Abstract
The hydrophobicity and aggregation of zein, a biopolymer, limit its application as an effective drug delivery carrier. Here, we developed a zein-induced polyelectrolyte (ZiP) complex and investigated its efficiency in delivering 1% hydrolyzed ginseng saponin, a compound K-rich fraction derived from the root of Panax ginseng. The ZiP complex was formulated by incorporating the self-assembled amphiphilic prolamin zein into the aqueous phase. The physical properties, encapsulation efficiency, and stability of the encapsulation system at room temperature (25 °C) and 45 °C were assessed. The effects of different ratios of zein, pullulan, and pectin on the formation of the ZiP complex, the encapsulation stability, and the cellular efficacy of ZiP complexes were also assessed. The ZiP complex was surface-modified with hydrophilic pullulan and pectin polysaccharides in a mass ratio of 1:2:0.2 through electrostatic interactions. The primary hydrophilic modification of the ZiP complex was formed by the adsorption of pullulan, which enhanced the encapsulation stability. The outermost hydrophilic layer comprised the gelling polysaccharide pectin, which further improved the stability of the macro-sized oil-encapsulated complex, reaching sizes over 50 μm. The size of the ZiP complex increased when the concentration of pectin or the total content of the ZiP complex increased to 2:4:0.2. Compound K was successfully encapsulated with a particle size of 294.8 nm and an encapsulation efficiency of 99.6%. The ZiP complex demonstrated stability at high temperatures and long-term stability of the encapsulated saponin over 24 weeks. These results revealed the potency of ZiP complexes that enhance the in vivo absorption of phytochemicals as effective drug delivery carriers that can overcome the limitations in industrial formulation development as a delivery system.
1. Introduction
Phytochemicals exert several health benefits and improve the immune system; however, their efficacy is limited by their low solubility and adsorption. To overcome these limitations and accelerate their application in drug delivery systems, research interests in various innovative technologies, including emulsification,1,2 encapsulation,3,4 and nanoemulsion systems,5,6 have increased. Over the past few decades, conventional phytochemical encapsulation systems using biopolymers have been developed to improve the bioavailability, aqueous solubility, and targeted delivery of phytochemicals.7,8 Among various biopolymers, the prolamin storage protein zein is widely used for industrial applications because of its biodegradability, biocompatibility, and economic advantages.9 Zein, a water-insoluble prolamin stored in corn kernels, is generally recognized as safe by the Food and Drug Administration.9 The potential of zein that exhibits a specific brick shape in the aqueous phase has been explored in fabricating encapsulation systems and delivery of hydrophobic drugs.9 Furthermore, zein overcomes the disadvantages of hydrophilic protein-based delivery systems because they do not require hardening by chemical or physical treatment to continuously increase the stability of the system.10 Zein nanoparticles have evolved as an efficient encapsulation and delivery system for hydrophobic materials in core/shell or hybrid forms.3,5 Native zein nanoparticles have been utilized for the oxidation stability of tocopherol.2 Zhang et al. used hyaluronic acid to coat honokiol-loaded zein nanoparticles that target tumors overexpressing CD44 receptors.11 Song et al. exploited a hollow nanoparticle zein complex to achieve the sustained release of crocin with a dual coat effect.12 Although zein has been considered a smart vehicle for drug delivery systems, its hydrophobicity and aggregation are major hurdles that limit its application in phytochemical delivery in industrial applications.
Polysaccharides, such as pullulan and pectin, are used as hydrophilic materials to coat the surfaces of protein vectors.13 Pullulan produced by Aureobasidium pullulans is a film-forming shell polysaccharide with maltotriose units (α-1,4-; α-1,6-glucan′) adsorption properties.14−16 Pectin forms a cellulose–hemicellulose network hydrogel held together by hydrogen bonding and acts as a physical support fixture in a tree-grid-like structure.13,17,18 Pectin is negatively charged in acidic environments,18 and low-methoxyl pectin has more carboxyl groups than high-methoxyl pectin.19 Therefore, it allows electrostatic repulsion15 and coassembly interactions with positively charged zein in the nanocomposites.9 Additionally, pectin has been demonstrated to enhance the absorption of phytochemicals, such as flavonoids, in the body by increasing mucosal adhesion and residence time through the protein–polysaccharide complex while enabling sustained release and targeted delivery.20 Therefore, these polysaccharides can enhance the physical encapsulation stability and water solubility of the zein-based delivery system. Previous studies have shown that proline-rich proteins can form hydrogen bonds and hydrophobic interactions with polyphenols and triterpenoids owing to the aromatic residues present in proline.21 Furthermore, pullulan has ideal gel-forming properties in water14 and is combined with aligned hydroxyl groups; therefore, it may have an appropriate immobilization ability between zein and negatively charged hydrophilic pectin. We hypothesized that formulating a polyelectrolyte complex by combining pullulan, pectin, and zein can increase the water solubility of phytochemicals and improve the storage stability of the complex.
To test the above hypothesis, we formulated a polyelectrolyte complex called a zein-induced polyelectrolyte (ZiP) complex by combining pullulan, pectin, and zein in different ratios. We quantitatively and qualitatively characterized the physical properties of the ZiP complex, including hydrodynamic size, ζ-potential, encapsulation efficiency (EE; %), and stability. The plant protein zein can self-assemble9,22 with carboxylic-group triterpenoids, such as compound K and oleanolic acid, through hydrophobic interactions.21,23,24 Compound K, derived from ginseng saponin, exerts anti-inflammatory effects7 and has been extensively researched as a health-promoting material.25 Therefore, to analyze the EE of the ZiP complex, we used compound K as the target molecule and assessed the encapsulation stability of compound K in the zein delivery system.
2. Results and Discussion
2.1. Formulation of ZiP Complexes
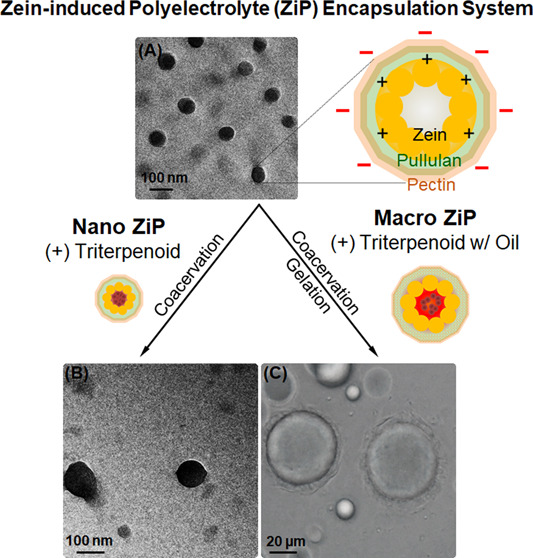
The ZiP complex reported here was developed by using biodegradable biopolymers. The core of this complex contains zein, which serves as an amphiphilic biopolymer at the interface between the hydrophobic and hydrophilic phases. It comprised zein, pullulan, and pectin at a mass ratio of 1:2:0.2. This ratio was selected based on preliminary trials using mass ratios of 1:2:0.1, 1:2:0.2, and 1:2:0.4, among which the dispersion prepared at a mass ratio of 1:2:0.2 exhibited a stable nanosized structure. As illustrated in Figure 1A, zein was phase-separated from propylene glycerol (80%) by mixing with pullulan solution (2% w/v), followed by the sequential addition of pectin solution (0.2% w/v) to form the ZiP complex. Subsequently, the triterpenoid compound was added to obtain the nanosized ZiP complex, and both the triterpenoid and squalene oil were added to obtain the macro-sized ZiP complex (Figure 1B). Mechanically, in acidic dispersions, zein becomes protonated and loses its solubility. The hydrophobic interaction between zein nanoparticles allows triterpenoids to be encapsulated in the proline-rich regions of the zein protein owing to the aromatic residues present in proline by self-assembly. Zein nanoparticles become positively charged under acidic conditions and exhibit strong adsorption between immiscible phases, resulting in the formation of hydrophobic zein coated with hydrophilic layers.26
Figure 1.
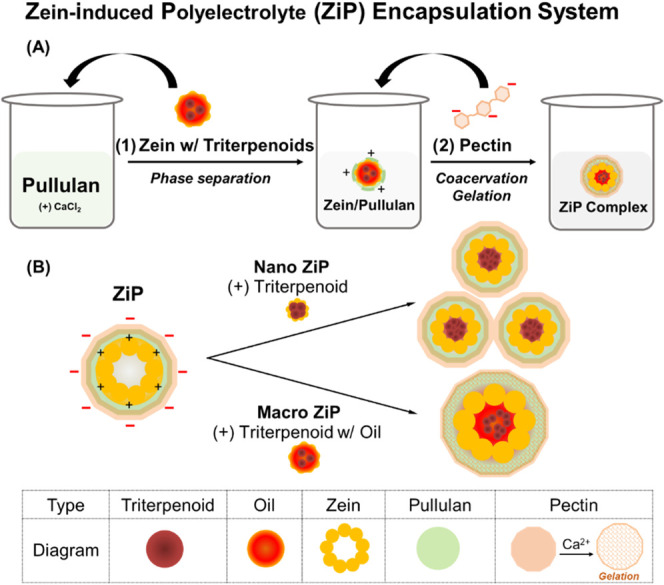
Scheme of the zein-induced polyelectrolyte (ZiP) complex formulation through the interfacial complexation of zein, pullulan, and pectin via electrostatic interactions.
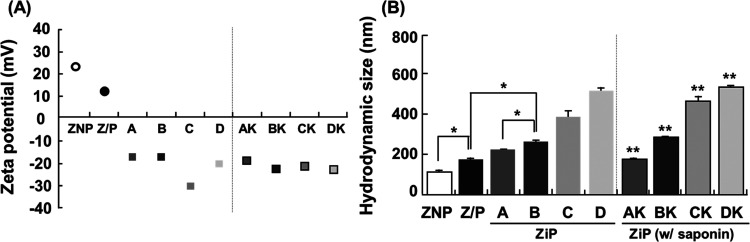
To evaluate the effects of the proportions of its components, four types of ZiP complexes were formulated, in which the mass ratio of zein to pullulan was set at 1:2 and that of pectin varied from 0.1 to 0.5: Type A: 1:2:0.1; Type B: 1:2:0.2; Type C: 1:2:0.5; Type D: 2:4:0.2 (Table 1). When a 1:1 mass ratio of zein to pullulan was used, the formation of nanosized complexes did not improve (not shown). Instead, these samples grew to macroscopic sizes and partially precipitated within 24 h of storage at room temperature, which could be because of the random occurrence of zein nanoparticle agglomeration owing to hydrophobic interactions. This result suggests that the surface modification of pullulan may not have been sufficient. Additionally, to evaluate the stability of the EE of 1% hydrolyzed ginseng saponin, type D was prepared by doubling the amount of type A.
Table 1. Effects of Zein, Pullulan, and Pectin Mass Ratio on Particle Size, Polydispersity Index (PDI), and ζ-Potential of ZiP Complexesa.
| types | zein | pullulan | pectin | size (nm) | PDI | ζ-potential (mV) | |
|---|---|---|---|---|---|---|---|
| ZNP | 1 | 129.6 | 0.215 | 23.2 | |||
| Z/P | 1 | 2 | 189.0 | 0.172 | 11.7 | ||
| ZiP | A | 1 | 2 | 0.1 | 237.3 | 0.241 | –18.2 |
| B | 1 | 2 | 0.2 | 276.4 | 0.235 | –18.0 | |
| C | 1 | 2 | 0.5 | 396.1 | 0.438 | –31.4 | |
| D | 2 | 4 | 0.2 | 527.3 | 0.372 | –21.5 | |
| ZiP | AK | 1 | 2 | 0.1 | 191.0 | 0.108 | –19.6 |
| (w/saponin) | BK | 1 | 2 | 0.2 | 294.8 | 0.167 | –23.9 |
| CK | 1 | 2 | 0.5 | 465.9 | 0.238 | –22.2 | |
| DK | 2 | 4 | 0.2 | 538.8 | 0.279 | –23.7 |
The types AK to DK correspond to the encapsulation of 1% hydrolyzed ginseng saponin, specifically containing the triterpenoid compound K. All samples were prepared at pH 6.0
(A) Schematic illustration of formulation of two hydrophilic layer coated zein complex using phase separation and coacervation: zein solution (2% w/v) was mixed with a hydrophilic pullulan solution (2% w/v) in water, and pectin solution (0.2% w/v) was added sequentially. For the encapsulation test, 1% hydrolyzed ginseng saponin was added to the zein stock solution during the encapsulation step. (B) The hydrophobic interaction of proline-rich zein in the aqueous phase enables the self-assembly and encapsulation of triterpenoids within the amphiphilic protein zein. The nanosized ZiP complex is stabilized through the adsorption of pullulan onto zein, which has a cationic surface charge, and the incorporation of negatively charged hydrophilic pectin layers. This stabilization mechanism allows the encapsulation of triterpenoids such as compound K or oleanolic acid within the complex. The macro-sized ZiP complex is stabilized by the gelation of the outer pectin layer with CaCl2, facilitating the encapsulation of liquid squalene and triterpenoids.
The size and surface charge of the nanoparticles could be adjusted by varying the ratio of zein to pullulan and pectin. Pullulan-coated zein nanoparticles have a positive surface charge, resulting in a negative charge in the coacervate with pectin (pH 6.0; Figure 2A). The incorporation of pullulan and pectin significantly altered the surface properties of the hydrophilic zein colloidal nanoparticles. In the absence of hydrophilic polysaccharides, the zein nanoparticles tended to form larger particles and agglomerates, making them difficult to disperse in water. The incorporation of pullulan resulted in a denser core/shell structure owing to its hydrophilic adsorption onto the zein nanoparticles. When only pectin coacervates interacted with the zein nanoparticles, the size of the resulting zein/pectin composite particles became much larger at the microscale, and the system became unstable, making it unsuitable for encapsulating 1% hydrolyzed ginseng saponin at the nanoscale (not shown).
Figure 2.
Characteristics and stability of zein nanoparticles (ZNP), zein/pullulan (Z/P) complexes, and ZiP complexes prepared with 1% hydrolyzed ginseng saponin. The ζ-potential (A) and hydrodynamic size (B) of the complexes were determined using dynamic light scattering (DLS) analysis. * p < 0.01 vs ZiP complexes with ZNP or Z/P complexes. ** p < 0.01 vs ZiP complexes without hydrolyzed ginseng saponin.
The vector size and surface charge at various formulation ratios were characterized for each condition. The size and charge between zein and pullulan (Z/P) and the ZiP complex structures differed. The average particle size increased from 237.3 to 396.1 nm as the mass ratio of zein, pullulan, and pectin increased from type A to type C (Figure 2B). The size of the ZiP complex was 237.3 nm when pectin was added to type A, which was 1.83 times larger than that of the Z/P nanoparticles without pectin. When pectin was added, the electrostatic interactions became stronger, forming stabilized ZiP complexes. Stable self-assembled ternary complexes were produced for type B with a mass ratio of pectin that was two times higher than that of type A (Table 1). These results indicate that the increased pectin ratio may improve the complex stability and suitability for various applications. However, the results for type D showed that doubling the amount of type A did not improve the formation of stabilized nanosized complexes. Instead, the size of the particles increased significantly to over 500 nm and the polydispersity index (PDI) increased to over 0.3 (Table 1). As shown in Table 1, the size of the AK nanoparticles (191.0 nm) was smaller than that of type A (237.3 nm). This could be because zein in the polyelectrolyte complex also decreased with increased precipitation because of the inability to form a good trapping film of pullulan and pectin after the hydrophobic binding of zein and hydrolyzed ginseng saponin.
No precipitation occurred immediately after the formation of the type BK complex; however, the type BK complex was bigger (294.8 nm) than type B (276.4 nm). This indicated that the active phytochemical compound K in hydrolyzed ginseng saponin maintained its shape in a denser state because of its hydrophobic interactions with zein. The zein content of type D increased 2-fold compared to that of type A, and its size increased to 527.3 nm, confirming the formation of an unstable nanocomplex. These data indicated that at a higher mass ratio of pectin (>0.2), the ZiP complex formed nanoparticles linked by a galacturonic acid chain rather than a single nanocarrier, owing to the higher concentration of galacturonic acid chains in pectin. Additionally, electron microscopy revealed that in type B, where the pectin content was twice that of type A, the core size did not increase, but relatively uniform and abundant particles were formed (Figure S1). It is believed that type B represents the optimal ratio for stable complex formation due to the increased pectin content, potentially driven by electrostatic forces, compared to type A. On the contrary, for type D, where the overall content was doubled compared to type A, the core particle size increased by more than 2 times. This result suggests that an increase in zein content led to the enlargement of the core size and a hydrophilic layer linkage between each complex, as confirmed when compared to hydrodynamic size values.
Measurement of the ζ-potential revealed the successful formation of the ZiP complexes through the physicochemical combination of zein, pullulan, and pectin. For the Z/P nanoparticles, pullulan was adsorbed onto the surface of zein but positively charged, indicating electrostatic reactivity on the surface. Later, it was confirmed that nanosized ZiP complexes of types A–D were negatively charged because of the binding of pectin (Figure 2B). In particular, in types A and B, the mass ratio of pectin content was 0.1 to 0.2, respectively, leading to a negative surface charge of around −18 mV. When the mass ratio of pectin was increased to 0.5 (type C), the charge decreased to −31.4 mV. In type D, the mass ratio of pectin was 0.2, with a 2-fold increase in the total mass ratio compared to type A. This resulted in an inability to maintain a sufficiently negative surface charge, which fell to −21.5 mV. A slight positive charge was observed when the mass ratio of zein to pullulan exceeded 1:2. This could be due to the excess adsorption of pullulan onto the zein surface, forming a film on the exterior of the Z/P nanoparticles. This film could have inhibited their interaction with pectin and prevented the formation of the outermost layer of pectin, which is necessary for the proper encapsulation of hydrolyzed ginseng saponins. The negative surface charge of type CK was decreased (−22.2 mV) compared to that of type C (−31.4 mV). On the contrary, the negative charge of type BK was higher (−23.9 mV) than that of type B (−18.0 mV). The data demonstrated that after encapsulating compound K, the ZiP complex remains stably formulated at a nanosized scale, measuring 294.8 nm with a PDI value of 0.167. Together, these findings suggest that the ratio of the components is an important factor in maintaining the stability and proper functioning of zein-based polyelectrolyte ternary complexes.
2.2. Characterization of ZiP Complexes
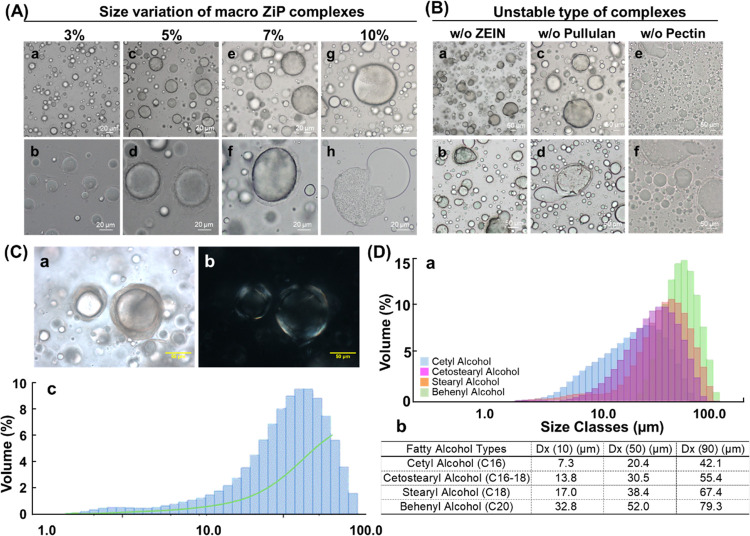
The ZiP system was also applied to squalene oil encapsulation, a phytochemical-enriched liquid. The simultaneous stable encapsulation of oil with hydrophobic triterpenoids on a macroscopic scale in ZiP systems requires hardening of the outer pectin layer compared with the encapsulation of triterpenoids alone. Low-methoxy pectin was gelled by adding calcium chloride under slightly acidic conditions (pH 6.0) to control particle size based on the oil content. Its shape is flexible, similar to that of an elastic liposome,27 forming a double membrane with different phases and serving as a stable barrier against internal and external ruptures. The B-type ZiP complexes were optimized by adding calcium chloride in a 0.2 ratio with pectin for outer surface gelation. The macro-sized ZiP complexes had a homogeneous spherical shape, with an average size of 59.9 μm. The size of the oil-encapsulated complexes increased with increasing oil loading; however, the stability deteriorated when the oil loading exceeded 5% (Figure 3A).
Figure 3.
Characteristics of the oil-encapsulated ZiP complexes. (A) Size distribution of ZiP complexes prepared with oil content variations of 3% (a, b), 5% (c, d), 7% (e, f), and 10% (g, h). Scale bar: 20 μm. (B) Instability of complexes without zein (a, b), pullulan (c, d), or pectin (e, f). Scale bar: 50 μm. (C) Macroscopic optical (a) and polarized (b) microscopy images of ZiP complexes with 5% oil content and 1% hydrolyzed ginseng saponin encapsulation. The gelation outer layer of the ZiP complex was detected in both optical and polarized imaging. Scale bar: 50 μm. (D) (a) Characteristics of ZiP complexes prepared with different types of fatty alcohols and 5% oil content; (b) Size distribution of the macro-sized ZiP complexes analyzed using a Master Sizer instrument.
The amphiphilic zein protein was used as a surfactant-like material in the ZiP system to stabilize the hydrophobic and hydrophilic interfaces. When macro-sized ZiP complexes were prepared without zein, the outermost gelation layer did not form and the oil separated from the complex immediately after preparation. This may be due to the loss of electrostatic interactions between zein and pectin, which maintain the stability of the complex. However, pullulan was adsorbed onto the zein surface even without pectin, forming a thin inner film and resulting in a stable oil-encapsulated complex with fewer particles and a relatively small size. When the complex was made with zein and pectin without pullulan, the gelation layer was visible, but the particle size was relatively large and oil leaked owing to rupture. This indicates that it is difficult to maintain a sufficiently stable state of a complex that encapsulates liquid oil and triterpenoids in a single pectin layer on a macroscopic scale. Together, the findings suggest that the zein-induced ternary polyelectrolyte complex is optimal for the encapsulation of liquid oil with triterpenoids of a macroscopic complex size. Figure 1 indicates that the stable and uniform loading of oil on the macro-sized ZiP complex was due to the presence of two different hydrophilic layers in the complex. This is because pullulan was adsorbed onto the zein surface, providing primary hydrophilic immobilization,42 whereas the outermost pectin gelation layer physically maintained complementary interactions with pullulan. The structure of the ZiP complex and the mechanism of complex formation are shown in Figure 1 based on these results.
2.3. Encapsulation Efficiency (EE) of ZiP Complexes
Previous studies have attempted to develop encapsulation technology to achieve efficient delivery and release of drugs for industrial applications.2,28,29 In this study, we quantitatively evaluated the EE of a ZiP system with potential applications in industrial products. To evaluate the EE of the ZiP complex, 1% hydrolyzed ginseng saponin was added to the samples. The optimal ratio of the ZiP complex content was determined by quantitative analysis of the encapsulated compound K in hydrolyzed ginseng saponin. We studied the mass ratio of pectin within the range of 0.1, 0.2, and 0.5 for samples AK to DK while maintaining a fixed concentration of hydrolyzed ginseng saponin at 1%. In the preparation of type AK, precipitation of the complex was observed and removed, leading to significant losses and insufficient encapsulation of 1% hydrolyzed ginseng saponin. Type A was unsuitable for the stable encapsulation of saponins, as the complexes separated and precipitated within 48 h. The EEs of saponin in types BK-DK ranged from 99.6% in BK to 78.74% in CK and 75.29% in DK complexes. The content of compound K in 1% hydrolyzed ginseng saponin was found to be 329.83 ppm, whereas that in type BK was 328.56 ppm, which was 99.6% of the original content. This data showed that high EE was stably maintained owing to the initial hydrophobic interaction between compound K and zein, as well as the electrostatic interaction between zein and pectin. Type BK had a higher negative charge than type B, resulting in a more stable packing structure with successful encapsulation of compound K and no precipitation. However, in type CK, increasing the pectin content to a mass ratio of 0.5 limited the encapsulation of compound K, resulting in a decreased negative charge and a high PDI value of 0.438. In type DK, in which the content of each component was twice that in AK, the size increased to 538.8 nm. This can be attributed to the excessive amount of each component in the self-assembled structures, which may have caused precipitation. No precipitation or active stability issues were observed during the long-term storage of compound K within the type BK ZiP complex. These results provide evidence that the EE of the ZiP system is influenced by the mass ratio of its components, with the highest EE observed at a mass ratio of 0.2 pectin and a 1:2 ratio of zein to pullulan (Figure 3). These findings suggest that adjusting the mass ratio of each component is a viable strategy for optimizing the EE of the ZiP system.
2.4. Encapsulation Stability of ZiP Complexes
Next, to optimize the stability of compound K within the oil-encapsulated system, a liquid phytochemical-enriched hydrocarbon oil (squalene) was added to type BK. After oil encapsulation, the sizes increased macroscopically and the stability was evaluated, as shown in Figure 3. Long-term stability was not guaranteed when the oil content in the complex exceeded 5%. Fatty alcohols with long carbon chains and hydroxyl groups are used in the industry to enhance oil encapsulation stability.28 We hypothesized that fatty alcohols could improve long-term stability when more than 5% oil and poorly soluble phytochemicals are loaded simultaneously. To test this hypothesis, we tested the suitability of various fatty alcohols at a concentration of 0.5% in the 5% oil-encapsulated ZiP system. The oil encapsulation size depended on the carbon chain length of the fatty alcohol added to the ZiP system and increased with an increasing carbon number (Figure 3D). Stearyl alcohol (C18) and behenyl alcohol (C20), with a size above 67.4 μm, led to inconsistent size distribution depending on manufacturing mixing speed. Cetostearyl alcohol (C16–C18) had over 90% of particles within 55.4 μm, and cetyl alcohol (C16) led to smaller gelation layer sizes and an average complex size of 20.4 μm.
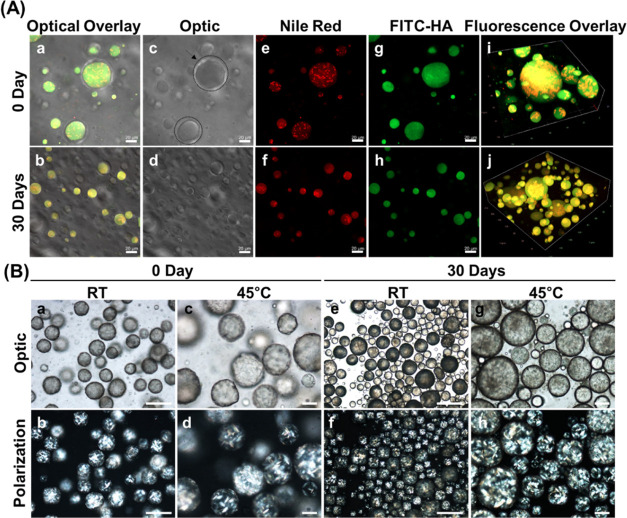
In the macro-sized ZiP system, the outermost gelation layer acts as a quasi-solid phase, serving as a physical barrier to prevent the release of hydrophobic particles. The results showed that the prevention of precipitation by the aqueous gel of the outermost layer stabilized the ZiP complex within the same phase (Figure 4A). Fluorescent dyes confirmed the hypothesis that pullulan, a key component of the inner surface layer, plays a crucial role in loading crystallized triterpenoids and oil. In other words, Fit C-tagged hyaluronic acid30 was simultaneously formed with pullulan, confirming the stability of the encapsulated triterpenoids and the successful formation of the inner pullulan surface layer. Furthermore, the analysis showed that the leakage of Nile Red dye contained in the oil did not release the hydrophobic core into the water phase (Figure 4A). Together, these results demonstrated that negatively charged hydrophilic active materials, such as hyaluronic acid, can be loaded into the ZiP system. Based on the EE of the ZiP complex, 1% hydrolyzed ginseng saponins were successfully encapsulated in 5% squalene (Figure 4A). To confirm the stability of encapsulated compound K, its content was analyzed using HPLC on 8, 12, and 24 weeks of storage. The stability of compound K was maintained for an extended period at 101.3, 98.0, and 96.1% on 8, 12, and 24 weeks, respectively. These results confirm the long-term storage stability of the complex, indicating the effectiveness of the potency stability test (Figure S2).
Figure 4.
Visualization of macro-sized ZiP complexes prepared with fluorescence dye tagging. (A) Confocal images taken in the Z-direction present the encapsulated 1% hydrolyzed ginseng saponin and 5% squalene in type BK (a, b). Optical images show the transparent gelation outermost pectin layer pointed with black arrows (c, d). Red hydrophobic Nile Red with squalene and triterpenoid core part (e, f). Green hydrophilic Fit C-tagged inner pullulan layer (g, h), which coexist simultaneously at 0 and 30 days. The overlay of all images (i, j). Scale bar: 20 μm. (B) Visualization of BK type ZiP prepared with 0.05% oleanolic acid for 30 days at room temperature (25 °C) and high temperature (45 °C). The optical and polarization images of macro-sized ZiP complexes were determined using microscopy to confirm long-term encapsulation stability; Scale bar: 50 μm.
Moreover, oleanolic acid was incorporated into the macro-scale encapsulated ZiP complex to validate its stability in other types of triterpenoid encapsulation systems. Oleanolic acid is a well-known phytochemical.31 However, its use has been limited because of its low aqueous solubility. In addition, formulation stability studies are required to prevent aggregation or precipitation in encapsulation complexes.32 Oleanolic acid inhibits encapsulation stability owing to its high melting point and less water solubility during long-term storage.33 This study also tested the ability of the gelation layer to maintain the encapsulated complex stability under high-temperature conditions. As shown in Figure 4B, the crystallized oleanolic acid did not leak to the outer layer of the complex for 30 days at 25 and 45 °C and was stably encapsulated within the gelation layer. Thus, the oleanolic acid encapsulated inside the external water layer did not precipitate and was confined within the pectin layer. These findings indicate that the outermost gelation layers and primary pullulan inner layers can effectively act as barriers to ensure long-term encapsulation stability. These results showed that adopting pullulan with zein is an effective barrier for encapsulation stability within the outermost gelation layer. In addition, the ability of the hybrid vector to effectively encapsulate both hydrophobic and hydrophilic materials and maintain their stability over time is also evident. Collectively, the ZiP complex formulated in this study is promising for encapsulating and stabilizing different types of materials for various applications.
2.5. Wound Healing Efficacy of ZiP Complexes
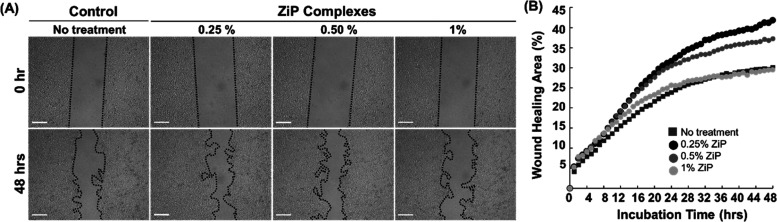
The wound healing assay is used to evaluate the two-dimensional effects of cell–cell interactions and the cell matrix on cell migration.34 In this study, the wound healing assay revealed significant regeneration efficacy in 0.5% ZiP complex-treated HaCaT cells compared to the negative control within 2 h, and the efficacy showed a significant difference after 16 h. In contrast, in the 0.25% ZiP complex treatment condition, increased wound healing efficacy was observed after 2 h of treatment compared to the negative control; however, it was significant only after 32 h of treatment. A quantitative comparison of the wound healing area at each hour (Table S1) revealed an increased wound healing area within 2 h of treatment with the 0.25% ZiP complex (Figure 5). This experiment confirmed that the ZiP complex at 0.25 and 0.5% exhibited cell regeneration efficacy in vitro.
Figure 5.
Wound scratch assay in the presence of the ZiP complex. (A) Representative images of migrated cells. Scale bar: 100 μm. (B) Quantitative analysis of the wound healing area by time-lapse microscopy imaged for 48 at 1 h intervals.
3. Conclusions
In this study, we strategically exploited the potential of sustainable drug delivery systems for the effective stabilization and delivery of phytochemicals using biopolymers. The adsorption capacity of pullulan on zein colloidal nanoparticles under aqueous conditions allowed the formation of self-assembled Z/P nanocomposites through phase separation, while simultaneously modifying the surface of the zein particles. The hydrophilic and film-forming pullulan component is the key ingredient of the ZiP complex, which can compete with pectin molecules for dissolution in water, reducing the hydration of pectin chains and encouraging their interaction. This trend is believed to increase the stability of both nano- and macro-ZiP complexes. The ZiP complex stably encapsulated triterpenoids, such as compound K and oleanolic acid. In addition, when oleanolic acid was encapsulated, the shape of the crystalline phase was stable without long-term precipitation. The double hydrophilic coating enhanced the solubility and encapsulation efficiency of the complex, ensuring the long-term stability of nonaqueous soluble triterpenoid phytochemicals in the inner phase owing to the primary barrier of the inner membrane and the gelation of the outermost film. In particular, macro-sized ZiP complexes were developed in squalene oil encapsulation with the gelation of the pectin layer. The ZiP complex showed cell regeneration efficacy within 2 h in vitro. These findings may serve as a foundation for further research on the ZiP system on a large scale to evaluate its cellular efficacy for potential industrial applications and to understand the mechanisms of phytochemical release from the ZiP system. In summary, this newly designed encapsulation technology is expected to contribute significantly to numerous sustainable industrial applications as a platform technology and overcome the limitations in industrial formulation development as a delivery system that enhances the in vivo absorption of phytochemicals.
4. Materials and Methods
4.1. Materials
Zein [>99%, powder, average molecular weight (MW) 20 kDa], pullulan (fermented polysaccharides from A. pullulans, MW 50–100 kDa), oleanolic acid (>97%, powder), pectin (from citrus peels, MW 195 kDa), calcium chloride (≥97%, anhydrous powder), and squalene oil (>96%, liquid) were purchased from Sigma-Aldrich (St. Louis, MO). Hydrolyzed ginseng saponin, a compound K-rich fraction containing approximately 30% ginseng from the roots of Panax ginseng, was produced by the Amorepacific R&D unit (Yongin, Korea).35 Standard compound K (≤100%, MW 622.87 g/mol) was purchased from the Ambo Institute. Cetyl alcohol (C16), stearyl alcohol (C18), cetostearyl alcohol (C16–18), and behenyl alcohol (C20) were provided from BASF Care Creations (Monheim, Germany), and Carbomer was obtained from SEPPIC, Inc. (Paris, France).
4.2. Formulation of ZiP Complexes
To prepare the ZiP complex, different mass ratios of zein and hydrophobic triterpenoids were mixed with 80% (w/v) ethanol or liquid polyalcohol.36 A stock solution of 2% (w/v) zein was prepared by using a binary mixture of propylene glycol and water at a mass ratio of 8:2 at 55 °C. Pullulan and pectin stock solutions were prepared at concentrations of 2 and 0.2% (w/v), respectively, in distilled water by gentle stirring at room temperature (25 °C). Each stock solution was stored at 4 °C before use. Afterward, 1 mL of pullulan stock solution was added dropwise to 49.4 mL of deionized water and mixed on a magnetic stirrer plate at 500 rpm (pH 6.0). Subsequently, 500 μL of the zein stock solution and 100 μL of pectin solution were added to the pullulan dispersion to obtain zein:pullulan:pectin mass ratio of 1:2:0.2. This ratio was selected based on preliminary trials using mass ratios of 1:2:0.1, 1:2:0.2, and 1:2:0.4, among which the dispersion prepared at a mass ratio of 1:2:0.2 formed stable nanosized complexes. For triterpenoid encapsulation, a 10% hydrolyzed ginseng saponin or 5% oleanolic acid stock solution was prepared using a binary mixture of propylene glycol and water at a mass ratio of 8:2. Five milliliters of the stock solution was added to zein during the encapsulation step. To evaluate the triterpenoid and oil EE of the ZiP complex, squalene oil was added at various concentrations (3, 5, 7, and 10%) to the ZiP complex obtained by combining zein, pullulan, and pectin in a mass ratio of 1:2:0.2. The outermost component, pectin, and gels in a semisolid form13,37,38 to enclose the oil inside the ZiP complex with calcium chloride (1:0.2 ratio with pectin) of macroscopic size, and 0.5% fatty alcohols were added to enhance the oil encapsulation stability by a physical protection effect39 of the gel phase formed in the ZiP complex. To evaluate the stability of the complexes, macro-sized ZiP complexes were dispersed in a 1% concentrated aqueous thickening solution of carbomer to prevent particle aggregation, and long-term stability at room temperature and 45 °C was evaluated for one month.
4.3. Characteristics and Morphological Analysis of ZiP Complexes
The particle size and surface charge of nanosized ZiP complex with encapsulating compound K were measured at 25 °C using a dynamic laser scattering instrument (Malvern). The intensity was measured at an angle of 90°. ζ-Potential measurements were conducted using a ζ-potential analyzer (Zetasizer Nano ZS, Malvern) at a pH of 6.0. All samples were 100 times diluted with deionized water to prevent isoelectrostatic precipitation and to minimize the interaction between the nanoparticles. The pH was adjusted by using an HCl solution, and the average value of three measurements was reported for each sample. Measurements were performed immediately after the preparation of the nanosized ZiP complexes. They were monitored for 8 weeks and stored at room temperature. The transmission electron microscopy (TEM) analysis of the nanosized ZiP complexes was performed at the Sungkyunkwan University for Cooperative Center for Research Facilities using a JEM-3010 transmission electron microscope (JEOL Ltd., Tokyo, Japan). Briefly, a carbon-coated grid was dipped into each sample (types A, B, BK, D, and DK) and dried overnight at room temperature. Afterward, TEM images were acquired under 200 kV with 200,000× magnification. The stability of the macro-sized ZiP complexes was visualized using a confocal microscope (LSM 980 NLO, ZEISS), and the size distribution of the macro-sized ZiP complexes (1–100 μm) of the oil-encapsulated complex was determined using a Master Sizer instrument (Master Sizer 3000, Malvern). The stability of the complex was evaluated for one month at room temperature using an optical microscope (Eclipse 80i, Nikon). Furthermore, we also performed optical and polarization imaging to visualize the macro-sized ZiP complexes. The sample was prepared using 0.05% oleanolic acid and 1% hydrolyzed ginseng saponin encapsulated in 5% squalene oil and stored at room temperature and 45 °C for 30 days before acquiring the optical and polarization images.
4.4. EE of ZiP Complexes
The 1% hydrolyzed ginseng saponin, a compound K-rich fraction, was encapsulated in each type of ZiP complex (AK–DK). To remove the unencapsulated complexes that did not form ZiP complexes during the manufacturing process, the supernatant was transferred and freeze-dried. Encapsulated compound K (d-glucopyranosyl-20(S)-protopanaxadiol) was released into an 80% ethanol solution in ZiP complexes (AK–DK) after ultracentrifugation at 10,000g for 10 min (Micro Centrifuge 5415C, Eppendorf). The samples were filtered using a 0.22 μm nylon membrane and analyzed by reversed-phase high-performance liquid chromatography (HPLC) using an Alliance e2695 XC HPLC system following a previously established method commonly used to analyze phenolic compounds.40 The separation was carried out in a gradient phase; the mobile phase comprised a mixture of acetonitrile and water at pH 6.0 to avoid ionization analysis. Ultrapure water was prepared by using a Direct-Q3 water purification machine (Merck Millipore, Inc.). Mobile phase A (acetonitrile: water = 60:40) was used at a flow rate of 1 mL/min, and the column was kept at 30 °C. The ultraviolet (UV) detector was set to 203 nm, and the following equation was used for the calculations5,41
| 1 |
where A is the total amount of compound K added to the ZiP complexes and a is the total amount of compound K in the encapsulated ZiP complexes, determined by measuring the amount released as the supernatant of 80% ethanol solution in freeze-dried samples.
4.5. Cellular Efficacy of ZiP Complexes
The human keratinocyte cell line (HaCaT) was obtained from CLS (Cell Lines Service GmbH; cat. no. 300493) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Lonza, #12–604F). The cells (2.0 × 105 cells/600 μL) were seeded in a 24-well cell culture plate and incubated for 24 h at 37 °C in a 5% CO2 incubator. The medium was then replaced with DMEM supplemented with 1% fetal bovine serum (FBS), and the cells were cultured for an additional 16 h. A linear scratch was made on a fully grown cell culture dish using a scratcher (SPL Company, #SPL201925), washed twice, and treated with the test sample in DMEM containing 1% FBS. The culture dish was then placed on the stage of a time-lapse microscope (JuLI Stage, NanoEnTek, Seoul, Korea) in a CO2 incubator, and the same location was photographed hourly for 48 h. Based on the images captured, the wound healing area (%) was calculated using the Wound Healing tool, an image J plugin.
4.6. Statistical Analysis
The experiments were performed in triplicate, and the results were expressed as the mean and standard deviation (SD). Statistical analysis was performed using a one-way analysis of variance (ANOVA) with a post-hoc Dunnett’s test using the SPSS 18.0 software package (IBM Corporation, Somers, NY). A p-value of <0.05 was considered significant.
Acknowledgments
The authors are grateful to the collaborators Joon Young Hwang, Soon Ae An, Sung-Il Park, Jae Won Yoo, Heung-Soo Beak, Won-Seok Park, Misook Shin, Somi Lee, and Byung-Fhy Suh of AMOREPACIFIC Corporation.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c05157.
Additional experimental details; materials, and methods; including photographs of experimental setup (PDF) (PDF)
Special Issue
Published as part of the ACS Omegavirtual special issue “Phytochemistry”.
Author Contributions
Y.J.K. was responsible for conceptualization, methodology, writing, and funding acquisition. E.-S.L. performed the methodology, investigation, writing, formal analysis, and visualization. J.C. was responsible for methodology, investigation, and formal analysis. S.H.P. performed the conceptualization, methodology, and funding acquisition. B.C. was responsible for conceptualization, supervision, project administration, and funding acquisition. E.K. performed the conceptualization, methodology, investigation, writing, supervision, and project administration.
This research was financially supported by the AMOREPACIFIC Corporation (R23L900008).
The authors declare no competing financial interest.
Special Issue
Published as part of the ACS Omegavirtual special issue “Phytochemistry”.
Supplementary Material
References
- Ganea G. M.; Fakayode S. O.; Losso J. N.; van Nostrum C. F.; Sabliov C. M.; Warner I. M. Delivery of phytochemical thymoquinone using molecular micelle modified poly(D, L lactide-co-glycolide) (PLGA) nanoparticles. Nanotechnology 2010, 21, 285104 10.1088/0957-4484/21/28/285104. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Khan M. A.; Cheng H.; Liang L. Co-encapsulation of α-tocopherol and resveratrol within zein nanoparticles: impact on antioxidant activity and stability. J. Food Eng. 2019, 247, 9–18. 10.1016/j.jfoodeng.2018.11.021. [DOI] [Google Scholar]
- Song J.; Sun C.; Gul K.; Mata A.; Fang Y. Prolamin-based complexes: Structure design and food-related applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1120–1149. 10.1111/1541-4337.12713. [DOI] [PubMed] [Google Scholar]
- Hassan E. A.; Hathout R. M.; Gad H. A.; Sammour O. A. A holistic review on zein nanoparticles and their use in phytochemicals delivery. J. Drug Delivery Sci. Technol. 2022, 73, 103460 10.1016/j.jddst.2022.103460. [DOI] [Google Scholar]
- Wang L.; Zhang Y. Eugenol Nanoemulsion Stabilized with Zein and Sodium Caseinate by Self-Assembly. J. Agric. Food Chem. 2017, 65 (14), 2990–2998. 10.1021/acs.jafc.7b00194. [DOI] [PubMed] [Google Scholar]
- Atanase L. I. Micellar Drug Delivery Systems Based on Natural Biopolymers. Polymers 2021, 13, 477 10.3390/polym13030477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baky M. H.; Elsaid M. B.; Farag M. A. Phytochemical and biological diversity of triterpenoid saponins from family Sapotaceae: A comprehensive review. Phytochemistry 2022, 202, 113345 10.1016/j.phytochem.2022.113345. [DOI] [PubMed] [Google Scholar]
- Baars R. J.; Leeuwen Y. M.; Hendrix Y.; Velikov K. P.; Kegel W. K.; Philipse A. P. Morphology-controlled functional colloids by heterocoagulation of zein and nanoparticles. Colloids Surf., A 2015, 483, 209–215. 10.1016/j.colsurfa.2015.04.042. [DOI] [Google Scholar]
- Gao Z.; Chen G.; Lu W.; Wu Y.; Hu B.; Xu L.; Fang Y.; Nishinari K.; Phillips G. O. Interfacial and emulsion-stabilizing properties of zein nanoparticles: differences among zein fractions (α-, β-, and γ-zein). Food Funct. 2021, 12, 1361–1370. 10.1039/D0FO02536D. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Luo Y.; Wang Q. Antioxidant and antimicrobial properties of essential oils encapsulated in zein nanoparticles prepared by liquid–liquid dispersion method. LWT-Food Sci. Technol. 2012, 48, 283–290. 10.1016/j.lwt.2012.03.027. [DOI] [Google Scholar]
- Zhang Q.; Wang J.; Liu D.; Zhu W.; Guan S.; Fan L.; Cai D. Targeted delivery of honokiol by zein/hyaluronic acid core-shell nanoparticles to suppress breast cancer growth and metastasis. Carbohydr. Polym. 2020, 240, 116325 10.1016/j.carbpol.2020.116325. [DOI] [PubMed] [Google Scholar]
- Song G.; Liu J.; Wang Q.; Wang D.; Chu B.; Li L.; Xiao G.; Gong J.; Zheng F. Layer-by-layer self-assembly of hollow dextran sulfate/chitosan-coated zein nanoparticles loaded with crocin: fabrication, structural characterization and potential biological fate. Food Hydrocoll. 2022, 125, 107420 10.1016/j.foodhyd.2021.107420. [DOI] [Google Scholar]
- Jiang Y.; Zhu Y.; Li F.; Du J.; Huang Q.; Sun-Waterhouse D.; Li D. Antioxidative pectin from hawthorn wine pomace stabilizes and protects Pickering emulsions via forming zein-pectin gel-like shell structure. Int. J. Biol. Macromol. 2020, 151, 193–203. 10.1016/j.ijbiomac.2020.02.164. [DOI] [PubMed] [Google Scholar]
- Jayeoye T. J.; Supachettapun C.; Muangsin N. Toxic Ag+ detection based on Au@Ag core shell nanostructure formation using Tannic acid assisted synthesis of Pullulan stabilized gold nanoparticles. Sci. Rep. 2023, 13, 1844 10.1038/s41598-023-27406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kicková E.; Salmaso S.; Mastrotto F.; Caliceti P.; Urtti A. Pullulan Based Bioconjugates for Ocular Dexamethasone Delivery. Pharmaceutics 2021, 13, 791 10.3390/pharmaceutics13060791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabasum S.; Noreen A.; Maqsood M. F.; Umar H.; Akram N.; Nazli Z. I.; Chatha S. A. S.; Zia K. M. A review on versatile applications of blends and composites of pullulan with natural and synthetic polymers. Int. J. Biol. Macromol. 2018, 120, 603–632. 10.1016/j.ijbiomac.2018.07.154. [DOI] [PubMed] [Google Scholar]
- Gawkowska D.; Cybulska J.; Zdunek A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers 2018, 10, 762 10.3390/polym10070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. Q.; Li J.; Dong H. L.; Li X.; Zhang J. Q.; Ramaswamy S.; Xu F. Pectin in biomedical and drug delivery applications: A review. Int. J. Biol. Macromol. 2021, 185, 49–65. 10.1016/j.ijbiomac.2021.06.088. [DOI] [PubMed] [Google Scholar]
- Wan L.; Wang H.; Zhu Y.; Pan S.; Cai R.; Liu F.; Pan S. Comparative study on gelling properties of low methoxyl pectin prepared by high hydrostatic pressure-assisted enzymatic, atmospheric enzymatic, and alkaline de-esterification. Carbohydr. Polym. 2019, 226, 115285 10.1016/j.carbpol.2019.115285. [DOI] [PubMed] [Google Scholar]
- Nižić L.; Potaś J.; Winnicka K.; Szekalska M.; Erak I.; Gretić M.; Jug M.; Hafner A. Development, characterisation and nasal deposition of melatonin-loaded pectin/hypromellose microspheres. Eur. J. Pharm. Sci. 2020, 141, 105115 10.1016/j.ejps.2019.105115. [DOI] [PubMed] [Google Scholar]
- Charlton A. J.; Baxter N. J.; Khan M. L.; Moir A. J.; Haslam E.; Davies A. P.; Williamson M. P. Polyphenol/peptide binding and precipitation. J. Agric. Food Chem. 2002, 50, 1593–1601. 10.1021/jf010897z. [DOI] [PubMed] [Google Scholar]
- Gomes A.; Sobral P. J. D. A. Plant Protein-Based Delivery Systems: An Emerging Approach for Increasing the Efficacy of Lipophilic Bioactive Compounds. Molecules 2022, 27, 60 10.3390/molecules27010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malík M.; Velechovský J.; Tlustoš P. Natural pentacyclic triterpenoid acids potentially useful as biocompatible nanocarriers. Fitoterapia 2021, 151, 104845 10.1016/j.fitote.2021.104845. [DOI] [PubMed] [Google Scholar]
- Zondlo N. J. Aromatic-proline interactions: electronically tunable CH/π interactions. Acc. Chem. Res. 2013, 46, 1039–1049. 10.1021/ar300087y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A.; Lee H. J. Ginsenoside Compound K: Insights into Recent Studies on Pharmacokinetics and Health-Promoting Activities. Biomolecules 2020, 10, 1028 10.3390/biom10071028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.; Singh R.; Vyas S. P. Multiple emulsion-based systems carrying insulin: development and characterization. J. Microencapsulation 1995, 12 (6), 609–615. 10.3109/02652049509006791. [DOI] [PubMed] [Google Scholar]
- Hussain A.; Singh S.; Sharma D.; Webster T. J.; Shafaat K.; Faruk A. Elastic liposomes as novel carriers: recent advances in drug delivery. Int. J. Nanomed. 2017, Volume 12, 5087–5108. 10.2147/IJN.S138267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj G. N.; Parakh S. R.; Devraj R.; Apte S. S.; Rao B. R.; Rambhau D. Release studies on niosomes containing fatty alcohols as bilayer stabilizers instead of cholesterol. J. Colloid Interface Sci. 2002, 251, 360–365. 10.1006/jcis.2002.8399. [DOI] [PubMed] [Google Scholar]
- Paliwal R.; Palakurthi S. Zein in controlled drug delivery and tissue engineering. J. Controlled Release 2014, 189, 108–122. 10.1016/j.jconrel.2014.06.036. [DOI] [PubMed] [Google Scholar]
- Gerecht S.; Burdick J. A.; Ferreira L. S.; Townsend S. A.; Langer R.; Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 11298–11303. 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollier J.; Goossens A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. 10.1016/j.phytochem.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Tong H. H.; Wu H. B.; Zheng Y.; Xi J.; Chow A. H.; Chan C. K. Physical characterization of oleanolic acid nonsolvate and solvates prepared by solvent recrystallization. Int. J. Pharm. 2008, 355, 195–202. 10.1016/j.ijpharm.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Gao S.; Yang M.; Luo Z.; Ban Z.; Pan Y.; Tu M.; Ma Q.; Lin X.; Xu Y.; Li L. Soy protein/chitosan-based microsphere as Stable Biocompatible Vehicles of Oleanolic Acid: An Emerging Alternative Enabling the Quality Maintenance of Minimally Processed Produce. Food Hydrocolloids 2022, 124, 107325 10.1016/j.foodhyd.2021.107325. [DOI] [Google Scholar]
- Liang C. C.; Park A. Y.; Guan J. L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Hossen M. J.; Hong Y. D.; Baek K. S.; Yoo S.; Hong Y. H.; Kim J. H.; Lee J. O.; Kim D.; Park J.; Cho J. Y. In vitro antioxidative and anti-inflammatory effects of the compound K-rich fraction BIOGF1K, prepared from Panax ginseng. J. Ginseng Res. 2017, 41, 43–51. 10.1016/j.jgr.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.; Zhong Q. A novel method of preparing stable zein nanoparticle dispersions for encapsulation of peppermint oil. Food Hydrocoll. 2015, 43, 593–602. 10.1016/j.foodhyd.2014.07.018. [DOI] [Google Scholar]
- Pliszczak D.; Bordes C.; Bourgeois S.; Marote P.; Zahouani H.; Tupin S.; Mattei C. P.; Lantéri P. Mucoadhesion evaluation of polysaccharide gels for vaginal application by using rheological and indentation measurements. Colloids Surf., B 2012, 92, 168–174. 10.1016/j.colsurfb.2011.11.039. [DOI] [PubMed] [Google Scholar]
- MacDougall A. J.; Brett G. M.; Morris V. J.; Rigby N. M.; Ridout M. J.; Ring S. G. The effect of peptide-pectin interactions on the gelation behaviour of a plant cell wall pectin. Carbohydr. Res. 2001, 335, 115–126. 10.1016/S0008-6215(01)00221-X. [DOI] [PubMed] [Google Scholar]
- Suzuki T.Effect of Molecular Assembly for Emulsion and Gel Formulations. In Cosmetic Science and Technology: Theoretical Principles and Applications; Sakamoto K.et al. , Ed.; Elsevier Inc., 2017; pp 519–537. [Google Scholar]
- Hossen M. J.; Hong Y. D.; Baek K. S.; Yoo S.; Hong Y. H.; Kim J. H.; Lee J. O.; Kim D.; Park J.; Cho J. Y. In vitro antioxidative and anti-inflammatory effects of the compound K-rich fraction BIOGF1K, prepared from Panax ginseng. J. Ginseng Res. 2017, 41, 43–51. 10.1016/j.jgr.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.; Zhang Z.; Hou J.; Jin X.; Ke Z.; Liu D.; Du M.; Jia X.; Lv H. Targeted delivery of ginsenoside compound K using TPGS/PEG-PCL mixed micelles for effective treatment of lung cancer. Int. J. Nanomed. 2017, 12, 7653–7667. 10.2147/IJN.S144305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evageliou V.; Richardson R. K.; Morris E. R. Effect of pH, sugar type and thermal annealing on high-methoxy pectin gels. Carbohydr. Polym. 2000, 42, 245–259. 10.1016/S0144-8617(99)00191-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.