Abstract
Background:
Deregulation in the urokinase-type plasminogen activator receptor (uPA/uPAR) system is reported in many diseases where the immune system is activated. During SARS-CoV-2 infection, a rise in soluble uPAR (suPAR) levels has been detected and its concentration above 6 µg/L predicts worsening to severe respiratory failure 14 days earlier, with a positive predictive value of 85.9%, and was the prerequisite for a treatment with anakinra, a recombinant IL-1 receptor antagonist that blocks the activity of both IL-1α and IL-1β.
Objectives:
To compare suPAR concentrations measured by CHORUS suPAR on CHORUS TRIO instrument of DIESSE with the commercially available suPARnostic (ViroGates) ELISA assay.
Design:
A single-centre, non-pharmacological, diagnostic study was performed.
Results:
A total of 522 serum samples from patients with COVID-19 were tested for suPAR. CHORUS suPAR resulted accurate and reliable, with a high grade of specificity (97.9%), accuracy (97.3%) and sensitivity (96.9%). The median concentration of suPAR, as determined with CHORUS suPAR, was 6.8 µg/L (interquartile range 4.5-9.7) in patients with moderate disease (n = 465) and 8.5 µg/L (interquartile range 5.4-10.6) in patients with severe disease. Among patients with moderate and severe disease, 60.6% and 71.9%, respectively, reached the cut-off concentration of suPAR ⩾6 µg/L, defining their illness severity and suggesting eligibility to anakinra treatment.
Conclusion:
CHORUS suPAR kit resulted as sensitive, specific, accurate and able to quantify suPAR concentrations in patients with moderate and severe COVID-19.
Keywords: suPAR, COVID-19, anakinra, ELISA assay, biomarker
Introduction
Urokinase receptor or urokinase plasminogen activator receptor (uPAR, CD87) is a glycosylphosphatidylinisotol-anchored (GPI–anchored) protein that acts as a receptor for prourokinase and facilitates the generation of activated plasmin. uPAR presents 3 domains (D-I, D-II and D-III) and the GPI-anchor composed of a lipid moiety, linked to the protein through a phosphodiester bond, and of a carbohydrate moiety. 1 When the membrane-anchoring GPI portion is cleaved from the DIII, the soluble form of uPAR (suPAR) is released from the cell membrane. In its membrane-bound form uPAR is present in various immunologically active cells including monocytes, activated T-lymphocytes and macrophages, but also endothelial cells, keratinocytes, fibroblasts, smooth muscle cells, megakaryocytes and certain tumour cells, while its soluble form it is found in various body fluids, including plasma, urine and cerebrospinal fluid. 1
As a causative agent, an upregulated uPA/uPAR system increases proinflammatory cytokines/chemokines levels and induces epithelial and endothelial cell proliferation with impaired tissue remodelling and loss of adequate TJ junctions. Evidence associates uPA/uPAR deregulation with increased epithelial and endothelial cell apoptosis, aquaporins dysregulation, hypoxia and VEGF-dependent microvasculature impairment.
Several diseases, including infections, cancer, pulmonary fibrosis, kidney disease, coronary artery disease, rheumatoid arthritis, systemic sclerosis, bone destructive disease, lupus erythematosus, psoriasis and endometriosis, show deregulation in the urokinase-type plasminogen activator receptor (uPA/uPAR) system.2,3 For instance, in chronic kidney disease, a higher suPAR level at baseline was associated with a greater decline in the estimated glomerular filtration rate. 4 Similarly, high suPAR levels were associated with acute kidney injury, characterized by a rapid (hours to days) decrease in renal excretory function, in various clinical and experimental context, empathizing the role of suPAR biomarker in early diagnosis, that can be essential in improving the prognosis and treatment of these patients. 5 On the other hand, people with kidney disease are disproportionately affected by atherosclerosis for unclear reasons. 6 It has been found that suPAR levels to be predictive of coronary artery calcification and cardiovascular events. A missense variant in the plasminogen activator, urokinase receptor (PLAUR) gene (rs4760) has been identified and it was experimentally confirmed that it led to higher suPAR levels, thus characterizing suPAR as a pathogenic factor for atherosclerosis. 6
In other context, suPAR can be used as biomarker as its concentration reflects the level of immune system activation, regardless of its etiology (viral, bacterial, parasitic or other). 1
Even during SARS-CoV-2 infection, there is a rise in suPAR levels. The pooled analysis of 5 studies demonstrated a positive difference in suPAR values in patients with critical COVID-19 compared to those without, with an increase of 55% (95% CI 35%-75%). 7 Among patients with a chest infection requiring hospitalization, suPAR was statistically significantly higher on admission in patients with COVID-19 when compared to those with non-COVID-19. The 2 groups did not differ in mortality, length of hospital stay, age, gender and disease severity as measured by the ratio of the arterial partial oxygen pressure divided by the fraction of inspired oxygen (PaO2/FiO2). 8 However, almost all the patients diagnosed with COVID-19 who died had elevated plasma levels of suPAR on admission. Therefore, a single measurement of suPAR on admission could provide prognostic information for patients with suspected COVID-19 pneumonia.
Data also showed that suPAR levels increased as the disease worsens and positively correlated with high-sensitivity C-reaction protein, neutrophil/leukocyte ratio and lymphocyte count. 9 Therefore, the suPAR as a COVID-19 prognostic biomarker may assist in the early triage of SARS-CoV-2-infected persons and represents a valuable tool for improving risk stratification accuracy, helping to predict the risk of developing severe consequences, especially acute kidney injury, along with micro- and macro thrombosis. 10
suPAR concentrations above 6 µg/L predicted worsening to severe respiratory failure 14 days earlier; the positive predictive value was as high as 85.9%. 11 These observations were the prerequisite to propose a two-step strategy for managing patients with COVID-19, which used elevated suPAR levels to first identify patients at risk of progressing to severe respiratory failure or death and then to initiate early targeted treatment with anakinra, a recombinant IL-1 receptor antagonist that blocks the activity of both IL-1α and IL-1β. 10
The open-label, phase II SAVE study was conducted as a proof of concept for this approach. Results showed a 70% decrease in the relative risk of progression to severe respiratory failure and a significant reduction in 28-day mortality with anakinra treatment compared to standard of care. 12 The SAVE-MORE double-blind, randomized, controlled, phase III trial confirmed the efficacy and safety of anakinra in 594 patients with COVID-19 at risk of progressing to respiratory failure as identified by plasma suPAR ⩾6 µg/L. 13 In the individual patient-level meta-analysis, after adjusting for age, comorbidities, PaO2/FiO2, C-reactive protein concentrations and lymphopenia, mortality was significantly lower in patients treated with anakinra (38 [11%] of 342) than in those receiving standard of care with or without placebo (137 [25%] of 553; adjusted odds ratio [OR] 0.32 [95% CI 0.20-0.51]). 14
The suPAR cut-off ⩾6 µg/L was granted by Italian Drug Agency (AIFA) based on literature evidence. 15 Both SAVE and SAVE-MORE trials used the cut-off of 6 µg/L of suPAR to identify those patients who were at risk to clinically progress to respiratory failure. The cut-off had been previously described in the Hellenic Sepsis Study Group in a cohort of 57 subjects with COVID-19 related pneumonia. 14 SuPAR levels ⩾6 µg/L resulted as the most appropriate value with sensitivity as 85.7% and specificity as 91.7.12,13
Given the prognostic value of suPAR in determining the risk of progression and mortality of COVID-19, an automated and easy-to-use method for effective quantification of suPAR would help to identify patients with a higher probability of developing severe COVID-19 and act promptly with the appropriate anakinra treatment. Therefore, this study was aimed at evaluating the performance of the CHORUS suPAR kit and the CHORUS TRIO instrument in quantifying inflammatory suPAR in samples derived from patients hospitalized with COVID-19.
Methods
Study design and samples
This single-centre, non-pharmacological, diagnostic study was carried out at Azienda Ospedaliera Universitaria Senese to compare the suPAR levels, measured by the CHORUS TRIO instrument, with the commercially available ELISA assay suPARnostic (ViroGates).
The study was conducted according to the ethical principles reported in the Declaration of Helsinki in its latest revision and the protocol was approved by the local ethics committee (protocol CHOR-VIT; Comitato Etico Regionale per la Sperimentazione Clinica della Regione Toscana) on April 11th, 2022.
Due to the study’s specific objectives regarding the comparison of CHORUS suPAR with ViroGates, no patient data or personal information was deemed essential; therefore, according to ethics committee, participation in the study did not require informed consent.
Serum from patient diagnosed with moderate or severe COVID-19, 16 collected for routine clinical analysis from January 2022 to the end of April 2022 were then analysed. All samples have been anonymized. Samples were aggregated and provided for analysis without any reference to collection time, patient’s prognosis status (except that for active disease), ongoing treatment.
All samples used were derived from intensive care, sub-intensive care and other wards where patients with moderate to severe COVID-19 were admitted. After the withdrawal, the samples were frozen and kept at −80°C.
Reagents
suPARnostic® was a CE/IVD marked product range applied for the determination of suPAR in human EDTA, heparin-plasma or serum. Briefly, the suPARnostic® AUTO Flex ELISA was a simplified double monoclonal antibody sandwich assay where samples and peroxidase-conjugated anti-suPAR were mixed in the mixing plate before incubation in the anti-suPAR precoated optically clear microwells. The assay utilized monoclonal mouse and rat antibodies designed against human suPAR DI-III. The CHORUS suPAR test was a ‘COMPANION DIAGNOSTIC’ device utilized to identify patients of medium or high severity COVID-19 who are most likely to benefit from anakinra treatment. It was based on the principle of the sandwich ELISA method; this novel test was developed by DIESSE in collaboration with ViroGates company (Birkeroed, Denmark) by using suPARnostic reagents. CHORUS suPAR and ViroGates utilized the same monoclonal antibodies and recombinant suPAR used as standard and for control curve, supplied by Virogates; the other CHORUS suPAR’ reagents were manufactured by DIESSE. The suPAR in the test sample was bound to specific antibodies present in the solid phase and conjugates; unbound components were removed by washing, while the bound enzyme activity was evaluated colourimetrically by a transformation of a chromogenic substrate. The intensity of colour developed was directly proportional to the concentration of suPAR in the test sample. Disposable devices contained all reagents to perform the test when applied to CHORUS TRIO instruments.
In both assays, suPAR concentrations determined were expressed in µg/L. The dosing range of CHORUS suPAR was 1.5 to 20 µg/L; values upper to 20 µg/L were already considered as severe, therefore all values upper to 20 µg/L were classified as >20. The ViroGates assay did not present a prefixed dosing range.
The test could be done on plasma and serum. The variability between plasma and serum samples, observed during kit validation, ranged from 10% to 15%, that was within the precision range.
Endpoint
The primary endpoint was to determine accuracy and reliability in quantify total and suPAR with CHORUS suPAR, compared with the reference method suPARnostic®, on serum samples of patients with COVID-19.
Sample size and statistical analysis
Using the two-sided test of the angular coefficient of the univariate regression to be equal to 1 -the 2 tests must give the same concentration value and the regression line must result as a bisector-, with alpha = .05 and a power of 0.90, a sample size of 500 was estimated (G*Power software).
Qualitative variables were summarized with absolute frequencies and percentages, while quantitative ones with median and interquartile range (IQR). To compare CHORUS suPAR and suPARnostic® methods, Spearman’s correlation coefficient, Passing Bablok and Bland-Altman analyses were performed. Cohen kappa was used to estimate the degree of concordance. Sensitivity, specificity, positive predictive value and negative predictive value were estimated with their 95% confidence interval (95% CI). A P-value <.05 was considered statistically significant. All analyses were performed using MedCalc® Statistical Software version 20.115 (MedCalc Software Ltd, Ostend, Belgium).
Results
A total of 522 serum and plasma samples derived from patients with COVID-19 were tested for suPAR. The median concentration was 6.8 µg/L (range 4.5-9.7) in patients with moderate disease (n = 465) and 8.5 µg/L (range 5.4-10.6) in patients with severe disease (n = 57) (Figure 1).
Figure 1.
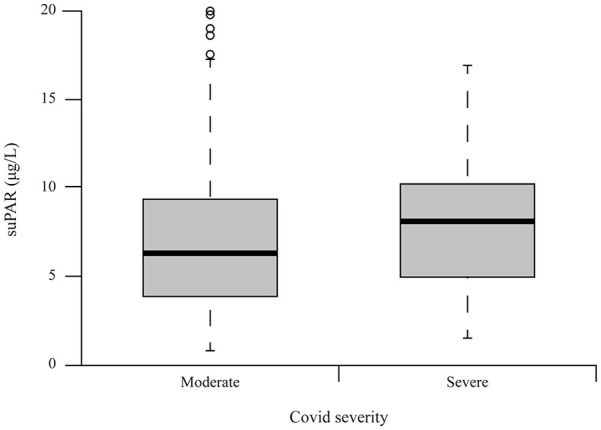
suPAR concentrations relative to illness severity, as resulted by CHORUS assay.
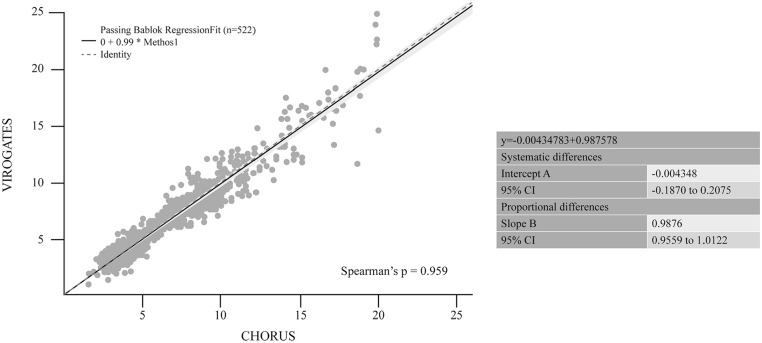
suPAR quantification was assessed with both CHORUS and the manual method ViroGates suPARnostic ELISA. Spearman’s correlation coefficient showed near perfect correlation between the 2 methods (0.959, P < .0001). As reported in Figure 2, Passing Bablock regression analyses showed no significant deviation from identity, the intercept was not statistically different from 0 and the regression coefficient from 1. Bland-Altman plots revealed no significant differences between the assays, as represented in Figure 3. The mean difference was −0.077 (95% CI −0.180 to 0.026) with upper and lower limits of 2.270 and −2.425, respectively. Some outliers were seen but did not show substantial differences considering that they are much higher than the threshold limit of 6 µg/L.
Figure 2.
Passing Bablock regression between CHORUS suPAR and ViroGates suPARnostic ELISA.
Figure 3.
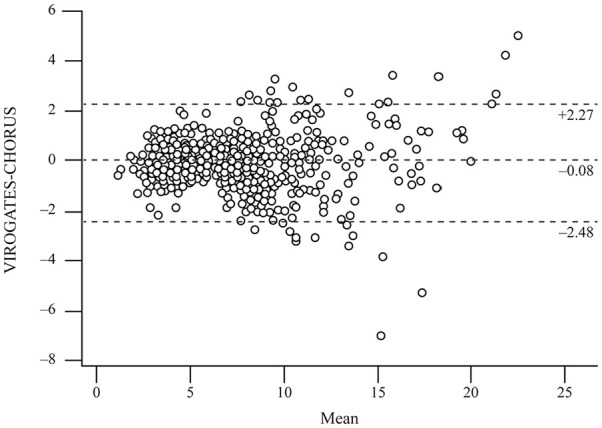
Bland-Altman plot showing differences between CHORUS suPAR and ViroGates suPARnostic ELISA.
Of 522 samples, 14 had discordant results between ViroGates and CHORUS suPAR values: 10 false negative and 4 false positives (Table 1).
Table 1.
Frequency table. Concordant/discordant values detected ViroGates or CHORUS suPAR.
| CHORUS suPAR | suPARnostic ViroGates | ||
|---|---|---|---|
| Negative | Positive | ||
| Negative | 189 | 10 | 199 (38.1%) |
| Positive | 4 | 319 | 323 (61.9%) |
| 193 (37.0%) | 329 (63.0%) | 522 | |
The value of sensitivity was 96.9% (range 94.4%-98.5%) and the specificity was 97.9% (range 94.7%-99.4%). The positive predictive value was 98.7% (range 96.7%-99.5%), the negative predictive value 94.9% (range 61.1%-97.2%), with an accuracy of 97.3% (range 95.5%-98.5%). The high degree of concordance was also confirmed by a Cohen’s kappa of 0.942 (95% CI 0.913-0.972).
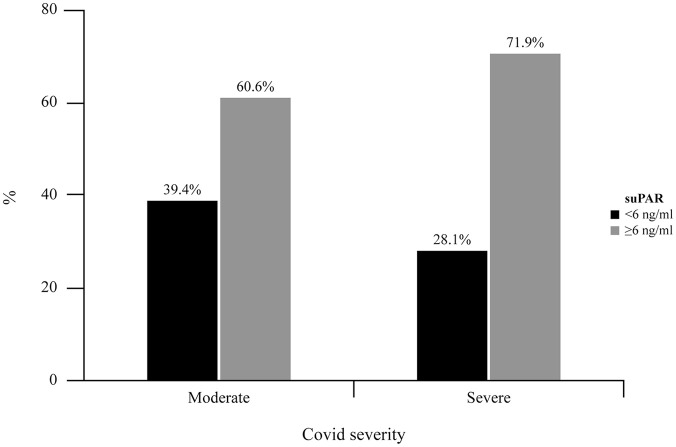
Once compared the 2 assays for validation, suPAR concentrations determined with the CHORUS assay were used to hypothesize which patients with moderate to severe disease could be candidates for anakinra treatment. Among patients with moderate and severe disease, 60.6% and 71.9%, respectively, reached the cut-off concentration of suPAR ⩾6 µg/L to be potential candidates for anakinra treatment (Figure 4).
Figure 4.
Percentage of patients eligible to anakinra treatment, relative to the levels of suPAR, for moderate to severe prognosis.
Discussion
This study validated the CHORUS suPAR assay to determine suPAR concentrations, by comparison with the commercially available assay suPARnostic® ELISA. CHORUS suPAR assay was an automated test developed in collaboration with ViroGates, using the same antibodies of suPARnostic® assay. We compared these 2 assays for suPAR quantification. The study results confirmed a similar assays’ performance, in term of accuracy and reliability, with an equivalent, high grade of specificity, precision and sensitivity.
In addition to the elements of validation that are common to all commercially available kits, the choice of a diagnostic assay could be driven by other parameters, such as ease of use and cost savings. CHORUS suPAR is an automated, user-friendly and ready-to-use kit, in which the possibility of manual errors is minimized as the operator should only dispense the sample into a well. Each sample is singularly processed in the CHORUS TRIO Instrument in a 75-minute procedure, thus overcoming the need to use a 96-well plate with a potential cost and time savings.
SuPAR quantification is usually used in many diseases in which the uPA/uPAR system is deregulated. However, CHORUS suPAR is a ‘COMPANION DIAGNOSTIC’ device and is specifically designed to study a peculiar population with COVID-19.
This device could be used in all wards, especially in the emergency room and intensive care units to identify those patients who could develop severe symptoms of COVID-19, given the association well described in the literature between elevated suPAR levels and COVID-19 severity.
Of 522 analysed samples, few false positives or false negatives were retrieved compared to suPARnostic ELISA. All these samples’ values were into the range from 5 to 7 µg/L, namely around the threshold (6 µg/L) that divided negative from positive result. Therefore, minimal quantitative differences in the suPAR concentration may affect qualitatively the results, establishing the positivity (or negativity) of the sample. On the other hand, the Bland Altman test indicated that there was some variability between CHORUS suPAR assay and suPARnostic® among values higher than 20, which were quantified as >20 in the CHORUS suPAR assay, even if, this variability has a low clinical significance.
Consequently, from a clinical point of view, test results must be evaluated without disregard those patient’s clinical parameters that could help to determine the disease severity or an unfavourable prognosis; the suPAR level should always be evaluated together with data from the patient’s history and/or other diagnostic tests, representing, at the same time, a key biomarker for the following patient management.
CHORUS suPAR was used to quantify suPAR concentration ⩾6 µg/L in patients with moderate or severe COVID-19; the percentages of patients with elevated suPAR concentrations were similar to those observed in previous trials (63.5% in open-label, phase II trial and 57.2% in the SAVE-MORE trial).12,13 Therefore, based on these preliminary data, the assay could be suitable and reliable to identify those patients with moderate to severe COVID-19 who could be potential candidates for anakinra treatment. The availability of a reliable assay that can accurately quantify suPAR is important because some inaccuracies in determining disease severity are still present. Further research is needed to determine the most sensitive modalities in identifying patients with higher mortality. 17
Conclusion
CHORUS suPAR kit resulted as rapid, sensitive, specific, accurate and able to quantify suPAR concentrations in patients with moderate and severe COVID-19. All these features support its use in clinical practice for suPAR quantification in all hospital wards, especially in emergency rooms and intensive care units. The easy-to-use and minimal possibility to make operator-related mistakes thanks to the complete automation of the system may represent further drivers of choice in favour of the use of these devices.
Acknowledgments
The authors thank Content Ed Net for editorial support, with the helpful contribution of medical writer Elisa Sala, Ph.D.
Declarations
Ethical approval and consent to participate: The study was approved by the Comitato Etico Regionale per la Sperimentazione Clinica della Regione Toscana on 11 April 2022. The informed consent was not required for this study.
Consent for publication: Not applicable.
Author contributions: Cerutti Helena: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Writing – original draft; Writing – review & editing. Tesi Giulia: Data curation; Investigation; Writing – review & editing. Cartocci Alessandra: Data curation; Investigation; Writing – review & editing. Guerranti Roberto: Data curation; Investigation; Writing – review & editing. Silvestrini Caterina: Data curation; Investigation; Writing – review & editing. Gori Sabrina: Data curation; Investigation; Writing – review & editing. Bianciardi Simone: Data curation; Investigation; Writing – review & editing. Bandini Tommaso: Data curation; Investigation; Methodology; Writing – review & editing. Brogi Alessandra: Conceptualization; Formal analysis; Methodology; Supervision; Writing – review & editing. Leoncini Roberto: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Supervision; Writing – review & editing.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: DIESSE Diagnostica Senese S.p.A. Società Benefit funded the study and editorial assistance.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Cerutti Helena, Tesi Giulia, Bianciardi Simone, Bandini Tommaso, Brogi Alessandra declare to be ‘DIESSE Diagnostica Senese S.p.A. Società Benefit’ employees. No other conflict of interest has been reported.
Availability of data and materials: Further data will be available upon request.
References
- 1. Thuno M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27:157-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cammalleri M, Dal Monte M, Pavone V, De Rosa M, Rusciano D, Bagnoli P. The uPAR system as a potential therapeutic target in the diseased eye. Cells. 2019;8:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. D’Alonzo D, De Fenza M, Pavone V. COVID-19 and pneumonia: a role for the uPA/uPAR system. Drug Discov Today. 2020;25:1528-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hayek SS, Sever S, Ko YA, et al. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hayek SS, Leaf DE, Samman Tahhan A, et al. Soluble urokinase receptor and acute kidney injury. N Engl J Med. 2020;382:416-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hindy G, Tyrrell DJ, Vasbinder A, et al. Increased soluble urokinase plasminogen activator levels modulate monocyte function to promote atherosclerosis. J Clin Invest. 2022;132:e158788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lippi G, Henry BM, Favaloro EJ. Elevated soluble urokinase plasminogen activator receptor (suPAR) in COVID-19 patients. Clin Chem Lab Med. 2021;59:e413-e415. [DOI] [PubMed] [Google Scholar]
- 8. Velissaris D, Lagadinou M, Paraskevas T, et al. Evaluation of plasma soluble urokinase plasminogen activator receptor levels in patients with COVID-19 and non-COVID-19 pneumonia: an observational cohort study. J Clin Med Res. 2021;13:474-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang M, Li L, Shen J, et al. Plasma levels of the active form of suPAR are associated with COVID-19 severity. Crit Care. 2020;24:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Enocsson H, Idoff C, Gustafsson A, et al. Soluble urokinase plasminogen activator receptor (suPAR) independently predicts severity and length of hospitalisation in patients with COVID-19. Front Med (Lausanne). 2021;8:791716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rovina N, Akinosoglou K, Eugen-Olsen J, Hayek S, Reiser J, Giamarellos-Bourboulis EJ. Soluble urokinase plasminogen activator receptor (suPAR) as an early predictor of severe respiratory failure in patients with COVID-19 pneumonia. Crit Care. 2020;24:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kyriazopoulou E, Panagopoulos P, Metallidis S, et al. An open label trial of anakinra to prevent respiratory failure in COVID-19. Elife. 2021;10:e66125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kyriazopoulou E, Poulakou G, Milionis H, et al. Author correction: early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. 2021;27:1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kyriazopoulou E, Huet T, Cavalli G, et al. Effect of anakinra on mortality in patients with COVID-19: a systematic review and patient-level meta-analysis. Lancet Rheumatol. 2021;3:e690-e697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. AIFA. Anakinra nella terapia dei pazienti adulti con COVID-19. 2021. https://www.aifa.gov.it/documents/20142/1123276/Anakinra_28.09.2021.pdf
- 16. WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192-e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matuszewski M, Ladny J, Rafique Z, et al. Prediction value of soluble urokinase plasminogen activator receptor (suPAR) in COVID-19 patients - a systematic review and meta-analysis. Ann Agric Environ Med. 2023;30:142-147. [DOI] [PubMed] [Google Scholar]