Graphical Abstract
CLINICAL CONTEXT
Pancreatic ductal adenocarcinoma (PDAC) is the fourth-leading cause of cancer-related mortality in the United States, with a 5-year survival rate of only 11%.1 While surgery remains the bedrock in the management of local disease, growing evidence demonstrates the benefits for neoadjuvant (NAT) therapy.2 NAT, inclusive of both chemotherapy and radiotherapy, offers several advantages over the standard “resection and adjuvant” approach. First, it ensures that chemoradiation (CRT) is delivered to all resectable patients, as more than 25% patients fail to receive the intended adjuvant therapy following resection due to complications, morbidity, or patient refusal.3 Furthermore, this strategy avoids the morbidity of an unnecessary operative intervention in patients with systemic progression. It also provides the benefit of delivering radiation to well-oxygenated tissues as decreased oxygenation of the resection bed postoperatively could theoretically limit effectiveness of radiation therapy (RT).3 Lastly, there is potential for NAT to increase the rates of margin-negative resections, particularly for patients with borderline/locally advanced disease.3
The addition of RT to chemotherapy in the neoadjuvant setting has been explored in several studies with mixed results.4,5 Currently, due to lack of data from prospective, randomized trials, there is no established preoperative RT strategy with proven benefit in PDAC. The types of RT typically used to treat pancreatic cancer include fractionated external beam radiation therapy (EBRT) and stereotactic body radiotherapy (SBRT). EBRT uses a linear accelerator to generate photons that target the tumor. SBRT allows for highly conformal dose distribution, which results in the delivery of higher doses of radiation to small tumor volumes with precise image guidance and motion management and is delivered over a shorter period of time.
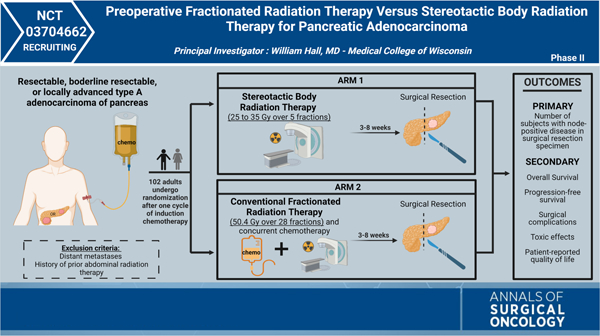
This prospective, randomized, double-arm, phase II, clinical trial, led by William Hall, compared preoperative SBRT with preoperative, conventionally fractionated radiation and chemotherapy (chemo-RT) for patients with resectable, borderline resectable, or locally advanced PDAC. Study participants will be randomized after a minimum of one cycle of induction chemotherapy into two arms. All participants will undergo repeat staging studies before randomization and should have no distant disease. Arm one will include patients who undergo SBRT (25–35 Gy over 5 fractions), and arm two will consist of patients who receive conventional concurrent chemotherapy and RT (50.4 Gy over 28 fractions).
INVESTIGATOR INSIGHT
There is a lack of randomized data that has compared different strategies of giving preoperative radiation therapy for patients with PDAC. Data are mounting that some type of NAT is an optimal strategy for both borderline and resectable pancreatic cancer, and much of that data demonstrating benefit to NAT has included some type of radiation. The existing radiation therapy strategies are rather heterogenous in both volumes treated and dose delivered. The purpose of this trial was to compare two different types of preoperative radiation: SBRT and conventionally fractionated chemo-RT. The SBRT developed and being given on this trial is different than prior strategies in that it includes some nodal areas in close proximity to the primary tumor that are being treated to 25 Gy in 5 fractions (a moderate dose intended to sterilize microscopic disease). All subjects will undergo surgical resection within 3 to 8 weeks following treatment, and the primary outcome measure is the number of patients who present with node-positive disease following surgical resection. This study not only provides a direct comparison between two different radiation modalities for PDAC but also aims to determine if SBRT results in similar nodal downstaging to preoperative, conventionally fractionated chemo-RT.
Through this trial, the investigators anticipate gaining a robust understanding of the response of pancreatic tumors to different types of preoperative RT. Once completed, this trial will be able to optimally inform future larger phase trials in pancreatic cancer.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge the additional contributors to this study: Ben George, MD, Kathleen Christians, MD, Kulwinder Dua, MD, and Parag Tolat, MD.
Footnotes
METHODS
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1245/s10434-022-12742-3.
DISCLOSURE Dr. William A. Hall reports institutional research and travel support from Elekta AB; all other authors have no conflicts to report.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.Hall WA, Dawson LA, Hong TS, et al. Value of neoadjuvant radiation therapy in the management of pancreatic adenocarcinoma. J Clin Oncol 2021;39(34):3773–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White RR, Tyler DS. Neoadjuvant therapy for pancreatic cancer: the Duke experience. Surg Oncol Clin North Am 2004;13(4):675–84. [DOI] [PubMed] [Google Scholar]
- 4.Katz MHG, Shi Q, Meyers JP, et al. Alliance A021501: preoperative mFOLFIRINOX or mFOLFIRINOX plus hypofractionated radiation therapy (RT) for borderline resectable (BR) adenocarcinoma of the pancreas. J Clin Oncol 2021;39(3_suppl):377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eijck CHJV, Versteijne E, Suker M, et al. Preoperative chemoradiotherapy to improve overall survival in pancreatic cancer: long-term results of the multicenter randomized phase III PREOPANC trial. J Clin Oncol 2021;39(15_suppl):4016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


