Abstract
Objective
There are many injectable treatments for knee osteoarthritis with different characteristics and effects, the aim is to understand which one can lead to better and safer results.
Methods
The PRISMA principles were followed when doing the literature search. Web of Science databases, Embase, the Cochrane Library, PubMed, and the Wanfang database were searched to identified randomized controlled trials that assessed the efficacy of corticosteroids (CSC), platelet-rich plasma (PRP), hyaluronic acid (HA), and combination therapy in treating KOA. Risk of bias was assessed using the relevant Cochrane tools (version 1.0). The outcome measure included the visual analog scale (VAS) score, the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) score, and treatment-related adverse events. The network meta-analysis was performed using STATA17 software and a Bayesian stratified random effects model.
Results
Network meta-analysis using the Bayesian random-effects model revealed 35 studies with 3104 participants. PRP showed the best WOMAC score at a 3-month follow-up, followed by PRP + HA, HA, placebo, and CSC; PRP + HA scored the highest VAS, followed by PRP, CSC, HA, and placebo. PRP, CSC, HA, and placebo had the highest WOMAC scores six months following treatment; PRP + HA showed the best VAS scores. PRP showed the best WOMAC score at 12 months, followed by PRP + HA, HA, placebo, and CSC; The best VAS score was obtained with PRP, followed by PRP + HA, HA, and CSC. No therapy demonstrated a rise in adverse events linked to the treatment in terms of safety.
Conclusions
The current study found that PRP and PRP + HA were the most successful in improving function and alleviating pain after 3, 6, and 12 months of follow-up. CSC, HA, PRP, and combination therapy did not result in an increase in the incidence of treatment-related side events as compared to placebo.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-023-06925-6.
Keywords: Knee osteoarthritis, Corticosteroids, Hyaluronic acid, Platelet-rich plasma, Meta-analysis
Introduction
Knee osteoarthritis (KOA) is a chronic joint condition characterized by cartilage degeneration and an increase in bone growth in the knee joint [1, 2]. The knee joint’s primary symptoms include discomfort, swelling, and mobility problems. As the population ages, more people are developing KOA, which has a major impact on middle-aged and older people’s health and quality of life [3–5]. As a multifactorial disease that develops over a long period of time [6], KOA has always been a huge burden on individuals and society as a whole due to its high disability rate [7].
Currently, intra-articular injection (IAI) remains the primary element of non-surgical therapy for KOA [8]. The evidence that is now available demonstrates that this therapy can significantly reduce short-term pain for patients with KOA and improve joint function while also having a minimal risk of patient injury [9, 10]. Interestingly, botulinum toxin and ozone have also been proven to be used for injection into joints to treat KOA [11, 12]. HA, a naturally occurring glycosaminoglycan, serves as a crucial component of synovial fluid in joints, functioning as a lubricant and a shock absorber with elastic properties during joint movement [13]. In addition, A has the following functions: proteoglycan and glycosaminoglycan synthesis, anti-inflammatory, mechanical, subchondral, and analgesic actions [14]. HA is a widely used conservative treatment for OA because of both its indirect and direct analgesic effects on joints. Many clinical studies have shown that HA supplementation has a good effect on KOA, but HA may increase the risk of adverse events, such as transient pain at the injection site [15]. Knee Joint injection of CSC has a lasting effect of weeks to months [16]. The anti-inflammatory and immunosuppressive effects of corticosteroids are obvious [17], and CSC can raise the knee joint’s relative viscosity and HA concentration [18]. Regarding the intra-articular CSC’s effective duration, there is disagreement. IAI of PRP has gained widespread attention in recent years as a novel and successful alternative therapy for patients with KOA [19]. The mechanism of local injection of PRP is that it can relieve joint pain and reduce synovial hyperplasia and effusion in the joint cavity [20]. PRP is considered to have a variety of important physiological functions, such as anti-inflammation, analgesia, and promoting chondrocyte proliferation and cartilage repair. Besides, PRP can also regulate the progression of KOA by regulating WNT and IL-1 signaling [21]. In recent years, scholars have combined them to investigate the possibility of dual therapy [22]. Wang et al. discovered that individuals taking hyaluronan and corticosteroids together had pain alleviation and improved knee function faster than either medication alone. At 6 months, however, there was no discernible difference [23]. Huang et al. discovered that whereas corticosteroids and hyaluronan were equivalent in terms of pain alleviation after three months, PRP injections were superior in terms of long-term pain relief [24]. John et al.‘s study found that PRP has better efficacy than HA [13], but another study found no difference between the two [25]. Overall, there are still many controversies in this field, and there is an urgent need for an article to integrate all the evidence and provide a credible recommendation.
In this study, a Bayesian network meta-analysis of randomized controlled trials (RCTs) was conducted to evaluate the effectiveness and safety of CSC, HA, PRP, and their combination in treating KOA.
Materials and methods
Ethical approval
This meta-analysis did not need ethical approval since no new clinical raw data were collected or used; rather, the analysis was conducted based only on previously published research that had already been granted ethical approval.
Literature search
In accordance with the PRISMA checklist [26], a comprehensive search was carried out in the Web of Science databases, Embase, the Cochrane Library, PubMed, and the Wanfang database to collect English publications until December 2022. The search criteria consisted of keywords such as “corticosteroids OR steroids OR hyaluronic acid OR platelet rich plasma OR PRP OR placebo (PLA)” and the condition of interest, “knee OR osteoarthritis OR KOA”. To find more pertinent literature, a manual search and literature tracking techniques were also performed. Supplemental File 1 provides details of the search strategy.
Inclusion and exclusion criteria of literature
The following were the study’s inclusion criteria: (1) RCTs involving patients with KOA; (2) original research; (3) studies that reported at least two of the following treatments: HA, CSC, PRP, combination therapy, and/or placebo; and (4) includes VAS OR WOMAC outcome scores or the proportion of patients who had adverse effects. The following were the exclusion criteria: (1) literature review; (2) non-randomized studies; (3) failure to get original data; and (4) low-quality or duplicate publications. Two authors conducted an independent search of all references and any disagreements were resolved by a vote of all authors.
Data extraction
Two authors (XQ and LY) conducted data extraction independently, discussed their findings, and reached an agreement in case of any disagreements. Each qualifying study’s first author, publication year, country, methods of treatment, length of time, sample size, outcome measures, and follow-up time points were all recorded.
Methodological quality assessment
Two authors (XL and LY) independently evaluated the quality of the included literature, and a third researcher was invited to help resolve any differences. Review Manager Software5.4 (The Nordic Cochrane Collaboration, Copenhagen)’s risk of bias summary was used to examine the following biases: sequence generation, allocation concealment, blinding, incomplete outcome data, no selective outcome reporting, and other sources of bias. Each criterion was judged to have a low, unclear, or high risk of bias.
Statistical analysis
Data synthesis
Stata 17.0 was used for data processing and analysis, and to draw related graphs [27]. For dichotomous variable data, we estimated the odds ratio (OR) with 95% confidence intervals (CIs), and for continuous variable data, we estimated the standardized mean differences (SMD) with 95% CIs. The initial model update iteration number was set to 10,000, and the continuous update iteration number was set to 10,000. To mitigate the impact of the starting value, the first 10,000 annealing times were utilized, and sampling began after 10,001 times. We calculated the relative ranks of the intervention groups using a consistency model and then displayed the percentages of the surface under the cumulative ranking curve (SUCRA). We conducted a network meta-analysis for each outcome only when the intervention groups could be connected to create a network; however, comparisons of support surfaces allocated to the same group were not excluded from the overall systematic review.
Assessing the certainty of evidence
A detailed review of the completeness of the literature search was used to estimate the possibility of publication bias. This involved creating funnel plots for each paired meta-analysis that contained more than 10 studies, as well as a network-adjusted funnel plot. Furthermore, the depth of the literature search and the amount of unpublished data acquired were considered.
Results
Literature search
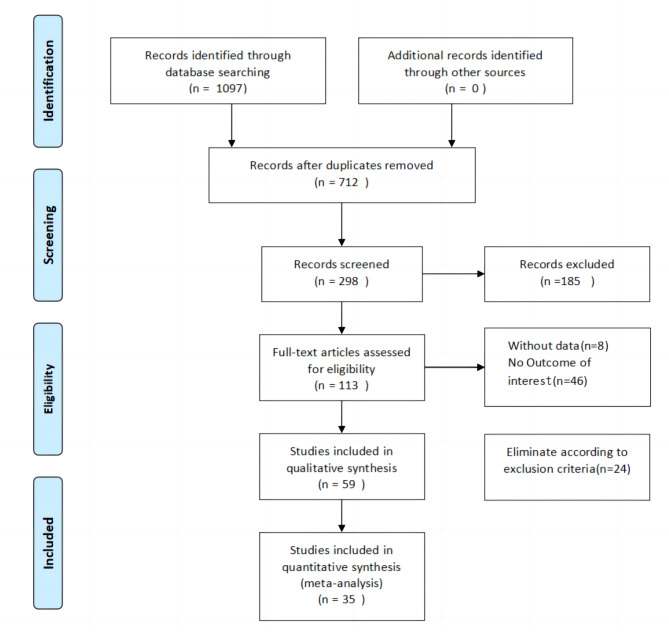
Out of 1097 RCTS pertaining to KOA identified through the database search, 1062 were eliminated for diverse reasons, including 385 duplicates, while 712 articles were screened by title and abstract, thereby resulting in the exclusion of 599 irrelevant studies. Afterward, a thorough examination of 113 articles led to the elimination of 8 articles that lacked an index of existing data, 46 articles that did not present the outcome of interest, and 24 articles that were not connected to the outcome. This ultimately brought the meta-analysis down to 35 studies. Figure 1 illustrates the particulars of the literature search.
Fig. 1.
Flowchart of the study procedure
Patient demographics and methodological quality assessment
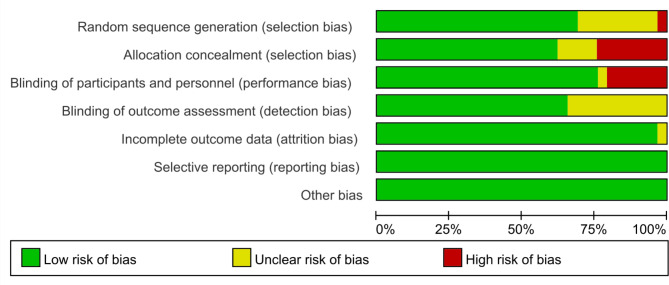
Included were 35 RCTs with 3104 patients from 16 countries in total. The mean age of the enrolled patients was 59.1 years, and 61.3% of them were female. The course of treatment ranged from 3 to 24 months. Follow-up time reached 3 months in 35 studies, 6 months in 31 studies, 9 months in 14 studies, 12 months in 14 studies. Table 1 presents a comprehensive list of included studies along with their characteristics. The majority of studies utilized blinding techniques. Furthermore, the hazards of attrition, reporting, and unidentified bias are minimal. Methodological evaluations had a minimal risk of bias and were of high quality. Figure 2 depicts the methodological quality evaluation.
Table 1.
Characteristics of 36 studies included in the meta-analysis
| Study | Country | Intervention | Duration | Sample | Age,mean (SD) | Gender(male/female) | OUTCOME | ||
|---|---|---|---|---|---|---|---|---|---|
| EXP | CON | EXP | CON | ||||||
| Askari et al., 2016 | Iran | CSC VS HA | 3months | 140 | 57.0 ± 1.9 | 58.5 ± 8.3 | 12/57 | 9/62 | VAS |
| Buendía-Lópe et al., 2018 | Spain | PRP VS HA | 52weeks | 65 | 56.15 ± 3.001 | 56.63 ± 2.9 | 16/17 | 15/17 | WOMAC、VAS、Adverse effects |
| Cerza et al., 2012 | Italy | PRP VS HA | 24weeks | 120 | 66.5 ± 11.3 | 66.2 ± 10.6 | 25/35 | 28/32 | WOMAC |
| Cole et al., 2016 | USA | PRP VS HA | 52weeks | 99 | 55.9 ± 10.4 | 56.8 ± 10.5 | 28/21 | 20/30 | WOMAC、VAS |
| Raeissadat et al., 2021 | Iran | PRP VS HA | 12months | 101 | 56.09 ± 6.0 | 57.91 ± 6.7 | 13/39 | 13/39 | WOMAC、VAS |
| Park et al., 2021 | Korea | PRP VS HA | 6months | 110 | 60.6 ± 8.2 | 62.3 ± 9.6 | 16/39 | 8/47 | WOMAC、VAS、Adverse effects |
| Dório et al., 2021 | Brazil | PRP VS PLA | 24weeks | 41 | 66.4 ± 5.6 | 66.1 ± 7.5 | 1/19 | 2/19 | WOMAC、VAS、Adverse effects |
| Elik et al., 2019 | Turkey | PRP VS PLA | 6months | 57 | 61.30 ± 7.91 | 60.19 ± 6.80 | 1/29 | 3/24 | WOMAC、VAS、Adverse effects |
| Lana et al., 2016 | Brazil | PRP VS HA VS PRP + HA | 12months | 105 | PRP: 60.9 ± 7,HA:60 ± 6.6,PRP + HA:62 ± 6.1 | PRP: 29/7,HA:33/3,PRP + HA:27/6 | VAS | ||
| Xu et al., 2020 | China | PRP VS HA VS PRP + HA | 24months | 122 | PRP:56.9 ± 4.2,HA:57.1 ± 3.4,PRP + HA:57.9 ± 4.1 | PRP: 10/20,HA:5/15,PRP + HA:8/20 | Adverse effects | ||
| Sun et al., 2021 | China | PRP VS PRP + HA | 6months | 85 | 60.6 ± 8.4 | 58.4 ± 8.1 | 18/21 | 22/17 | WOMAC 、VAS |
| Yu et al., 2018 | China | PRP VS HA VS PRP + HA VS PLA | 52weeks | 360 | PRP: 46.2 ± 8.6,HA:51.5 ± 9.3,PRP + HA:46.5 ± 7.5,PLA:56.2 ± 8.4 | PRP:50/54,HA:48/40,PRP + HA:50/46,PLA:42/30 | WOMAC、Adverse effects | ||
| Elksniņš-Finogejevs et al., 2020 | Latvia | PRP VS CSC | 12months | 40 | 66.4 ± 8.4 | 70.2 ± 9.2 | 3/17 | 5/15 | VAS、Adverse effects |
| Yan et al., 2020 | China | HA VS PLA | 26weeks | 440 | 61.5 ± 7.9 | 61.6 ± 7.8 | 50/170 | 48/172 | WOMAC、Adverse effects |
| Petterson et al., 2018 | USA | HA VS PLA | 26weeks | 369 | 59.5 ± 8.0 | 58.7 ± 9.2 | 75/109 | 79/106 | WOMAC、VAS、Adverse effects |
| Huang et al., 2019 | China | PRP VS HA VS CSC | 12months | 120 | PRP:54.5 ± 1.2,HA:54.8 ± 1.1,CSC:54.3 ± 1.4 | PRP:19/21,HA:21/19,CSC:35/15 | WOMAC、VAS、Adverse effects | ||
| Kesiktas et al., 2020 | Turkey | PRP VS HA | 3months | 36 | 52.7 ± 8.3 | 55.1 ± 10.3 | 4/14 | 2/16 | WOMAC、VAS |
| Wang et al., 2021 | China | CSC + HA VS HA | 3months | 57 | 61.7 ± 15.3 | 59.2 ± 13.8 | 12/16 | 12/17 | Adverse effects |
| Davalillo et al., 2015 | Mexico | HA VS CSC | 12months | 200 | 62.7 ± 0.6 | 62.8 ± 0.6 | 59/38 | 57/41 | WOMAC、Adverse effects |
| Martino et al., 2018 | Italy | PRP VS HA | 24months | 192 | 52.7 ± 13.2 | 57.5 ± 11.7 | 53/32 | 47/35 | VAS |
| Duymus et al., 2015 | Turkey | PRP VS HA | 12months | 102 | 60.4 ± 5.1 | 60.3 ± 9.1 | 1/32 | 1/33 | WOMAC、VAS |
| Elsawy et al., 2017 | Egypt | HA VS CSC | 6months | 60 | 52.5 ± 12.5 | 50.2 ± 11.4 | 18/42 | WOMAC、VAS | |
| GÜVENDİ et al., 2018 | Turkey | CSC VS PRP | 6months | 57 | 62.8 ± 1.7 | 62.3 ± 1.6 | 2/15 | 1/18 | WOMAC |
| Ismaiel et al., 2019 | Egypt | CSC VS PRP | 6months | 92 | 61.1 ± 11.6 | 62.9 ± 11.6 | 9/31 | 23/29 | VAS |
| Jubert et al., 2017 | Spain | CSC VS PRP | 6months | 65 | 68 ± 7.17 | 65.56 ± 8.6 | 6/24 | 6/23 | VAS |
| Khongwir et al., 2018 | India | HA VS CSC | 6months | 45 | 70.8 ± 4.82 | 71.2 ± 5.22 | — | — | WOMAC |
| Lin et al., 2019 | China | PRP VS HA VS PLA | 12months | 87 | PRP: 61.17 ± 13.08,HA:62.53 ± 9.9, PLA:62.2 ± 11.71 | PRP: 9/22,HA:10/19, PLA:10/17 | WOMAC | ||
| Louis et al.,2018 | France | PRP VS HA | 3months | 54 | 53.2 ± 11.7 | 48.5 ± 11.5 | 14/10 | 11/13 | WOMAC、VAS、Adverse effects |
| McAlindon et al.,2017 | USA | CSC VS PLA | 24months | 140 | 59.1 ± 8.3 | 57.2 ± 7.6 | 33/37 | 38/32 | Adverse effects |
| Naderi et al.,2018 | Iran | PRP VS CSC | 6months | 77 | 58.55 ± 8.79 | 59.09 ± 7.79 | 7/27 | 5/28 | VAS |
| Patel et al., 2013 | India | PRP VS PLA | 6months | 78 | 53.11 ± 11.55 | 53.65 ± 8.17 | 11/16 | 6/17 | VAS、Adverse effects |
| Spakova et al., 2012 | Slovakia | PRP VS HA | 6months | 120 | 52.80 ± 12.43 | 53.20 ± 14.53 | 33/27 | 31/29 | WOMAC、Adverse effects |
| Su et al., 2018 | China | PRP VS HA | 18months | 86 | 54.16 ± 6.56 | 53.13 ± 6.41 | 11/14 | 12/18 | WOMAC、VAS、Adverse effects |
| Tammachote et al., 2016 | Thailand | HA VS CSC | 6months | 99 | 62.6 | 61 | 7/43 | 13/46 | WOMAC、VAS |
| Wang et al., 2022 | China | PRP VS HA | 6months | 110 | 61.87 ± 5.46 | 63.00 ± 5.33 | 12/42 | 16/40 | WOMAC |
CSC: corticosteroids;HA: yaluronic acid ; PRP:latelet-rich plasma ; PLA: Placebo ; WOMAC:Western Ontario and McMaster Universities Osteoarthritis; VAS:visual analogue scale
Fig. 2.
Summary of the risk of bias
WOMAC scores
At the 3-month follow-up, 1319 patients were included in the study, with 15 reported WOMAC scores. The PRP groups performed the best in terms of the outcomes (SMD=-8.79; 95% CI-15.69~-1.89), followed by PRP + HA (SUCRA value, 61.2; mean rank, 2.6); HA (SUCRA value, 48.9; mean rank, 3); PLA (SUCRA value, 38.2; mean rank, 3.5); and CSC (SUCRA value, 17.3; mean rank, 4.3).
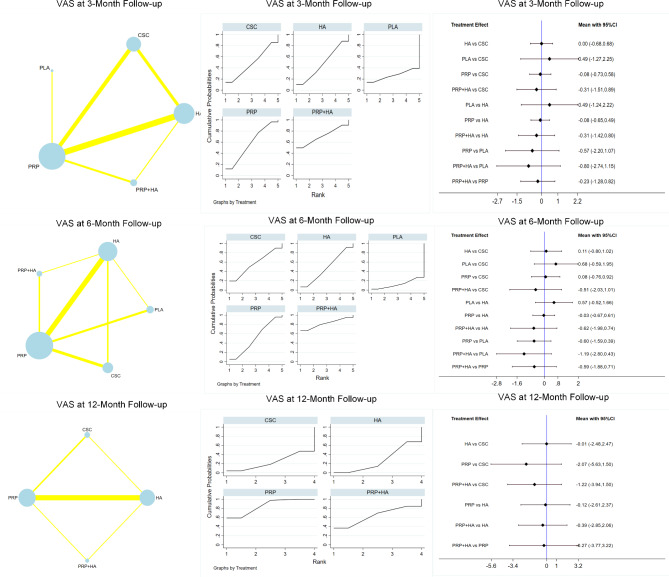
Twenty reported WOMAC scores at 6 months of follow-up, including a total of 2310 patients, the best outcomes were shown in the PRP groups (SMD=-11.92; 95% CI: -19.16~-4.69), which were followed by PRP + HA (SUCRA value, 64.2; mean rank, 2.4), HA (SUCRA value, 50.2; mean rank, 3.0), PLA (SUCRA value, 39.9; mean rank, 3.4), and CSC (SUCRA value, 6.7; mean rank, 4.7). Ten reported WOMAC scores at 12 months of follow-up, including a total of 1148 patients, the PRP groups performed the best (SMD=-7.04;95% CI: -9.38~-4.70), followed by PRP + HA (SUCRA value, 69.0; mean rank 2.2), HA (SUCRA value, 42.8; mean rank, 3.3), PLA (SUCRA value, 42.0; mean rank, 3.3), CSC (SUCRA value, 0.0; mean rank, 5.0). Table 2; Fig. 3 provide summaries of the network meta-analysis findings. No discrepancy between the direct and indirect effects of any intervention was observed as per the nodal analysis of the intervention measures (P > 0.05). Figure 3 compares the results based on the WOMAC scores at the 3, 6, and 12-month follow-ups.
Table 2.
Network meta-analysis treatment ranking results for each of WOMAC scores, VAS scores and adverse effects
| Treatment | WOMAC scores | VAS scores | Adverse effects | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SURCA (3month) |
Mean Rank | SURCA (6month) |
Mean Rank | SURCA (12month) |
Mean Rank | SURCA (3month) |
Mean Rank | SURCA (6month) |
Mean Rank | SURCA (12month) |
Mean Rank | SURCA | Mean Rank | |
| CSC | 17.3 | 4.3 | 6.7 | 4.7 | 0.0 | 5.0 | 48.2 | 3.1 | 56.7 | 2.7 | 23.3 | 3.3 | 64.2 | 2.8 |
| HA | 48.9 | 3.0 | 50.2 | 3.0 | 42.8 | 3.3 | 47.4 | 3.1 | 48.0 | 3.1 | 27.5 | 3.2 | 43.2 | 3.8 |
| PLA | 38.2 | 3.5 | 39.9 | 3.4 | 42.0 | 3.3 | 26.3 | 3.9 | 12.7 | 4.5 | 81.8 | 1.9 | ||
| PRP | 84.4 | 1.6 | 88.9 | 1.4 | 96.2 | 1.2 | 57.6 | 2.7 | 50.7 | 3.0 | 85.5 | 1.4 | 22.6 | 4.9 |
| PRP + HA | 61.2 | 2.6 | 64.2 | 2.4 | 69.0 | 2.2 | 70.5 | 2.2 | 81.8 | 1.7 | 63.7 | 2.1 | 81.2 | 1.9 |
| CSC + HA | 7.0 | 5.7 | ||||||||||||
Note: CSC :corticosteroids; HA: hyaluronic acid ; PRP: platelet-rich plasma; PLA: placebo
Fig. 3.
Overall network comparisons using WOMAC scores
VAS scores
In total, 1099 patients reported 15 VAS scores after 3 months of follow-up. PRP + HA had the best outcomes, with a SUCRA value of 70.5 and a mean rank of 2.2, followed by PRP (SUCRA value, 57.6; mean rank, 2.7), CSC (SUCRA value, 48.2; mean rank, 3.1), HA (SUCRA value, 47.4; mean rank, 3.1), and PLA (SUCRA value, 26.3; mean rank, 3.9). Eighteen reported VAS scores at 6 months of follow-up, including a total of 1732 patients, the PRP + HA groups showed the best outcomes (SUCRA value, 81.8; mean rank 1.7), followed by CSC (SUCRA value 56.7; mean rank, 2.7), PRP (SUCRA value, 50.7; mean rank, 3.0), HA (SUCRA value, 48.0; mean rank, 3.1), PLA (SUCRA value, 12.7; mean rank, 4.5). At the 12-month follow-up, a total of 656 patients reported 8 VAS scores, with the PRP + HA groups displaying the most favorable outcomes (SUCRA value, 85.5; mean rank, 1.4), followed by PRP + HA (SUCRA value, 63.7; mean rank, 2.1), HA (SUCRA value, 27.5; mean rank, 3.2), and CSC (SUCRA value, 23.3; mean rank, 3.3). The results of the network meta-analysis are summarized in Table 2; Fig. 4. No discrepancy between the direct and indirect effects of any intervention was observed as per the nodal analysis of the intervention measures (P > 0.05). Figure 4 compares the results based on the VAS scores at the 3, 6, and 12-month follow-ups.
Fig. 4.
Overall network comparisons using VAS scores
Safety
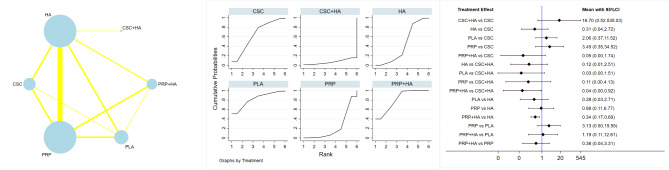
Among the 2576 patients with reported adverse effects, the PRP groups demonstrated the most favorable outcomes with a SUCRA value (81.8) and a mean rank (1.9), followed by PRP + HA (SUCRA value, 81.2; mean rank, 1.9), CSC (SUCRA value, 64.2; mean rank, 2.8), HA (SUCRA value, 27.5; mean rank, 3.2), PRP (SUCRA value, 22.6; mean rank, 4.9), and CSC + HA (SUCRA value, 7.0; mean rank 5.7). The results of the network meta-analysis are summarized in Table 2; Fig. 5. No discrepancy between the direct and indirect effects of any intervention was observed as per the nodal analysis of the intervention measures (P > 0.05). Figure 5 showed a comparison of results based on adverse effects.
Fig. 5.
Overall network comparisons using adverse effects
Discussion
After 3, 6, and 12 months of follow-up, the Bayesian network meta-analysis revealed that PRP and PRP + HA IAIs were superior to CSC, HA, and placebo in alleviating pain and improving joint function. However, no discernible changes between CSC, HA, and placebo were found. Regarding safety, the incidence of adverse events associated with the other interventions was not significantly higher than that of the placebo.
According to this study, PRP proved to be superior to PRP + HA, CSC, HA, and PLA in enhancing joint function. Additionally, PRP + HA was found to be better than PRP, CSC, HA, and PLA in reducing pain. The incidence of adverse events did not significantly increase with other interventions, as compared to placebo. According to a prior network meta-analysis, the PRP group was more effective than CSC, HA, and placebo [28]. In the research by Zhao and his colleagues, the PRP + HA scheme was shown to be more effective than PRP alone in alleviating knee pain and raising the WOMAC overall score [29]. Compared with lower-molecular-weight hyaluronic acid, the highest-molecular-weight hyaluronic acid may be more efficacious in treating knee OA [30]. However, viscosupplementation is associated with an increased risk for serious adverse events [31]. Another study showed that intraarticular CS is more effective on pain relief than intraarticular HA in short term (up to 1 month), while HA is more effective in long term (up to 6 months) [32]. Autologous blood can be subjected to centrifugation to extract PRP, which can increase the platelet concentration by nearly ten times [33]. Upon activation, it exhibits the ability to discharge macrophages and growth factors, consequently promoting the elimination of necrotic tissue, reducing the inflammatory reaction, and facilitating the repair and regeneration of articular cartilage [34, 35]. HA, an essential element of synovial fluid and articular cartilage [10], is a polysaccharide with a high molecular weight. Injecting HA into the knee joint cavity can physically lubricate the joint surface, reduce erosion, biologically nourish the articular cartilage, and stimulate the production of endogenous HA, thereby delaying the onset of additional joint disease [36, 37]. Besides, HA has also been proven effective in obese individuals [38]. According to Marmotti et al. [39], the incorporation of HA into PRP has been found to significantly enhance the growth of chondrocytes and enhance cartilage regeneration capabilities. PRP and HA have been shown in studies to synergistically increase the functioning of signaling molecules such as inflammatory molecules, catabolic enzymes [40], cytokines, and growth factors, thus contributing to the successful treatment of KOA [41].
Some studies are consistent with the results of this study [42, 43], which found that CSC and HA showed similar results compared to placebo. However, there are also other studies that have reached different conclusions [30, 44, 45], finding that CSC and HA are more effective than placebo. The study demonstrated that the analgesic efficacy of the two therapies varied with time. Particularly, the VAS score of the intra-articular CSC group was considerably lower than that of the intra-articular HA group after 1 month, suggesting that CSC had a higher short-term analgesic impact than HA. However, in the long run, HA exhibited a greater analgesic effect than CSC [32]. No significant difference in pain relief was found between HA and placebo(saline)by Colen et al. [46]. According to a meta-analysis, intra-articular corticosteroid injection is an effective treatment for pain relief with no increase in treatment-related adverse reactions when compared to placebo [47]. Najm et al. discovered that CSC decreased pain and increased function early after administration (≤ 6 weeks) compared to placebo. However, there were no clinical improvements when compared to HA [10]. Based on our analysis, the only treatments that clinically showed improvement in both cases were PRP and PRP + HA. The effectiveness of CCS and HA is uncertain. Although treating KOA with PRP and HA combination may be more expensive and difficult, it may still be a preferable option to the expenses and risks of surgery. Nevertheless, there is still a shortage of cost-effectiveness studies that examine the combination of PRP and HA for KOA treatment, as well as studies that investigate PRP or HA alone, indicating a need for further research.
There are several limitations to this study: First of all, the main limiting factor is the lack of available data between the included studies. Secondly, some authors conducted a single injection, whereas others performed repeated injections. Thirdly, the duration of treatment and follow-up was diverse. Fourthly, we only included studies written in English, which may result in the loss of some research data. Lastly, the use of different formulations in different studies of HA may lead to bias.
Conclusions
The study’s SUCRA value backs the application of PRP and PRP + HA for appropriate patients with KOA. PRP is likely the most effective pain-relieving treatment with the lowest incidence of adverse effects, followed by PRP + HA. The differences in treatment effects were minor and might not have any significant impact on clinical outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
To the best of our knowledge, no conflict of interest, financial or other.
Authors’ contributions
XQ, XY and ZT conceived and designed the study. LY and YF developed the search strategy and did the literature search. XL and KZ assessed the quality of study. LY ZL, CX and XQ collected the data and performed all analysis. XQ, LY, FL and SZ contributed to writing of original manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of Shanxi Province [20210302124410; 201901D111373; 201903D321156], The Second Hospital of Shanxi Medical University, and four research funding projects within the hospital [2020001-10].
Data Availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaochen Qiao, Lei Yan and Yi Feng have contributed equally to this work.
Contributor Information
Xihua Yang, Email: yangxihua@126.com.
Zhi Tian, Email: drtianzh@sxmu.edu.cn.
References
- 1.Wang AT, Feng Y, Jia HH, Zhao M, Yu H. Application of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee: a concise review. World J Stem Cells. 2019;11:222–35. doi: 10.4252/wjsc.v11.i4.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An S, Li J, Xie W, Yin N, Li Y, Hu Y. Extracorporeal shockwave treatment in knee osteoarthritis: therapeutic effects and possible mechanism. Biosci Rep. 2020;40(11):BSR20200926. doi: 10.1042/BSR20200926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michael JW, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107:152–62. doi: 10.3238/arztebl.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma L. Osteoarthritis of the knee. N Engl J Med. 2021;384:51–9. doi: 10.1056/NEJMcp1903768. [DOI] [PubMed] [Google Scholar]
- 5.Kan HS, Chan PK, Chiu KY, Yan CH, Yeung SS, Ng YL, et al. Non-surgical treatment of knee osteoarthritis. Hong Kong Med J. 2019;25:127–33. doi: 10.12809/hkmj187600. [DOI] [PubMed] [Google Scholar]
- 6.Siddiq MAB, Clegg D, Jansen TL, Rasker JJ. Emerging and New Treatment options for knee osteoarthritis. Curr Rheumatol Rev. 2022;18:20–32. doi: 10.2174/1573397117666211116111738. [DOI] [PubMed] [Google Scholar]
- 7.Cross M, Smith E, Hoy D, Nolte S, AcKerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–30. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 8.Nunes-Tamashiro JC, Natour J, Ramuth FM, Toffolo SR, Mendes JG, Rosenfeld A, et al. Intra-articular injection with platelet-rich plasma compared to triamcinolone hexacetonide or saline solution in knee osteoarthritis: a double blinded randomized controlled trial with one year follow-up. Clin Rehabil. 2022;36:900–15. doi: 10.1177/02692155221090407. [DOI] [PubMed] [Google Scholar]
- 9.Wang R, Ma C, Han Y, Tan M, Lu L. Effectiveness of Denervation Therapy on Pain and joint function for patients with refractory knee osteoarthritis: a systematic review and Meta-analysis. Pain Physician. 2019;22:341–52. [PubMed] [Google Scholar]
- 10.Anil U, Markus DH, Hurley ET, Manjunath AK, Alaia MJ, Campbell KA, et al. The efficacy of intra-articular injections in the treatment of knee osteoarthritis: a network meta-analysis of randomized controlled trials. Knee. 2021;32:173–82. doi: 10.1016/j.knee.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Sconza C, Leonardi G, Carfì C, Kon E, Respizzi S, Scaturro D, et al. Intra-articular injection of Botulinum Toxin for the treatment of knee osteoarthritis: a systematic review of Randomized controlled trials. Int J Mol Sci. 2023;24. 10.3390/ijms24021486 [DOI] [PMC free article] [PubMed]
- 12.Sconza C, Di Matteo B, Queirazza P, Dina A, Amenta R, Respizzi S, et al. Ozone therapy versus Hyaluronic Acid Injections for Pain Relief in patients with knee osteoarthritis: preliminary findings on Molecular and Clinical Outcomes from a Randomized Controlled Trial. Int J Mol Sci. 2023;24. 10.3390/ijms24108788 [DOI] [PMC free article] [PubMed]
- 13.Belk JW, Kraeutler MJ, Houck DA, Goodrich JA, Dragoo JL, McCarty EC. Platelet-Rich plasma Versus Hyaluronic acid for knee osteoarthritis: a systematic review and Meta-analysis of Randomized controlled trials. Am J Sports Med. 2021;49:249–60. doi: 10.1177/0363546520909397. [DOI] [PubMed] [Google Scholar]
- 14.Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi: 10.1186/s12891-015-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arrich J, Piribauer F, Mad P, Schmid D, Klaushofer K, Müllner M. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. CMAJ. 2005;172:1039–43. doi: 10.1503/cmaj.1041203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325:568–78. doi: 10.1001/jama.2020.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578–89. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu M, Higuchi H, Takagishi K, Shinozaki T, Kobayashi T. Clinical and biochemical characteristics after intra-articular injection for the treatment of osteoarthritis of the knee: prospective randomized study of sodium hyaluronate and corticosteroid. J Orthop Sci. 2010;15:51–6. doi: 10.1007/s00776-009-1421-0. [DOI] [PubMed] [Google Scholar]
- 19.Szwedowski D, Szczepanek J, Paczesny Ł, Zabrzyński J, Gagat M, Mobasheri A, et al. The effect of platelet-rich plasma on the intra-articular microenvironment in knee osteoarthritis. Int J Mol Sci. 2021;22. 10.3390/ijms22115492 [DOI] [PMC free article] [PubMed]
- 20.Huang G, Hua S, Yang T, Ma J, Yu W, Chen X. Platelet-rich plasma shows beneficial effects for patients with knee osteoarthritis by suppressing inflammatory factors. Exp Ther Med. 2018;15:3096–102. doi: 10.3892/etm.2018.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tonutti A, Granata V, Marrella V, Sobacchi C, Ragusa R, Sconza C, et al. The role of WNT and IL-1 signaling in osteoarthritis: therapeutic implications for platelet-rich plasma therapy. Front Aging. 2023;4:1201019. doi: 10.3389/fragi.2023.1201019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peck J, Slovek A, Miro P, Vij N, Traube B, Lee C, et al. A Comprehensive Review of Viscosupplementation in Osteoarthritis of the knee. Orthop Rev. 2021;13:25549. doi: 10.52965/001c.25549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang SZ, Wu DY, Chang Q, Guo YD, Wang C, Fan WM. Intra-articular, single-shot co-injection of hyaluronic acid and corticosteroids in knee osteoarthritis: a randomized controlled trial. Exp Ther Med. 2018;16:1928–34. doi: 10.3892/etm.2018.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y, Liu X, Xu X, Liu J. Intra-articular injections of platelet-rich plasma, hyaluronic acid or corticosteroids for knee osteoarthritis: a prospective randomized controlled study. Orthopade. 2019;48:239–47. doi: 10.1007/s00132-018-03659-5. [DOI] [PubMed] [Google Scholar]
- 25.Han SB, Seo IW, Shin YS. Intra-articular injections of Hyaluronic acid or steroids Associated with Better Outcomes Than platelet-rich plasma, adipose mesenchymal stromal cells, or placebo in knee osteoarthritis: a Network Meta-analysis. Arthroscopy. 2021;37:292–306. doi: 10.1016/j.arthro.2020.03.041. [DOI] [PubMed] [Google Scholar]
- 26.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 27.Shim S, Yoon BH, Shin IS, Bae JM. Network meta-analysis: application and practice using Stata. Epidemiol Health. 2017;39:e2017047. doi: 10.4178/epih.e2017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Migliorini F, Driessen A, Quack V, Sippel N, Cooper B, Mansy YE, et al. Comparison between intra-articular infiltrations of placebo, steroids, hyaluronic and PRP for knee osteoarthritis: a bayesian network meta-analysis. Arch Orthop Tauma Surg. 2021;141:1473–90. doi: 10.1007/s00402-020-03551-y. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Huang H, Liang G, Zeng LF, Yang W, Liu J. Effects and safety of the combination of platelet-rich plasma (PRP) and hyaluronic acid (HA) in the treatment of knee osteoarthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2020;21:224. doi: 10.1186/s12891-020-03262-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo GH, LaValley M, McAlindon T, Felson DT. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA. 2003;290:3115–21. doi: 10.1001/jama.290.23.3115. [DOI] [PubMed] [Google Scholar]
- 31.Rutjes AW, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157:180–91. doi: 10.7326/0003-4819-157-3-201208070-00473. [DOI] [PubMed] [Google Scholar]
- 32.He WW, Kuang MJ, Zhao J, Sun L, Lu B, Wang Y, et al. Efficacy and safety of intraarticular hyaluronic acid and corticosteroid for knee osteoarthritis: a meta-analysis. Int J Surg. 2017;39:95–103. doi: 10.1016/j.ijsu.2017.01.087. [DOI] [PubMed] [Google Scholar]
- 33.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158–67. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Mascarenhas R, Saltzman BM, Fortier LA, Cole BJ. Role of platelet-rich plasma in articular cartilage injury and disease. J Knee Surg. 2015;28:3–10. doi: 10.1055/s-0034-1384672. [DOI] [PubMed] [Google Scholar]
- 35.Shahid M, Kundra R. Platelet-rich plasma (PRP) for knee disorders. EFORT Open Rev. 2017;2:28–34. doi: 10.1302/2058-5241.2.160004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee CL, Wang YC, Huang HT, Chen CH, Chang KL, Tien YC. Efficacy of Intra-Articular Injection of Biofermentation-Derived High-Molecular Hyaluronic acid in knee osteoarthritis: an Ultrasonographic Study. Cartilage. 2022;13:19476035221077404. doi: 10.1177/19476035221077404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komatsu S, Iwata H, Nabeshima T. Studies on the kinetics, metabolism and re-utilisation after intra-articular administration of hyaluronan to rabbits. Arzneimittelforschung. 1999;49:427–33. doi: 10.1055/s-0031-1300438. [DOI] [PubMed] [Google Scholar]
- 38.Scaturro D, Vitagliani F, Terrana P, Cuntrera D, Falco V, Tomasello S, et al. Intra-articular hybrid Hyaluronic Acid Injection treatment in overweight patients with knee osteoarthritis: a Single-Center, Open-Label, prospective study. Appl Sciences-Basel. 2021;11. 10.3390/app11188711
- 39.Marmotti A, Bruzzone M, Bonasia DE, Castoldi F, Rossi R, Piras L, et al. One-step osteochondral repair with cartilage fragments in a composite scaffold. Knee Surg Sports Traumatol Arthrosc. 2012;20:2590–601. doi: 10.1007/s00167-012-1920-y. [DOI] [PubMed] [Google Scholar]
- 40.Lana JF, Weglein A, Sampson SE, Vicente EF, Huber SC, Souza CV, et al. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J Stem Cells Regen Med. 2016;12:69–78. doi: 10.46582/jsrm.1202011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saturveithan C, Premganesh G, Fakhrizzaki S, Mahathir M, Karuna K, Rauf K, et al. Intra-articular hyaluronic acid (HA) and platelet Rich plasma (PRP) injection versus hyaluronic acid (HA) injection alone in patients with Grade III and IV knee osteoarthritis (OA): a retrospective study on functional outcome. Malays Orthop J. 2016;10:35–40. doi: 10.5704/MOJ.1607.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Askari A, Gholami T, NaghiZadeh MM, Farjam M, Kouhpayeh SA, Shahabfard Z. Hyaluronic acid compared with corticosteroid injections for the treatment of osteoarthritis of the knee: a randomized control trail. Springerplus. 2016;5:442. doi: 10.1186/s40064-016-2020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leighton R, Akermark C, Therrien R, Richardson JB, Andersson M, Todman MG, et al. NASHA hyaluronic acid vs. methylprednisolone for knee osteoarthritis: a prospective, multi-centre, randomized, non-inferiority trial. Osteoarthritis Cartilage. 2014;22:17–25. doi: 10.1016/j.joca.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Gaffney K, Ledingham J, Perry JD. Intra-articular triamcinolone hexacetonide in knee osteoarthritis: factors influencing the clinical response. Ann Rheum Dis. 1995;54:379–81. doi: 10.1136/ard.54.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones A, Doherty M. Intra-articular corticosteroids are effective in osteoarthritis but there are no clinical predictors of response. Ann Rheum Dis. 1996;55:829–32. doi: 10.1136/ard.55.11.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colen S, van den Bekerom MP, Mulier M, Haverkamp D. Hyaluronic acid in the treatment of knee osteoarthritis: a systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs. 2012;26:257–68. doi: 10.2165/11632580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 47.Saltychev M, Mattie R, McCormick Z, Laimi K. The magnitude and duration of the Effect of Intra-articular Corticosteroid Injections on Pain Severity in knee osteoarthritis: a systematic review and Meta-analysis. Am J Phys Med Rehabil. 2020;99:617–25. doi: 10.1097/PHM.0000000000001384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].