Abstract
This study was designed to provide evidence of the neuroprotective of human adipose–derived mesenchymal stem cells (hADSCs) in oxygen-induced retinopathy (OIR). In vivo, hADSCs were intravitreally injected into OIR mice. Various assessments, including HE (histological evaluation), TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) staining, electroretinogram (ERG) analysis, and retinal flat-mount examination, were performed separately at postnatal days 15 (P15) and 17 (P17) to evaluate neurological damage and functional changes. Western blot analysis of ciliary neurotrophic factor (CNTF), glial cell line–derived neurotrophic factor (GDNF), and brain-derived neurotrophic factor (BDNF) was conducted at P17 to elucidate the neuroprotective mechanism. The P17 OIR group exhibited a significant increase in vascular endothelial cell nuclei and neovascularization that breached the ILM (inner limiting membrane) to the P17 control group. In addition, the retinal nonperfusion areas in the P17 OIR group and the number of apoptotic retinal cells in the P15 OIR group were significantly higher than in the corresponding hADSCs treatment group and control group. There was no significant thickness change in the inner nuclear layer (INL) but the outer nuclear layer (ONL) in the P17 OIR treatment group compared with the P17 OIR group. The cell density in the INL and ONL at P17 in the hADSCs treatment group was not significantly different from the OIR group. The amplitude of a-wave and b-wave in scotopic ERG analysis for the P17 OIR group was significantly lower than in the P17 hADSCs treatment group and the P17 control group. Furthermore, the latency of the a-wave and b-wave in the P17 OIR group was significantly longer than in the P17 hADSCs treatment group and the P17 control group. In addition, the expression levels of CNTF and BDNF in the P17 OIR group were statistically higher than those in the P17 control group, whereas the expression of GDNF was statistically lower in the P17 OIR group, compared with the P17 control group. The expression of CNTF and GDNF in the P17 hADSCs treatment group was statistically higher than in the P17 OIR group. However, the expression of BDNF in the P17 hADSCs treatment group was statistically lower than in the P17 OIR group. This study provides evidence for the neuroprotective effects of hADSCs in OIR.
Keywords: human adipose–derived mesenchymal stem cells, oxygen-induced retinopathy, histological evaluation, TUNEL, electroretinogram, neuroprotection, ciliary neurotrophic factor, glial cell line–derived neurotrophic factor, brain-derived neurotrophic factor
Introduction
Diabetic retinopathy (DR), retinal vein occlusion (RVO), ROP (retinopathy of prematurity), and other ischemic retinopathy can cause irreversible vision loss, and the incidence of these diseases is increased yearly1,2. Ischemic retinopathy’s pathological mechanism involves hemorrhage and edema resulting from vascular leakage3,4 and neuronal death due to glycolysis and reduced oxidative phosphorylation rates 5 . Although anti-vascular endothelial growth factor (VEGF) therapy effectively inhibits neovascularization, reduces edema 6 , and is widely used in clinical trials, studies have shown that 15% to 20% of DR patients 7 do not respond adequately or fully to anti-VEGF therapy. Moreover, the extent of VEGF’s role in neural cell survival is crucial8,9, and chronic inhibition of VEGF-A has been linked to a significant retinal ganglion cell loss 10 . Considering the need for repetitive injections and associated risks, there is a critical need to develop new treatments to reduce neovascularization and neural damage.
Mesenchymal stem cell (MSC) therapy, owing to its unique advantages11,12, such as cell replacement, paracrine, and lack of immune rejection, holds promise for treating retinal diseases. It has also been proven safe for intraocular use in oxygen-induced retinopathy (OIR) 13 . Among the various sources of MSCs, adipose-derived mesenchymal stem cells (ADSCs) are relatively easy to obtain and have higher proliferation rates than bone marrow–derived mesenchymal stem cells (BMSCs), making them particularly attractive14,15. Studies have already demonstrated that MSCs can reduce neovascularization in ischemic retinopathy16,17. However, whether the MSC offers neuroprotection in ischemic retinopathy has yet to be thoroughly investigated. In this study, we intravitreally injected human adipose–derived mesenchymal stem cells (hADSCs) into OIR mice, a well-established model for ischemic retinopathy. Histological evaluation (HE), retina flat mount, TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling), and electroretinogram (ERG) were performed to assess changes in neuro function. In addition, Western blot analysis was used to evaluate the expression of the ciliary neurotrophic factor (CNTF), the glial cell line–derived neurotrophic factor (GDNF), and the brain-derived neurotrophic factor (BDNF) to elucidate the underlying mechanism of hADSCs action. This study provides valuable data supporting the neuroprotective potential of hADSCs in ischemic retinopathy.
Methods
Culture and Characterization of HADSCs
The second passage of hADSCs was obtained from the Tissue Engineering Center of Peking Union Medical College, China. The hADSCs were cultured in a humidified incubator, and 48 h later, half of the culture medium was replaced. Subculturing was performed when the cells reached 80% confluence. Flow cytometry was used to characterize the third passage of hADSCs by analyzing specific surface antigens, including CD29, CD34, CD44, CD105, Flk-1, and HLA-DR (BD Biosciences, Franklin Lakes, NJ, USA), according to the manufacturer’s instructions. Data analysis was conducted using SPSS 23.0 (IBM Corp., Armonk, NY).
Oxygen-Induced Retinopathy Model
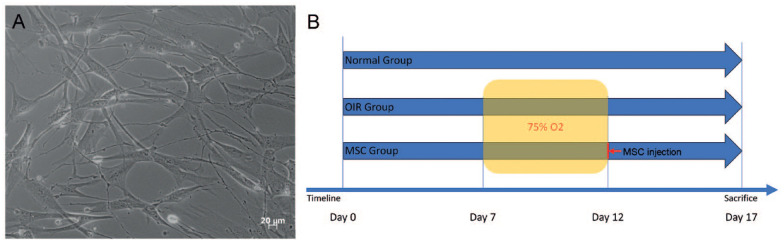
Pregnant C57BL/6J mice were provided by the Laboratory Animal Center of Southern Medical University, China. All animal experiments followed the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and received approval from the local animal welfare committee. Mice pups were maintained in a normal environment without any treatment (control group) or exposed to 75% ± 2% oxygen for 5 days from P7 to P12 (OIR group) along with their mothers as described by Smith et al. 18 After exposure, half of the OIR mice were maintained in a normal environment, whereas the other half received intravitreal injections of hADSCs at P12 (hADSCs treatment group). Relevant tests were conducted at P17. A schematic diagram illustrates the treatment and examination time points (Fig. 1B).
Figure 1.
Culture of hADSCs and experimental timeline. (A) The third passage of hADSCs displays spindle and polygonal shapes. (B) A schematic diagram illustrating the experimental time points. hADSCs: human adipose–derived mesenchymal stem cells; OIR: oxygen-induced retinopathy; MSC: mesenchymal stem cell.
Intravitreal Injection
Mice were anesthetized using intraperitoneal injection of 10% chloral hydrate (2.5 mL/kg). The eyes were dilated with 0.5% tropicamide. Following anterior chamber paracentesis to release aqueous humor, 1 µL hADSCs (1 × 105/mL) was intravitreally injected using a 33-G Hamilton syringe (Hamilton Company, Reno, NV, USA), positioned 1 mm behind the corneoscleral limbus and removed after 30 s. Erythromycin ointment was applied to prevent infection.
Histological Evaluation
Enucleated eyes were fixed with 4% paraformaldehyde, dehydrated in gradient ethanol and xylene, and embedded in paraffin wax. Retinal slices, 4 μm in thickness and parallel to the sagittal axis of the optic nerve, were obtained and processed for HE. Vascular endothelial cell (VEC) nuclei that broke the retina’s inner limiting membrane (ILM) were counted under 10X magnification in one slice. The cell density described previously by Lee et al. 19 and the thickness of inner nuclear layer (INL) and outer nuclear layer (ONL) of the central retinal area were analyzed. In addition, HE was performed on five eyes in each control, OIR, and hADSCs treatment group at P17.
Retinal Flat Mount
Mice pups were anesthetized and received retro-orbital injections of fluorescein isothiocyanate dextran 20 . Thereafter, 10 s after injection, the mice were euthanized with pentobarbital. Enucleated eyes were fixed in 4% paraformaldehyde for 30 min at room temperature. The retinas were separated, cut into four parts, and mounted. Retinal flat mounts were photographed using a fluorescence microscope (Zeiss Axioplan 2 Imaging, Zeiss, Gottingen, Germany) to generate images of the entire retina. Retinal flat mount from five eyes in each control, OIR, and hADSCs treatment group were performed at P17.
Terminal Deoxynucleotidyl Transferase Dutp Nick End Labeling (TUNEL)
Retina assays were performed according to the manufacturer’s instructions (Fluorescein Kit, Roche, Roche, Switzerland, 11684795910). The assay utilized the green channel, with DAPI staining in the blue channel. Images were captured using a fluorescence microscope (Zeiss Axioplan 2 Imaging, Zeiss, Gottingen, Germany). The TUNEL assay was performed on five eyes in each control, OIR, and hADSCs treatment group at P15.
Electroretinogram
After dark adaptation for 12 h, mice were anesthetized using intraperitoneal injection of 10% chloral hydrate (2.5 mL/kg). The eyes were dilated with 0.5% tropicamide. Following the application of a small drop of 2.5% hypromellose, a ring-shaped contact electrode was gently positioned on the cornea. Reference and ground electrodes were appropriately placed under the tongue and tail, respectively. Stimulation and recording of the ERGs were performed using the RETIscan system (Roland Consult, Brandenburg, Germany). The scotopic flash ERG was recorded with a white flash at an intensity ranging from 0.01 to 3.7 cd.s/m2. The ERG recordings were obtained from five eyes in each control, OIR, and hADSCs treatment group performed at P17.
Western Blot Analysis
Retinal protein was extracted and eluted using Laemmli buffer, then separated on a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Subsequently, the proteins were transferred onto a polyvinylidene fluoride membrane. The membrane was then blocked with 5% dried nonfat milk and incubated in Tris-buffered saline containing 0.1% Tween-20 (TBST) at room temperature for 2 h. After three TBST washes (10 min each time), the membrane was incubated overnight at 4°C with primary antibodies. The primary antibodies used in this study included Anti-CNTF antibody (1:200, Santa Cruz Biotechnology, TX, USA, sc-365210), Anti-GDNF antibody (1:1,000, Santa Cruz Biotechnology, TX, USA, sc-13147), Anti-BDNF antibody (1:500, Santa Cruz Biotechnology, TX, USA, sc-546), and Anti-beta Actin antibody (1:5,000, Abcam, MA, USA, ab6276). Subsequently, the membrane underwent three additional TBST washes (10 min each time) and was then incubated with goat anti-mouse secondary antibody (1:10,000, Invitrogen, CA, USA, 31430) at room temperature for 1 h. Finally, the membrane was subjected to enhanced chemiluminescence and detected using photographic film. Western blot analysis was performed on samples from the control, OIR, and hADSCs treatment groups at P17.
Statistical Analysis
Data are expressed as mean ± standard deviation. Statistical analysis was conducted using SPSS 23.0 (IBM Corp., Armonk, NY). Differences were conducted using one-way analysis of variance (ANOVA) followed by Fisher least significant difference (LSD) test. P < 0.05 was considered statistically significant.
Results
Culture and Characterization of HADSCs
The third passage of HADSCs exhibited healthy growth and displayed spindle and polygonal shapes (Fig. 1A). Flow cytometry showed that the hADSCs expressed high levels of CD29 (95.3%), CD44 (99.8%), CD105 (99.7%), and Flk-1 (99.0%), and low levels of the endothelial marker CD34 (0.8%) and HLA-DR (0.5%).
Histological Evaluation
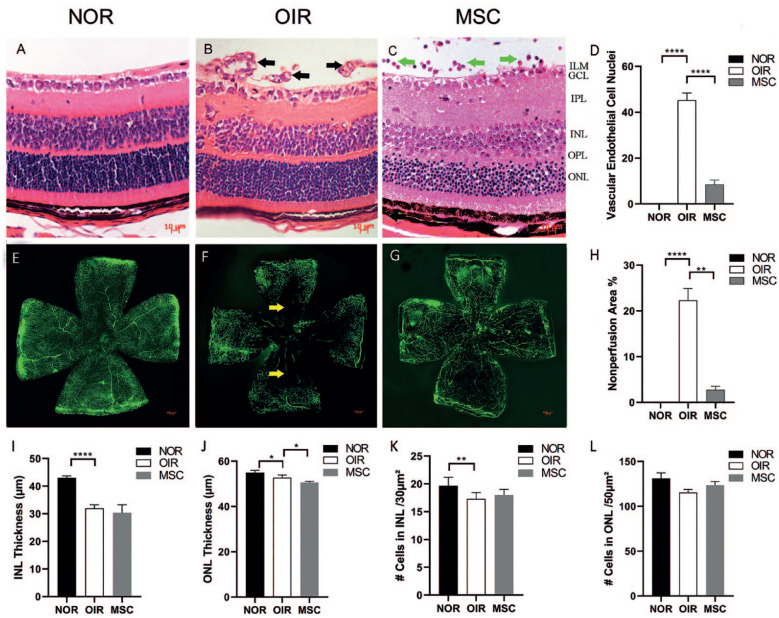
In the P17 control group, no VEC nuclei or neovascularization that breached the ILM of the retina were observed (Fig. 2A). The P17 OIR group showed that numerous VEC nuclei and neovascularization breached the ILM, with the VEC nuclei numbering 45.3 ± 3.06 (Fig. 2B). In the P17 hADSCs treatment group, VEC nuclei and neovascularization breaking through the retina ILM were infrequent, with the number of VEC nuclei being 8.3 ± 1.53 (Fig. 2C). The injected cells were located in the vitreous cavity and above the retina. A few number of infiltrating cells were discernible within the retinal tissue. Compared with the P17 control group, the P17 OIR group showed a significant increase in the number of VEC nuclei that broke through the ILM (P < 0.0001). The P17 hADSCs treatment group exhibited a significant decrease in the number of VEC nuclei breaking through the ILM compared with the P17 OIR group (P < 0.0001; Fig. 2D). The cells density and thickness of the INL in the P17 normal group, the OIR group, and hADSCs treatment group were 19.67 ± 1.53 cells/30 µm², 17.33 ± 1.15 cells/30 µm², and 18 ± 1 cells/30 µm², and 43.04 ± 0.76 µm, 31.94 ± 1.39 µm, and 30.37 ± 2.93 µm, respectively. The cells density and thickness of the ONL in the P17 normal group, the OIR group, and hADSCs treatment group were 131 ± 6.23 cells/50 µm², 115.3 ± 3.51 cells/50 µm², and 123.67 ± 4.04 cells/50 µm², and 54.94 ± 1.02 µm, 52.7 ± 1.25 µm, and 50.67 ± 0.49 µm, respectively. Statistical analysis revealed a significant reduction in both INL and ONL thickness in the P17 OIR group compared with the P17 normal group (P = 0.000 and P = 0.030; Fig. 2I, 2J). There was no significant change in INL thickness, but ONL thickness was reduced in the P17 OIR treatment group compared with the P17 OIR group (P = 0.358 and P = 0.043; Fig. 2I, J). Moreover, the cell density in the INL at P17 in the OIR group was significantly lower than in the P17 normal group (P = 0.007; Fig. 2K), with no significant difference compared with the P17 hADSCs treatment group (P = 0.075; (Fig. 2K). Furthermore, there were no significant differences in cell density within the ONL between the P17 control group and the hADSCs treatment group compared with the P17 OIR group (P = 0.062 and P = 0.537; Fig. 2L).
Figure 2.
The HE and retinal flat mount results. (A) The HE of the P17 control group. No VEC nuclei and neovascularization breached the retina’s ILM. (B) The HE of the P17 OIR group. Numerous VEC nuclei and neovascularization breached the ILM (black arrows). (C) The HE of the P17 hADSCs treatment group. Few VEC nuclei and neovascularization broke through the ILM, and the injected cells were observed in the vitreous cavity and above the retina (green arrows). (D) Quantification of VEC nuclei that breached the retina`s ILM. The P17 OIR group exhibited a significant increase compared with the P17 control group (P < 0.0001) and the P17 hADSCs treatment group (P < 0.0001). (n = 5) (E) Retinal flat mount of the P17 control group. No apparent neovascular fluorescence and nonperfusion areas were observed. (F) Retinal flat mount of the P17 OIR group. Extensive neovascular fluorescence and nonperfusion areas (yellow arrows) were evident. (G) Retinal flat mount of the P17 hADSCs treatment group. Slight neovascular fluorescence and no apparent nonperfusion areas were detected. (H) The extent of retinal nonperfusion areas. The P17 OIR group exhibited a significant increase compared with the P17 control group (P < 0.0001) and a significant decrease compared with the P17 hADSCs treatment group (P < 0.0001). (I, J) INL and ONL thickness. Significant reduction in both INL and ONL thickness in the P17 OIR group compared with the P17 normal group (P = 0.000 and P = 0.030; Fig. 2I, J). There was no significant change in INL thickness, but ONL thickness was reduced in the P17 OIR treatment group compared with the P17 OIR group (P = 0.358 and P = 0.043). (K, L) Cells in INL and ONL. The cell density in the INL at P17 OIR group was significantly lower than in the P17 normal group (P = 0.007), with no significant difference compared with the P17 hADSCs treatment group (P = 0.075). There were no significant differences within the ONL between the P17 control group and the hADSCs treatment group compared with the P17 OIR group (P = 0.062 and P = 0.537; n = 5). HE: Histological evaluation; VEC: vascular endothelial cell; ILM: inner limiting membrane; NOR: normal group; OIR: oxygen-induced retinopathy; hADSCs: human adipose–derived mesenchymal stem cells; INL: inner nuclear layer; ONL: outer nuclear layer; MSC: mesenchymal stem cell; GCL: ganglion cell layer; IPL: inner plexiform layer; OPL: outer plexiform layer; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Retinal Nonperfusion Areas
In the P17 control group, no apparent neovascular fluorescence or nonperfusion areas were observed (Fig. 2E). The P17 OIR group displayed extensive neovascular fluorescence and nonperfusion areas, with nonperfusion areas measuring 22.3% ± 1.20% (Fig. 2F). In the P17 hADSCs treatment group, slight neovascular fluorescence was observed, and nonperfusion areas were not apparent, measuring only 2.79% ± 0.30% (Fig. 2G). The retinal nonperfusion areas in the P17 OIR group significantly increased compared with the P17 control group (P < 0.0001) but significantly decreased compared with the P17 hADSCs treatment group (P < 0.0001; Fig. 2H).
Terminal Deoxynucleotidyl Transferase Dutp Nick End Labeling (TUNEL)
In the P15 control group, no apparent TUNEL-positive cells were observed (Fig. 3B), with only 0.333 ± 0.577 positive cells per field. The OIR group showed numerous TUNEL-positive cells, primarily located in the INL (Fig. 3E), with the number of positive cells reaching 21.667 ± 1.528 per field. In the hADSCs treatment group, some TUNEL-positive cells were present (Fig. 3H), with the number of positive cells amounting to 9.176 ± 1.023 per field. The number of positive cells in the P15 OIR group was significantly higher than in both the P15 control (P < 0.0001) and the hADSCs treatment group (P < 0.0001).
Figure 3.

TUNEL results. (A, B, C) TUNEL results from the P15 control group. No apparent TUNEL-positive cells were observed. (D, E, F) TUNEL results from the P15 OIR group. A substantial number of TUNEL-positive cells were evident (white arrows), primarily localized in the INL. (G, H, I) TUNEL results from the P15 hADSCs treatment group. Some TUNEL-positive cells were observed. OIR: oxygen-induced retinopathy; INL: inner nuclear layer; hADSCs: human adipose–derived mesenchymal stem cells; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling.
Electroretinogram
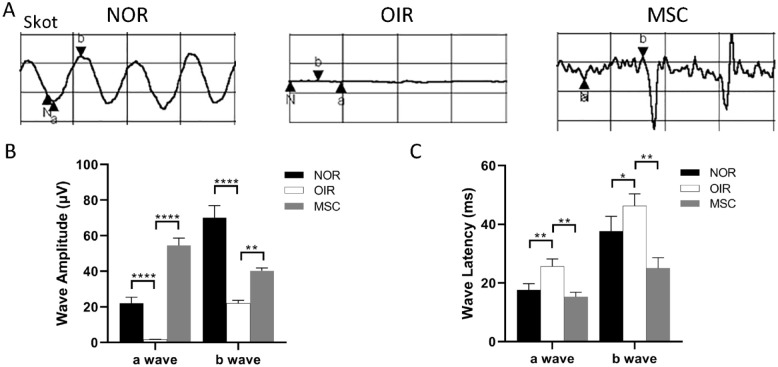
Scotopic ERG measurements showed that, in the P17 control group, P17 OIR group, and P17 hADSCs treatment group, the amplitudes of a-wave were 22 ± 2.89 μν, 1.7 ± 0.173 μν, and 54.67 ± 4.041 μν, respectively, while the amplitudes of b-wave were 70 ± 6.03 μν, 22.2 ± 1.732 μν, 40.33 ± 1.528 μν, respectively. The amplitudes of both the a-wave and b-wave in the P17 OIR group were significantly lower than in the P17 control group (P < 0.0001 for both). The amplitudes of both the a-wave and b-wave in the P17 hADSCs treatment group were significantly higher than in the P17 OIR group (P < 0.0001 for a-wave, P < 0.001 for b-wave; Fig. 4A, B). The latencies of a-wave and b-wave in the P17 control group, P17 OIR group, and P17 hADSCs treatment group were as follows: a-wave—18 ± 2.00 ms, 25.67 ± 2.52 ms, and 15.33 ± 1.53 ms; b-wave—38 ± 5.03 ms, 46 ± 3.79 ms, and 25.33 ± 3.61 ms, respectively. The latencies of both the a-wave and b-wave in the P17 OIR group were significantly longer than in the P17 control group (P = 0.003 for a-wave, P = 0.047 for b-wave). The latencies of both the a-wave and b-wave in the P17 hADSCs treatment group were significantly shorter than in the P17 OIR group (P = 0.001 for both; Fig. 4A, C).
Figure 4.
Electroretinogram results. (A, B) Amplitudes of the a-wave and b-wave. The amplitudes of the a-wave and b-wave in the P17 OIR group were significantly lower than in the P17 control group (P: < 0.0001, < 0.0001). The amplitudes of the a-wave and b-wave in the P17 hADSCs treatment group were significantly higher than in the P17 OIR group (P: < 0.0001, = 0.001). (n = 5) (A, C) Latencies of the a-wave and b-wave. The latencies of the a-wave and b-wave in the P17 OIR group were significantly longer than in the P17 control group (P: = 0.003, = 0.001). The latencies of the a-wave and b-wave in the P17 hADSCs treatment group were significantly shorter than in the P17 OIR group (P: =0.047, = 0.001). (n = 5). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. NOR: normal group; OIR: oxygen-induced retinopathy; hADSCs: human adipose–derived mesenchymal stem cells; MSC: mesenchymal stem cell.
Expression of CNTF, GDNF, and BDNF
The expression of CNTF and BDNF in the P17 OIR group was statistically higher than in the P17 control group (P < 0.0001). The expression of GDNF in the P17 OIR group was statistically lower than in the P17 control group (P < 0.0001). The expression of CNTF and GDNF in the P17 hADSCs treatment group was statistically higher than in the P17 OIR group (P < 0.0001). The expression of BDNF in the P17 hADSCs treatment group was statistically lower than in the P17 OIR group (P < 0.0001; Fig. 5).
Figure 5.
Expression of CNTF, GDNF, and BDNF. (A) Western blot analysis of the protein expressions of CNTF, GDNF, and BDNF in the P17 control, OIR, and hADSCs treatment groups. The relative protein expression levels were normalized to β-Actin. (B) Quantification illustrating trends in the expressions of CNTF, GDNF, and BDNF in the P17 control, OIR, and hADSCs treatment group. The expression of CNTF and BDNF in the P17 OIR group was statistically higher than in the P17 control group (P < 0.0001). The expression of GDNF in the P17 OIR group was statistically lower than in the P17 control group (P < 0.0001). The expression of CNTF and GDNF in the P17 hADSCs treatment group was statistically higher than in the P17 OIR group (P < 0.0001). The expression of BDNF in the P17 hADSCs treatment group was statistically lower than in the P17 OIR group (P < 0.0001). CNTF: ciliary neurotrophic factor; GDNF: glial cell line–derived neurotrophic factor; BDNF: brain-derived neurotrophic factor; NOR: normal group; OIR: oxygen-induced retinopathy; hADSCs: human adipose–derived mesenchymal stem cells; MSC: mesenchymal stem cell.
Discussion
Ischemic retinopathy can lead to retinal neuronal dysfunction 21 . Our study was designed to provide evidence for the neuroprotective effects of hADSCs in OIR. We used HE, retina flat mount, TUNEL assays, ERG, and Western blot analysis to assess neuroprotection. The results from this study provide neuroprotective evidence for the intraocular use of hADSCs in OIR.
Our results revealed that the thickness and cell density of the INL and ONL in the P17 OIR group were significantly lower than in the P17 normal group. These findings align with our ERG results, further corroborating the detrimental impact on retinal neurofunction. Intriguingly, the INL and ONL thickness in the P17 hADSCs treatment group was lower than that in the P17 OIR group. This observation diverges from a prior study that reported an increase in INL and ONL thickness following treatment 22 . Despite no statistically significant difference, it is noteworthy that the cell density within INL and ONL of the P17 hADSCs treatment group was slightly higher, compared with that of the P17 OIR group. This alignment with the ERG results underscores the potentially influential role of cell density in shaping retinal function. It strengthens the suggestion that hADSC treatment can potentially enhance neurological function.
The occurrence of retinal nonperfusion areas signifies inadequate perfusion in the retina, leading to altered neuronal and dopaminergic neurotransmitter signaling 23 . It also reduces the expression of synaptic protein 24 and disrupts glial function 25 . In our study, the nonperfusion areas in the P17 hADSCs treatment group were significantly smaller than those in the P17 OIR group, suggesting that hADSCs improved neuronal retina function. The TUNEL analysis, another morphological test for neurological changes in our study, provides insight into cell apoptosis and the extent of tissue function damage. Existing reports indicate that ischemic retinopathy induces neuronal death in the inner retina, particularly around P14 and P1526,27, leading to retinal neuronal dysfunction 28 . Our results demonstrate a significantly higher number of apoptotic cells in the P15 OIR group compared with the P15 control group, primarily within the INL. Fletcher`s research suggests that neurochemical changes in OIR can be attributed to the alterations in Müller cells 29 . Interestingly, Müller cells constitute the INL, aligning with our observation of apoptotic cells predominantly in the INL. Furthermore, our study reveals a significantly reduced number of apoptotic cells in the P15 hADSCs treatment group compared with the P15 OIR group. These findings suggest that the hADSCs effectively mitigate damage and protect the retinal neurofunction in OIR.
The neuroprotective effects of hADSCs in OIR mice were further confirmed by the ERG analysis in our study. Both scotopic and photopic ERG parameters minimally reflect inner retinal function 30 . The a- and b-waves of ERG reflect the function of photoreceptor potentials, bipolar cells, and Müller cells. Studies emphasized the clinical significance of changes in amplitude 31 . In our research, the amplitudes of a-wave and b-wave in the P17 OIR group were significantly reduced, compared with the P17 control group, indicating impaired neuronal function in the OIR model. This finding aligns with reports of retinal function defects in the rat OIR model 32 . Notably, the P17 hADSCs treatment group exhibited significantly higher a-wave and b-wave amplitudes than the P17 OIR group, suggesting that the hADSCs improved the retinal neurofunction. This effect is consistent with the report that BMSCs enhance ERG amplitude 33 . In addition, we observed the latencies of the a- and b-waves, finding a significant prolongation in the OIR group, akin to studies reporting prolonged latency in retinal ischemia 34 . Furthermore, the lat-encies of a-wave and b-wave in the P17 hADSCs treatment group were shorter than the P17 OIR group, indicating improved retinal function.
The CNTF, GDNF, and BDNF mediate neuroprotective retina functions and play pivotal roles in the nervous system development and function35–37. In our study, we examined the expression of these neurotrophic factors using Western blot analysis to uncover the potential mechanisms of hADSCs’ neuroprotection in OIR. Our results revealed that CNTF expression was significantly increased in P17 OIR mice, compared with the P17 control group, in line with reports of increased CNTF levels in OIR models38,39. The BDNF showed a similar expressive pattern to CNTF, with many reports confirming increased BDNF and fibroblast growth factor 2 (FGF2) during the retinal damage40,41. Our findings also indicated that the expression of GDNF was significantly decreased in P17 OIR mice, compared with the P17 control group, consistent with reports of reduced GDNF in damaged retinopathy 42 . Following hADSCs’ treatment, our study showed a significant increase in the expression of both CNTF and GDNF. Numerous studies have confirmed that elevated CNTF and GDNF exert neuroprotective effects on the retina43,44, suggesting that increased expression of CNTF and GDNF may be one of the mechanisms underlying hADSCs’ neuroprotection in OIR. However, BDNF expression de-creased, differing from reports of increased BDNF after MSC application in traumatic optic neuropathy 45 . Given the absence of reference related to the OIR model, we speculate that hADSCs’ intravitreal injection may not have a significant short-term effect on BDNF in the OIR model.
The one limitation of our study is that we primarily investigated three neurotrophic factors affecting the retina and did not investigate whether the effects of hADSCs are autocrine or paracrine. The mechanism underlying hADSCs’ effects is likely complex and involves multiple factors. Identifying additional neuroprotective factors and exploring downstream factors will be essential. Another limitation is that it is important to recognize that, while our research has corroborated the notion that enhancing non-perfusion areas can lead to neuronal alterations, it is crucial to acknowledge that “improvement of vascularization” cannot supplant the fundamental concept of “neuroprotection.” Moreover, while the experiments have proved that intraocular injection of stem cells inhibits inflammation46,47 and the HE staining of the hADSCs group showed the appearance of infiltrating cells, it is essential to acknowledge that we did not conduct a comprehensive follow-up study to definitively confirm the nature and extent of the inflammatory response associated with xenogeneic MSC transplantation. Further research is warranted to address these questions before considering the clinical application of hADSCs.
In conclusion, to the best of our knowledge, this study is the first attempt to evaluate the neuroprotection of hADSCs in OIR. We provide preliminary theoretical data supporting the neuroprotective function and potential mechanisms of hADSCs in OIR. Using hADSCs to enhance retinal neurofunction could be a promising strategy for clinical treatment.
Supplemental Material
Supplemental material, sj-docx-1-cll-10.1177_09636897231213309 for Neuroprotective Effects of Human Adipose–Derived Mesenchymal Stem Cells in Oxygen-Induced Retinopathy by Jifu Xin, Lvlv Zhou, Ling Zhang, Kai Guo and Dayong Yang in Cell Transplantation
Supplemental material, sj-jpg-2-cll-10.1177_09636897231213309 for Neuroprotective Effects of Human Adipose–Derived Mesenchymal Stem Cells in Oxygen-Induced Retinopathy by Jifu Xin, Lvlv Zhou, Ling Zhang, Kai Guo and Dayong Yang in Cell Transplantation
Supplemental material, sj-tif-3-cll-10.1177_09636897231213309 for Neuroprotective Effects of Human Adipose–Derived Mesenchymal Stem Cells in Oxygen-Induced Retinopathy by Jifu Xin, Lvlv Zhou, Ling Zhang, Kai Guo and Dayong Yang in Cell Transplantation
Supplemental material, sj-tif-4-cll-10.1177_09636897231213309 for Neuroprotective Effects of Human Adipose–Derived Mesenchymal Stem Cells in Oxygen-Induced Retinopathy by Jifu Xin, Lvlv Zhou, Ling Zhang, Kai Guo and Dayong Yang in Cell Transplantation
Footnotes
Author Contributions: J.X., L. Zhou, and K.G. performed experiments and data analysis. J.X., L. Zhou, L. Zhang, K.G., and D.Y. planned the research, discussed the data analysis, and wrote the manuscript. All authors approved the final version of the manuscript to be published.
Publisher’s Note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article or claim made by its manufacturer is not guaranteed or endorsed by the publisher.
Data Availability: The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethical Approval: The studies involving human participants were reviewed and approved by the ethics committee of Inner Mongolia Medical University (YKD2016075).
Statement of Human and Animal Rights: This animal study was reviewed and approved by the Institutional Animal Care and Use Committee of Inner Mongolia Medical University. All experiments adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Statement of Informed Consent: The patients/participants provided written informed consent to participate in this study. No potentially identifiable human images or data are presented in this study.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (No. 81760173) and the Natural Science Foundation of Inner Mongolia (No. 2019MS08014).
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60(5):428–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Song P, Xu Y, Zha M, Zhang Y, Rudan I. Global epidemiology of retinal vein occlusion: a systematic review and meta-analysis of prevalence, incidence, and risk factors. J Glob Health. 2019;9(1):010427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res. 2015;49:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fortmann SD, Grant MB. Molecular mechanisms of retinal ischemia. Curr Opin Physiol. 2019;7:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23(1):91–147. [DOI] [PubMed] [Google Scholar]
- 6. Rosenfeld PJ, Schwartz SD, Blumenkranz MS, Miller JW, Haller JA, Reimann JD, Greene WL, Shams N. Maximum tolerated dose of a humanized anti–vascular endothelial growth factor antibody fragment for treating neovascular age-related macular degeneration. Ophthalmology. 2005;112(6):1048–53. [DOI] [PubMed] [Google Scholar]
- 7. Bressler NM, Beaulieu WT, Glassman AR, Blinder KJ, Bressler SB, Jampol LM, Melia M, Wells JA, Network DRCR. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018;136(3):257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004;26(9):943–54. [DOI] [PubMed] [Google Scholar]
- 9. Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111(12):1843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nishijima K, Ng Y-S, Zhong L, Bradley J, Schubert W, Jo N, Akita J, Samuelsson SJ, Robinson GS, Adamis AP. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171(1):53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nava MM, Raimondi MT, Pietrabissa R. Controlling self-renewal and differentiation of stem cells via mechanical cues. J Biomed Biotechnol. 2012;2012:797410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ding S, Schultz PG. A role for chemistry in stem cell biology. Nat Biotechnol. 2004;22(7):833–40. [DOI] [PubMed] [Google Scholar]
- 13. Zhou L, Zhang H, Wu S, He Y, Guo K. In vitro induction and intraocular application in oxygen-induced retinopathy of adipose-derived mesenchymal stem cells. Mol Vis. 2022;28:432–40. [PMC free article] [PubMed] [Google Scholar]
- 14. Pendleton C, Li Q, Chesler DA, Yuan K, Guerrero-Cazares H, Quinones-Hinojosa A. Mesenchymal stem cells derived from adipose tissue vs bone marrow: in vitro comparison of their tropism towards gliomas. PLoS ONE. 2013;8(3):e58198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qi Z, Zhang Y, Liu L, Guo X, Qin J, Cui G. Mesenchymal stem cells derived from different origins have unique sensitivities to different chemotherapeutic agents. Cell Biol Int. 2012;36(9):857–62. [DOI] [PubMed] [Google Scholar]
- 16. Noueihed B, Rivera JC, Dabouz R, Abram P, Omri S, Lahaie I, Chemtob S. Mesenchymal stromal cells promote retinal vascular repair by modulating Sema3e and Il-17a in a model of ischemic retinopathy. Front Cell Dev Biol. 2021;9:630645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu W, Cheng W, Cui X, Xu G. Therapeutic effect against retinal neovascularization in a mouse model of oxygen-induced retinopathy: bone marrow-derived mesenchymal stem cells versus Conbercept. BMC Ophthalmol. 2020;20(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith LEH, Wesolowski E, McLellan A, Kostyk SK, D’Amato RJ, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–11. [PubMed] [Google Scholar]
- 19. Lee SC, Martin PR, Grünert U. Topography of neurons in the rod pathway of human retina. Invest Ophthalmol Vis Sci. 2019;60(8):2848–59. [DOI] [PubMed] [Google Scholar]
- 20. Li S, Li T, Luo Y, Yu H, Sun Y, Zhou H, Liang X, Huang J, Tang S. Retro-orbital injection of FITC-dextran is an effective and economical method for observing mouse retinal vessels. Mol Vis. 2011;17:3566–73. [PMC free article] [PubMed] [Google Scholar]
- 21. Downie LE, Pianta MJ, Vingrys AJ, Wilkinson-Berka JL, Fletcher EL. Neuronal and glial cell changes are determined by retinal vascularization in retinopathy of prematurity. J Comp Neurol. 2007;504(4):404–17. [DOI] [PubMed] [Google Scholar]
- 22. Su W, Li Z, Jia Y, Zhu Y, Cai W, Wan P, Zhang Y, Zheng SG, Zhuo Y. microRNA-21a-5p/PDCD4 axis regulates mesenchymal stem cell-induced neuroprotection in acute glaucoma. J Mol Cell Biol. 2017;9(4):289–301. [DOI] [PubMed] [Google Scholar]
- 23. Aung MH, Na Park H, Han MK, Obertone TS, Abey J, Aseem F, Thule PM, Iuvone PM, Pardue MT. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J Neurosci. 2014;34(3):726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D’Cruz TS, Weibley BN, Kimball SR, Barber AJ. Post-translational processing of synaptophysin in the rat retina is disrupted by diabetes. The Penn State Retina Research Group. PLoS ONE. 2012. 7(9):e44711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lieth E, LaNoue KF, Antonetti DA, Ratz M. Diabetes reduces glutamate oxidation and glutamine synthesis in the retina. Exp Eye Res. 2000;70(6):723–30. [DOI] [PubMed] [Google Scholar]
- 26. Sennlaub F, Courtois Y, Goureau O. Inducible nitric oxide synthase mediates retinal apoptosis in ischemic proliferative retinopathy. J Neurosci. 2002;22(10):3987–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou H, Ding Y, Li S, Sun G, Yang C, Zhang X, Wei L, Ge J, Liang X. TMP prevents retinal neovascularization and imparts neuroprotection in an oxygen-induced retinopathy model. Invest Ophthalmol Vis Sci. 2012;53(14):2554–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lachapelle P, Dembinska O, Rojas LM, Benoit J, Almazan G, Chemtob S. Persistent functional and structural retinal anomalies in newborn rats exposed to hyperoxia. Can J Physiol Pharmacol. 1999;77(1):48–55. [DOI] [PubMed] [Google Scholar]
- 29. Fletcher EL, Downie LE, Hatzopoulos K, Vessey KA, Ward MM, Chow CL, Pianta MJ, Vingrys AJ, Kalloniatis M, Wilkinson-Berka JL. The significance of neuronal and glial cell changes in the rat retina during oxygen-induced retinopathy. Doc Ophthalmol. 2010;120(1):67–86. [DOI] [PubMed] [Google Scholar]
- 30. Smith BJ, Wang X, Chauhan BC, Côté PD, Tremblay F. Contribution of retinal ganglion cells to the mouse electroretinogram. Doc Ophthalmol. 2014;128(3):155–68. [DOI] [PubMed] [Google Scholar]
- 31. Seeliger M, Jurklies B, Kellner U, Palmowski A, Bach M, Kretschmann U. Multifokale Elektroretinographie (mfERG). Der Ophthalmologe. 2001;98:1112–30. [DOI] [PubMed] [Google Scholar]
- 32. Liu K, Akula JD, Hansen RM, Moskowitz A, Kleinman MS, Fulton AB. Development of the electroretinographic oscillatory potentials in normal and ROP rats. Invest Ophthalmol Vis Sci. 2006;47(12):5447–52. [DOI] [PubMed] [Google Scholar]
- 33. Gramlich OW, Brown AJ, Godwin CR, Chimenti MS, Boland LK, Ankrum JA, Kardon RH. Systemic mesenchymal stem cell treatment mitigates structural and functional retinal ganglion cell degeneration in a mouse model of multiple sclerosis. Transl Vis Sci Technol. 2020;9(8):16–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qin Y, Ji M, Deng T, Luo D, Zi Y, Pan L, Wang Z, Jin M. Functional and morphologic study of retinal hypoperfusion injury induced by bilateral common carotid artery occlusion in rats. Sci Rep. 2019;9(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wen R, Tao W, Li Y, Sieving PA. CNTF and retina. Prog Retin Eye Res. 2012;31(2):136–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ibáñez CF, Andressoo J-O. Biology of GDNF and its receptors—relevance for disorders of the central nervous system. Neurobiol Dis. 2017;97:80–89. [DOI] [PubMed] [Google Scholar]
- 37. Liu X, Grishanin RN, Tolwani RJ, Rentería RC, Xu B, Reichardt LF, Copenhagen DR. Brain-derived neurotrophic factor and TrkB modulate visual experience-dependent refinement of neuronal pathways in retina. J Neurosci. 2007;27(27):7256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xin J, He Y, Guo K, Yang D. Expression of the neuroprotective factors BDNF, CNTF, and FGF-2 in normal and oxygen induced retinopathy. Front Neurosci. 2022;16:971952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dorfman AL, Polosa A, Joly S, Chemtob S, Lachapelle P. Functional and structural changes resulting from strain differences in the rat model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2009;50(5):2436–50. [DOI] [PubMed] [Google Scholar]
- 40. Okoye G, Zimmer J, Sung J, Gehlbach P, Deering T, Nambu H, Hackett S, Melia M, Esumi N, Zack DJ. Increased expression of brain-derived neurotrophic factor preserves retinal function and slows cell death from rhodopsin mutation or oxidative damage. J Neurosci. 2003;23(10):4164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fang L, Barber AJ, Shenberger JS. Regulation of fibroblast growth factor 2 expression in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2015;56(1):207–15. [DOI] [PubMed] [Google Scholar]
- 42. Dong A, Shen J, Krause M, Hackett SF, Campochiaro PA. Increased expression of glial cell line-derived neurotrophic factor protects against oxidative damage-induced retinal degeneration. J Neurochem. 2007;103(3):1041–52. [DOI] [PubMed] [Google Scholar]
- 43. Wang W-J, Jin W, Yang A-H, Chen Z, Xing Y-Q. Protective effects of ciliary neurotrophic factor on the retinal ganglion cells by injure of hydrogen peroxide. Int J Ophthalmol. 2018;11(6):923–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hauck SM, Kinkl N, Deeg CA, Swiatek-de Lange M, Schöffmann S, Ueffing M. GDNF family ligands trigger indirect neuroprotective signaling in retinal glial cells. Mol Cell Biol. 2006;26(7):2746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Junyi L, Na L, Yan J. Mesenchymal stem cells secrete brain-derived neurotrophic factor and promote retinal ganglion cell survival after traumatic optic neuropathy. J Craniofac Surg. 2015;26(2):548–52. [DOI] [PubMed] [Google Scholar]
- 46. Hermankova B, Kossl J, Bohacova P, Javorkova E, Hajkova M, Krulova M, Zajicova A, Holan V. The immunomodulatory potential of mesenchymal stem cells in a retinal inflammatory environment. Stem Cell Rev Rep. 2019;15(6):880–91. [DOI] [PubMed] [Google Scholar]
- 47. Mathew B, Poston JN, Dreixler JC, Torres L, Lopez J, Zelkha R, Balyasnikova I, Lesniak MS, Roth S. Bone-marrow mesenchymal stem-cell administration significantly improves outcome after retinal ischemia in rats. Graefes Arch Clin Exp Ophthalmol. 2017;255:1581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cll-10.1177_09636897231213309 for Neuroprotective Effects of Human Adipose–Derived Mesenchymal Stem Cells in Oxygen-Induced Retinopathy by Jifu Xin, Lvlv Zhou, Ling Zhang, Kai Guo and Dayong Yang in Cell Transplantation
Supplemental material, sj-jpg-2-cll-10.1177_09636897231213309 for Neuroprotective Effects of Human Adipose–Derived Mesenchymal Stem Cells in Oxygen-Induced Retinopathy by Jifu Xin, Lvlv Zhou, Ling Zhang, Kai Guo and Dayong Yang in Cell Transplantation
Supplemental material, sj-tif-3-cll-10.1177_09636897231213309 for Neuroprotective Effects of Human Adipose–Derived Mesenchymal Stem Cells in Oxygen-Induced Retinopathy by Jifu Xin, Lvlv Zhou, Ling Zhang, Kai Guo and Dayong Yang in Cell Transplantation
Supplemental material, sj-tif-4-cll-10.1177_09636897231213309 for Neuroprotective Effects of Human Adipose–Derived Mesenchymal Stem Cells in Oxygen-Induced Retinopathy by Jifu Xin, Lvlv Zhou, Ling Zhang, Kai Guo and Dayong Yang in Cell Transplantation