Abstract

The preparation method of hydrogels has a significant effect on their structural and physicochemical properties. In this report, physically and chemically cross-linked poly(vinyl alcohol) (PVA) networks containing humic acid (HA) were alternatively prepared by autoclaving (AC) and through glutaraldehyde (GA) addition, respectively, for agricultural purposes. PVA/HA hydrogels were comparatively characterized by Fourier transform infrared spectroscopy, thermogravimetric analysis, mechanical assays, scanning electron microscopy, swelling kinetics measurements, and water retention tests in soil. AC hydrogels showed a more homogeneous porous microstructure, higher swelling levels, and a better capacity to preserve the humidity of soil than those obtained by adding GA. Both PVA/HA hydrogels exhibited no phytotoxicity on cultivation trials of Sorghum sp., but the plant growth was promoted with the GA-cross-linked network as compared to the effect of the AC sample. The release behavior of urea was modified according to the preparation method of the PVA/HA hydrogels. After 3 days of sustained urea release, 91% of the fertilizer was delivered from the AC hydrogel, whereas a lower amount of 56% was released for the GA-cross-linked hydrogel. Beyond the advantages of applying PVA/HA hydrogels in the agricultural field, an appropriate method of preparing these materials endows them with specific properties according to the requirements of the target crop.
Introduction
Hydrogels are three-dimensional (3D) hydrophilic polymer networks, which can be formed by physical or chemical cross-linking methods.1 These materials are capable of absorbing and retaining large amounts of water without being solubilized, as well as their polymer networks can expand or contract according to their environmental conditions.2 Hydrogels have been extensively applied in controlled drug delivery,3 tissue engineering,4 biosensors,5 and agriculture,6 among other fields. The physicochemical properties of hydrogels depend on the inherent characteristics of polymer matrixes, proportion of monomers or polymers, their cross-linking nature, and the experimental setup for their preparation.7
In the agricultural area, hydrogels have been intended to retain water in crops, for moisture conservation, to mitigate plant drought stress, in seed coating, and for the controlled release of fertilizers. The porous structure of these materials promotes the availability of oxygen and water in the root system, stimulating the physiological parameters of the plant and promoting its growth.8−10
Poly(vinyl alcohol) (PVA) is a candidate for plant growth substrate uses.11 PVA-based hydrogels have been widely developed owing to the feasible tune of their preparation methods, low toxicity, high water absorption, and mechanical stability.7 Typically, PVA hydrogels have been prepared by physical cross-linking, through a series of freeze–thaw cycles and anneal-swell, or by adding chemical cross-linking agents, such as bifunctional aldehydes.7,12−14
The preparation method modulates the physicochemical properties of PVA hydrogels and their behavior in the agricultural applications.15,16 For example, Sarkar and Sen synthesized physically cross-linked PVA hydrogels with poly(ethylene glycol) and sodium sulfate in the presence of urea.17 The hydrogel showed a sustained release of urea and a high absorption of Fe3+ ions from soil. Hakim et al.18 prepared chemically cross-linked PVA hydrogels containing a nanoclay filler and loading with a potassium phosphate fertilizer. They reported that the addition of the nanoclay improved the dispersion of potassium phosphate in the PVA matrix, controlling the rate and amount of fertilizer release.
Humic acid (HA) macromolecules have been used in agricultural fields as the nutrient and promoter of plant growth.19 This material is formed by macromolecules derived from humic substances, which are organic matter distributed in the soil, natural water, and sediments, resulting from the decomposition of plants and natural residues.20 Due to their amphiphilic character, HA molecules form micelle-like structures under neutral to acidic conditions, which are useful in agriculture,21 pollution remediation,22 medicine,23 and the pharmaceutical industry.24 HA molecules have undefined compositions that vary according to their origin and production process. The typical composition of HA includes high proportions of ionizable phenol and carboxyl groups, containing also quinones as well as sugar and peptide residues.20 Some reports have mentioned that the addition of HA to hydrogels can beneficially modulate their mechanical behavior.25,26
Combining PVA frameworks with HA molecules seems to be a convenient alternative to form a multifunctional material for agricultural purposes. However, few studies have dealt with this approach. A previous report showed the synthesis of PVA-based supramolecular hydrogels containing HA nanoparticles extracted from leonardite using a cyclic freeze–thaw method. In this study, it was shown that HA nanoparticles physically interact with PVA chains and interconnect polymer chains as a functional cross-linker.13
Considering the potential of the PVA/HA combination as precursors of biomaterials with advantages for the agricultural area, this work presents the preparation of HA-containing PVA hydrogels by alternative processes of autoclaving (AC) and glutaraldehyde (GA) addition. The hydrogels were comparatively characterized by Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), compression test, scanning electron microscopy (SEM), swelling kinetic measurements, and water retention evaluation in soil. The effect of the different PVA/HA hydrogels on the growth and physiological traits of sorghum plants was also evaluated. Finally, the release kinetics of urea from the hydrogels were evaluated in a soil aqueous extract. Urea is a common fertilizer that is typically lost due to volatilization and leaching, increasing the eutrophication of aqueous environments.27 The absence of phytotoxicity of PVA/HA hydrogels and their sustained urea release profiles evidenced the potential of the materials for agricultural applications.
Materials and Methods
Materials
PVA (Mw 85,000–124,000, 99% hydrolyzed); HA sodium salt, technical grade; acetic acid, 99.7%; urea, 98%, and GA solution grade II, 25% in H2O, were purchased from Sigma-Aldrich. All reagents were of analytical grade and used as received without further purification. The aqueous solutions were prepared with deionized water and purified by a Milli-Q Organex system (Millipore, Molsheim, France).
Preparation of Hydrogels by the Autoclave Process
A PVA solution (5 wt %) was prepared by dissolving PVA in deionized water for 3 h at 85 °C. An amount of HA powder (9 or 12 wt % for AC9 or AC12, respectively) was dispersed in 2 mL of the PVA solution. The pH of the mixture solution was adjusted to 4.0 using acetic acid. Then, the solution was poured into cylindrical molds and placed in a vertical autoclave (CVQ-B50L) at 120 °C and 0.12 MPa for 90 min.28 Subsequently, the solution was allowed to cool at room temperature and placed at −12 °C for 12 h. Finally, hydrogels AC9 and AC12 were removed from the mold, washed with deionized water, and dried by lyophilization in a Labconco FreeZone freeze-dryer of 4.5 L.
Preparation of Hydrogels by Adding Glutaraldehyde
To prepare the GA cross-linked hydrogels, an amount of HA powder (9 or 12 wt % for GA9 or GA12, respectively) was dispersed in 2 mL of a 5 wt % PVA solution. The pH of the mixture solution was adjusted to 4.0 using acetic acid, followed by the addition of 50 μL of GA with further stirring. The resultant suspension was poured into cylindrical molds and allowed to cure at room temperature for 24 h.14 The hydrogels GA9 and GA12 were removed from molds, washed with deionized water, and dried by lyophilization.
Table 1 summarizes the component portions used for the preparation of each hydrogel type. The hydrogel code numeral indicates the HA wt % in dried samples. Figure 1 illustrates the preparation methods for PVA/HA hydrogels.
Table 1. Feed Compositions in the Preparation of Hydrogels.
| sample | PVA 5 wt % (mL) | HA (mg) | GA (μL) |
|---|---|---|---|
| AC9 | 2 | 9.89 | |
| AC12 | 2 | 13.63 | |
| GA9 | 2 | 9.89 | 50 |
| GA12 | 2 | 13.63 | 50 |
Figure 1.

PVA/HA hydrogel preparation methods.
Hydrogel Characterization
FTIR spectra were recorded in a Frontier spectrometer (PerkinElmer, Beaconsfield, UK) by the KBr pellet technique in the range of 4000–500 cm–1. The spectra of commercial reagents were also included to identify their inherent peaks in the hydrogel spectra.
TGA experiments were carried out under a nitrogen flow until 800 °C and a heating rate of 10 °C min–1 using a Pyris 1 apparatus (PerkinElmer, Llantrisant, UK).
Mechanical properties of hydrated hydrogels were evaluated in compression tests using a TA ElectroForce 5500 BioDynamic equipment with a 200 N load cell. Cylindrical-shaped hydrogels of 10 mm diameter × 7 mm height were cyclic-loaded 5 times up to 50% deformation at a constant strain rate of 3.5 mm s–1.29 GA and AC hydrogels without adding HA were prepared and labeled as GA0 and AC0, respectively.
SEM was used to study the internal morphology of the hydrogel samples. The analyses were performed using a scanning electron microscope model JEOL JSM-5410LV (JEOL-LTD, Tokyo, Japan) operated with an acceleration voltage of 15 kV. Cross-sectional samples were frozen and lyophilized. Then, the dried samples were fixed on carbon ribbon and gold sputtered prior to SEM examination.
Swollen Polymer Network Modeling Calculations
Thermodynamic equations were used to estimate structural parameters of the GA hydrogels. A density kit was 3D-printed using poly(lactic acid) on a Dremel 3D40 printer (3PI Tech Solutions, Illinois, USA).30 Polymer volume fractions in swollen (ϕs) and relaxed (ϕr) states were calculated by the following equations:
where Wa,d is the hydrogel weight in the dry state in air, Wh,d is the weight in the dry state in heptane, Wa,s is the weight in the swollen state in air, Wh,s is the weight in the swollen state in heptane, Wa,r is the weight in the relaxed state in air, and Wh,r is the weight in the relaxed state in heptane.
The effective molecular weight between cross-links (M̅c) was determined by the Bray–Peppas–Merrill modified equation of Flory–Rehner as follows31,32:
 |
The frequency of chain-end defects (γ) was also calculated according to the following equation30:
The shear modulus (G) was calculated according to the rubberlike elasticity theory using the following equation30,33:
The hydrogel mesh size (ξ), which defines the linear distance between consecutive cross-links, was calculated using a modified Canal–Peppas equation34:
The parameters used for modeling calculations are provided in Table S1.
Swelling Properties
The swelling capacity of hydrogels was evaluated in deionized water and in a soil aqueous extract (pH 7.05, EC = 239 μS cm–1) by the gravimetric method. The soil extract was prepared according to Durpekova et al.35 Commercial garden soil (Happy Flower-prepared potting soil mix) was autoclaved at 120 °C and 0.12 MPa for 40 min. Then, 20 g of soil was added to 1 L of deionized water, and the resultant suspension was stirred for 24 h. Afterward, the suspension was centrifuged at 3540 rpm for 10 min. The supernatant was used in the swelling experiments.
Freeze-dried samples of known weight (W0) were immersed in aqueous media. At specific times (t), the samples were removed from the swelling medium, blotted, weighed (Wt), and placed in the same bath until a constant weight was reached. The swelling percent at time t was calculated from the following relation:
Data of swelling kinetics were fitted to the Korsmeyer–Peppas model to elucidate the mechanism of swelling in the hydrogels:
In this equation, Mt/M∞ represents the fractional uptake of solvent normalized with respect to the equilibrium conditions. The variables k and n are constants which can be related to diffusion coefficients and the specific transport mechanism.36
Water Retention Capacity
The ability of hydrogels to retain water in soil was assessed by measuring the water evaporation ratio (WER). Commercial garden soil was dried at 60 °C for 48 h in a mechanical convection laboratory incubator (Thermo Scientific Precision 3511, USA). The dried hydrogel samples were buried in 20 g (W0) of the dried soil in a plastic pot. Pure soil was used as control. Then, 20 mL of deionized water was added to each pot, and their weights (W1) were monitored. The samples were stored at room temperature and their weights monitored at different times (Wt) until no detectable weight loss was observed.35,37 The WER percent at time t was calculated from the following relation:
Effect of Hydrogels on Plant Growth
A phytotoxicity test was carried out to evaluate the effect of the hydrogel on growth and physiological traits of plants. The test was performed according to the methodology reported by Montesano et al.6 Seeds of sorghum (Sorghum sp.) were placed in conical centrifuge tubes with common tissue paper or with hydrogel samples. The paper was moistened with deionized water (control), whereas hydrogel samples were saturated with deionized water. The tubes were maintained at 27 ± 1 °C and a photoperiod of 12 h light using a plant growth chamber (Percival Scientific model CU36L4, Iowa, USA). Plant growth was observed after 3, 5, and 7 days. An amount of 30 replications (tubes) was performed per each treatment.
After the growth observation time, the seedlings were cut crosswise, separating the aerial part from the roots. Then, both parts were dried in a mechanical convection laboratory incubator (Thermo Scientific Precision 3511, USA) for 48 h at 60 °C. Once dry, samples were weighed, and the average dry weight of the aerial part and the roots is herein reported. For comparative purposes, analysis of variance (ANOVA) was carried out with an acceptable level of significance of P < 0.05 using the statistical package Minitab19.
Loading and Controlled Release of the Fertilizer from the Hydrogel
The loading of urea in the hydrogels was carried out by the swelling equilibrium method. Freeze-dried samples were loaded by sorption in 0.5 mL of an aqueous solution of urea (0.1 M) for 48 h followed by lyophilization.37 The urea concentration was quantified with an enzymatic kit (Randox Laboratories Limited, Crumlin, UK), measuring the absorbance at 685 nm in an UV–Vis spectrophotometer, model 8453 (Agilent Technologies, Shangai, China).
The urea release kinetics were obtained in the soil aqueous extract (pH 7.05, EC 239 μS cm–1). Urea-loaded hydrogels were immersed in 30 mL of the release medium at a controlled temperature of 25 °C using a shaking water bath model LSB-030s (LabTech, Gyeonggi-do, Korea) with continuous orbital stirring of 60 rpm. Aliquots of 20 μL were withdrawn at specific time intervals and replaced with equal volumes of fresh medium.38,39 The urea concentration was quantified as previously mentioned.
Mathematical Modeling of Urea Release
Data obtained in the urea release studies were fitted to the Korsmeyer–Peppas model, as previously specified, to evaluate the mechanism of release of the chemical compound from the hydrogels. In this case, Mt and M∞ were the absolute cumulative amounts of urea released at time t and equilibrium, respectively; k was the release rate coefficient, and n was the diffusional exponent that can be related to the chemical compound transport mechanism.14,40
Results and Discussion
FTIR Spectroscopy
Figure 2a shows the FTIR spectra of the PVA/HA hydrogels and those of their neat PVA and HA components. Table 2 summarizes the assignments of FTIR bands of PVA and HA samples.
Figure 2.
FTIR spectra of PVA, HA, and composite hydrogels; full spectra from 4000 to 500 cm–1 (a) and spectral details from 1400 to 800 cm–1 (b).
Table 2. Assignment of the FTIR Bandsa.
| sample | wavenumber (cm–1) | functional groups |
|---|---|---|
| PVA | 3700–3180 | ν (O–H) |
| 2941 | ν (C–H) | |
| 1430 | δ (CH2) | |
| 1099 | ν (C–O) | |
| HA | 3385 | ν (O–H) |
| 2927 | ν (C–H) | |
| 1690 | ν (COO–) | |
| 1585 | ν (C=C) | |
| 1394 | ν (COO–) | |
| 1106 | ν (CO) | |
| 1035 | ν (C–N) |
Abbreviations: ν, stretching; δ, bending.
PVA spectrum shows the typical broadband attributed to the stretching vibration of the O–H bond in the 3700–3180 cm–1 region. The signals corresponding to the stretching vibration of the C–H bond are located around 2941 cm–1. The peak related to the wagging of CH2 group appears at 1430 cm–1, while the peak at 1099 cm–1 is attributed to the stretching vibration of the C–OH moiety.14
The HA spectrum shows a broadband at 3385 cm–1 attributed to the O–H vibrational stretching of the hydrogen-bonded carboxyl, alcohol, and phenol groups. The band at 2927 cm–1 is attributed to the asymmetric C–H stretching vibration of the methyl and/or methylene groups. A small shoulder at 1690 cm–1 is related to the COO– stretching vibration corresponding to carboxyl groups, and a band at 1585 cm–1 is due to the C=C vibrational stretching mode. The signals at 1394, 1106, and 1035 cm–1 are attributed to COO– moieties, C–O stretching vibration of phenolic groups, and C–N stretching vibration, respectively.13
Most of the absorptions of PVA and HA overlap in the spectra of composite hydrogels; however, some individual signals are also distinguished. It is noticed that the peak intensity ratio of O–H and C–O signals (IO–H/IC–O) was higher in PVA/HA spectra, with values of 1.011, 1.029, 1.015, and 1.017 for AC9, AC12, GA9, and GA12, respectively, than for the PVA sample (1.000).
In the case of hydrogels prepared by AC, individual peaks of HA moieties are observed, particularly those signals attributed to C=C (1572 cm–1) and COO– (1332–1326 cm–1) stretching vibrations. Furthermore, this band of carboxyl groups appeared at lower wavenumbers in AC hydrogel spectra with respect to that of the HA sample (1394 cm–1) indicating physical interactions between components, for example, hydrogen bonding between carboxyl groups of the HA and the hydroxyl side groups of the PVA chains.13 During the AC stage, the thermal and pressure conditions promoted the intermolecular contact between PVA and HA. Next, a self-supporting hydrogel was built by phase separation of the PVA/HA mixture under water-freezing conditions, leading to a polymer-rich phase in which the close interchain contact promoted intermolecular hydrogel bonding and crystallite formation. The crystalline regions remained intact after the sample was thawed at room temperature and a three-dimensional supramolecular network of PVA was formed in which the HA molecules were trapped by additional physical interactions between the active sites of both components.13,41
In the case of spectra of hydrogels formed by adding GA, the peak at 1120 cm–1 is attributed to the symmetric C–O–C stretching of the acetal ring resulting from the cross-linking reaction between PVA and GA14 (Figure 2b). Figure 3 illustrates the interactions between the components of the hydrogels prepared by both methods.
Figure 3.
Schematic representation of AC PVA/HA hydrogel (a) and GA PVA/HA hydrogel (b) formation.
TG Analysis
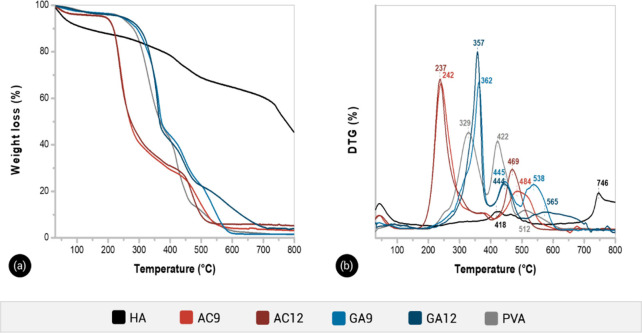
Figure 4a shows the thermograms of PVA, HA, and hydrogel samples of different compositions. Figure 4b shows the temperatures of the maximum rate of weight loss (Tmax) for each weight loss step for different materials.
Figure 4.
Thermogravimetric (a) and first derivative curves (b) for hydrogel composites and their individual components.
All samples lost mass at temperatures below 100 °C, which was associated with the evaporation of residual moisture. PVA exhibited a three-step degradation process (Tmax of 328.6, 421.9, and 512.0 °C) as previously reported.42 The first stage of degradation was related to the elimination reactions that occur in linear and aliphatic polymers, yielding polyene compounds through dehydration, including the degradation of the hydroxyl side groups. Since the degradation temperature of the first stage was not high enough to break all main-chain bonds, polyene intermediates were degraded into low-molecular-weight products in the next degradation step. The second thermal degradation step involved a complex set of reactions that include chain cleavage, side, and cyclization reactions as well as the continuous degradation of residual acetate groups due to the breakdown of the polymer backbone. The last stage of degradation was related to the decomposition of carbon and hydrocarbons residues formed during the second stage.43
The HA exhibited a multistep degradation process, showing a higher thermal stability than the PVA at high temperatures (i.e., more than 50% of HA mass is preserved after heating up to 800 °C). Different decomposition reactions can occur during HA heating according to its heterogeneous chemical composition. Thermal-induced reactions involved the decomposition of carboxyl, methyl, methylene, and alcohol groups, as well as the decomposition of carbohydrate units and oxidation/polycondensation of aromatic structures.44
Composite hydrogels showed degradation temperatures similar to those of their counterparts. The Tmax of the first step of weight loss of AC formed hydrogels shifted more than 100 °C to lower temperatures as compared to the neat PVA sample. The low thermal stability of AC hydrogels was straightly associated with the decrease of the molecular weight of PVA due to the AC conditions (120 °C, 120 kPa).45
Conversely, the main weight loss step of GA9 and GA12 samples occurred at Tmax of 357 and 362 °C, respectively, that was at least 28 °C higher than the Tmax of neat PVA (329 °C). The shift of the degradation peaks to higher temperatures for samples GA9 and GA12 indicated that the chemical cross-linking effectively improved the thermal stability of the hydrogels as compared to linear PVA and physically cross-linked samples. The acetal bridge between PVA chains increased the thermal requirements for scission reactions.
Mechanical Behavior of Hydrogels
All hydrogels exhibited J-shaped stress–strain behavior with high compliance at low strains and high strength at high strains. This behavior can be suitable for the mechanical adaptation of materials to the action of external forces, like those occurring in soil. At 50% deformation, none of the hydrogels showed fracture, and all samples recovered their original shape once the applied load was released (Figure S1).
The compressive stress–strain curves are displayed in Figure 5. For AC hydrogels, the compressive strength at 50% strain increased with an increase in the HA content in the hydrogel (Figure 5a). This results evidenced that HA molecules strengthened the supramolecular network of PVA by additional interactions with PVA moieties, increasing the interconnections of PVA chains.13
Figure 5.
Representative compressive stress–strain curves for AC (a) and GA hydrogels (b).
GA hydrogels exhibited higher strength values than the AC hydrogels in the whole strain range (Figure 5b). Moreover, the compressive strength of GA samples at 50% strain decreased with an increase in the HA content in hydrogel samples. It should be noticed that the maximum compressive strength of the GA12 hydrogel was 52% lower than that of the GA0 sample. It is suggested that the presence of HA molecules during the chemical formation of the 3D-network of PVA partially hindered the reaction between the GA cross-linker and the hydroxyl side groups of PVA chains.
SEM Analysis
Figure 6 shows SEM micrographs of cross sections of freeze-dried PVA/HA hydrogels at different magnifications. A porous structure in the form of a well-defined network is observed in all samples. This interconnected porous structure has been observed in chemically and physically cross-linked PVA hydrogels.13,14,16,46
Figure 6.
SEM micrographs of cross sections of AC9 (a,e), AC12 (b,f), GA9 (c,g), and GA12 (d,h) hydrogels at 500X (a–d) and 75X (e–h) magnifications.
For autoclaved hydrogels, the increase of HA content increased the pore dimensions (Figure 6a,b,e,f), indicating a reorganization of PVA phase by adding HA. During freezing, ice crystals were formed, leading to PVA and HA-rich domains.7,46 These results differ from those obtained by Sirousazar and Khodamoradi who found that by increasing the HA ratio in freeze-thawed PVA/HA hydrogels, their pore size was reduced.13Figure S2a,b shows the pore size distribution of AC9 and AC12 hydrogels, respectively.
The pore dimensions of GA-cross-linked PVA/HA hydrogels were larger than those of their analogous autoclaved hydrogels (Figure S2c,d). This effect was more noticeable in hydrogels containing 9 wt % HA (Figure 6c,d,g,h). Furthermore, GA-cross-linked PVA/HA hydrogels exhibited regions of different porous sizes, evidencing a less homogeneous porous microstructure as compared to autoclaved PVA/HA hydrogels. The nonuniform structures of chemical PVA hydrogels have been associated with the rapid kinetics of cross-linking reactions and the inhomogeneous dispersion of the cross-linker.7 The SEM results suggested that the preparation method of the PVA/HA hydrogels played a significant role in controlling the size and shape of their porous structure.
Evaluation of Hydrogel Structural Parameters
The poor thermal stability of the CA samples showed that the autoclave conditions strongly affected the molecular weight of the PVA chains; thus, the polymer network modeling calculations were carried out only for GA hydrogels.
Table 3 summarizes the results obtained from the swollen polymer network modeling calculations. The values of M̅c and mesh size indicated that the GA9 sample exhibited a lower distance between network junctions compared to that of the GA0 and GA12 samples. The mesh size of the GA12 sample (5.06 nm) was higher than that calculated for the GA9 network (3.98 nm); however, without adding HA, the mesh size of the PVA network was the highest (5.67 nm). The higher values of mesh sizes in the GA0 and GA12 samples compared to that of GA9 hydrogels may favor the loading of high molecular weight compounds within their network structures.47
Table 3. GA Hydrogel Formulation Structural Parameters at 25 °C.
| sample | ϕs | ϕr | M̅c (g mol–1) | γ | G (kPa) | ξ (nm) |
|---|---|---|---|---|---|---|
| GA0 | 0.092 | 0.058 | 1463.81 | 0.028 | 70.70 | 5.67 |
| GA9 | 0.067 | 0.027 | 585.71 | 0.011 | 97.15 | 3.98 |
| GA12 | 0.071 | 0.032 | 982.15 | 0.018 | 65.69 | 5.06 |
The theoretical shear modulus of GA hydrogels was determined to compare the relative behavior of samples with the experimental results obtained in compression mode. The theoretical shear modulus increased for the samples in the order: GA12, GA0, and GA9. Both theoretical and experimental results agreed that the GA12 hydrogel is the sample with the lowest stiffness. However, no correlation between the theoretical and experimental results was found for the GA0 and GA9 samples. The differences between theorical estimations and experimental results may be attributed to a nonideal cross-linking behavior.47
Swelling Measurements
The swelling profiles were obtained for each hydrogel at room temperature in deionized water and a soil extract. Figure 7 shows the swelling kinetics of the PVA/HA hydrogels in both media. A rapid swelling occurred for all samples within the first hour due to the surface hydrophilicity and capillarity of pores of the hydrogels. Both samples AC9 and AC12 reached the swelling equilibrium at 12 and 15 h in deionized water and soil extract, respectively. The swelling equilibrium of hydrogels GA9 and GA12 was reached more faster (3 h in deionized water and soil extract) than in the case of autoclaved hydrogels. Several studies have explained that hydrogel water uptake depends on various prevailing conditions such as cross-linking density, ionic strength, temperature, and preparation method, among others.15,48,49
Figure 7.
Swelling kinetics of composite hydrogels in deionized water (a) and soil extract (b) at 25 °C.
Autoclaved hydrogels exhibited a higher swelling capacity in both media than those obtained by GA cross-linking. In deionized water, hydrogels AC9 and AC12 reached swelling levels of 546 and 530%, respectively, during the first hour, while samples GA9 and GA12 reached both around 489% (Figure 7a). Furthermore, the equilibrium swelling values were 744 and 657% for AC9 and AC12, respectively, whereas the values decreased up to 484 and 454% for GA9 and GA12, respectively. This comparative swelling behavior was consistent with the SEM observations. The pore density of lyophilized AC samples was higher than that of GA hydrogels; thereby, a higher inner network space allowed the containing of more water molecules. The comparative swelling results between AC and GA samples were also consistent with their experimental mechanical behavior, evidencing that the cross-linking degree of GA hydrogels was higher than that for AC hydrogels. Increasing the cross-linking density decreased the ability of the polymer network to swell, which in turn disfavored the water uptake.13
Similar findings for the swelling behavior of hydrogels were observed in the soil extract (Figure 7b), although the AC12 sample reached the highest value of swelling at 24 h (500%) followed by AC9, GA9, and GA12 hydrogels with 379, 174, and 155%, respectively. The swelling level of all samples was slightly reduced as compared to those measured in deionized water. At high ionic strength, the concentration of ions increases the osmotic pressure of the hydrogel, causing water to desorb from the network.48 This behavior (Gibbs Donnan effect) is a consequence of a charge imbalance between the inner and outer environments of the hydrogel.50 In the soil extract, the concentration gradient of ion species between the hydrogel and the external solution was lower than in water medium; thus, the water uptake decreased.35
To further examine the swelling mechanism of the hydrogels, experimental data were fitted to the power law model. For cylinder-shaped gels, when the n value is less than 0.45, the solvent uptake mechanism is governed by Fickian diffusion, and a n value between 0.45 and 0.89 indicates that the process follows an anomalous solvent uptake mechanism. The model is also capable of predicting Case II (n = 1.0) transport in anomalous diffusion and Super Case II transport (n > 1.0).36 The linear fit of the plot ln Mt/M∞ against ln t yields the diffusion exponent (n), the Pearson coefficient (r2), and the diffusion constant (k). The results are summarized in Table 4.
Table 4. Fitting Parameters of Swelling Data to the Power Law Equation.
| hydrogel sample | conditions | fitting parameters | release mechanism | ||
|---|---|---|---|---|---|
| n | k | r2 | |||
| AC9 | deionized water | 0.3617 | 1.826 | 0.9671 | Fickian diffusion |
| soil extract | 0.4279 | 2.0548 | 0.9716 | Fickian diffusion | |
| AC12 | deionized water | 0.3579 | 1.7825 | 0.995 | Fickian diffusion |
| soil extract | 0.3057 | 1.6176 | 0.9915 | Fickian diffusion | |
| GA9 | deionized water | 0.1869 | 0.8421 | 0.8627 | Fickian diffusion |
| soil extract | 0.1886 | 1.326 | 0.9693 | Fickian diffusion | |
| GA12 | deionized water | 0.1061 | 0.6086 | 0.8936 | Fickian diffusion |
| soil extract | 0.1966 | 1.2369 | 0.9523 | Fickian diffusion | |
The values of n indicated that the solvent sorption through the hydrogels was controlled by a Fickian diffusion mechanism in all samples under both conditions. It was also observed that the k values in deionized water were higher for AC samples than for the GA hydrogels, indicating a faster diffusion of water in the AC hydrogels. Furthermore, the k values in soil extract were higher than those obtained in deionized water, excepting for the AC12 hydrogel, in which the k value in soil extract was 10.2% lower than in deionized water. This behavior evidenced that the conditions of the swelling medium can modulate the diffusion of solvents in PVA/HA hydrogels.
Water Retention Study in Soil
The water evaporation from soil is mediated by ambient conditions such as air temperature, relative humidity, and the capacity of the soil to retain water.35 The water retention capacity of soils produces positive effects on the survival rate of seedlings and the growth of plants.51,52 Hydrogels hinder the exchange of water vapor between the porous soil and atmosphere, decreasing the evaporation rate. The presence of hydrogels also helps to preserve the moisture content of agricultural soils after irrigation or rain since water is gradually released over time.53−55
Figure 8 shows the WER values of hydrogel-added soils and the hydrogel-free soil over 9 days. The presence of 0.5 wt % of hydrogels enhanced the amount of water retained in all samples, but the AC hydrogels demonstrated a better capacity to preserve the humidity of soil. After 9 days, the WER values decreased in the following order: pure soil (98.80%), GA12-containing soil (85.90%), GA9-containing soil (78.00%), AC9-containing soil (74.90%), and AC12-containing soil (68.00%). This feature agrees with the swelling relative behavior of hydrogels; a higher swelling capacity of AC hydrogels (Figure 7b) induced a higher water retention of soil.
Figure 8.

Water evaporation ratio of pure soil and soil containing the AC and GA hydrogels.
Effects of Hydrogels on Plant Growth
The absence of phytotoxicity is the first requirement for using any material as a component of the growth medium of plants. Hydrogel samples with the highest HA content were used in the phytotoxicity test. The assays revealed that 83% of sorghum seeds germinated in the control sample and in the presence of GA12 hydrogel; meanwhile, 80% of the seeds germinated with the AC12 hydrogel. In commercial seeds, a value close to 90% is accepted.56 However, due to the seeds nature and the conditions of the assays, it is not expected that all seeds achieve germination. According to Montesano et al.,6 the lower limit of germination to consider a polymeric material as nonphytotoxic is 60%. From this point of view, the use of both GA12 and AC12 hydrogels is safe for plants. PVA-based hydrogels have been evaluated for agricultural applications, mixed with soil or for seed coating, evidencing a high water retention over long periods and a positive impact on the survival rate of plants.52 Lopez-Velazquez et al.54 have also reported that PVA hydrogels act either as water containers or as protective means for plants.
Figure 9 shows the average dry weight of the roots and aerial parts of sorghum plants that grew in the presence of GA12 and AC12 hydrogels. Statistical analysis showed significant differences in the dry weight of roots and aerial parts of sorghum according to the method used to prepare the PVA/HA hydrogels. The plant growth was promoted in the presence of the GA12 hydrogel as compared to the effect observed with the AC12 sample. No significant differences were detected in the dry weight of roots and aerial parts between the GA12 hydrogel and the control sample. The weight parameters were significantly lower when the AC12 hydrogel was used as compared to the control condition. It is expected that the microstructure of hydrogels improves the water availability and airflow through the system, reducing the drought stress and aeration problems for plants, respectively.11 For PVA/HA hydrogels, the heterogeneous porous structure of the PVA network formed by adding GA seems to promote the growth and storage of seedlings, as compared to the behavior of autoclaved hydrogels with lower pore dimensions and a highly uniform pore distribution.
Figure 9.

Effect of composite hydrogels on dry weight of root (a) and aerial part (b) of sorghum (Sorghum sp.). Different letters in the columns indicate significant differences at P < 0.05.
Urea Release
Most of traditional agrochemicals including fertilizers fail to reach the target site due to adverse processes such as leaching, volatilization, and air-drift.27,57 For instance, the nitrogen (N) use efficiency is around 40% for conventional urea.58 Significant N losses cause economic and environmental problems such as N2O emissions. In Mexican agriculture, N2O from cultivated soils represents one of the largest sources of emissions, eutrophication, and loss of soil fertility.57 Therefore, the use of urea-controlled release systems can be a potential solution to the problem of urea loss in agricultural crops.27,35,59
Figure 10 shows the urea release kinetics from AC12 and GA12 hydrogels in a soil aqueous extract at room temperature. The preparation method of the PVA/HA hydrogel influenced the urea release behavior. The urea release profiles revealed an initial burst delivery up to the first 2 h, reaching 33% of urea cumulative release for the AC12 sample and a lower value of 21% for the GA12 hydrogel. It is notorious that the amount of urea released at equilibrium varied with respect to the sample, indicating a correlation with the preparation method. The AC12 hydrogel released 91% of the urea content within the first 72 h, while the amount achieved by the GA12 hydrogel was 56%. Both hydrogels exhibited a sustained release of urea up to 3 days. The release kinetic of urea and the total amount of the fertilizer at equilibrium were consistent with the swelling relative behavior of hydrogels. The AC12 hydrogel showed a more efficient urea release as compared to the GA12 hydrogel. This feature can be attributed to the flexibility of PVA chains in the hydrogels obtained by the autoclave process, in contrast to the chain mobility restrictions of the GA-cross-linked hydrogels.7
Figure 10.

Urea release kinetics of AC12 and GA12 hydrogels; the experiments were performed in soil extract media at 25 °C.
Kinetic Model
To further examine the release mechanism of urea from the hydrogels, the experimental data obtained in the release studies were fitted to the model developed by Korsmeyer–Peppas. This model has been applied to evaluate the mechanisms involved in the chemical release process, as well as the possible coupling between the relaxation swelling of polymer systems and the diffusion phenomena of the model compound through the polymer matrix.14,40 Fitting parameters are summarized in Table 5.
Table 5. Fitting Parameters of Urea Release Data to the Korsmeyer–Peppas Equation.
| hydrogel sample | fitting parameters | release mechanism | ||
|---|---|---|---|---|
| n | k (10–3) | r2 | ||
| AC12 | 0.24 | 7.99 | 0.983 | Fickian diffusion |
| GA12 | 0.29 | 4.92 | 0.942 | Fickian diffusion |
The values of n indicated that the transport of urea through the hydrogels was controlled by a Fickian diffusion mechanism in both hydrogels, in agreement with the swelling mechanism of samples in aqueous media. It is also observed that the k value was 62% higher for the AC12 sample than for the GA12 hydrogel, evidencing the possibility of modulating the urea release kinetic from PVA/HA hydrogels by choosing between the preparation method of AC or GA cross-linking.
Conclusions
PVA hydrogels were successfully synthesized by AC or by adding GA in the presence of different proportions of HA. FTIR confirmed the formation of a 3D-supramolecular network of PVA by the autoclaving/freezing process, as well as the presence of acetal bridges in the GA-mediated cross-linked hydrogels. The thermal stability of the AC hydrogels was affected by the autoclaving process, whereas the thermal behavior of the GA samples was improved due to the chemical cross-linking. All hydrogels showed an interconnected porous structure, though GA samples displayed a less homogeneous morphology than the AC samples. PVA/HA hydrogels increased the water content of soils; a higher swelling capacity of AC hydrogels induced a higher water retention of the soil. Both AC and GA hydrogels showed nonphytotoxicity during the growth test of Sorghum sp.; however, the plant growth was promoted in the presence of the GA hydrogel as compared to the effect observed with the AC sample, evidencing the key role of the hydrogel morphology in the agricultural application. PVA/HA hydrogels showed a sustained delivery of urea at 25 °C in soil extract by a classical diffusion mechanism, although the total amount delivered of the fertilizer was closely influenced by the hydrogel structure. Besides the compositional concerns, this work demonstrated that the preparation method is an important criterion in the design of hydrogels intended for the agricultural field. AC or GA-cross-linked PVA/HA hydrogels showed suitable characteristics for agricultural applications, and particular requirements of crops can be addressed by choosing the appropriate method for the preparation of material.
Acknowledgments
The authors are grateful to the Consejo Nacional de Humanidades Ciencia y Tecnología (CONAHCYT), Mexico, grant number A1-S-26204, Ciencia Basica 2017–2018 for support. Ana Valeria Torres acknowledges CONAHCYT for her scholarship during this study. The authors thank Irela Santos Sauceda for the thermal analysis and Silvia Burruel for the SEM studies.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c05868.
Values considered for network parameters of PVA hydrogels, photographs of compression–decompression test, and graphics of pore size distribution (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ahmed E. M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6 (2), 105–121. 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah F.; Othman M. B. H.; Javed F.; Ahmad Z.; Akil H. M. Classification, Processing and Application of Hydrogels: A Review. Mater. Sci. Eng., C 2015, 57, 414–433. 10.1016/j.msec.2015.07.053. [DOI] [PubMed] [Google Scholar]
- Torres-Figueroa A. V.; Pérez-Martínez C. J.; Carmelo Encinas J.; Burruel-Ibarra S.; Silvas-García M. I.; García Alegría A. M.; Del Castillo-Castro T. Thermosensitive Bioadhesive Hydrogels Based on Poly(N-Isopropylacrilamide) and Poly(Methyl Vinyl Ether-Altmaleic Anhydride) for the Controlled Release of Metronidazole in the Vaginal Environment. Pharmaceutics 2021, 13 (8), 1284. 10.3390/pharmaceutics13081284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell J. A. Synthetic Biodegradable Polymers for Tissue Engineering and Drug Delivery. Curr. Opin. Solid State Mater. Sci. 1998, 3 (3), 246–251. 10.1016/S1359-0286(98)80098-3. [DOI] [Google Scholar]
- Herrmann A.; Haag R.; Schedler U. Hydrogels and Their Role in Biosensing Applications. Adv. Healthcare Mater. 2021, 10 (11), 1–25. 10.1002/adhm.202100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano F. F.; Parente A.; Santamaria P.; Sannino A.; Serio F. Biodegradable Superabsorbent Hydrogel IncreasesWater Retention Properties of Growing Media and Plant Growth. Agric. Agric. Sci. Procedia 2015, 4, 451–458. 10.1016/j.aaspro.2015.03.052. [DOI] [Google Scholar]
- Wang M.; Bai J.; Shao K.; Tang W.; Zhao X.; Lin D.; Huang S.; Chen C.; Ding Z.; Ye J. Poly(Vinyl Alcohol) Hydrogels: The Old and New Functional Materials. Int. J. Polym. Sci. 2021, 2021, 2225426 10.1155/2021/2225426. [DOI] [Google Scholar]
- Oladosu Y.; Rafii M. Y.; Arolu F.; Chukwu S. C.; Salisu M. A.; Fagbohun I. K.; Muftaudeen T. K.; Swaray S.; Haliru B. S. Superabsorbent Polymer Hydrogels for Sustainable Agriculture: A Review. Horticulturae 2022, 8 (7), 1–17. 10.3390/horticulturae8070605. [DOI] [Google Scholar]
- Patra S. K.; Poddar R.; Brestic M.; Acharjee P. U.; Bhattacharya P.; Sengupta S.; Pal P.; Bam N.; Biswas B.; Barek V.; Ondrisik P.; Skalicky M.; Hossain A. Prospects of Hydrogels in Agriculture for Enhancing Crop and Water Productivity under Water Deficit Condition. Int. J. Polym. Sci. 2022, 2022, 4914836 10.1155/2022/4914836. [DOI] [Google Scholar]
- Abobatta W. Impact of Hydrogel Polymer in Agricultural Sector. Adv. Agric. Environ. Sci. Open Access 2018, 1 (2), 59–64. 10.30881/aaeoa.00011. [DOI] [Google Scholar]
- Ma L.; Chai C.; Wu W.; Qi P.; Liu X.; Hao J. Hydrogels as the Plant Culture Substrates: A Review. Carbohydr. Polym. 2023, 305 (September 2022), 120544 10.1016/j.carbpol.2023.120544. [DOI] [PubMed] [Google Scholar]
- Kanmaz N.; Saloglu D.; Hizal J. Humic Acid Embedded Chitosan/Poly (Vinyl Alcohol) PH-Sensitive Hydrogel: Synthesis, Characterization, Swelling Kinetic and Diffusion Coefficient. Chem. Eng. Commun. 2019, 206 (9), 1168–1180. 10.1080/00986445.2018.1550396. [DOI] [Google Scholar]
- Sirousazar M.; Khodamoradi P. Freeze-Thawed Humic Acid/Polyvinyl Alcohol Supramolecular Hydrogels. Mater. Today Commun. 2020, 22 (October 2019), 100719 10.1016/j.mtcomm.2019.100719. [DOI] [Google Scholar]
- Orduño Rodríguez A. M.; Pérez Martínez C. J.; del Castillo Castro T.; Castillo Ortega M. M.; Rodríguez Félix D. E.; Romero García J. Nanocomposite Hydrogel of Poly(Vinyl Alcohol) and Biocatalytically Synthesized Polypyrrole as Potential System for Controlled Release of Metoprolol. Polym. Bull. 2020, 77 (3), 1217–1232. 10.1007/s00289-019-02788-x. [DOI] [Google Scholar]
- Jiang X.; Li C.; Han Q. Modulation of Swelling of PVA Hydrogel by Polymer and Crosslinking Agent Concentration. Polym. Bull. 2023, 80 (2), 1303–1320. 10.1007/s00289-022-04116-2. [DOI] [Google Scholar]
- Bates N. M.; Puy C.; Jurney P. L.; McCarty O. J. T.; Hinds M. T. Evaluation of the Effect of Crosslinking Method of Poly(Vinyl Alcohol) Hydrogels on Thrombogenicity. Cardiovasc. Eng. Technol. 2020, 11 (4), 448–455. 10.1007/s13239-020-00474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar K.; Sen K. Polyvinyl Alcohol Based Hydrogels for Urea Release and Fe(III) Uptake from Soil Medium. J. Environ. Chem. Eng. 2018, 6 (1), 736–744. 10.1016/j.jece.2018.01.004. [DOI] [Google Scholar]
- Hakim S.; Darounkola M. R. R.; Talari Hanieh; Barghemadi M.; Parvazinia M. Fabrication of PVA/Nanoclay Hydrogel Nanocomposites and Their Microstructural Effect on the Release Behavior of a Potassium Phosphate Fertilizer. J. Polym. Environ. 2019, 27 (12), 2925–2932. 10.1007/s10924-019-01580-2. [DOI] [Google Scholar]
- Canellas L. P.; Olivares F. L.; Aguiar N. O.; Jones D. L.; Nebbioso A.; Mazzei P.; Piccolo A. Humic and Fulvic Acids as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 15–27. 10.1016/j.scienta.2015.09.013. [DOI] [Google Scholar]
- De Melo B. A. G.; Motta F. L.; Santana M. H. A. Humic Acids: Structural Properties and Multiple Functionalities for Novel Technological Developments. Mater. Sci. Eng., C 2016, 62, 967–974. 10.1016/j.msec.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Tahir M. M.; Khurshid M.; Khan M. Z.; Abbasi M. K.; Kazmi M. H. Lignite-Derived Humic Acid Effect on Growth of Wheat Plants in Different Soils. Pedosphere 2011, 21 (1), 124–131. 10.1016/S1002-0160(10)60087-2. [DOI] [Google Scholar]
- Kaschl A.; Chen Y.. Interactions of Humic Substances with Trace Metals and Their Stimulatory Effects on Plant Growth; 2005; Vol. 52, 10.1007/1-4020-3252-8_4. [DOI] [Google Scholar]
- Hajdrik P.; Pályi B.; Kis Z.; Kovács N.; Veres D. S.; Szigeti K.; Budán F.; Hegedüs I.; Kovács T.; Bergmann R.; Máthé D. In Vitro Determination of Inhibitory Effects of Humic Substances Complexing Zn and Se on SARS-CoV-2 Virus Replication. Foods 2022, 11 (5), 1–12. 10.3390/foods11050694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza M. A.; Ahmad N.; Agarwal S. P.; Mahmood D.; Anwer M. K.; Iqbal Z. Comparative Evaluation of Humic Substances in Oral Drug Delivery. Results Pharma Sci. 2011, 1 (1), 16–26. 10.1016/j.rinphs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venezia V.; Verrillo M.; Avallone P. R.; Silvestri B.; Cangemi S.; Pasquino R.; Grizzuti N.; Spaccini R.; Luciani G. Waste to Wealth Approach: Improved Antimicrobial Properties in Bioactive Hydrogels through Humic Substance-Gelatin Chemical Conjugation. Biomacromolecules 2023, 24, 2691. 10.1021/acs.biomac.3c00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venezia V.; Avallone P. R.; Vitiello G.; Silvestri B.; Grizzuti N.; Pasquino R.; Luciani G. Adding Humic Acids to Gelatin Hydrogels: A Way to Tune Gelation. Biomacromolecules 2022, 23 (1), 443–453. 10.1021/acs.biomac.1c01398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.; Chen L.; Niu Y.; Ruan H. Sustained Urea Release Performance of Humic Acid Hydrogel for Green Vegetable Growth Environment Evaluation. J. Porous Mater. 2022, 29 (6), 1747–1758. 10.1007/s10934-022-01282-6. [DOI] [Google Scholar]
- Caló E.; Barros J. M. S. D.; Fernández-Gutiérrez M.; San Román J.; Ballamy L.; Khutoryanskiy V. V. Antimicrobial Hydrogels Based on Autoclaved Poly(Vinyl Alcohol) and Poly(Methyl Vinyl Ether-: Alt -Maleic Anhydride) Mixtures for Wound Care Applications. RSC Adv. 2016, 6 (60), 55211–55219. 10.1039/C6RA08234C. [DOI] [Google Scholar]
- Liu J.; Bao S.; Ling Q.; Fan X.; Gu H. Ultra-Fast Preparation of Multifunctional Conductive Hydrogels with High Mechanical Strength, Self-Healing and Self-Adhesive Properties Based on Tara Tannin-Fe3+ Dynamic Redox System for Strain Sensors Applications. Polymer 2022, 240 (January), 124513 10.1016/j.polymer.2021.124513. [DOI] [Google Scholar]
- Richbourg N. R.; Wancura M.; Gilchrist A. E.; Toubbeh S.; Harley B. A. C.; Cosgriff-Hernandez E.; Peppas N. A. Precise Control of Synthetic Hydrogel Network Structure via Linear, Independent Synthesis-Swelling Relationships. Sci. Adv. 2021, 7 (7), abe3245 10.1126/sciadv.abe3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray J. C.; Merrill E. W. Poly(Vinyl Alcohol) Hydrogels. Formation by Electron Beam Irradiation of Aqueous Solutions and Subsequent Crystallization. J. Appl. Polym. Sci. 1973, 17 (12), 3779–3794. 10.1002/app.1973.070171219. [DOI] [Google Scholar]
- Peppas N. A.; Merrill E. W. Crosslinked Poly(Vinyl Alcohol) Hydrogels as Swollen Elastic Networks. J. Appl. Polym. Sci. 1977, 21 (7), 1763–1770. 10.1002/app.1977.070210704. [DOI] [Google Scholar]
- Richbourg N. R.; Peppas N. A. The Swollen Polymer Network Hypothesis: Quantitative Models of Hydrogel Swelling, Stiffness, and Solute Transport. Prog. Polym. Sci. 2020, 105, 101243 10.1016/j.progpolymsci.2020.101243. [DOI] [Google Scholar]
- Canal T.; Peppas N. A. Correlation between Mesh Size and Equilibrium Degree of Swelling of Polymeric Networks. J. Biomed. Mater. Res. 1989, 23 (10), 1183–1193. 10.1002/jbm.820231007. [DOI] [PubMed] [Google Scholar]
- Durpekova S.; Bergerova E. D.; Hanusova D.; Dusankova M.; Sedlarik V. Eco-Friendly Whey/Polysaccharide-Based Hydrogel with Poly(Lactic Acid) for Improvement of Agricultural Soil Quality and Plant Growth. Int. J. Biol. Macromol. 2022, 212 (May), 85–96. 10.1016/j.ijbiomac.2022.05.053. [DOI] [PubMed] [Google Scholar]
- Brazel C. S.; Peppas N. A. Modeling of Drug Release from Swellable Polymers. Eur. J. Pharm. Biopharm. 2000, 49 (1), 47–58. 10.1016/S0939-6411(99)00058-2. [DOI] [PubMed] [Google Scholar]
- Durpekova S.; Di Martino A.; Dusankova M.; Drohsler P.; Sedlarik V. Biopolymer Hydrogel Based on Acid Whey and Cellulose Derivatives for Enhancement Water Retention Capacity of Soil and Slow Release of Fertilizers. Polymers 2021, 13 (19), 3274. 10.3390/polym13193274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreras-Urbina C. G.; Plascencia-Jatomea M.; Wong-Corral F. J.; Pérez-Tello M.; Ledesma-Osuna A. I.; Tapia-Hernández J. A.; Castro-Enríquez D. D.; Rueda-Puente E. O.; Rodríguez-Félix F. Simple Method to Obtaining a Prolonged-Release System of Urea Based on Wheat Gluten: Development and Characterization. Polym. Bull. 2020, 77 (12), 6525–6541. 10.1007/s00289-019-03074-6. [DOI] [Google Scholar]
- Tapia-Hernández J. A.; Madera-Santana T. J.; Rodríguez-Félix F.; Barreras-Urbina C. G. Controlled and Prolonged Release Systems of Urea from Micro-and Nanomaterials as an Alternative for Developing a Sustainable Agriculture: A Review. J. Nanomater. 2022, 2022, 5697803 10.1155/2022/5697803. [DOI] [Google Scholar]
- Torres-Figueroa A. V.; Pérez-Martínez C. J.; Del Castillo-Castro T.; Bolado-Martínez E.; Corella-Madueño M. A. G.; García-Alegría A. M.; Lara-Ceniceros T. E.; Armenta-Villegas L. Composite Hydrogel of Poly(Acrylamide) and Starch as Potential System for Controlled Release of Amoxicillin and Inhibition of Bacterial Growth. J. Chem. 2020, 2020, 5860487 10.1155/2020/5860487. [DOI] [Google Scholar]
- Waresindo W. X.; Luthfianti H. R.; Priyanto A.; Hapidin D. A.; Edikresnha D.; Aimon A. H.; Suciati T.; Khairurrijal K. Freeze-Thaw Hydrogel Fabrication Method: Basic Principles, Synthesis Parameters, Properties, and Biomedical Applications. Mater. Res. Express 2023, 10 (2), 024003 10.1088/2053-1591/acb98e. [DOI] [Google Scholar]
- Oliveira A. S.; Silva J. C.; Loureiro M. V.; Marques A. C.; Kotov N. A.; Colaço R.; Serro A. P. Super-Strong Hydrogel Composites Reinforced with PBO Nanofibers for Cartilage Replacement. Macromol. Biosci. 2023, 23 (2), 1–18. 10.1002/mabi.202200240. [DOI] [PubMed] [Google Scholar]
- Sonker A. K.; Rathore K.; Nagarale R. K.; Verma V. Crosslinking of Polyvinyl Alcohol (PVA) and Effect of Crosslinker Shape (Aliphatic and Aromatic) Thereof. J. Polym. Environ. 2018, 26 (5), 1782–1794. 10.1007/s10924-017-1077-3. [DOI] [Google Scholar]
- Song J.; Li L.; Niu Y. H.; Ke R. Y.; Zhao X. Preparation of Humic Acid Water-Retaining Agent-Modified Polyurethane Sponge as a Soilless Culture Material. J. Appl. Polym. Sci. 2022, 139 (20), 52182. 10.1002/app.52182. [DOI] [Google Scholar]
- Sugama T.; Pyatina T. Utilization of PVA Flakes in Promoting Self-Degradation of Temporary Cementitious Fracture Sealing Material. Trans. - Geotherm. Resour. Counc. 2014, 38 (October), 331–338. [Google Scholar]
- Chang C.; Lue A.; Zhang L. Effects of Crosslinking Methods on Structure and Properties of Cellulose/PVA Hydrogels. Macromol. Chem. Phys. 2008, 209 (12), 1266–1273. 10.1002/macp.200800161. [DOI] [Google Scholar]
- Hewawasam R. S.; Blomberg R.; Šerbedžija P.; Magin C. M. Chemical Modification of Human Decellularized Extracellular Matrix for Incorporation into Phototunable Hybrid-Hydrogel Models of Tissue Fibrosis. ACS Appl. Mater. Interfaces 2023, 15 (12), 15071–15083. 10.1021/acsami.2c18330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero S. M. M.; Ponce F R. V.; Cremona M.; Triques A. L. C.; d’Almeida A. R.; Braga A. M. B. Swelling and Morphological Properties of Poly(Vinyl Alcohol) (PVA) and Poly(Acrylic Acid) (PAA) Hydrogels in Solution with High Salt Concentration. Polymer 2010, 51 (4), 953–958. 10.1016/j.polymer.2009.12.016. [DOI] [Google Scholar]
- Ou K.; Dong X.; Qin C.; Ji X.; He J. Properties and Toughening Mechanisms of PVA/PAM Double-Network Hydrogels Prepared by Freeze-Thawing and Anneal-Swelling. Mater. Sci. Eng., C 2017, 77, 1017–1026. 10.1016/j.msec.2017.03.287. [DOI] [PubMed] [Google Scholar]
- Demitri C.; Scalera F.; Madaghiele M.; Sannino A.; Maffezzoli A. Potential of Cellulose-Based Superabsorbent Hydrogels as Water Reservoir in Agriculture. Int. J. Polym. Sci. 2013, 2013, 435073 10.1155/2013/435073. [DOI] [Google Scholar]
- Du M.; Xiao Z.; Luo Y. Advances and Emerging Trends in Cultivation Substrates for Growing Sprouts and Microgreens toward Safe and Sustainable Agriculture. Curr. Opin. Food Sci. 2022, 46, 100863 10.1016/j.cofs.2022.100863. [DOI] [Google Scholar]
- Sikder A.; Pearce A. K.; Parkinson S. J.; Napier R.; O’Reilly R. K. Recent Trends in Advanced Polymer Materials in Agriculture Related Applications. ACS Appl. Polym. Mater. 2021, 3 (3), 1203–1217. 10.1021/acsapm.0c00982. [DOI] [Google Scholar]
- Rabat N. E.; Hashim S.; Majid R. A. Effect of Different Monomers on Water Retention Properties of Slow Release Fertilizer Hydrogel. Procedia Eng. 2016, 148, 201–207. 10.1016/j.proeng.2016.06.573. [DOI] [Google Scholar]
- López-Velázquez J. C.; Rodríguez-Rodríguez R.; Espinosa-Andrews H.; Qui-Zapata J. A.; García-Morales S.; Navarro-López D. E.; Luna-Bárcenas G.; Vassallo-Brigneti E. C.; García-Carvajal Z. Y. Gelatin–Chitosan–PVA Hydrogels and Their Application in Agriculture. J. Chem. Technol. Biotechnol. 2019, 94 (11), 3495–3504. 10.1002/jctb.5961. [DOI] [Google Scholar]
- Miljković V.; Gajić I.; Nikolić L. Waste Materials as a Resource for Production of Cmc Superabsorbent Hydrogel for Sustainable Agriculture. Polymers 2021, 13 (23), 4115. 10.3390/polym13234115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepho H. P.; Kruse M.; Deplewski P. M. Expected Variance between Seed Germination Test Replicate Results. Seed Sci. Technol. 2018, 46 (2), 197–206. 10.15258/sst.2018.46.2.01. [DOI] [Google Scholar]
- Rojas-Padilla J.; de-Bashan L. E.; Parra-Cota F. I.; Rocha-Estrada J.; de los Santos-Villalobos S. Microencapsulation of Bacillus Strains for Improving Wheat (Triticum Turgidum Subsp. Durum) Growth and Development. Plants 2022, 11 (21), 1–15. 10.3390/plants11212920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindasamy P.; Muthusamy S. K.; Bagavathiannan M.; Mowrer J.; Jagannadham P. T. K.; Maity A.; Halli H. M.; G K. S.; Vadivel R.; T K. D.; Raj R.; Pooniya V.; Babu S.; Rathore S. S.; L M.; Tiwari G. Nitrogen Use Efficiency—a Key to Enhance Crop Productivity under a Changing Climate. Front. Plant Sci. 2023, 14 (3), 1–19. 10.3389/fpls.2023.1121073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman A.; Sharma A. K.; Bhardwaj D.; Agrawal G. Biodegradable Dual Stimuli Responsive Alginate Based Microgels for Controlled Agrochemicals Release and Soil Remediation. Int. J. Biol. Macromol. 2023, 228 (November 2022), 323–332. 10.1016/j.ijbiomac.2022.12.225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.