Abstract
Background
Emergence agitation (EA) is a prevalent complication in children following general anesthesia. Several studies have assessed the relationship between melatonin or its analogs and the incidence of pediatric EA, yielding conflicting results. This meta-analysis aims to assess the effects of premedication with melatonin or its analogs on preventing EA in children after general anesthesia.
Methods
PubMed, EMBASE, the Cochrane Library, ProQuest Dissertations & Theses Global, Web of Science, CNKI, Wanfang Data, clinicaltrials.gov, and WHO International Clinical Trials Registry Platform were searched until 25 November 2022. We included randomized controlled trials that assessed EA in patients less than 18 years old who underwent general anesthesia. We excluded studies that did not use a specific evaluation to assess EA.
Results
Nine studies (951 participants) were included in this systematic review. Melatonin significantly reduced the incidence of EA compared with placebos (risk ratio 0.40, 95% CI 0.26 to 0.61, P < 0.01) and midazolam (risk ratio 0.48, 95% CI 0.32 to 0.73, P < 0.01). Dexmedetomidine remarkably decreased the incidence of EA compared with melatonin (risk ratio 2.04, 95% CI 1.11 to 3.73, P = 0.02).
Conclusions
Melatonin premedication significantly decreases the incidence of EA compared with placebos and midazolam. Dexmedetomidine premedication has a stronger effect than melatonin in preventing EA. Nevertheless, further studies are warranted to reinforce and validate the conclusion on the efficacy of melatonin premedication in mitigating EA in pediatric patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12871-023-02356-x.
Keywords: Emergence agitation, Melatonin, Pediatrics
Introduction
Emergence agitation (EA) is a prevalent complication in children after general anesthesia with a reported incidence of 10–80% [1]. Characterized by perceptual disturbances and psychomotor agitation, EA may be distressing for children and their parents as it delays wound healing and prolongs the length of the hospital stay [2]. A lack of premedication is a risk factor for the development of EA [2], and pharmacological premedication is considered effective in preventing EA [3].
Melatonin, a neurohormone secreted by the pineal gland in the human brain, has several important physiological functions [4]. Low serum melatonin levels have been demonstrated to be associated with delirium in adult patients [5]. Melatonin has been implicated as having anti-inflammatory [6], anxiolytic [7], and analgesic properties [4], which may reduce the precipitating factors of EA [1]. Exogenous melatonin is considered beneficial in various pediatric therapies due to its high therapeutic safety and few adverse effects [8]. Recently, researchers have paid increasing attention to melatonin premedication for children undergoing general anesthesia. Several studies have evaluated melatonin and the incidence of EA in children, with conflicting results. Some studies reported that melatonin, compared with placebos or midazolam, significantly reduced EA [7, 9], and one study demonstrated that dexmedetomidine had a stronger effect than melatonin in reducing EA [10]. Meanwhile, another study comparing melatonin with clonidine and dexmedetomidine found no significant differences [11].
In the context of melatonin’s limited oral bioavailability and short half-life, scientists have developed several melatonin analogs to improve its duration of action, bioavailability, and receptor affinity [12]. Notable among these analogs are ramelteon, tasimelteon, and agomelatine. Specifically, ramelteon, a tricyclic synthetic analog, selectively targets melatonin receptor 1 and melatonin receptor 2 [13]. Studies have confirmed that ramelteon has a superior receptor affinity and longer half-life than melatonin [13]. Ramelteon has demonstrated efficacy in reducing delirium risk among hospitalized adult patients [14]. Furthermore, in pediatric surgical settings, a clinical trial has explored ramelteon as a premedication to assess its potential to mitigate EA [15].
A meta-analysis [16] in 2015 reported that melatonin premedication could prevent EA in children who had undergone general anesthesia. However, as it only included four studies the authors could not draw conclusions on the comparison between melatonin and midazolam or dexmedetomidine and did not assess publication bias. In addition, this meta-analysis [16] did not include studies on melatonin analogs, which have been used to prevent EA in children [15]. In light of the inconsistent results of recent studies [9–11], there is a need to update the association between premedication with melatonin or its analogs and the incidence of pediatric EA.
Thus, we performed a comprehensive updated meta-analysis and systematic review to evaluate the effects of premedicated melatonin or its analogs in preventing EA in children who have undergone general anesthesia.
Method
This meta-analysis was conducted according to the PRISMA guidelines [17]. The study protocol was prospectively registered with PROSPERO (registration no. CRD42022355915).
Eligibility criteria
The following inclusion criteria were adopted: (1) Patients less than 18 years old who underwent general anesthesia; (2) Prophylactic use of melatonin or its analogs, including ramelteon, tasimelteon, and agomelatine; (3) Use of placebos or alternative premedication drugs as control; (4) Outcomes including the assessment of EA or emergence delirium; and (5) Randomized controlled trials (RCTs).
The following studies were excluded from the systematic review: (1) Those not assessing EA using a specific evaluation tool; (2) Case reports, reviews, editorial letters, and animal studies; (3) Ongoing clinical trials; and (4) Redundant publications and repeated studies from the same trial.
Search strategy
Relevant research published until 25 November 2022 was searched on MEDLINE (PubMed), Excerpta Medica Database (EMBASE), Web of Science, the Cochrane Library, ProQuest Dissertations & Theses Global, China National Knowledge Infrastructure (CNKI), and Wanfang Data. Study registrations published in the WHO International Clinical Trials Registry Platform and ClinicalTrials.gov were also searched. We searched Google Scholar to identify gray literature and checked the first 300 results [18]. The references of included studies were also hand-searched. No language restriction was applied. The search strategy for the electronic databases is demonstrated in eTable 1 in the Supplement. Two authors (D.Z. and X.J.) independently screened the title and abstract of each study to identify eligible studies. Potential eligible studies were retrieved as full-text studies. Any discrepancy was resolved through discussion with a third author (D.L.).
Outcomes
The primary outcome was the incidence of EA, comparing melatonin and its analogs against controls, including placebos and other premedication types. The secondary outcome was the incidence of the adverse effects of premedication.
Data extraction
Two investigators (D.Z. and X.J.) independently reviewed the retrieved studies and extracted relevant data using the data extraction tables. Discrepancies between the extractions were resolved through discussion. Data such as author, publication date, sample size, age, gender, type of surgery, American Society of Anesthesiologists (ASA) physical status, anesthetic agents, EA diagnosis tool, administration mode, given dose of melatonin, type of control, postoperative analgesia, EA incidence rate, adverse effects of premedication, and funding sources were collected. If any of these data were missing, the corresponding authors were contacted through email. For studies with multiple dosages of an alternative premedication drug, the group with the recommended dose was selected as the control group (e.g., midazolam at least 0.5 mg kg− 1 [19, 20] in children below 10 years old). When the multiple-time-points EA incidence was reported without the total EA incidence across various groups, the closest time point after emergence was selected to collect data. For studies with multiple melatonin dosage groups, all melatonin groups were combined into a single group.
Evaluating the studies’ risk of bias
Two investigators (D.Z. and X.J.) independently evaluated the risk of bias using the Cochrane Collaboration Risk of Bias Tool (Version 2.0) [21]. Each study was categorized as low risk, some concerns, or high risk. Any disagreement between the reviewers was resolved through consensus or discussion with a third reviewer (D.L.).
Statistical analysis
Any studies with a high risk of bias were excluded from the meta-analysis. Data were expressed using the pooled risk ratio (RR) with a 95% confidence interval (CI). Heterogeneity between studies was evaluated using an I2 test. The I2 value of 0% corresponded to no degree and 25% to low, 50% to moderate, and 75% to high degrees of heterogeneity. The pooled effect size was measured by a random effects model (DerSimonian and Laird) if I2 was larger than 50%; otherwise, a fixed effects model (Mantel–Haenszel) was employed. A continuity correction of 0.5 was applied if zero events were reported in one group. When heterogeneity was observed, a subgroup analysis was performed to further identify heterogeneity sources by type of melatonin (melatonin vs. ramelteon). Trial sequential analysis (TSA) was performed to calculate the adjusted CI and quantify the required information size (RIS) and monitoring boundaries. In the TSA model, the type I and type II error rates were 5% and 20%, respectively. Relative risk reduction was defined as 25% [16], and the incidence of the control arm was calculated based on the overall incidence of the corresponding control groups. Meta-regression was used to test the dose–response associations. Visual inspection of asymmetry in the funnel plots was applied to evaluate the publication bias. Statistical tests were conducted with Stata Version 17. TSA was carried out using TSA Viewer Version 0.9 Beta (www.ctu.dk/tsa). P < 0.05 was deemed significant.
Grading of the evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines (GRADEpro software; http://gradepro.org/) were used to judge the certainty of the evidence. Certainty was initially assessed as high and downgraded or upgraded according to the risk of bias, imprecision, indirectness, inconsistency, publication bias, and dose-response gradient. The certainty of the evidence was defined as very low, low, moderate, or high.
Results
Study selection
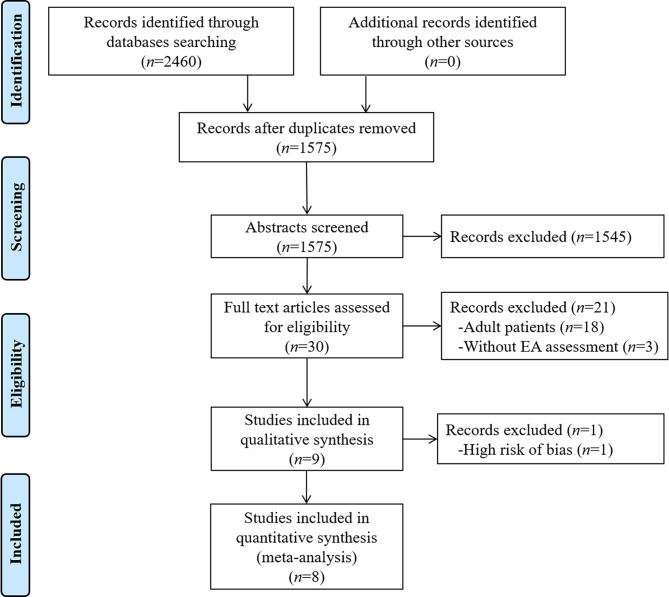
A flow diagram of the search and selection processes is shown in Fig. 1. In total, 2460 studies were identified from the databases and other sources. After duplicates were excluded (n = 885), 1575 studies were subjected to title and abstract screening, and 30 full-text studies were then evaluated for eligibility. Twenty-one further studies were excluded due to considering adult patients (n = 18) or lacking an EA assessment (n = 3). Nine studies were subjected to qualitative analysis. After one study with a high risk of bias was excluded, eight studies were included in this meta-analysis.
Fig. 1.
Flow diagram of the literature search and study selection
Study characteristics
The study characteristics are shown in Table 1. A total of 951 participants was included in nine RCTs evaluating the preventive effects of melatonin premedication on pediatric EA [7, 9–11, 15, 22–25]. Eight studies were peer-reviewed publications [7, 9–11, 15, 22–24], while one study was an unpublished master’s thesis [25]. The sample size ranged from 48 to 163, and the children ranged in age from 1.5 to 9 years old. Eight studies used oral administration of 0.05 to 0.5 mg kg− 1 melatonin, and one study used a melatonin analog (ramelteon 0.1 mg kg− 1) [15]. The comparators included placebos [7, 9, 15, 23–25], midazolam [7, 9, 22, 24, 25], dexmedetomidine [10, 11, 24], and clonidine [11]. Anesthesia was induced by propofol in two studies [11, 25] and sevoflurane with or without N2O in the remaining studies [7, 9, 10, 15, 22–24]. Anesthesia was maintained under sevoflurane anesthesia with or without N2O in all studies. No study reported total intravenous anesthesia. The surgery types were minor elective surgery [7], elective ambulatory surgery [9, 22], tonsillectomy [15, 25] or adenoidectomy [25], ophthalmic surgery [10], elective infraumbilical surgery [11], and oesophageal dilatation procedures [24]. The diagnosis tools of EA included the Watcha scale [9, 11], pediatric anesthesia emergence delirium scale [10, 15, 25], Aono’s scale [15], Keegan scale [22], five-point scale [23], EA scale [24], and pain/discomfort scale [7]. One study did not provide a cut-off value for the Watcha scale [11]. The corresponding author was consulted by email, and it was determined that the patients were considered to have EA when the Watcha scale score was > 2 in this study.
Table 1.
Clinical characteristics of the included studies
| Study | Sample size | ASA | Age | Sex (M/F) | Type of surgery | Anesthetic agents | Postoperative analgesia | Melatonin dosage | Diagnosis tool for EA | Control | Foundation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ali [11] 2020 | 105 | 1 to 2 | 3 to 8 years | 91/14 | Elective infraumbilical surgical procedure |
Induce: propofol + fentanyl+atracurium; Maintain: sevoflurane + N2O; |
Paracetamol | 0.2 mg kg− 1 | Watcha scale | Dexmedetomidine or clonidine | Not mentioned |
| Jangra [10] 2022 | 120 | 1 to 2 | 3 to 9 years | 75/45 | Ophthalmic surgery |
Induce: sevoflurane+fentanyl; Maintain: sevoflurane + N2O; |
Fentanyl for postoperative pain rescue | 0.5 mg kg− 1 | PAED scale | Dexmedetomidine | Departmental funds |
| Kain [22] 2009 | 148 | 1 to 2 | 2 to 8 years | 82/66 | Outpatient elective surgery |
Induce: sevoflurane + N2O; Maintain: not mentioned; |
Not mentioned | 0.05, 0.2, 0.4 mg kg− 1 |
Keegan scale (3-point scale) |
Midazolam | Not mentioned |
| Khalifa [23] 2013 | 60 | not reported | 3 to 6 years | 31/29 | Not reported |
Induce: sevoflurane + cisatracurium; Maintain: sevoflurane; |
Paracetamol | 0.1 mg kg− 1 | Five-point scale | Placebo (saline) | Not mentioned |
| Komazaki [15] 2020 | 48 | 1 to 2 | 18 to 119 months | 37/11 | Tonsillectomy |
Induce: sevoflurane + N2O +fentanyl+rocuronium; Maintain: sevoflurane; |
Paracetamol | 0.1 mg kg− 1 | PAED scale, Aono’s scale | Placebo (syrup) | National Funds |
| Ozcengiz [24] 2011 | 100 | 1 to 2 | 3 to 9 years | 50/50 | Oesophageal dilatation procedures |
Induce: sevoflurane + N2O+vecuronium; Maintain: sevoflurane + N2O; |
Paracetamol | 0.1 mg kg− 1 |
EAS (4-point scale) |
Placebo (saline), midazolam, or dexmedetomidine | Not mentioned |
| Samarkandi [7] 2005 | 75 | 1 | 2 to 5 years | 50/25 | Minor elective surgery |
Induce: sevoflurane + N2O; Maintain: sevoflurane + N2O; |
Paracetamol + caudal block | 0.1, 0.25, 0.5 mg kg− 1 |
Pain/discomfort scale (0 to 6 points) |
Placebo (acetaminophen)a or midazolam | Departmental funds |
| Singla [9] 2021 | 132 | 1 to 2 | 3 to 8 years | 93/39 | Elective ambulatory procedure |
Induce: sevoflurane + N2O + fentanyl; Maintain: sevoflurane + N2O; |
Fentanyl or regional block if required | 0.3 mg kg− 1 | Watcha scale | Placebo (honey) or midazolam | Departmental funds |
| Song [25] 2020 | 163 | 1 to 2 | 2 to 6 years | 99/64 | Elective adenotonsillectomy or adenoidectomy |
Induce: propofol + fentanyl + succinylcholine; Maintain: sevoflurane + N2O |
Ketorolac | 0.5 mg kg− 1 | PAED scale | Placebo (syrup) or midazolam | Not mentioned |
a. In this study, melatonin or midazolam was orally administered mixed with acetaminophen, and the researchers identified oral acetaminophen alone as the placebo comparator
Abbreviations: ASA, American Society of Anesthesiologists; PAED, Pediatric anesthesia emergence delirium; EAS, Emergence Agitation Scale
Risk of bias in the studies
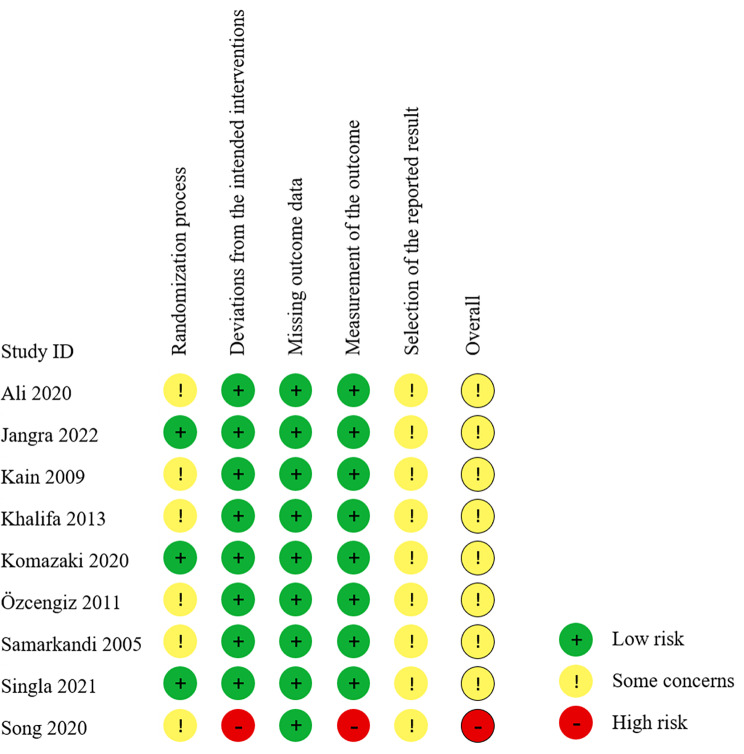
The risk of bias is shown in Fig. 2. One study [25] was considered to have a high risk because the researcher reported in the trial registry record that it was an open-label study and the thesis did not provide any blinding information. Six studies [7, 11, 22–25] raised some concerns regarding the randomization process because the allocation concealment was not described. There were some concerns regarding the selection of the reported results in all the studies: prospectively registered protocols were missing in five studies [7, 11, 22–24], and the multiple time points of EA assessment were not mentioned in the registered protocols of the remaining four studies [9, 10, 15, 25].
Fig. 2.
Risk bias of the included studies
Incidence of EA
Melatonin or its analogs vs. placebos
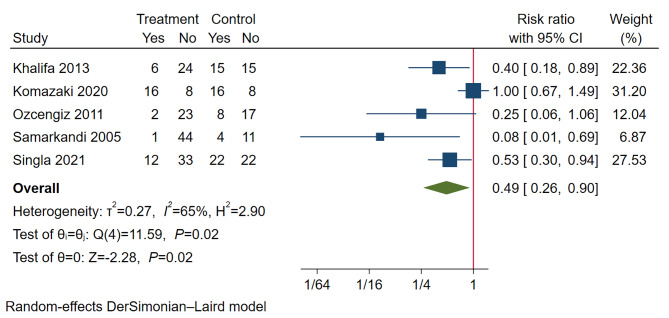
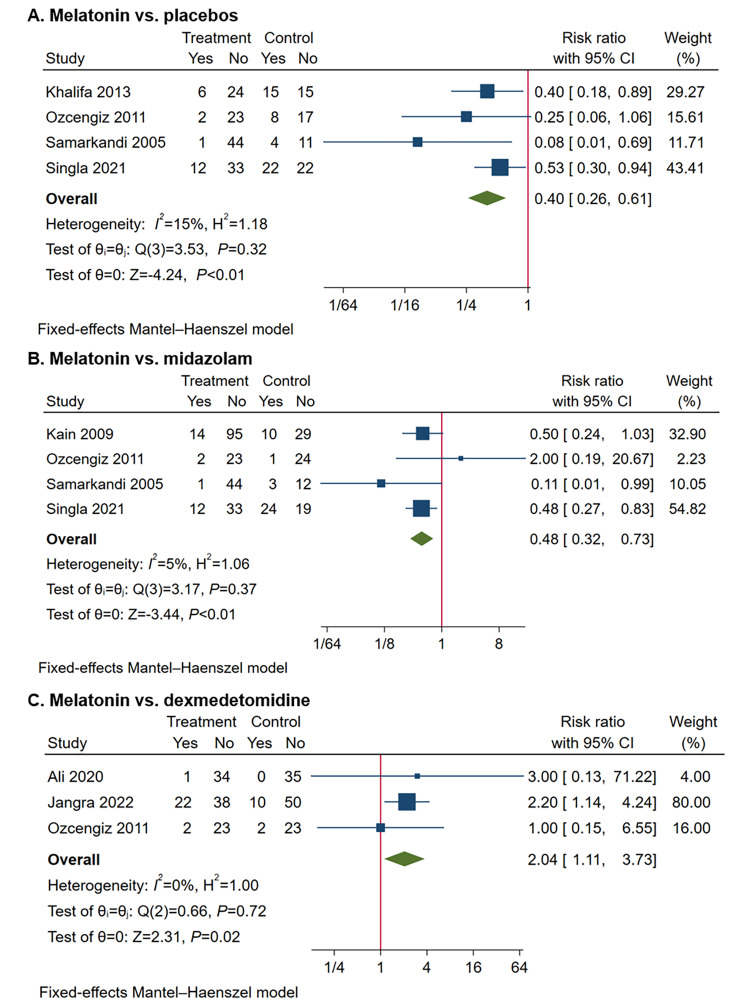
In studies comparing melatonin with placebos, the incidence of EA was 21.9% in the melatonin and its analogs group and 47.1% in the placebos group. Melatonin and its analogs remarkably decreased EA incidence compared with placebos (RR 0.49, 95% CI 0.26 to 0.90, P = 0.02; TSA-adjusted CI 0.03 to 7.30; participants n = 307; studies n = 5) (Fig. 3). In the TSA, the cumulative Z-curve did not pass through the TSA boundary, with 10.7% of RIS cases (n = 2873) accrued (eFig. 1 in the Supplement). The statistical heterogeneity was substantial (I2 = 65%, P = 0.02). One study [15] using a melatonin analog (ramelteon) instead of melatonin had a major impact on the heterogeneity. Excluding this study obviously reduced the heterogeneity (I2 = 15%, P = 0.32) with no change in the meta-analysis results (RR 0.40, 95% CI 0.26 to 0.61, P < 0.01; TSA-adjusted CI 0.18 to 0.88; participants n = 259; studies n = 4) (Fig. 4A). In the TSA of melatonin premedication, the cumulative Z-curve passed through the TSA boundary before reaching the RIS (n = 755) (eFig. 2 in the Supplement). In addition, the meta-regression analysis showed no significant effect modification by dose of melatonin compared with placebo (regression coefficient 0.99, 95% CI -3.02 to 5.00, P = 0.63).
Fig. 3.
A forest plot comparing the incidence of pediatric emergence agitation between melatonin or its analogs and placebo groups
Fig. 4.
Forest plots comparing the incidence of pediatric emergence agitation between melatonin and control groups. A, Melatonin vs. placebos; B, Melatonin vs. midazolam; C, Melatonin vs. dexmedetomidine
Melatonin vs. midazolam
In studies comparing melatonin with midazolam, the incidence of EA was 12.9% in the melatonin group and 31.1% in the midazolam group. Melatonin significantly decreased EA incidence compared with midazolam (RR 0.48, 95% CI 0.32 to 0.73, P < 0.01; TSA-adjusted CI 0.21 to 1.12; participants n = 346; studies n = 4) (Fig. 4B). In the TSA, the cumulative Z-curve did not pass through the TSA boundary, with 29.4% of RIS cases (n = 1175) accrued (eFig. 3 in the Supplement). Heterogeneity was not detected (I2 = 5%, P = 0.37). In addition, the meta-regression analysis showed no significant effect modification by dose of melatonin compared with midazolam (regression coefficient − 3.85, 95% CI -7.84 to 0.13, P = 0.06).
Melatonin vs. dexmedetomidine
In studies comparing melatonin with dexmedetomidine, EA incidence was 20.8% in the melatonin group and 10.0% in the dexmedetomidine group, with a significant difference (RR 2.04, 95% CI 1.11 to 3.73, P = 0.02; TSA-adjusted CI 0.17 to 24.04; participants n = 240; studies n = 3) (Fig. 4C). In the TSA, the cumulative Z-curve did not pass through the TSA boundary, with 6.0% of RIS cases (n = 4011) accrued (eFig. 4 in the Supplement). No obvious heterogeneity (I2 = 0%, P = 0.72) was found. In addition, the meta-regression analysis showed no significant effect modification by dose of melatonin compared with dexmedetomidine (regression coefficient 1.50, 95% CI -3.13 to 6.12, P = 0.53).
Melatonin vs. clonidine
Only one study performed this comparison. Hence, no meta-analysis or TSA was performed. Melatonin did not attenuate the incidence of EA compared with clonidine (RR 3.0, 95% CI 0.13 to 71.22, P = 0.50). The wide 95% CI reveals the statistical imprecision.
Adverse effects
One study reported that no melatonin-relevant adverse effects were observed [24]. The corresponding authors of the other studies were contacted by email. Two authors [9, 11] responded that no adverse effects related to melatonin were found. One author [7] responded to a previous meta-analysis [16] stating that no melatonin-related adverse effects were found, but they did not respond to our contact. Two studies [10, 11] focusing on melatonin and dexmedetomidine reported a lower heart rate after dexmedetomidine premedication compared with melatonin. One of these [10] reported that no participants had symptomatic bradycardia requiring pharmacological intervention.
Reporting biases
The funnel plots of melatonin compared with placebos and midazolam are shown in eFig. 5 in the Supplement. Funnel plots of melatonin compared with dexmedetomidine or clonidine could not be generated because the paucity of studies precluded meaningful analysis. A visual inspection of the melatonin and placebo funnel plots indicated obvious asymmetry, suggesting the existence of publication bias (eFig. 5 A in the Supplement). No publication bias was observed in the melatonin and midazolam funnel plots (eFig. 5B in the Supplement).
Certainty of evidence
A summary of the findings is presented in Table 2, and the certainty of the evidence was assessed as very low or moderate in all outcomes.
Table 2.
Summary of the findings
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with control | Risk with melatonin | ||||
| Incidence of EA | |||||
| Melatonin and its analogs vs. placebos | 471 per 1000 |
240 fewer per 1000 (from 349 fewer to 47 fewer) |
0.49 (0.26 to 0.90) |
307 (5) |
⨁◯◯◯ Very low abc |
| Melatonin vs. placebos | 430 per 1000 | 258 fewer per 1000 (from 318 fewer to 168 fewer) |
0.40 (0.26 to 0.61) |
259 (4) |
⨁⨁⨁◯ Moderate c |
| Melatonin vs. midazolam | 311 per 1000 |
162 fewer per 1000 (from 212 fewer to 84 fewer) |
0.48 (0.32 to 0.73) |
346 (4) |
⨁⨁⨁◯ Moderate b |
| Melatonin vs. dexmedetomidine | 100 per 1000 |
104 more per 1000 (from 11 more to 273 more) |
2.04 (1.11 to 3.73) |
240 (3) |
⨁⨁⨁◯ Moderate b |
a. For high I2 scores, the level of certainty was downgraded to “serious” for “inconsistency.”
b. For the wide range of TSA-adjusted 95% CI, the certainty of evidence was downgraded to “serious” for “imprecision.”
c. Publication bias was strongly suspected
Abbreviations: EA, Emergence agitation; CI, Confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation
Discussion
This meta-analysis suggests that the prophylactic use of melatonin significantly decreases EA incidence compared with the use of placebos and midazolam. Dexmedetomidine premedication has a stronger effect than melatonin in preventing EA. The TSA indicates that more RCTs are needed to confirm the findings. No melatonin-related adverse effects were found in this meta-analysis.
A previous meta-analysis reported that melatonin premedication decreased EA incidence in children after general anesthesia [16]. However, that systematic review extended only until April 2014 and considered four RCTs with 358 participants. Nor did it include studies on melatonin analogs, and it was unable to compare melatonin with dexmedetomidine as only one study associated with dexmedetomidine was included. In contrast, our systematic review used a more comprehensive search strategy, including searching for gray literature and undertaking a manual search of reference lists. We updated the search to include papers published until November 2022 and found nine RCTs with 951 participants. The present meta-analysis also used rigorous methodological and quality-of-evidence assessments. Studies with a high risk of bias were excluded from the primary analysis to avoid impairing credibility, reduce the overall bias, and increase the homogeneity of this meta-analysis [26–28].
It is found that melatonin, compared to the use of a placebo, decreases the incidence of pediatric EA after general anesthesia, which is similar to the findings of the previous meta-analysis [16]. Unlike the previous meta-analysis, the TSA in our meta-analysis showed that a significant result has been achieved. However, it is paramount to approach this result with caution. The RIS has yet to be achieved, and thus there is a risk of random errors. Such findings underscore the importance of continuing research comparing melatonin and placebos to ensure the robustness of the observed outcomes.
The present systematic review included one study [15] on a melatonin analog, which reported that 0.1 mg kg− 1 ramelteon could not prevent EA in children after general anesthesia. The authors deemed that 0.1 to 0.5 mg kg− 1 of melatonin effectively prevented EA in children, and as ramelteon had a higher affinity than melatonin, 0.1 mg kg− 1 of ramelteon was chosen. However, higher affinity does not necessarily mean a greater effect [29]. The effects of drugs on the human body are potentially influenced by various factors, including drug efficacy [30] and pharmacokinetic properties [31]. Besides, melatonin exerts its effects via both receptor-dependent and receptor-independent mechanisms [32]. While ramelteon exhibits superior receptor affinity to melatonin, this does not necessarily guarantee enhanced efficacy in preventing EA. Two studies [33, 34] performed on adult participants indicated the preventive effects of ramelteon on delirium. The dose of ramelteon in these two studies was higher than the common dose of melatonin in preventing delirium (8 mg d− 1 ramelteon [33, 34] vs. 3 mg d− 1 to 5 mg d− 1 melatonin [35]). Thus, ramelteon may have the potential to prevent EA in children, but higher doses may be required. Additional studies are evidently needed to confirm the effect of ramelteon and other melatonin analogs in preventing pediatric EA.
To our knowledge, this is the first study to draw tentative conclusions on melatonin premedication compared with midazolam and dexmedetomidine. The previous meta-analysis [16] reached no conclusion on the effects of melatonin compared with dexmedetomidine or midazolam because of the small number of participants included. In our meta-analysis, melatonin showed a greater effect than midazolam, and dexmedetomidine showed a greater effect than melatonin, in preventing EA. However, the results of the comparison between melatonin and midazolam or dexmedetomidine should be interpreted cautiously, as both TSA results suggest that further studies are warranted to confirm the findings.
No adverse effects of melatonin predication were reported in the meta-analysis. According to the previous studies, melatonin appears to be well tolerated and safe at a high dose (10 mg kg− 1 [36, 37]) or over a long course [38]. Previous studies reported no addiction or detrimental effect on children’s growth [38]. The possible side effects of melatonin include dizziness, headache, nausea, and sleepiness [39], which were reported in children who made long-term use of melatonin [38]. No studies reported severe adverse effects of exogenous melatonin in children. However, the paucity of complete adverse effects data prevents us from drawing any conclusions regarding the safety of melatonin premedication.
Our review has certain limitations. First, the exclusion of studies with a high risk of bias decreased the sample size, thus reducing the overall precision. Second, although we searched multiple databases and the gray literature without language restriction, the TSA indicates that more RCTs are needed to confirm the findings on melatonin premedication in mitigating EA in pediatric patients. Third, the funnel plots indicated that there was a publication bias in the comparison between melatonin or its analogs and placebos. Finally, our meta-analysis revealed inconsistencies across the included studies, spanning several confounding factors. These inconsistencies encompassed aspects such as placebos, diversity in anesthesia types, surgical procedures, and EA diagnostic tools. Due to the limited number of studies available, we were unable to perform a detailed subgroup analysis to account for these potential confounders. In light of this, the conclusions presented in this paper should be interpreted with caution. We strongly recommend further research on melatonin premedication in pediatric populations with a particular emphasis on an exploration of different anesthesia modalities, surgical interventions, and diagnostic approaches for EA.
Conclusion
In summary, the prophylactic use of melatonin significantly decreases EA incidence compared with placebos and midazolam. Premedication with dexmedetomidine has a stronger effect than that with melatonin in preventing EA. The results from the TSA suggest that additional research is essential to conclusively determine the efficacy of melatonin premedication in mitigating EA in pediatric patients. More research is necessary to confirm the effect of melatonin analogs in preventing pediatric EA.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- EA
Emergence agitation
- EMBASE
Excerpta Medica Database
- CNKI
China National Knowledge Infrastructure
- ASA
American Society of Anesthesiologists
- RR
risk ratio
- CI
confidence interval
- TSA
Trial sequential analysis
- RIS
required information size
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
Authors’ contributions
Concept and design: DZ, XJ, JM. Data acquisition: DZ, XJ, DL. Data analysis: DZ, XJ, DL. Data interpretation: All authors. Drafting of the manuscript: DZ, XJ. Revising of the manuscript: All authors. Supervision: DL, JM. All authors read and approved the final manuscript.
Funding
The study was supported by the grant from the National Natural Science Foundation of China (No. 81871592).
Data availability
All data generated or analyzed during this study are included in this published study [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moore AD, Anghelescu DL. Emergence delirium in Pediatric Anesthesia. Paediatr Drugs. 2017;19(1):11–20. doi: 10.1007/s40272-016-0201-5. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Sung TY. Emergence agitation: current knowledge and unresolved questions. Korean J Anesthesiol. 2020;73(6):471–85. doi: 10.4097/kja.20097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang HY, Chen TY, Li DJ, Lin PY, Su KP, Chiang MH, Carvalho AF, Stubbs B, Tu YK, Wu YC, et al. Association of pharmacological prophylaxis with the risk of pediatric emergence delirium after sevoflurane anesthesia: an updated network meta-analysis. J Clin Anesth. 2021;75:110488. doi: 10.1016/j.jclinane.2021.110488. [DOI] [PubMed] [Google Scholar]
- 4.Marseglia L, D’Angelo G, Manti S, Aversa S, Arrigo T, Reiter RJ, Gitto E. Analgesic, anxiolytic and anaesthetic effects of melatonin: new potential uses in pediatrics. Int J Mol Sci. 2015;16(1):1209–20. doi: 10.3390/ijms16011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen QH, Li HF, Zhou XY, Lu YP, Yuan XZ. Relation of serum melatonin levels to postoperative delirium in older patients undergoing major abdominal Surgery. J Int Med Res. 2020;48(3):300060520910642. doi: 10.1177/0300060520910642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Won E, Na KS, Kim YK. Associations between Melatonin, Neuroinflammation, and brain alterations in Depression. Int J Mol Sci 2021, 23(1). [DOI] [PMC free article] [PubMed]
- 7.Samarkandi A, Naguib M, Riad W, Thalaj A, Alotibi W, Aldammas F, Albassam A. Melatonin vs. midazolam premedication in children: a double-blind, placebo-controlled study. Eur J Anaesthesiol. 2005;22(3):189–96. doi: 10.1097/00003643-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Andersen LP, Gögenur I, Rosenberg J, Reiter RJ. The safety of melatonin in humans. Clin Drug Investig. 2016;36(3):169–75. doi: 10.1007/s40261-015-0368-5. [DOI] [PubMed] [Google Scholar]
- 9.Singla L, Mathew PJ, Jain A, Yaddanapudi S, Peters NJ. Oral melatonin as part of multimodal anxiolysis decreases emergence delirium in children whereas midazolam does not: a randomised, double-blind, placebo-controlled study. Eur J Anaesthesiol. 2021;38(11):1130–7. doi: 10.1097/EJA.0000000000001561. [DOI] [PubMed] [Google Scholar]
- 10.Jangra S, Ashok V, Sethi S, Ram J. Atomised intranasal dexmedetomidine versus oral melatonin in prevention of emergence delirium in children undergoing ophthalmic Surgery with sevoflurane: a randomised double-blind study. Eur J Anaesthesiol 2022. [DOI] [PubMed]
- 11.Ali ST, Asthana V, Gupta D, Singh SK. A comparative evaluation of oral clonidine, Dexmedetomidine, and Melatonin as premedicants in Pediatric patients undergoing Subumbilical surgeries. Rom J Anaesth Intensive Care. 2020;27(1):35–42. doi: 10.2478/rjaic-2020-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivara S, Pala D, Bedini A, Spadoni G. Therapeutic uses of melatonin and melatonin derivatives: a patent review (2012–2014) Expert Opin Ther Pat. 2015;25(4):425–41. doi: 10.1517/13543776.2014.1001739. [DOI] [PubMed] [Google Scholar]
- 13.Sateia MJ, Kirby-Long P, Taylor JL. Efficacy and clinical safety of ramelteon: an evidence-based review. Sleep Med Rev. 2008;12(4):319–32. doi: 10.1016/j.smrv.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Yu CL, Carvalho AF, Thompson T, Tsai TC, Tseng PT, Tu YK, Yang SN, Yang FC, Chang CH, Hsu CW, et al. Ramelteon for delirium prevention in hospitalized patients: an updated meta-analysis and trial sequential analysis of randomized controlled trials. J Pineal Res. 2023;74(3):e12857. doi: 10.1111/jpi.12857. [DOI] [PubMed] [Google Scholar]
- 15.Komazaki M, Mihara T, Nakamura N, Ka K, Goto T. Preventive effect of ramelteon on emergence agitation after general anaesthesia in paediatric patients undergoing tonsillectomy: a randomised, placebo-controlled clinical trial. Sci Rep. 2020;10(1):21996. doi: 10.1038/s41598-020-79078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mihara T, Nakamura N, Ka K, Oba MS, Goto T. Effects of melatonin premedication to prevent emergence agitation after general anaesthesia in children: a systematic review and meta-analysis with trial sequential analysis. Eur J Anaesthesiol. 2015;32(12):862–71. doi: 10.1097/EJA.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haddaway NR, Collins AM, Coughlin D, Kirk S. The role of Google Scholar in evidence reviews and its applicability to Grey Literature Searching. PLoS ONE. 2015;10(9):e0138237. doi: 10.1371/journal.pone.0138237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerman J. Preoperative assessment and premedication in paediatrics. Eur J Anaesthesiol. 2013;30(11):645–50. doi: 10.1097/EJA.0b013e328360c3e2. [DOI] [PubMed] [Google Scholar]
- 20.Manso MA, Guittet C, Vandenhende F, Granier LA. Efficacy of oral midazolam for minimal and moderate sedation in pediatric patients: a systematic review. Paediatr Anaesth. 2019;29(11):1094–106. doi: 10.1111/pan.13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 22.Kain ZN, MacLaren JE, Herrmann L, Mayes L, Rosenbaum A, Hata J, Lerman J. Preoperative melatonin and its effects on induction and emergence in children undergoing anesthesia and Surgery. Anesthesiology. 2009;111(1):44–9. doi: 10.1097/ALN.0b013e3181a91870. [DOI] [PubMed] [Google Scholar]
- 23.Khalifa OSM, Hassanin AAM. Melatonin, ketamine and their combination in half doses for management of sevoflurane agitation in children undergoing adenotonsillectomy. Egypt J Anaesth. 2013;29(4):337–41. doi: 10.1016/j.egja.2013.05.006. [DOI] [Google Scholar]
- 24.Özcengiz D, Gunes Y, Ozmete O. Oral melatonin, dexmedetomidine, and midazolam for prevention of postoperative agitation in children. J Anesth. 2011;25(2):184–8. doi: 10.1007/s00540-011-1099-2. [DOI] [PubMed] [Google Scholar]
- 25.Song T. The effects of melatonin versus midazolam on postoperative behavioral disturbances in pediatric patients undergoing general anesthesia. MD dissertation China Medical University; 2020. doi: 10.27652/d.cnki.gzyku.2020.000602. https://oversea.cnki.net/KCMS/detail/detail.aspx?dbcode=CMFD&dbname=CMFD202101&filename=1020382874.nh&uniplatform=OVERSEA&v=mUgWPqPMgdDcQwGEQzohPfAYo1vZWkLJPl6NEG5UCHdHi43l8-dJ842Up368nme2. Accessed 23 Nov. 2022.
- 26.Detweiler BN, Kollmorgen LE, Umberham BA, Hedin RJ, Vassar BM. Risk of bias and methodological appraisal practices in systematic reviews published in anaesthetic journals: a meta-epidemiological study. Anaesthesia. 2016;71(8):955–68. doi: 10.1111/anae.13520. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johns G, Samuel V, Freemantle L, Lewis J, Waddington L. The global prevalence of depression and anxiety among doctors during the covid-19 pandemic: systematic review and meta-analysis. J Affect Disord. 2022;298(Pt A):431–41. doi: 10.1016/j.jad.2021.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strange PG. Agonist binding, agonist affinity and agonist efficacy at G protein-coupled receptors. Br J Pharmacol. 2008;153(7):1353–63. doi: 10.1038/sj.bjp.0707672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galandrin S, Oligny-Longpré G, Bouvier M. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci. 2007;28(8):423–30. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Liu X. Transporter-mediated drug-drug interactions and their significance. Adv Exp Med Biol. 2019;1141:241–91. doi: 10.1007/978-981-13-7647-4_5. [DOI] [PubMed] [Google Scholar]
- 32.Reiter RJ, Tan DX, Galano A. Melatonin: exceeding expectations. Physiol (Bethesda) 2014;29(5):325–33. doi: 10.1152/physiol.00011.2014. [DOI] [PubMed] [Google Scholar]
- 33.Hatta K, Kishi Y, Wada K, Takeuchi T, Odawara T, Usui C, Nakamura H. Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA Psychiatry. 2014;71(4):397–403. doi: 10.1001/jamapsychiatry.2013.3320. [DOI] [PubMed] [Google Scholar]
- 34.Nishikimi M, Numaguchi A, Takahashi K, Miyagawa Y, Matsui K, Higashi M, Makishi G, Matsui S, Matsuda N. Effect of Administration of Ramelteon, a melatonin receptor agonist, on the duration of stay in the ICU: a single-Center Randomized Placebo-Controlled Trial. Crit Care Med. 2018;46(7):1099–105. doi: 10.1097/CCM.0000000000003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaiswal SJ, McCarthy TJ, Wineinger NE, Kang DY, Song J, Garcia S, van Niekerk CJ, Lu CY, Loeks M, Owens RL. Melatonin and sleep in preventing hospitalized delirium: a Randomized Clinical Trial. Am J Med. 2018;131(9):1110–1117e1114. doi: 10.1016/j.amjmed.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gitto E, Reiter RJ, Amodio A, Romeo C, Cuzzocrea E, Sabatino G, Buonocore G, Cordaro V, Trimarchi G, Barberi I. Early indicators of chronic lung Disease in preterm infants with respiratory distress syndrome and their inhibition by melatonin. J Pineal Res. 2004;36(4):250–5. doi: 10.1111/j.1600-079X.2004.00124.x. [DOI] [PubMed] [Google Scholar]
- 37.Aly H, Elmahdy H, El-Dib M, Rowisha M, Awny M, El-Gohary T, Elbatch M, Hamisa M, El-Mashad AR. Melatonin use for neuroprotection in perinatal Asphyxia: a randomized controlled pilot study. J Perinatol. 2015;35(3):186–91. doi: 10.1038/jp.2014.186. [DOI] [PubMed] [Google Scholar]
- 38.Malow BA, Findling RL, Schroder CM, Maras A, Breddy J, Nir T, Zisapel N, Gringras P. Sleep, growth, and Puberty after 2 years of prolonged-release melatonin in Children with Autism Spectrum Disorder. J Am Acad Child Adolesc Psychiatry. 2021;60(2):252–261e253. doi: 10.1016/j.jaac.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esposito S, Laino D, D’Alonzo R, Mencarelli A, Di Genova L, Fattorusso A, Argentiero A, Mencaroni E. Pediatric sleep disturbances and treatment with melatonin. J Transl Med. 2019;17(1):77. doi: 10.1186/s12967-019-1835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published study [and its supplementary information files].