements
This article is dedicated to Prof. Dr. Rolf Garms.
Abbreviations
- Bti
Bacillus thuringiensis israelensis
- CHIKV
Chikungunya virus
- DENV
Dengue virus
- USUV
Usutu virus
- WNV
West Nile virus
- ZIKV
Zika virus
Author contributions
RL, NB, NB wrote the manuscript.
Funding
RL are funded by the Federal Ministry of Education and Research of Germany (BMBF) under the project NEED (Grant no. 01Kl2022).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Becker N, Petrić D, Zgomba M, Boase C, Madon MB, Dahl C, et al. Mosquitoes: identification, ecology and control. Cham: Springer Nature; 2020. [Google Scholar]
- 2.World Health Organization: Vector-borne diseases. https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (2023). Accessed 31 Mar 2023.
- 3.Boualam MA, Pradines B, Drancourt M, Barbieri R. Malaria in Europe: a historical perspective. Front Med. 2021;8:691095. doi: 10.3389/fmed.2021.691095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weyer F. Bemerkungen zum Erlöschen der ostfriesischen malaria und zur Anopheles-Lage in Deutschland. Z Tropenmed Parasitol. 1956;7:219–228. [PubMed] [Google Scholar]
- 5.Pluskota B, Storch V, Braunbeck T, Beck M, Becker N. First record of Stegomyia albopicta (Skuse) (Diptera: Culicidae) in Germany. Eur Mosq Bull. 2008;26:1–5. [Google Scholar]
- 6.Conraths FJ, Gethmann JM, Staubach C, Mettenleiter TC, Beer M, Hoffmann B. Epidemiology of bluetongue virus serotype 8, Germany. Emerg Infect Dis. 2009;15:433–435. doi: 10.3201/eid1503.081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beer M, Conraths FJ, van der Poel WHM. “Schmallenberg virus”—a novel orthobunyavirus emerging in Europe. Epidemiol Infect. 2013;141:1–8. doi: 10.1017/S0950268812002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker N, Geier M, Balczun C, Bradersen U, Huber K, Kiel E, et al. Repeated introduction of Aedes albopictus into Germany, july to october 2012. Parasitol Res. 2013;112:1787–1790. doi: 10.1007/s00436-012-3230-1. [DOI] [PubMed] [Google Scholar]
- 9.Tappe D, Plauth M, Bauer T, Muntau B, Dießel L, Tannich E, et al. A case of autochthonous human Dirofilaria infection, Germany, march 2014. Euro Surveill. 2014;19:20790. doi: 10.2807/1560-7917.ES2014.19.17.20790. [DOI] [PubMed] [Google Scholar]
- 10.Czajka C, Becker N, Jöst H, Poppert S, Schmidt-Chanasit J, Krüger A, et al. Stable transmission of Dirofilaria repens nematodes, northern Germany. Emerg Infect Dis. 2014;20:328–331. doi: 10.3201/eid2002.131003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kronefeld M, Kampen H, Sassnau R, Werner D. Molecular detection of Dirofilaria immitis, Dirofilaria repens and Setaria tundra in mosquitoes from Germany. Parasit Vectors. 2014;7:30. doi: 10.1186/1756-3305-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bezerra-Santos MA, Dantas-Torres F, Benelli G, Otranto D. Emerging parasites and vectors in a rapidly changing world: from ecology to management. Acta Trop. 2023;238:106746. doi: 10.1016/j.actatropica.2022.106746. [DOI] [PubMed] [Google Scholar]
- 13.Ibáñez-Justicia A, Smitz N, den Hartog W, van de Vossenberg B, De Wolf K, Deblauwe I, et al. Detection of exotic mosquito species (Diptera: Culicidae) at international airports in Europe. Int J Environ Res Public Health. 2020;17:3450. doi: 10.3390/ijerph17103450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giunti G, Becker N, Benelli G. Invasive mosquito vectors in Europe: from bioecology to surveillance and management. Acta Trop. 2023;239:106832. doi: 10.1016/j.actatropica.2023.106832. [DOI] [PubMed] [Google Scholar]
- 15.Medlock JM, Hansford KM, Versteirt V, Cull B, Kampen H, Fontenille D, et al. An entomological review of invasive mosquitoes in Europe. Bull Entomol Res. 2015;105:637–663. doi: 10.1017/S0007485315000103. [DOI] [PubMed] [Google Scholar]
- 16.Vavassori L, Saddler A, Müller P. Active dispersal of Aedes albopictus: a mark-release-recapture study using self-marking units. Parasit Vectors. 2019;12:583. doi: 10.1186/s13071-019-3837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker N, Schön S, Klein A-M, Ferstl I, Kizgin A, Tannich E, et al. First mass development of Aedes albopictus (Diptera: Culicidae)—its surveillance and control in Germany. Parasitol Res. 2017;116:847–858. doi: 10.1007/s00436-016-5356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lühken R, Heitmann A, Jansen S, Schmidt-Chanasit J, Börstler J, Werner D, et al. Microsatellite typing of Aedes albopictus (Diptera: Culicidae) populations from Germany suggests regular introductions. Infect Genet Evol. 2020;81:104237. doi: 10.1016/j.meegid.2020.104237. [DOI] [PubMed] [Google Scholar]
- 19.Versteirt V, Schaffner F, Garros C, Dekoninck W, Coosemans M, Van Bortel W. Introduction and establishment of the exotic mosquito species Aedes japonicus japonicus (Diptera: Culicidae) in Belgium. J Med Entomol. 2009;46:1464–1467. doi: 10.1603/033.046.0632. [DOI] [PubMed] [Google Scholar]
- 20.Becker N, Huber K, Pluskota B, Kaiser A, et al. Ochlerotatus japonicus japonicus – a newly established neozoan in Germany and a revised list of the German mosquito fauna. Eur Mosq Bull. 2011;29:102. [Google Scholar]
- 21.Koban MB, Kampen H, Scheuch DE, Frueh L, Kuhlisch C, Janssen N, et al. The Asian bush mosquito Aedes japonicus japonicus (Diptera: Culicidae) in Europe, 17 years after its first detection, with a focus on monitoring methods. Parasit Vectors. 2019;12:109. doi: 10.1186/s13071-019-3349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibañez-Justicia A, Teekema S, den Hartog W, Jacobs F, Dik M, Stroo A. The effectiveness of Asian bush mosquito (Aedes japonicus japonicus) control actions in colonised peri-urban areas in the Netherlands. J Med Entomol. 2018;55:673–680. doi: 10.1093/jme/tjy002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaffner F, Kaufmann C, Hegglin D, Mathis A. The invasive mosquito Aedes japonicus in Central Europe. Med Vet Entomol. 2009;23:448–451. doi: 10.1111/j.1365-2915.2009.00825.x. [DOI] [PubMed] [Google Scholar]
- 24.Wagner S, Guidi V, Torgerson PR, Mathis A, Schaffner F. Diversity and seasonal abundances of mosquitoes at potential arboviral transmission sites in two different climate zones in Switzerland. Med Vet Entomol. 2018;32:175–185. doi: 10.1111/mve.12292. [DOI] [PubMed] [Google Scholar]
- 25.Kurucz K, Kiss V, Zana B, Schmieder V, Kepner A, Jakab F, et al. Emergence of Aedes koreicus (Diptera: Culicidae) in an urban area, Hungary, 2016. Parasitol Res. 2016;115:4687–4689. doi: 10.1007/s00436-016-5229-5. [DOI] [PubMed] [Google Scholar]
- 26.Kalan K, Šušnjar J, Ivović V, Buzan E. First record of Aedes koreicus (Diptera, Culicidae) in Slovenia. Parasitol Res. 2017;116:2355–2358. doi: 10.1007/s00436-017-5532-9. [DOI] [PubMed] [Google Scholar]
- 27.Capelli G, Drago A, Martini S, Montarsi F, Soppelsa M, Delai N, et al. First report in Italy of the exotic mosquito species Aedes (Finlaya) koreicus, a potential vector of arboviruses and filariae. Parasit Vectors. 2011;4:188. doi: 10.1186/1756-3305-4-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Versteirt V, Pecor JE, Fonseca DM, Coosemans M, Van Bortel W. Confirmation of Aedes koreicus (Diptera: Culicidae) in Belgium and description of morphological differences between Korean and Belgian specimens validated by molecular identification. Zootaxa. 2012;3191:21–32. doi: 10.11646/zootaxa.3191.1.2. [DOI] [Google Scholar]
- 29.Pfitzner WP, Lehner A, Hoffmann D, Czajka C, Becker N. First record and morphological characterization of an established population of Aedes (Hulecoeteomyia) koreicus (Diptera: Culicidae) in Germany. Parasit Vectors. 2018;11:662. doi: 10.1186/s13071-018-3199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huber K, Jansen S, Leggewie M, Badusche M, Schmidt-Chanasit J, Becker N, et al. Aedes japonicus japonicus (Diptera: Culicidae) from Germany have vector competence for Japan encephalitis virus but are refractory to infection with West Nile virus. Parasitol Res. 2014;113:3195–3199. doi: 10.1007/s00436-014-3983-9. [DOI] [PubMed] [Google Scholar]
- 31.Jansen S, Heitmann A, Lühken R, Jöst H, Helms M, Vapalahti O, et al. Experimental transmission of Zika virus by Aedes japonicus japonicus from southwestern Germany. Emerg Microbes Infect. 2018;7:192. doi: 10.1038/s41426-018-0195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansen S, Cadar D, Lühken R, Pfitzner WP, Jöst H, Oerther S, et al. Vector competence of the invasive mosquito species Aedes koreicus for arboviruses and interference with a novel insect specific virus. Viruses. 2021;13:2507. doi: 10.3390/v13122507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonizzoni M, Gasperi G, Chen X, James AA. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol. 2013;29:460–468. doi: 10.1016/j.pt.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faraji A, Egizi A, Fonseca DM, Unlu I, Crepeau T, Healy SP, et al. Comparative host feeding patterns of the Asian tiger mosquito, Aedes albopictus, in urban and suburban Northeastern USA and implications for disease transmission. PLoS Negl Trop Dis Public. 2014;8:e3037. doi: 10.1371/journal.pntd.0003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medlock JM, Hansford KM, Schaffner F, Versteirt V, Hendrickx G, Zeller H, et al. A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012;12:435–447. doi: 10.1089/vbz.2011.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaffner F, Medlock JM, Van Bortel W. Public health significance of invasive mosquitoes in Europe. Clin Microbiol Infect. 2013;19:685–692. doi: 10.1111/1469-0691.12189. [DOI] [PubMed] [Google Scholar]
- 37.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 38.Delisle E, Rousseau C, Broche B, Leparc-Goffart I, L’Ambert G, Cochet A, et al. Chikungunya outbreak in Montpellier, France, september to october 2014. Euro Surveill. 2015;20:21108. doi: 10.2807/1560-7917.ES2015.20.17.21108. [DOI] [PubMed] [Google Scholar]
- 39.Grandadam M, Caro V, Plumet S, Thiberge JM, Souarès Y, Failloux A-B, et al. Chikungunya virus, southeastern France. Emerg Infect Dis. 2011;17:910–913. doi: 10.3201/eid1705.101873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venturi G, Di Luca M, Fortuna C, Remoli ME, Riccardo F, Severini F, et al. Detection of a chikungunya outbreak in Central Italy, august to september 2017. Euro Surveill. 2017;22:17–00646. doi: 10.2807/1560-7917.ES.2017.22.39.17-00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gjenero-Margan I, Aleraj B, Krajcar D, Lesnikar V, Klobučar A, Pem-Novosel I, et al. Autochthonous dengue fever in Croatia, august-september 2010. Euro Surveill. 2011;16:19805. doi: 10.2807/ese.16.09.19805-en. [DOI] [PubMed] [Google Scholar]
- 42.La Ruche G, Souarès Y, Armengaud A, Peloux-Petiot F, Delaunay P, Desprès P, et al. First two autochthonous dengue virus infections in metropolitan France, september 2010. Euro Surveill. 2010;15:19676. doi: 10.2807/ese.15.39.19676-en. [DOI] [PubMed] [Google Scholar]
- 43.Marchand E, Prat C, Jeannin C, Lafont E, Bergmann T, Flusin O, et al. Autochthonous case of dengue in France, october 2013. Euro Surveill. 2013;18:20661. doi: 10.2807/1560-7917.ES2013.18.50.20661. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt-Chanasit J, Haditsch M, Schoneberg I, Gunther S, Stark K, Frank C. Dengue virus infection in a traveller returning from Croatia to Germany. Euro Surveill. 2010;15:19677. doi: 10.2807/ese.15.40.19677-en. [DOI] [PubMed] [Google Scholar]
- 45.Succo T, Leparc-Goffart I, Ferré J-B, Roiz D, Broche B, Maquart M, et al. Autochthonous dengue outbreak in Nîmes, South of France, july to september 2015. Euro Surveill. 2016;21:pii=30240. doi: 10.2807/1560-7917.ES.2016.21.21.30240. [DOI] [PubMed] [Google Scholar]
- 46.European Centre for Disease Prevention and Control: Annual Epidemiological Report. https://www.ecdc.europa.eu/en/publications-data/monitoring/all-annual-epidemiological-reports (2023). Accessed 31 Mar 2023.
- 47.Giron S, Franke F, Decoppet A, Cadiou B, Travaglini T, Thirion L, et al. Vector-borne transmission of Zika virus in Europe, southern France, August 2019. Euro Surveill. 2019;24:1900655. doi: 10.2807/1560-7917.ES.2019.24.45.1900655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Luca M, Severini F, Toma L, Boccolini D, Romi R, Remoli ME, et al. Experimental studies of susceptibility of Italian Aedes albopictus to Zika virus. Euro Surveill. 2016;21:pii=30223. doi: 10.2807/1560-7917.ES.2016.21.18.30223. [DOI] [PubMed] [Google Scholar]
- 49.Heitmann A, Jansen S, Lühken R, Leggewie M, Badusche M, Pluskota B, et al. Experimental transmission of Zika virus by mosquitoes from central Europe. Euro Surveill. 2017;22:30437. doi: 10.2807/1560-7917.ES.2017.22.2.30437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heitmann A, Jansen S, Lühken R, Helms M, Pluskota B, Becker N, et al. Experimental risk assessment for chikungunya virus transmission based on vector competence, distribution and temperature suitability in Europe, 2018. Euro Surveill. 2018;23:pii=1800033. doi: 10.2807/1560-7917.ES.2018.23.29.1800033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Severini F, Boccolini D, Fortuna C, Di Luca M, Toma L, Amendola A, et al. Vector competence of Italian Aedes albopictus populations for the chikungunya virus (E1–226V) PLoS Negl Trop Dis. 2018;12:e0006435. doi: 10.1371/journal.pntd.0006435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaufman MG, Fonseca DM. Invasion biology of Aedes japonicus japonicus (Diptera: Culicidae) Annu Rev Entomol. 2014;59:31–49. doi: 10.1146/annurev-ento-011613-162012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molaei G, Farajollahi A, Scott JJ, Gaugler R, Andreadis TG. Human bloodfeeding by the recently introduced mosquito, Aedes japonicus japonicus, and public health implications. J Am Mosq Control Assoc. 2009;25:210–214. doi: 10.2987/09-0012.1. [DOI] [PubMed] [Google Scholar]
- 54.Schönenberger AC, Wagner S, Tuten HC, Schaffner F, Torgerson P, Furrer S, et al. Host preferences in host-seeking and blood-fed mosquitoes in Switzerland. Med Vet Entomol. 2016;30:39–52. doi: 10.1111/mve.12155. [DOI] [PubMed] [Google Scholar]
- 55.Turell MJ, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- 56.Sardelis MR, Turell MJ, Andre RG. Experimental transmission of St. Louis encephalitis virus by Ochlerotatus j. japonicus. J Am Mosq Control Assoc. 2003;19:159–62. [PubMed] [Google Scholar]
- 57.Sardelis MR, Turell MJ, Andre RG. Laboratory transmission of La Crosse virus by Ochlerotatus j. japonicus (Diptera: Culicidae) J Med Entomol. 2002;39:635–9. doi: 10.1603/0022-2585-39.4.635. [DOI] [PubMed] [Google Scholar]
- 58.Schaffner F, Vazeille M, Kaufmann C, Failloux AB, Mathis A. Vector competence of Aedes japonicus for chikungunya and dengue viruses. Eur Mosq Bull. 2011;29:141–2. [Google Scholar]
- 59.Montarsi F, Ciocchetta S, Devine G, Ravagnan S, Mutinelli F, di Frangipane RA, et al. Development of Dirofilaria immitis within the mosquito Aedes (Finlaya) koreicus, a new invasive species for Europe. Parasit Vectors. 2015;8:177. doi: 10.1186/s13071-015-0800-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ciocchetta S, Prow NA, Darbro JM, Frentiu FD, Savino S, Montarsi F, et al. The new European invader Aedes (Finlaya) koreicus: a potential vector of chikungunya virus. Pathog Glob Health. 2018;112:107–114. doi: 10.1080/20477724.2018.1464780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miles JA. Some ecological aspects of the problem of arthropod-borne animal viruses in the Western Pacific and South-East Asia regions. Bull World Health Organ. 1964;30:197–210. [PMC free article] [PubMed] [Google Scholar]
- 62.Suvanto MT, Uusitalo R, Otte IKE, Vuorinen T, Kurkela S, Vapalahti O, et al. Sindbis virus outbreak and evidence for geographical expansion in Finland, 2021. Euro Surveill. 2022;27:pii=2200580. doi: 10.2807/1560-7917.ES.2022.27.31.2200580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ziegler U, Santos PD, Groschup MH, Hattendorf C, Eiden M, Höper D, et al. West Nile virus epidemic in Germany triggered by epizootic emergence, 2019. Viruses. 2020;12:448. doi: 10.3390/v12040448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cadar D, Lühken R, van der Jeugd H, Garigliany M, Ziegler U, Keller M, et al. Widespread activity of multiple lineages of Usutu virus, western Europe, 2016. Euro Surveill. 2017;22:30452. doi: 10.2807/1560-7917.ES.2017.22.4.30452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ziegler U, Fischer D, Eiden M, Reuschel M, Rinder M, Müller K, et al. Sindbis virus – a wild bird associated zoonotic arbovirus circulates in Germany. Vet Microbiol. 2019;239:108453. doi: 10.1016/j.vetmic.2019.108453. [DOI] [PubMed] [Google Scholar]
- 66.Eiden M, Ziegler U, Keller M, Müller K, Granzow H, Jöst H, et al. Isolation of Sindbis virus from a hooded crow in Germany. Vector Borne Zoonotic Dis. 2014;14:220–222. doi: 10.1089/vbz.2013.1354. [DOI] [PubMed] [Google Scholar]
- 67.Scheuch DE, Schäfer M, Eiden M, Heym EC, Ziegler U, Walther D, et al. Detection of Usutu, Sindbis, and Batai viruses in mosquitoes (Diptera: Culicidae) collected in Germany, 2011–2016. Viruses. 2018;10:389. doi: 10.3390/v10070389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jöst H, Bialonski A, Storch V, Günther S, Becker N, Schmidt-Chanasit J. Isolation and phylogenetic analysis of Sindbis viruses from mosquitoes in Germany. J Clin Microbiol. 2010;48:1900–1903. doi: 10.1128/JCM.00037-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jansen S, Lühken R, Helms M, Pluskota B, Pfitzner WP, Oerther S, et al. Vector competence of mosquitoes from Germany for Sindbis Virus. Viruses. 2022;14:2644. doi: 10.3390/v14122644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jöst H, Bialonski A, Schmetz C, Günther S, Becker N, Schmidt-Chanasit J. Isolation and phylogenetic analysis of Batai virus. Germany Am J Trop Med Hyg. 2011;84:241–243. doi: 10.4269/ajtmh.2011.10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cichon N, Eiden M, Schulz J, Günther A, Wysocki P, Holicki CM, et al. Serological and molecular investigation of Batai virus infections in ruminants from the state of Saxony-Anhalt, Germany, 2018. Viruses. 2021;13:370. doi: 10.3390/v13030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kampen H, Tews BA, Werner D. First evidence of West Nile virus overwintering in mosquitoes in Germany. Viruses. 2021;13:2463. doi: 10.3390/v13122463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cadar D, Simonin Y. Human Usutu virus infections in Europe: a new risk on horizon? Viruses. 2022;15:77. doi: 10.3390/v15010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weissenböck H, Bakonyi T, Rossi G, Mani P, Nowotny N. Usutu virus, Italy, 1996. Emerg Infect Dis. 2013;19:274–277. doi: 10.3201/eid1902.121191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rijks JM, Kik ML, Slaterus R, Foppen R, Stroo A, IJzer J, et al. Widespread Usutu virus outbreak in birds in the Netherlands, 2016. Euro Surveill. 2016;21:30391. doi: 10.2807/1560-7917.ES.2016.21.45.30391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nikolay B. A review of West Nile and Usutu virus co-circulation in Europe: how much do transmission cycles overlap? Trans R Soc Trop Med Hyg. 2015;109:609–618. doi: 10.1093/trstmh/trv066. [DOI] [PubMed] [Google Scholar]
- 77.Engel D, Jöst H, Wink M, Börstler J, Bosch S, Garigliany M-M, et al. Reconstruction of the evolutionary history and dispersal of Usutu Virus, a neglected emerging arbovirus in Europe and Africa. MBio. 2016;7:e01938–e2015. doi: 10.1128/mBio.01938-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Becker N, Jöst H, Ziegler U, Eiden M, Höper D, Emmerich P, et al. Epizootic emergence of Usutu virus in wild and captive birds in Germany. PLoS One. 2012;7:e32604. doi: 10.1371/journal.pone.0032604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weissenböck H, Kolodziejek J, Url A, Lussy H, Rebel-Bauder B, Nowotny N. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg Infect Dis. 2002;8:652–656. doi: 10.3201/eid0807.020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jöst H, Bialonski A, Maus D, Sambri V, Eiden M, Groschup MH, et al. Isolation of Usutu virus in Germany. Am J Trop Med Hyg. 2011;85:551–553. doi: 10.4269/ajtmh.2011.11-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lühken R, Jöst H, Cadar D, Thomas SM, Bosch S, Tannich E, et al. Distribution of Usutu virus in Germany and its effect on breeding bird populations. Emerg Infect Dis. 2017;23:1994–2001. doi: 10.3201/eid2312.171257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Michel F, Sieg M, Fischer D, Keller M, Eiden M, Reuschel M, et al. Evidence for West Nile Virus and Usutu virus infections in wild and resident birds in Germany, 2017 and 2018. Viruses. 2019;11:674. doi: 10.3390/v11070674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Börstler J, Engel D, Petersen M, Poggensee C, Jansen S, Schmidt-Chanasit J, et al. Surveillance of maternal antibodies against West Nile virus in chicken eggs in South-West Germany. Trop Med Int Health. 2016;21:687–690. doi: 10.1111/tmi.12676. [DOI] [PubMed] [Google Scholar]
- 84.Ziegler U, Jöst H, Müller K, Fischer D, Rinder M, Tietze DT, et al. Epidemic spread of Usutu virus in southwest Germany in 2011 to 2013 and monitoring of wild birds for Usutu and West Nile viruses. Vector Borne Zoonotic Dis. 2015;15:481–488. doi: 10.1089/vbz.2014.1746. [DOI] [PubMed] [Google Scholar]
- 85.Ziegler U, Angenvoort J, Klaus C, Nagel-Kohl U, Sauerwald C, Thalheim S, Horner S, Braun B, Kenklies S, Tyczka J, Keller M, Groschup MH. Use of competition ELISA for monitoring of West Nile virus infections in horses in Germany. Int J Environ Res Public Health. 2013;10:3112–3120. doi: 10.3390/ijerph10083112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ziegler U, Lühken R, Keller M, Cadar D, van der Grinten E, Michel F, et al. West Nile virus epizootic in Germany, 2018. Antiviral Res. 2019;162:39–43. doi: 10.1016/j.antiviral.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 87.Fynmore N, Lühken R, Maisch H, Risch T, Merz S, Kliemke K, et al. Rapid assessment of West Nile virus circulation in a German zoo based on honey-baited FTA cards in combination with box gravid traps. Parasit Vectors. 2021;14:449. doi: 10.1186/s13071-021-04951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jansen S, Heitmann A, Lühken R, Leggewie M, Helms M, Badusche M, et al. Culex torrentium: a potent vector for the transmission of West Nile virus in Central Europe. Viruses. 2019;11:492. doi: 10.3390/v11060492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Becker N. Influence of climate change on mosquito development and mosquito-borne diseases in Europe. Parasitol Res. 2008;103(Suppl 1):S19–28. doi: 10.1007/s00436-008-1210-2. [DOI] [PubMed] [Google Scholar]
- 90.Fischer D, Thomas SM, Neteler M, Tjaden NB, Beierkuhnlein C. Climatic suitability of Aedes albopictus in Europe referring to climate change projections: comparison of mechanistic and correlative niche modelling approaches. Euro Surveill. 2014;19:20696. doi: 10.2807/1560-7917.ES2014.19.6.20696. [DOI] [PubMed] [Google Scholar]
- 91.Verdonschot PFM, Besse-Lototskaya AA. Flight distance of mosquitoes (Culicidae): a metadata analysis to support the management of barrier zones around rewetted and newly constructed wetlands. Limnologica. 2014;45:69–79. doi: 10.1016/j.limno.2013.11.002. [DOI] [Google Scholar]
- 92.Becker N, Langentepe-Kong SM, Tokatlian Rodriguez A, Oo TT, Reichle D, Lühken R, et al. Integrated control of Aedes albopictus in southwest Germany supported by the sterile insect technique. Parasit Vectors. 2022;15:9. doi: 10.1186/s13071-021-05112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bellini R, Michaelakis A, Petrić D, Schaffner F, Alten B, Angelini P, et al. Practical management plan for invasive mosquito species in Europe: I. Asian tiger mosquito (Aedes albopictus). Travel Med Infect Dis. 2020;35:101691. [DOI] [PubMed]
- 94.World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control. World Health Organization; 2009. [PubMed]
- 95.European Mosquito Control Association in collaboration with the World Health Organization. Guidelines for the Control of Mosquitoes of Public Health Importance in Europe. 2013.
- 96.Bellini R, Carrieri M, Burgio G, Bacchi M. Efficacy of different ovitraps and binomial sampling in Aedes albopictus surveillance activity. J Am Mosq Control Assoc. 1996;12:632–636. [PubMed] [Google Scholar]
- 97.Carrieri M, Angelini P, Venturelli C, Maccagnani B, Bellini R. Aedes albopictus (Diptera: Culicidae) population size survey in the 2007 chikungunya outbreak area in Italy. II: Estimating epidemic thresholds. J Med Entomol. 2012;49:388–99. [DOI] [PubMed]
- 98.Webb CE, Russell RC. A laboratory investigation of the mosquito control potential of the monomolecular film Aquatain® mosquito formula against immature stages of Aedes aegypti and Culex quinquefasciatus. J Am Mosq Control Assoc. 2009;25:106–109. doi: 10.2987/08-5750.1. [DOI] [PubMed] [Google Scholar]
- 99.Becker N. Community participation in the operational use of microbial control agents in mosquito control programs. Bull Soc Vector Ecol. 1992;17:114–118. [Google Scholar]
- 100.Donati L, Carrieri M, Bellini R. A door-to-door strategy for Aedes albopictus control in Northern Italy: efficacy, cost-analysis and public perception. Vector Biol J. 2020;5:1. [Google Scholar]
- 101.Romeo B, Alessandro A, Marco C, Roberta C, Luciano D, Maurizio M, et al. Efficacy and lasting activity of four IGRs formulations against mosquitoes in catch basins of northern Italy. Eur Mosq Bull. 2009;127:33–46. [Google Scholar]
- 102.Bellini R, Medici A, Puggioli A, Balestrino F, Carrieri M. Pilot field trials with Aedes albopictus irradiated sterile males in Italian urban areas. J Med Entomol. 2013;50:317–325. doi: 10.1603/ME12048. [DOI] [PubMed] [Google Scholar]
- 103.Balestrino F, Puggioli A, Gilles JRL, Bellini R. Validation of a new larval rearing unit for Aedes albopictus (Diptera: Culicidae) mass rearing. PLoS ONE. 2014;9:e91914. doi: 10.1371/journal.pone.0091914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Puggioli A, Carrieri M, Dindo ML, Medici A, Lees RS, Gilles JRL, et al. Development of Aedes albopictus (Diptera: Culicidae) larvae under different laboratory conditions. J Med Entomol. 2017;54:142–149. doi: 10.1093/jme/tjw127. [DOI] [PubMed] [Google Scholar]
- 105.Puggioli A, Balestrino F, Damiens D, Lees RS, Soliban SM, Madakacherry O, et al. Efficiency of three diets for larval development in mass rearing Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2013;50:819–825. doi: 10.1603/ME13011. [DOI] [PubMed] [Google Scholar]
- 106.Focks DA. An improved separator for the developmental stages, sexes, and species of mosquitoes (Diptera: Culicidae) J Med Entomol. 1980;17:567–568. doi: 10.1093/jmedent/17.6.567. [DOI] [PubMed] [Google Scholar]
- 107.Balestrino F, Puggioli A, Carrieri M, Bouyer J, Bellini R. Quality control methods for Aedes albopictus sterile male production. PLoS Negl Trop Dis. 2017;11:e0005881. doi: 10.1371/journal.pntd.0005881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Balestrino F, Medici A, Candini G, Carrieri M, Maccagnani B, Calvitti M, et al. γ Ray Dosimetry and mating capacity studies in the laboratory on Aedes albopictus males. J Med Entomol. 2010;47:581–591. doi: 10.1093/jmedent/47.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bellini R. Safety, regulatory and environmental issues with sterile insect technique-based mosquito vector control in European countries. Rev Sci Tech. 2022;41:170–177. doi: 10.20506/rst.41.1.3314. [DOI] [PubMed] [Google Scholar]
- 110.de Castro Poncio L, Apolinário Dos Anjos F, de Oliveira DA, de Oliveira da Rosa A, Piraccini Silva B, Rebechi D, et al. Prevention of a dengue outbreak via the large-scale deployment of Sterile Insect Technology in a Brazilian city: a prospective study. Lancet Reg Health Am. 2023;21:100498. [DOI] [PMC free article] [PubMed]
- 111.Pauly I, Jakoby O, Becker N. Efficacy of native cyclopoid copepods in biological vector control with regard to their predatory behavior against the Asian tiger mosquito Aedes albopictus. Parasit Vectors. 2022;15:351. doi: 10.1186/s13071-022-05460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lacey LA. Microbial control of insect and mite pests: from theory to practice. Elsevier: Science; 2016. [Google Scholar]
- 113.Rivière F, Thirel R. La prédation du copépode Mesocyclops leuckarti pilosa (Crustacea) sur les larves de Aedes (Stegomyia) aegypti et Ae. (St.) polynesiensis (Diptera: Culicidae): essais préliminaries d' utilisation comme agent de lutte biologique. Entomophaga. 1981;26:427–439. doi: 10.1007/BF02374717. [DOI] [Google Scholar]
- 114.Russell MC, Qureshi A, Wilson CG, Cator LJ. Size, not temperature, drives cyclopoid copepod predation of invasive mosquito larvae. PLoS ONE. 2021;16:e0246178. doi: 10.1371/journal.pone.0246178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rey JR, Connell OS, Suárez S, Menéndez Z, Lounibos LP, Byer G. Laboratory and field studies of Macrocyclops albidus (Crustacea: Copepoda) for biological control of mosquitoes in artificial containers in a subtropical environment. J Vector Ecol. 2004;29:124–34. [PubMed] [Google Scholar]
- 116.Marten GG, Bordes ES, Nguyen M. Use of cyclopoid copepods for mosquito control. Ecology and Morphology of Copepods. Hydrobiologia. 1994;292–293:491–6. doi: 10.1007/BF00229976. [DOI] [Google Scholar]
- 117.Marten GG, Reid JW. Cyclopoid copepods. J Am Mosq Control Assoc. 2007;23:65–92. doi: 10.2987/8756-971X(2007)23[65:CC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 118.Baldacchino F, Bruno MC, Visentin P, Blondel K, Arnoldi D, Hauffe HC, et al. Predation efficiency of copepods against the new invasive mosquito species Aedes koreicus (Diptera: Culicidae) in Italy. Eur Zool J. 2017;84:43–48. doi: 10.1080/11250003.2016.1271028. [DOI] [Google Scholar]
- 119.Baldacchino F, Caputo B, Chandre F, Drago A, della Torre A, Montarsi F, , et al. Control methods against invasive Aedes mosquitoes in Europe: a review. Pest Manag Sci. 2015;71:1471–1485. doi: 10.1002/ps.4044. [DOI] [PubMed] [Google Scholar]
- 120.Walsh JR, Carpenter SR, Vander Zanden MJ. Invasive species triggers a massive loss of ecosystem services through a trophic cascade. Proc Natl Acad Sci. 2016;113:4081–4085. doi: 10.1073/pnas.1600366113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Früh L, Kampen H, Schaub GA, Werner D. Predation on the invasive mosquito Aedes japonicus (Diptera: Culicidae) by native copepod species in Germany. J Vector Ecol. 2019;44:241–247. doi: 10.1111/jvec.12355. [DOI] [PubMed] [Google Scholar]
- 122.Nie D, Li J, Xie Q, Ai L, Zhu C, Wu Y, et al. Nanoparticles: a potential and effective method to control insect-borne diseases. Bioinorg Chem Appl. 2023 doi: 10.1155/2023/5898160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mohanta YK, Nayak D, Mishra AK, Chakrabartty I, Ray MK, Mohanta TK, et al. Green Synthesis of endolichenic fungi functionalized silver nanoparticles: the role in antimicrobial, anti-cancer, and mosquitocidal activities. Int J Mol Sci. 2022;23(18):10626. doi: 10.3390/ijms231810626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Malla RK, Chandra G. Diospyros montana mediated reduction, stabilization, and characterization of silver nanoparticles and evaluation of their mosquitocidal potentiality against dengue vector Aedes albopictus. Sci Rep. 2023;13:17202. doi: 10.1038/s41598-023-44442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sujitha V, Murugan K, Paulpandi M, Panneerselvam C, Suresh U, Roni M, et al. Green-synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitol Res. 2015;114(9):3315–3325. doi: 10.1007/s00436-015-4556-2. [DOI] [PubMed] [Google Scholar]
- 126.Yen JH, Barr AR. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature. 1971;232:657–658. doi: 10.1038/232657a0. [DOI] [PubMed] [Google Scholar]
- 127.Dobson SL. Reversing Wolbachia-based population replacement. Trends Parasitol. 2003;19:128–133. doi: 10.1016/S1471-4922(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 128.Xi Z, Dean JL, Khoo C, Dobson SL. Generation of a novel Wolbachia infection in Aedes albopictus (Asian tiger mosquito) via embryonic microinjection. Insect Biochem Mol Biol. 2005;35:903–910. doi: 10.1016/j.ibmb.2005.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 130.Mains JW, Brelsfoard CL, Rose RI, Dobson SL. Female adult Aedes albopictus suppression by Wolbachia-infected male mosquitoes. Sci Rep. 2016;6:33846. doi: 10.1038/srep33846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ogunlade ST, Adekunle AI, Meehan MT, McBryde ES. Quantifying the impact of Wolbachia releases on dengue infection in Townsville, Australia. Sci Rep. 2023;13(1):14932. doi: 10.1038/s41598-023-42336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Caputo B, Moretti R, Virgillito C, Manica M, Lampazzi E, Lombardi G, et al. A bacterium against the tiger: further evidence of the potential of noninundative releases of males with manipulated Wolbachia infection in reducing fertility of Aedes albopictus field populations in Italy. Pest Manag Sci. 2023;79(9):3167–3176. doi: 10.1002/ps.7495. [DOI] [PubMed] [Google Scholar]
- 133.Soh S, Ho SH, Ong J, Seah A, Dickens BS, Tan KW, et al. Strategies to mitigate establishment under the Wolbachia incompatible insect technique. Viruses. 2022;14(6):1132. doi: 10.3390/v14061132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Osario J, Villa-Arias S, Camargo C, Ramírez-Sánchez LF, Barrientos LM, Bedoya C, et al. wMel Wolbachia alters female post-mating behaviors and physiology in the dengue vector mosquito Aedes aegypti. Commun Biol. 2023;21(6):865. doi: 10.1038/s42003-023-05180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rainey SM, Geoghegan V, Lefteri DA, Ant TH, Martinez J, McNamara CJ, et al. Differences in proteome perturbations caused by the Wolbachia strain wAu suggest multiple mechanisms of Wolbachia-mediated antiviral activity. Sci Rep. 2023;13(1):11737. doi: 10.1038/s41598-023-38127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Edwards B, Ghedin E, Voronin D. Wolbachia interferes with Zika virus replication by hijacking cholesterol metabolism in mosquito cells. Microbiol Spectr. 2023;9:e0218023. doi: 10.1128/spectrum.02180-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Praulins G, McDermott DP, Spiers A, Lees RS. Reviewing the WHO tube bioassay methodology: accurate method reporting and numbers of mosquitoes are key to producing robust results. Insects. 2022;13:544. doi: 10.3390/insects13060544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.