ABSTRACT
CD8 T cells are important tools for protection against intracellularly replicating pathogens such as viruses. Previous studies showed that a discrete population of HLADR and CD38-expressing CD8 T cells expands massively during the acute febrile phase of human dengue virus infection—but very few of these cells secrete IFNγ upon in vitro stimulation with dengue peptides. To gain a better understanding of what other cytokines/chemokines do these massively expanding HLADR+CD38+ CD8 T cells express, we performed RNA seq of sorted HLADR+CD38+ CD8 T cell subsets after peptide stimulation. A majority of these peptide-stimulated HLADR+CD38+ CD8 T cells were CD69-IFNγ-, nearly a third were CD69+IFNγ-, whereas very few (<10%) were CD69+IFNγ+. The CD69-IFNγ- subset was enriched for the expression of key genes implicated in the negative regulation of T cell receptor (TCR) signaling and T-cell exhaustion, attraction of B cells and other lymphocytes, and cytokines related to Tc17/T-reg lineages or those that are implicated in immunosuppression/immunomodulatory and anti-inflammatory activities and angiogenesis. The CD69+IFNγ- subset showed enriched transcription of key genes implicated in cytotoxic effector functions as well as costimulatory and signaling adaptors implicated in fine balancing of T cell receptor signaling. The CD69+IFNγ+ subset largely shared the transcriptional profile with the CD69+IFNγ- subset—but with relatively more pronounced expression along with additional genes such as chemokines XCL1/XCL2. Our findings showing distinct functional subsets among these massively expanding CD8 T cells in dengue CD8 T cells warrant further studies to carefully examine the precise role of these T cell subsets in protection against dengue.
IMPORTANCE
CD8 T cells play a crucial role in protecting against intracellular pathogens such as viruses by eliminating infected cells and releasing anti-viral cytokines such as interferon gamma (IFNγ). Consequently, there is significant interest in comprehensively characterizing CD8 T cell responses in acute dengue febrile patients. Previous studies, including our own, have demonstrated that a discrete population of CD8 T cells with HLADR+ CD38+ phenotype undergoes massive expansion during the acute febrile phase of natural dengue virus infection. Although about a third of these massively expanding HLADR+ CD38+ CD8 T cells were also CD69high when examined ex vivo, only a small fraction of them produced IFNγ upon in vitro peptide stimulation. Therefore, to better understand such functional diversity of CD8 T cells responding to dengue virus infection, it is important to know the cytokines/chemokines expressed by these peptide-stimulated HLADR+CD38+ CD8 T cells and the transcriptional profiles that distinguish the CD69+IFNγ+, CD69+IFNγ-, and CD69-IFNγ- subsets.
KEYWORDS: CD8 T cells, dengue, transcriptomics, IFNγ production, human
INTRODUCTION
Dengue is a global epidemic with an estimated 390 million infections and over 100 million clinical cases annually (1). The clinical disease can range from mild febrile dengue fever to more severe forms of the disease, which include dengue hemorrhagic fever and/or shock syndrome (2). Heightened vascular permeability is central to these severe disease manifestations (3 – 5). Historical epidemiological studies showed that individuals who had a past dengue virus exposure were more likely to develop these severe forms of the disease (6 – 11) and several hypotheses have been proposed to explain this. One of the main hypotheses is based on a phenomenon called antibody-dependent enhancement of infection (12 – 14). This hypothesis suggests that cross-reactive yet poorly cross-neutralizing antibodies that are either produced against a previously encountered serotype or transferred from a dengue immune mother to their infants, can enhance infection (4, 15 – 18). Moreover, intense infection may produce and release high concentrations of viral nonstructural protein 1 (NS1) antigen that can increase vascular permeability resulting in endothelial cell damage (19 – 22). Another proposed hypothesis is that a potent T cell-mediated recall response produces massive amounts of effector mediators resulting in a so-called cytokine storm, which is responsible for the observed immunopathology (23 – 25). This hypothesis gained traction when it was observed that activated CD8 T cells peaked in dengue patients around the time of commencement of capillary leakage (26). However, more recent studies have shown a protective role of CD8 T cells in murine models of dengue as well as an HLA allelic-associated multifunctional protective T-cell memory in humans (27 – 30). Considering these and given that the T-cell responses are emerging as a key issue for vaccine design and evaluation (31 – 33), interest in a comprehensive characterization of the T-cell responses during dengue natural infection as well as vaccination is emerging (34 – 37).
Previously, we and others have shown that a discrete population of HLADR and CD38-expressing CD8 T cells expands massively during the acute febrile phase of dengue natural infection (26, 38, 39). Interestingly, only a small proportion of these massively expanding cells made IFNγ even when stimulated with dengue peptide pools spanning the entire proteome (38, 40).
These findings opened several knowledge gaps: are there any other functional subsets within these massively expanding but non-IFNγ producing HLADR+CD38+ CD8 T cell populations? If they are, then what other cytokines/chemokines do these massively expanding cells express in place of IFNγ? How different are the transcriptional profiles of these massively expanding non-IFNγ producing HLADR+CD38+ CD8 T cell populations compared to the smaller population that produces IFNγ?
To address these knowledge gaps, here we performed RNA seq analysis of HLADR+CD38+ CD8 T cells that were stimulated with dengue peptides. We found that nearly half of these peptide stimulated HLADR+CD38+ CD8 T cells were CD69-IFNγ-, nearly a third were CD69+IFNγ-, whereas very few (<10%) were CD69+IFNγ+. The CD69-IFNγ- subset was enriched in pathways involved in inflammatory homing and extravasation. This subset downregulated genes positively associated with TCR signaling and was enriched for key genes implicated in the negative regulation of TCR signaling (PTPRF), attraction of B cells (CXCL13), and other lymphocytes (IL-16) and cytokines related to Tc17 (IL-17B) and T-reg (IL-34) lineages or those that are implicated in immunosuppression/immunomodulatory and anti-inflammatory activities and angiogenesis (IL-19, IL-36RN, IL-37, PDGFB, and TGFA). The CD69+IFNγ- subset showed enrichment in the transcription of key genes implicated in cytotoxic effector functions (GZMA, GZMB, GZMH, PRF1, GNLY, CTSW, CRTAM, and IGF2R) as well as costimulatory and signaling adaptors (DUSP4, SH2D2A, PTPN22, and JAK2) implicated in fine balancing of T cell receptor signaling. The smallest population of CD69+IFNγ+ largely shared the transcriptional profile of the CD69+IFNγ- subset and showed more heightened expression of common genes—and additionally also expressed other immune function genes that included chemokines XCL1/XCL2. Taken together, these results show that distinct functional subsets are present within the massively expanding HLADR+CD38+ CD8 T cells during natural dengue virus infections and provide valuable insights into the inherent diversity of these effector CD8 T cell responses.
MATERIALS AND METHODS
Dengue confirmation
For dengue confirmation, Dengue NS1 Elisa (J Mitra, IR031096) and Dengue IgM Elisa (Pan Bio, 01PE20) were used in combination. Based on WHO recommendations (2), only patients who were positive either for dengue NS1 and/or dengue IgM were analyzed in this study. Using these criteria allowed for the identification of dengue NS1 positive patients who may not have detectable antibody response at the time of symptom onset, and simultaneously also identify individuals who have an IgM response in response to either homologous (in case of primary) or heterologous (in case of secondary) infection. Additionally, we also ascertained that these patients were negative for malaria (SD Bioline, 05EK40) and chikungunya (J Mitra, IR061010).
Characterization of primary and secondary infection status
Primary and secondary infections were classified using the standard Pan bio capture ELISAs (Cat# 01PE10/01PE20) that quantify IgM and IgG ratios in plasma diluted at 1:100. Using WHO criteria (2), patients were characterized as primary when the IgM/IgG ratio was >1.2 and as secondary when IgM/IgG ratio was <1.2.
Whole blood processing and PBMC/ plasma isolation
The Vacutainer CPT tube (Becton Dickenson, Cat# 362761) containing blood samples was centrifuged at 1,500 g for 25 min without brake at room temperature. After centrifugation, the uppermost layer containing plasma was aspirated and then transferred to cryo-vial tubes and stored at −80°C. The white buffy coat above the gel plug containing the peripheral blood mononuclear cells (PBMCs) was transferred to a sterile 15 mL falcon tube and filled with phosphate-buffered saline (PBS) (HiMedia #TL1099) and centrifuged at 1,800 RPM for 8 min at 4°C. After centrifugation, the supernatant was discarded, the pellet was re-suspended and washed twice in PBS. After washing, the PBMC pellet was re-suspended in red blood cell lysis buffer (HiMedia Cat#R075) and incubated for 2 min to remove any remaining RBCs. The 15 mL tube was then filled with RPMI 1640 media (HiMedia #AL162S) containing 1% fetal bovine serum (FBS) and centrifuged at 1,500 RPM for 5 min at 4°C and washed twice. The pellet was re-suspended in 1 mL of RPMI media containing 10% FBS and the cells were counted using 0.1% trypan blue (HiMedia #TCL046). The cells were either used immediately or cryopreserved in liquid nitrogen in FBS (Hyclone #SV30014.03) with 10% dimethyl sulfoxide (MP #196055) for later analysis.
Analytical flow cytometry
PBMCs were washed and surface stained for 30 min in ice-cold FACS buffer at 4°C (1× PBS containing 0.25% bovine serum albumin). Fixable viability dye e-Fluor 780 (eBioscience, 65086518) was used for excluding dead cells at the time of analysis. For CD8 T cell subset analysis, surface staining on PBMCs was performed with antibodies against CD3 (clone UCHTI), CD8 (clone SK1), CD38 (clone H1T2), and HLADR (clone L243) antibodies. For phenotypic analysis, subsets of the samples were co-stained with antibodies against CD45RA (clone HI100), CCR7 (clone 150503), CD127 (clone EbioRDR5), CX3CR1 (clone 2A91), and CCR5 (clone 3A9). For intracellular detection of cytotoxic effector molecules, subsets of the samples were fixed and permeabilized using the Cytofix/Cytoperm Kit (BD, 554714) and co-stained with antibodies against perforin (clone deltaG9) or Granzyme A (clone CB9), and Granzyme B (clone GB11) or granulysin (clone RB1).
For intracellular cytokine staining, peptide-stimulated and unstimulated cells were first surface stained using antibodies against CD3, CD8, CD38, and HLADR, followed by treatment with fixation buffer, and then permeabilized with Cytofix/Cytoperm (e-Bioscience) and stained with antibodies against IFNγ that were diluted in 1× perm buffer (BD, 554723) for 60 min. Flow cytometry data acquisition was performed on BD LSR Fortessa X-20. Data were analyzed using FlowJo software (TreeStar Inc.). CD8 T cells were gated as those that co-expressed CD3 and CD8 and were analyzed for phenotypes and functions.
Ex vivo stimulation and functional assessment of CD8 T cells
Isolated PBMCs were plated at 1 × 106 cells/well in 96-well U-bottom plates. Unless otherwise mentioned, the cells were cultured with a pool of 511 overlapping peptides spanning entire dengue proteome (BEI resources catalog numbers: NR506, PreM protein; NR505, Capsid protein; NR507, Envelope protein; NR508, NS1 protein; NR2747, NS2a protein; NR2748, NS2b protein; NR509, NS3 protein; NR2749, NS4a protein; NR2750, NS4b protein; NR2746, NS5 protein) at a final concentration of 1 µg/mL of each peptide along with co-stimulation with purified anti-human CD28 (clone L293) and CD49D (clone L25). Unstimulated cells were used as a negative control. As a positive control, cells were also stimulated with 1× cell stimulation cocktail containing phorbol 12-myristate 13-acetate (PMA) and ionomycin (eBioscience, 00-4970-03). Cells were then cultured for 2 hours at 37°C in a 5% CO2 incubator followed by the addition of protein secretion inhibitor cocktail containing brefeldin A and monensin (GolgiPlug, BD, 555029) for another 4 hours. The cells were then harvested, surface stained with antibody cocktail containing fixable viability dye, CD3, CD8, CD38, and HLA-DR, and then fixed and permeabilized using Cytofix/Cytoperm Kit. Cells were then processed for intracellular staining with antibodies against IFNγ (clone 4S.B3). Cells were acquired on BD Canto II or BD LSRFortessa X-20 and analyzed using FlowJo (TreeStar).
IFNγ capture assay to identify and sort IFNγ-producing CD8 T cells
For the identification and sorting of viable IFNγ-secreting cells, we used a human IFNγ secretion assay detection kit (PE) (Miltenyi Biotec, #130-054-202) as per the manufacturer’s protocol. Briefly, PBMCs were cultured in 12-well flat-bottom plate and stimulated with dengue CD8 megapools (a kind gift from Dr. Daniela Weiskopf and contain 268 immunodominant peptides that span most commonly represented HLAs) (28), which were prepared at a final concentration of 1 µg/mL for each peptide. Unstimulated cells were used as controls. Cells were cultured in a tissue culture incubator at 37°C for 3 hours in the presence of 5% CO2. The cells were then mixed with IFNγ catch reagent (PE-conjugated anti-human IFNγ mouse monoclonal IgG1 conjugated to anti-human CD45 mouse IgG2a). The cells were then allowed to secrete IFNγ for an additional 45 min and then IFNγ-secreting cells were identified by labeling with PE-conjugated IFNγ detection antibody (anti-human IFNγ mouse IgG1) conjugated to PE.
Flow cytometric cell sorting for IFNγ producing and non-producing CD8 T cells
After staining the cells with IFNγ capture reagent as described in the section above, the cells were surface stained with fixable viability dye, CD3 (clone UCHT1), CD4 (clone RPA-T4/OKT-4), CD8 (clone SK1), CD38 (clone H1T2), HLADR (clone L243), and CD69 (clone FN50). Cells were then analyzed in BD FACS Aria Fusion II. After lymphocyte and doublet discrimination, we first gated on CD3+, then excluded CD4+ cells. After this, the gated CD8+CD3+ population was analyzed to distinguish HLADR+CD38+CD69+IFNγ+, HLADR+ CD38+CD69+IFNγ-, and HLADR+CD38+CD69-IFNγ- and then sorted in lysis buffer at 4°C using BD FACS Aria Fusion II cell sorter. As controls, we also sorted the total HLADR+CD38+CD8 T cells and HLADR-CD38-CD8 T cells that were not stimulated with the dengue peptide pool.
RNA isolation and library preparation for RNA seq
RNA sequencing libraries from the bulk sorted cell subsets were prepared with Illumina compatible SMARTer Stranded Total RNA-Seq Kitv2—Pico Input Mammalian (TakaraBio, Inc. CA, USA) at Genotypic Technology Pvt. Ltd., Bangalore, India. For each condition, approximately 200–500 cells were sorted and collected in 10× lysis buffer of SMART-Seq v4 ultra-low input RNA seq kit. From this, 8 µL was taken as input for SMARTer Stranded Total RNA-Seq Kit v2—Pico Input Mammalian for fragmentation (with slight modification) and first strand cDNA synthesis followed by the addition of Illumina adaptors and indexes via five cycles of PCR. Indexed libraries were purified using JetSeq SPRI magnetic beads (Bio, # 68031). To remove rRNA fragments, the cDNA was cleaved by ZapR with the libraries hybridized to R-probes. The resulting library fragments that were ribo-depleted were further enriched by 16–18 cycles of PCR. The final ribo-depleted libraries were purified using JetSeq SPRI magnetic beads (Bio, # 68031). The concentration of the libraries was measured using a Qubit fluorometer (Thermo Fisher Scientific, MA, USA) and then analyzed on an Agilent 2100 Bioanalyzer for fragment size distribution.
RNA seq data analysis
The transcriptome analysis was performed by processing the raw data for the removal of low-quality data and adaptor sequences. The raw reads were processed using FastQC for quality assessment and pre-processing, which includes removing the adapter sequences and low-quality bases (<q30) using TrimGalore. The high-quality reads were considered for alignment with the reference genome using a spliced aligner. Only the high-quality data were aligned to reference (Homo sapiensGRCh38.p13 build genome downloaded from Ensembl database) using Hisat2 with the default settings to identify the alignment percentage. Reads were classified into aligned and unaligned reads depending on whether they aligned to the reference genome. Hisat2 was used to calculate raw read counts for each gene after alignment. For differential gene expression analysis, low count genes with a mean raw read count <100 per subset were excluded. All differential gene expression analysis was performed using R package DESeq2. Genes with Benjamini-Hochberg (B-H) adjusted P-value < 0.05 were considered significant. Furthermore, genes with log2 fold change of ≥+1 were considered upregulated, whereas ≤−1 were considered downregulated genes. For heatmaps, log normalized counts transformed to their Z-scores were used. Z-score was calculated by subtracting normalized counts from the mean of the entire data set and then dividing it by the standard deviation (σ) of the data set. Ward.D2 method was used for hierarchal clustering, which employs the sum of squares of Euclidian distances to perform clustering. R packages clusterProfiler, ggplot2, and dplyr were used for data transformation and plotting.
Pathway enrichment analysis
Gene set enrichment analysis (41) was performed using 346 Blood transcription modules (BTMs) (42). Differentially expressed genes (DEGs) for each HLADR+CD38+ subset compared to HLADR-CD38- CD8 T cells (DN) were used as input with default parameters (number of permutations: 1,000 and enrichment statistics: weighted). Each of the positively and negatively enriched modules with normalized enrichment score (NES) of >+1.5 or <−1.5, respectively, false discovery rate < 25%, and nominal P-value < 0.05 were selected for downstream analysis.
Statistical analysis
The data were curated in MS Excel and statistical analysis was performed on GraphPad Prism and by using R programming language. For analysis of groups, an unpaired two-tailed t test was used and P values were interpreted as *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; and ****P < 0.0001.
RESULTS
The massively expanding HLADR+ CD38+ CD8 T cell population found in the acute febrile dengue patients constitutes functionally distinct subsets
To determine the expansion, phenotypes, and functional subsets of the CD8 T cell responses, we first analyzed PBMC from dengue-confirmed acute febrile patients. The patient characteristics are shown in Table 1. HLADR and CD38 are well-defined surface markers for the identification of activated CD8 T cells (38, 43, 44). Previously, we and others have shown that these HLADR+CD38+ CD8 T cells expand massively during the acute febrile phase of natural dengue virus infection—but that very few of these cells produce IFNγ when stimulated with dengue peptides (38, 40). Consistent with this, we found that the HLADR+CD38+ CD8 T cell population expanded massively in the dengue patients who were recruited in this study (Fig. 1A), with frequencies reaching as high as 80% of the total CD8 T cells (Fig. 1B, left) and the absolute numbers reaching million cells/mL of the blood (Fig. 1C, right). The expansion was comparable (P = 0.69) between patients with primary (21.44 ± 2.23, n = 63) versus secondary dengue infections (22.77 ± 2.14, n = 37), and also comparable (P = 0.5003) between males (22.32 ± 1.489, n = 63) and females (20.78 ± 1.553, n = 37). These massively expanding HLADR+CD38+ CD8 T cell population was blasting as seen by high forward scatter and side scatter and robustly expressed proliferating cell marker (Ki67), downregulated markers of naive cells (CD45RA, CCR7, and CD127), upregulated inflammatory homing receptors (CX3CR1 and CCR5), and upregulated several markers indicative of cytotoxic T-cell effector functions (granzyme A, granzyme B, and granulysin) (Fig. 1D). Consistent with the previous studies, we also found that only a small proportion of these massively expanding HLADR+CD38+ CD8 T cells made IFNγ even when stimulated with overlapping peptides spanning the entire dengue proteome (Fig. 1E). The highest frequency of the IFNγ+ cells among the gated HLADR+CD38+ CD8 T population was <5% with mean ± SEM being 0.75 ± 0.18 (Fig. 1F). The IFNγ production was comparable (P = 0.502) between patients with primary (1.48 ± 0.75, n = 25) versus secondary dengue infections (0.76 ± 0.41, n = 19), and also comparable (P = 0.6203) between males (1.08 ± 0.34, n = 42) and females (1.4 ± 0.68, n = 14). Similar results were obtained when we employed a well-characterized CD8 T-cell epitope mega pool (kind gift from Dr. Daniela Weiskopf) and a sensitive IFNγ capture assay for the detection of the IFNγ-producing cells. Even by using this sensitive assay, we found that a majority of these peptides stimulated HLADR+CD38+ CD8 T cells was CD69-IFNγ- phenotype, nearly a third was CD69+IFNγ- phenotype, and only <10% was CD69+ IFNγ+ phenotype.
TABLE 1.
Characteristics of dengue-confirmed adult acute febrile patients
| Total number of patients | 100 |
|---|---|
| No. of females/males | 37/63 |
| Average age of patients in years (range) | 28.42 (17–63) |
| Average days post onset of clinical symptoms (range) | 7.5 (3-12) |
| Number of primary/secondary dengue infections a | 63/37 |
Primary and secondary infections were classified using capture ELISA (PanBio) according to WHO guidelines. Primary infections were defined as those with an IgM/IgG ratio > 1.2 and secondary infections were defined as those with an IgM/IgG ratio < 1.2.
Fig 1.
Phenotype analysis of the CD8 T cell responses in acute dengue febrile patients. (A) Example of flow cytometry plot gated on total CD8 T cell showing the evaluation of the HLADR+CD38+ CD8 T cells in healthy (left) and dengue febrile patients (right). (B) The scatter plots show the frequencies (percentages) of HLADR+CD38+CD8 T cells among the gated CD8 T cell population and (C) absolute numbers per milliliter of blood in healthy and dengue febrile adults. (D) The histogram plots represent the pattern of the expression of various phenotypic markers studied. HLADR+CD38+ CD8 T population is red color, and all other CD8 T cells are green. (E) Flow cytometry plots show examples of side scatter and IFNγ production in unstimulated and peptide-stimulated HLADR+CD38+ CD8 T cells. (F) Scatter plots compare the frequencies of HLADR+CD38+ CD8 T cells and IFNγ-producing cells within the HLADR+CD38+ CD8 T cells.
Transcriptional profiling of the sorted CD69-IFNγ-, CD69+IFNγ-, and CD69+IFNγ+ subsets from the peptide-stimulated HLADR+CD38+ CD8 T cell population
To more rigorously address potential qualitative differences between the subsets of the peptide-stimulated HLADR+CD38+ population beyond the IFNγ and CD69 expression, we sorted CD69-IFNγ-, CD69+IFNγ-, and CD69+IFNγ+ subsets from the patients described in Table 2 and performed bulk RNA sequencing analysis. As controls, we also sorted total HLADR+CD38+ CD8 T cells that were not peptide stimulated (hereafter referred to as unstimulated double positive, unstim DP) and total HLADR-CD38- cells (hereafter referred to as unstimulated double negative, unstim DN) from four additional patients. Figure 2A shows the gating strategy. The post-sort purity was >96%.
TABLE 2.
Frequency of CD69-IFNγ-, CD69+IFNγ-, and CD69+IFNγ+ subsets in dengue patients stimulated with peptide megapools and stained by IFNγ capture assay
| Days post onset of clinical symptoms | Gender | Frequencies (%) | ||||
|---|---|---|---|---|---|---|
| HLADR+CD38+ CD8 T cells | CD69+IFNγ+ cells within HLADR+CD38+ CD8 T cells | CD69+IFNγ cells within HLADR+CD38+ CD8 T cells | CD69-IFNγ- cells within HLADR+CD38+ CD8 T cells | |||
| Subject 1 | 6 | Male | 35 | 9.87 | 32.1 | 57.4 |
| Subject 2 | 8 | Male | 24 | 4.49 | 34.7 | 60.5 |
| Subject 3 | 7 | Female | 20 | 3.30 | 34.8 | 61.4 |
Fig 2.
Global transcriptional profiling/analysis using RNA-seq of distinct functional subsets of HLADR+CD38+ CD8 T cells. (A) The subsets of activated CD8 T cells were sorted using flow cytometry based on the expression of CD38, HLADR, CD69, and IFNγ following ex vivo stimulation for 3 hours with and without dengue peptides. Examples of the sorting strategy and purity of sort are shown. (B) PCA of 14,959 genes in the five subsets of CD8 T cells analyzed—unstimulated HLADR-CD38- (DN, n = 4, gray), unstimulated HLADR+CD38+ (Unstim DP, n = 4, red), stimulated HLADR+CD38+ CD69-IFNγ- (CD69-IFNγ-, n = 3, violet), stimulated HLADR+CD38+ CD69+IFNγ- (CD69+IFNγ-, n = 3, brown), and stimulated HLADR+CD38+ CD69+IFNγ- (CD69+IFNγ+, n = 3, blue). The gender of the individual patients from whom the samples were derived is indicated (male, M and female, F). Samples from patients with secondary dengue infection are differentiated from primary dengue infection with a yellow outline. (C) Volcano plots highlighting differentially expressed genes in the following comparisons: Unstim DP versus DN (first from left), CD69-IFNγ- versus DN (second from left), CD69+IFNγ- versus DN (third from left), and CD69+IFNγ+ versus DN (right). Each dot represents a gene with log2 fold change (Log2FC) on the x-axis and negative log10 B-H adjusted P-value (-Log10 adjusted P) on the y-axis. Upregulated genes (Log2FC > 1 and adjusted P-value < 0.05) are shown in red, downregulated genes (Log2FC < −1 and adjusted P-value < 0.05) are shown in blue, and genes that are not significant are shown in gray. (D) Heatmap showing hierarchal clustering of all DEGs from the comparisons shown in panel (C) based on Ward.D2 algorithm. Normalized gene expression was converted into Z-scores for plotting. (E) Enrichment analysis of DEGs of HLADR+CD38+ subsets compared to HLADR-CD38- with BTMs. Only modules with FDR < 25% are highlighted. The color of each bubble represents NES ranging from red to blue for positive to negative enrichment, respectively. The size of the bubble represents the frequency of genes in a module that showed positive or negative enrichment.
The DEGs of the unstim DP compared to unstim DN were largely consistent with our previous study (38) in which we performed microarray analysis of the ex vivo-sorted DP (Table S1), suggesting a high degree of similarity between the transcriptional patterns of the ex vivo-sorted HLADR+CD38+ CD8 T cells versus those that were cultured without any peptide stimulation in vitro (unstim DP in this study) suggesting that no major transcriptional change occurs due to a few hours of culture.
Principal component analysis (PCA) of 14,957 genes that were obtained after filtering out low count genes is shown in Fig. 2B. The DN population formed a distinct cluster compared to all the other four subsets. This result is consistent with our previous study (38) and further indicated that these massively expanding HLADR+CD38+ CD8 T cells, regardless of their peptide stimulation status or their IFNγ production status, were undergoing global transcriptional changes compared to the non-activated cells. Within these massively expanding HLADR+CD38+ CD8 T cells, the CD69-IFNγ- subset, which constituted the bulk of the HLADR+CD38+ population, clustered together with the total unstim DP. This result indicated that the CD69-IFNγ- subset was either refractory or blind to peptide stimulation. Interestingly, the CD69+IFNγ+ subset and the CD69+IFNγ- subsets of the peptide-stimulated HLADR+CD38+ population formed distinct clusters suggesting that these two subsets differed in their global transcriptional programs beyond the IFNγ and CD69. The DEGs in the unstim DP, CD69-IFNγ- subset, CD69+IFNγ- subset, and the CD69+IFNγ+ subset as compared to the DN subset is shown as volcano plots in Fig. 2C and as a heat map in Fig. 2D; and the raw data on expression counts of these DEGs are provided in Table S2A through D. Consistent with the PCA analysis, the DEGs found in the unstim DP were highly similar to the DEGs found in the CD69-IFNγ- subset (Fig. 2C, first and second plots and Fig. 2D, second and third columns; Table S2A through D). The DEGs of these two subsets largely differed from the DEGs of the CD69+IFNγ- subset and the CD69+IFNγ+ subsets. Also, there were some differences in the DEGs found in the CD69+IFNγ- subset from the DEGs found in the CD69+IFNγ+ subset (Fig. 2C, third and fourth plots and Fig. 2D, third and fourth columns; Table S2E through H).
The analysis of key biological pathways enriched in each of these subsets is summarized in Fig. 2E and the raw data on the genes expressed in each of these pathways are elaborated in Table S3. All the four populations (i.e., unstim DP, stimulated CD69-IFNγ-, CD69+IFNγ-, and CD69+IFNγ+) were enriched for proliferation and DNA repair (Fig. 2E, first box from top). Only the unstim DP and the CD69-IFNγ- subsets were highly enriched for pathways associated with adhesion and tissue extravasation (Fig. 2E, second box from top). These two subsets also downregulated several pathways associated with T-cell activation and signaling (Fig. 2E, third box from top). By contrast, the CD69+IFNγ- and the CD69+IFNγ+ subsets did not substantially downregulate these TCR signaling pathways, and moreover, highly enriched for other pathways associated with TCR signaling (Fig. 2E, fourth box from top). Interestingly, only the CD69+IFNγ+ subset showed an additional enrichment for RNA processing and protein translation (Fig. 2E, fifth box from top).
Notable findings with a focus on expression differences in the key genes of interest related to cytokines/chemokines and their receptors, cytotoxic and effector functions, TCR signaling, and metabolism are elaborated in the following sections.
Cytokines/chemokines
Notable findings regarding subset-specific expression differences among the cytokine/chemokines are summarized in Fig. 3A. Raw count data along with statistical values are shown in Table S4A. The unstim DP and the stimulated CD69-IFNγ- subsets showed a bias toward the expression of chemokines involved in the attraction of B cells (CXCL13) (45) and other lymphocytes (IL-16) (46), and cytokines related to Tc17 (IL-17B) (47) and T-reg (IL-34) (48) lineages or those that are implicated in promoting the formation of inducible T-regs (GRN) or in immunosuppression/immunomodulatory and anti-inflammatory activities, and angiogenesis (IL-19, IL-36RN, IL-37, PDGFB, and TGFA) (49 – 54). There was very little or no expression of these cytokines/chemokines in the CD69+IFNγ- and the CD69+IFNγ+ subsets. Interestingly, only the peptide-stimulated CD69+IFNγ+ subset and no other subset robustly expressed chemokines that are implicated in dendritic cell cross talk (XCL1) (55) and CD8 T cell migration (XCL2) (56).
Fig 3.
Key (A) cytokine/chemokine and (B) their receptors differentially expressed in functionally distinct HLADR+CD38+ CD8 T cell subsets. Heatmap showing the expression profile of key genes of interest (left). Color gradient represents the normalized gene expression transformed into z-scores from blue (low expression) to red (high expression). For each gene, bar plots representing average normalized counts of DN, Unstim DP, CD69-IFNγ-, CD69+IFNγ-, and CD69+IFNγ+ subsets are shown. Error bar represents standard error (SEM). Significance values between the groups are provided in Table S5.
Cytokine/chemokine receptors
Expression differences among the cytokine/chemokine receptors are summarized in Fig. 3B. Raw count data along with statistical values are shown in Table S4B. The DN and the CD69-IFNγ- subsets showed enriched expression of receptors for prostaglandin E2 (PTGER3) (57), receptors for pro-inflammatory cytokines IL-12/IL-23 (IL-12RB1), IL-17 (IL17RE), and IL-3/IL-5 (CSF2RB) (58), and receptor for adipose tissue chemokine-like chemerin (CMKLR1) (59). By contrast, the CD69+IFNγ- subset and the CD69+IFNγ+ subsets showed enriched expression of receptors IFNγ synthesis promoting cytokine IL-18 (Il-18R1 and IL-18RAP) (60) and IFNγ inducible chemokines MIG/CXCL9, IP-10/CXCL10, and ITAC/CXCL11 (CXCR3) (61).
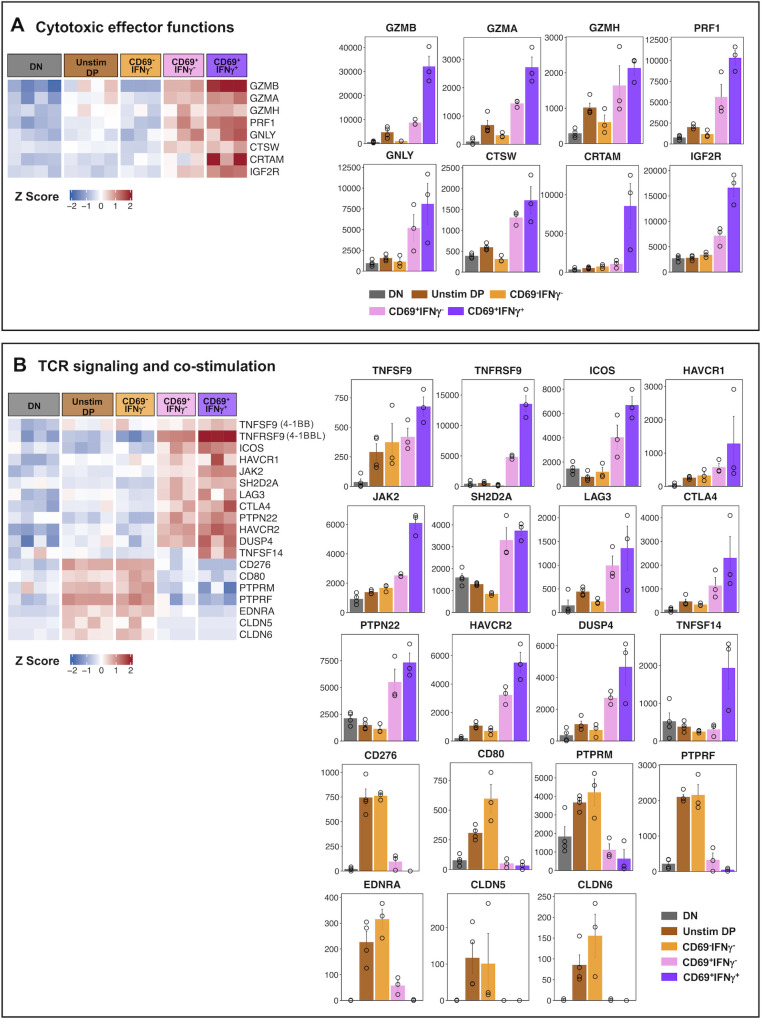
Cytotoxic effector functions
We observed that several key cytotoxic effector genes were upregulated in both the CD69+IFNγ- and CD69+IFNγ+ subsets, with the latter having more robust expression (Fig. 4A; Table S4C). Notable were the well-known genes involved in cytotoxic effector functions such as granzyme B (GZMB), granzyme A (GZMA), granzyme H (GZMH), perforin (PRF1), granulysin (GNLY), cathepsin W (CTSW), cytotoxic regulatory T cell molecule (CRTAM) (62), and the insulin growth factor receptor-2 (IGF2R) that is known to be involved in intracellular trafficking of lysosomal enzymes (63). By contrast, all these genes associated with cytotoxic function were strikingly low in the CD69-IFNγ- subset.
Fig 4.
Notable (A) cytotoxic effector genes and genes involved in (B) TCR signaling and co-stimulation differentially expressed in functionally distinct HLADR+CD38+ CD8 T cell subset. Heatmap showing the expression profile of notable genes, which were upregulated in CD69+IFNγ- and CD69+IFNγ+ subset as compared to DN (left). Genes are segregated based on their functions. Color gradient represents the normalized gene expression transformed into z-scores from blue (low expression) to red (high expression). For each gene, bar plots representing average normalized counts of DN, Unstim DP, CD69-IFNγ-, CD69+IFNγ-, and CD69+IFNγ+ subsets are shown. Error bar represents standard error (SEM). Significance values between the groups are provided in Table S5.
TCR signaling and co-stimulation
Consistent with the results presented above, the CD69+IFNγ+ and the CD69+IFNγ- subsets showed robust expression of several key genes involved in TCR signaling and co-stimulation (Fig. 4B; Table S4D). Notable among these include ligands for B7H2 (ICOS/CD278) (64), CD80/CD86 (CTLA4), FGL1 (LAG3) (65), and bidirectional ligands for TNFSF9/4-1-BBL (TNFRSF9/4-1-BB) and TNFRSF9/4-1-BB (TNFSF9/4-1-BBL) (66), which are implicated in balancing TCR mediated signals. Additionally, these cells also upregulated signaling components JAK2, SH2D2A (67), DUSP4, HAVCR2 (68), TNFSF14 (69), and PTPN22 (70), which are implicated in fine balancing of the TCR and or cytokine signaling. By contrast, the CD69-IFNγ- subset showed preferential expression of genes encoding a different set of costimulatory molecules that regulate T cell responses including the ligands for B7H3 (CD276) and CD28/CTLA4 (CD80), genes belonging to the tyrosine phosphatase family—PTPRM, which is required for cell growth (71), and PTPRF that is known to negatively regulate TCR signaling by dephosphorylation of LCK and Fyn (72). Interestingly, these cells also upregulated genes implicated in regulating extravasation including Endothelin 1 (EDNRA), which plays a role in vasoconstriction (73), and Claudin 5 and 6 (CLDN5 and CLDN6), which are components of tight junctions and are required for extravasation of immune cells (74).
Transcription, translation, metabolism, and proliferation
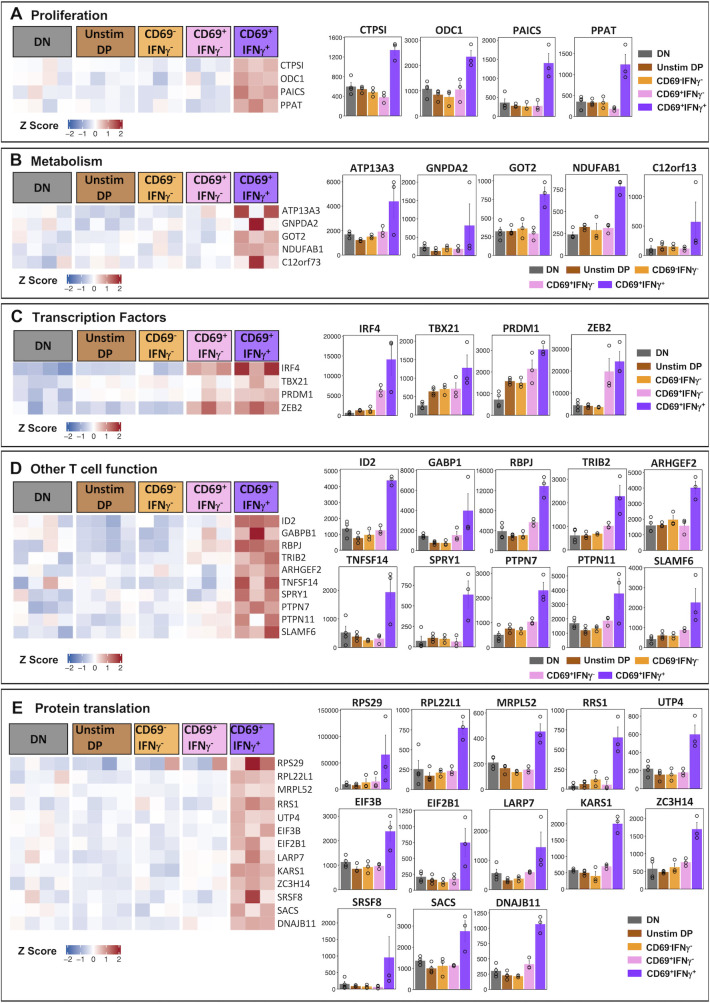
The above results show that the CD69-IFNγ- subset is likely to be a different lineage since it differed substantially from the CD69+IFNγ- and CD69+IFNγ+ subsets in the expression of several cytokines, chemokines, their receptors, and cytotoxic effector function. By contrast, the CD69+IFNγ- and CD69+IFNγ+ subsets showed nearly similar qualitative expression patterns—with the exception that the CD69+IFNγ+ subset additionally expressed XCL1 and XCL2. Therefore, we were interested to examine if there are any other genes that are specifically expressed in the CD69+IFNγ+ subset, which may give us a hint about their functionally distinct nature. A careful comparison of the CD69+IFNγ+ subset against all the four other populations sorted revealed 70 genes that were specifically and uniquely upregulated only in the cells that were capable of making IFNγ in response to dengue peptides (Fig. 5; Table S5A). Of these were genes associated with proliferation, energy metabolism, certain lineage-defining transcription factors, genes involved in TCR signaling, inflammation, and protein translation (Table S5B through F). Notable genes involved in DNA replication and proliferation found to be expressed uniquely in the CD69+IFNγ+ were: CTPS1 (CTP Synthase 1), a part of the pyrimidine metabolism pathway is an enzyme essential for the biosynthesis of UTP to CTP (75); ODC1, a rate-limiting enzyme in the polyamine biosynthesis pathway, which catalyzes the conversion of ornithine to putrescine and thus is highly expressed during cell growth and proliferation (76); PAICS, a key enzyme in purine biosynthesis and PPAT, which is also another enzyme that is of the phosphoribosyl transferase family (77) both of which contribute to the de novo biosynthesis of purines that are essential building blocks of DNA and RNA (Fig. 5A; Table S5B).
Fig 5.
Genes uniquely overexpressed in CD69+IFNγ+ subset categorized by function (A-E). Heatmap showing the expression profile of genes uniquely overexpressed in CD69+IFNγ+ subset (left). The genes are categorized into putative functions such as (A) DNA replication and proliferation, (B) metabolism, (C) transcription factors, (D) T cell functions, and (E) protein translation. Color gradient represents the normalized gene expression transformed into z-scores from blue (low expression) to red (high expression). For each gene, bar plots representing average normalized counts of DN, Unstim DP, CD69-IFNγ-, CD69+IFNγ-, and CD69+IFNγ+ subsets are shown. Error bar represents standard error (SEM). Significance values between the groups are provided in Table S5.
High metabolic activity is an indicator of rapidly proliferating and differentiating effector T cells. On these lines, we also found that the CD69+IFNγ+ HLADR+CD38+ CD8 T cells also uniquely expressed genes that participate in cellular metabolism such as APT13A3, a p-type ATPase; GNPDA2, an enzyme that converts glucosamine-6-phosphate to fructose-6-phosphate (78); GOT2, which is known to be expressed in activated CD8 T cells and is required for amino acid metabolism and TCA cycle (79); and NDUFAB1 and C12orf73, both of which are involved in the mitochondrial respiratory chain complex (80) (Fig. 5B; Table S5C).
Interestingly, we also observed that the CD69+IFNγ+ had significantly higher expression of lineage commitment transcription factors like IRF4, TBX21 (T-Bet), PRDM1 (Blimp1), and ZEB2, which are well known to regulate CD8 cytotoxic T cell responses (81, 82) (Fig. 5C; Table S5D). IRF4 has a critical role in the activation and differentiation of effector CD8 T cells (83). IRF4 also promotes expression of transcription factors TBX21 and PRDM1, which in turn drive terminal differentiation of CD8 T cells and regulate IFNγ production. The transcription factor ZEB2 cooperates with TBX21 to program cytotoxic T cell differentiation. Coordinated expression of all four of these transcription factors in the CD69+IFNγ+ perhaps allows for the upregulation of pathways involved in the activation and differentiation of IFNγ-producing terminally differentiated cytotoxic effector CD8 T cells.
We also observed many key genes of interest, which are necessary for T cell function, such as transcription factor ID2, which promotes survival and differentiation of CD8 T cells (84); and GABPB1, an Ets transcription factor shown to be critically responsible for antigen-stimulated T cell responses (85). Other genes associated with T cell function such as RBPJ, which augments notch signaling (86); TRIB2, involved in MAPK signaling (87); ARHGEF2, shown to promote IL-6 and TNFα secretion (88); TNFSF14/LTg (69), which promotes co-stimulation, and SPRY1 (89), which has a dual effect on cytokine production depending on the state of the T cell were also found to be robustly expressed in the CD69+IFNγ+ subset. Interestingly, we also observed that the CD69+IFNγ+ HLADR+CD38+ CD8 T cells also expressed genes that probably regulate the overabundance of TCR-mediated immune activation. Phosphatases such as PTPN7 and PTPN11 that downregulate TCR signaling were increased (72), along with SLAMF6, which is a known immune checkpoint inhibitor (90) (Fig. 5D; Table S5E).
Lastly, in line with our data on biological pathways, we observed that CD69+IFNγ+ HLADR+CD38+ CD8 T cells expressed a number of genes that were involved in protein translation such as components of the ribosome RPS29, RPL22L1, and MRPL52; ribosome biogenesis regulators such as RRS1 and UTP4; translational initiation factors EIF3B, EIF2B1, and LARP7; KARS1, a lysyl-tRNA-synthetase; ZC3H14, critically required for mRNA stability; SRSF8, required for mRNA splicing and protein chaperones SACS and DNAJB11 (Fig. 5E; Table S5F). These data indicated that the CD69+IFNγ+ were enriched in protein translation machinery, which is necessary for effector T cell responses.
Overall, this analysis of several key genes that are uniquely enriched by the CD69+IFNγ+ subset suggests that CD69+IFNγ+ HLADR+CD38+ CD8 T cells are classical effector CD8 T cells that have a high demand for energy and biosynthetic precursors to support their high proliferative potential and lineage defining effector cytotoxic and cytokine-producing CD8 T cell responses.
DISCUSSION
In summary, the results presented in this study show that the massively expanding population of the HLADR+CD38+ CD8 T cells observed in response to acute febrile dengue virus infection constitutes heterogeneous functional populations. At least three distinct subsets can be distinguished based on the pattern of the CD69 expression and the pattern of the IFNγ production upon in vitro peptide stimulation: the CD69-IFNγ- subset, the CD69+IFNγ- subset, and the CD69+IFNγ+ subset.
The CD69-IFNγ- subset, which constitutes nearly half of these massively proliferating HLADR+CD38+ CD8 T cells, appears refractory to TCR signaling as evidenced by downregulation of gene expression pathways associated with T-cell activation/signaling. Interestingly, this subset was notably low in the transcriptional machinery associated with cytotoxic T-cell effector functions—but high in transcriptional machinery associated with lymphocyte adhesion and extravasation as compared to the other two subsets. Additionally, these cells showed a bias toward the expression of chemokines involved in the attraction of B cells and other lymphocytes and cytokines related to Tc17 and T-reg lineages or those that are implicated in promoting the formation of inducible T-regs or in immunosuppression/immunomodulatory and anti-inflammatory activities and angiogenesis. Taken together, these results suggest that the CD69-IFNγ- subset is likely to be homing to tissues to exert immunoregulatory functions rather than the cytotoxic/cytokine-mediated effector functions—and thus is likely to represent a distinct lineage of the T cell population responding to the dengue virus infection. The expression of these immunoregulators in the CD69-IFNγ- subset is likely to be constitutive and unlikely to require in vitro peptide stimulation since the expression of these same genes was also observed in the unstimulated HLADR+CD38+ CD8 T cells. These interesting observations warrant further studies to address some very important questions: do these CD69-IFNγ-cells represent a distinct lineage? Are they truly dengue peptide-specific population that became refractory to TCR signals or are they a bystander population that is involved in immunoregulatory functions by constitutive secretion of these immuno-regulatory cytokines/chemokines? Are they involved in dialing down of the CD8 T cells response; and with their ability to home to tissues and make cytokines implicated in immunoregulatory and angiogenesis functions, perhaps, are they also involved in tissue repair and remodeling? What is the relative enrichment of these cells compared to the other two subsets in blood versus tissues? What is the role of these cells in dengue protection and or immunopathology? In-depth studies are needed to address these important questions since they will have important implications in understanding the role of the CD8 T cell responses in dengue.
The CD69+IFNγ- subset, which constitutes nearly a third of these massively proliferating HLADR+CD38+ CD8 T cells, appears well equipped with gene expression machinery associated with cytotoxic effector functions—but largely failed to produce any of the signature cytokines of either the CD69-IFNγ- subset or the CD69+IFNγ+ subset in response to in vitro peptide stimulation. This observation raises many important questions: is this CD69+IFNγ- subset capable of recognizing and killing the infected cells but incapable of secreting cytokines upon peptide stimulation? What is the role of this subset in dengue protection and/or immunopathology? Further studies are needed to address these important questions.
The CD69+IFNγ+ subset, which constitutes only a minority of these massively proliferating HLADR+CD38+ CD8 T cells, showed a robust gene expression machinery associated with efficient transcriptional, translational, and metabolic pathways. They are also well equipped with gene expression associated with cytotoxic effector functions as well as the transcriptional machinery associated with balancing of the TCR signals. Consistent with this observation, these cells were expressing not only IFNγ but also XCL1/XCL2. These interesting observations raise many important questions: do these minority populations of the cytotoxic plus IFNγ/XCL1/XCL2 co-expressing cells represent a separate lineage, or are they part of the same lineage as the CD69+IFNγ- subset but represent a transitory population fated to differentiate into memory cells following the resolution of the acute infection? Do these cells home to tissues, and if so what cytokines/chemokines do they produce when these tissue-derived cells are stimulated? What is the role of these cells in dengue protection and/or immunopathology? Further studies are needed to address these important questions.
It is important to note that a recent study reported the transcriptional profiles of the dengue patient blood-derived CD8 T cells that could produce IFNγ upon in vitro peptide stimulation (40). The transcriptional profiles of the CD69+IFNγ-+ subset from our study closely match with the transcriptional profiles of the IFNγ+ subset reported in this study. In addition to confirming these previous studies, here we provide a detailed understanding of the unique and common transcriptional signatures associated with each of the functionally distinct subsets within the massively expanding HLADR+CD38+ population in the dengue patients (i.e., CD69-IFNγ- versus the CD69+IFNγ- versus the CD69+IFNγ+ subsets). These results suggest that there could be distinct functional lineages of CD8 T cells within these highly activated and massively expanding HLADR+CD38+ CD8 T cells. Of these three subsets, the CD69+IFNγ+ subset can be undoubtedly interpreted as antigen specific since they are responding to in vitro peptide stimulation. However, this CD69+IFNγ+ subset represents only a minor fraction of these massively expanding HLADR+CD38+ CD8 T cells. Whether the CD69+IFNγ- subset and the CD69-IFNγ- subsets (which together form >80% of these massively expanding CD8 T cells) truly represent antigen-specific populations or bystander responses remains to be addressed.
Interestingly, a recent study by Waickman et al. (44) showed that people who were vaccinated with live attenuated dengue virus (TAK-003) also elicited a massive expansion of the HLADR+CD38+ CD8 T cells but only a small proportion of them made IFNγ upon in vitro stimulation. Further studies are warranted to determine whether the massively expanding HLADR+CD38+ CD8 T cells in the vaccinated individuals also comprised the CD69-IFNγ-, CD69+IFNγ-, and CD69+IFNγ+ subsets, whether each of these subsets from the vaccinated individuals express similar transcriptional profiles as what we found in dengue natural infection, and whether the protective efficacy correlates with one or more of these individual subsets of the CD8 T cells remain to be determined.
It would also be noteworthy to understand whether the proportions of these functionally distinct subsets differ in patients with different grades of clinical disease. In this perspective, it is important to note that a recent study by Grifoni et al. (40) showed that the overall frequencies of the IFNγ-producing subset did not significantly differ in patients with mild febrile dengue illness versus those with dengue hemorrhagic fever. Given our observations showing that the massively expanding HLADR+CD38+ CD8 T cell population actually comprised multiple functional subsets, further studies are needed to address the expansion, transcription, and behavior of each of these functional subsets in the blood and tissues of the patients with different grades of the disease severity ranging from asymptomatic to mild dengue, dengue hemorrhagic fever, and shock syndrome.
Previous studies in human hepatitis virus or cytomegalovirus natural infections and influenza/yellow fever live attenuated vaccination showed that a substantial fraction of the antigen-specific CD8 T cells that expand in response to viral infection are of stunned phenotype as assessed by their inability to produce IFNγ upon in vitro peptide stimulation (38, 91 – 93). A recent study showed that those antigen-specific CD8 T cells that are capable of expressing the IFNγ gene upon in vitro peptide stimulation reliably co-express chemokines such as XCL1 and XCL2 (62). Our result showing co-expression of XCL1 and XCL2 among the IFNγ-producing cells is consistent with these previous studies and suggests that, perhaps, the cytokine functional response properties of the CD8 T cells in dengue natural infection are likely to be similar to those in other human viral infections. Interestingly, we also found that the CD69+IFNγ+ subset, in addition to expressing IFNγ, XCL1, XCL2, and other genes associated with cytotoxic effector functions, also upregulated several other genes that contribute to enhanced proliferation, protein translation, and metabolic cellular programs involving mitochondrial respiratory chains. In this perspective, it is important to note that memory T cell development involves the expression of a large number of metabolism-related genes (94 – 96). In light of our findings, it is intriguing to wonder whether memory precursors are restricted to the CD69+IFNγ+ subset, which makes up a small portion of the massively expanding HLADR+CD38+ CD8 T cells in dengue infection since these frequencies are comparable to those of the cells that are anticipated to survive once the infection is resolved (97 – 99). This is more likely to be the case given the observations from a recent study in people vaccinated with live attenuated dengue vaccine showing that the TCR clonotypes found among the IFNγ-producing CD8 T cells were similar at the peak of the CD8 expansion and memory phase (44). Similar studies are needed in unvaccinated and vaccinated people following dengue natural infection to understand the precursors of memory development in dengue natural infection.
It is also interesting to note that both the CD69+IFNγ- and CD69+IFNγ+ subsets among these massively expanding HLADR+CD38+ CD8 T cells shared a number of DEGs that are directly responsible for cytotoxic functions, chemokines and their receptors, and co-stimulatory and inhibitory molecules that perfectly align with an antigen-responsive phenotype. These phenotypes were very similar to those observed in single-cell analysis of Flu and CMV-specific CD8 T cells (62, 100). Additionally, we found that the CD69+IFNγ+ subset expressed several unique genes. These observations raise the question of whether CD69+IFNγ- subset also has any unique gene signature. Analysis of the DEGs that especially filter to this subset revealed only four genes—SLFN12L, TENT5C, GGA2, and F2R (PAR1), suggesting that this subset is very similar to the CD69+IFNγ+ subset in cytotoxic function but does have a unique gene signature not expressed by any other subset, which allows it to make any distinct cytokine.
An unexpected, intriguing observation from our study is that the CD69-IFNγ- subset, which constitutes the bulk of the massively expanding HLADR+CD38+ CD8 T cells seen in response to acute dengue infection, expresses CXCL13. The CXCL13 is classically considered a B cell chemoattractant that promotes germinal center formation and antibody maturation (45). However, many emerging studies from cancer patients suggest that CXCL13 was highly expressed in tumor-infiltrating follicular helper cells as well as the tumor-infiltrating exhausted CD8 T cells, and that the CXCL13 may serve as a potential biomarker for prognosis prediction in renal cell carcinoma, colorectal cancer, prostate cancer, lymphoma, and non-small cell lung carcinomas (101 – 103). Additionally, a recent study showed that patients who succumbed to Sars-CoV-2 infection produced high plasma CXCL13. In light of these observations, our finding that the CD69-IFNγ- subset, which constitutes the bulk of the massively expanding HLADR+CD38+ CD8 T cells in dengue, expresses CXCL13 is important and warrants further studies to carefully examine whether these cells are dengue specific but exhausted and what is the precise role of these cells in dengue antibody production, protection, and or immunopathology.
Taken together, our study while improving our understanding of CD8 T cell responses in dengue also sheds light on the diversity of the expanding CD8 T cell population and warrants further studies to understand and examine the precise role of different functional lineages of CD8 T cells in dengue natural infection and/or vaccines.
ACKNOWLEDGMENTS
We thank Dr. Guruprasad Medigeshi, Translational Health Sciences and Technology Institute (THSTI), Faridabad, India, for Dengue IgM, IgG, and NS1 antigen ELISAs performed in the NABL-accredited THSTI bioassay lab. We also extend our thanks to Aditya Rathi (ICGEB-TACF) for FACS sorting of the PBMCs, and Dr. Daniela Weiskopf, La Jolla Institute of Immunology, for CD8 megapools. The authors thank Mr. Satendra Singh and Mr. Ajay Singh, ICGEB, New Delhi, for their technical support.
This work was funded by the Department of Biotechnology, Government of India, grant number BT/PR28416/MED/29/1310/2018, and the U.S. National Institutes of Health grant ICIDR 1UO1A/115654.
Experimental work, data acquisition, and analysis were performed by P. Singh, P.B., D.M., Y.M.C., K.S., E.S.R., K.N., S.J., and P. Singla. Conceptualization and implementation were done by P.S., P.B., D.M., Y.M.C., K.S., E.S.R., K.N., S.J., M.S., N.W., K.M.-K., and A.C. Manuscript was written by P.S., P.B., K.M.-K., and A.C. All authors contributed to reviewing and editing of the manuscript.
AFTER EPUB
[This article was published on 19 October 2023 with typos in the last sentence of the abstract and in the description of panel E in the legend of Fig. 1. These items were corrected in the current version, posted on 1 November 2023.]
Contributor Information
Naveet Wig, Email: naveetwig@gmail.com.
Kaja Murali-Krishna, Email: Murali.Kaja@emory.edu.
Anmol Chandele, Email: chandeleanmol@gmail.com.
Bryan R. G. Williams, Hudson Institute of Medical Research, Clayton, Victoria, Australia
DATA AVAILABILITY
The dengue RNA seq dataset is deposited in Gene Expression Omnibus (GEO) with the accession code GSE212034.
ETHICS APPROVAL
This study uses peripheral blood mononuclear cells (PBMC) and plasma samples obtained from dengue confirmed acute febrile patients who were recruited at the Department of Medicine, All India Institute of Medical Sciences (AIIMS, New Delhi), India, during the years 2018–2021. After informed consent was taken by the doctor on call, a portion of the blood sample collected for routine clinical tests at the time of enrollment, when available in sufficient quantity, was sent to the research laboratory for T cell analysis. The study was approved by institutional ethical boards of the participating institutions (AIIMS, IEC-411/03.08.2018, RP-21/2018; ICGEB, ICGEB/IEC/2018/04/version 3).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/jvi.00746-23.
Comparison of previous microarray analysis of gene signature of HLADR+CD38+ CD8 T cells and CD45RA+CCR7+ CD8 T cells from dengue confirmed children.
Total differentially expressed genes in DN, Unstim DP, CD69-IFNγ-, CD69+IFNγ- and CD69+IFNγ+ subsets.
List of Blood transcription modules (BTMs) enriched for each HLADR+ CD38+ CD8 T cell subsets.
Differences in the expression of key genes of interests in HLADR+CD38+ CD8 T cell subsets.
Mean expression counts of genes uniquely upregulated in CD69+IFNγ+ subset, mean expression counts and their pairwise comparison of select key genes involved in proliferation, metabolism, transcription regulation, T cell function and translation.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO/TDR. 2009. Dengue: guidelines for diagnosis, treatment, prevention and control. [PubMed]
- 3. Halstead SB. 2013. Dengue vascular permeability syndrome: what, no T cells? Clin Infect Dis 56:900–901. doi: 10.1093/cid/cis1047 [DOI] [PubMed] [Google Scholar]
- 4. Halstead SB. 2015. Pathogenesis of dengue: dawn of a new era. F1000Res 4:1353. doi: 10.12688/f1000research.7024.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Malavige GN, Ogg GS. 2017. Pathogenesis of vascular leak in dengue virus infection. Immunology 151:261–269. doi: 10.1111/imm.12748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burke DS, Scott RMcN, Johnson DE, Nisalak A. 1988. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg 38:172–180. doi: 10.4269/ajtmh.1988.38.172 [DOI] [PubMed] [Google Scholar]
- 7. Graham RR, Juffrie M, Tan R, Hayes CG, Laksono I, Ma’roef C, Porter KR, Halstead SB. 1999. A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia I. studies in 1995-1996. Am J Trop Med Hyg 61:412–419. doi: 10.4269/ajtmh.1999.61.412 [DOI] [PubMed] [Google Scholar]
- 8. SABIN AB. 1952. Research on dengue during World War II. Am J Trop Med Hyg 1:30–50. doi: 10.4269/ajtmh.1952.1.30 [DOI] [PubMed] [Google Scholar]
- 9. Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, Salitul V, Phanthumachinda B, Halstead SB. 1984. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong. Am J Epidemiol 120:653–669. doi: 10.1093/oxfordjournals.aje.a113932 [DOI] [PubMed] [Google Scholar]
- 10. Snow GE, Haaland B, Ooi EE, Gubler DJ. 2014. Review article: research on dengue during World War II revisited. Am J Trop Med Hyg 91:1203–1217. doi: 10.4269/ajtmh.14-0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thein S, Aung MM, Shwe TN, Aye M, Zaw A, Aye K, Aye KM, Aaskov J. 1997. Risk factors in dengue shock syndrome. Am J Trop Med Hyg 56:566–572. doi: 10.4269/ajtmh.1997.56.566 [DOI] [PubMed] [Google Scholar]
- 12. Halstead SB. 1979. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis 140:527–533. doi: 10.1093/infdis/140.4.527 [DOI] [PubMed] [Google Scholar]
- 13. Halstead SB, Nimmannitya S, Cohen SN. 1970. Observations related to pathogenesis of dengue hemorrhagic fever. IV. relation of disease severity to antibody response and virus recovered. Yale J Biol Med 42:311–328. [PMC free article] [PubMed] [Google Scholar]
- 14. Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. 1989. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg 40:444–451. doi: 10.4269/ajtmh.1989.40.444 [DOI] [PubMed] [Google Scholar]
- 15. Guzmán MG, Kouri G, Bravo J, Valdes L, Susana V, Halstead SB. 2002. Effect of age on outcome of secondary dengue 2 infections. Int J Infect Dis 6:118–124. doi: 10.1016/S1201-9712(02)90072-X [DOI] [PubMed] [Google Scholar]
- 16. Cohen SN, Margiotta MR, Nimmannitya S, Halstead SB. 1969. Dengue and chikungunya virus infection in man in Thailand, 1962-1964. I. observations on hospitalized patients with hemorrhagic fever. Am J Trop Med Hyg 18:954–971. doi: 10.4269/ajtmh.1969.18.954 [DOI] [PubMed] [Google Scholar]
- 17. Gould DJ, Russell PK, Winter PE, Udomsakdi S, Yuill TM, Nisalak A. 1968. An insular outbreak of dengue hemorrhagic fever. II. virologic and serologic studies. Am J Trop Med Hyg 17:600–608. doi: 10.4269/ajtmh.1968.17.600 [DOI] [PubMed] [Google Scholar]
- 18. Nantapanich S, Gould D, Russell PK, Udomsakdi S, Yuill TM, Winter PE. 1968. An insular outbreak of dengue hemorrhagic fever. I. epidemiologic observations. Am J Trop Med Hyg 17:590–599. doi: 10.4269/ajtmh.1968.17.590 [DOI] [PubMed] [Google Scholar]
- 19. Falconar AK. 1997. The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to human endothelial cells: potential implications in haemorrhagic fever pathogenesis. Arch Virol 142:897–916. doi: 10.1007/s007050050127 [DOI] [PubMed] [Google Scholar]
- 20. Glasner DR, Puerta-Guardo H, Beatty PR, Harris E. 2018. The good, the bad, and the shocking: the multiple roles of dengue virus nonstructural protein 1 in protection and pathogenesis. Annu Rev Virol 5:227–253. doi: 10.1146/annurev-virology-101416-041848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paranavitane SA, Gomes L, Kamaladasa A, Adikari TN, Wickramasinghe N, Jeewandara C, Shyamali NLA, Ogg GS, Malavige GN. 2014. Dengue NS1 antigen as a marker of severe clinical disease. BMC Infect Dis 14:570. doi: 10.1186/s12879-014-0570-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tricou V, Minh NN, Farrar J, Tran HT, Simmons CP. 2011. Kinetics of viremia and NS1 antigenemia are shaped by immune status and virus serotype in adults with dengue. PLoS Negl Trop Dis 5:e1309. doi: 10.1371/journal.pntd.0001309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mangada MM, Rothman AL. 2005. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J Immunol 175:2676–2683. doi: 10.4049/jimmunol.175.4.2676 [DOI] [PubMed] [Google Scholar]
- 24. Mongkolsapaya J, Dejnirattisai W, Xu X, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S, Yenchitsomanus P, McMichael A, Malasit P, Screaton G. 2003. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med 9:921–927. doi: 10.1038/nm887 [DOI] [PubMed] [Google Scholar]
- 25. Pang T, Cardosa MJ, Guzman MG. 2007. Of cascades and perfect storms: the Immunopathogenesis of dengue haemorrhagic fever-dengue shock syndrome (DHF/DSS). Immunol Cell Biol 85:43–45. doi: 10.1038/sj.icb.7100008 [DOI] [PubMed] [Google Scholar]
- 26. Dung NTP, Duyen HTL, Thuy NTV, Ngoc TV, Chau NVV, Hien TT, Rowland-Jones SL, Dong T, Farrar J, Wills B, Simmons CP. 2010. Timing of CD8+ T cell responses in relation to commencement of capillary leakage in children with dengue. J Immunol 184:7281–7287. doi: 10.4049/jimmunol.0903262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elong Ngono A, Chen HW, Tang WW, Joo Y, King K, Weiskopf D, Sidney J, Sette A, Shresta S. 2016. Protective role of cross-reactive CD8 T cells against dengue virus infection. EBioMedicine 13:284–293. doi: 10.1016/j.ebiom.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weiskopf D, Angelo MA, de Azeredo EL, Sidney J, Greenbaum JA, Fernando AN, Broadwater A, Kolla RV, De Silva AD, de Silva AM, Mattia KA, Doranz BJ, Grey HM, Shresta S, Peters B, Sette A. 2013. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci U S A 110:E2046–53. doi: 10.1073/pnas.1305227110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yauch LE, Zellweger RM, Kotturi MF, Qutubuddin A, Sidney J, Peters B, Prestwood TR, Sette A, Shresta S. 2009. A protective role for dengue virus-specific CD8+ T cells. J Immunol 182:4865–4873. doi: 10.4049/jimmunol.0801974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zellweger RM, Tang WW, Eddy WE, King K, Sanchez MC, Shresta S. 2015. CD8+ T cells can mediate short-term protection against heterotypic dengue virus reinfection in mice. J Virol 89:6494–6505. doi: 10.1128/JVI.00036-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elong Ngono A, Shresta S. 2019. Cross-reactive T cell immunity to dengue and zika viruses: new insights into vaccine development. Front Immunol 10:1316. doi: 10.3389/fimmu.2019.01316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rivino L. 2016. T cell immunity to dengue virus and implications for vaccine design. Expert Rev Vaccines 15:443–453. doi: 10.1586/14760584.2016.1116948 [DOI] [PubMed] [Google Scholar]
- 33. Weiskopf D, Yauch LE, Angelo MA, John DV, Greenbaum JA, Sidney J, Kolla RV, De Silva AD, de Silva AM, Grey H, Peters B, Shresta S, Sette A. 2011. Insights into HLA-restricted T cell responses in a novel mouse model of dengue virus infection point toward new implications for vaccine design. J Immunol 187:4268–4279. doi: 10.4049/jimmunol.1101970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chu H, George SL, Stinchcomb DT, Osorio JE, Partidos CD. 2015. CD8+ T-cell responses in flavivirus-naive individuals following immunization with a live-attenuated tetravalent dengue vaccine candidate. J Infect Dis 212:1618–1628. doi: 10.1093/infdis/jiv258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guy B, Nougarede N, Begue S, Sanchez V, Souag N, Carre M, Chambonneau L, Morrisson DN, Shaw D, Qiao M, Dumas R, Lang J, Forrat R. 2008. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine 26:5712–5721. doi: 10.1016/j.vaccine.2008.08.019 [DOI] [PubMed] [Google Scholar]
- 36. Tian Y, Grifoni A, Sette A, Weiskopf D. 2019. Human T cell response to dengue virus infection. Front Immunol 10:2125. doi: 10.3389/fimmu.2019.02125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Der Most RG, Murali-Krishna K, Ahmed R, Strauss JH. 2000. Chimeric yellow fever/dengue virus as a candidate dengue vaccine: quantitation of the dengue virus-specific CD8 T-cell response. J Virol 74:8094–8101. doi: 10.1128/jvi.74.17.8094-8101.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chandele A, Sewatanon J, Gunisetty S, Singla M, Onlamoon N, Akondy RS, Kissick HT, Nayak K, Reddy ES, Kalam H, Kumar D, Verma A, Panda H, Wang S, Angkasekwinai N, Pattanapanyasat K, Chokephaibulkit K, Medigeshi GR, Lodha R, Kabra S, Ahmed R, Murali-Krishna K, Diamond MS. 2016. Characterization of human CD8 T cell responses in dengue virus-infected patients from India. J Virol 90:11259–11278. doi: 10.1128/JVI.01424-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Matos AM, Carvalho KI, Rosa DS, Villas-Boas LS. 2015. CD8+ T lymphocyte expansion, proliferation and activation in dengue fever. PLoS Negl Trop Dis 9:e0003520. doi: 10.1371/journal.pntd.0003520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grifoni A, Voic H, Yu ED, Mateus J, Yan Fung KM, Wang A, Seumois G, De Silva AD, Tennekon R, Premawansa S, Premawansa G, Tippalagama R, Wijewickrama A, Chawla A, Greenbaum J, Peters B, Pandurangan V, Weiskopf D, Sette A. 2022. Transcriptomics of acute DENV-specific CD8+ T cells does not support qualitative differences as drivers of disease severity. Vaccines 10:612. doi: 10.3390/vaccines10040612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102:15545–15550. doi: 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li S, Rouphael N, Duraisingham S, Romero-Steiner S, Presnell S, Davis C, Schmidt DS, Johnson SE, Milton A, Rajam G, Kasturi S, Carlone GM, Quinn C, Chaussabel D, Palucka AK, Mulligan MJ, Ahmed R, Stephens DS, Nakaya HI, Pulendran B. 2014. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat Immunol 15:195–204. doi: 10.1038/ni.2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Akondy RS, Monson ND, Miller JD, Edupuganti S, Teuwen D, Wu H, Quyyumi F, Garg S, Altman JD, Del Rio C, Keyserling HL, Ploss A, Rice CM, Orenstein WA, Mulligan MJ, Ahmed R. 2009. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J Immunol 183:7919–7930. doi: 10.4049/jimmunol.0803903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Waickman AT, Victor K, Li T, Hatch K, Rutvisuttinunt W, Medin C, Gabriel B, Jarman RG, Friberg H, Currier JR. 2019. Dissecting the heterogeneity of DENV vaccine-elicited cellular immunity using single-cell RNA sequencing and metabolic profiling. Nat Commun 10:3666. doi: 10.1038/s41467-019-11634-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ebert LM, Schaerli P, Moser B. 2005. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol 42:799–809. doi: 10.1016/j.molimm.2004.06.040 [DOI] [PubMed] [Google Scholar]
- 46. Cruikshank W, Little F. 2008. Interleukin-16: the ins and outs of regulating T-cell activation. Crit Rev Immunol 28:467–483. doi: 10.1615/critrevimmunol.v28.i6.10 [DOI] [PubMed] [Google Scholar]
- 47. Casalegno Garduño R, Däbritz J. 2021. New insights on CD8(+) T cells in inflammatory bowel disease and therapeutic approaches. Front Immunol 12:738762. doi: 10.3389/fimmu.2021.738762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bézie S, Picarda E, Ossart J, Tesson L, Usal C, Renaudin K, Anegon I, Guillonneau C. 2015. IL-34 is a Treg-specific cytokine and mediates transplant tolerance. J Clin Invest 125:3952–3964. doi: 10.1172/JCI81227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen W, Xing J, Liu X, Wang S, Xing D. 2021. The role and transformative potential of IL-19 in atherosclerosis. Cytokine Growth Factor Rev 62:70–82. doi: 10.1016/j.cytogfr.2021.09.001 [DOI] [PubMed] [Google Scholar]
- 50. Leker RR, Toth ZE, Shahar T, Cassiani-Ingoni R, Szalayova I, Key S, Bratincsák A, Mezey E. 2009. Transforming growth factor alpha induces angiogenesis and neurogenesis following stroke. Neuroscience 163:233–243. doi: 10.1016/j.neuroscience.2009.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peluzzo AM, Autieri MV. 2022. Challenging the paradigm: anti-inflammatory interleukins and angiogenesis. Cells 11:587. doi: 10.3390/cells11030587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Queen D, Ediriweera C, Liu L. 2019. Function and regulation of IL-36 signaling in inflammatory diseases and cancer development. Front Cell Dev Biol 7:317. doi: 10.3389/fcell.2019.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang M, Wei J, Shang F, Zang K, Ji T. 2019. Platelet-derived growth factor B attenuates lethal sepsis through inhibition of inflammatory responses. Int Immunopharmacol 75:105792. doi: 10.1016/j.intimp.2019.105792 [DOI] [PubMed] [Google Scholar]
- 54. Zhao M, Hu Y, Jin J, Yu Y, Zhang S, Cao J, Zhai Y, Wei R, Shou J, Cai W, Liu S, Yang X, Xu GT, Yang J, Corry DB, Su SB, Liu X, Yang T. 2017. Interleukin 37 promotes angiogenesis through TGF-beta signaling. Sci Rep 7:6113. doi: 10.1038/s41598-017-06124-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Matsuo K, Kitahata K, Kawabata F, Kamei M, Hara Y, Takamura S, Oiso N, Kawada A, Yoshie O, Nakayama T. 2018. A highly active form of XCL1/lymphotactin functions as an effective adjuvant to recruit cross-presenting dendritic cells for induction of effector and memory CD8(+) T cells. Front Immunol 9:2775. doi: 10.3389/fimmu.2018.02775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang M, Windgassen D, Papoutsakis ET. 2008. Comparative analysis of transcriptional profiling of CD3+, CD4+ and CD8+ T cells identifies novel immune response players in T-cell activation. BMC Genomics 9:225. doi: 10.1186/1471-2164-9-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sreeramkumar V, Fresno M, Cuesta N. 2012. Prostaglandin E2 and T cells: friends or foes? Immunol Cell Biol 90:579–586. doi: 10.1038/icb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hoeve MA, Savage NDL, de Boer T, Langenberg DML, de Waal Malefyt R, Ottenhoff THM, Verreck FAW. 2006. Divergent effects of IL-12 and IL-23 on the production of IL-17 by human T cells. Eur J Immunol 36:661–670. doi: 10.1002/eji.200535239 [DOI] [PubMed] [Google Scholar]
- 59. Rennier K, Shin WJ, Krug E, Virdi G, Pachynski RK. 2020. Chemerin Reactivates PTEN and suppresses PD-L1 in tumor cells via modulation of a novel CMKLR1-mediated signaling cascade. Clin Cancer Res 26:5019–5035. doi: 10.1158/1078-0432.CCR-19-4245 [DOI] [PubMed] [Google Scholar]
- 60. Tominaga K, Yoshimoto T, Torigoe K, Kurimoto M, Matsui K, Hada T, Okamura H, Nakanishi K. 2000. IL-12 synergizes with IL-18 or IL-1β for IFN-γ production from human T cells. Int Immunol 12:151–160. doi: 10.1093/intimm/12.2.151 [DOI] [PubMed] [Google Scholar]
- 61. Groom JR, Luster AD. 2011. CXCR3 in T cell function. Exp Cell Res 317:620–631. doi: 10.1016/j.yexcr.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fuchs YF, Sharma V, Eugster A, Kraus G, Morgenstern R, Dahl A, Reinhardt S, Petzold A, Lindner A, Löbel D, Bonifacio E. 2019. Gene expression-based identification of antigen-responsive CD8(+) T cells on a single-cell level. Front Immunol 10:2568. doi: 10.3389/fimmu.2019.02568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sohar I, Sleat D, Gong Liu C, Ludwig T, Lobel P. 1998. Mouse mutants lacking the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor are impaired in lysosomal enzyme transport: comparison of cation-independent and cation-dependent mannose 6-phosphate receptor-deficient mice. Biochem J 330 (Pt 2):903–908. doi: 10.1042/bj3300903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wikenheiser DJ, Stumhofer JS. 2016. ICOS co-stimulation: friend or foe? Front Immunol 7:304. doi: 10.3389/fimmu.2016.00304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DAA, Wherry EJ. 2009. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 10:29–37. doi: 10.1038/ni.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Croft M. 2009. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol 9:271–285. doi: 10.1038/nri2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kolltveit KM, Granum S, Aasheim H-C, Forsbring M, Sundvold-Gjerstad V, Dai K-Z, Molberg Ø, Schjetne KW, Bogen B, Shapiro VS, Johansen F-E, Schenck K, Spurkland A. 2008. Expression of SH2D2A in T-cells is regulated both at the transcriptional and translational level. Mol Immunol 45:2380–2390. doi: 10.1016/j.molimm.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 68. Ferris RL, Lu B, Kane LP. 2014. Too much of a good thing? Tim-3 and TCR signaling in T cell exhaustion. J Immunol 193:1525–1530. doi: 10.4049/jimmunol.1400557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wan X, Zhang J, Luo H, Shi G, Kapnik E, Kim S, Kanakaraj P, Wu J. 2002. A TNF family member LIGHT transduces costimulatory signals into human T cells. J Immunol 169:6813–6821. doi: 10.4049/jimmunol.169.12.6813 [DOI] [PubMed] [Google Scholar]
- 70. Zhang X, Yu Y, Bai B, Wang T, Zhao J, Zhang N, Zhao Y, Wang X, Wang B. 2020. PTPN22 interacts with EB1 to regulate T-cell receptor signaling. FASEB J 34:8959–8974. doi: 10.1096/fj.201902811RR [DOI] [PubMed] [Google Scholar]
- 71. Barazeghi E, Hellman P, Westin G, Stålberg P. 2019. PTPRM, a candidate tumor suppressor gene in small intestinal neuroendocrine tumors. Endocr Connect 8:1126–1135. doi: 10.1530/EC-19-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Stanford SM, Rapini N, Bottini N. 2012. Regulation of TCR signalling by tyrosine phosphatases: from immune homeostasis to autoimmunity. Immunology 137:1–19. doi: 10.1111/j.1365-2567.2012.03591.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kowalczyk A, Kleniewska P, Kolodziejczyk M, Skibska B, Goraca A. 2015. The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch Immunol Ther Exp 63:41–52. doi: 10.1007/s00005-014-0310-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Son HJ, An CH, Yoo NJ, Lee SH. 2020. Tight junction-related CLDN5 and CLDN6 genes, and gap junction-related GJB6 and GJB7 genes are somatically mutated in gastric and colorectal cancers. Pathol Oncol Res 26:1983–1987. doi: 10.1007/s12253-020-00806-2 [DOI] [PubMed] [Google Scholar]
- 75. Martin E, Palmic N, Sanquer S, Lenoir C, Hauck F, Mongellaz C, Fabrega S, Nitschké P, Esposti MD, Schwartzentruber J, Taylor N, Majewski J, Jabado N, Wynn RF, Picard C, Fischer A, Arkwright PD, Latour S. 2014. CTP synthase 1 deficiency in humans reveals its central role in lymphocyte proliferation. Nature 510:288–292. doi: 10.1038/nature13386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bachmann AS, Geerts D. 2018. Polyamine synthesis as a target of MYC oncogenes. J Biol Chem 293:18757–18769. doi: 10.1074/jbc.TM118.003336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Goswami MT, Chen G, Chakravarthi BVSK, Pathi SS, Anand SK, Carskadon SL, Giordano TJ, Chinnaiyan AM, Thomas DG, Palanisamy N, Beer DG, Varambally S. 2015. Role and regulation of coordinately expressed de novo purine biosynthetic enzymes PPAT and PAICS in lung cancer. Oncotarget 6:23445–23461. doi: 10.18632/oncotarget.4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Arreola R, Valderrama B, Morante ML, Horjales E. 2003. Two mammalian glucosamine-6-phosphate deaminases: a structural and genetic study. FEBS Lett 551:63–70. doi: 10.1016/s0014-5793(03)00896-2 [DOI] [PubMed] [Google Scholar]
- 79. Hickman TL, Choi E, Whiteman KR, Muralidharan S, Pai T, Johnson T, Parikh A, Friedman T, Gilbert M, Shen B, Barron L, McGinness KE, Ettenberg SA, Motz GT, Weiss GJ, Jensen-Smith A. 2022. BOXR1030, an anti-GPC3 CAR with exogenous GOT2 expression, shows enhanced T cell metabolism and improved anti-cell line derived tumor xenograft activity. PLoS One 17:e0266980. doi: 10.1371/journal.pone.0266980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hou T, Zhang R, Jian C, Ding W, Wang Y, Ling S, Ma Q, Hu X, Cheng H, Wang X. 2019. NDUFAB1 confers cardio-protection by enhancing mitochondrial bioenergetics through coordination of respiratory complex and supercomplex assembly. Cell Res 29:754–766. doi: 10.1038/s41422-019-0208-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dominguez CX, Amezquita RA, Guan T, Marshall HD, Joshi NS, Kleinstein SH, Kaech SM. 2015. The transcription factors ZEB2 and T-bet cooperate to program cytotoxic T cell terminal differentiation in response to LCMV viral infection. J Exp Med 212:2041–2056. doi: 10.1084/jem.20150186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. 2007. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27:281–295. doi: 10.1016/j.immuni.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Huber M, Lohoff M. 2014. IRF4 at the crossroads of effector T-cell fate decision. Eur J Immunol 44:1886–1895. doi: 10.1002/eji.201344279 [DOI] [PubMed] [Google Scholar]
- 84. Cannarile MA, Lind NA, Rivera R, Sheridan AD, Camfield KA, Wu BB, Cheung KP, Ding Z, Goldrath AW. 2006. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat Immunol 7:1317–1325. doi: 10.1038/ni1403 [DOI] [PubMed] [Google Scholar]
- 85. Luo CT, Osmanbeyoglu HU, Do MH, Bivona MR, Toure A, Kang D, Xie Y, Leslie CS, Li MO. 2017. Ets transcription factor GABP controls T cell homeostasis and immunity. Nat Commun 8:1062. doi: 10.1038/s41467-017-01020-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lake RJ, Tsai P-F, Choi I, Won K-J, Fan H-Y, Fortini M. 2014. RBPJ, the major transcriptional effector of notch signaling, remains associated with chromatin throughout mitosis, suggesting a role in mitotic bookmarking. PLoS Genet 10:e1004204. doi: 10.1371/journal.pgen.1004204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Johnston J, Basatvat S, Ilyas Z, Francis S, Kiss-Toth E. 2015. Tribbles in inflammation. Biochem Soc Trans 43:1069–1074. doi: 10.1042/BST20150095 [DOI] [PubMed] [Google Scholar]
- 88. Lu G, Tian S, Sun Y, Dong J, Wang N, Zeng J, Nie Y, Wu K, Han Y, Feng B, Shang Y. 2021. NEK9, a novel effector of IL-6/STAT3, regulates metastasis of gastric cancer by targeting ARHGEF2 phosphorylation. Theranostics 11:2460–2474. doi: 10.7150/thno.53169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Choi H, Cho SY, Schwartz RH, Choi K. 2006. Dual effects of Sprouty1 on TCR signaling depending on the differentiation state of the T cell. J Immunol 176:6034–6045. doi: 10.4049/jimmunol.176.10.6034 [DOI] [PubMed] [Google Scholar]
- 90. Yigit B, Wang N, ten Hacken E, Chen S-S, Bhan AK, Suarez-Fueyo A, Katsuyama E, Tsokos GC, Chiorazzi N, Wu CJ, Burger JA, Herzog RW, Engel P, Terhorst C. 2019. SLAMF6 as a regulator of exhausted CD8+ T cells in cancer. Cancer Immunol Res 7:1485–1496. doi: 10.1158/2326-6066.CIR-18-0664 [DOI] [PubMed] [Google Scholar]
- 91. Ahmed R, Akondy RS. 2011. Insights into human CD8(+) T-cell memory using the yellow fever and smallpox vaccines. Immunol Cell Biol 89:340–345. doi: 10.1038/icb.2010.155 [DOI] [PubMed] [Google Scholar]
- 92. Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med 191:1499–1512. doi: 10.1084/jem.191.9.1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Welsh RM. 2001. Assessing CD8 T cell number and dysfunction in the presence of antigen. J Exp Med 193:F19–22. doi: 10.1084/jem.193.5.f19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Buck MD, O’Sullivan D, Pearce EL. 2015. T cell metabolism drives immunity. J Exp Med 212:1345–1360. doi: 10.1084/jem.20151159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Corrado M, Pearce EL. 2022. Targeting memory T cell metabolism to improve immunity. J Clin Invest 132:e148546. doi: 10.1172/JCI148546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Soriano-Baguet L, Brenner D. 2023. Metabolism and epigenetics at the heart of T cell function. Trends Immunol 44:231–244. doi: 10.1016/j.it.2023.01.002 [DOI] [PubMed] [Google Scholar]
- 97. Kaech SM, Hemby S, Kersh E, Ahmed R. 2002. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111:837–851. doi: 10.1016/s0092-8674(02)01139-x [DOI] [PubMed] [Google Scholar]
- 98. Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol 4:1191–1198. doi: 10.1038/ni1009 [DOI] [PubMed] [Google Scholar]
- 99. Kaech SM, Wherry EJ. 2007. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity 27:393–405. doi: 10.1016/j.immuni.2007.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chng MHY, Lim MQ, Rouers A, Becht E, Lee B, MacAry PA, Lye DC, Leo YS, Chen J, Fink K, Rivino L, Newell EW. 2019. Large-scale HLA tetramer tracking of T cells during dengue infection reveals broad acute activation and differentiation into two memory cell fates. Immunity 51:1119–1135. doi: 10.1016/j.immuni.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 101. Adorno Febles VR, Hao Y, Ahsan A, Wu J, Qian Y, Zhong H, Loeb S, Makarov DV, Lepor H, Wysock J, Taneja SS, Huang WC, Becker DJ, Balar AV, Melamed J, Deng FM, Ren Q, Kufe D, Wong KK, Adeegbe DO, Deng J, Wise DR. 2023. Single-cell analysis of localized prostate cancer patients links high Gleason score with an immunosuppressive profile. Prostate 83:840–849. doi: 10.1002/pros.24524 [DOI] [PubMed] [Google Scholar]
- 102. Lee SH, Kim Y, Jeon BN, Kim G, Sohn J, Yoon Y, Kim S, Kim Y, Kim H, Cha H, Lee NE, Yang H, Chung JY, Jeong AR, Kim YY, Kim SG, Seo Y, Park S, Jung HA, Sun JM, Ahn JS, Ahn MJ, Park H, Yoon KW. 2023. Intracellular adhesion molecule-1 improves responsiveness to immune checkpoint inhibitor by activating CD8(+) T cells. Adv Sci (Weinh) 10:e2204378. doi: 10.1002/advs.202204378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pichler R, Siska PJ, Tymoszuk P, Martowicz A, Untergasser G, Mayr R, Weber F, Seeber A, Kocher F, Barth DA, Pichler M, Thurnher M. 2023. A chemokine network of T cell exhaustion and metabolic reprogramming in renal cell carcinoma. Front Immunol 14:1095195. doi: 10.3389/fimmu.2023.1095195 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of previous microarray analysis of gene signature of HLADR+CD38+ CD8 T cells and CD45RA+CCR7+ CD8 T cells from dengue confirmed children.
Total differentially expressed genes in DN, Unstim DP, CD69-IFNγ-, CD69+IFNγ- and CD69+IFNγ+ subsets.
List of Blood transcription modules (BTMs) enriched for each HLADR+ CD38+ CD8 T cell subsets.
Differences in the expression of key genes of interests in HLADR+CD38+ CD8 T cell subsets.
Mean expression counts of genes uniquely upregulated in CD69+IFNγ+ subset, mean expression counts and their pairwise comparison of select key genes involved in proliferation, metabolism, transcription regulation, T cell function and translation.
Data Availability Statement
The dengue RNA seq dataset is deposited in Gene Expression Omnibus (GEO) with the accession code GSE212034.