Abstract
Hypertension has become a global threat and is one of the greatest risk factors for chronic kidney disease. Fenchyl acetate is a monoterpene that has been assessed for its various pharmacological activities in the past, but no study has evaluated its diuretic potential and the mechanism involved in the diuretic activity after prolonged administration in rats. Therefore, this study aimed to measure the safety and diuretic profile of fenchyl acetate in rats. For evaluating the acute toxicity, a single dose of 2000 mg/kg was administered as per the OECD guideline no. 425, and the rats were observed for 14 days. After 14 days, blood samples were assessed for biochemical, hematological, and oxidative stress parameters. For the acute diuretic study, fenchyl acetate was given in doses of 100, 200, and 400 mg/kg, and urine samples after 8 h were assessed for sodium, potassium, creatinine, uric acid excretion, and urinary output. A single dose of fenchyl acetate (F.A) was selected for prolonged diuretic activity, and furosemide was taken as the standard drug in a repeated dose administration for 7 days. Rats’ urine was assessed for pH, sodium, potassium, creatinine, and uric acid excretion along with urinary volume excretion. Furthermore, blood was withdrawn by cardiac puncture, and selected organs like the heart, liver, kidney, and spleen were analyzed for oxidative stress biomarkers. Using pharmacological antagonists or inhibitors, the involvement of L-NAME, acetylcholine, or prostaglandin in F.A.-induced diuresis was determined. Mitochondrial respiratory chain enzyme complexes were also assessed in the kidney homogenates. The acute toxicity results showed F.A to be safe as its LD50 was greater than 2000 mg/kg and there were no signs of mortality or toxicity. The acute diuretic study showed that F.A resulted in a significant and dose-dependent increase in sodium, potassium, creatinine, and uric acid excretion along with urinary output, and these results were comparable to the standard drug furosemide. Prolonged administration with F.A (400 mg/kg) resulted in a comparable excretion of sodium, potassium, creatinine, uric acid, and urine output with furosemide (15 mg/kg). The oxidative stress parameters revealed that F.A (400 mg/kg) resulted in reducing the formation of free radicals. The results from the mechanism-based studies showed the involvement of NO in inducing diuresis. Furthermore, F.A (400 mg/kg) significantly increased the mitochondrial complexes I, II, III, IV, I + III, and II + III in the kidney homogenates, thus restoring the mitochondrial enzymes and improving the renal function. The current study suggests that F.A is safe with a significant diuretic potential with the involvement of NO in its mechanism of action.
Introduction
International regulations concerning human well-being necessitate the importance of toxicity profiling of newly formulated drugs before their use in humans because it helps in determining the impending hazards and characterization of actions of the compound under study.1 Acute toxicity studies serve as the battery of these toxicological studies as they provide preliminary data on the nature of the test drug for which no other information is available.2 This short-term evaluation involves the administration of a single dose of the test drug, followed by an assessment of the potential risks. Acute toxicity provides information on LD50 that is utilized for estimating the toxicity of the test substance.3 Toxicity studies also provide an easy valuation of tissues along with various pathophysiological and biochemical markers.4 Furthermore, the results from the extensive toxicity studies encourage the researchers to decide whether to incorporate the drug in clinical trials or not.5
Hypertension is one of the greatest risk factors for the development of chronic kidney disease (CKD) and it escalates the incidence of cardiovascular disease in individuals having CKD than in individuals with normal kidney functioning.6 Moreover, patients having CKD are more prone to cardiovascular mortality and fatality.7 Diuretics are used either alone or in combination with other therapeutic agents for managing conditions like hypertension, congestive heart failure (CHF), pulmonary edema, etc., but their irrational utilization results in various side effects, thus demanding the need for innovative agents from natural sources.8,9
Mitochondria have a noticeable role in energy generation for cellular metabolism by the process of oxidative phosphorylation (OXPHOS). The electrons derived from cell metabolism pass through the electron transport chain (ETC) that comprises five protein complexes localized in the inner membrane of mitochondria. During this process, electrons might escape from the ETC causing the transformation of oxygen to superoxide radicals. Thus, mitochondria are the main source of reactive oxygen species. The findings from recent studies highlight the association of mitochondria in the advancement of CKD principally either due to a decrease in mitochondrial DNA or mitochondrial membrane potential along with a decrease in ATP formation.10 Therapies targeting mitochondria have gained attention for prevailing research with the idea that the preservation of mitochondrial homeostasis can halt renal disease progression and pathogenesis.11
Phytochemicals are naturally occurring secondary plant metabolites having innate biological activity and thus provide beneficial effects to humans.12 Phytochemicals offer various benefits, including low toxicity with cost and easy access to availability accompanied by biological effects.13 Terpenoids are one of the foremost secondary metabolites among plant species that constitute a major part of essential oils. Monoterpenes have numerous therapeutic and biological effects, including antihypertensive, antioxidant, antimicrobial, and antispasmodic properties.14
Fenchyl acetate is a monoterpene that is naturally present as a secondary plant metabolite in various plants species like Croton matourensis and Croton micans and various Alpinia genus, where different parts of the plant have been evaluated for various pharmacological activities like antimicrobial, larvicidal, insecticidal, anti-inflammatory, cytotoxic, antioxidant, and antiasthmatic activities.15,16 Therefore, this study was designed to evaluate the acute toxicity and diuretic potential of fenchyl acetate in a rat model.
Materials and Methods
Chemicals
Carboxymethylcellulose (CMC), atropine, indomethacin, N-ω-nitro-l-arginine methyl ester (L-NAME), sodium chloride (NaCl), furosemide, and fenchyl acetate (CAS no:13851–11–1) were purchased from Sigma-Aldrich Chemicals.
Animals
Adult Wistar albino rats (150–250 g) were placed in the animal house of The University of Lahore, Lahore, Pakistan. All animals were housed under normal environmental conditions, i.e., 12 h light–dark cycle and 25 ± 1 °C, and provided with water and feed ad libitum. Accepted principles for laboratory animal use and care (NIH publication No. 85–23, revised in 1985) were followed for keeping the animals. Maximum effort was made to minimize animal distress and the number of animals used. All of the experimental procedures were approved (IREC-2022–17) by the Institutional Ethical Committee of the Faculty of Pharmacy, The University of Lahore, Lahore, Pakistan.
Acute Oral Toxicity
For assessing acute oral toxicity, OECD guidelines 425 were followed.17 Healthy nonpregnant female albino rats weighing 150–250 g were selected randomly. The rats fasted overnight before the procedure but had free access to water. A limit test for fenchyl acetate (F.A) was performed by administering a single dose of 2000 mg/kg to a single female rat according to the body weight. The rat was monitored closely for the first 30 min and then for 4 h. After the treated rat survived, the same dose was administered to four additional rats under the same settings. The same procedure opted for the vehicle control group of five rats, who were given 0.5% carboxymethylcellulose (CMC) in the same way as the treatment group. The normal and the treatment groups were observed for any sign of toxicity initially for the first 6 h and then at regular intervals for 14 days. The rats were weighed throughout the study period. After the completion of 14 days, animals were weighed, blood was withdrawn by cardiac puncture, and serum was isolated for hematological and biochemical estimation. Rats were killed by cervical dislocation followed by the excision of vital organs, which were then weighted and kept in 10% formalin for histopathological examination and for evaluating oxidative markers.18
Study Design
Healthy adult Wistar albino rats of both sexes weighing 150–250 g were divided into 5 groups (n = 5) for an acute diuretic study and 3 groups for a prolonged diuretic study of 7 days. Rats were fasted for 12 h before the experiment but had free access to water ad libitum. Rats were acclimatized to the environmental conditions 7 days before the experimental procedure by placing them in individual metabolic cages.19
Assessment of Acute Diuretic Activity
All of the rats were administered 5 mL/100 g of 0.9% NaCl by oral gavage to ensure a uniform salt and water load. After 45 min, the control group was administered 0.5% CMC, the standard group was administered 15 mg/kg furosemide, and treatment groups were administered 100, 200, and 400 mg/kg F.A consecutively through oral gavage. Immediately after dosing, the rats were placed in metabolic cages, and urine was collected and urine volume was recorded at regular intervals for up to 24 h. At the end of the acute diuretic study, the urine sample was analyzed for sodium (Na+), potassium (K+), uric acid, creatinine, and pH. Urinary pH was measured by a pH meter, Na+, K+, uric acid, and creatinine were measured by Bioactive Diagnostic System kits.1
Determination of Na+/K+ Ratio, Diuretic Index, Saluretic index, and Lipschitz Value
Urinary electrolytes (Na+ and K+) were used for calculating the Na+/K+ ratio. Similarly, urinary volume and urinary electrolyte concentration were utilized for evaluating diuretic index, saluretic index, and lipschitz value. They were calculated by using the following formulas.
Assessment of the Prolonged Diuretic Activity
Based on the acute diuretic activity, a single dose of F.A was selected for the prolonged diuretic activity. Rats were divided into 3 groups (n = 5) and were kept fasted overnight. The control group was administered 0.5% CMC, the standard group was administered 15 mg/kg furosemide, and the treatment group was administered 400 mg/kg F.A for 7 consecutive days. Urine samples were gathered on the first and seventh days for the estimation of sodium, potassium, creatinine, and uric acid. Blood was drawn on the seventh day by cardiac puncture, and serum was isolated by centrifugating the blood at 2000 rpm for 10 min. Serum was analyzed for the estimation of electrolytes (Na+, K+), uric acid, creatinine, and biochemical parameter analysis.20 Vital organs such as the heart, liver, kidney, and spleen were isolated after the decapitation of animals for the estimation of oxidative stress parameters.
Assessment of the Underlying Mechanism in the Diuretic Activity of Fenchyl Acetate
Involvement of the Role of Nitric Oxide, Prostaglandins, and Acetylcholine in the Diuretic Activity of F.A
The overnight fasted rats were divided into 5 groups having five rats in each group. All of the rats received 5 mL/100 g of 0.9% normal saline. After 45 min, animals in group I received 0.5% CMC. Group II received 400 mg/kg F.A, groups III, IV, and V received L-Name (60 mg/kg), indomethacin (5 mg/kg), and atropine (1 mg/kg), respectively, followed by administration of 400 mg/kg F.A after 1 h. After dosing, rats were placed in individual metabolic cages and urine was collected for 8 h and subjected to pH and electrolyte analysis.20
Assessment of Mitochondrial Respiratory Chain Enzymatic Activity of Fenchyl Acetate
Estimation of Protein
For the estimation of protein in the tissue homogenate, rats were sacrificed and kidneys were excised. The protein content was analyzed by utilizing Lowry’s method and the standard curve of bovine serum albumin was drawn to determine the protein concentration in the tissue homogenate, which was expressed in mg/mL.21 The protein estimation values were then utilized for calculating the mitochondrial enzyme complexes.
Estimation of Mitochondrial Complex Activity
Respiratory chain mitochondrial complexes were estimated in the kidney homogenates. The protocol method described by Spinazzi et al. was followed for measuring complex-1 (NADH: ubiquinone oxidoreductase at 340 nm), complex II (succinate dehydrogenase at 600 nm), complex III (decylubiquinol cytochrome c oxidoreductase at 550 nm), complex IV (cytochrome c oxidase at 550 nm), complex I + III (NADH cytochrome c oxidoreductase at 550 nm), and complex II+III (succinate cytochrome c reductase at 412 nm) spectrophotometrically.22
In Silico Studies
Retrieval and Preparation of the Ligand and Target Proteins
The three-dimensional (3D) structure of phytochemical fenchyl acetate (PubChem CID 107217) and the selected reference drug furosemide (PubChem CID 3440) were retrieved from the PubChem database in SDF format. X-ray crystal structures of target proteins superoxide dismutase (PDB id:1cb4) and glutathione (PDB id:7fc2) were retrieved from the Protein Data Bank (www.rcsb.org) and downloaded in PDB format. The obtained protein was prepared with Discovery studio visualizer. For this, residues, such as ligands, water, and heteroatoms attached to the target protein, were removed to avoid unwanted interactions during the docking process. Subsequently, polar hydrogens were added to the target structure in DSV.23 After the retrieval and preparation of protein targets and ligands, molecules were uploaded to pyrx virtual docking software. Ligand file.sdf was minimized and converted to pdbqt using OpenBabel.
Molecular Docking
Autodock vina4.2 suit of pyrx was used for the virtual docking of selected ligands against the desired targets. The number of runs was set to 200 for each docking. A grid map was resized to cover the active sites of the target protein and had an exhaustiveness of 8.24 The docking scores resulted in the generated.log files. The output docking scores were defined as affinity binding (kcal/mol). The interactions between ligands and target protein complexes after docking were visualized through DSV.25
Statistical Analysis
All of the results were evaluated using GraphPad Prism version 5. The data were presented as mean ± SEM. For statistically evaluating the data, both one-way and two-way analysis of variance (ANOVA) were used, followed by Tukey’s comparison test and Bonferroni and Dunnett’s post hoc tests. P < 0.05 was considered significant. P < 0.01 and p < 0.001 showed moderate and highly significant levels, respectively.
Results
Acute Toxicity Study
Effect of F.A on Behavioral Changes
Administration of a single dose of F.A (2000 mg/kg) did not induce any morbidity or mortality nor there was any sign or symptom of toxicity during the 14 days study period. Various observations regarding the behavioral pattern show no significant difference as compared to the control group, as shown in Table 1.
Table 1. Behavioral Parameters in Acute Toxicity Study vs Control Group Following Treatment with a Single Dose of FA (2000 mg/kg)a.
| parameters | CG | F.A (2000 mg/kg) dose |
|---|---|---|
| fur & skin | N | N |
| mucous membrane | N | N |
| convulsions & tremors | N. F | N. F |
| itching | N. F | P |
| eyes | N | N |
| salivation | N | N |
| somatomotor activity and behavior pattern | N | N |
| sleep | N | N |
| urination (color) | N | N |
| respiration | N | N |
| feces consistency | N | N |
| mortality | N. F | N. F |
C.G: control group, F.A: fenchyl acetate, N: normal, N.F: not found, P: present.
Changes in Body and Organ Weight
The body weight of rats was observed throughout the study period of 14 days, and at the end of the study period, vital organs like the heart, liver, kidney, and spleen were excised and weighed. Tables 2 and 3 show that there was no significant difference in the body weight as well as the organ weights of rats throughout the acute toxicity study as compared to the control group.
Table 2. Change in Body Weight of Ratsa.
| groups | body weight (gm) day 1 | body weight (gm) day 7 | body weight (gm) day 14 |
|---|---|---|---|
| CG | 168 ± 7.36 | 168.7 ± 7.31 | 169 ± 6.9 |
| TG FA (2000 mg/kg) | 158 ± 2.58 | 154 ± 3.48 | 158 ± 2.21 |
Values are presented as mean ± SEM; N = 6.
Table 3. Effects of F.A (2000 mg/kg) on Individual Organ Weighta.
| organ | normal control (g) | F.A (2000 mg/kg) |
|---|---|---|
| heart | 0.53 ± 0.02 | 0.53 ± 0.02 |
| kidney | 1.69 ± 0.04 | 1.70 ± 0.006 |
| liver | 6.70 ± 0.34 | 7.04 ± 0.007 |
| spleen | 0.50 ± 0.03 | 0.45 ± 0.02 |
Values are shown as mean ± SEM, N = 6.
Effect of F.A on Biochemical and Hematological Parameters
Rats were sacrificed at the end of a 14-day period by cardiac puncture to assess the effect of the F.A (2000 mg/kg) dose on lipid profile, hepatic profile, and kidney functioning in an acute toxicity study. Cholesterol levels were significantly decreased compared to the control group. Furthermore, there was a significant decrease in creatinine and uric acid levels in the F.A treatment group as shown in Table 4. Liver enzymes like ALT, AST, and bilirubin were also increased in the F.A.-treated group. There was no significant difference in the hematological profile in the F.A (2000 mg/kg)-treated group as shown in Table 5.
Table 4. Effect of FA on Biochemical Markersa.
| biochemical markers | units | C.G | F.A (2000 mg/kg) | |
|---|---|---|---|---|
| lipid profile | triglycerides | mg/dL | 196 ± 1.15 | 190 ± 0.57 |
| cholesterol | mg/dL | 221.33 ± 1.45 | 153.66 ± 3.93*** | |
| renal function test | creatinine | mg/dL | 23.77 ± 0.34 | 22.44 ± 0.12 |
| uric acid | mg/dL | 31.39 ± 0.70 | 25.41 ± 1.10 | |
| liver function test | ALP | U/L | 184 ± 0.57 | 191 ± 0.88** |
| ALT | U/L | 46 ± 0.57 | 57.66 ± 0.88*** | |
| AST | U/L | 73.33 ± 0.8 | 81 ± 1.15** | |
| bilirubin | mg/dL | 0.97 ± 0.02 | 1.26 ± 0.009 | |
Data are presented as mean ± SEM, N = 6.
Table 5. Effect of F.A (2000 mg/kg) on Hematological Parameters.
| parameters | units | C.G | F.A (2000 mg/kg) |
|---|---|---|---|
| Hb | g/dL | 11.9 ± 0.23 | 15.76 ± 0.17 |
| RBCs | X106/μL | 3.71 ± 0.03 | 3.92 ± 0.13 |
| PCV | % | 43.0 ± 0.57 | 41.0 ± 0.57 |
| MCV | FL | 97.46 ± 2.37 | 50.66 ± 1.20*** |
| MCH | Pg | 23.53 ± 0.83 | 19.7 ± 0.23 |
| MCHC | g/dL | 25.35 ± 0.10 | 37.86 ± 0.87*** |
| WBCs | X103/μL | 7.08 ± 0.13 | 7.16 ± 0.12 |
Analysis of Oxidative Stress Markers
Oxidative markers were assessed in vital organs like the heart, liver, kidney, and spleen at the end of the study for assessing any signs of toxicity at the tissue level. The results showed that there was a significant increase in the SOD and GSH levels in the heart, kidney, and spleen, whereas MDA levels were significantly decreased in the heart, kidney, and spleen as shown in Figure 1
Figure 1.

Effect of F.A (2000 mg/kg) dose on oxidative stress parameters in acute toxicity. Oxidative stress parameter analysis in rats in the acute toxicity study. Data are presented as mean ± SEM, N = 3. ***p <. 001 represents a significant increase, where ‴p <. 001 represents a significant decrease vs the control group.
Histopathological Analysis
Histopathological analysis shows changes in the architecture of cells (Figure 2).
Figure 2.
Histopathology of selected organs treated with F.A (2000 mg/kg) in acute toxicity study (H&E stain) at 10×.
Acute Diuretic Activity of F.A
Acute diuretic activity results with different doses of F.A (100, 200, and 400 mg/kg) and furosemide (15 mg/kg) are shown in Table 6. F.A (400 mg/kg) produced a pronounced effect on urinary volume after 8 h, which was 4.90 ± 0.11, as compared to the control group, where it came out to be 2.33 ± 0.12. Moreover, there was a significant increase in the excretion of Na and K (182.26 ± 0.72 and 44.51 ± 0.75), respectively, in the F.A (400 mg/kg) group as compared to the control group, where it was 136.10 ± 0.55 and 25.0 ± 1.04, and it can be evident through the Na+/K+ ratio as well and it was comparable to the standard drug furosemide. Furthermore, urinary creatinine and uric acid excretion were the highest with F.A (400 mg/kg dose) as compared to the control group. These values were 2.21 ± 0.11 and 7.9 ± 0.17 vs 1.37 ± 0.01 and 4.37 ± 0.09 mg/dL, respectively, and there was a nonsignificant difference between F.A (400 mg/kg) and furosemide (15 mg/kg).
Table 6. Effect of Different Doses of F.A on pH, Urinary Volume, Na+ and K+ Excretion, Na+/K+ Ratio, Creatinine, and Uric Acid in Acute Diuretic Activitya.
| treatment groups | pH | urine volume mL/100 g 8 h | Na+(mmol/L) | K+(mmol/L) | Na+/K+ | creatinine (mg/dL) | uric acid (mg/dL) |
|---|---|---|---|---|---|---|---|
| C.G | 7.27 ± 0.10 | 2.33 ± 0.12 | 136.10 ± 0.55 | 25.0 ± 1.04 | 5.45 ± 0.21 | 1.37 ± 0.01 | 4.37 ± 0.09 |
| furosemide (15 mg/kg) | 7.74 ± 0.079* | 4.76 ± 0.35*** | 182.56 ± 0.76*** | 44.03 ± 0.49*** | 4.14 ± 0.03*** | 2.14 ± 0.02*** | 7.78 ± 0.19*** |
| F.A (100 mg/kg) | 7.63 ± 0.12* | 3.06 ± 0.20 | 142.16 ± 1.48** | 29.51 ± 0.82* | 4.82 ± 0.16* | 0.91 ± 0.04** | 6.86 ± 0.18*** |
| F.A (200 mg/kg) | 7.76 ± 0.10* | 3.50 ± 0.17 ** | 173.13 ± 1.04*** | 33.96 ± 0.98*** | 5.10 ± 0.17 | 1.63 ± 0.08 | 7.23 ± 0.03*** |
| F.A (400 mg/kg) | 7.90 ± 0.05** | 4.90 ± 0.11*** | 182.26 ± 0.72*** | 44.51 ± 0.75*** | 4.09 ± 0.08*** | 2.21 ± 0.11*** | 7.9 ± 0.17*** |
Results are stated as mean ± SEM, where *p < 0.05, **p < 0.01, and ***p < 0.001, as when compared to the control group. All data are subjected to one-way ANOVA followed by Dunnett’s post-test; C.G = Control group, Na+/K+ = Urinary excretion of sodium/urinary excretion of potassium.
Effect of F.A on Diuretic index, Saluretic Index, and Lipchitz Values
The information gathered regarding the urinary excretion of electrolytes (Na and K) and urine output in the control group, standard group, and treatment groups was utilized for estimating the diuretic index, saluretic index, and Lipchitz value as shown in Table 7. All of the results were quite significant with the highest dose of F.A (400 mg/kg) and were comparable to furosemide.
Table 7. Effect of Different Doses of F.A on Diuretic Index, Saluretic Index, and Lipschitz Valuesa.
| saluretic
index (SI) |
|||||
|---|---|---|---|---|---|
| treatment groups | diuretic index (DI) | lipschitz value (LV) | SINa+ | SIK+ | saluretic index (SI) |
| C.G | 1.00 | 0.49 ± 0.02 | 0.99 ± 0.006 | 1 | 1 |
| furosemide (15 mg/kg) | 2.03 ± 0.08*** | 1.00*** | 1.33 ± 0.01*** | 1.76 ± 0.06*** | 1.35 |
| F.A (100 mg/kg) | 1.31 ± 0.02** | 0.64 ± 0.03 | 1.04 ± 0.006** | 1.18 ± 0.08 | 1.02 |
| F.A (200 mg/kg) | 1.49 ± 0.02*** | 0.73 ± 0.03** | 1.26 ± 0.003*** | 1.45 ± 0.14* | 1.23 |
| F.A (400 mg/kg) | 2.10 ± 0.06*** | 1.03 ± 0.06*** | 1.33 ± 0.006*** | 1.77 ± 0.06*** | 1.35 |
Results are stated as mean ± SEM, where *p < 0.05, **p < 0.01, and ***p < 0.001, as when compared to the control group. All data are subjected to one-way ANOVA followed by Dunnett’s post-test.
Prolonged Diuretic Activity of F.A
Administration of F.A (400 mg/kg) for 7 consecutive days resulted in a significant increase in the excretion of electrolytes (Na and K) on the seventh day. Correspondingly, urinary uric acid and creatinine excretion were also increased at the last day of treatment and these results were similar to the standard drug furosemide (Table 8 and Figure 3).
Table 8. Effect of Daily Administration of F.A (400 mg/kg) for 7 Days on Oxidative Stress Biomarkersa.
| oxidative stress markers | treatment groups | heart | liver | kidney | spleen |
|---|---|---|---|---|---|
| SOD (μg/mg of tissue protein) | C.G | 4.16 ± 0.064 | 0.966 ± 0.021 | 5.664 ± 0.055 | 9.882 ± 0.069 |
| furosemide (15 mg/kg) | 22.54 ± 0.303*** | 0.984 ± 0.039 | 5.052 ± 0.099** | 6.758 ± 0.108*** | |
| F.A (400 mg/kg) | 24.64 ± 0.232*** | 0.948 ± 0.024 | 6.758 ± 0.108*** | 12.58 ± 0.091*** | |
| NO (μg/mg of tissue protein) | C.G | 26.26 ± 0.422 | 2.79 ± 0.037 | 9.38 ± 0.027*** | 39.18 ± 0.108 |
| furosemide (15 mg/kg) | 36.45 ± 0.33*** | 3.32 ± 0.044 | 19.75 ± 0.85*** | 34.51 ± 1.039*** | |
| F.A (400 mg/kg) | 36.33 ± 0.33*** | 3.88 ± 0.031 | 18.64 ± 0.624*** | 46.71 ± 0.626*** | |
| GSH (μg/mg of tissue protein) | C.G | 54.26 ± 0.58 | 3.64 ± 0.058 | 20.14 ± 0.59 | 69.38 ± 0.87 |
| furosemide (15 mg/kg) | 41.95 ± 0.57*** | 4.54 ± 0.24 | 26.14 ± 0.59*** | 45.37 ± 0.31*** | |
| F.A (400 mg/kg) | 46.14 ± 0.59*** | 6.86 ± 0.10*** | 28.76 ± 0.14*** | 87.55 ± 0.23*** | |
| MDA (μmol/mg of tissue protein) | C.G | 0.95 ± 0.012 | 0.14 ± 0.017 | 1.20 ± 0.14 | 2.07 ± 0.06 |
| furosemide (15 mg/kg) | 0.94 ± 0.023 | 0.14 ± 0.009 | 0.907 ± 0.022** | 1.55 ± 0.020*** | |
| F.A (400 mg/kg) | 0.85 ± 0.023 | 0.13 ± 0.023 | 0.633 ± 0.012*** | 1.86 ± 0.009* |
All of the results are shown as mean ± SEM, whereas ** P < 0.01 and *** P < 0.001 when compared to the control group. All data are subjected to two-way ANOVA followed by Bonferroni’s post-test.
Figure 3.
Effect of daily administration of F.A (400 mg/kg) for 7 days on (A) Na+ excretion, (B) K+ excretion, (C) urinary uric acid, and (D) urinary creatinine. All of the results are shown as mean ± SEM, whereas ** P < 0.01 and *** P < 0.001 when compared to the control group. All data are subjected to two-way ANOVA followed by Bonferroni’s post-test.
Assessment of Oxidative Stress Markers in Selected Organs
Mechanism(s) Underlying the Diuretic Potential of F.A
The mechanistic evaluation study results for F.A suggest the involvement of L-NAME, which is a nitric oxide synthetase inhibitor. Pretreatment with L-NAME resulted in a significant decrease in F.A-induced diuresis and electrolyte excretion, whereas atropine and indomethacin did not alter the capability of F.A in inducing diuresis as shown in Figure 4.
Figure 4.
Effect of F.A (400 mg/kg) on (A) Na+ excretion, (B) K+ excretion, (C) uric acid excretion, (D) creatinine excretion, (E) urine output, and (F) urine pH in the presence of different antagonists. Data are presented as mean ± SEM, N = 5 ***p <. 001 represents a significant increase, where ’p <. 001 represents a significant decrease vs control group.
Effect of Mitochondrial Respiratory Chain Enzyme Complexes with F.A Dosing
Mitochondrial respiratory chain enzymes were assessed in the kidney homogenates, and the results showed that complex-1 activity (NADH: ubiquinine oxidoreductase) was significantly increased with F.A (400 mg/kg) dosing. Similarly, complex II (succinate dehydrogenase) activity was also increased in the F.A group when compared to furosemide as well as the control group. However, there was no significant change in the complex -III (deylubiquinone cytochrome c oxidoreductase) activity when compared to furosemide. Complex IV (cytochrome c oxidase), complex I + III (NADH cytochrome c oxidoreductase), and complex II + III (succinate cytochrome c reductase) activities were also increased significantly as compared to the control group (Figure 5).
Figure 5.
Effect of F.A (400 mg/kg) on mitochondrial respiratory enzyme complexes: (A) complex I, (B) complex II, (C) complex-III, (D) complex IV, (E) complex I + III, and (F) complex II + III. Data are presented as mean ± SEM, N = 5, ***p <. 001 represents a significant increase, where ’p <. 001 represents a significant decrease vs control group.
F.A Showed Strong Interaction with SOD and GSH in In Silico Studies
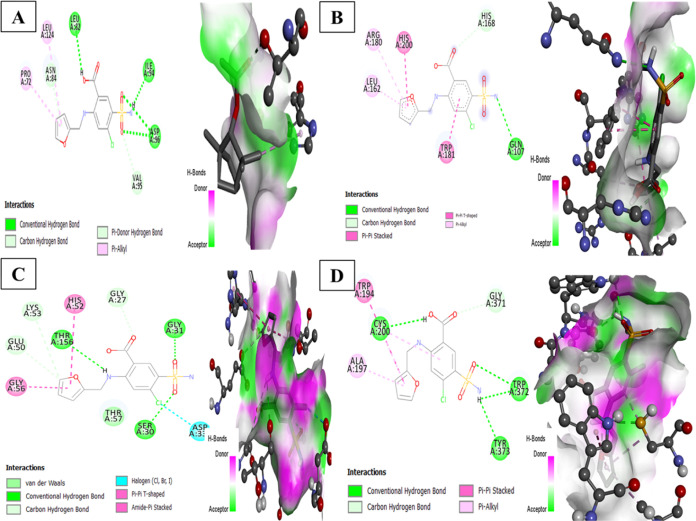
Molecular docking is a widely utilized computational approach that aids to comprehend the binding orientation of one molecule with another.26 The reference drug furosemide formed stable hydrogen bonds with SOD, glutathione peroxidase (GPx), glutathione reductase (GR), and nitric oxide synthase (NOS) with binding energies of −5.6, −5.8, −7.8, and −7.2 kcal/mol (Table 9) (Figure 1A–D).
Table 9. Results of Molecular Docking in Terms of Hydrogen Bonds and Binding Affinity.
| target protein | PDB id | compound | number of hydrogen bonds | binding affinity |
|---|---|---|---|---|
| SOD | 1cb4 | furosemide | Leu A 82, Ile A94, Asp A 96 | –5.6 |
| GSH peroxidase | 7fc2 | Gln A 107 | –5.8 | |
| GSH reductase | 1XAN | Ser A 30, Gly A 31, Thr A 156 | –7.8 | |
| iNOS | 3e7g | TRP A:372, Cys A:200, Tyr A:373 | –7.2 | |
| SOD | 1cb4 | fenchyl acetate | Thr A135, Gly A 139 | –4.2 |
| GSH peroxidase | 7fc2 | 0 | –5.6 | |
| GSH reductase | 1XAN | Thr A 379, Gly A 381 | –5.4 | |
| NOS | 3e7g | 0 | –5.7 |
In the same way, F.A interacted through hydrogen bonding and hydrophobic interaction to amino acid residues of SOD and glutathione reductase having binding energies of −4.2 and −5.4 kcal/mol. Through visualization of 2d and 3d interactions of the ligand–target complex, F.A was found to form 2 hydrogen bonds with residues Thr A135 and Gly A 139 of target protein SOD having hydrogen bond lengths 2.88 and 3.09 Å (Figure 6). It also interacted with SOD through alkyl bonds at residues His A118 and ARG A141. Moreover, it interacted with glutathione reductase target protein at residues Thr A 379 and Gly A 381 having bond lengths 2.27 and 2.99 and Pi alkyl and Pi σ bonds with PHE A226 (Figure 7).
Figure 6.
2D and 3D representation of docking complexes: (A) SOD, (B) GPx, (C) GR, and (D) iNOS.
Figure 7.
2D and 3D representation of docking complexes: (A) SOD, (B) GPX, (C) GR, and (D) iNOS.
Discussion
Medicinal plants have become the cynosure of scientific allure owing to their extensive utilization as alternative medicine in developing countries.27 Toxicological findings have verified that several plants can be detrimental due to their toxic effects. Therefore, a thorough scientific toxicology assessment is an essential prerequisite before utilizing a plant or its secondary metabolite for pharmacological effects.27 So, based on this, the present study evaluated the toxicological profile of fenchyl acetate (F.A) by in vivo assessment of the hematological and biochemical parameters relating it to the chemical dynamics of the compound.
For assessing the acute toxicity study, a single dose of F.A (2000 mg/kg) was administered orally to adult Wistar albino rats for 14 days, and the results revealed that there was neither mortality nor any change in the behavioral pattern or body organ weight as these are the main pointers for toxicity.28 As per the OECD guideline 423,17 since all of the animals survived by the end of the experiment, it can be anticipated that F.A has lower toxicity with an estimated LD50 greater than 2000 mg/kg. The hematological and biochemical parameters provide a vivid picture of toxicity and organ dysfunction. There was no significant change in the hematological parameters when compared with the control group. However, regarding biochemical parameters, there was a significant decrease in the cholesterol levels in the F.A-treated group as compared to the control group and this might be accredited to the leptin receptor expression;29 these results propose that F.A might be beneficial in treating pathologies related to dyslipidemia. The liver and kidney are believed to be the key organs responsible for metabolizing and eliminating the drug flowing in the body30 and any change in the hepatic markers (ALT, ALP, AST, and bilirubin) or renal parameters (creatinine and uric acid) displays the hepatotoxic and renal toxic nature of the compound.31 Damage to hepatic cells may cause the liberation of aminotransferases in the bloodstream owing to the increased permeability of the cell membrane.32 There was a moderate increase in ALP, AST, and ALT levels in the F.A-treated group as compared to the control group, which is consistent with the centrilobular necrosis seen during histopathological analysis, whereas all other biochemical markers were within the range when compared to the control group.
Continuous production of reactive oxygen species may cause damage to nucleic acids, lipids, and proteins by burdening the cell with oxidative stress, thus leading to perpetual cell damage. Endogenous antioxidants (SOD, GSH, and NO) and oxidants (MDA) were analyzed in specific organs to access any cellular damage induced by F.A dosing. The results of the acute toxicity study showed that there was a significant increase in the SOD levels in the heart, kidney, and spleen, increased NO levels in the heart, and elevated GSH levels in the kidney, showing the protective effect of F.A in rats.33 MDA is the end product of lipid peroxidation of membranes causing the generation of free radicals by the attack of unsaturated free radicals in the cell membranes.34 The current study shows a significant decrease in MDA levels in the heart and kidneys that can aid in recovery. These results are further corroborated through our in silico study, where F.A formed strong hydrogen bonds with SOD and GSH and showed good binding affinity.
Hypertension has become a global issue owing to its recurrent occurrence, ceaseless, and unrestrained threat for accompanying cardiovascular and renal morbidities.35 Diuretics have become the cornerstone for the treatment of hypertension along with edematous conditions exemplified by excessive extracellular fluid. Diuretics enhance water and salt excretion from the body, thereby reducing blood volume and blood flow resistance. Despite their availability, these drugs pose several metabolic complaints enforcing the development of newer diuretic agents with superior efficacy and fewer side effects.19 Data obtained from the present study revealed that F.A in the dose of 400 mg/kg resulted in a significant and dose-dependent increase in the diuretic activity. A single dose of F.A resulted in a remarkable increase in the urine output that is 4.90 ± 0.11 when compared to the control group (2.33 ± 0.12) with the maximum effect at 8 h intervals, and this was comparable to the standard drug furosemide (15 mg/kg), and it was 4.76 ± 0.35. The diuretic activity may be attributed either to the enhanced blood flow or dilatation of vessels or inhibition of tubular reabsorption of water or salts.36 Moreover, the study also revealed that F.A (400 mg/kg) resulted in a significant increase in the urinary electrolytes Na+ (182.26 ± 0.72) and K+ (44.51 ± 0.75) in comparison to the standard drug furosemide, which was Na+ (182.56 ± 0.76) and K+ (44.03 ± 0.49), exhibiting both saluretic and kaliuretic potential owing to the involvement of polar groups.37 Creatinine has been considered a reliable marker and diagnostic tool for assessing kidney functions. Urinary creatinine excretion was the highest with F.A (400 mg/kg) is 2.21 ± 0.11 as compared to the standard drug furosemide, which is 2.14 ± 0.02, considering it to be safe for patients having renal insufficiencies.38 Elevation in the levels of uric acid has been associated with several risk factors like cardiovascular diseases, renal disease, diabetes, hypertension, and gout.39 The present data revealed that F.A caused a dose-dependent effect with the highest uric acid excretion with 400 mg/kg dose; thus, it can be used in treating these conditions.
Based on the acute diuretic activity, a single dose of F.A (400 mg/kg) was selected for the prolonged diuretic activity of 7 days for assessing the natriuretic, kaliuretic, and diuretic potential. All of the findings revealed that F.A in the dose of 400 mg/kg dose resulted in a significant excretion of Na+, K+, creatinine, and uric acid as well as in urine output, and these were comparable to the standard drug furosemide. Moreover, oxidative stress biomarkers like SOD, NO, GSH, and MDA were analyzed in the selected organs like the heart, liver, kidney, and spleen, and the results revealed the protective effect of F.A (400 mg/kg) in reducing oxidative stress.
Furthermore, to evaluate the diuretic potential of F.A., mechanism-based studies were performed. Nitric oxide (NO) is synthesized by nitric oxide synthetase and plays a crucial role in regulating renal function. Nitric oxide not only controls the glomerular filtration rate and vascular tone but also regulates the dilatation of renal arterioles. Both NO and prostaglandin I2 (PGI2) play a vital role in regulating arteriolar tone and maintaining blood pressure in the vessel. NO liberated by the endothelial cells also helps in controlling the blood pressure as well as urination, and its production is diminished in patients having CKD, thus resulting in renal and cardiovascular complications. Thus, any factor capable of causing either increase or decrease in NO production can affect the urinary output and salt excretion.19,20 Atropine (muscarinic receptor blocker) and indomethacin (prostaglandin inhibitor) had little effect on the diuretic potential of F.A. The diuretic activity of F.A (400 mg/kg) was significantly decreased in the presence of L-NAME, which is an inhibitor of NO synthetase, reinforcing the involvement of NO in the diuretic activity induced by F.A.
Mitochondria have a noticeable role in energy generation for cellular metabolism by the process of oxidative phosphorylation (OXPHOS) and are the main sources for generating reactive oxygen species. The electrons derived from cell metabolism then pass through the electron transport chain that comprises five protein complexes localized in the inner membrane of mitochondria. Electrons pass through all of these complexes and reach complex five, where this energy is converted for ATP formation. During this process, electrons might escape from the ETC causing the transformation of oxygen to superoxide radicals. The findings from recent studies highlight the association of mitochondria in the advancement of CKD principally either due to a decrease in mitochondrial DNA or mitochondrial membrane potential along with a decrease in ATP formation.40 The present study showed that F.A (400 mg/kg) significantly increased the mitochondrial complexes I, II, III, IV, I + III, and II + III in the kidney homogenates, thus restoring the mitochondrial enzymes and improving the renal function.10 Therapies targeting mitochondrial functioning can thus aid in preserving the mitochondria along with halting renal disease progression and pathogenesis.11
Acknowledgments
The authors thank The University of Lahore and Riphah International University for providing research facilities to carry out the research. The authors would like to extend their sincere appreciation to the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia for funding this work through the project number (RSP2023R457).
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The authors declare no competing financial interest.
References
- Bashir A.; Mushtaq M. N.; Younis W.; et al. Fenchone a monoterpene: Toxicity and diuretic profiling in Rats. Front. Pharmacol. 2023, 14, 1119360 10.3389/fphar.2023.1119360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asare G.; Addo P.; Bugyei K.; et al. Acute toxicity studies of aqueous leaf extract of. Interdiscip. Toxicol. 2011, 4 (4), 206–210. 10.2478/v10102-011-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arome D.; Chinedu E. The importance of toxicity testing. J. Pharm. BioSci. 2013, 4, 146–148. [Google Scholar]
- Dandekar P.; Dhumal R.; Jain R.; et al. Toxicological evaluation of pH-sensitive nanoparticles of curcumin: acute, sub-acute and genotoxicity studies. Food Chem. Toxicol. 2010, 48 (8–9), 2073–2089. 10.1016/j.fct.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Aneela S.; De S.; Kanthal L.; et al. Acute oral toxicity studies of Pongamia Pinnata and Annona squamosa on albino wister rats. Int. J. Res. Pharm. Chem. 2011, 1 (4), 820–824. [Google Scholar]
- Kokubo Y.; Nakamura S.; Okamura T.; et al. Relationship between blood pressure category and incidence of stroke and myocardial infarction in an urban Japanese population with and without chronic kidney disease: the Suita Study. Stroke 2009, 40 (8), 2674–2679. 10.1161/STROKEAHA.109.550707. [DOI] [PubMed] [Google Scholar]
- Cachofeiro V.; Goicochea M.; de Vinuesa S. G.; et al. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease: New strategies to prevent cardiovascular risk in chronic kidney disease. Kidney Int. 2008, 74, S4–S9. 10.1038/ki.2008.516. [DOI] [PubMed] [Google Scholar]
- Lahlou S.; Tahraoui A.; Israili Z.; et al. Diuretic activity of the aqueous extracts of Carum carvi and Tanacetum vulgare in normal rats. J. Ethnopharmacol. 2007, 110 (3), 458–463. 10.1016/j.jep.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Schlickmann F.; Boeing T.; Mariano L. N. B.; et al. Gallic acid, a phenolic compound isolated from Mimosa bimucronata (DC.) Kuntze leaves, induces diuresis and saluresis in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 649–655. 10.1007/s00210-018-1502-8. [DOI] [PubMed] [Google Scholar]
- Granata S.; Gassa A. D.; Tomei P.; et al. Mitochondria: a new therapeutic target in chronic kidney disease. Nutr. Metab. 2015, 12 (1), 49 10.1186/s12986-015-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann P.; Lin P.-H.. Mitochondria Damage and Kidney Disease. In Mitochondrial Dynamics in Cardiovascular Medicine; Springer, 2017; Vol. 982, pp 529–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A.; Alqurainy F. Activities of antioxidants in plants under environmental stress. Lutein-Prev. Treat. Dis. 2006, 187–256. [Google Scholar]
- Andre C. M.; Larondelle Y.; Evers D. Dietary antioxidants and oxidative stress from a human and plant perspective: a review. Curr. Nutr. Food Sci. 2010, 6 (1), 2–12. 10.2174/157340110790909563. [DOI] [Google Scholar]
- Razzaq M. A.; Younis W.; Malik M. N. H.; et al. Pulegone Prevents Hypertension through Activation of Muscarinic Receptors and Cyclooxygenase Pathway in L-NAME-Induced Hypertensive Rats. Cardiovasc. Ther. 2023, 2023, 8166840 10.1155/2023/8166840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van H. T.; Thang T. D.; Luu T. N.; et al. An overview of the chemical composition and biological activities of essential oils from Alpinia genus (Zingiberaceae). RSC Adv. 2021, 11 (60), 37767–37783. 10.1039/D1RA07370B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone R. S.; Chavez K.; Mateu E.; et al. Composition and cytotoxic activity of essential oils from Croton matourensis and Croton micans from Venezuela. Rec. Nat. Prod. 2010, 4 (2), 101–108. [Google Scholar]
- Oecd O.Guidelines for the Testing of Chemicals. Acute oral toxicity—acute toxic class method: test no-423. Organization for Economic Co-operation and Development, 2001.
- Saleem U.; Amin S.; Ahmad B.; et al. Acute oral toxicity evaluation of aqueous ethanolic extract of Saccharum munja Roxb. roots in albino mice as per OECD 425 TG. Toxicol. Rep. 2017, 4, 580–585. 10.1016/j.toxrep.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis W.; Schini-Kerth V. B.; Nocchi S. R.; et al. Involvement of Muscarinic Receptors in Hypotensive and Diuretic Effects of Aqueous Soluble Fraction from Asphodelus tenuifolius Cav. Evidence-Based Complementary Altern. Med. 2021, 2021, 6653270 10.1155/2021/6653270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prando T. B. L.; Barboza L. N.; de Oliveira Araújo V.; et al. Involvement of bradykinin B2 and muscarinic receptors in the prolonged diuretic and antihypertensive properties of Echinodorus grandiflorus (Cham. & Schltdl.) Micheli. Phytomedicine 2016, 23 (11), 1249–1258. 10.1016/j.phymed.2015.10.020. [DOI] [PubMed] [Google Scholar]
- Hira S.; Saleem U.; Anwar F.; et al. In silico study and pharmacological evaluation of Eplerinone as an Anti-Alzheimer’s drug in STZ-induced Alzheimer’s disease model. ACS Omega 2020, 5 (23), 13973–13983. 10.1021/acsomega.0c01381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinazzi M.; Casarin A.; Pertegato V.; et al. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 2012, 7 (6), 1235–1246. 10.1038/nprot.2012.058. [DOI] [PubMed] [Google Scholar]
- Durhan B.; Yalçın E.; Çavuşoğlu K.; et al. Molecular docking assisted biological functions and phytochemical screening of Amaranthus lividus L. extract. Sci. Rep. 2022, 12 (1), 4308 10.1038/s41598-022-08421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usman H.; et al. Identification of novel and potential PPARγ stimulators as repurposed drugs for MCAO associated brain degeneration. Toxicol. Appl. Pharmacol. 2022, 446, 116055 10.1016/j.taap.2022.116055. [DOI] [PubMed] [Google Scholar]
- Chigurupati S.; Al-murikhy A.; Almahmoud S. A.; et al. Molecular docking of phenolic compounds and screening of antioxidant and antidiabetic potential of Moringa oleifera ethanolic leaves extract from Qassim region, Saudi Arabia. Saudi J. Biol. Sci. 2022, 29 (2), 854–859. 10.1016/j.sjbs.2021.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig M. H.; Ahmad K.; Rabbani G.; et al. Computer Aided Drug Design and its Application to the Development of Potential Drugs for Neurodegenerative Disorders. Curr. Neuropharmacol. 2018, 16 (6), 740–748. 10.2174/1570159X15666171016163510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Kandhare A. D.; Mukherjee A. A.; et al. Acute and sub-chronic oral toxicity studies of hesperidin isolated from orange peel extract in Sprague Dawley rats. Regul. Toxicol. Pharmacol. 2019, 105, 77–85. 10.1016/j.yrtph.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Variya B. C.; Bakrania A. K.; Madan P.; et al. Acute and 28-days repeated dose sub-acute toxicity study of gallic acid in albino mice. Regul. Toxicol. Pharmacol. 2019, 101, 71–78. 10.1016/j.yrtph.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Zakernezhad F.; Barati M.; Sanadgol N.; et al. The Association Between Fennel Extract, Serum Lipid Profile, and Leptin Receptor Expression. Basic Clin. Neurosci. 2021, 12 (6), 711–720. 10.32598/bcn.2021.998.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariweni M. W.; Yibala O. I.; Ozolua R. I. Toxicological studies on the aqueous leaf extract of Pavetta crassipes (K. Schum) in rodents. J. Pharm. Pharmacogn. Res. 2018, 6 (1), 1–16. [Google Scholar]
- Abdelkader N. F.; Elyamany M.; Gad A. M.; et al. Ellagic acid attenuates liver toxicity induced by valproic acid in rats. J. Pharmacol. Sci. 2020, 143 (1), 23–29. 10.1016/j.jphs.2020.01.007. [DOI] [PubMed] [Google Scholar]
- Friedman L.; Martin P.; Munoz S. Liver function tests and the objective evaluation of the patient with liver disease. Hepatology 1996, 1, 791–833. [Google Scholar]
- Wang J.; Zhu H.; Wang K.; et al. Protective effect of quercetin on rat testes against cadmium toxicity by alleviating oxidative stress and autophagy. Environ. Sci. Pollut. Res. 2020, 27 (20), 25278–25286. 10.1007/s11356-020-08947-2. [DOI] [PubMed] [Google Scholar]
- Ni H.; Peng L.; Gao X.; et al. Effects of maduramicin on adult zebrafish (Danio rerio): acute toxicity, tissue damage and oxidative stress. Ecotoxicol. Environ. Saf. 2019, 168, 249–259. 10.1016/j.ecoenv.2018.10.040. [DOI] [PubMed] [Google Scholar]
- Kearney P. M.; Whelton M.; Reynolds K.; et al. Global burden of hypertension: analysis of worldwide data. Lancet 2005, 365 (9455), 217–223. 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- de Fitoquímica S. L. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas. BLACPMA 2004, 3 (6), 66–79. [Google Scholar]
- Martín-Herrera D.; Abdala S.; Benjumea D.; et al. Diuretic activity of Withania aristata: An endemic Canary Island species. J. Ethnopharmacol. 2007, 113 (3), 487–491. 10.1016/j.jep.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Prathibhakumari P.; Prasad G. Pharmacological investigation on the diuretic activity of the aqueous fruit extract of Neolamarckia cadamba (Roxb) Bosser. J. Pharm. Res. 2014, 8 (2), 130–135. [Google Scholar]
- Zhang Y.; Jin L.; Liu J.; et al. Effect and mechanism of dioscin from Dioscorea spongiosa on uric acid excretion in animal model of hyperuricemia. J. Ethnopharmacol. 2018, 214, 29–36. 10.1016/j.jep.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Granata S.; Gassa A. D.; Tomei P.; Lupo A.; Zaza G. Mitochondria: A New Therapeutic Target in Chronic Kidney Disease. Nutr. Metab. 2015, 12, 49 10.1186/s12986-015-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.