Abstract
Airway function is under constant neurophysiological control, in order to maximize airflow and gas exchange and to protect the airways from aspiration, damage, and infection. There are multiple sensory nerve subtypes, whose disparate functions provide a wide array of sensory information into the CNS. Activation of these subtypes triggers specific reflexes, including cough and alterations in autonomic efferent control of airway smooth muscle, secretory cells, and vasculature. Importantly, every aspect of these reflex arcs can be impacted and altered by local inflammation caused by chronic lung disease such as asthma, bronchitis, and infections. Excessive and inappropriate activity in sensory and autonomic nerves within the airways is thought to contribute to the morbidity and symptoms associated with lung disease.
INTRODUCTION
The airways are densely innervated by both sensory/afferent nerves and autonomic (motor/efferent) nerves, which regulate both respiratory rhythms and organ function within the airways. In this chapter, we provide a review of the sensory and autonomic innervation of the lower airways (from the trachea and bronchi through to the intrapulmonary bronchioles, alveoli, and blood vessels) and their physiological role in health, as well as the contribution of these nerves to the pathophysiology of airway disease including inflammation and cough. In the context of this chapter, we use the term “lower airways” to refer to the airway between the larynx and alveoli. The literature pertaining to nasal airways will not be reviewed.
SENSORY AFFERENT INNERVATION OF THE LOWER AIRWAYS
In mammals, the majority of airway sensory innervation is supplied by primary afferent nerves projected from neurons residing in the vagal ganglia (Xth cranial nerve) (Fig. 14.1). This heterogeneous population of sensory nerves expresses a variety of receptors for mechanical, thermal, and chemical stimuli, and serves to detect the local environment of the lower airways and initiate local and central reflexes that can evoke defensive reflexes and also serve to regulate respiratory rhythm and cardiovascular function (Coleridge and Coleridge, 1984; Mazzone and Undem, 2016). The sensory innervation of the airways is supplied by branches of the vagus nerve; the branches that innervate the trachea are referred to as the superior laryngeal nerve (SLN) and the recurrent laryngeal nerve (RLN). In guinea pigs and larger mammals, the vagal ganglia are comprised of two distinct structures: the nodose or inferior vagal ganglion and the jugular or superior vagal ganglion (Ricco et al., 1996; Undem et al., 2004). In mice and rats, these two structures are partially fused into the jugular–nodose complex (McGovern et al., 2015b; Kim et al., 2020b). Regardless of mammalian species, neurons within the nodose ganglia are embryologically derived from the epibranchial placodes, whereas the jugular neurons are embryologically derived from the neural crest (Taylor-Clark, 2021). The embryological source of these neurons is a major determinant of their gene expression, sensitivity to growth factors, anatomical connectivity and function. Vagal afferents project their central axons to the brainstem medulla, where they synapse with second-order neurons in the nucleus tractus solitarius (nTS) and, in some cases, the paratrigeminal complex (Pa5) (Kubin et al., 2006; Behrens et al., 2021; Taylor-Clark, 2021). In this way, afferent activity regulates CNS networks that control breathing rhythms and autonomic efferent outflow to the lungs and cardiovascular organs.
Fig. 14.1.
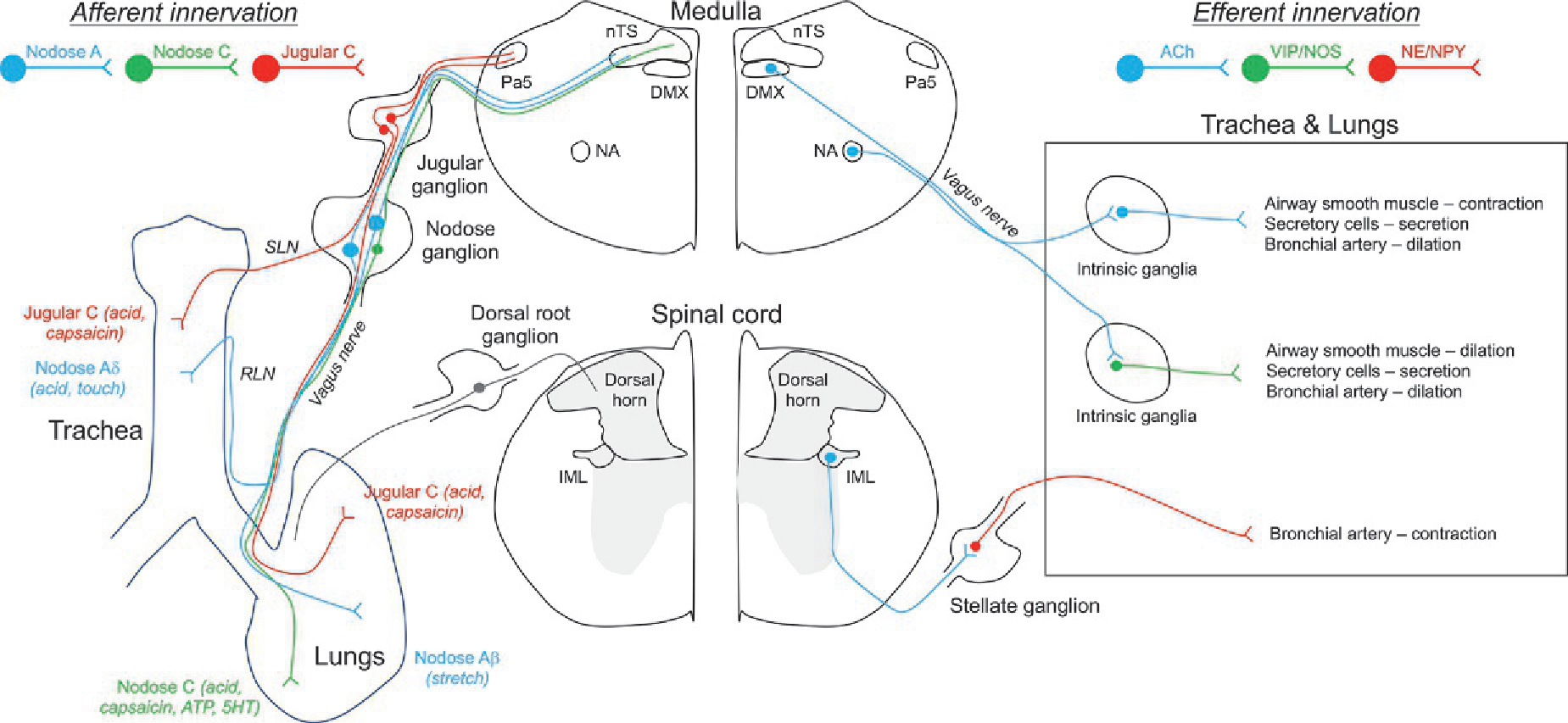
Schematic illustrating the basic sensory afferent and autonomic efferent innervation of the airways. Left, sensory fibers projecting from neurons within the nodose and jugular ganglia project to the trachea and intrapulmonary airways, via the vagus nerve and its branches the superior laryngeal nerve (SLN) and the recurrent laryngeal nerve (RLN). Sensory afferents have been grouped based upon their conduction velocity and their sensitivity to specific stimuli. Nodose sensory fibers project to the nTS in the medulla and jugular fibers project to the Pa5 in the medulla, where they synpase with second order neurons involved in networks regulating cardiopulmonary reflexes. Also shown is the sparse sensory innervation projected from the dorsal root ganglion. Little is known about the sensitivity profile, anatomical pathways and function of these DRG afferents, but it is likely that many are C fibers that project to the dorsal horn lamina. Right, extrinsic and intrinsic autonomic innervation of the airways. Cholinergic preganglionic parasympathetic fibers project from the dorsal motor nucleus of the vagus (DMX) and nucleus ambiguus (NA) to the airways where they synapse with neurons within the intrinsic ganglia. Such postganglionic fibers are either cholinergic or NANC fibers (expressing VIP and/or NOS), and they project to airway smooth muscle cells, secretory cells and bronchial arteries. Cholinergic preganglionic sympathetic fibers project from the intermediolateral nucleus of the spinal cord to neurons within the stellate and thoracic sympathetic ganglia. These adrenergic and NPY-expressing ganglionic neurons project to the airways and mainly innervate bronchial arteries.
The majority of vagal sensory nerves express receptors for stimuli that are considered noxious, such as nonphysiological temperatures, pH, osmolarity or mechanical forces, or inflammatory mediators (e.g., bradykinin) or irritants (e.g., capsaicin, the pungent ingredient in chili peppers) (Coleridge and Coleridge, 1984; Mazzone and Undem, 2016). The activation of these so-called “nociceptive” sensory nerves (or “nociceptors”) evokes defensive responses/reflexes such as apnea, tachypnea, bronchospasm, mucus secretion, cough and the sensation of dyspnea, as well as changes in heart rate and blood pressure. These reflexes serve to protect the airways but if they are triggered excessively or chronically can contribute to the morbidity of airway diseases such as asthma and chronic obstructive pulmonary disease (see below). Nociceptors are typically quiescent during eupneic breathing (Ho et al., 2001), indicating such mechanical forces fail to reach threshold for nociceptor activation. Instead, lung stretch during eupnea activates nonnociceptive stretch-sensitive mechanoreceptor fibers (Widdicombe, 1954; Ho et al., 2001), whose activation finely regulates inspiration and expiration.
Afferent activation
Afferent nerves express ion channel receptors on their peripheral nerve terminals within the airways that are sensitive to specific local stimuli (see Table 14.1) (Mazzone and Undem, 2016). For example, most nociceptive afferents express transient receptor potential (TRP) vanilloid 1 (V1) (Ho et al., 2001; Undem et al., 2004), which is activated by capsaicin, extracellular acidification and excessive heat (Caterina et al., 1997). Binding of capsaicin to TRPV1 causes the channel’s cation-permeable pore to open, leading to Na+ and Ca2+ influx. These fluxes evoke a graded depolarization of the afferent terminal that, if of sufficient magnitude, can reach the activation threshold of voltage-gated sodium channels (NaV). Depolarization-evoked activation of NaV induces rapid influx of Na+, resulting in the upstroke of the all-or-nothing action potential (AP). This sets off a wave of depolarization along the axon—i.e., AP conduction. In this way, the sensory stimulus is encoded by AP discharge frequency. Electrical excitability of airway afferents is in part determined by the number and biophysical properties of NaV and potassium channels (both voltage-gated and Ca2+–gated) which contribute to the various parts of the AP waveform (Schild et al., 2005; Kwong et al., 2008a; Gover et al., 2009; Sun et al., 2019; Sun, 2021). These parameters are sensitive to cell signaling cascades that can be initiated by inflammatory mediators (Undem et al., 1991, 1993; Kwong and Lee, 2005). Thus inflammation can increase AP discharge independently of the receptor-mediated graded depolarization.
Table 14.1.
Sensitivity of vagal sensory nerve subtypes innervating the lower airways
| Fiber | Nodose Aβ | Nodose Aδ | Nodose C | Jugular C |
|---|---|---|---|---|
|
| ||||
| Terminal location | Intrapulmonary | Extrapulmonary | Mainly intrapulmonary | Both extrapulmonary and intrapulmonary |
| Conduction velocity (m/s) | 12–50 | 2–8 | >1.2 | >1.2 |
| Mechanical sensitivity | Low-threshold stretch | Low-threshold punctate | High-threshold stretch and punctate | High-threshold stretch and punctate |
| Chemical sensitivitya | ATP, α,β mATP (P2X2/3) | Acid (not TRPV1 channel) Hypotonic saline | Acid, capsaicin (TRPV1 channel) AITC, cinnamaldehyde (TRPA1 channel) Bradykinin (B2 receptor) ATP, α,β mATP (P2X2/3 channel) Adenosine (A1 and A2A receptors) Serotonin (5HT3 channel) | Acid, capsaicin (TRPV1 channel) AITC, cinnamaldehyde (TRPA1 channel) Bradykinin (B2 receptor) |
| Physiological effect following activation | Inhibition of inspiration, decrease parasympathetic drive (SAR fibers) Increases inspiratory effort and parasympathetic drive (RAR fibers) | Cough | Modulation of respiratory rhythms Increase parasympathetic drive Decrease cough reflex sensitivity | Cough Increase cough reflex sensitivity Increase parasympathetic drive Neurogenic-mediated bronchospasm and secretion Modulation of respiratory rhythms |
Denotes direct sensitivity of the nerve to the substance. Does not include sensitivity of the nerve to any indirect effects of the substance (e.g., bronchospasm). There are likely species distinctions in the sensitivity to chemical mediators. Much of this summary stems from work on guinea pigs.
Many afferents also express a host of nonchannel forming receptors for inflammatory mediators that are commonly produced during airway inflammation (Mazzone and Undem, 2016). These receptors are typically G-protein–coupled receptors, such as the bradykinin B2 receptor, histamine H1 receptor, adenosine A1 and A2 receptors and the spingosine-1-phosphate S1PR3 receptor. In addition, some cytokine receptors (e.g., IFN γ receptor) can modulate afferent activity. Activation of metabotropic receptors can modulate afferent excitability via second messenger signaling cascades that target channels involved in the AP. For example, activation of Gs-coupled receptors can cause protein kinase A-dependent increases in NaV membrane expression and NaV voltage sensitivity (Kwong and Lee, 2005). However, in order to directly induce AP discharge the inflammatory mediator-sensitive metabotropic receptor must directly regulate ion channels responsible for graded potentials in the afferent terminal. Many of the specifics are currently unclear, but multiple ion channels have been implicated in these “receptor-operated channel” pathways (Undem and Sun, 2020). For example, bradykinin-evoked activation of airway nociceptors has been shown to be mediated by TRPV1 (possibly via Gq-mediated hydrolysis of the inhibitory phosphatidylinositol bisphosphate) (Carr et al.,2003) and byCa2+-sensitive chloride channels (whose activation causes depolarization of vagal afferents as their reversal potential for chloride is more positive than the resting membrane potential) (Lee et al., 2005).
Afferent subsets
Vagal afferents are heterogeneous and considerable efforts have been made to characterize this heterogeneity at the functional, electrophysiological, pharmacological, anatomical, biochemical and genetic levels (Coleridge and Coleridge, 1984; Mazzone and Undem, 2016). Electrophysiological recordings of single fibers indicate that vagal afferents innervating the airways range from slow conducting (~1 m/s) unmyelinated C fibers to fast conducting myelinated A fibers. Myelination of afferents correlates with the size of the afferent cell body within the vagal ganglia, and with neurofilament expression in these neurons. Expression of neuropeptides such as substance P, calcitonin gene-related peptide (CGRP) and neurokinin A is more prevalent in small diameter, neurofilament-negative neurons. Below is a list of the major afferent subsets innervating the lower airways.
Intrapulmonary A fibers
Approximately 10% to 20% of vagal afferents innervating the mammalian lung are myelinated afferents (conduction velocity ~12–50m/s, mostly Aβ range) that are highly sensitive to mechanical stimulation (Widdicombe, 1954; Bergren and Peterson, 1993; Ho et al., 2001; Schelegle and Green, 2001; Mazzone and Undem, 2016). Eupneic breathing induces lung stretch that is sufficient to activate these fibers, and their responses have been characterized based upon their adaptation: slowly adapting receptors (SARs), also known as pulmonary stretch receptors (PSRs), and rapidly adapting receptors (RARs). RARs tend to conduct slower than SAR, but there is substantial overlap. The rapid adaptation of RARs is unlikely to be due to intrinsic electrophysiological properties (McAlexander et al., 1999), but rather to an adaptation of the stimulus. Activation of the mechanoreceptors regulates central respiratory networks within the nTS. SARs central projections terminate within the nTS at about the rostracaudal level of the area postrema, in particular the ventral and lateral subnuclei, where they synapse with pump cells (whose activation parallel SAR activity) and inspiratory-β cells (Kubin et al., 2006). These second order neurons then project to the parabrachial nucleus and Kölliker–Fuse nucleus in the dorsolateral pons and the ventral respiratory column, synapsing with multiple levels of the respiratory pattern generator network. SAR activation leads to the inhibition of inspiration and the facilitation of expiratory drive (Hering–Breuer reflex) (Widdicombe and Nadel, 1963; Chang et al., 2015). In mice, lung stretch-induced apnea is dependent on the activation of the Piezo2 channel expressed on myelinated nodose afferents innervating the lung (Nonomura et al., 2017). SAR activation also causes a decrease in parasympathetic drive to the airways and heart, resulting in bronchodilation and tachycardia (Widdicombe and Nadel, 1963; Kubin et al., 2006; Mazzone and Undem, 2016). Intrapulmonary RAR fibers project to more caudal and medial nTS regions than SARs, where they synapse with so-called “RAR cells” (Lipski et al., 1991). RAR cells provide inputs into the respiratory premotor networks within the nucleus ambiguus, parabrachial nucleus and Kölliker–Fuse nucleus (Otake et al., 2001; Kubin et al., 2006). RAR activation increases inspiratory effort and rate, as well as increasing parasympathetic drive to the airways, resulting in bronchospasm (Widdicombe, 1954; Mazzone and Undem, 2016). In addition to their antagonistic effects on respiration and autonomic output, SAR/pump cell activity leads to direct inhibition of RAR cells within the nTS (Kubin et al., 2006).
SARs and RARs do not typically express nociceptor-associated receptors such as TRPV1 and the bradykinin B2 receptor, nor do they typically express neuropeptides (Bergren and Peterson, 1993; Ho et al., 2001; Mazzone and Undem, 2016). Therefore these mechanoreceptors are not directly activated by nociceptive stimuli. However, some mechanoreceptor afferents, particularly RARs, are indirectly activated by agents that cause bronchospasm or edema or decreases in lung compliance, and this phenomenon induced some studies to refer to these fibers as “irritant receptors.” Thus, local bronchospastic autacoids can modulate respiratory reflexes: histamine evokes tachypnea in anesthetized guinea pigs, which is reduced by the bronchodilator albuterol, suggesting the involvement of the mechanosensitive RAR fibers (Chou et al., 2008).
Many mechanoreceptors express P2X2 and P2X3 and display sensitivity to ATP and α,β methylene ATP (α,β mATP), the selective ligand of P2X2/3 heteromeric channels, indicating that they are derived from nodose neurons, which express, as expected, high levels of neurofilament (Canning et al., 2004; Hooper et al., 2016).
Extrapulmonary nodose Aδ fibers
In guinea pigs, the majority of nodose afferents innervating the extrapulmonary airways are partially myelinated Aδ fibers that conduct APs at 2 to 8 m/s (Ricco et al., 1996; Canning et al., 2004). These fibers, which project to the trachea and bronchi via the RLN, are exquisitely sensitive to punctate stimulation but are insensitive to stretch. Nodose Aδ fibers innervating the trachea and bronchi are also activated by acid, but are insensitive to other nociceptive stimuli such as bradykinin and capsaicin (Ricco et al., 1996). Aδ fiber responses to acid are not mediated by TRPV1, which they lack. Instead, acid sensing ion channels (ASICs) have been implicated (Canning et al., 2006), but their role has yet to be definitely demonstrated. Consistent with their nodose (i.e., placodal) origin, nodose neurofilament-positive neurons traced from the trachea lack neuropeptide content. Recent evidence suggests that these afferents express both tetrodotoxin-sensitive NaV1.3 and NaV1.7 and tetrodotoxin-insensitive NaV1.8 and NaV1.9 (Kollarik et al., 2018). Nevertheless, AP discharge from the activated afferent terminal is abolished by tetrodotoxin or by an NaV1.7-selective inhibitor.
Punctate mechanical stimulation of the trachea and bronchi evokes cough under anesthetic conditions in multiple mammalian species, including guinea pigs, rabbits and cats (Canning et al., 2004, 2014; Canning, 2008). There is substantial evidence, best characterized in the guinea pig, that extrapulmonary nodose Aδ fibers are responsible for the cough evoked by either punctate mechanical stimulation, electrical stimulation or citric acid in anesthetized animals (Ricco et al., 1996; Canning et al., 2004; Mazzone et al., 2009; Muroi et al., 2011, 2013): firstly, section of the RLN but not the SLN ablates such tracheal cough responses; secondly, only stimuli (e.g., punctate mechanical, citric acid, electrical) shown in single fiber electrophysiological recordings to activate nodose Aδ fibers evoke cough when applied to the trachea of anesthetized guinea pigs, whereas other stimuli such as capsaicin, bradykinin and ATP fail to evoke cough; and thirdly, selective reduction of electrical activity in nodose neurons (induced by knockdown of the tetrodotoxin-sensitive NaV1.7) eliminates cough evoked by citric acid or mechanical stimulation in anesthetized guinea pigs. Extrapulmonary nodose Aδ fibers project axons to an area just rostral of obex and lateral to the commissural subnucleus of the nTS, where they synapse with second-order neurons that regulate cough-associated networks (Canning and Mori, 2010). The signaling at this first synapse is mediated by glutamate.
Nodose Aδ fibers innervating the trachea respond to punctate stimuli with rapidly adapting AP discharge, and this has sometimes led to these extrapulmonary Aδ fibers being functionally grouped with the faster conducting intrapulmonary RARs (conduction velocity 10–20m/s) (Mazzone and Undem, 2016). Nevertheless, evidence suggests that these are probably two distinct subsets (Canning et al., 2004): (1) activation of extrapulmonary Aδ fibers causes cough but activation of intrapulmonary RARs fails to evoke cough, and (2) intrapulmonary RARs are activated by α,β mATP, but extrapulmonary Aδ fibers are insensitive to P2X2/3 agonists (despite the fact that they are nodose afferents).
Bronchopulmonary C fibers
Vagal C fibers arise from every level of the lower airways from the trachea through to the alveoli (Dey et al., 1990; Baluk et al., 1992; Watanabe et al., 2005, 2006; Su et al., 2021). C fibers are unmyelinated axons that conduct APs at ~0.3 to 1.2 m/s, and they comprise the majority of vagal airway afferents (Coleridge and Coleridge, 1984; Ricco et al., 1996; Ho et al., 2001; Undem et al., 2004). C fibers are derived from neurons in both nodose and jugular ganglia. C fiber innervation of the trachea is largely derived from jugular neurons (many via the SLN), whereas the intrapulmonary airways are preferentially innervated by nodose C fibers. Almost all C fibers innervating the lower airways of guinea pigs and rats are activated by the classic nociceptive stimuli capsaicin, due to their expression of TRPV1. The vast majority of guinea pig and rat C fibers are also activated by bradykinin (via B2 receptors) and allyl isothiocyanate or cinnamaldehyde (selective agonists for TRP ankyrin 1 (A1)) (Ho et al., 2001; Undem et al., 2004; Hooper et al., 2016). As such C fibers are robustly activated by stimuli perceived as noxious by mammals and thus can be considered to be nociceptive, according to Sherrington’s definition (Sherrington, 1906). Interestingly, only slowly conducting C fibers (0.3–0.7 m/s) innervating the mouse airways are activated by these classic nociceptive stimuli; faster C fibers are insensitive (Kollarik et al., 2003). Airway C fibers in all mammals studied are also mechanically sensitive to either punctate or stretch stimuli, although their threshold for mechanical stimulation is high enough that they are typically quiescent during eupnea (Coleridge and Coleridge, 1984; Ho et al., 2001).
Nodose and jugular C fibers have distinct stimuli sensitivities, and this has been shown to be dependent on their distinct gene expression profiles (Taylor-Clark, 2021). While both nodose and jugular C fibers express P2X3, only nodose C fibers also express P2X2, and it is the expression of the P2X2/3 heteromeric channel which is necessary for robust activation of C fibers by ATP or α,β mATP (Undem et al., 2004; Kwong et al., 2008b; Nassenstein et al., 2010; Kim et al., 2020a). Furthermore, nodose C fibers are activated by adenosine (A1 and A2A receptors) and 2-methyl-5HT (5HT3 receptors), whereas jugular C fibers are insensitive to these agents (Chuaychoo et al., 2005b, 2006). The cell bodies of airway-specific nociceptors (typically identified by TRPV1 expression and/or a lack of neurofilament expression) in the nodose ganglion lack substance P expression, whereas those in the jugular ganglion widely express substance P (Ricco et al., 1996; Undem et al., 2004; Kim et al., 2020b). This is consistent with the robust innervation of the tracheal subepithelial layer by neuropeptide-expressing terminals (Dey et al., 1990; Baluk et al., 1992).
Nodose C fibers terminate within the medial and caudal subnuclei of the nTS (Kim et al., 2020a,b; Su et al., 2021). There is some conflicting evidence for the central terminations of jugular C fibers (Behrens et al., 2021; Taylor-Clark, 2021). Multiple studies have shown using chemical tracers and viral vectors that both vagal neuropeptide-expressing afferents in mice and jugular afferents of rats and guinea pigs terminate within the Pa5 (Driessen et al., 2015, 2020; McGovern et al., 2015a, b; Kim et al., 2020b). Although tracing from the airways itself has identified airway-specific terminations within the Pa5 of rats and guinea pigs, this was not seen in mice (Kim et al., 2020b; Su et al., 2021). Furthermore, it is not clear if the jugular-Pa5 connectivity is exclusive—there is evidence that jugular/peptidergic afferents also terminate within the nTS (Mazzone and Canning, 2002; Kim et al., 2020b). Importantly, a recent study demonstrated that capsaicin inhalation (activates both nodose and jugular C fibers) causes neural activity in both the nTS and Pa5 regions of human subjects, whereas ATP (selective nodose stimulant) only increased activity in then TS, suggesting that these pathways are present in humans as well as in animal models (Farrell et al., 2020).
Capsaicin, the canonical C fiber stimulant, evokes nocifensive reflexes from the lower airways including modulation of respiratory rhythms (apnea, bradypnea, tachypnea, and cough), modulation of central autonomic efferent output (bronchospasm, mucus secretion and bradycardia) and local neurogenic-mediated bronchospasm (Coleridge and Coleridge, 1984; Mazzone and Undem, 2016). The specific role of nodose and jugular C fibers in these nocifensive reflexes is incompletely understood. This is in part due to the lack of selective jugular C fiber stimuli. Nevertheless, some elegant studies have implicated specific reflex function in some cases, particularly in the guinea pig (Muroi et al., 2013; Chou et al., 2018), which has a robust cough and bronchospastic reflex compared to the mouse and rat (Mazzone and Undem, 2016).
As mentioned above, C fiber stimulants such as capsaicin, bradykinin or allyl isothiocyanate fail to evoke cough in anesthetized guinea pigs, despite their robust modulation of breathing rates (Canning et al., 2004). However, these noxious stimuli do evoke cough in conscious guinea pigs, rabbits, cats and humans (Taylor-Clark, 2015). Two lines of evidence suggest such responses are mediated entirely by jugular C fibers: firstly, selective agonists of nodose C fibers (e.g., adenosine, 2-methyl 5HT) fail to evoke cough (Muroi et al., 2013); and secondly, selective reduction of electrical activity in nodose neurons (NaV1.7 knockdown) has no effect on capsaicin-evoked cough (Muroi et al., 2013). Interestingly, in anesthetized guinea pigs, capsaicin- and bradykinin-evoked C fiber activation sensitizes the cough reflex initiated by extrapulmonary nodose Aδ fiber activation (Mazzone et al., 2005). This C fiber-mediated sensitization is sensitive to inhibition of neuropeptide signaling in the medulla, suggesting a role of jugular C fibers. Whereas selective activation of nodose C fibers (with adenosine or 2-methyl 5HT) dramatically inhibited nodose Aδ fiber-mediated cough (Chou et al., 2018), indicting distinct functional effects of nodose and jugular C fibers on the cough reflex.
Capsaicin and other C fiber stimulants evoke central reflex modulation of autonomic output to the airways and heart (Coleridge and Coleridge, 1984; Mazzone and Undem, 2016). Capsaicin and bradykinin induce atropine-sensitive bronchospasm, mucus secretion and bradycardia, indicating a predominant role of parasympathetic signaling (Davis et al., 1982; Schultz et al., 1985; Canning et al., 2001; Mazzone and Canning, 2002; Hooper et al., 2016). Nevertheless, the airways have a complex autonomic innervation, which will be discussed in sections below. Importantly, the net effect of airway C fiber stimulation (bronchospasm) is abolished by central administration of neurokinin receptor antagonists, suggesting that neurotransmission of neuropeptide-containing jugular afferents play an important role inthis reflex (Mazzone and Undem, 2016). Nevertheless, such evidence is not definitive as some nTS neurons also express neuropeptides (Kim et al., 2020b), and there is evidence that the 5HT3 agonist phenylbiguanide (nodose selective stimulant) also evokes reflex bronchospasm in some species (Szarek et al., 1995; Taylor-Clark, 2021).
What is clear is that the activation of jugular C fibers in the airways also induces bronchospasm via a local neurogenic axon-reflex mechanisms in some mammals such as rats and guinea pigs (although the evidence is lacking in humans) (Ellis and Undem, 1990, 1994; Ellis et al., 1993). Capsaicin-induced depolarization of jugular C fiber terminals evokes release of neuropeptides such as the tachykinins substance P and neurokinin A that activate NK1 and NK2 receptors on bronchial smooth muscle, inducing contraction. Such responses are independent of the autonomic innervation of the airways. Nodose C fibers lack neuropeptides and so do not contribute to this neurogenic bronchospasm (Undem et al., 2004; Nassenstein et al., 2010). Neuropeptides released from jugular C fibers also activate NK receptors on airway glands and parasympathetic ganglionic neurons, increasing mucus secretion (Hayes et al., 1995; Rogers, 1995) and synaptic transmission (Myers and Undem, 1993), respectively.
C fiber modulation of respiratory rhythm suggests a complex interaction of multiple pathways, which can be preferential activated by different agents and by distinct routes of administration (e.g., inhalation vs. topical vs. intravenous injection) (Coleridge and Coleridge, 1984; Palecek et al., 1989; Chou et al., 2008, 2018). Capsaicin evokes tachypnea when inhaled, bradypnea when applied to the trachea, and tachypnea followed by apnea/bradypnea when given intravenously in guinea pigs, rats and dogs. In the anesthetized guinea pig, both adenosine and 2-methyl 5HT (activate nodose C fibers selectively) evoke tachypnea when inhaled or given intravenously, and these responses were abolished by vagotomy, suggesting that nodose C fibers are responsible (Chou et al., 2008, 2018). This is consistent with tachypnea responses during optogenic stimulation of nociceptors in mice (Chang et al., 2015). However, adenosine and/or selective 5HT3 receptor agonists have been reported to evoke apnea in anesthetized rats, mice and cats (Coleridge and Coleridge, 1984; Hsu et al., 2019). As such, the specific role of nodose and jugular C fiber in modulating respiratory rhythms is currently unresolved.
Many of the studies investigating the differential role of nodose and jugular C fibers in respiratory reflexes have been performed in guinea pig and, now with genetic-based approaches, in mice. How these studies relate to earlier studies of afferent neurophysiology in larger animals which identified C fiber subsets based upon preferential stimulation by application of stimuli via the pulmonary and bronchial circulations remains to be seen. Of note, fast C fibers innervating the mouse bronchopulmonary tree lack responsiveness to most noxious stimuli (Kollarik et al., 2003) and the function of these of afferents is currently unknown.
Extrapulmonary jugular Aδ fibers
Approximately 50% of jugular afferents innervating the trachea are partially myelinated Aδ fibers (Ricco et al., 1996). Like the extrapulmonary jugular C fibers, these jugular Aδ fibers have a high threshold for mechanical activation and are sensitive to capsaicin and bradykinin. Unlike jugular C fibers, their cell soma expresses neurofilament but do not express neuropeptides. There are no studies that have selectively activated extrapulmonary jugular Aδ fibers, thus their reflex function is currently unknown.
Dorsal root ganglia afferents
There is a minor sensory innervation of the airways from the dorsal root ganglia (C8 to T5) (Dinh et al., 2004; Oh et al., 2006) (Fig. 14.1). DRG neurons are derived from the same embryological source as jugular afferents: the neural crest (Taylor-Clark, 2021). Consistent with this, many DRG airway afferents express neuropeptides but few express P2X2 (Dinh et al., 2004; Nassenstein et al., 2010; Kim et al., 2020a). Few DRG airway afferents express neurofilament, suggesting that most project unmyelinated C fiber axons. However, the expression of TRPV1 in DRG airway afferents varies across species: from <12% in mice to >61% in guinea pig. Stimulation of airway DRG nociceptors increases sympathetic drive to the cardiopulmonary system (i.e., evokes reflex tachycardia) (Oh et al., 2006; Shanks et al., 2018). These responses are more evident following vagotomy.
AUTONOMIC EFFERENT INNERVATION OF THE LOWER AIRWAYS
Both parasympathetic and sympathetic nerves project to the lower airways and regulate organ function, including airway patency, mucus secretions and blood flow (Karlsson et al., 1988; Widdicombe, 1993; Rogers, 2001; Canning, 2006; Undem and Potenzieri, 2012). Parasympathetic nerves are the predominant regulator of airway patency and mucus secretions, whereas blood flow is regulated by both autonomic branches. Anatomically, autonomic pathways consist of two neuron populations in series: a preganglionic neuron, which resides in the CNS and projects axons to the peripheral autonomic ganglia, where it synapses with the postganglionic neurons that projects axons to effector cells within the lungs (e.g., smooth muscle cells, goblet cells, etc.).
Parasympathetic innervation
As shown in Fig. 14.1, preganglionic cholinergic neurons within the nucleus ambiguus and dorsal motor nucleus of the vagus (DMX) project axons, via the vagus nerve, to airway parasympathetic ganglia (Kalia and Mesulam, 1980; Kalia and Sullivan, 1982; Canning, 2006; Undem and Potenzieri, 2012). Studies in cat, dog and guinea pig demonstrate that many axons projected from the nucleus ambiguus are myelinated A fibers, whereas most projected from the DMX are unmyelinated C fibers (McAllen and Spyer, 1978; Haselton et al., 1992; Canning and Undem, 1993a). The majority of ganglia are found in the extrapulmonary airways, but there is substantial variety in the number of neurons within each ganglia, ranging from 2 to 100, depending on the species. Ganglia are associated in plexi superficial to and within the smooth muscle layers (Baker et al., 1986; Bałuk and Gabella, 1989; Dey et al., 1996, 1999). Guinea pig airways also receive input from parasympathetic ganglia within the myenteric plexus of the esophagus (Canning and Undem, 1993a; Mazzone and McGovern, 2010). In the lungs, ganglia are found in the large peribronchial plexus and the smaller perivascular plexus (Honjin, 1956; Fontán et al., 2000; Pérez Fontán and Velloff, 2001). Despite ganglia only being located within the first 2 or 3 generation of intrapulmonary airways, postganglionic fibers project down to the smallest bronchioles. Parasympathetic ganglion neurons have substantial dendritic structures, and these are the sites which receive synaptic input from the preganglionic cholinergic nerves (Mitchell et al., 1987; Myers et al., 1990, 1996). Activity in preganglionic nerves induces fast excitatory postsynaptic potentials (fEPSPs) via acetylcholine-mediated nicotinic channel activation. Ganglionic neurons receive input from multiple preganglionic axons, and the evoked fEPSPs converge onto the axon hillock, eliciting APs if of sufficient magnitude. Often moderate stimulation of preganglionic APs produces fEPSPs in ganglionic neurons that fail to evoke APs—i.e., ganglionic neurons robustly filter preganglionic input (Myers et al., 1990; Myers and Undem, 1991; Kajekar et al., 2001). Ganglionic neurons also adapt to prolonged depolarizing currents, although heterogeneity in this property has been observed across guinea pig and rat neurons. Many ganglionic neurons are cholinergic, but some instead express vasoactive intestinal peptide (VIP) and nitric oxide synthase (NOS) (Baker et al., 1986; Bałuk and Gabella, 1989; Dey et al., 1996, 1999). Very few choline acetyltransferase (ChAT)-positive neurons express VIP/NOS, indicating there are distinct parasympathetic postganglionic subsets. VIP/NOS-positive neurons are predominantly found in the superficial muscle plexus in the ferret and in the esophageal myenteric plexus in the guinea pig. Postganglionic fibers project to tracheal and bronchial smooth muscle, mucous and serous cells in submucosal glands, goblet epithelial cells and blood vessels.
Chemical stimulation of airway preganglionic parasympathetic neurons or electrical stimulation of the distal end of the vagus nerve (sectioned at the cervical level) causes robust bronchospasm and mucus secretion in all mammals studied (Karlsson et al., 1988; Rogers, 2001; Canning, 2006; Undem and Potenzieri, 2012). These responses are abolished by hexamethonium (nicotinic acetylcholine receptor antagonist) and atropine (muscarinic acetylcholine receptor inhibitor), and are mimicked by muscarinic agonists—indicating the predominant role of cholinergic parasympathetic signaling (Ueki et al., 1980; Tokuyama et al., 1990; Undem et al., 1990; Fung et al., 1992; Canning and Undem, 1993a). Both airway smooth muscle and secretory cells express the Gq-coupled muscarinic M3, whose activation is responsible for the cholinergic-mediated contraction and mucus secretion (Mak and Barnes, 1990; Roffel et al., 1994; Watson et al., 1995; Rogers, 2001). These cells also express Gi-coupled M2 receptors, whose physiological role is less clear. Electrical vagal stimulation-evoked smooth muscle contraction is abolished by the NaV blocker tetrodotoxin in ex vivo studies of human, mouse and guinea pig tissue (Kocmalova et al., 2017; Kollarik et al., 2021). Responses in human and guinea pig tissue were also abolished by selective NaV1.7 inhibition, but in mouse tissue both NaV1.7 and NaV1.1 and/or NaV1.3 were capable of conducting APs.
Interestingly, in the presence of atropine, electrical stimulation of vagal efferents causes relaxation of precontracted airway smooth muscle (Canning and Undem, 1993a,b). This muscarinic-independent mechanism is mediated by the nonadrenergic, noncholinergic (NANC) parasympathetic fibers expressing VIP and NOS. In human airways, this branch of the parasympathetic nervous system is the only neural pathway that relaxes airway smooth muscle (Canning, 2006; Undem and Potenzieri, 2012). NANC-mediated relaxation is abolished by hexamethonium (blocks ganglionic transmission) (Canning and Undem, 1993a,b), is reduced by inhibition of VIP or nitric oxide (NO) signaling (Belvisi et al., 1992; Ellis and Undem, 1992; Hasaneen et al., 2003), and is mimicked by either exogenous VIP or NO (Ellis and Undem, 1992; Schmidt et al., 2001; Berisha et al., 2002). VIP is a neuropeptide that binds VPAC1 and VPAC2 receptors, both of which are found in the lung (Busto et al., 2000; Schmidt et al., 2001) and both of which increase adenylate cyclase activity and cyclic AMP levels (Groneberg et al., 2006). Cyclic AMP causes relaxation of airway smooth muscle, and there is evidence that VPAC2 predominates in VIP-evoked relaxation. NO is a gas that is synthesized on demand by NOS following AP-dependent Ca2+ influx, diffuses from the NANC efferent and stimulates guanylate cyclase activity in airway smooth muscle cells, inducing cyclic GMP-dependent relaxation (Esplugues, 2002). There is some evidence in guinea pig and human airway that NANC-mediated relaxations are more dependent on NO than VIP, particularly in the peripheral airways (Li and Rand, 1991; Ellis and Undem, 1992; Bai and Bramley, 1993; Groneberg et al., 2006). NANC-mediated relaxation is slower and more long-lasting than cholinergic-mediated contraction, and requires much higher frequency of vagal electrical stimulation (Canning and Undem, 1993a,b). Consistent with the observation that airway-projecting VIP/NOS-positive parasympathetic neurons are found in the esophageal myenteric plexus in the guinea pig, NANC-mediated relaxations were abolished by esophageal removal (Canning and Undem, 1993a).
Electric field stimulation of ferret tracheal rings under cholinergic and adrenergic blockage causes mucus secretion that is abolished by an antibody targeting endogenous VIP (Liu et al., 1999). Similarly, vagal stimulation in anesthetized cats evokes secretion that is only minimally reduced by cholinergic and adrenergic blockade (Peatfield and Richardson, 1983). Micromolar concentrations of exogenous VIP increases mucus secretion, likely via the activation of VPAC2 receptors on secretory cells (although VPAC1 is also expressed) (Liu et al., 1999; Rogers, 2001). However, in the ferret nanomolar concentrations of VIP have been shown to inhibit cholinergic-mediated mucus secretion, and cholinergic-mediated secretions evoked by low stimulus electric field stimulation are potentiated when VIP is scavenged (Liu et al., 1999). These studies suggest that VIP can act prejunctionally to inhibit acetylcholine release from cholinergic postganglionic neurons. NO is not thought play a substantial role in NANC-mediated modulation of mucus secretion.
Bronchial (i.e., systemic) arteries receive innervation from both cholinergic and NANC postganglionic parasympathetic neurons (Widdicombe, 1993; Coleridge and Coleridge, 1994). Vagal stimulation in large mammals (dogs, cats, pigs) induces vasodilation of bronchial arteries, predominantly in an atropine-sensitive manner (Laitinen et al., 1987b; Pisarri et al., 1999). Consistent with this, exogenous acetylcholine also dilates these vessels via the activation of muscarinic M3 on vascular endothelial cells, which leads to NO generation and subsequent NO-mediated relaxation of vascular smooth muscle cells (Laitinen et al., 1987a; O’Rourke and Vanhoutte, 1990).
Sympathetic innervation
The airways receive postganglionic sympathetic innervation from neurons within the stellate and thoracic sympathetic ganglia (Widdicombe, 1993; Canning, 2006; Undem and Potenzieri, 2012). Most expressed tyrosine hydroxylase, indicating they are adrenergic, and the neurotransmitter neuropeptide Y (NPY) (Kummer et al., 1992). Some ganglionic neurons only express NPY. Ganglionic sympathetic neurons are activated by cholinergic input from preganglionic neurons within the intermediolateral nucleus of the spinal cord. As in the parasympathetic system, acetylcholine release from active preganglionic fibers activates nicotinic receptors on the ganglionic neurons, evoking fEPSPs that will, if of sufficient magnitude, evoke APs along the postganglionic axon (McLachlan, 2003). Unlike parasympathetic ganglionic synapses, there is little filtering of preganglionic input by sympathetic ganglionic neurons.
Humans, primates and rabbits have no direct sympathetic innervation of airway smooth muscle cells (Canning, 2006; Undem and Potenzieri, 2012). However, guinea pig, cat and dog have direct innervation of airway smooth muscle and electrical stimulation of the sympathetic trunk induces relaxation of precontracted airway muscle (Canning and Undem, 1993b). Such responses are abolished by propranolol and are mediated by smooth muscle β adrenoceptors in a cAMP-dependent manner. All mammals express β adrenoceptors on smooth muscle that can be activated by circulating epinephrine released from the adrenal medulla, evoking bronchodilation (Barnes, 1986, 2004). In humans, catecholamine-evoked bronchodilation is mediated exclusively by smooth muscle β2 adrenoceptors.
There is anatomical evidence that sympathetic nerves innervate submucosal glands, but not epithelial goblet cells. Adrenergic agonists can evoke mucus secretion, although the response is limited compared to parasympathetic stimulation (Ueki et al., 1980; Rogers, 2001). In both cats and ferrets, sympathetic stimulation increases mucus secretion and this is blocked by α and β adrenoceptor antagonists in the ferret, but by only β adrenoceptor inhibitors in the cat.
There is substantial sympathetic innervation of pulmonary arteries and bronchial arteries. Innervation occurs throughout the arterial vasculature down to ~10 to 30 μm in larger mammals (Widdicombe, 1993; Coleridge and Coleridge, 1994). Electrical stimulation of sympathetic nerves causes vasoconstriction in these vessels, predominantly via activation of smooth muscle α1 adrenoceptors, although a slower and more longer lasting α1-independent vasoconstriction also occurs, which is mediated by NPY (Laitinen et al., 1987a). Sympathetic innervation is considered the predominant branch of the autonomic nervous system controlling arterial blood flow within the airways. There is limited sympathetic innervation of pulmonary veins or bronchial veins.
Basal and reflex autonomic control
The airways are under basal autonomic control (Widdicombe, 1966; McAllen and Spyer, 1978; Roffel et al., 1994; Kesler and Canning, 1999; Canning, 2006). Sectioning of the vagal parasympathetic efferents in vivo causes airway smooth muscle dilation and increased airflow. This is also the case when the afferent nerves innervating the lower airways are selectively resected, indicating that baseline parasympathetic drive stems, at least in part, from activation of afferent subsets, most likely low-threshold mechanosensitive A fibers (Kesler and Canning, 1999). This sectioning also interrupts preganglionic fibers connected to NANC postganglionic fibers. As such the net effect of basal parasympathetic activity on airway smooth muscle at rest is partial contraction. Consistent with the role of cholinergic fibers in maintaining airway patency, muscarinic inhibitors evoke bronchodilation in vivo (Canning, 2006; Peters et al., 2010). Interruption of vagal efferents decreased blood flow in bronchial arteries in dogs (Horisberger and Rodbard, 1960) but pharmacological studies suggest that both cholinergic and NANC contributions to basal dilation of these vessels is variable and inconsistent (Baile et al., 1987; Laitinen et al., 1987a,b; Widdicombe, 1993; Coleridge and Coleridge, 1994; Pisarri et al., 1999; Zimmerman and Pisarri, 2000). Despite the robust vasoconstrictor responses of bronchial and pulmonary arteries to α adrenoceptor-dependent sympathetic stimulation, most studies suggest a lack of basal sympathetic-mediated vasoconstriction of either vessel type (Williams and Drummond, 1983; Baile et al., 1987; Widdicombe, 1993; Hennessy et al., 1993; Coleridge and Coleridge, 1994; Zimmerman and Pisarri, 2000), although this could be due to particular anesthetic use (Hennessy 93). Measurement of basal mucus secretion in vivo is challenging (Undem and Potenzieri, 2012). Nevertheless, there is evidence that muscarinic inhibitors reduce basal secretions in the cat and dog (Ueki et al., 1980; Johnson et al., 1983; Padda et al., 2001), indicating a role of cholinergic parasympathetic signaling—although other studies have failed to demonstrate this effect (Tokuyama et al., 1990; Fung et al., 1992).
Airway-projecting preganglionic parasympathetic neurons within the nucleus ambiguus and DMX receive input from multiple sources including the nTS (input from sensory afferents), other nuclei in the ventral respiratory group, pons, periaqueductal gray and the hypothalamus (Haselton et al., 1992; Haxhiu et al., 2005). Activation of lung afferents modulates parasympathetic preganglionic neuron activity. Thus activation of C fibers and RAR fibers increases parasympathetic drive, whereas SAR fiber activation decreases parasympathetic drive (Kubin et al., 2006; Mazzone and Undem, 2016).
AIRWAY INFLAMMATION-INDUCED NEUROMODULATION
Pulmonary inflammation can lead to changes in airway neural function at all levels of the reflex circuit; from the afferent nerve terminals, to processing in the CNS, to activity in the efferent autonomic nervous system (summarized in Fig. 14.2). This modulation can directly contribute to the symptoms of airway diseases. Such symptoms include chronic coughing, excessive secretions, bronchospasm, and sensations of dyspnea.
Fig. 14.2.
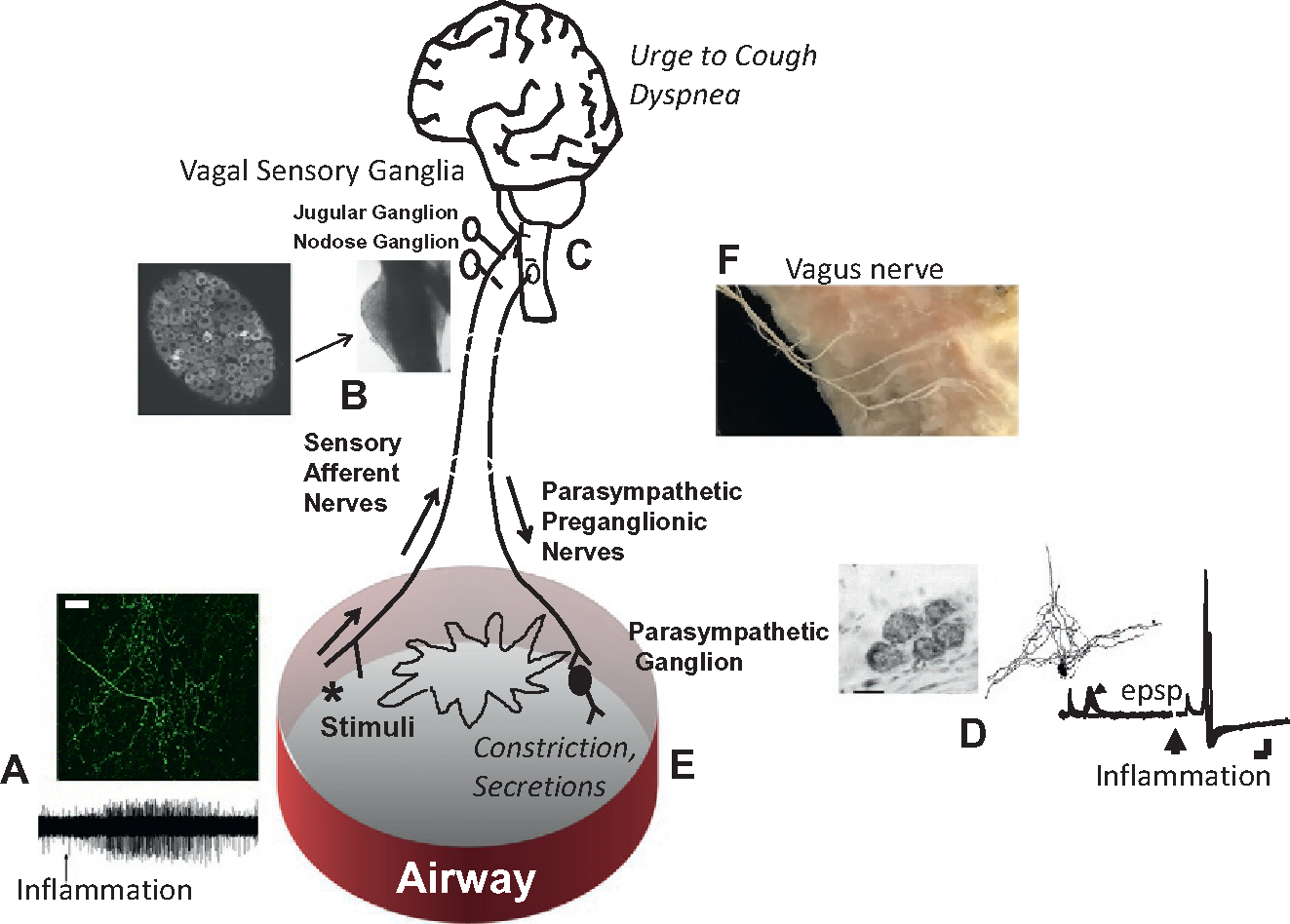
Inflammation-induced airway neuromodulation. Inflammation can lead to activation and sensitization of C-fiber terminals in the airways; (A) above, a C fiber terminating in the airways of a mouse; below electrophysiological recording of action potentials evoked in a C fiber terminal caused by the application of bradykinin. Inflammation can cause phenotypic changes in the sensory nerves by altering gene expression; (B) photograph of the nodose ganglion along the cervical vagus nerve of a mouse, also a cross-section showing the large number of visceral sensory neurons packed within the ganglion. (C) Inflammation can eventually lead to sensitization of neural activation within the brainstem of the CNS. Inflammation can lead to increases in synaptic efficacy within the parasympathetic bronchial ganglia; (D) shows an image of a small bronchial parasympathetic ganglion containing 5 neurons, a cameral lucida drawing of a single neuron and its dendritic tree, and synaptic recording of excitatory post synaptic potentials before and after inflammation (allergen challenge ex vivo), these images are from a guinea pig bronchial ganglion. (E) Inflammation can also increase transmitter release at the neuroeffector junction leading to increased airway narrowing and secretions. (F) Dissection showing the branches of the vagus nerve innervating a human bronchus.
Modulation of afferent nerves
Most investigations regarding airway inflammation and afferent nerve terminals have focused on the vagal C fibers (Coleridge and Coleridge, 1984; Mazzone and Undem, 2016). Although the receptors for only a minority of inflammatory mediators are expressed by vagal sensory neurons, receptors are expressed for several inflammatory mediators that are prevalent in airway inflammatory diseases (Wang et al., 2017; Mazzone et al., 2019). Chemical mediators can interact with their receptors to lead to an overt activation of C fibers; i.e., result in a membrane depolarization (generator potential) that exceeds the voltage threshold of action potential discharge. Chemical mediators can also lead to a “sensitization” of the nerve terminal without overt activation; i.e., they render the nerve terminal more sensitive to activating stimuli such that the nerve responds at lower intensities of stimulation, or responds to the same level of stimulation with a greater action potential discharge frequency.
Some mediators interact with ionotropic receptors such as ATP acting on P2X3 receptors, 5-HT acting on 5-HT3 receptor, and various stimuli that activate TRPV1 and TRPA1 receptors. Mediators that act on GCPRs also activate and sensitize airway C fibers. These mediators can include various eicosanoids, histamine, protease activated receptor agonists, bradykinin, etc. (Coleridge and Coleridge, 1984; Mazzone and Undem, 2016). Finally, activation of certain cytokine receptors has been found to activate and sensitize vagal airway C-fiber terminals. These receptors include type 1 and type 2 interferons and Tumor Necrosis Factor α (TNFα) receptors (Hsu et al., 2017; Patil et al., 2020).
In addition to producing mediators that directly interact with primary afferent nerves, inflammation can set up conditions where the afferent nerves are indirectly activated. For example, tissue edema associated with plasma extravasation can lead to mechanical stimulation of afferent nerves and drops in pH in the biophase of the afferent terminals can lead to afferent stimulation secondary to activation of acid-sensitive ion channels. Afferent nerves in the airways may also be activated indirectly via accessory cells. Both simple unmyelinated nerve terminals and more complex myelinated terminals are often found around the basal pole of collections of neuroendocrine cells known as neuroepithelial bodies (NEBs). The NEBs can release chemicals such as serotonin and ATP that can in turn activate the afferent nerve terminals (Brouns et al., 2012). The stimuli by which NEBs are activated and their role in airway physiology and disease remains poorly understood. Similarly, in the larger airways afferent nerve terminals have been found to be anatomically associated epithelial brush cells; an arrangement somewhat analogous to taste buds. It is envisaged that activation of the brush cells can release chemicals that activate the juxtaposed afferent nerve terminals. Little is known about the relevant brush cell activators or the specific chemical transmitter(s) involved in brush cell-mediated afferent nerve activation, although cholinergic mechanisms have been proposed (Krasteva et al., 2011).
Airway inflammation can also lead to phenotypic changes in airway afferent neurons. Allergic inflammation as well as respiratory virus infections induces the expression of genes in airway Aδ and Aβ fibers that are normally associated with C fibers. For example, airway inflammation induces de novo expression of genes for tachykinins and certain TRP channels (TRPV1 and TRPA1) in vagal airway A-fibers (Chuaychoo et al., 2005a; Zaccone and Undem, 2016). This leads to a situation where stretch receptors such as SARs become activatable via TRPV1 channel stimulation (Zhang et al., 2008). In the somatosensory system, this has been referred to as inflammation-induced phenotypic switching of sensory nerves and is thought to play an important role in hyperalgesia and allodynia (Neumann et al., 1996).
Modulation in the CNS
Airway inflammation is associated with an increase in the excitability of secondary neurons situated in the nTS, i.e., central sensitization (Behrens et al., 2021). Allergic inflammation associated with house dust mite in nonhuman primates can lead to a decrease in activation threshold and an increase in peak action potential discharge frequency in nTS neurons (Chen et al., 2009). Activation of afferent C fibers within the lungs, or esophagus of guinea pigs is associated with an increase in the sensitivity of coughing evoked by tracheal Aδ fiber activation (Canning and Chou, 2009). The C fiber-induced increase in A fiber cough is a manifestation of central sensitization (Singh et al., 2020).
Modulation of autonomic nerves
By increasing the activity of nociceptive afferent nerves, airway inflammation can indirectly lead to an increase in efferent autonomic outflow via vagal-vagal reflexes. Airway inflammation can also directly interact with parasympathetic nerve function within the airways. As described above, the filtering by parasympathetic ganglionic neurons results in decreased postganglionic APs compared to their input from preganglionic fibers. Allergic inflammation increases the synaptic efficacy in the airway ganglia thereby reducing the filtering activity (Myers et al., 1991; Kajekar et al., 2003). Thus, there is more postganglionic nerve activation for a given amount of presynaptic input.
As the action potentials invade the postganglionic terminals, acetylcholine, and other parasympathetic transmitters such as nitric oxide and VIP are released onto effector cells. Acetylcholine has been found to negatively feedback on the terminals via the interaction with muscarinic M2 receptors to inhibit further transmitter release. This M2-receptor inhibitory system is downregulated by airway inflammation (Pincus et al., 2021). These mechanisms, acting in concert, can lead to an exaggeration of parasympathetic activities including bronchoconstriction and mucus secretions.
Structural modulation
In addition to neuromodulation relating to electrophysiology, synaptic efficacy and gene expression, airway inflammatory diseases have also been associated with changes in nerve density and architecture (Shapiro et al., 2021). In general, morphometric studies point to an increase in airway nerve density in biopsies of airways obtained from those suffering from airway diseases like chronic cough and asthma (Drake et al., 2018). This is likely due to the prevalence of eosinophils and various nerve growth factors. Little is known about how airway inflammation alters the architecture of specific subtypes of afferent and efferent nerves within the airway, but an increase in sprouting and branching has been observed in nerves within the mucosal layer; a location where nociceptive afferent C fibers predominate (Drake et al., 2018).
ROLE OF AIRWAY NERVES IN ASTHMA, AIRWAY HYPERREACTIVITY, COPD, AND CHRONIC COUGH
Asthma/COPD
The idea that the nervous system is intimately involved in asthma is not new. About 150 years ago Henry Hyde Salter penned his classic treatise “On Asthma” (Salter, 1864). Without the benefit of our present understanding of airway physiology and neurology, Salter used his keen mind and his acute sense of observation to conclude a common feature of his asthmatic patients was morbid proclivity of the nervous system:
“A morbid proclivity of the musculo-nervous system of his bronchial tubes to be thrown into a state of activity; the stimulus may be either immediately or remotely applied, but in either case would not normally be attended by any such result. There is no peculiarity in the stimulus, the air breathed is the same to the asthmatic and non-asthmatic;…nor probably is there any peculiarity in the irritability of the bronchial muscle; the peculiarity is confined to the link that connects these two—the nervous system—and consists in its perverted sensibility in its receiving and transmitting on to the muscle, as a stimulus to contraction, that which it should take no cognizance.”
This treatise provides eight lines of evidence for a role of nerves in asthma that remain relevant today. Experimental evidence to support a neural role in asthma was filtering in by the early 1900s when surgeons were selectively denervating the lungs and finding a substantial relief is those suffering from severe asthma. These studies were poorly controlled by today’s standards and must be interpreted with an abundance of suspicion; indeed, most of these studies are best ignored. Nevertheless, in 1929 Phillips and Scott wrote what was in essence a meta-analysis of the burgeoning literature in this area that sprouted up during the first 20 or so years of the 20th century (Phillips and Scott, 1929). They reviewed some 300 cases but considered informative only those cases in which asthma was accurately diagnosed and the subject was followed and evaluated for a minimum of 6 months. With this filter in mind, they found 29 of the 300 cases that are of scientific value. They summarize by stating that…” there are a few brilliant cures in extremely severe forms of asthma. Roughly one half of the patients definitely improved, while frequently the other half, after temporary improvement, are in no better condition than before the operation. The patients who were cured have been followed on average almost two years.” Among these 29 cases there was little consistency in the surgical procedure, and some of the surgeries described can be questioned based on present knowledge of the physiology and extrinsic innervation of the airways. Later, the surgical technique to selectively denervate the lungs was refined by Reinhoff working at The Johns Hopkins Hospital. He astutely teamed up with a noted allergist/asthmologist in Leslie Gay to provide a careful analysis of the benefit of lung denervation in asthma (Rienhoff and Gay, 1938). The patients were selected on the basis of an unquestionable diagnosis of bronchial asthma, and on severity of disease with only those totally incapacitated by their disease being included. The report covers 11 patients economically, physically, and socially incapacitated by their disease. All the subjects were then followed for 1.75 to 2.75 years postoperatively. They concluded that approximately 2 years postsurgery:
Of the 10 patients discharged from the hospital, 1 was entirely unimproved; 1 improved for 3 months, finally succumbing to what seemed to be cardiac failure; 4 are completely well as the time of writing, having been free of attacks since the operation or a short time later, and are able to resume their former work; 4 have occasional mild attacks of asthma, all of which are amenable to control by means of small doses of ephedrine.
From physiological perspective this should not come as a surprise. Asthma is a disease of airway narrowing. The immediate relief obtained upon inhaling a β adrenoceptor agonist indicates that much of the airway narrowing in asthma is secondary to smooth muscle contraction. Studies reveal that when a maximally effective dose of a cholinergic muscarinic receptor antagonist is delivered to the airways the resistance to airflow is decreased to the extent that adding a β adrenoceptor agonist provides little additive relief (Ullah et al., 1981). In other words, the chemical responsible for nearly all the airway smooth muscle contraction that can be reversed with a functional antagonist is acetylcholine, derived from the postganglionic parasympathetic nerves that densely innervated bronchial smooth muscle. Accordingly, in clinical studies newer antimuscarinic drugs provide as much relief as a β adrenoceptor agonist (Peters et al., 2010).
COPD involves dyspnea, incessant coughing, airway narrowing, and excessive secretions. These symptoms are consistent with an overactive nervous system, with the latter two likely involving an enhanced cholinergic drive. Indeed, the mainstay of treatment to maximally dilate the airways and decrease secretions is an anticholinergic drug (Niewoehner et al., 2005). Dyspnea is a complex symptom that can be caused by many disparate neurological mechanisms. It is not clear that the airway sensory nervous system contributes to the dyspnea associated with COPD, but it is worth noting that inflammatory mediators present in the inflamed airways such as adenosine led to sensations of dyspnea in human volunteers in the absence of bronchoconstriction, and this can be reversed by topical (inhaled) delivery of adenosine receptor antagonist (Burki and Lee, 2010). Strategic airway denervation studies are underway for the treatment of severe asthma and COPD (Slebos et al., 2020; Hartman et al., 2021).
Airway hyperreactivity
Airway hyperreactivity is a hallmark of asthma. This is typically quantified as the concentration of an inhaled bronchoconstrictor (usually methacholine) required to increase airflow resistance (FEV1) by 20%. Those suffering with asthma often reach this level of increased resistance at doses <1% of that required in healthy volunteers. The mechanisms underlying airway hyper-reactivity associated with asthma remain largely obscure, but it is known that anti-inflammatory treatments in use today have very little influence on the underlying airway hyperreactivity (Szefler et al., 2000).
When the airways of laboratory animals are allergically inflamed, they also reveal airway hyperreactivity to an applied bronchoconstrictor. This is most often studied in mice, where it is clear that the nervous system plays a key role. In one study, cutting the vagus nerves just before the cholinergic challenge took place, had no effect on airway inflammation, but eliminated the airway hyperreactivity (McAlexander et al., 2015). In another study, using genetic tools to selectively remove only the C-fiber neurons within the vagal ganglia prevented the hyperreactivity, without noticeably influencing the allergen-induced inflammation (Trankner et al., 2014).
Pathological cough
There are fundamentally two types of induced cough. One type of cough is triggered by stimuli that threaten our airways. This protective cough is typified by the violent cough induced by aspiration. The other type of cough does not necessarily provide any protective role and can be considered a pathological cough. Virtually all adult human beings have experienced both types of coughs, the difference between them being self-evident. The protective cough is very immediate, involuntary, and often a violent cough. The pathological cough is, at least up to a point, a voluntary maneuver aimed at reducing the sensation of an irritable itch in the throat (an itch you cannot scratch). These irritation-induced coughs generally require an awakened state. Cough monitors show this to be true for the coughing associated with diseases like chronic cough and COPD (Satia et al., 2016). Substances known to activate C fibers, such as capsaicin, bradykinin, and citric acid, especially when delivered to the airways in low concentrations can accurately mimic this itchy urge to cough. When the concentration is increased the irritation is so severe that the ensuing cough becomes all but involuntary.
Chronic cough is an incessant cough that has lasted for at least 2 months. In some cases, treating an underlying disorder such as acid reflux, asthma, rhinitis, solid tumor, can effectively treat chronic cough. In many cases, however, treating all underlying disease does not block the cough. These subjects are said to suffer from chronic idiopathic cough hypersensitivity syndrome (Satia et al., 2016). These subjects are considered hypersensitive, because they cough at doses of a tussive stimuli (e.g., capsaicin) much lower than that required to evoke cough the healthy population. This “hypertussivity” has obvious similarities to airway hyperreactivity, discussed above, as well as hyperalgesia and allodynia associated with chronic pain (O’Neill et al., 2013). These chronic coughers often present to cough clinics with a cough that has lasted many years. Local anesthetics can cause a temporary relief, indicating that the cough most likely arises from inappropriate input from the airway vagal sensory nervous system. This would also be consistent with the finding that a peripheral acting drug named gefapixant, that blocks the purinergic P2X3 and P2X2/3 receptors, has been found in clinical trials to relieve the cough of severe chronic coughers (Abdulqawi et al., 2015;Ford et al., 2021). The expression of purinergic P2X3 and P2X2/3 receptors is limited largely to sensory nociceptors indicating that, at least in some cases, chronic cough is secondary to overactive vagal afferent nerves (Ford et al., 2021).
SUMMARY
Like all visceral organs the airways are richly innervated by sensory (afferent) and autonomic (efferent) nerves. The majority of both sensory and autonomic nerves in the airways are carried in the vagus nerves. These nerves provide key regulatory functions that are important in host-defense and respiratory homeostasis. Uniquely, among visceral organs is the fact that the airway mucosa, where most afferents terminate, is exposed to the ~10,000 L of air an adult breathes every day; air that includes a complex mixture of particles and substances that, depending on the environment, may be harmful and potentially lethal. This can set up conditions of over-activation of vagal afferent nerves, in particular vagal nociceptors. The consequence of their activation, including coughing, dyspnea, parasympathetic reflex bronchoconstriction and secretions, may contribute directly to airway diseases such as COPD, asthma and chronic cough.
References
- Abdulqawi R, Dockry R, Holt K et al. (2015). P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 385: 1198–1205. [DOI] [PubMed] [Google Scholar]
- Bai TR, Bramley AM (1993). Effect of an inhibitor of nitric oxide synthase on neural relaxation of human bronchi. Am J Physiol 264: L425–L430. [DOI] [PubMed] [Google Scholar]
- Baile EM, Osborne S, Paré PD (1987). Effect of autonomic blockade on tracheobronchial blood flow. J Appl Physiol (1985) 62: 520–525. [DOI] [PubMed] [Google Scholar]
- Baker DG, McDonald DM, Basbaum CB et al. (1986). The architecture of nerves and ganglia of the ferret trachea as revealed by acetylcholinesterase histochemistry. J Comp Neurol 246: 513–526. [DOI] [PubMed] [Google Scholar]
- Bałuk P, Gabella G (1989). Innervation of the guinea pig trachea: a quantitative morphological study of intrinsic neurons and extrinsic nerves. J Comp Neurol 285:117–132. [DOI] [PubMed] [Google Scholar]
- Baluk P, Nadel JA, McDonald DM (1992). Substance P-immunoreactive sensory axons in the rat respiratory tract: a quantitative study of their distribution and role in neurogenic inflammation. J Comp Neurol 319: 586–598. [DOI] [PubMed] [Google Scholar]
- Barnes PJ (1986). Neural control of human airways in health and disease. Am Rev Respir Dis 134: 1289–1314. [DOI] [PubMed] [Google Scholar]
- Barnes PJ (2004). Distribution of receptor targets in the lung. Proc Am Thorac Soc 1: 345–351. [DOI] [PubMed] [Google Scholar]
- Behrens R, McGovern AE, Farrell MJ et al. (2021). Mini review: central organization of airway afferent nerve circuits. Neurosci Lett 744: 135604. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Stretton CD, Yacoub M et al. (1992). Nitric oxide is the endogenous neurotransmitter of bronchodilator nerves in humans. Eur J Pharmacol 210: 221–222. [DOI] [PubMed] [Google Scholar]
- Bergren DR, Peterson DF (1993). Identification of vagal sensory receptors in the rat lung: are there subtypes of slowly adapting receptors? J Physiol 464: 681–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berisha HI, Bratut M, Bangale Y et al. (2002). New evidence for transmitter role of VIP in the airways: impaired relaxation by a catalytic antibody. Pulm Pharmacol Ther 15: 121–127. [DOI] [PubMed] [Google Scholar]
- Brouns I, Pintelon I, Timmermans JP et al. (2012). Novel insights in the neurochemistry and function of pulmonary sensory receptors. Adv Anat Embryol Cell Biol 211: 1–115, vii. [PubMed] [Google Scholar]
- Burki NK, Lee LY (2010). Mechanisms of dyspnea. Chest 138: 1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busto R, Prieto JC, Bodega G et al. (2000). Immunohistochemical localization and distribution of VIP/PACAP receptors in human lung. Peptides 21: 265–269. [DOI] [PubMed] [Google Scholar]
- Canning BJ (2006). Reflex regulation of airway smooth muscle tone. J Appl Physiol 101: 971–985. [DOI] [PubMed] [Google Scholar]
- Canning BJ (2008). The cough reflex in animals: relevance to human cough research. Lung 186: S23–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Chou YL (2009). Cough sensors. I. Physiological and pharmacological properties of the afferent nerves regulating cough. Handb Exp Pharmacol 187: 23–47. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Mori N (2010). An essential component to brainstem cough gating identified in anesthetized guinea pigs. FASEB J 24: 3916–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Undem BJ (1993a). Evidence that distinct neural pathways mediate parasympathetic contractions and relaxations of guinea-pig trachealis. J Physiol 471: 25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Undem BJ (1993b). Relaxant innervation of the guinea-pig trachealis: demonstration of capsaicin-sensitive and -insensitive vagal pathways. J Physiol 460: 719–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Reynolds SM, Mazzone SB (2001). Multiple mechanisms of reflex bronchospasm in guinea pigs. J Appl Physiol (1985) 91: 2642–2653. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Mazzone SB, Meeker SN et al. (2004). Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 557: 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Farmer DG, Mori N (2006). Mechanistic studies of acid-evoked coughing in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol 291: R454–R463. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Chang AB, Bolser DC et al. (2014). Anatomy and neurophysiology of cough: CHEST guideline and expert panel report. Chest 146: 1633–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MJ, Kollarik M, Meeker SN et al. (2003). A role for TRPV1 in bradykinin-induced excitation of vagal airway afferent nerve terminals. J Pharmacol Exp Ther 304: 1275–1279. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M et al. (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824. [DOI] [PubMed] [Google Scholar]
- Chang RB, Strochlic DE, Williams EK et al. (2015). Vagal sensory neuron subtypes that differentially control breathing. Cell 161: 622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Joad JP, Bric J et al. (2009). Central mechanisms I: plasticity of central pathways. Handb Exp Pharmacol 187–201. [DOI] [PubMed] [Google Scholar]
- Chou YL, Scarupa MD, Mori N et al. (2008). Differential effects of airway afferent nerve subtypes on cough and respiration in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol 295: R1572–R1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YL, Mori N, Canning BJ (2018). Opposing effects of bronchopulmonary C-fiber subtypes on cough in guinea pigs. Am J Physiol Regul Integr Comp Physiol 314: R489–r498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuaychoo B, Hunter DD, Myers AC et al. (2005a). Allergen-induced substance P synthesis in large-diameter sensory neurons innervating the lungs. J Allergy Clin Immunol 116: 325–331. [DOI] [PubMed] [Google Scholar]
- Chuaychoo B, Lee MG, Kollarik M et al. (2005b). Effect of 5-hydroxytryptamine on vagal C-fiber subtypes in guinea pig lungs. Pulm Pharmacol Ther 18: 269–276. [DOI] [PubMed] [Google Scholar]
- Chuaychoo B, Lee MG, Kollarik M et al. (2006). Evidence for both adenosine A1 and A2A receptors activating single vagal sensory C-fibres in guinea pig lungs. J Physiol 575: 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleridge JC, Coleridge HM (1984). Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99: 1–110. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JC (1994). Neural regulation of bronchial blood flow. Respir Physiol 98: 1–13. [DOI] [PubMed] [Google Scholar]
- Davis B, Roberts AM, Coleridge HM et al. (1982). Reflex tracheal gland secretion evoked by stimulation of bronchial C-fibers in dogs. J Appl Physiol Respir Environ Exerc Physiol 53: 985–991. [DOI] [PubMed] [Google Scholar]
- Dey RD, Altemus JB, Zervos I et al. (1990). Origin and colocalization of CGRP- and SP-reactive nerves in cat airway epithelium. J Appl Physiol (1985) 68: 770–778. [DOI] [PubMed] [Google Scholar]
- Dey RD, Altemus JB, Rodd A et al. (1996). Neurochemical characterization of intrinsic neurons in ferret tracheal plexus. Am J Respir Cell Mol Biol 14: 207–216. [DOI] [PubMed] [Google Scholar]
- Dey RD, Satterfield B, Altemus JB (1999). Innervation of tracheal epithelium and smooth muscle by neurons in airway ganglia. Anat Rec 254: 166–172. [DOI] [PubMed] [Google Scholar]
- Dinh QT, Groneberg DA, Peiser C et al. (2004). Substance P expression in TRPV1 and trkA-positive dorsal root ganglion neurons innervating the mouse lung. Respir Physiol Neurobiol 144: 15–24. [DOI] [PubMed] [Google Scholar]
- Drake MG, Scott GD, Blum ED et al. (2018). Eosinophils increase airway sensory nerve density in mice and in human asthma. Sci Transl Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen AK, Farrell MJ, Mazzone SB et al. (2015). The role of the paratrigeminal nucleus in vagal afferent evoked respiratory reflexes: a neuroanatomical and functional study in Guinea pigs. Front Physiol 6: 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen AK, McGovern AE, Behrens R et al. (2020). A role for neurokinin 1 receptor expressing neurons in the paratrigeminal nucleus in bradykinin-evoked cough in guinea-pigs. J Physiol 598: 2257–2275. [DOI] [PubMed] [Google Scholar]
- Ellis JL, Undem BJ (1990). Non-adrenergic, non-cholinergic contractions in the electrically field stimulated guinea-pig trachea. Br J Pharmacol 101: 875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JL, Undem BJ (1992). Inhibition by L-NG-nitro-L-arginine of nonadrenergic-noncholinergic-mediated relaxations of human isolated central and peripheral airway. Am Rev Respir Dis 146: 1543–1547. [DOI] [PubMed] [Google Scholar]
- Ellis JL, Undem BJ (1994). Pharmacology of non-adrenergic, non-cholinergic nerves in airway smooth muscle. Pulm Pharmacol 7: 205–223. [DOI] [PubMed] [Google Scholar]
- Ellis JL, Undem BJ, Kays JS et al. (1993). Pharmacological examination of receptors mediating contractile responses to tachykinins in airways isolated from human, guinea pig and hamster. J Pharmacol Exp Ther 267: 95–101. [PubMed] [Google Scholar]
- Esplugues JV (2002). NO as a signalling molecule in the nervous system. Br J Pharmacol 135: 1079–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MJ, Bautista TG, Liang E et al. (2020). Evidence for multiple bulbar and higher brain circuits processing sensory inputs from the respiratory system in humans. J Physiol 598: 5771–5787. [DOI] [PubMed] [Google Scholar]
- Fontán JJ, Diec CT, Velloff CR (2000). Bilateral distribution of vagal motor and sensory nerve fibers in the rat’s lungs and airways. Am J Physiol Regul Integr Comp Physiol 279: R713–R728. [DOI] [PubMed] [Google Scholar]
- Ford AP, Dillon MP, Kitt MM et al. (2021). The discovery and development of gefapixant. Auton Neurosci 235: 102859. [DOI] [PubMed] [Google Scholar]
- Fung DC, Beacock DJ, Richardson PS (1992). Vagal control of mucus glycoconjugate secretion into the feline trachea. J Physiol 453: 435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gover TD, Moreira TH, Weinreich D (2009). Role of calcium in regulating primary sensory neuronal excitability. Handb Exp Pharmacol 563–587. [DOI] [PubMed] [Google Scholar]
- Groneberg DA, Rabe KF, Fischer A (2006). Novel concepts of neuropeptide-based drug therapy: vasoactive intestinal polypeptide and its receptors. Eur J Pharmacol 533: 182–194. [DOI] [PubMed] [Google Scholar]
- Hartman JE, Srikanthan K, Caneja C et al. (2021). Bronchoscopic targeted lung denervation in patients with severe asthma: preliminary findings. Respiration 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasaneen NA, Foda HD, Said SI (2003). Nitric oxide and vasoactive intestinal peptide as co-transmitters of airway smooth-muscle relaxation: analysis in neuronal nitric oxide synthase knockout mice. Chest 124: 1067–1072. [DOI] [PubMed] [Google Scholar]
- Haselton JR, Solomon IC, Motekaitis AM et al. (1992). Bronchomotor vagal preganglionic cell bodies in the dog: an anatomic and functional study. J Appl Physiol (1985) 73: 1122–1129. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Kc P, Moore CT et al. (2005). Brain stem excitatory and inhibitory signaling pathways regulating bronchoconstrictive responses. J Appl Physiol (1985) 98: 1961–1982. [DOI] [PubMed] [Google Scholar]
- Hayes JP, Kuo HP, Rohde JA et al. (1995). Neurogenic goblet cell secretion and bronchoconstriction in guinea pigs sensitised to trimellitic anhydride. Eur J Pharmacol 292: 127–134. [DOI] [PubMed] [Google Scholar]
- Hennessy E, White S, van der Touw T et al. (1993). Control of resting bronchial hemodynamics in the awake dog. Am J Physiol 265: H649–H660. [DOI] [PubMed] [Google Scholar]
- Ho CY, Gu Q, Lin YS et al. (2001). Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol 127: 113–124. [DOI] [PubMed] [Google Scholar]
- Honjin R (1956). On the nerve supply of the lung of the mouse, with special reference to the structure of the peripheral vegetative nervous system. J Comp Neurol 105: 587–625. [DOI] [PubMed] [Google Scholar]
- Hooper JS, Hadley SH, Morris KF et al. (2016). Characterization of cardiovascular reflexes evoked by airway stimulation with allylisothiocyanate, capsaicin, and ATP in Sprague-Dawley rats. J Appl Physiol (1985) 120: 580–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger B, Rodbard S (1960). Direct measurement of bronchial arterial flow. Circ Res 8: 1149–1156. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Lin YS, Lin RL et al. (2017). Immediate and delayed potentiating effects of tumor necrosis factor-α on TRPV1 sensitivity of rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 313: L293–l304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CC, Ruan T, Lee LY et al. (2019). Stimulatory effect of 5-hydroxytryptamine (5-HT) on rat capsaicin-sensitive lung vagal sensory neurons via activation of 5-HT(3) receptors. Front Physiol 10: 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson HG, Chinn RA, Chow AW et al. (1983). Leukotriene-C4 enhances mucus production from submucosal glands in canine trachea in vivo. Int J Immunopharmacol 5: 391–396. [DOI] [PubMed] [Google Scholar]
- Kajekar R, Rohde HK, Myers AC (2001). The integrative membrane properties of human bronchial parasympathetic Ganglia neurons. Am J Respir Crit Care Med 164: 1927–1932. [DOI] [PubMed] [Google Scholar]
- Kajekar R, Undem BJ, Myers AC (2003). Role of cyclooxy-genase activation and prostaglandins in antigen-induced excitability changes of bronchial parasympathetic ganglia neurons. Am J Physiol Lung Cell Mol Physiol 284: L581–L587. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mesulam MM (1980). Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol 193: 435–465. [DOI] [PubMed] [Google Scholar]
- Kalia M, Sullivan JM (1982). Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol 211: 248–265. [DOI] [PubMed] [Google Scholar]
- Karlsson JA, Sant’Ambrogio G, Widdicombe J (1988). Afferent neural pathways in cough and reflex bronchoconstriction. J Appl Physiol 65: 1007–1023. [DOI] [PubMed] [Google Scholar]
- Kesler BS, Canning BJ (1999). Regulation of baseline cholinergic tone in guinea-pig airway smooth muscle. J Physiol 518: 843–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Bahia PK, Patil M et al. (2020a). Development of a mouse reporter strain for the purinergic P2X(2) receptor. eNeuro 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Hadley SH, Maddison M et al. (2020b). Mapping of sensory nerve subsets within the vagal ganglia and the brainstem using reporter mice for Pirt, TRPV1, 5HT3 and Tac1 expression. eNeuro 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocmalova M, Kollarik M, Canning BJ et al. (2017). Control of neurotransmission by NaV1.7 in human, Guineapig, and mouse airway parasympathetic nerves. J Pharmacol Exp Ther 361: 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollarik M, Dinh QT, Fischer A et al. (2003). Capsaicin-sensitive and -insensitive vagal bronchopulmonary C-fibres in the mouse. J Physiol 551: 869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollarik M, Sun H, Herbstsomer RA et al. (2018). Different role of TTX-sensitive voltage-gated sodium channel (NaV 1) subtypes in action potential initiation and conduction in vagal airway nociceptors. J Physiol 596: 1419–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollarik M, Ru F, Pavelkova N et al. (2021). Role of Na(V) 1.7 in action potential conduction along human bronchial vagal afferent C-fibres. Br J Pharmacol 179: 242–251. [DOI] [PubMed] [Google Scholar]
- Krasteva G, Canning BJ, Hartmann P et al. (2011). Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci U S A 108: 9478–9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ et al. (2006). Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol 101: 618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer W, Fischer A, Kurkowski R et al. (1992). The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience 49: 715–737. [DOI] [PubMed] [Google Scholar]
- Kwong K, Lee LY (2005). Prostaglandin E2 potentiates a TTX-resistant sodium current in rat capsaicin-sensitive vagal pulmonary sensory neurones. J Physiol 564: 437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong K, Carr MJ, Gibbard A et al. (2008a). Voltage-gated sodium channels in nociceptive versus non-nociceptive nodose vagal sensory neurons innervating guinea pig lungs. J Physiol 586: 1321–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong K, Kollarik M, Nassenstein C et al. (2008b). P2X2 receptors differentiate placodal vs neural crest C-fiber phenotypes innervating Guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol 295: L858–L865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen LA, Laitinen MA, Widdicombe JG (1987a). Dose-related effects of pharmacological mediators on tracheal vascular resistance in dogs. Br J Pharmacol 92: 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen LA, Laitinen MV, Widdicombe JG (1987b). Parasympathetic nervous control of tracheal vascular resistance in the dog. J Physiol 385: 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Macglashan DW Jr, Undem BJ (2005). Role of chloride channels in bradykinin-induced guinea pig airway vagal C-fibre activation. J Physiol 566: 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CG, Rand MJ (1991). Evidence that part of the NANC relaxant response of guinea-pig trachea to electrical field stimulation is mediated by nitric oxide. Br J Pharmacol 102: 91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Ezure K, Wong She RB (1991). Identification of neurons receiving input from pulmonary rapidly adapting receptors in the cat. J Physiol 443: 55–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC, Patel HJ, Khawaja AM et al. (1999). Neuroregulation by vasoactive intestinal peptide (VIP) of mucus secretion in ferret trachea: activation of BK(Ca) channels and inhibition of neurotransmitter release. Br J Pharmacol 126: 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak JC, Barnes PJ (1990). Autoradiographic visualization of muscarinic receptor subtypes in human and guinea pig lung. Am Rev Respir Dis 141: 1559–1568. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Canning BJ (2002). Synergistic interactions between airway afferent nerve subtypes mediating reflex bronchospasm in guinea pigs. Am J Physiol Regul Integr Comp Physiol 283: R86–R98. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Canning BJ (2002). Synergistic interactions between airway afferent nerve subtypes mediating reflex bronchospasm in guinea pigs. Am J Physiol Regul Integr Comp Physiol 283: R86–R98. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Undem BJ (2016). Vagal afferent innervation of the airways in health and disease. Physiol Rev 96: 975–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone SB, Mori N, Canning BJ (2005). Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol 569: 559–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone SB, Reynolds SM, Mori N et al. (2009). Selective expression of a sodium pump isozyme by cough receptors and evidence for its essential role in regulating cough. J Neurosci 29: 13662–13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone SB, Tian L, Moe AAK et al. (2019). Transcriptional profiling of individual airway projecting vagal sensory neurons. Mol Neurobiol 57: 949–963. [DOI] [PubMed] [Google Scholar]
- McAlexander MA, Myers AC, Undem BJ (1999). Adaptation of guinea-pig vagal airway afferent neurones to mechanical stimulation. J Physiol 521: 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlexander MA, Gavett SH, Kollarik M et al. (2015). Vagotomy reverses established allergen-induced airway hyperreactivity to methacholine in the mouse. Respir Physiol Neurobiol 212–214: 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen RM, Spyer KM (1978). Two types of vagal preganglionic motoneurones projecting to the heart and lungs. J Physiol 282: 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern AE, Davis-Poynter N, Yang SK et al. (2015a). Evidence for multiple sensory circuits in the brain arising from the respiratory system: an anterograde viral tract tracing study in rodents. Brain Struct Funct 220: 3683–3699. [DOI] [PubMed] [Google Scholar]
- McGovern AE, Driessen AK, Simmons DG et al. (2015b). Distinct brainstem and forebrain circuits receiving tracheal sensory neuron inputs revealed using a novel conditional anterograde transsynaptic viral tracing system. J Neurosci 35: 7041–7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan EM (2003). Transmission of signals through sympathetic ganglia—modulation, integration or simply distribution? Acta Physiol Scand 177: 227–235. [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Herbert DA, Baker DG et al. (1987). In vivo activity of tracheal parasympathetic ganglion cells innervating tracheal smooth muscle. Brain Res 437: 157–160. [DOI] [PubMed] [Google Scholar]
- Muroi Y, Ru F, Kollarik M et al. (2011). Selective silencing of NaV1.7 decreases excitability and conduction in vagal sensory neurons. J Physiol 589: 5663–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroi Y, Ru F, Chou YL et al. (2013). Selective inhibition of vagal afferent nerve pathways regulating cough using Nav 1.7 shRNA silencing in guinea pig nodose ganglia. Am J Physiol Regul Integr Comp Physiol 304: R1017–R1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers AC, Undem BJ (1991). Analysis of preganglionic nerve evoked cholinergic contractions of the guinea pig bronchus. J Auton Nerv Syst 35: 175–184. [DOI] [PubMed] [Google Scholar]
- Myers AC, Undem BJ (1993). Electrophysiological effects of tachykinins and capsaicin on guinea-pig bronchial parasympathetic ganglion neurones. J Physiol 470: 665–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers AC, Undem BJ, Weinreich D (1990). Electrophysiological properties of neurons in guinea pig bronchial parasympathetic ganglia. Am J Physiol 259: L403–L409. [DOI] [PubMed] [Google Scholar]
- Myers AC, Undem BJ, Weinreich D (1991). Influence of antigen on membrane properties of guinea pig bronchial ganglion neurons. J Appl Physiol 71: 970–976. [DOI] [PubMed] [Google Scholar]
- Myers A, Undem B, Kummer W (1996). Anatomical and electrophysiological comparison of the sensory innervation of bronchial and tracheal parasympathetic ganglion neurons. J Auton Nerv Syst 61: 162–168. [DOI] [PubMed] [Google Scholar]
- Nassenstein C, Taylor-Clark TE, Myers AC et al. (2010). Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. J Physiol 588: 4769–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Doubell TP, Leslie T et al. (1996). Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature 384:360–364. [DOI] [PubMed] [Google Scholar]
- Niewoehner DE, Rice K, Cote C et al. (2005). Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med 143: 317–326. [DOI] [PubMed] [Google Scholar]
- Nonomura K, Woo SH, Chang RB et al. (2017). Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 541: 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh EJ, Mazzone SB, Canning BJ et al. (2006). Reflex regulation of airway sympathetic nerves in guinea-pigs. J Physiol 573: 549–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, McMahon SB, Undem BJ (2013). Chronic cough and pain: Janus faces in sensory neurobiology? Pulm Pharmacol Ther 26: 476–485. [DOI] [PubMed] [Google Scholar]
- O’Rourke ST, Vanhoutte PM (1990). Adrenergic and cholinergic responsiveness of isolated canine bronchial arteries. Am J Physiol 259: H156–H161. [DOI] [PubMed] [Google Scholar]
- Otake K, Nakamura Y, Tanaka I et al. (2001). Morphology of pulmonary rapidly adapting receptor relay neurons in the rat. J Comp Neurol 430: 458–470. [DOI] [PubMed] [Google Scholar]
- Padda GS, Kishioka C, Rubin BK (2001). Propofol and methohexital have no significant effect on mucus secretion or clearance in the anesthetized dog. Crit Care Med 29: 1045–1048. [DOI] [PubMed] [Google Scholar]
- Palecek F, Sant’Ambrogio G, Sant’Ambrogio FB et al. (1989). Reflex responses to capsaicin: intravenous, aerosol, and intratracheal administration. J Appl Physiol 67: 1428–1437. [DOI] [PubMed] [Google Scholar]
- Patil MJ, Ru F, Sun H et al. (2020). Acute activation of bronchopulmonary vagal nociceptors by type I interferons. J Physiol 598: 5541–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peatfield AC, Richardson PS (1983). Evidence for non-cholinergic, non-adrenergic nervous control of mucus secretion into the cat trachea. J Physiol 342: 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez Fontán JJ, Velloff CR (2001). Labeling of vagal motoneurons and central afferents after injection of cholera toxin B into the airway lumen. Am J Physiol Lung Cell Mol Physiol 280: L152–L164. [DOI] [PubMed] [Google Scholar]
- Peters SP, Kunselman SJ, Icitovic N et al. (2010). Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med 363: 1715–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips EW, Scott WJM (1929). The surgical treatment of bronchial asthma. Arch Surg 19: 1425–1456. [Google Scholar]
- Pincus AB, Fryer AD, Jacoby DB (2021). Mini review: neural mechanisms underlying airway hyperresponsiveness. Neurosci Lett 751: 135795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisarri TE, Zimmerman MP, Adrian TE et al. (1999). Bronchial vasodilator pathways in the vagus nerve of dogs. J Appl Physiol (1985) 86: 105–113. [DOI] [PubMed] [Google Scholar]
- Ricco MM, Kummer W, Biglari B et al. (1996). Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol 496: 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rienhoff WF Jr, Gay LN (1938). Treatment of intractable bronchial asthma by bilateral resection of the posterior pulmonary plexus. Arch Surg 37: 456–469. [Google Scholar]
- Roffel AF, Meurs H, Zaagsma J (1994). Muscarinic acetylcholine receptors and control of smooth muscle tone. Trends Pharmacol Sci 15: 407–408. [DOI] [PubMed] [Google Scholar]
- Rogers DF (1995). Neurokinin receptors subserving airways secretion. Can J Physiol Pharmacol 73: 932–939. [DOI] [PubMed] [Google Scholar]
- Rogers DF (2001). Motor control of airway goblet cells and glands. Respir Physiol 125: 129–144. [DOI] [PubMed] [Google Scholar]
- Salter HH (1864). On asthma: its pathology and treatment, Philedelphia, Blanchard & Lea. [Google Scholar]
- Satia I, Badri H, Al-Sheklly B et al. (2016). Towards understanding and managing chronic cough. Clin Med (Lond) 16: s92–s97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelegle ES, Green JF (2001). An overview of the anatomy and physiology of slowly adapting pulmonary stretch receptors. Respir Physiol 125: 17–31. [DOI] [PubMed] [Google Scholar]
- Schild JH, Alfrey KD, Li BY (2005). Voltage-gated ion channels in vagal afferent neurons. In: Undem BJ, Weinreich D (Eds.), Advances in vagal afferent neurobiology. CRC Press. [Google Scholar]
- Schmidt DT, Rühlmann E, Waldeck B et al. (2001). The effect of the vasoactive intestinal polypeptide agonist Ro 25–1553 on induced tone in isolated human airways and pulmonary artery. Naunyn Schmiedebergs Arch Pharmacol 364: 314–320. [DOI] [PubMed] [Google Scholar]
- Schultz HD, Roberts AM, Bratcher C et al. (1985). Pulmonary C-fibers reflexly increase secretion by tracheal submucosal glands in dogs. J Appl Physiol (1985) 58: 907–910. [DOI] [PubMed] [Google Scholar]
- Shanks J, Xia Z, Lisco SJ et al. (2018). Sympatho-excitatory response to pulmonary chemosensitive spinal afferent activation in anesthetized, vagotomized rats. Physiol Rep 6: e13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro CO, Proskocil BJ, Oppegard LJ et al. (2021). Airway sensory nerve density is increased in chronic cough. Am J Respir Crit Care Med 203: 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington C (1906). The integrative action of the nervous system. Yale University Press, New Haven. [Google Scholar]
- Singh N, Driessen AK, McGovern AE et al. (2020). Peripheral and central mechanisms of cough hypersensitivity. J Thorac Dis 12: 5179–5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slebos DJ, Degano B, Valipour A et al. (2020). Design for a multicenter, randomized, sham-controlled study to evaluate safety and efficacy after treatment with the Nuvaira® lung denervation system in subjects with chronic obstructive pulmonary disease (AIRFLOW-3). BMC Pulm Med 20: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Barr J, Jaquish A et al. (2021). Identification of lung innervating sensory neurons and their target specificity. Am J Physiol Lung Cell Mol Physiol 322: L50–L63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H (2021). Different sensitivity of action potential generation to the rate of depolarization in vagal afferent A-fiber versus C-fiber neurons. J Neurophysiol 125: 2000–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Lin AH, Ru F et al. (2019). KCNQ/M-channels regulate mouse vagal bronchopulmonary C-fiber excitability and cough sensitivity. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarek JL, Zhang JZ, Gruetter CA (1995). Mechanisms of 5-hydroxytryptamine-induced contraction of isolated rat intrapulmonary bronchi. Pulm Pharmacol 8: 273–281. [DOI] [PubMed] [Google Scholar]
- Szefler S, Weiss S, Tonascia J et al. (2000). Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 343: 1054–1063. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE (2015). Peripheral neural circuitry in cough. Curr Opin Pharmacol 22: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE (2021). Molecular identity, anatomy, gene expression and function of neural crest vs. placode-derived nociceptors in the lower airways. Neurosci Lett 742:135505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama K, Kuo HP, Rohde JA et al. (1990). Neural control of goblet cell secretion in guinea pig airways. Am J Physiol 259: L108–L115. [DOI] [PubMed] [Google Scholar]
- Trankner D, Hahne N, Sugino K et al. (2014). Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci U S A 111: 11515–11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki I, German VF, Nadel JA (1980). Micropipette measurement of airway submucosal gland secretion. Autonomic effects. Am Rev Respir Dis 121: 351–357. [DOI] [PubMed] [Google Scholar]
- Ullah MI, Newman GB, Saunders KB (1981). Influence of age on response to ipratropium and salbutamol in asthma. Thorax 36: 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undem BJ, Potenzieri C (2012). Autonomic neural control of intrathoracic airways. Compr Physiol 2: 1241–1267. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Sun H (2020). Molecular/ionic basis of vagal bronchopulmonary C-fiber activation by inflammatory mediators. Physiology (Bethesda) 35: 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undem BJ, Myers AC, Barthlow H et al. (1990). Vagal innervation of guinea pig bronchial smooth muscle. J Appl Physiol 69: 1336–1346. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Myers AC, Weinreich D (1991). Antigen-induced modulation of autonomic and sensory neurons in vitro. Int Arch Allergy Appl Immunol 94: 319–324. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Hubbard W, Weinreich D (1993). Immunologically induced neuromodulation of guinea pig nodose ganglion neurons. J Auton Nerv Syst 44: 35–44. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Chuaychoo B, Lee MG et al. (2004). Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol 556: 905–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Kollarik M, Ru F et al. (2017). Distinct and common expression of receptors for inflammatory mediators in vagal nodose versus jugular capsaicin-sensitive/TRPV1-positive neurons detected by low input RNA sequencing. PLoS One 12: e0185985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Horie S, Michael GJ et al. (2005). Immunohistochemical localization of vanilloid receptor subtype 1 (TRPV1) in the guinea pig respiratory system. Pulm Pharmacol Ther 18: 187–197. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Horie S, Michael GJ et al. (2006). Immunohistochemical co-localization of transient receptor potential vanilloid (TRPV)1 and sensory neuropeptides in the guinea-pig respiratory system. Neuroscience 141: 1533–1543. [DOI] [PubMed] [Google Scholar]
- Watson N, Magnussen H, Rabe KF (1995). Pharmacological characterization of the muscarinic receptor subtype mediating contraction of human peripheral airways. J Pharmacol Exp Ther 274: 1293–1297. [PubMed] [Google Scholar]
- Widdicombe JG (1954). Respiratory reflexes excited by inflation of the lungs. J Physiol 123: 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe JG (1966). Action potentials in parasympathetic and sympathetic efferent fibres to the trachea and lungs of dogs and cats. J Physiol 186: 56–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe J (1993). The airway vasculature. Exp Physiol 78: 433–452. [DOI] [PubMed] [Google Scholar]
- Widdicombe JG, Nadel JA (1963). Reflex effects of lung inflation on tracheal volume. J Appl Physiol 18: 681–686. [DOI] [PubMed] [Google Scholar]
- Williams BJ, Drummond WH (1983). The effect of alpha-adrenergic blockade on the pulmonary vascular response to dopamine in neonatal lambs. Pediatr Res 17: 464–467. [DOI] [PubMed] [Google Scholar]
- Zaccone EJ, Undem BJ (2016). Airway vagal neuroplasticity associated with respiratory viral infections. Lung 194: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Lin RL, Wiggers M et al. (2008). Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. J Physiol 586: 5771–5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman MP, Pisarri TE (2000). Bronchial vasodilation evoked by increased lower airway osmolarity in dogs. J Appl Physiol (1985) 88: 425–432. [DOI] [PubMed] [Google Scholar]


