Abstract

To provide precise medical regimens, photonics technologies have been involved in the field of nanomedicine. Phototriggered liposomes have been cast as promising nanosystems that achieve controlled release of payloads in several pathological conditions such as cancer, autoimmune, and infectious diseases. In contrast to the conventional liposomes, this photoresponsive element greatly improves therapeutic efficacy and reduces the adverse effects of gene/drug therapy during treatment. Recently, cancer immunotherpay has been one of the hot topics in the field of oncology due to the great success and therapeutic benefits that were well-recognized by the patients. However, several side effects have been encountered due to the unmonitored augmentation of the immune system. This Review highlights the most recent advancements in the development of photoresponsive liposome nanosystems in the field of oncology, with a specific emphasis on challenges and opportunities in the field of cancer immunotherapy.
1. Introduction
The development and use of novel nanomaterials for theranostic (diagnosis and therapeutic) purposes is known as nanomedicine.1−3 These nanoscale carriers promote the targeted controlled delivery of various therapeutic molecules via increasing endocytosis, improving tumor cellular uptake, and enhancing the permeability and retention effect (EPR). Furthermore, these nanocarriers improve the pharmacokinetic properties and half-lives of the different payloads while minimizing their toxic off-target impact on healthy tissues.2,4−7
Liposomes are among the nanoscale carriers that represent well-known and reliable drug and gene delivery systems that are frequently employed in treatment of cancer, and the well-known liposomal doxorubicin (Doxil, Janssen Biotech, Inc.) represents the best example of a selective tumor targeting agent with decreased toxicity. It was the first FDA-approved liposomal medication for the treatment of several malignant tumors such as Kaponsi’s sarcoma, ovarian cancer, and multiple myeloma.
Liposomes that are triggered by external physical stimuli such as photoirradiation, magnetic fields, or X-ray irradiation represent novel nanocarrier systems that have an advantage over existing liposome drug delivery systems in that they allow on-demand payload release in response to external stimuli.1,8 Using light as an external stimulus for different photoresponsive nanocarriers has several benefits compared to other external stimulants, such as noninvasiveness, sharp spatial resolution, simplicity, and affordability.9 Light of an optimized wavelength is utilized as a stimulus for the photothermal therapy (PTT), photodynamic therapy (PDT), and controlled release of loaded drugs/nucleic acids from photosensitive nanosystems containing light-dissociable compounds, such as coumarin derivatives, o-nitrobenzyl, or others.10 Upon exposure to light at the optimized wavelength, the nanostructures release their payloads via either the disassembly of the nanosystem by the photothermal effect, photocleavage, or the structural deformations of the nanosystem caused by photoisomerization.9 Three light wavelength ranges are used as triggers: (i) ultraviolet (UV, 200–400 nm), visible light (400–650), and near-infrared (NIR, 650–900 nm).11 Even though UV stimulation generates more effective photochemical reactions and potent energy that are effective in both photodynamic and photothermal therapies (PDT and PTT), NIR stimulation is commonly used for the reasons presented in Table 1.
Table 1. Comparison between the Significant Features of Ultraviolet and near-Infrared Light Irradiations in Photodynamic and Photothermal Therapies.
| UV stimulation | NIR stimulation | |
|---|---|---|
| wavelength range (nm) | 200–400 | 650–900 |
| energy | more energetic | less energetic |
| photochemical reactions | more effective | less effective |
| penetration | poor body tissue penetration (<1 mm) | deep body tissue penetration (1–4 mm) |
| effectiveness against deep tumors | not effective against deep tumors | effective against deep tumors |
| effective against superficial tumors | ||
| applicability in PTT and PDT | can be transformed into heat (for PTT) or reactive oxygen species (for PDT) | can be transformed into heat (for PTT) or reactive oxygen species (for PDT) |
| safety | hazardous to healthy tissues (could be absorbed by lipids and hemoglobin) | safer to healthy tissues |
Lately, recent studies have exploited the advantages of both UV, such as low wavelengths, achieving high energy and effective photochemical reactions, and NIR, such as safety and deep tissue penetration irradiations.12 In this regard, few strategies have been employed to convert NIR light to UV or visible light after passing the body tissues, such as using the two-photon absorption strategy and lanthanide-based nanostructures.13 Visudyne (liposome for injection, Novartis AG) was the first to earn FDA approval in 2000 as a photoengineered liposome for the treatment of myopia and age-related macular degeneration. Verteporfin (VP) is a synthetic porphyrin that is activated to produce highly hazardous reactive oxygen species when exposed to light at 690 nm, causing local damage to the neovascular endothelium.14
Immuno-oncology is the new era of cancer treatment.15−17 It focuses mainly on exploiting this intrinsic tendency of the mammalian immune system to combat cancer.18−20 The process of distinguishing cancer cells from healthy cells is known as immunosurveillance and is mainly carried out by the tumor-infiltrating lymphocytes such as adaptive cytotoxic T lymphocytes (CTLs) and innate natural killer cells (NK cells),21,22 as shown in Figure 1. However, despite the apparent immunogenicity of cancer, the body often fails to eradicate it or prevent metastasis on its own. This is partly due to the evident evolution of malignant cells, which evade the immune system by various mechanisms, including the exploitation of immune checkpoints and T cell exhaustion.23
Figure 1.
A schematic diagram illustrating the tumor microenvironment. Created by Biorender.com.
It has been evidenced that a liposome-based combination of phototherapy and immunotherapy can increase the therapeutic index of these modalities by improving the stability and biocompatibility of the cargo as well as minimizing the adverse effects.24,25 In the same context, various studies have suggested that using nanocarriers in conjunction with immunotherapy and photothermal therapy could result in more significant antitumor immunological effects.26,27
Although scientists have spent the last ten years focusing on light-triggered liposomes in drug/gene delivery, the shallow depth of light irradiation still limits the spectrum of therapeutic indications because the light is applied externally. Furthermore, if free medications are freely delivered, they are likely to be activated in nontarget sites. As a result, the authors in this Review address the most recent achievements in light-triggered liposomes with respect to their structure and encapsulation power for chemotherapeutic, immunotherapeutic, and oncological molecular targets, with a special emphasis on the potential alternative routes to these present obstacles for future research and clinical use.
2. Liposomes as a Drug Delivery System
2.1. Structure and Properties
Liposomes are developed by self-assembling biocompatible and biodegradable phospholipids and cholesterol in an aqueous medium to form concentric nanovesicles comprised of lipophilic tails (fatty acids chains) and polar heads (phosphate ester groups).28
The unique structure of liposomes, an aqueous center entrapped within lipid bilayers, facilitates the chaperoning of various hydrophilic and hydrophobic therapeutic molecules such as phytochemicals, chemotherapeutic or immunotherapeutic agents, and RNA molecules for cancer therapy (Figure 2A).4,29,30 Charged therapeutic moieties could bind to the liposomal membranes through electrostatic interactions (Figure 2A). Additionally, liposomes can cross the tumor cell membrane through endocytosis or membrane fusion (Figure 2B), expediting the preferential cellular uptake and passive accumulation of loaded drugs and/or photosensitizers (PS) inside cancer cells.31,32 In addition, using liposomes as nanocarriers in cancer therapy is auspicious because their surfaces could be tailored with different targeting moieties, such as folic acid, hyaluronic acid, antibodies, and peptides, ensuring the active targeting of liposomes to their target site of action (Figure 1C).33,34 Additionally, using liposomes as nanocarriers for drug/RNA/PS delivery would improve the pharmacokinetics of the loaded therapeutic molecules and prolong their half-lives (>72 h) via surface coating with polyethylene glycol (PEG, Figure 2C).
Figure 2.
Schematic diagram illustrating (A) drug loading, (B) modes of crossing the tumor cell membrane, and (C) surface functionalization of liposomes. Created by Biorender.com.
The composition of the liposomes is crucial in controlling the integrity of the liposomes, as it gives the liposomes their unique features such as particle size, stiffness, fluidity, stability, and electrical charge.35,36 The phospholipids (PLs) involved in designing liposomes might be natural, such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylserine (PS), or synthetic, such as 1,2-dipalmitoyl-sn-glycero-3-phosphorylethanolamine (DPPE), 1,2-distearoyl-sn-glycero-3-phosphorylethanolamine (DSPE), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC). In addition, cholesterol steroid (Chol) is used in combination with the PLs to control the fluidity of the liposomal membrane. Surfactants (Span 60 and Tween 60) are also used in the formulation of liposomes to lower the surface tension between various immiscible phases, improving the drug/PS encapsulation and release profiles. Finally, polymers, such as PEG (polyethylene glycol), are used to decorate the liposomal surface to shield the liposomes from circulating proteins, increasing systemic circulation time and lowering immunogenicity while increasing therapeutic efficacy.35 The major components used in the formulation of liposomes and their chemical structures are summarized in Table 2.
Table 2. Major Components and Their Chemical Structures Used in the Design of Liposomes.
3. Phototriggered Liposomes (PTLs)
3.1. Design, Optimization, And Physicochemical Properties
Since the FDA approved the first photoresponsive liposomes, Visudyne, in 2000, several studies reported the engineering and optimization of light-sensitive liposomes loaded with various therapeutic moieties to achieve controlled and convenient cancer therapy.37,38 Numerous physical and chemical properties should be considered when fabricating photoresponsive liposomes, as they can influence the drug encapsulation capacity, liposomal stability in body fluids, payload release percentage in response to light stimulation, and, eventually, the therapeutic activity.39 These factors are lipid membrane fluidity,40 lipid phase transition (Tm),41 and lipid polymerization.42
Lipid membrane permeability is an essential factor that should be optimized to achieve the maximum payload release capacity upon exposure to a light stimulus. The components used in formulating PTLs are key players that control liposome’s physicochemical and biological features. For instance, using DSPC as one component of the PTLs led to an increase in the stability and entrapment efficiency of the liposomes as compared to using either dipalmitoylphosphatidylcholine (DPPC) or EPC. The decoration of the PTLs with the DPPE mPEG5000 polymer generates a steric hindrance around the liposomal membrane, minimizing the reticuloendothelial system uptake. Additionally, the inclusion of DPPG in the design of PTLs was reported to enhance the capability of the liposomes to penetrate the tumor cells due to its fusogenic features.37,43 Finally, it was reported that increasing the concentration of Chol led to increasing the stiffness of the liposomal membranes while decreasing their fluidity. Additionally, increasing the molar ratio of Chol was found to reduce the membrane’s permeability.41
The lipid phase transition (Tm) is another critical factor affecting the cargo release from the lipid bilayer after light irradiation. It is affected by the composition of the phospholipids. Light illumination causes a photothermal effect, increasing the temperature above the Tm.
Consequently, a phase transition of the lipid bilayer occurs from the orderly solid phase to the disassembled one, improving the selective diffusion of the loaded therapeutic moiety out of the liposomal membranes at the tumor site (Figure 3).41 For instance, a study reported the design of photoresponsive liposomes containing DPPC (Tm = 41 C) and gold nanoparticles. Upon irradiation with NIR radiation of 760 nm, the incorporated gold nanoparticles emit thermal energy (that exceeds the Tm of DPPC) and thus lead to the release of the loaded drugs at the site of action.44
Figure 3.
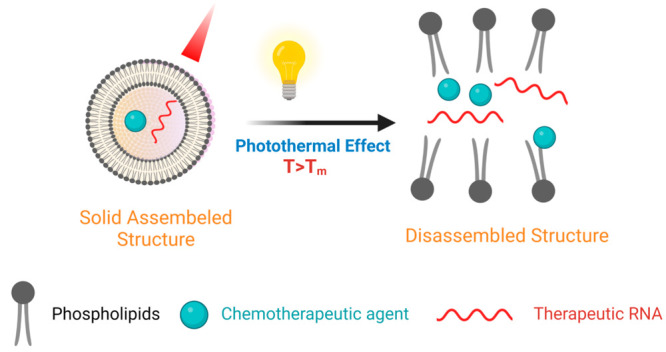
Photothermal effect on the payload release from the photoresponsive liposomes. Created by Biorender.com.
Finally, lipid polymerization is another important property that affects the efficient release of loaded therapeutic agents upon light triggering. Photopolymerizable phospholipids, such as DPPC, are essential for disassembling the liposomal membranes, upon light irradiation, through the photo-cross-linking phenomenon. This leads to the selective release of the loaded drugs in the tumor tissues.36
3.2. Payload Release Modalities from the Phototriggered Liposomes (PTLs)
The design of PTLs involves incorporating light-responsive entities (such as azobenzene) or PS (such as porphyrins derivatives) within optimized ratios of phospholipids using several approaches, such as thin-film hydration, ultrasonic dispersion, injection, freeze-drying, and microfluidic methods.45 The typical structure of liposomes is exploited where the light-responsive molecules (PS) are loaded based on their water-solubility; the hydrophilic ones are encapsulated in the aqueous core, while the hydrophobic ones are incorporated in the lipid bilayer.46 Upon light irradiation, the PTLs are disassembled via either physical or chemical activation of the incorporated light-responsive molecules, leading to the efflux of the payloads at the site of action. Table 3 summarizes the release modalities of the loaded therapeutic agents through either photophysical or photochemical activation.
Table 3. Release Modalities of the Payloads from Photoresponsive Liposomes.
| payload release modality | example | mechanism | ref |
|---|---|---|---|
| A. photophysical activation | |||
| 1. photothermal conversion | indocyanine green (ICG, FDA-approved) | introducing molecular dyes (ICG) into the liposomes leads to the conversion of NIR into heat upon light irradiation at a definite wavelength, destabilizing the liposomal structure and, eventually, releasing the payloads at the tumor site | (49) |
| 2. incorporation of plasmonic nanostructures | plasmonic nanoparticles such as gold nanoparticles (Au NPs) | including plasmonic nanoparticles (Au NPs) in the liposomal system | (50) |
| upon light illumination (at a specific wavelength), the plasmonic nanoparticles induce heat (surpassing the lipid phase transition (Tm) through the surface plasmon resonance (SPR) effect, dismantling the liposomal lipid bilayers and releasing the loaded therapeutic moieties | |||
| 3. incorporation of inorganic nanostructures | upconverting nanoparticles (UCNPs) such as graphene oxide (GO) | using UCNPs during the engineering of PTL can absorb NIR radiation and convert it to UV–vis energy | (51) |
| for instance, GO (a two-dimensional (2D) nanostructure) can absorb NIR radiation and transform it into strong thermal energy via delocalizing the electron | |||
| the released thermal energy exceeds the Tm of the phospholipids and leads to the disassembly of the liposomes upon phototriggering and light-controlled effluxing of their cargos | |||
| B. photochemical activation | |||
| 1. photocleavage | photocleavable molecules such as coumarin derivatives and the o-nitrobenzyl group | the photocleavable entities included in the light-responsive liposomes can irreversibly disrupt the integrity of the liposomes through photocleavage, rearrangement, and electron transfer reactions upon light illumination | (52) |
| 2. photoisomerization | photoisomerizable entities such as azobenzene | the photoisomerizable compound incorporated in the liposomal lipid bilayer leads to photoinduced conformational modifications when irradiated by NIR radiation | (53) |
| these conformational changes then result in the disassembly of the liposomal bilayer membrane and consequent payload release | |||
| 3. photochemical internalization | photosensitizers (PS) such as porphyrins derivatives (verteporfin) | when exposed to light stimulation, the PS incorporated in the liposomes generate reactive oxygen species, which oxidize the liposomal phospholipids | (54) |
| this leads to the rupture of the lipid bilayer and the subsequent release of the loaded therapeutic molecules. | |||
The liposomes coloaded by PS and anticancer agents are accumulated in the tumor site via passive targeting through the enhanced permeability and retention (EPR) effect. In addition, PTLs could be actively targeted to the intended site of action by functionalizing their surfaces with target moieties that bind to specific overexpressed receptors in the tumor cells (folate receptors, for instance). Both passive and active targeting could decrease the off-target effects and help in the preferential accumulation of PS and payloads in the tumor tissues. Then, upon the light irradiation of the desired site of action, activating the PS leads to the PTT (or PDT) effects.47
For instance, a study reported the design of GE11 peptide conjugated liposomes coloaded with curcumin, a natural chemotherapeutic, and indocyanine green (ICG), a photosensitizer. The presence of GE11 peptide facilitates the selective targeting of liposomes via binding to the epidermal growth factor receptor (EGFR) on the lung tumor surface. Then, upon NIR irradiation, the liposomes released their payloads, which synergistically generated reactive oxygen species (ROS) and induced apoptotic pathways, improving the anticancer effects while reducing off-target effects.48
4. Phototriggered Liposomes in Cancer Chemotherapy and Immunotherapy
4.1. Phototriggered Liposomes Encapsulating Chemotherapeutic and/or Immunotherapeutic Agents
As previously mentioned, cancer nanomedicine is a hot topic that has been highly tackled by researchers from different disciplines.55 This displays an arsenal of nanotherapeutics with proven better efficiency in the sustained delivery of several anticancer agents.56 Phototriggered liposomes have been on top of the list of nanocarriers tailored for cancer patients, prompting the development of personalized nanomedicine among cancer patients.57,58 PDT and PTT are less invasive local therapy methods that eliminate cancerous cells by producing highly toxic reactive oxygen species (ROS) or hyperthermia upon light illumination.59 For instance, Hyp-HPbCD-loaded liposomes containing hypericin (Hyp) as a photosensitizer showed a phototoxic effect against ovarian adenocarcinoma only after irradiation.60 Additionally, the encapsulation of indocyanine Green (ICG) in chitosan-coated liposomes gave stable liposomes, enhanced their uptake by B16-F10 melanoma cancer cells, and increased and improved phototoxicity.61 According to another study, a novel multimodal delivery system using curcumin-loaded lipopolyplexes (LPPs) in combination with PDT was created, and it enhanced the delivery of RNA molecules to the SKOV-3 cancer cell.62 In a different study, curcumin was loaded into liposomes made of HSPC and DPPE-mPEG5000, which improved the stiffness of the liposomal bilayer membrane, prevented curcumin from leaking outside the lipid membranes, and led to increased cytotoxicity toward cancer cells.63 Another study reported the fabrication of photoresponsive liposomes that contained both the anticancer medication cisplatin and ICG.64 Through a photothermal action mediated by ICG under NIR illumination, cisplatin was selectively released to tumor tissues, demonstrating an improved anticancer efficacy either with PTT alone or in conjunction with chemotherapy.64 Furthermore, Wu et al.’s study described the creation of zwitterionic liposomes containing methylene blue (MB) PS, which may produce reactive oxygen species (ROS) in response to light exposure and induce the death of cancer cells.65 This work encapsulated MB into zwitterionic liposomes to protect loaded medicines from degradation in the bloodstream, prolong systemic circulation, and improve MB cellular uptake into cancer cells. DSPC was used to self-assemble a zwitterionic polymer–lipid, poly(12-(methacryloyloxy)dodecyl phosphorylcholine), in a 1:4 molar ratio, which showed enhanced cytotoxicity on breast cancer cells through the release of ROS65 (Table 4).
Table 4. Phototriggered Liposomes Encapsulating Chemotherapeutic and Immunotherapeutic Agents.
| liposome system | liposome components | photosensitizer (PS) | in vitro/in vivo model | therapy model | outcome | ref |
|---|---|---|---|---|---|---|
| NIL-IM-Lip | 1-MT, IR780, NGR motif, Mal-MMP2 sensitive pep-IL-15, DPPC, HSPC, and DSPE PEG2000 | IR780 | B16F10, CT-26, MC38, and HUVEC cell lines | PTT | enhances the effectiveness of T and NK cells, induces immunogenic cell death, and suppresses regulatory T cells | (70) |
| poly I:C- and ICG-containing TRLs (piTRLs) | DPPC, MPPC, DSPE-PEG2000, and poly I:C | ICG | B16F10 CT-26 cell lines | PTT | activates dendritic cells (DCs) and cytotoxic T cells and accelerates cancer cell death | (68) |
| ICG thermosensitive liposome | DPPC, ICG, and cisplatin | ICG | HeLa 4T1 cell lines | PTT | enhances efficacy either for PTT alone or with chemotherapy | (64) |
| FA-L@MD@CAT liposome | SPC, DSPE-mPEG2k or FA-DSPE-mPEG2k, cholesterol, MBDP, and Dox | MBDP | 4T1MCF7 cell lines | PDT | reduces hypoxic conditions in the tumor microenvironment and boosts the efficacy of PDT | (66) |
| Hyp-HPbCD-loaded liposomes and hypericin TEL liposomes | DSPC, DPPC, TEL Hypericin, and HPbCD | hypericin | SK-OV-3 cell lines | PDT | more precise delivery of the photosensitizer to the tumor site | (60) |
| AQ4N-64Cu-hCe6 liposome | DPPC, DSPEmPEG5k, AQ4N, and hCe6 | hCe6 (hexadecylamine-conjugated chlorin e6) | Balb/c mice bearing 4T1 tumors | PDT | improves the elimination of cancer in a mice model via sequential PDT and hypoxia-activated chemotherapy AQ4N | (67) |
| zwitterionic liposomes | poly(12-methacryloyloxy)dodecyl phosphorylcholine DSPC | methylene blue (MB) | 4T1 cell lines | PDT | increases cytotoxicity against breast cancer cells | (65) |
| chitosan-coated liposomes | DMPC, Cholesterol Chitosan | ICG | melanoma cell lines | PDT | increases permeability and phototoxicity against melanoma cell lines | (61) |
| lipopolyplexes (LPPs) | DOPC DPPC Cholesterol | curcumin | SK-OV-3 B16F10 cell lines | PDT | increases and improves gene delivery to SK-OV-3 | (62) |
| stealth liposomes (decorated with PEG) | HSPC DPPE mPEG5000 | curcumin | MUG-Mel2 | PDT/PTT | increased cytotoxicity against cancer cells and decreased cytotoxicity for normal keratinocytes. | (63) |
| SCC-25 cell lines | ||||||
| GNOL (gold nanoshell-coated liposomes) | oleanolic acid cholesterol chitosan | gold | 143B cell lines | PTT | increases target specificity and improved therapeutic efficacy of the encapsulated drug | (71) |
The hypoxia caused by the tumor microenvironment is one of the issues that has been identified as an impeding factor for PDT’s effectiveness. Consequently, numerous methods have been devised to address these issues, such as coloading nanoparticles to provide adequate oxygen self-generation, which improved the effectiveness of PDT by reducing tumor microenvironment (TME)-induced hypoxia. Another idea is creating a unique liposomal platform encapsulating the catalase (CAT) enzyme for combinatory photo/chemotherapies to tackle tumor hypoxia.66 For instance, doxorubicin (Dox), catalase, and NIR photosensitizer-loaded liposomes were invented with an improved lysosome targeting capability. Catalase reduced the amount of in the hypoxic TME and boosted the PDT’s efficacy by successfully causing the breakdown of H2O2 to produce in situ O2.66 Another intriguing work created novel light-triggered liposomes by coloading PS, chlorin e6, and the prodrug AQ4N, demonstrating a therapeutic potential in treating cancer under hypoxic conditions.67 Under hypoxic conditions, the targeted delivered AQ4N was preferentially activated inside cancer tissues to release the more cytotoxic active form. This liposomal delivery system produced both the PDT effect and hypoxia-activated chemotherapy when exposed to LED light at 660 nm, which significantly improved the overall treatment results for the 4T1 tumor-bearing mouse model, as shown in Table 4.67 Moreover, to test the viability and potential of combining PTT and immunotherapy, PTT-responsive liposomes coloaded with the immunostimulatory drug polyinosinic:polycytidylic acid (poly I:C) and indocyanine green were formulated.68 The liposomal structure was disassembled upon irradiation with 808 nm laser light, inducing a photothermal therapeutic impact against B16 melanoma tumor and CT-26 colorectal carcinoma models and accelerating cancer cell death. The released poly I:C from the liposomes clearly activates dendritic cells (DCs) in nearby lymph nodes.
Additionally, a new triple-sensitive liposomal system called NIL-IM-Lip (pH/MMP2/temperature triple-sensitive) was reported to remodel the repressed tumor lymph node immune microenvironment (TLIME) by simultaneously mobilizing the adaptive and the innate immune arms at the TME, namely, cytotoxic T cells and natural killer (NK) cells, respectively.69 Following the pH-sensitive release of the NGR motif (that binds to CD13) and the MMP2-responsive release of IL-15, the temperature-sensitive NIL-IM-Lip is directed to the lymph nodes. In addition, it contains IR780 (photosensitizer) and 1-MT (IDO inhibitor) that simultaneously induce immunogenic cell death and suppress regulatory T cells during photothermal stimulation.70
4.2. Phototriggered Liposomes Modulating Tumor Microenvironment and Cancer Cellular Behaviors through Encapsulating Genetic Material
The application of gene therapy to combat cancer oncological signaling pathways has been prompted by a growing body of knowledge in regard to its modulatory role for tumor cells and their surrounding TME. Gene therapy includes exogenous nucleic acids, such as genes, gene segments, oligonucleotides, microRNAs (miRNAs), or small interfering RNAs (siRNAs). Such genetic materials are delivered through ex vivo or in vivo modalities through several carriers, such as liposomes, nanoparticles, viral vectors, and many others.72 In this section, the authors will shed light on the use of phototriggered liposomes in delivering genetic materials into cancer cells.
For instance, a study reported the usage of noncationic photoresponsive liposomes—Al(III) phthalocyanine chloride disulfonic acid (AlPcS2a)—and siRNAs ,where it was reported that the noncationic LPS results in minimizing off-target effects and improving the selective uptake of the siRNAs by cancer cells such as HepG2 and SK-HEP-1.73 Several studies have recently reported the successful delivery of the CRISPER/Cas 9 system using liposomes. For example, the fabrication of liposomes loaded with photosensitizer verteporfin (VP) and sgRNA against the TNFAIP3 gene (that encodes the A20 protein, which is a tumor promoter in numerous cancer types, such as breast and liver cancers, and glioblastoma) was reported. The usage of these phototriggered liposomes resulted in a decrease in the TNFAIP3 mRNA level and increased knockdown efficiency.74 Another multifunctional vehicle was reported for the delivery of Cas9-sgRNA. It was based on a lipid/AuNPs complex coated by TAT peptide for nucleus targeting and sgRNA targeting Plk-1 (a master mitotic regulator that is frequently overexpressed in tumor cells). This multifunctional system was successful enough to produce a PlK-1 knockout model. The designed liposomes led to effective tumor (melanoma) target gene (Plk-1) knockouts and tumor suppression owing to the synergic effect and the interaction between the photothermal-mediated intracellular release of the CP and the CP-induced cell death.75Table 5 summarizes the photoresponsive liposomes encapsulating genetic materials used in cancer therapy.
Table 5. Photoresponsive Liposomes Encapsulating Genetic Materials Used for Cancer Therapy.
| liposomes | liposomes component | photosensitizer | cargo | in vitro/in vivo model | outcomes | ref |
|---|---|---|---|---|---|---|
| noncationic LPs | DOPC | Al(III) phthalocyanine chloride disulfonic acid (AlPcS2a) | siRNA | HepG2 SK-HEP-1 cell lines | minimizing off-target effects and providing selectivity to hepatoma cancer cells | (73) |
| CRISPER/Cas 9 liposomes | DOTAP, DOPE, cholesterol | verteporfin (VP) | CRISPER/CAS 9 sgRNA against TNFAIP3 | HEK293 cell lines | increased knockdown efficiency | (74) |
| TAT peptide-modified Lipid Encapsulated Gold Nanoparticles | AuNPs (DOTAP, DOPE, cholesterol, PEG2000-DSPE | gold | CRISPER/CAS 9sgRNA-targeting Plk-1 | Melanoma cell lines and in vivo tumor model | Plk-1 knockout and tumor suppression in vitro and in vivo | (75) |
| light-triggerable liposome (lipVP) | DOPC DOTAP | verteporfin (VP) | antisense oligonucleotides against PAC1R | PC12 cell lines | increased PAC1R repression efficiency | (76) |
| X-ray triggerable liposomes | DOPC DOTAP | verteporfin (VP) | antisense oligonucleotides Against PAC1R | PC12 HCT 116 cell lines | increased PAC1R repression efficiency | (77) |
| liposome–polycation–DNA (LPD) nanocomplexes | DOTAP, PEGylated neutral lipid and cholesterol | Verteporfin (VP) | polyethylenimine (PEI)/plasmid DNA (pDNA) encoding EGFP | HCT116 cell lines | improves transfection efficiency | (46) |
4.3. Phototriggered Liposomes As Carriers for Novel Immunotherapeutic Agents and TME-Remodeling Agents
Immune surveillance is the process by which different immune system elements safeguard the host against the establishment of primary tumors, promote tumor escape, or both, either by sculpting tumor immunogenicity or attenuating antitumor immune responses and forming what is called the TME.15,78
Cancer immune editing or immune escape is the process by which cancer cells evade the immune system using many tactics, such as defective antigen presentation, inducing apoptosis for cytotoxic T cells and NK cells, immune suppressive mediators, immune checkpoints, and other mechanisms, and the best way to restore the antitumor effect of the immune system is by immunotherapy.16,17
Various immunological elements are found upon cellular examination of a typical TME, indicating an immune battle against the tumor.21,78−80 The appearance of multiple cytokines, macrophages, neutrophils, and tumor-infiltrating lymphocytes (TILs), among others, signify an attempt of the host immune system to eradicate the tumor.22,81−84
As previously mentioned, the recent introduction of immunotherapeutic agents in the field of oncology has been considered a revolution that restored faith in many desperate cancer victims.19,21,85 Cancer immunotherapy has significantly improved patients’ chances of survival and quality of life as compared to earlier standards of care (such as chemotherapy, radiation, and surgery), and there are many strategies for cancer immunotherapy like vaccination therapy, adoptive T cell therapy, and immune checkpoint inhibition on the molecular and cellular scale.84 In this section, the authors will highlight the recent literature discussing the role of phototriggered liposomes in immunotherapy.
4.3.1. Liposome-Mediated Delivery of Immune Checkpoint Inhibitors
Immune checkpoints, by definition, are signals received by immune cells to either stimulate or repress their action. In a functional scenario, these direct the immune system to properly aggress during the initial stages of response before prompting it to regress during later stages to prevent autoimmune damage/prolonged inflammation. One example of an immune checkpoint dynamic is the interaction between the costimulatory T-cell receptor CD28 and the inhibitory cytotoxic T lymphocyte-associated antigen 4 (CTLA4), a receptor expressed in relatively low numbers only after T-cell activation. While CD28 prompts T-cell amplification upon binding to its B7 ligands (CD80 and CD86), CTLA4 counteracts this due to its higher affinity to the identical ligands and resultant competition with CD28, as shown in Figure 4.86
Figure 4.
Mechanism of action of antibodies targeting overexpressed immune checkpoint proteins on cancer cells. Created by Biorender.com.
As the other key player in terms of immune checkpoints, PD-1 is a chief target of immune checkpoint blockade. A notable aspect of PD-1 is that its ligand, PD-L1, is commonly overexpressed on malignant cell surfaces to serve as another method for suppressive immune evasion method. The interaction between PD-1 and PD-L1 is characteristic of T-cell exhaustion and thus the abrogation of this interaction has been proposed to effectively “reverse” this exhaustion, as shown in Figure 4. Henceforth, treatments by either anti-PD-1 mAb or anti-PD-L1 mAb have been tested and showed promising results.87,88
Liposomes have been recently introduced to immune checkpoint blockade therapy, especially targeting PD-1/PD-L1 inhibitors, increasing their efficiency, and reducing their previously mentioned possible side effects. A double-layer system liposome has been employed by Lang et al.89 to encapsulate the PD-1 inhibitor HY19991 and thioridazine. The liposomes successfully increased the accumulation of the HY19991 and thioridazine drugs inside the tumor tissue.89
From another perspective, Merino et al. employed liposomes coupled with PD-L1 mAbs to modulate the immune system from the other side of the cancer–immune synapse.90 Such approach results showed that liposomes containing 5% PEG showed the highest hindering of the PD-L1 suppressor effects. Furthermore, Hei et al. developed another type of liposomes coupled with anti-PD-L1 on their surface, yet they also contain the catalase enzyme inside them; those liposomes showed better therapeutic effects and, most importantly, low general toxicity.91 Gu et al. also reported the reduced systemic toxicity and tissue damage of PD-L1 antibodies when formulated with liposomes in mice melanoma models.92
It is worth noting that a combination of phototriggered liposomes and immune checkpoint inhibitors has been reported to be effective in improving the immunogenic profile of cancer cells. Lan et al. designed a new phototriggered liposome called Lp(DHA)@CP (composed of a polyunsaturated fatty acid-doped liposomal hydrogel) loaded with the PD-L1 antibody (αPD-L1) and photosensitizer chlorin e6 (Ce6). In response to the NIR light irradiation, abundant ROS were produced by the integrated Ce6, which then caused the liposomal hydrogel to disintegrate for the on-demand sustained release of PD-L1. Additionally, the produced ROS in TME was able to make low-immunogenic “cold” tumors “hot” by boosting the T cell infiltration in tumors, which in turn increased their potential responses to ICB treatments.93
Another liposomal system coloaded with a photothermal agent (IR780), folic acid (FA) (that increases tumor targeting and also increases the enhanced permeability and retention (EPR) effect of the liposomal system), oxaliplatin (OXA), and PD-L1 inhibitors (BMS-1) was fabricated. The created liposomal system demonstrated advantageous tumor tissue biodistribution and tumor cell endocytosis, boosting the photothermal and anticancer effects, as well as improved dendritic cell maturation and infiltration of antigen-specific T lymphocytes into the residual tumors and blocking the immune checkpoint as summarized in Table 6.94
Table 6. Photoresponsive Liposomes Encapsulating Immunotherapeutic and TME-Modulating Agents.
| liposomes | immunotherapeutic agent | photosensitizers | immunotherapy strategy | effector cells | cancer cell | outcomes | ref |
|---|---|---|---|---|---|---|---|
| Lp(DHA)@CP | αPD-L1 (Ab) | Ce6 | PD-L1 inhibition | CD4+ T cells | 4T1 cells | activated tumor-infiltrating lymphocytes | (93) |
| CD8+ T cells | |||||||
| macrophages | |||||||
| FOIB@Lip | FA, BMS-1 oxaliplatin | IR780 | PD-L1 inhibition | CD8+ T cells | CT26 cells | improves dendritic cell maturation and infiltration of antigen-specific T cells into the tumor site | (94) |
| dendritic cells | |||||||
| IND@RAL | indoleamine-2,3-dioxygenase (IDO) inhibitor | porphyrin | TIME-remodeling agent | CD8+ T cells | 4T1 cells | activates innate and adaptive immune arms | (103) |
| CD4+ T cells | promotes antigen presentation | ||||||
| NK cells | increases T cell infiltration | ||||||
| FA-L@MD@CAT | encapsulated catalase (CA) and doxorubicin (Dox) | MBDP | TIME-remodeling agent | tumor-associated macrophage | 4T1 cells | boosts the efficacy of the PDT by decreasing hypoxia | (66) |
| decreases M2 tumor-associated macrophages (TAM) | |||||||
| PGIL | galectin-3 inhibitor (low molecular citrus pectin (LCP)) | chlorin e6 (Ce6) | TIME-remodeling agent | NK cells | A375 cells | Inhibition of migration and invasion capacities of tumor cells | (104) |
| Immune activation of NK cells |
4.3.2. Liposome-Mediated delivery of Immunogene Therapy
Fusogenic liposomes represent an optimum RNA delivery vehicle, since they protect the RNA moiety from endocytic sequestration and lysosomal destruction.95 Anionic fusogenic liposomes were created by Stremersch et al. and were assessed for their ability to transport siRNA to the monocyte/DC (JAWSII) cell line and B16F10 cancer cells.96 Another interesting delivery method for DC targeting is RNA lipoplexes. When delivered systemically to lymphoid organs, they can shield RNA from extracellular ribonucleases and permit the selective expression of their RNA cargo in local APCs. By enhancing in situ CD8 T cell immunity, Salomon et al. revealed RNA lipoplexes encoding CD4 T cell-recognizable neoantigens and created powerful adaptive T cell responses.97 Hybrid lipopolyplexes containing N1-methyl pseudouridine nucleoside-modified mRNA has been reported by Thielemans et al. to diminish inflammatory responses without impairing T-cell immunity. Compared to mRNA and lipoplexes in this investigation, immunization with lipopolyplexes demonstrated robust T cell immunity and higher efficacy in suppressing tumor growth.98
4.3.3. Phototriggered Liposomes: TME Remolding Agents
In addition to the conventional photosensitive liposomal effects in the ablation of tumors, PTT and PDT have been recently employed to alleviate the immunosuppressive TME intrinsically and, at the same time, act as a carrier for several immunotherapeutic agents.99
On the mechanistic level, the produced heat during either PTT or PDT radiation can induce the eradication of cancer cells by activating the involved immune cells at the TME. This is mainly accompanied by the release of tumor-associated antigens, ATP, and high mobility group box 1 protein into the TME and the translocation of calreticulin to the cell surface.100 Such release will result in the uptake of the antigens by antigen presentation cells and the activation of effector T cells and thus initiation signals for antitumor immunosurveillance episodes.101 In addition, PTT-mediated intrinsic immunogenic effects can further enhance antitumor immune responses of immunotherapeutic agents, leading to combinational action for effectively eradicating cancer cells and alleviating the immune-suppressive TME.102
Liu and colleagues formulated a photosensitive liposomal system coloaded with porphyrin as a photosensitizer and indoleamine-2,3-dioxygenase (IDO) (which is an enzyme that changes tryptophan (Trp) to kynurenine (Kyn), which could impair the survival and activity of CD8+ T cells) inhibitor as an immune-metabolic adjuvant. This designed liposomes were reported to initiate the cytotoxic T cell-mediated eradication of cancer cells through the induction of the immunogenic cell death (ICD) cascade and the simultaneous blockage of the IDO pathway.103 This highlights the synergistic spectacles that could happen upon combining PDT and immunotherapeutic agents and the potent reversal of the immunosuppressive TME, fighting against metastatic cancers.103 Shi et al. developed a liposome-based immune activation method that dramatically enhanced tumor growth inhibition when paired with photodynamic therapy. Specifically, they developed a novel liposomal-encapsulated catalase (CAT), lyso-targeted NIR photosensitizer (MBDP), and doxorubicin (Dox) to achieve combinatory chemo-PDT to promote tumor oxygenation and antagonize the immunosuppressive effects of the TME.66
Another study by Wang et al. reported that a chlorine e6 (Ce6) liposome formulation encapsulating galectin-3 inhibitor low molecular citrus pectin (LCP) was used against melanoma by combining PDT with immunotherapy.104 It was speculated that Ce6 induced PDT therapeutic effects against melanoma via apoptosis, inhibition of migration and invasion capacities of tumor cells, and eventually immune activation effects, as shown in Figure 5. Kim et al. reported the potent immunomodulatory effects of light-triggered liposomes in a xenograft mouse model bearing human liver bile duct carcinoma cell line.105 The Kim group formulated the liposomes using a PS-conjugated lipid encapsulating gemcitabine as a conventional chemotherapeutic agent. A confirmation of immunomodulatory cells at the TME recruited as a result of light-triggered liposomes has been done using immunohistochemical analysis and also helped in the maturation of dendritic cells, production of proinflammatory cytokines and, finally, activation of T cells.105
Figure 5.
Mechanistic role of chlorine e6 liposomes in halting cancer cells through oncological and immunological pathways. Created by Biorender.com.
Photoresponsive liposomes encapsulating immunotherapeutic and TME-modulating agents are summarized in Table 6.
5. Challenges and Opportunities of Translational Phototherapy
Although various photoresponsive liposomes are considered the mainstay of phototherapy, poor light penetration limits the applicability to cutaneous conditions.59 However, different solutions to this problem have been suggested, such as the creation of medical gadgets that make it easier to apply light endoscopically. In the case of Photofrin, an optic guide fiber diffuser was designed for employing endoscopically administered light to irradiate the lungs.106
Another option is to construct photosensitizer in nanoformulation, limiting systemic circulation and managing the photosensitizer’s action. A phase 1/2 clinical trial (NCT02367547) is currently being conducted to investigate using PDT against cancer utilizing a nanoscale lipid vesicle gel composition.106
A phase 3 clinical trial is currently being conducted in primary liver cancer patients to assess the efficacy of combining microwave hyperthermia, radiofrequency, thermal ablation, or high-intensity focused ultrasound with ThermoDox/OPTIMA, a liposomal drug that releases DOX sealed in lysolipid thermally sensitive liposomes at temperatures above 40 °C.37,107,108 This raises the question of whether the external stimulus that generates heat could be replaced with a light source, increasing the expandability of this nanoplatform.
6. Conclusions and Future Prospects
Despite the fact that phototriggered liposomes have been at the top of the list of nanocarriers that have prompted the development of precision cancer nanomedicine, there are still many challenges that limit the application of photoresponsive liposomes, particularly in solid tumors where the external shallow application of light may not be able to penetrate. This opens up the possibilities to new solutions, such as the development of nanoformulation photosensitizers that allow target-specific penetration or by embedding medical devices that allow for the use of endoscopically administered light.
This work is financially supported by the Special Research Account of the National and Kapodistrian University of Athens.
The authors declare no competing financial interest.
References
- Kim B. Y.; Rutka J. T.; Chan W. C. N Engl J Med. Nanomedicine. 2010, 363 (25), 2434–43. 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- Ramzy A. Drugless nanoparticles tune-up an array of intertwined pathways contributing to immune checkpoint signaling and metabolic reprogramming in triple-negative breast cancer. Biomed Mater. 2023, 18 (1), 015023. 10.1088/1748-605X/aca85d. [DOI] [PubMed] [Google Scholar]
- Fahmy S. A. Molecular Engines, Therapeutic Targets, and Challenges in Pediatric Brain Tumors: A Special Emphasis on Hydrogen Sulfide and RNA-Based Nano-Delivery. Cancers 2022, 14 (21), 5244. 10.3390/cancers14215244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy S. A.; Azzazy H. M. E.; Schaefer J. Liposome Photosensitizer Formulations for Effective Cancer Photodynamic Therapy. Pharmaceutics 2021, 13 (9), 1345. 10.3390/pharmaceutics13091345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy S. A. Ozonated Olive Oil: Enhanced Cutaneous Delivery via Niosomal Nanovesicles for Melanoma Treatment. Antioxidants (Basel) 2022, 11 (7), 1318. 10.3390/antiox11071318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy S. A.; et al. Stimuli-Responsive Amphiphilic Pillar[n]arene Nanovesicles for Targeted Delivery of Cancer Drugs. ACS Omega 2021, 6 (40), 25876–25883. 10.1021/acsomega.1c04297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy S. A.; et al. Peganum harmala Alkaloids Self-Assembled Supramolecular Nanocapsules with Enhanced Antioxidant and Cytotoxic Activities. ACS Omega 2021, 6 (18), 11954–11963. 10.1021/acsomega.1c00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.; Zhang C.-J.; Liu B. A Photoactivatable AIE Polymer for Light-Controlled Gene Delivery: Concurrent Endo/Lysosomal Escape and DNA Unpacking. Angewandte Chemie (International ed. in English) 2015, 54 (39), 11419–11423. 10.1002/anie.201503640. [DOI] [PubMed] [Google Scholar]
- Linsley C. S.; Wu B. M. Recent advances in light-responsive on-demand drug-delivery systems. Ther Deliv 2017, 8 (2), 89–107. 10.4155/tde-2016-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blersch J.; et al. A Light-Triggerable Nanoparticle Library for the Controlled Release of Non-Coding RNAs. Angew. Chem., Int. Ed. Engl. 2020, 59 (5), 1985–1991. 10.1002/anie.201911398. [DOI] [PubMed] [Google Scholar]
- Zhou S.; et al. UV-light cross-linked and pH de-cross-linked coumarin-decorated cationic copolymer grafted mesoporous silica nanoparticles for drug and gene co-delivery in vitro. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110469. 10.1016/j.msec.2019.110469. [DOI] [PubMed] [Google Scholar]
- Chen S.; et al. Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science 2018, 359 (6376), 679–684. 10.1126/science.aaq1144. [DOI] [PubMed] [Google Scholar]
- Chen G.; et al. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem. Rev. 2014, 114 (10), 5161–214. 10.1021/cr400425h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidgeon C.; Hunt C. A. LIGHT SENSITIVE LIPOSOMES. Photochem. Photobiol. 1983, 37 (5), 491–494. 10.1111/j.1751-1097.1983.tb04506.x. [DOI] [Google Scholar]
- ZeinElAbdeen Y. A.; AbdAlSeed A.; Youness R. A. Decoding Insulin-Like Growth Factor Signaling Pathway From a Non-coding RNAs Perspective: A Step Towards Precision Oncology in Breast Cancer. J. Mammary Gland Biol. Neoplasia 2022, 27 (1), 79–99. 10.1007/s10911-022-09511-z. [DOI] [PubMed] [Google Scholar]
- Kiriacos C. J.; et al. Prospective Medicinal Plants and Their Phytochemicals Shielding Autoimmune and Cancer Patients Against the SARS-CoV-2 Pandemic: A Special Focus on Matcha. Front Oncol 2022, 12, 837408. 10.3389/fonc.2022.837408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youness R. A.; et al. Heat Shock Proteins: Central Players in Oncological and Immuno-Oncological Tracks. Adv. Exp. Med. Biol. 2022, 1409, 193–203. 10.1007/5584_2022_736. [DOI] [PubMed] [Google Scholar]
- Elemam N. M.; et al. Expression of GPR68, an Acid-Sensing Orphan G Protein-Coupled Receptor, in Breast Cancer. Front Oncol 2022, 12, 847543. 10.3389/fonc.2022.847543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Latif M.; et al. MALAT-1/p53/miR-155/miR-146a ceRNA circuit tuned by methoxylated quercitin glycoside alters immunogenic and oncogenic profiles of breast cancer. Mol. Cell. Biochem. 2022, 477 (4), 1281–1293. 10.1007/s11010-022-04378-4. [DOI] [PubMed] [Google Scholar]
- Selem N. A.; et al. Let-7a/cMyc/CCAT1/miR-17–5p Circuit Re-sensitizes Atezolizumab Resistance in Triple Negative Breast Cancer through Modulating PD-L1. Pathol Res. Pract 2023, 248, 154579. 10.1016/j.prp.2023.154579. [DOI] [PubMed] [Google Scholar]
- Abdel-Latif M.; Youness R. A. Why natural killer cells in triple negative breast cancer?. World J. Clin Oncol 2020, 11 (7), 464–476. 10.5306/wjco.v11.i7.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafea H.; et al. LncRNA HEIH/miR-939–5p interplay modulates triple-negative breast cancer progression through NOS2-induced nitric oxide production. J. Cell Physiol 2021, 236 (7), 5362–5372. 10.1002/jcp.30234. [DOI] [PubMed] [Google Scholar]
- Allard B.; et al. Immuno-oncology-101: overview of major concepts and translational perspectives. Semin Cancer Biol. 2018, 52, 1–11. 10.1016/j.semcancer.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Zhu G.; et al. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat. Commun. 2017, 8, 1954. 10.1038/s41467-017-02191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto G. M.; et al. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21 (3), 342. 10.3390/molecules21030342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L.; et al. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano 2014, 8 (6), 5670–81. 10.1021/nn5002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.; et al. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv. Mater. 2014, 26 (48), 8154–62. 10.1002/adma.201402996. [DOI] [PubMed] [Google Scholar]
- El-Shafie S. Encapsulation of Nedaplatin in Novel PEGylated Liposomes Increases Its Cytotoxicity and Genotoxicity against A549 and U2OS Human Cancer Cells. Pharmaceutics 2020, 12 (9), 863. 10.3390/pharmaceutics12090863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y.; Siegwart D. J.; Anderson D. G. Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug Deliv Rev. 2019, 144, 133–147. 10.1016/j.addr.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H.; et al. Non-viral vectors for gene-based therapy. Nat. Rev. Genet 2014, 15 (8), 541–55. 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- Almeida B. Recent Progress in Bioconjugation Strategies for Liposome-Mediated Drug Delivery. Molecules 2020, 25 (23), 5672. 10.3390/molecules25235672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri A.; Grabowska A.; Stolnik S. Pathways of cellular internalisation of liposomes delivered siRNA and effects on siRNA engagement with target mRNA and silencing in cancer cells. Sci. Rep 2018, 8, 3748. 10.1038/s41598-018-22166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanasty R.; et al. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12 (11), 967–77. 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- Torchilin V. P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov 2014, 13 (11), 813–27. 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nsairat H.; et al. Liposomes: structure, composition, types, and clinical applications. Heliyon 2022, 8 (5), e09394 10.1016/j.heliyon.2022.e09394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhaei P. Liposomes: Structure, Biomedical Applications, and Stability Parameters With Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. 10.3389/fbioe.2021.705886. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bulbake U. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9 (2), 12. 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamala M.; et al. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–67. 10.1016/j.biomaterials.2016.01.061. [DOI] [PubMed] [Google Scholar]
- Youness R. A.; et al. Oral Delivery of Psoralidin by Mucoadhesive Surface-Modified Bilosomes Showed Boosted Apoptotic and Necrotic Effects against Breast and Lung Cancer Cells. Polymers 2023, 15 (6), 1464. 10.3390/polym15061464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter K. A.; et al. Porphyrin-phospholipid liposomes permeabilized by near-infrared light. Nat. Commun. 2014, 5, 3546. 10.1038/ncomms4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy S. A.; et al. Thermosensitive Liposomes Encapsulating Nedaplatin and Picoplatin Demonstrate Enhanced Cytotoxicity against Breast Cancer Cells. ACS Omega 2022, 7 (46), 42115–42125. 10.1021/acsomega.2c04525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavlovich A.; et al. A novel class of photo-triggerable liposomes containing DPPC:DC8,9PC as vehicles for delivery of doxorubcin to cells. Biochimica et Biophysica Acta (BBA) - Biomembranes 2011, 1808 (1), 117–126. 10.1016/j.bbamem.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi N.; et al. Influence of cholesterol inclusion on the doxorubicin release characteristics of lysolipid-based thermosensitive liposomes. Int. J. Pharm. 2018, 548 (2), 778–782. 10.1016/j.ijpharm.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Leung S. J.; Romanowski M. NIR-activated content release from plasmon resonant liposomes for probing single-cell responses. ACS Nano 2012, 6 (11), 9383–91. 10.1021/nn304434a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šturm L.; Poklar Ulrih N. Basic Methods for Preparation of Liposomes and Studying Their Interactions with Different Compounds, with the Emphasis on Polyphenols. Int. J. Mol. Sci. 2021, 22 (12), 6547. 10.3390/ijms22126547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.; et al. Photoresponsive endosomal escape enhances gene delivery using liposome–polycation–DNA (LPD) nanovectors. J. Mater. Chem. B 2018, 6 (32), 5269–5281. 10.1039/C8TB00994E. [DOI] [PubMed] [Google Scholar]
- Puri A. Phototriggerable liposomes: current research and future perspectives. Pharmaceutics 2014, 6 (1), 1–25. 10.3390/pharmaceutics6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.; et al. GE11 Peptide Conjugated Liposomes for EGFR-Targeted and Chemophotothermal Combined Anticancer Therapy. Bioinorg. Chem. Appl. 2021, 2021, 5534870. 10.1155/2021/5534870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M.; et al. Single-step assembly of DOX/ICG loaded lipid–polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano 2013, 7 (3), 2056–67. 10.1021/nn400334y. [DOI] [PubMed] [Google Scholar]
- Ding X.; et al. Designing Aptamer-Gold Nanoparticle-Loaded pH-Sensitive Liposomes Encapsulate Morin for Treating Cancer. Nanoscale Res. Lett. 2020, 15, 68. 10.1186/s11671-020-03297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi M.; et al. Layer-by-layer assembly of graphene oxide on thermosensitive liposomes for photo-chemotherapy. Acta Biomater 2018, 65, 376–392. 10.1016/j.actbio.2017.10.040. [DOI] [PubMed] [Google Scholar]
- Klán P.; et al. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013, 113 (1), 119–191. 10.1021/cr300177k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C.; et al. Near-Infrared-Triggered Azobenzene-Liposome/Upconversion Nanoparticle Hybrid Vesicles for Remotely Controlled Drug Delivery to Overcome Cancer Multidrug Resistance. Adv. Mater. 2016, 28 (42), 9341–9348. 10.1002/adma.201503799. [DOI] [PubMed] [Google Scholar]
- Bacellar I. O. L.; et al. Photodynamic Efficiency: From Molecular Photochemistry to Cell Death. International Journal of Molecular Sciences 2015, 16 (9), 20523–20559. 10.3390/ijms160920523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen K. A.; et al. Intracellular delivery of bioactive molecules using light-addressable nanocapsules. ACS Nano 2010, 4 (12), 7603–11. 10.1021/nn102345f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman J. A.; et al. Quantitative Profiling of Nanoscale Liposome Deformation by a Localized Surface Plasmon Resonance Sensor. Anal. Chem. 2017, 89 (2), 1102–1109. 10.1021/acs.analchem.6b02532. [DOI] [PubMed] [Google Scholar]
- Tomitaka A.; et al. Hybrid magneto-plasmonic liposomes for multimodal image-guided and brain-targeted HIV treatment. Nanoscale 2018, 10 (1), 184–194. 10.1039/C7NR07255D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; et al. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48 (7), 2053–2108. 10.1039/C8CS00618K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derycke A. S.; de Witte P. A. Liposomes for photodynamic therapy. Adv. Drug Deliv Rev. 2004, 56 (1), 17–30. 10.1016/j.addr.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Plenagl N.; et al. Photodynamic therapy - hypericin tetraether liposome conjugates and their antitumor and antiangiogenic activity. Drug Deliv 2019, 26 (1), 23–33. 10.1080/10717544.2018.1531954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. H.; Lim S. J.; Lee M. K. Chitosan-coated liposomes to stabilize and enhance transdermal delivery of indocyanine green for photodynamic therapy of melanoma. Carbohydr. Polym. 2019, 224, 115143. 10.1016/j.carbpol.2019.115143. [DOI] [PubMed] [Google Scholar]
- Bakowsky U. Photo-Enhanced Delivery of Genetic Material Using Curcumin Loaded Composite Nanocarriers. Clin. Oncol. 2017, 2, 1323. 10.25107/2474-1663.1323. [DOI] [Google Scholar]
- Woźniak M. The Comparison of In Vitro Photosensitizing Efficacy of Curcumin-Loaded Liposomes Following Photodynamic Therapy on Melanoma MUG-Mel2, Squamous Cell Carcinoma SCC-25, and Normal Keratinocyte HaCaT Cells. Pharmaceuticals (Basel) 2021, 14 (4), 374. 10.3390/ph14040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H. J.; et al. Photothermally Amplified Therapeutic Liposomes for Effective Combination Treatment of Cancer. ACS Appl. Mater. Interfaces 2018, 10 (7), 6118–6123. 10.1021/acsami.7b15996. [DOI] [PubMed] [Google Scholar]
- Wu P. T. Methylene-Blue-Encapsulated Liposomes as Photodynamic Therapy Nano Agents for Breast Cancer Cells. Nanomaterials (Basel) 2019, 9 (1), 14. 10.3390/nano9010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C.; et al. Catalase-based liposomal for reversing immunosuppressive tumor microenvironment and enhanced cancer chemo-photodynamic therapy. Biomaterials 2020, 233, 119755. 10.1016/j.biomaterials.2020.119755. [DOI] [PubMed] [Google Scholar]
- Feng L.; et al. Theranostic Liposomes with Hypoxia-Activated Prodrug to Effectively Destruct Hypoxic Tumors Post-Photodynamic Therapy. ACS Nano 2017, 11 (1), 927–937. 10.1021/acsnano.6b07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; et al. Indocyanine green and poly I:C containing thermo-responsive liposomes used in immune-photothermal therapy prevent cancer growth and metastasis. J. Immunother. Cancer 2019, 7 (1), 220. 10.1186/s40425-019-0702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; et al. Indocyanine green and poly I:C containing thermo-responsive liposomes used in immune-photothermal therapy prevent cancer growth and metastasis. J. Immunother. Cancer 2019, 7 (1), 220. 10.1186/s40425-019-0702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S.; et al. Temperature sensitive liposome based cancer nanomedicine enables tumour lymph node immune microenvironment remodelling. Nat. Commun. 2023, 14, 2248. 10.1038/s41467-023-38014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L.; et al. Combined Near Infrared Photothermal Therapy and Chemotherapy Using Gold Nanoshells Coated Liposomes to Enhance Antitumor Effect. Small 2016, 12 (30), 4103–12. 10.1002/smll.201503961. [DOI] [PubMed] [Google Scholar]
- Roma-Rodrigues C. Gene Therapy in Cancer Treatment: Why Go Nano?. Pharmaceutics 2020, 12 (3), 233. 10.3390/pharmaceutics12030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somiya M.; et al. Cytoplasmic delivery of small interfering RNA by photoresponsive non-cationic liposomes. Journal of Drug Delivery Science and Technology 2021, 63, 102488. 10.1016/j.jddst.2021.102488. [DOI] [Google Scholar]
- Aksoy Y. A.; et al. Spatial and Temporal Control of CRISPR-Cas9-Mediated Gene Editing Delivered via a Light-Triggered Liposome System. ACS Appl. Mater. Interfaces 2020, 12 (47), 52433–52444. 10.1021/acsami.0c16380. [DOI] [PubMed] [Google Scholar]
- Wang P.; et al. Thermo-triggered Release of CRISPR-Cas9 System by Lipid-Encapsulated Gold Nanoparticles for Tumor Therapy. Angew. Chem., Int. Ed. Engl. 2018, 57 (6), 1491–1496. 10.1002/anie.201708689. [DOI] [PubMed] [Google Scholar]
- Chen W.; Deng W.; Goldys E. M. Light-Triggerable Liposomes for Enhanced Endolysosomal Escape and Gene Silencing in PC12 Cells. Molecular Therapy - Nucleic Acids 2017, 7, 366–377. 10.1016/j.omtn.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W.; et al. Controlled gene and drug release from a liposomal delivery platform triggered by X-ray radiation. Nat. Commun. 2018, 9, 2713. 10.1038/s41467-018-05118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad A. R.; et al. An acetylated derivative of vitexin halts MDA-MB-231 cellular progression and improves its immunogenic profile through tuning miR- 20a-MICA/B axis. Nat. Prod Res. 2021, 35 (18), 3126–3130. 10.1080/14786419.2019.1686372. [DOI] [PubMed] [Google Scholar]
- Abdallah R. M.; et al. Hindering the Synchronization Between miR-486–5p and H19 lncRNA by Hesperetin Halts Breast Cancer Aggressiveness Through Tuning ICAM-1. Anticancer Agents Med. Chem. 2022, 22 (3), 586–595. 10.2174/1871520621666210419093652. [DOI] [PubMed] [Google Scholar]
- El Din G. S.; et al. miRNA-506–3p Directly Regulates rs10754339 (A/G) in the Immune Checkpoint Protein B7-H4 in Breast Cancer. Microrna 2021, 9 (5), 346–353. 10.2174/2211536609666201209152949. [DOI] [PubMed] [Google Scholar]
- Mekky R. Y.; et al. MALAT-1: Immunomodulatory lncRNA hampering the innate and the adaptive immune arms in triple negative breast cancer. Transl Oncol 2023, 31, 101653. 10.1016/j.tranon.2023.101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmoon M. A.; et al. MiR-615–5p depresses natural killer cells cytotoxicity through repressing IGF-1R in hepatocellular carcinoma patients. Growth Factors 2017, 35 (2–3), 76–87. 10.1080/08977194.2017.1354859. [DOI] [PubMed] [Google Scholar]
- Youness R. A.; et al. Contradicting interplay between insulin-like growth factor-1 and miR-486–5p in primary NK cells and hepatoma cell lines with a contemporary inhibitory impact on HCC tumor progression. Growth Factors 2016, 34 (3–4), 128–40. 10.1080/08977194.2016.1200571. [DOI] [PubMed] [Google Scholar]
- Elemam N. M.; et al. Editorial: Understanding the crosstalk between immune cells and the tumor microenvironment in cancer and its implications for immunotherapy. Front Med. (Lausanne) 2023, 10, 1202581. 10.3389/fmed.2023.1202581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youness R. A. Heat Shock Proteins: Central Players in Oncological and Immuno-Oncological Tracks. Adv. Exp. Med. Biol. 2022, 1409, 193. 10.1007/5584_2022_736. [DOI] [PubMed] [Google Scholar]
- Buchbinder E. I.; Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin Oncol 2016, 39 (1), 98–106. 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12 (4), 252–64. 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T.; et al. Cocktail Strategy Based on Spatio-Temporally Controlled Nano Device Improves Therapy of Breast Cancer. Adv. Mater. 2019, 31 (5), 1806202 10.1002/adma.201806202. [DOI] [PubMed] [Google Scholar]
- Lang T.; et al. Cocktail Strategy Based on Spatio-Temporally Controlled Nano Device Improves Therapy of Breast Cancer. Adv. Mater. 2019, 31 (33), 1903844 10.1002/adma.201903844. [DOI] [PubMed] [Google Scholar]
- Merino M.; et al. A new immune-nanoplatform for promoting adaptive antitumor immune response. Nanomedicine 2019, 17, 13–25. 10.1016/j.nano.2018.12.016. [DOI] [PubMed] [Google Scholar]
- Hei Y.; et al. Multifunctional Immunoliposomes Combining Catalase and PD-L1 Antibodies Overcome Tumor Hypoxia and Enhance Immunotherapeutic Effects Against Melanoma. Int. J. Nanomedicine 2020, 15, 1677–1691. 10.2147/IJN.S225807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z.; et al. Nanotechnology-mediated immunochemotherapy combined with docetaxel and PD-L1 antibody increase therapeutic effects and decrease systemic toxicity. J. Controlled Release 2018, 286, 369–380. 10.1016/j.jconrel.2018.08.011. [DOI] [PubMed] [Google Scholar]
- Lan X.; et al. Photo-manipulated polyunsaturated fatty acid-doped liposomal hydrogel for flexible photoimmunotherapy. Chin. Chem. Lett. 2023, 108616. 10.1016/j.cclet.2023.108616. [DOI] [Google Scholar]
- Yu J.; et al. Combining PD-L1 inhibitors with immunogenic cell death triggered by chemo-photothermal therapy via a thermosensitive liposome system to stimulate tumor-specific immunological response. Nanoscale 2021, 13 (30), 12966–12978. 10.1039/D1NR03288G. [DOI] [PubMed] [Google Scholar]
- Yuba E.; et al. The application of pH-sensitive polymer-lipids to antigen delivery for cancer immunotherapy. Biomaterials 2013, 34 (22), 5711–21. 10.1016/j.biomaterials.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Stremersch S.; et al. Comparing exosome-like vesicles with liposomes for the functional cellular delivery of small RNAs. J. Controlled Release 2016, 232, 51–61. 10.1016/j.jconrel.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Salomon N.; et al. A liposomal RNA vaccine inducing neoantigen-specific CD4(+) T cells augments the antitumor activity of local radiotherapy in mice. Oncoimmunology 2020, 9 (1), 1771925. 10.1080/2162402X.2020.1771925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Jeught K.; et al. Dendritic Cell Targeting mRNA Lipopolyplexes Combine Strong Antitumor T-Cell Immunity with Improved Inflammatory Safety. ACS Nano 2018, 12 (10), 9815–9829. 10.1021/acsnano.8b00966. [DOI] [PubMed] [Google Scholar]
- Xu C.; et al. Second Near-Infrared Light-Activatable Polymeric Nanoantagonist for Photothermal Immunometabolic Cancer Therapy. Adv. Mater. 2021, 33 (36), 2101410 10.1002/adma.202101410. [DOI] [PubMed] [Google Scholar]
- Shi Y.; Lammers T. Combining Nanomedicine and Immunotherapy. Acc. Chem. Res. 2019, 52 (6), 1543–1554. 10.1021/acs.accounts.9b00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X.; Chan C.; Lin W. Nanoparticle-Mediated Immunogenic Cell Death Enables and Potentiates Cancer Immunotherapy. Angew. Chem., Int. Ed. Engl. 2019, 58 (3), 670–680. 10.1002/anie.201804882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C.; Pu K. Second near-infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 2021, 50 (2), 1111–1137. 10.1039/D0CS00664E. [DOI] [PubMed] [Google Scholar]
- Liu D.; et al. Redox-Activated Porphyrin-Based Liposome Remote-Loaded with Indoleamine 2,3-Dioxygenase (IDO) Inhibitor for Synergistic Photoimmunotherapy through Induction of Immunogenic Cell Death and Blockage of IDO Pathway. Nano Lett. 2019, 19 (10), 6964–6976. 10.1021/acs.nanolett.9b02306. [DOI] [PubMed] [Google Scholar]
- Wang S.; et al. Chlorin-Based Photoactivable Galectin-3-Inhibitor Nanoliposome for Enhanced Photodynamic Therapy and NK Cell-Related Immunity in Melanoma. ACS Appl. Mater. Interfaces 2019, 11 (45), 41829–41841. 10.1021/acsami.9b09560. [DOI] [PubMed] [Google Scholar]
- Kim D. H.; et al. Gemcitabine-loaded DSPE-PEG-PheoA liposome as a photomediated immune modulator for cholangiocarcinoma treatment. Biomaterials 2018, 183, 139–150. 10.1016/j.biomaterials.2018.08.052. [DOI] [PubMed] [Google Scholar]
- Harewood G. C.; et al. Pilot study to assess patient outcomes following endoscopic application of photodynamic therapy for advanced cholangiocarcinoma. J. Gastroenterol Hepatol 2005, 20 (3), 415–20. 10.1111/j.1440-1746.2005.03582.x. [DOI] [PubMed] [Google Scholar]
- Lyon P. C.; et al. Clinical trial protocol for TARDOX: a phase I study to investigate the feasibility of targeted release of lyso-thermosensitive liposomal doxorubicin (ThermoDox) using focused ultrasound in patients with liver tumours. J. Ther Ultrasound 2017, 5, 28. 10.1186/s40349-017-0104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami T.; Ozeki T. Recent Trends in Clinical Trials Related to Carrier-Based Drugs. J. Pharm. Sci. 2017, 106 (9), 2219–2226. 10.1016/j.xphs.2017.02.026. [DOI] [PubMed] [Google Scholar]







