Abstract
Alopecia universalis is a severe, difficult to treat variant of alopecia areata that results in loss of hair on the scalp, eyebrows, eyelashes, and extremities. Deucravacitinib, a selective TYK2 inhibitor, has been recently approved in Canada, opening the door to novel uses of the drug. We present the case of a patient known for psoriasis who developed alopecia universalis resistant to many interventions (topical minoxidil and topical, intralesional, and systemic corticosteroids). We report the first case of successful rapid hair regrowth after starting deucravacitinib, which should prompt further inquiry into the use of TYK2 inhibitors in the management of alopecia areata.
Keywords: deucravacitinib, janus kinase inhibitors, alopecia universalis, alopecia areata, psoriasis
Alopecia areata (AA) is an autoimmune non-scarring hair loss. 1 Alopecia universalis (AU, complete scalp/body hair loss) is the most severe and hard-to-treat variant.1,2 AA is more common in psoriasis patients and may rarely be triggered by biologics used to treat psoriasis (particularly TNF-α inhibitors). 3 However, in drug-induced AA cases, spontaneous hair regrowth is common upon drug discontinuation. 3 We report a case of treatment-resistant AU arising in a psoriasis patient in whom biologic discontinuation did not result in hair regrowth, but a switch to deucravacitinib led to a rapid improvement of AU. This is the first case reporting successful use of deucravacitinib in AA.
A 50-year-old Caucasian female known for hypothyroidism and a well-controlled psoriasis developed AU in January 2021, 9 months into secukinumab treatment. She failed topical, intralesional and systemic corticosteroids in combination with topical minoxidil and was referred to our clinic for help in management. In September 2021, the SALT score was 97, with complete loss of bilateral eyebrows and eyelashes and almost complete loss of body hair (Figure 1). Multiple treatment options (janus kinase (JAK)inhibitors, secukinumab discontinuation, topical immunotherapy, and cyclosporine) were discussed. The patient opted to discontinue secukinumab and was switched to risankizumab. Cyclosporine (4 mg/kg for 1 month) and topicals were also given. One year later, there was no improvement of AU and she developed a few patches of vitiligo on the scalp. JAK inhibitors and topical immunotherapy were discussed, but declined by the patient due to fear of side effects. In March 2023, deucravacitinib was approved for psoriasis and became commercially available in Canada. The patient agreed to switch from risankizumab to deucravacitinib in an attempt to regrow hair while controlling psoriasis. She started deucravacitinib in April 2023 (6 mg daily). Rapidly, hair grew on the eyebrows followed by eyelashes, body hair, and finally the scalp. At the follow-up visit 6 weeks later, moderate regrowth of dark hair on the arms and legs, eyelashes, and eyebrows was noted. Short ~1 cm white-gray hairs were noted on the scalp with a SALT score of 45.2 (Figure 2).
Figure 1.
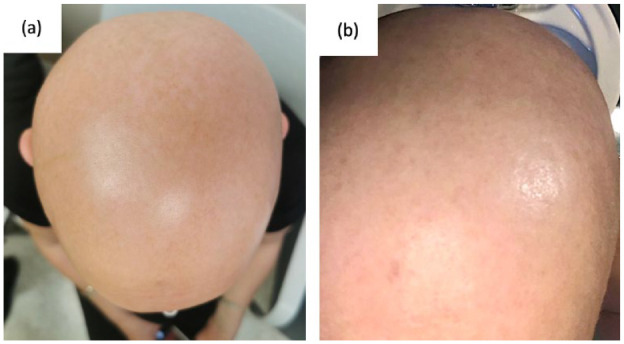
Clinical findings prior to treatment with deucravacitinib. (a) illustrates alopecia areata (AA) over the vertex of the scalp. (b) illustrates AA over the occiput of the scalp.
Figure 2.
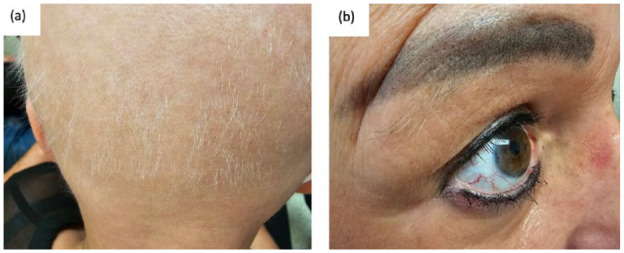
Clinical findings 6 weeks after initiating deucravacitinib. (a) illustrates regrowth of short white-gray hairs along the occipital region of the scalp. (b) illustrates regrowth of eyebrow hairs and eyelashes, with some dark hairs in the eyebrows.
Recent research advances highlighted the interferon (IFN) and IL-15 cytokines as the primary drivers of AA pathogenesis, placing the inhibition of the JAK/STAT pathway as a promising therapeutic intervention. 4 The JAK/STAT family is composed of four tyrosine kinases (JAK1/2/3 and TYK2) and seven transcription factors (STAT proteins). 5 Upon cytokine (e.g., IFN) binding to its receptor, JAK proteins are recruited and activated leading to STAT phosphorylation, translocation to the nucleus and modulation of target genes transcription.4,5 Baricitinib (JAK 1/2 inhibitor) and ritlecitinib (JAK 3 inhibitor) are the only Food and Drug Administration (FDA) approved treatments for severe AA. 4 While very promising, JAK1/2/3 inhibitors are associated with a black box warning, notably for an increased risk of serious infections, cancer and cardiovascular/thromboembolic events. Our patient declined the treatment for this reason.
Deucravacitinib, a selective TYK2 inhibitor, prevents signal transduction for the IL-12/23 and IFN cytokines. 5 While, a phase II clinical trial in AA is ongoing (NCT05556265), this is the first case report of successful use of deucravacitinib in AA to our knowledge. Deucravacitinib binds to an allosteric site of the TYK2 protein, allowing for high selectivity and negligible inhibition of JAK1/2/3 proteins. 5 Thereby it does not carry a black box warning nor does it require blood test monitoring (besides a baseline work up for latent tuberculosis). 5 Hence, exploring the efficacy of deucravacitinib in AA and other IFN-mediated dermatoses is of interest.
Footnotes
Data availability statement: The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.O. None to declare. S.M. None to declare. M.S. None to declare. E.N. has been an Advisory Board/Speaker/Consultant and/or received Investigator Initiated Educational and/or Research funding from AbbVie Inc., Arcutis, Bausch Health, Beiersdorf, Boehringer Ingelheim International, Bristol Myers Squibb, Eli Lilly, Galderma SA., Janssen Inc., LEO Pharma, Medexus, Novartis Pharmaceuticals, Pfizer Inc., Sanofi Genzyme, Sun Pharmaceuticals, and UCB.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics statement section: The patients in this manuscript have given written informed consent to publication of their case details.
ORCID iDs: Sarah Oliel  https://orcid.org/0009-0009-8875-7983
https://orcid.org/0009-0009-8875-7983
Elena Netchiporouk  https://orcid.org/0000-0002-6692-7787
https://orcid.org/0000-0002-6692-7787
References
- 1. Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol 2020; 82(3): 675–682. [DOI] [PubMed] [Google Scholar]
- 2. Wei D, Chen Y, Shen Y, et al. Efficacy and safety of different JAK inhibitors in the treatment of alopecia areata: a network meta-analysis. Front Immunol 2023; 14: 1152513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ezemma O, Devjani S, Jothishankar B, et al. Drug-induced alopecia areata: a systematic review. J Am Acad Dermatol 2023; S0190-9622(23)00906-4. [DOI] [PubMed] [Google Scholar]
- 4. Yan D, Fan H, Chen M, et al. The efficacy and safety of JAK inhibitors for alopecia areata: a systematic review and meta-analysis of prospective studies. Front Pharmacol 2022; 13: 950450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Estevinho T, Lé AM, Torres T. Deucravacitinib in the treatment of psoriasis. J Dermatolog Treat 2023; 34(1): 2154122. [DOI] [PubMed] [Google Scholar]


