Abstract
Objective
There is a lack of standard salvage treatment options for recurrent or metastatic nasopharyngeal carcinoma (RM-NPC) that has failed platinum-containing regimens. Breakthroughs in immunotherapy have opened up new options for these patients. However, the efficacy and safety of immunotherapy have not been clarified. This study aimed to summarize and assess the efficacy and safety of PD-1 inhibitors in patients with RM-NPC who failed platinum-containing chemotherapy.
Methods
Up to August 25, 2022, clinical trials of PD-1 inhibitors in RM-NPC patients who failed platinum-containing regimens were searched in the PubMed, Embase, Cochrane, and Web of Science databases. Retrieval subject terms included “nasopharyngeal carcinoma”, “metastatic”, “recurrence”, “PD-1”, and “PD-L1”. The clinical trials eligible for inclusion were systematically reviewed and meta-analyzed.
Results
A total of 9 studies including 842 patients with RM-NPC were included in this meta-analysis. The results showed that PD-1 inhibitors had promising efficacy in patients with RM-NPC who failed platinum-containing regimens: objective response rate (ORR) was 24% (95% confidence interval [CI] 21–26%), disease control rate (DCR) was 52% (95% CI 45–58%), 1-year progression-free survival (PFS) rate was 25% (95% CI 18–32%), and 1-year overall survival (OS) rate was 53% (95% CI 37–68%). In terms of treatment-related adverse events (AEs), the incidence of grade ≥ 3 treatment-related AEs was 19% (95% CI 13–24%). In addition, we found that PD-1 inhibitors were more effective in patients with PD-L1 positive than in patients with PD-L1 negative nasopharyngeal carcinoma who had failed platinum-containing regimens (ORR 31% (95%CI 26–35%) vs. 21% (95% CI 17–25%)).
Conclusion
PD-1 inhibitors may provide a survival benefit for patients with RM-NPC who have failed platinum-containing regimens and have the advantage of a good safety profile, making them a promising treatment option.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-023-11318-y.
Keywords: Recurrent or metastatic nasopharyngeal carcinoma, Platinum-containing regimens, PD-1, Efficacy, Safety
Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy with marked geographic and racial differences in incidence and is prevalent in southern China, East Asia, and Southeast Asia [1, 2]. The International Agency for Research on Cancer statistics showed that about 133,000 new cases of NPC were diagnosed in 2020 [3]. For newly diagnosed early localized NPC, radiotherapy has a good effect. However, for locally advanced nasopharyngeal carcinoma (LA-NPC), induction chemotherapy and concurrent chemoradiotherapy are the first treatment options, but about 15–30% of patients will still develop recurrence or metastasis after initial treatment [4, 5].
In general, local treatment is not appropriate for patients with recurrent or metastatic nasopharyngeal carcinoma (RM-NPC), for whom the mainstream treatment plan remains palliative systemic chemotherapy with platinum-containing regimens [6, 7]. In 2016, a landmark phase III randomized controlled trial comparing the efficacy and adverse events of gemcitabine plus cisplatin (GP) and fluorouracil plus cisplatin (PF) in RM-NPC showed that the former had superior efficacy, median progression-free survival (PFS, 7.0 vs. 5.6 months), overall survival (OS, 29.1 vs. 20.9 months), and objective response rate (ORR, 64% vs. 42%) [8]. No serious adverse events occurred in both groups. From then on, the best first-line plan for RM-NPC was established.
There is no consensus on the next salvage treatment option for patients with RM-NPC who have failed platinum-containing chemotherapy regimens. As scientists continue to deepen their understanding of the immune system, it has made significant progress in the field of cancer treatment [9–11]. On the one hand, NPC has the characteristics of high expression of PD-1 and PD-L1 [12, 13]; on the other hand, there are a large number of infiltrating lymphocytes in NPC tissues [14], which provides a theoretical basis for the immunotherapy of NPC. In 2020, a multicenter phase II trial (CAPTAIN) reported the results of camrelizumab for RM-NPC after multiple chemotherapy failures: ORR of 28.2%, median PFS of 3.7 months, and median OS of 17.1 months [15]. In 2021, another clinical trial (POLARIS-02) reported the efficacy of toripalimab for the treatment of RM-NPC who have failed first-line platinum-containing regimens, with an ORR of 20.5%, median PFS of 1.9 months and median OS of 17.4 months [16]. Subsequently, several multi-center clinical trials of PD-1 inhibitors for the treatment of RM-NPC have been implemented worldwide. Initially, these researchers looked at the effectiveness of PD-1 inhibitors in NPC. However, the results reported by different studies were not completely consistent, and there was a lack of focused analysis of the adverse events caused by immunotherapy.
Therefore, we conducted this systematic review and meta-analysis to systematically summarize and compare the therapeutic effect and adverse events of various PD-1 inhibitors for the treatment of RM-NPC patients who failed platinum-containing regimens, and to compile more comprehensive data to provide important reference values for clinicians in developing individualized treatment plans for RM-NPC patients.
Methods
This study is registered in the International Prospective Register of Systematic Reviews (PROSPERO) and the number is CRD42022373462. The conduct of this systematic review and meta-analysis adhered to PRISMA recommendations.
Search strategy
Studies up to 25 August 2022 were searched from PubMed, Embase, Cochrane, and Web of Science databases. Retrieval subject terms included “nasopharyngeal carcinoma”, “metastatic”, “recurrence” “PD-1”, and “PD-L1”. We only included studies where the language of publication was English. More detailed literature searches and screening steps are described in Supplementary Text 1.
Studies selection
Inclusion criteria: (1) Patients with pathologically diagnosed RM-NPC. (2) RM-NPC patients who failed platinum-containing regimens. (3) RM-NPC patients treated with PD-1 inhibitors alone. (4) Included studies were required to contain complete information on efficacy metrics and incidence of adverse events. Exclusion criteria include: (1) The patients also had a head and neck tumor other than NPC. (2) The treatment regimen includes drugs other than PD-1/PD-L1 inhibitors as combination therapy. (3) Article types of reviews, retrospective analyses, case reports, letters, editorials, and meta-analyses were also excluded.
Data acquisition and quality assessment
Two researchers independently extracted the efficacy indicators and anti-PD-1 treatment-related adverse events data of patients with RM-NPC. Efficacy evaluation indexes included objective response rate (ORR: complete response (CR) + partial response (PR)), OS, PFS, and disease control rates (DCR: CR + PR + stable disease (SD)). Adverse events (AEs) data included the incidence of treatment-related AEs of any grade and grade ≥ 3. The detailed information extracted includes first author, year of publication, trial design, treatment and dose, PD-L1 positive, line of therapy, median age, 1-year PFS, 1-year OS, ORR, DCR, any AEs, and grade ≥ 3 AEs. Since most of our included studies were single-arm or uncontrolled studies, we used the Newcastle-Ottawa Scale (NOS) tool to evaluate the quality of the studies [17]. Only studies with a score of more than four stars were included in our subsequent analysis. Studies with more than four stars were included for further analysis. Any disagreements arising from data extraction and literature quality evaluation were resolved in consultation with the third investigator.
Statistical analysis
Stata 14.0 software (Stata Corporation, College Station, Texas, UAS) was used to perform the statistical analysis. The chi-square test and I2 statistics were used to measure heterogeneity. The fixed effect model is used if P > 0.1 or I2 < 50% of the heterogeneity. On the other hand, the random effect model will be used if the heterogeneity is clear. Publication bias was evaluated using Egger’s test.
Results
Eligible studies and characteristics
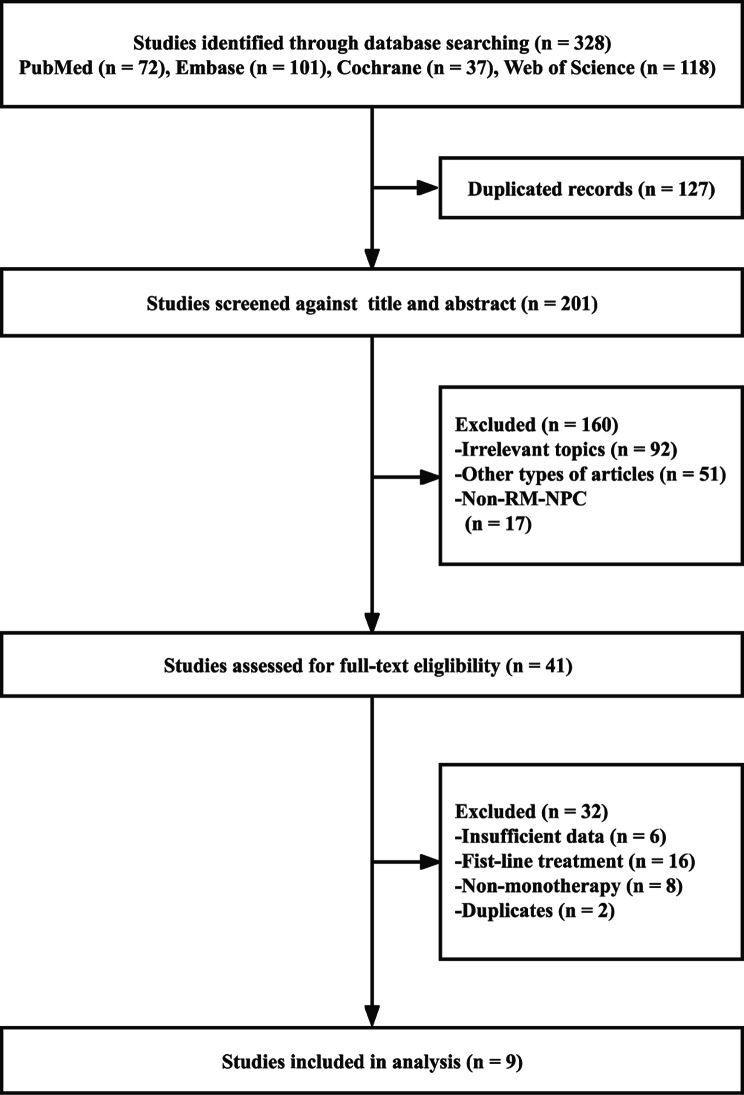
We obtained a total of 328 articles using the above-mentioned search subject phrases from the PubMed, Embase, Cochrane, and Web of Science databases; 201 articles remained after excluding duplicates. Next, 160 articles were further excluded by reading the titles and abstracts, leaving 41 articles for full-text reading. At last, just 9 articles remained which met the inclusion criteria were obtained for meta-analysis. The detailed process of literature screening is shown in Fig. 1.
Fig. 1.
Flow chart of the screening process in the meta-analysis
Finally, 842 patients with RM-NPC after the failure of platinum-containing regimens from 9 studies were included in this meta-analysis. Seven articles are single-arm studies [15, 16, 18–22], including two phase I studies, four phase II studies, and one phase I/II study. Two were randomized controlled studies [23, 24], including one Phase II study and one Phase III study. Anti-PD-1 agents include pembrolizumab, nivolumab, camrelizumab, toripalimab, penpulimab, and spartalizumab. Six studies reported the rate of positive PD-L1 expression, and three of them compared the difference in efficacy of PD-1 inhibitors in NPC patients with positive versus negative PD-L1 expression [15, 16, 25], showing that anti-PD-1 therapy could benefit regardless of PD-L1 expression status, and the benefit was more pronounced in those with positive PD-L1 expression (ORR, 31% vs. 21%). The results of the literature quality assessment showed that 8 of the 9 studies were assessed at 7–9 stars, and the remaining one was assessed at 5 stars. The detailed characteristics of the final nine included studies are described in Table 1.
Table 1.
The detailed characteristics of the final nine included studies
| First author | Year | Trial design | Treatment | Typle | PD-L1positive(%) | Line of therapy ≥ 2(%) | Patients enrolled | Dose | Median age | Median OS (months, 95% CI) | Median PFS (months, 95% CI) | 1-Year OS(%) | 1-Year PFS(%) | ORR (%) | DCR (%) | Any AEs | Grade ≥ 3 AEs | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. Hsu | 2017 | Single-arm phaseI | Pembrolizumab | PD-1 | NA | 81.5 | 27 | 10mg/kg q2w | 52 | 16.5 (10.1–NR) | 6.5(3.6–13.4) | 63 | 33.4 | 25.9 | 77.8 | 74.1 | 29.6 | 7 |
| Brigette B.Y. Ma | 2018 | Single-arm phaseII | Nivolumab | PD-1 | 40 | 100 | 44 | 3mg/kg q2w | 57 | 17.1(10.9-NR) | 2.8(1.8–7.4) | 59 | 19.3 | 20.5 | 54.5 | NA | 22.2 | 8 |
| Wenfeng Fang | 2018 | Single-arm phaseI | Camrelizumab | PD-1 | NA | 76 | 91 | 1 mg/kg, 3 mg/kg, and 10 mg/kg, and a bridging dose of 200 mg per dose once q2w | 45 | NA | 5.6(3.3–7.9) | NA | NA | 34 | 59 | 97 | 17 | 8 |
| Yunpeng Yang | 2021 | Single-arm phaseII | Camrelizumab | PD-1 | 73.1 | 100 | 156 | 200mg q2w | 48 | 17.4(15.2–21.9) | 3.7(2.0-4.1) | NA | NA | 28.2 | 54.5 | 99.4 | 33.3 | 8 |
| Feng-Hua Wang | 2021 | Single-arm phaseII | Toripalimab | PD-1 | 25.3 | 48.4 | 190 | 3mg/kg q2w | 46 | 17.4(11.7–22.9) | 1.9(1.8–3.5) | NA | NA | 20.5 | 40 | 74.2 | 14.2 | 8 |
| Delord, J. P. | 2017 | Single-arm phase I/II | Nivolumab | PD-1 | NA | NA | 24 | 240mg q2w | NA | NR | 2.4(1.5-NR) | NA | NA | 20.8 | 45.8 | NA | NA | 5 |
| Xiaozhong Chen | 2020 | Single-arm phase II | Penpulimab | PD-1 | 38.7 | 100 | 111 | 200mg q2w | NA | NA | NA | NA | NA | 27 | 49.5 | 79.2 | 14.6 | 7 |
| Caroline Even | 2021 | Randomized Phase II | Spartalizumab vs.Chemotherapy | PD-1 | 95.1 | 80.5 | 82 | 400mg q4w | 51 | 25.2(13.1-NR) | 1.9(1.8–3.6) | NA | 27.1 | 17.1 | 42.7 | 72 | 17.1 | 9 |
| A.T. Chan | 2021 | Randomized Phase III | Pembrolizumab vs.Chemotherapy | PD-1 | 74.4 | NA | 117 | 200mg q3w | NA | 17.2(11.7–22.9) | 4.1(2.1–5.6) | 40.2 | NA | 21.4 | 50.4 | 61.2 | 10.3 | 7 |
PFS: Progression-free survival; OS: Overall survival; ORR: Objective response rate; DCR: Disease control rate; NA: Not available; NR: Not reach; AEs: Adverse events
Efficacy
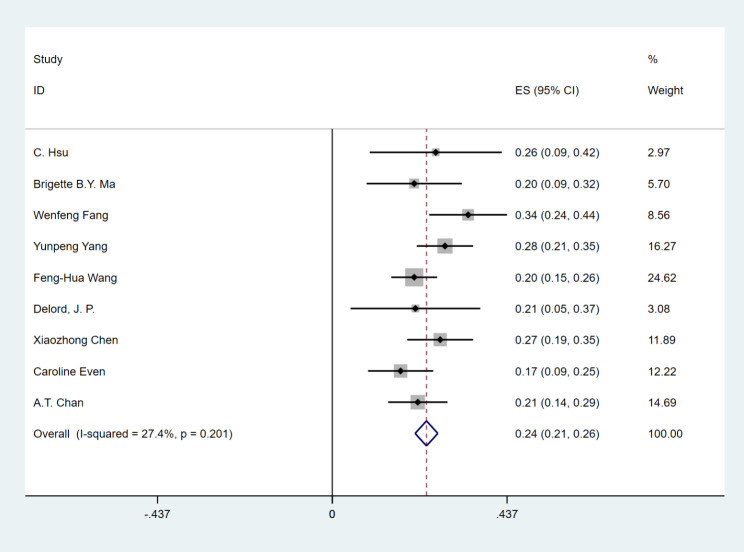
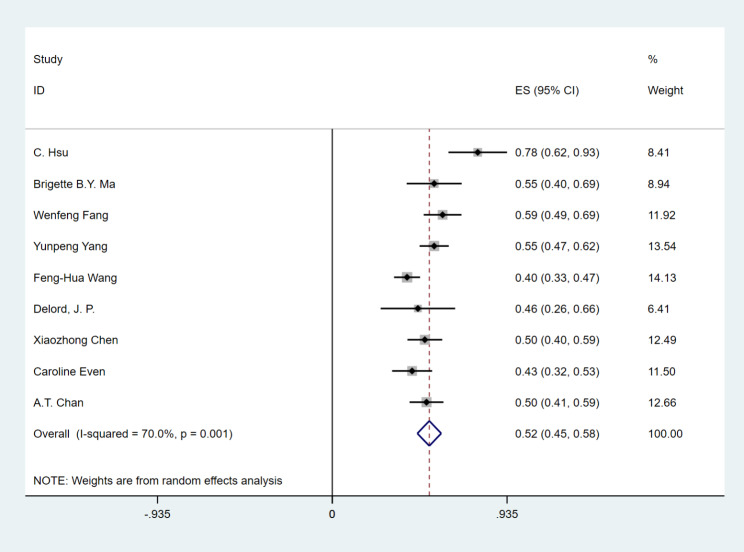
All 842 patients in the study reported ORR and DCR, with the range of ORR from 17.1 to 28.2% and DCR from 40 to 77.8%. PD-1 inhibitor in the treatment of patients with RM-NPC who failed platinum-containing regimen, the ORR was 24% (95% CI 21–26%), (I2 = 27.4%, P = 0.201) fixed effect model was used (Fig. 2), DCR was 52% (95% CI 45–58%), (I2 = 70%, P = 0.001), and random effect model was used (Fig. 3). This has achieved efficacy similar to that of single-agent chemotherapy (ORR:23.5%) [26].
Fig. 2.
Forest plots of objective response rate (ORR) in RM-NPC patients with platinum-containing regimen failure. ES: effect size; CI: confidence interval
Fig. 3.
Forest plots of disease control rates (DCR) in RM-NPC patients with platinum-containing regimen failure
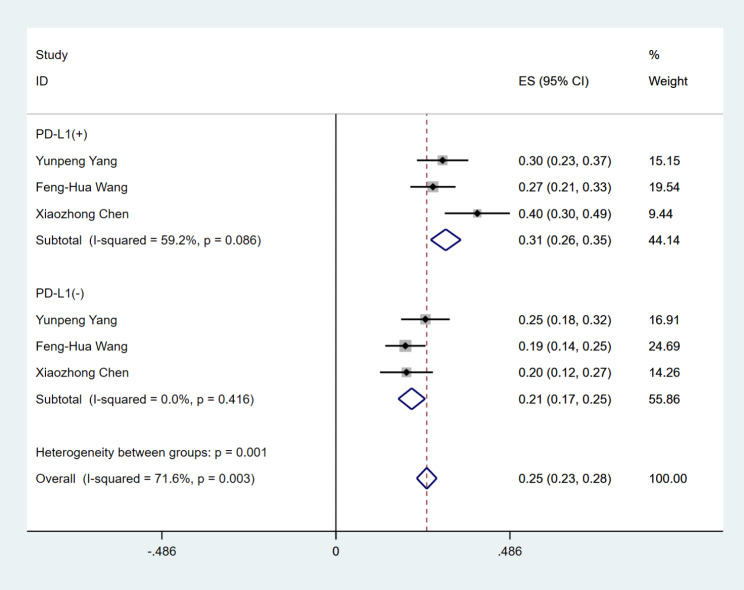
We evaluated the expression status of PD- L1 in tumor tissues of a total of 441 NPC patients in three studies, of which 205 were PD-L1 positive and 236 were PD-L1 negative. The ORR for PD-1 inhibitors used to treat PD-L1-positive RM-NPC patients was 31% (95%CI 26–35%), (I2 = 59.2%, P = 0.086), while the ORR for PD-L1-negative patients was 21% (95% CI 17–25%), (I2 = 0.00%, P = 0.416) (Fig. 4). These results suggest that PD-1 inhibitors used in PD-L1-positive RM-NPC patients have better ORR than PD-L1-negative patients.
Fig. 4.
The ORR of PD-L1 expression in RM-NPC patients with platinum-containing regimen failure
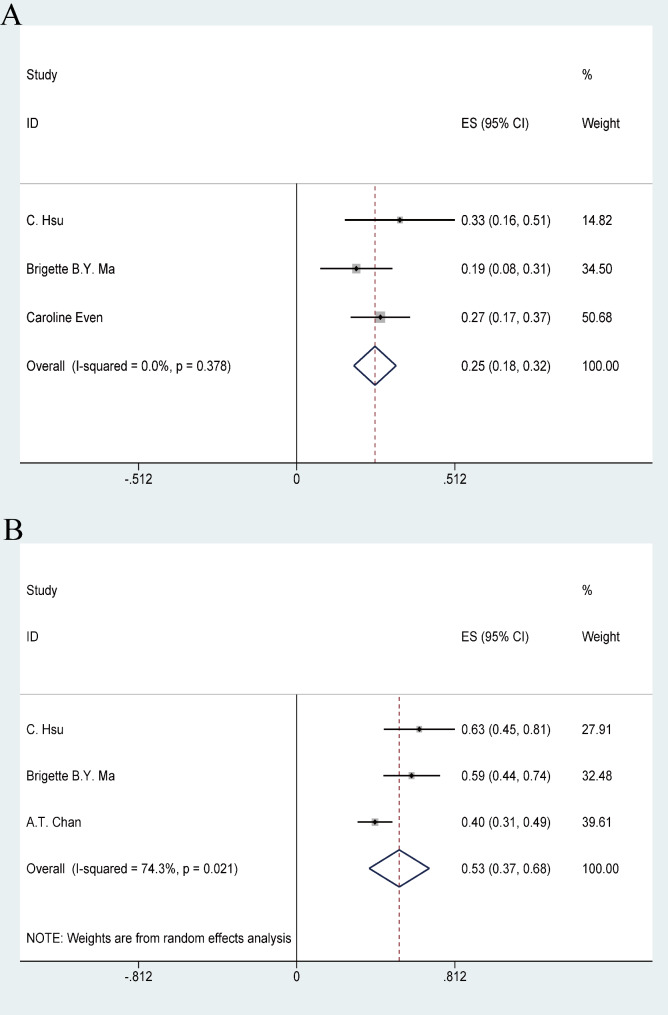
Three studies with 126 patients reported 1-year PFS ranging from 19.3 to 33.4%. 1-year PFS was 25% (95% CI 18–32%), (I2 = 0.0%, P = 0.378) using a fixed effect model (Fig. 5A). A total of 161 patients in three studies reported 1-year OS, ranging from 40.2 to 63%. 1-year OS was 53% (95% CI 37–68%), (I2 = 74.3%, P = 0.021) using a random effect model (Fig. 5B).
Fig. 5.
(A) Forest plots of 1-year progression-free survival (PFS) rate in RM-NPC patients with the platinum-containing regimen failure. (B) Forest plots of 1 1-year overall survival (OS) rate in RM-NPC patients with the platinum-containing regimen failure
Safety
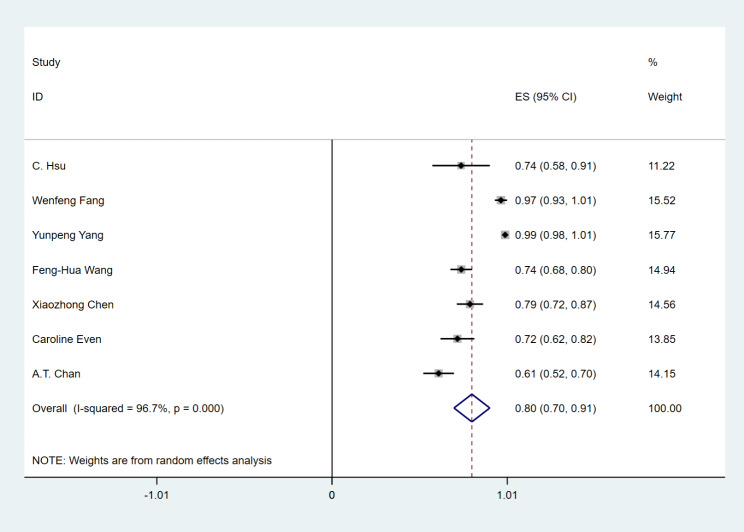
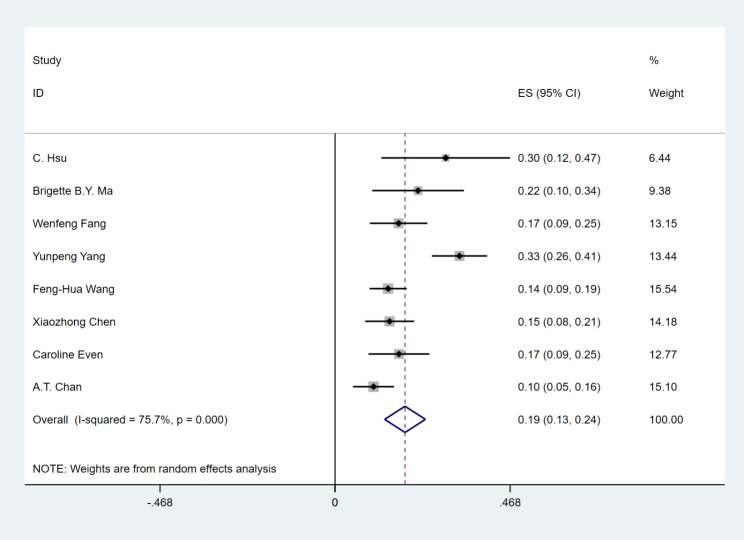
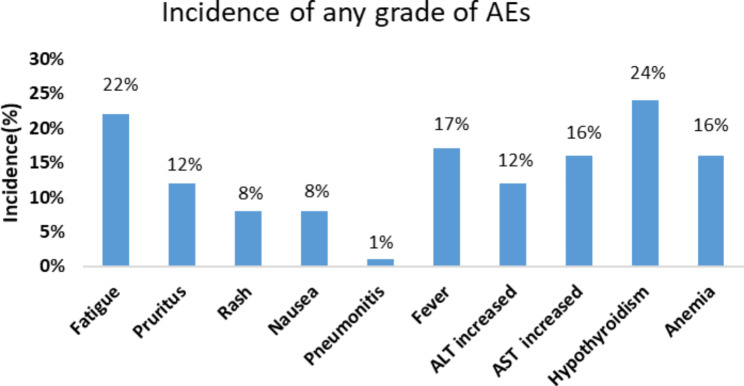
Seven studies with 774 patients reported any grade of treatment-related AEs. The incidence of any grade of treatment-related AEs was 80% (95%CI 70–91%), (I2 = 96.7%, P = 0.000), and the random effect model was used (Fig. 6). Eight studies reported treatment-related AEs with grade 3 or higher. The incidence of grade 3 or higher treatment-related AEs was 19% (95%CI 13–24%), (I2 = 75.7%, P = 0.000), and the random effect model was used (Fig. 7). The incidence of any grade of treatment-related AEs is shown in Fig. 8: hypothyroidism 24%, fatigue 22%, fever 17%, anemia 16%, AST increased 16%, ALT increased 12%, pruritus 12%, rash 8%, nausea 8%, pneumonitis 1%. From this result, we can see that PD-1 inhibitor monotherapy used to treat RM-NPC patients who have failed treatment with platinum-containing regimens still has a relatively high overall rate of treatment-related adverse events, but the rate of grade 3 or higher treatment-related adverse events is only 19%, which is much lower than the rate of 34.4% for single-agent chemotherapy [27].
Fig. 6.
Forest plots of the incidence of treatment-related any-grade adverse events in RM-NPC patients with platinum-containing regimen failure
Fig. 7.
Forest plots of the incidence of treatment-related grade ≥ 3 adverse events in RM-NPC patients with platinum-containing regimen failure
Fig. 8.
The incidence of any grade of treatment-related adverse events in RM-NPC patients with platinum-containing regimen failure. ALT: alanine aminotransferase; AST: aspartate aminotransferase
Publication bias
We assessed the publication bias of PD-1 inhibitors in R/M NPC patients after failure of platinum-containing regimens by Egger’s test of P > 0.01 and found that there was no significant publication bias in the relevant results, as shown in Table 2.
Table 2.
Results of publication bias
| Effect size | Egger’s test (P) |
|---|---|
| ORR | 0.696 |
| DCR | 0.204 |
| PFS | 0.733 |
| OS | 0.121 |
| Any grade of AEs | 0.048 |
| Grade ≥ 3 AEs | 0.171 |
ORR: Objective response rate; DCR: Disease control rate; PFS: Progression-free survival; OS: Overall survival; AEs: Adverse events
Discussion
With the increasing understanding of the immune system by scientists, immunotherapy is becoming a new therapy for many tumors, especially in the treatment of patients with advanced tumors that are not suitable for radical surgical resection treatment [28]. Immunotherapy for tumors includes tumor vaccines, adoptive immune cell therapy, immunomodulators, and immune checkpoint inhibitors [29–31]. Currently, PD-1 inhibitors are the most commonly used immunotherapies in treating NPC. With the publication of increasing clinical trial results and the formation of evidence-based medical evidence, the level of evidence of PD-1/PD-L1 inhibitors in the treatment of NPC is expected to be further improved in the future.
For patients with RM-NPC, the National Comprehensive Cancer Network guidelines recommended gemcitabine plus cisplatin (class 1 recommendation) as the first-line systemic treatment of choice. With the breakthroughs in immunotherapy, some studies have found that the efficacy of immunotherapy combined with chemotherapy is superior to chemotherapy alone in local RM-NPC. A phase III clinical trial (CAPTAIN-1) reported the efficacy of camrelizumab plus gemcitabine and cisplatin versus placebo plus gemcitabine and cisplatin, and the results suggested that the camrelizumab group was significantly more effective than the placebo control group: median PFS (10.8 vs. 6.9 months), DOR (9.9 vs. 5.7 months), ORR (88.1% vs. 80.6%), and both regimens were free of major adverse events [32]. Another phase III clinical trial found that toripalimab plus gemcitabine and cisplatin also had superior efficacy compared to placebo in combination with gemcitabine and cisplatin: median PFS (11.7 vs. 8.0 months), DOR (10.0 vs. 5.7 months), ORR (77.4% vs. 66.4%) [33]. These findings suggest that PD-1 inhibitors in combination with chemotherapy have superior efficacy and fewer adverse events than chemotherapy alone in local RM-NPC, making them more suitable as first-line standard treatment options.
For patients with RM-NPC who had failed treatment with a platinum-containing regimen, monotherapy was previously mainly performed with a new chemotherapeutic agent not used in the first-line regimen, including docetaxel, capecitabine, and gemcitabine [26, 27, 34]. With the development and wide application of immunotherapy, in recent years, some studies have tried to explore the efficacy and safety of immunotherapy for RM-NPC after the failure of platinum-containing regimens. A phase II clinical study (NCT03605967) comparing the efficacy of Spartalizumab and chemotherapy in RM-NPC patients who failed platinum-containing regimens showed a better Median OS (25.2 vs. 15.5 months) but worse Median PFS (1.9 vs. 6.6 months) and ORR (17.1% vs. 35.0%), Spartalizumab has a good safety profile like other PD-1 inhibitors [23]. Another phase III clinical trial (KEYNOTE-122) compared the efficacy and adverse events of pembrolizumab (n = 117) with standard single-agent chemotherapy (n = 116) and showed a median OS of 17.2 vs. 15.3 months, median PFS was (4.1 vs. 5.5 months), ORR was (21.4% vs. 23.3%), and the incidence of grade 3–5 adverse events between the two groups was (10.3% vs. 43.8%) [24]. These findings suggest that in patients with RM-NPC who have failed platinum-containing regimens, PD-1 inhibitors can achieve similar efficacy to single-agent chemotherapy, but the incidence of adverse reactions is significantly lower than chemotherapy, and the compliance and tolerance of patients are better than chemotherapy regiments.
Our meta-analysis also showed similar results as above: ORR 24% (95% confidence interval [CI] 21–26%), DCR 52% (95% CI 45–58%), 1-year PFS rate 25% (95% CI 18–32%), and 1-year OS rate 53% (95% CI 37–68%). The incidence of grade ≥ 3 treatment-related AEs were 19% (95% CI 13–24%), and the incidence of treatment-related any grade AEs was 80% (95% CI 70–91%). Among all AEs related to PD-1 inhibitor treatment, the highest incidence rate was 23% for hyperthyroidism and 22% for fatigue, followed by 17% for fever, 16% for anemia, 16% for AST increased, and finally, 12% for ALT increased, 12% for pruritus, 8% for rash, 8% for nausea, and 1% for pneumonia. Interestingly, a previous meta-analysis reported the efficacy and safety of PD-1/PD-L1 in the treatment of RM-NPC, and the results were similar to those in this study (ORR 25%, and DCR 60%) [35]. The difference with our study is that only 3 clinical trials were included in this study, and the subjects included in this study were different from our study. The subjects in this study were patients with RM-NPC, while the subjects in our study were patients with RM-NPC who failed the platinum-containing regimen. Overall, these findings suggest that PD-1 inhibitors in the treatment of patients with RM-NPC who have failed platinum-based regimens have similar efficacy as single-agent chemotherapy, but the incidence of adverse events is significantly lower than chemotherapy. Therefore, immunotherapy may be a promising approach for patients with RM-NPC who have failed platinum-containing regimens.
Programmed cell death-Ligand 1(PD-L1) is overexpressed in many types of tumor cells and is strongly associated with a patient’s prognosis. A meta-analysis by Huang ZL et al. compared the relationship between different PD-L1 expression statuses and the prognosis of patients with NPC and found no statistical difference between positive or negative PD-L1 expression in tumor tissues and the prognosis of NPC. However, in a subgroup analysis, it was found that those with positive PD-L1 expression in immune cells of NPC patients had a better prognosis, and the higher the expression level, the longer the OS [36]. To clarify whether the efficacy of PD-1 inhibitors treatment for RM-NPC is related to the PD-L1 expression status of the tumor, we compared the ORR of PD-1 inhibitors in patients with PD-L1-positive and PD-L1-negative expressing NPC and showed that PD-1 inhibitors can benefit regardless of PD-L1 expression status, and the benefit was more pronounced in those with positive PD-L1 expression (ORR, 31% vs. 21%). Accordingly, we speculate that PD-L1 expression status may correlate with PD-1 inhibitors efficacy, with better efficacy in those with positive PD-L1 expression, but more studies are needed to further confirm this conclusion.
Admittedly, this study has some limitations. On the one hand, most of the eligible studies included were single-arm clinical trials, some of which had small sample sizes, which may influence conclusions; on the other hand, there were differences in the systematic treatment received by these patients before treatment with PD-1 inhibitors, which may affect the overall survival of patients and lead to a large potential heterogeneity between studies.
Conclusion
The efficacy of PD-1 inhibitors for RM-NPC patients who failed treatment with platinum-containing regimens is similar to that of single-agent re-chemotherapy reported in previous studies, but PD-1 inhibitors have the advantages of fewer adverse effects and better tolerability. Of course, more large clinical studies are still needed to further confirm the above conclusions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Thanks for the help of the members of The First Affiliated Hospital of Guangdong Pharmaceutical University and Dongguan People’s Hospital.
Abbreviations
- AEs
Adverse events
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- CI
Confidence interval
- DCR
Disease control rate
- ES
Effect size
- GP
Gemcitabine plus cisplatin
- LA-NPC
Locally advanced nasopharyngeal carcinoma
- NA
Not available
- NPC
Nasopharyngeal carcinoma
- NR
Not reach
- ORR
Objective response rate
- OS
Overall survival
- PD-1
Programmed death-ligand 1
- PF
Fluorouracil plus cisplatin
- PFS
Progression-free survival
- RM-NPC
Recurrent or metastatic nasopharyngeal carcinoma
Authors’ contributions
Both XW and DW made significant contributions to the idea and design of the article, which they both critically revised for key intellectual elements. JL and WX made substantial contributions to data extraction and data analysis. FH and YC reviewed the data; JL wrote the manuscript. The final manuscript was reviewed and approved by all writers.
Funding
This study was founded by the Guangzhou Science and Technology Program key project (Grant No. 202103000041).
Data Availability
The authors declare that the data covered in this study can be found in the Supplementary file or requested from the corresponding author upon reasonable request.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dongxia Wang, Email: dongxiadg@163.com.
Xicheng Wang, Email: 13902400598@126.com.
References
- 1.Tang LL, Chen WQ, Xue WQ, He YQ, Zheng RS, Zeng YX, et al. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett. 2016;374(1):22–30. doi: 10.1016/j.canlet.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet (London England) 2019;394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Li AC, Xiao WW, Shen GZ, Wang L, Xu AA, Cao YQ, et al. Distant metastasis risk and patterns of nasopharyngeal carcinoma in the era of IMRT: long-term results and benefits of chemotherapy. Oncotarget. 2015;6(27):24511–21. doi: 10.18632/oncotarget.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee AW, Ma BB, Ng WT, Chan AT. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncology: Official J Am Soc Clin Oncol. 2015;33(29):3356–64. doi: 10.1200/JCO.2015.60.9347. [DOI] [PubMed] [Google Scholar]
- 6.Au E, Ang PT. A phase II trial of 5-fluorouracil and cisplatinum in recurrent or metastatic nasopharyngeal carcinoma. Annals of Oncology: Official Journal of the European Society for Medical Oncology. 1994;5(1):87–9. doi: 10.1093/oxfordjournals.annonc.a058703. [DOI] [PubMed] [Google Scholar]
- 7.Yeo W, Leung TW, Leung SF, Teo PM, Chan AT, Lee WY, et al. Phase II study of the combination of carboplatin and 5-fluorouracil in metastatic nasopharyngeal carcinoma. Cancer Chemother Pharmacol. 1996;38(5):466–70. doi: 10.1007/s002800050512. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Huang Y, Hong S, Yang Y, Yu G, Jia J, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet (London England) 2016;388(10054):1883–92. doi: 10.1016/S0140-6736(16)31388-5. [DOI] [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced squamous-cell non-small-cell Lung Cancer. N Engl J Med. 2015;373(2):123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14):1277–90. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Q, Cai MY, Chen CL, Hu H, Lin HX, Li M, et al. Tumor cells PD-L1 expression as a favorable prognosis factor in nasopharyngeal carcinoma patients with pre-existing intratumor-infiltrating lymphocytes. Oncoimmunology. 2017;6(5):e1312240. doi: 10.1080/2162402X.2017.1312240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee VH, Lo AW, Leung CY, Shek WH, Kwong DL, Lam KO, et al. Correlation of PD-L1 expression of Tumor cells with survival outcomes after Radical Intensity-Modulated Radiation Therapy for non-metastatic nasopharyngeal carcinoma. PLoS ONE. 2016;11(6):e0157969. doi: 10.1371/journal.pone.0157969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang YQ, Chen YP, Zhang Y, Jiang W, Liu N, Yun JP, et al. Prognostic significance of tumor-infiltrating lymphocytes in nondisseminated nasopharyngeal carcinoma: a large-scale cohort study. Int J Cancer. 2018;142(12):2558–66. doi: 10.1002/ijc.31279. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Zhou T, Chen X, Li J, Pan J, He X et al. Efficacy, safety, and biomarker analysis of Camrelizumab in previously treated recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN study). J Immunother Cancer. 2021;9(12). [DOI] [PMC free article] [PubMed]
- 16.Wang FH, Wei XL, Feng J, Li Q, Xu N, Hu XC, et al. Efficacy, Safety, and correlative biomarkers of Toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: a phase II clinical trial (POLARIS-02) J Clin Oncology: Official J Am Soc Clin Oncol. 2021;39(7):704–12. doi: 10.1200/JCO.20.02712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma BBY, Lim WT, Goh BC, Hui EP, Lo KW, Pettinger A, et al. Antitumor Activity of Nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742) J Clin Oncology: Official J Am Soc Clin Oncol. 2018;36(14):1412–8. doi: 10.1200/JCO.2017.77.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang W, Yang Y, Ma Y, Hong S, Lin L, He X, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 2018;19(10):1338–50. doi: 10.1016/S1470-2045(18)30495-9. [DOI] [PubMed] [Google Scholar]
- 20.Delord JP, Hollebecque A, De Boer JP, De Greve J, Machiels JPH, Leidner RS et al. An open-label, multicohort, phase I/II study to evaluate nivolumab in patients with virus-associated tumors (CheckMate 358): efficacy and safety in recurrent or metastatic (R/M) nasopharyngeal carcinoma (NPC). J Clin Oncol. 2017;35(15).
- 21.Chen X, Hu C, Wang W, Zou Q, Li J, Lin Q, et al. A phase II study of the anti-programmed cell death-1 (PD-1) antibody penpulimab in patients with metastatic nasopharyngeal carcinoma (NPC) who had progressed after two or more lines of chemotherapy: updated results. Ann Oncol. 2021;32:806. doi: 10.1016/j.annonc.2021.08.1319. [DOI] [Google Scholar]
- 22.Hsu C, Lee SH, Ejadi S, Even C, Cohen RB, Le Tourneau C, et al. Safety and Antitumor Activity of Pembrolizumab in patients with programmed death-ligand 1-Positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncology: Official J Am Soc Clin Oncol. 2017;35(36):4050–6. doi: 10.1200/JCO.2017.73.3675. [DOI] [PubMed] [Google Scholar]
- 23.Even C, Wang HM, Li SH, Ngan RKC, Dechaphunkul A, Zhang L, et al. Phase II, Randomized Study of Spartalizumab (PDR001), an Anti-PD-1 antibody, versus Chemotherapy in patients with Recurrent/Metastatic nasopharyngeal Cancer. Clin Cancer Res. 2021;27(23):6413–23. doi: 10.1158/1078-0432.CCR-21-0822. [DOI] [PubMed] [Google Scholar]
- 24.Chan AT, Lee VHF, Hong RL, Ahn MJ, Chong WQ, Kim SB, et al. Results of KEYNOTE-122: a phase III study of pembrolizumab (pembro) monotherapy vs chemotherapy (chemo) for platinum-pretreated, recurrent or metastatic (R/M) nasopharyngeal carcinoma (NPC) Ann Oncol. 2021;32:786. doi: 10.1016/j.annonc.2021.08.1268. [DOI] [Google Scholar]
- 25.Chen X, Wang W, Zou Q, Li J, Hu C, Lin Q, et al. A phase ii study of the anti-programmed cell death-1 (Pd-1) antibody penpulimab in patients with metastatic nasopharyngeal carcinoma (NPC) who had progressed after two or more lines of chemotherapy. J Immunother Cancer. 2020;8(SUPPL 3):A481. [Google Scholar]
- 26.Chua DT, Sham JS, Au GK. A phase II study of capecitabine in patients with recurrent and metastatic nasopharyngeal carcinoma pretreated with platinum-based chemotherapy. Oral Oncol. 2003;39(4):361–6. doi: 10.1016/S1368-8375(02)00120-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Zhang Y, Huang PY, Xu F, Peng PJ, Guan ZZ. Phase II clinical study of gemcitabine in the treatment of patients with advanced nasopharyngeal carcinoma after the failure of platinum-based chemotherapy. Cancer Chemother Pharmacol. 2008;61(1):33–8. doi: 10.1007/s00280-007-0441-8. [DOI] [PubMed] [Google Scholar]
- 28.Sharon E, Streicher H, Goncalves P, Chen HX. Immune checkpoint inhibitors in clinical trials. Chin J cancer. 2014;33(9):434–44. doi: 10.5732/cjc.014.10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chia WK, Wang WW, Teo M, Tai WM, Lim WT, Tan EH, et al. A phase II study evaluating the safety and efficacy of an adenovirus-∆LMP1-LMP2 transduced dendritic cell vaccine in patients with advanced metastatic nasopharyngeal carcinoma. Annals of Oncology: Official Journal of the European Society for Medical Oncology. 2012;23(4):997–1005. doi: 10.1093/annonc/mdr341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith C, Lee V, Schuessler A, Beagley L, Rehan S, Tsang J, et al. Pre-emptive and therapeutic adoptive immunotherapy for nasopharyngeal carcinoma: phenotype and effector function of T cells impact on clinical response. Oncoimmunology. 2017;6(2):e1273311. doi: 10.1080/2162402X.2016.1273311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rotte A, Jin JY, Lemaire V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Annals of Oncology: Official Journal of the European Society for Medical Oncology. 2018;29(1):71–83. doi: 10.1093/annonc/mdx686. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Qu S, Li J, Hu C, Xu M, Li W, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2021;22(8):1162–74. doi: 10.1016/S1470-2045(21)00302-8. [DOI] [PubMed] [Google Scholar]
- 33.Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen J, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med. 2021;27(9):1536–43. doi: 10.1038/s41591-021-01444-0. [DOI] [PubMed] [Google Scholar]
- 34.Ngeow J, Lim WT, Leong SS, Ang MK, Toh CK, Gao F, et al. Docetaxel is effective in heavily pretreated patients with disseminated nasopharyngeal carcinoma. Annals of Oncology: Official Journal of the European Society for Medical Oncology. 2011;22(3):718–22. doi: 10.1093/annonc/mdq425. [DOI] [PubMed] [Google Scholar]
- 35.Wang BC, Cao RB, Fu C, Chen WB, Li PD, Lin GH, et al. The efficacy and safety of PD-1/PD-L1 inhibitors in patients with recurrent or metastatic nasopharyngeal carcinoma: a systematic review and meta-analysis. Oral Oncol. 2020;104:104640. doi: 10.1016/j.oraloncology.2020.104640. [DOI] [PubMed] [Google Scholar]
- 36.Huang ZL, Liu S, Wang GN, Zheng SH, Ding SR, Tao YL, et al. The prognostic significance of PD-L1 and PD-1 expression in patients with nasopharyngeal carcinoma: a systematic review and meta-analysis. Cancer Cell Int. 2019;19:141. doi: 10.1186/s12935-019-0863-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data covered in this study can be found in the Supplementary file or requested from the corresponding author upon reasonable request.