Abstract
Purpose:
Alterations in retinal structure and function have been well documented in type 2 diabetes (T2DM). However, few studies have evaluated the eye in prediabetes (preDM), a precursor to T2DM. It is unknown which retinal deficits, if any, occur before T2DM diagnosis. This study evaluates retinal structure via optical coherence tomography (OCT) and retinal function via multifocal electroretinogram (mfERG) N1 and P1 in those with PreDM. The goal is to evaluate associations between structure and function across glucose dysfunction.
Methods:
85 subjects (aged 28–69yrs) were tested with VERIS mfERG and Heidelberg Spectralis OCT. Demographic and health information was collected. Subjects were grouped by HbA1c: 33 controls (HbA1c <5.7%), 31 with preDM (HbA1c 5.7–6.4%), and 21 with T2DM (HbA1c >6.4% at the time of testing or diagnosed by physician) and mild or no retinopathy. mfERG N1 and P1 latency and amplitude were measured for the right eye in the foveal hexagon (central 2.4°). Average macular thickness was also measured over the central 3.3°. Groups were compared with ANOVA and corrected t-tests. Models of these associations with diabetes diagnosis (in groups above) were created with backward multivariate regression.
Results:
The T2DM group was exceptionally well-controlled with an HbA1c of 7.0% ± 0.68 but also had elevated systolic blood pressure compared to other groups (P<0.01). The age of the control group was younger (P<0.01), so other testing was age controlled. There was a borderline but statistically significant difference in P1 between the control group and both the preDM and T2DM groups after Bonferroni corrections (P<0.03). There was also a difference in N1 latency between the control and other groups (P<0.001). A multivariate model demonstrated a significant relationship between T2DM/PreDM diagnosis and delayed N1 latency, reduced foveal thickness, and age.
Conclusions:
Structure and function together can provide an associative model of preDM or T2DM changes for patients. Based on this multivariate model, N1 is strongly associated with preDM and T2DM. N1 findings and decreasing foveal thickness are additive and can together inform ocular health related to preDM. Future longitudinal studies are needed to understand changes in function and structure in preDM and T2DM.
Keywords: Prediabetes, Type 2 diabetes, MfERG, OCT
Introduction
Diabetes mellitus (DM) is a rapidly growing epidemic that poses serious global public health concerns. DM consists of several metabolic conditions that are defined by a chronic state of hyperglycemia relating to insulin secretion and insulin resistance. [1] Diabetic retinopathy (DR) is the most common ocular complication of Type 2 Diabetes (T2DM) and results in microvascular damage to the retina [2]. DR is one of the most common causes of preventable yet irreversible blindness in adults aged 20–74 in the western world. [3,4]. Though DR does not occur in all persons with T2DM, there is an association between increased risk of DR and duration of T2DM. [5] Therefore, early detection of glucose dysregulation is key in reducing the risk of developing DR.
It is well-established in the literature that structural and functional changes in the retina occur in T2DM. Studies of function have shown that changes in the implicit time (IT) of the prominent positive wave, P1, measured in the mfERG occur in diabetes both with and without retinopathy. [6,7] Multifocal electroretinogram (mfERG) testing can be used to examine both cone photoreceptor and cone bipolar cell driven responses. In interpreting the mfERG waveform, N1, defined as the initial negative deflection is directly followed by P1, defined as the initial positive deflection of the waveform. Studies in non-human primates have shown that both cone photoreceptors and Off bipolar cells in the central retinal contribute to N1 [8]. Peripheral retinal areas have decreased N1 amplitudes due, in part, to a decrease in contribution from cone photoreceptors. The positive P1 response originates largely from On bipolar cells [8]. As noted above, P1 implicit time is a sensitive measure of altered retinal function in individuals with diabetes; less is known about effects on N1 in the diabetic retina [9]. Because the fovea contains the greatest density of cones in the retina, the N1 response that is derived from photoreceptors is expected to produce a stronger response with greater impact in the fovea than elsewhere in the retina.
Structurally, the inner retinal layers have been observed to decrease in thickness in diabetic individuals without diabetic retinopathy [6]. Some studies have shown that retinal ganglion cells may be lost in diabetic patients, contributing to the decreased inner retinal thickness in diabetic individuals without diabetic retinopathy. Outer retinal layers, specifically the outer nuclear and photoreceptor layers have also been observed to be thinner in diabetic subjects without diabetic retinopathy compared to normoglycemic subjects [10]. Thus, both structural and functional changes have been noted in diabetic eyes without retinopathy.
Prediabetes, an early stage of glucose dysregulation, is defined as an impairment in fasting plasma glucose, impaired glucose tolerance, or elevated HbA1c with blood glucose levels below diabetic levels but above normoglycemic levels. [11]. In 2017, the estimated global prevalence of prediabetes was 352 million, and this number was projected to rise to 587 million by the year 2045 [12]. Despite the predicted increase in prevalence of prediabetes, little is known about the changes that occur in the eye in individuals with prediabetes. The identification of individuals with prediabetes and early intervention may reduce the risk of developing ocular complications associated with T2DM.
Though some retinal changes have been shown to occur in prediabetes, there is limited knowledge of the extent to which structural and functional changes in the retina are detectable in prediabetic individuals. A significant difference in N1 cone response amplitude has been reported between individuals with prediabetes and healthy controls [6]. While some studies have examined structural and functional changes in individuals with pre-diabetes, our knowledge of the extent of these changes in the retina is limited.
In this study, we measured and analyzed average foveal thickness and the P1 and N1 cone-driven implicit times and amplitudes. The purpose of this study was to determine the extent to which structural and functional changes in the fovea are detectable in prediabetes and to expand on the limited research in this area.
Methods
Subjects
Subjects between the ages of 28–70 years old were recruited to participate in the study. Data was collected from two different study groups and the groups were de-identified and combined to make a single data set with the same tests completed for both groups. Subjects in the first group were recruited, consented, and data for this group was collected at Midwestern University Arizona College of Optometry (MWU-AZCOPT) between 2014 and 2017. Recruitment was later expanded to include subjects recruited and consented at the University of Houston College of Optometry between 2017 and 2022. Studies had IRB approval by the appropriate institution. The UH IRB also approved use of the deidentified spreadsheet of data from AZCOPT to be combined with UH data collection. Both studies were conducted by the same PI and used identical equipment. The final dataset consisted of 85 participants. The study data collection followed the Declaration of Helsinki.
Subjects were grouped by HbA1c into three categories (diabetes status): control group (HbA1c <5.7%), pre-diabetic group (HbA1c 5.7–6.4%) and type 2 diabetic group (HbA1c>6.4% or diagnosed by a physician). The determination of HbA1c cutoffs for each group was based on the classification by the American Diabetes Association.
Exclusion criteria in the analysis included subjects with eye diseases affecting the anterior or posterior segment health. This includes but is not limited to subjects who have been diagnosed with diabetic retinopathy greater than mild or any diabetic macular edema (3 subjects with a small number of early microaneurysms or dot hemes were included). Subjects with type 1 diabetes also were excluded from the study analysis. Broader exclusion criteria included: a history of ocular surgery, autoimmune diseases, active ocular inflammation, narrow angles (due to the risk of harm with dilation), and pregnancy.
History and demographics
Subjects were asked to self-report medical history, ocular history, and medications. Demographic information including race, ethnicity, and gender were also collected based on self-report. Subjects in the diabetes group were required to provide a history of their diabetes including onset, duration, and treatment. When necessary, medical records pertaining to the subject’s diabetic disease state were requested from the physician and eye health provider. Blood pressure measures were taken at the time of the study with an automated cuff.
Ocular health assessment
Assessments of both the anterior and posterior segment were conducted to determine ocular health of both the right and left eyes. A slit lamp examination was performed to assess anterior segment health. The subject’s right eye was dilated with one drop of 1% tropicamide and one drop of 2.5% phenylephrine. A retinal camera was used to obtain a 45-degree photograph of the retina. Fundus photographs were used to determine the presence or absence of diabetic retinopathy and any subjects with signs of diabetic retinopathy of any stage beyond mild were excluded from the study analysis.
Blood testing
The subject’s third or fourth finger was pricked with a standard commercial diabetes lancing device and <1.0 mL of blood was collected. The blood sample was used to quantify blood glucose and HbA1c for all subjects in the Houston data group. A small sample of blood was tested via a lipid panel to determine cholesterol, HDL, and LDL levels in the blood. A Siemens DCA HbA1c analyzer was used to determine HbA1c. HbA1c and previous doctor diagnosis for diabetic subjects were used to classify subjects into the control, pre-diabetic, and type 2 diabetic groups. In the Arizona group, the finger prick was used only for blood glucose and other blood used was drawn by a phlebotomist via venous arm draw from an accredited lab which included the same cholesterol and HbA1c testing. The subjects were not fasted at either site.
Multifocal electroretinogram (mfERG)
A ground electrode was placed on the subject’s left ear and was connected to an isolated amplifier. The subject’s dilated right eye was anesthetized with 0.5% proparacaine and a Burian-Allen lens filled with 1% carboxymethylcellulose Celluvisc gel eye drops was placed on the right eye; the left eye was patched. During testing, the subject was instructed to look at a red x target centered on the screen while 103 scaled hexagons flashed between black (< 5 cd/m2) and white (200 cd/m2) in a pseudo-random m-sequence for a total of four minutes. Recording segments were separated into eight segments of 30 s each. Overall, signals were amplified 100,000 times and a 10–300 Hz band pass filter was applied. The eye was monitored during recording and segments with issues were rejected and re-recorded. There was 10% averaging from neighbors and a single iteration of artifact rejection in the record processing. A VERIS Electrodiagnostic Imaging System (VERIS 6.3.4) was used to obtain the mfERG. Only first order kernels were analyzed. Though mfERG tests the central 45° of vision (see Fig. 1 for waveforms), amplitude and latency (N1 and P1) only of the central hexagon (2.4 deg) representing the fovea was analyzed. The central hexagon was chosen as it is the only hexagon that fits entirely within the OCT area measured. The selected area was one in which the amplitude was most robust due to the high cone density and minimal contribution from rods.
Fig. 1.
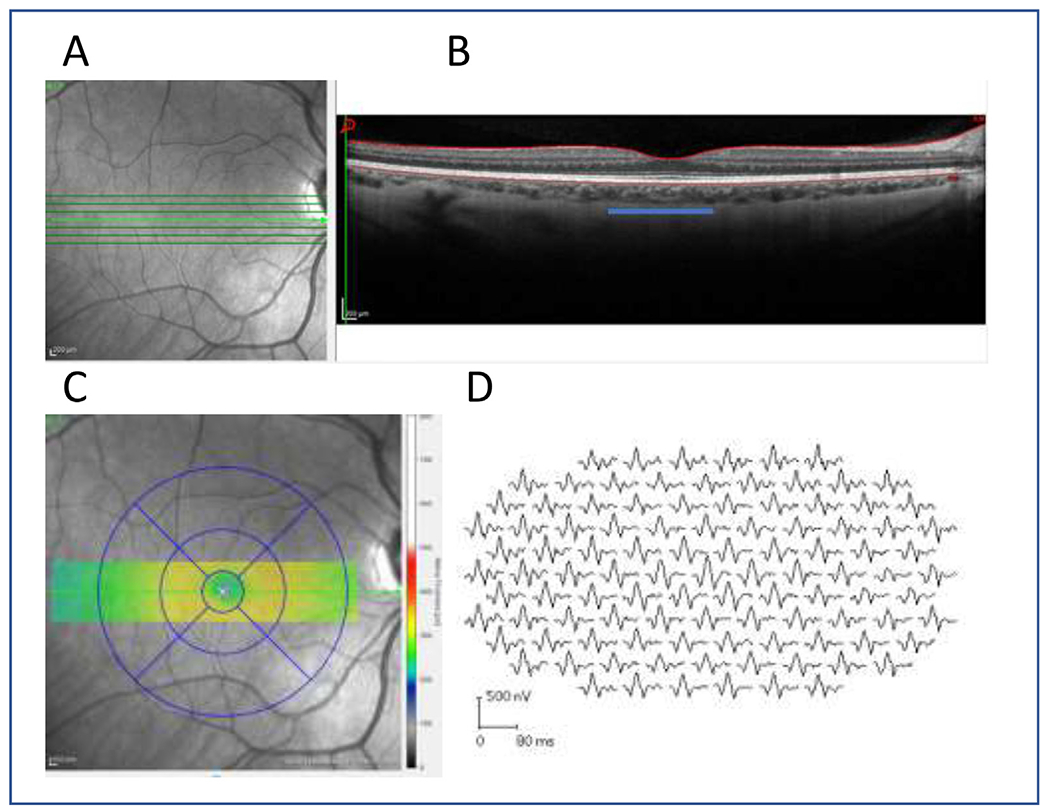
(A) The en face image of retinal area and (B) the central 1mm (3.33 deg) foveal area (blue line) as measured from the 7-line raster and (C) the corresponding foveal area represented by the OCT measure using the ETDRS grid. (D) the mfERG traces from the same subject are also shown. The central waveform was evaluated in this study.
Optical coherence tomography (OCT)
A Heidelberg-Spectralis OCT was used to measure the central macular area in each subject’s right eye. A 7-line raster and a 21-line raster were both measured for the right eye. The scans were examined to determine the presence of diabetic macular edema, an exclusion criterion for subjects participating in the study. Average thickness for the central 1 mm (3.33 deg) of the macula was recorded from the 7-line raster reading.
Statistics
In addition, differences between groups were evaluated using ANOVAs and t-tests with Bonferroni corrections as needed (for 3 comparisons). Backward regression models were used to evaluate the relationship between either HbA1c or diabetes status (normoglycemic, preDM, and DM) and structure and function factors. The factors included were retinal thickness, n1 amplitude, n1 implicit time, p1 amplitude, p1 implicit time, cholesterol, age, gender, and blood pressure. Factors that were not significant were removed from the regression one by one. The model was finalized when only significant or confounding factors remained. The prediabetes group was evaluated separately as well to further characterize changes in that group alone.
Results
Subjects
Eighty-five subjects were included in this study: thirty-three control subjects, thirty-one subjects with prediabetes, and twenty-one subjects with T2DM (Table 1). All subjects with complete datasets from both institutions were included. There was a greater number of females than males enrolled in the study; the control group was the only group that had an approximately equal number of females and males. Gender was included in our analyses but was not a significant confounding factor. The average age for the control group was statistically lower than the age for both the prediabetes and diabetes groups (p<0.001). While all groups contained mostly 40- and 50-year-old patients and are clinically similar, the difference in age is accounted for in the backwards regression model used to analyze the data as a confounder. There was no difference in cholesterol levels (p = 0.36) or diastolic blood pressure (p = 0.22) between the groups, but systolic blood pressure was higher on average (p = 0.007) in the group with diabetes. The average HbA1c in the diabetes group was 7% and indicates a well-controlled group of subjects. The prediabetes group was dominated by patients with lower HbA1c values around 5.8% which is close to control levels with mild glucose elevation and early dysfunction. Only 7 of the prediabetes group had HbA1c values in the 6.0–6.4% range. Although three subjects within the T2DM group had mild NPDR, the inclusion of these subjects did not significantly alter the results of this study. None of the 3 had retinopathy in the fovea but rather had microaneurysms or dot hemes elsewhere in the posterior pole. An analysis with the exclusion of subjects with DR did not have a significant impact on the results. As many microaneurysms are clinically transient, it is unclear in this cross-sectional study if other subjects with DM may have also previously had mild retinopathy changes or if the retinopathy changes seen were long standing or new [13].
Table 1.
Primary demographic information and structural and functional foveal outcomes of the study population. Values given in counts and mean [IQR].
| Control (HbA1c ≤5.6%) | Prediabetes (HbA1c 5.7–6.4%) | Diabetes (HbA1c≥6.5%) | p-value | |
|---|---|---|---|---|
| N | 33 | 31 | 21 | |
| Dx of Diabetes | No | No | Yes | |
| Gender (male:female) | 16:15 | 10:21 | 5:16 | |
| Age (years) | 43 [33–52] | 51 [42–58] | 56 [52–60] | <0.001* |
| HbA1c (%) | 5.3 [5.2–5.4] | 5.9 [5.8–5.9] | 7.0 [6.7–7.2] | <0.001 |
| Cholesterol (mg/dL) | 184 [154–222] | 200 [174–229] | 197 [165–214] | 0.36 |
| Systolic BP (mmHg) | 122 [115–132] | 123 [115–132] | 137 [123–144] | 0.007 |
| Diastolic BP(mmHg) | 80 [73–85] | 80 [74–86] | 85 [74–85] | 0.22 |
| mfERG Foveal N1 Latency (ms) | 15.6 [14.5–16.4] | 16.6[15.9–17.3] | 16.63 [15.9–17.6] | 0.001* |
| mfERG Foveal N1 Amplitude (nV deg2) | 29.3 [24.0–36.8] | 30.9 [25.7–37.6] | 28.77 [24.2–33.1] | 0.6 |
| mfERG Foveal P1 Latency (ms) | 28.7 [28.0–29.6] | 29.56 [28.3–30.9] | 29.83 [29.9–30.7] | 0.03* |
| mfERG Foveal P1 Amplitude (nV deg2) | 58.0 [49.9–67.8] | 56.3 [38.8–67.4] | 52.9 [35.1–57.0] | 0.7 |
| Foveal thickness (μm) | 282 [273–293] | 274 [266–282] | 267 [240–288] | 0.05* |
Indicates significant findings.
Retinal function
There was a significant difference between groups for foveal N1 latency (Fig. 2) and foveal P1 latency (Fig. 3) as measured by mfERG. Post-hoc analyses of N1 latency showed significant differences between the control and prediabetes group and between the control and diabetes groups. There was no significant difference between the prediabetes and T2DM groups. Post-hoc analyses of P1 latency showed similar differences between the control group and non-control groups. There was no significant difference found between groups for foveal N1 amplitude and P1 amplitude. Measures of retinal function including foveal N1 latency, N1 amplitude, P1 latency, and P1 amplitude were not found to be significantly associated with HbA1c across all groups
Fig. 2.
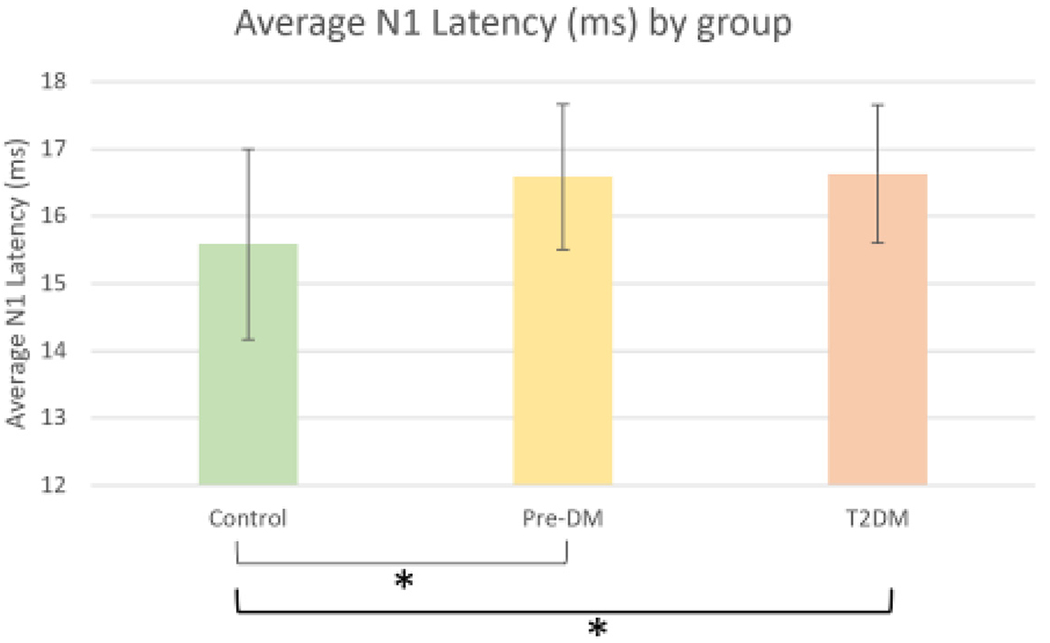
Average N1 Latency at the central foveal hexagon for Control, Prediabetes, and Type 2 Diabetes groups. A significant relationship (denoted by *) is present where the N1 latency is longer in the subjects with Diabetes and Prediabetes compared to controls. P<0.001 after controlling for age. Error bars indicated the 95% confidence interval.
Fig. 3.
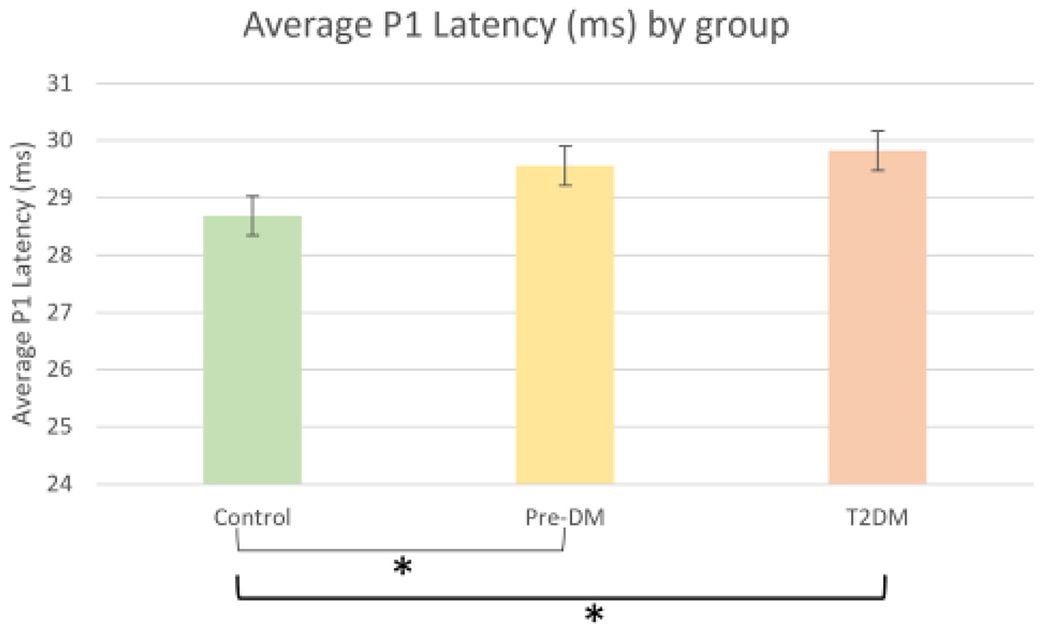
Average P1 Latency at the central foveal hexagon for Control, Prediabetes, and Type 2 Diabetes groups. A significant relationship (denoted by *) is present where the P1 latency is longer in the subjects with Diabetes and Prediabetes compared to controls. P<0.03 after controlling for age. Error bars indicated the 95% confidence interval.
Figure 3. Average P1 Latency at the central foveal hexagon for Control, Prediabetes, and Type 2 Diabetes groups. A significant relationship (denoted by *) is present where the P1 latency is longer in the subjects with Diabetes and Prediabetes compared to controls. P<0.03 after controlling for age. Error bars indicated the 95% confidence interval.
Structural differences between groups
There was a marginally significant difference between groups for average foveal thickness (p = 0.05). Post-hoc analysis of average foveal thickness differed slightly from the results found in post-hoc analyses of foveal function in that there was a significant difference for average foveal thickness between the control and diabetes groups but there was no significant difference found between the control group and the prediabetes group or between the prediabetes and the diabetes groups (Fig. 4).
Fig. 4.
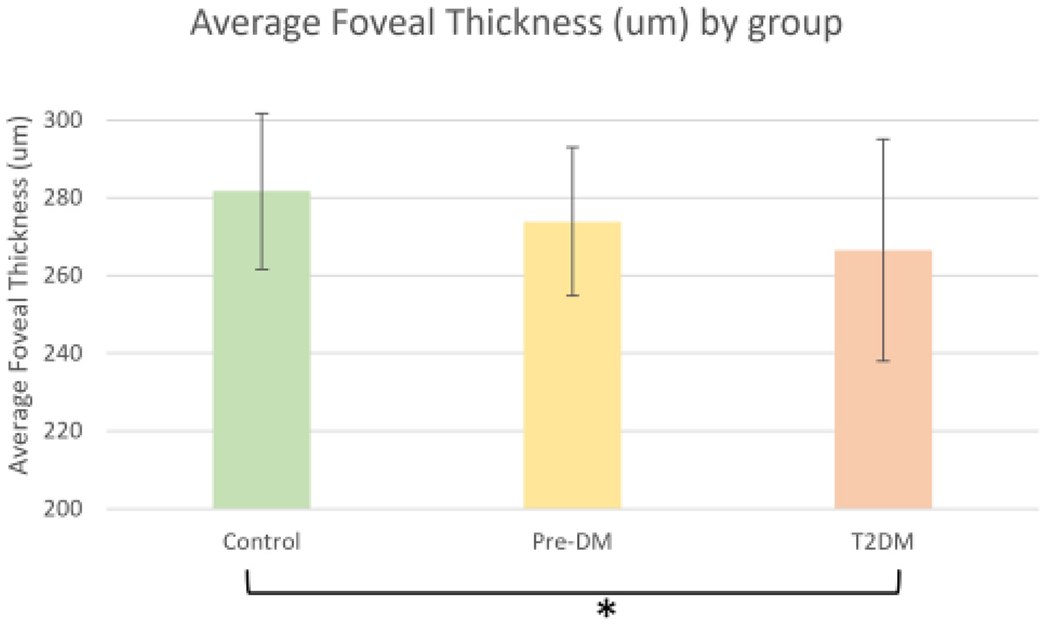
Average Foveal Thickness for Control, Prediabetes, and Type 2 Diabetes Groups. A borderline significant relationship is present (denoted by *) where average foveal thickness was thinner in subjects with Diabetes as compared to controls. p = 0.05 after controlling for age. Error bars indicated the 95% confidence interval.
Regression analysis
Backwards stepwise multivariate regression was conducted with two different outcomes as described. First the relationship between HbA1c and other factors and then the relationship between diabetes status and other factors was conducted. The models were generated separately as there was overlap in the HbA1c values between the well-controlled T2DM patients and the prediabetes group. In the model generated based on diabetic status, diabetic status was defined as the classification of subjects into control (normoglycemic), preDM, or T2DM based on HbA1c at the time of measurement. N1 latency, P1 latency, N1 amplitude, P1 Amplitude, average foveal thickness, age, gender, blood pressure and cholesterol levels were included.
Another model was generated based on HbA1c as the outcome without consideration for diabetic status. The same factors assessed in the first model were also included in the second model. The model evaluating HbA1c was not as successful and had all the factors drop out except for age. Ultimately the selected model was determined to be one based on the relationship between diabetic status and the following factors: age, N1 latency, and foveal thickness. The final selected model is shown in Table 2. This model demonstrates that age, N1 latency, and average foveal thickness were the strongest predictors of diabetic status.
Table 2.
Selected model and values.
| Diabetic status = | Age + | N1 latency + | Average foveal thickness |
|---|---|---|---|
| coefficient | 0.025 | 0.161 | −0.010 |
| p-value | 0.001 | 0.008 | 0.003 |
Discussion
We found that functional changes in the form of delayed cone response in the retina are detectable even in prediabetes. Most studies to date have evaluated these changes in diabetes. This work adds to the growing literature on changes in prediabetes and our results agree with previous studies that show changes in retinal function in prediabetes via mfERG and color vision testing. [14,15] Specifically, we also evaluated the mfERG N1 in the current study.
We are in partial agreement with a study by Ratra et al. in that we found alterations in cone function in subjects with prediabetes compared to the control group. Ratra et al. demonstrated a detectable difference in N1 amplitude without a significant difference in N1 implicit time for individuals with prediabetes [14]. However, our results show that there is a significant increase in implicit time for individuals with prediabetes versus the control group but no significant difference in N1 amplitude between individuals with prediabetes or T2DM versus the control group. However, our study evaluated metrics only for the central hexagon, which represents the area that contains the greatest density of cones as opposed to a greater area of the macula as was done in the previous work. Our study also expands on studies that have shown delayed photoreceptor latency in diabetic subjects who have no visible signs of diabetic retinopathy [12]. The results of our study show a detectable delay in photoreceptor latency in prediabetes, indicating that neuronal dysfunction may have already begun in the early stages of glucose dysregulation. While it has been shown that the delay in P1 implicit time is associated with duration of impaired glucose metabolism and poor glucose control, our understanding about the effect of glucose regulation in restoring delayed photoreceptor latency is limited [12].
There has been other work that shows retinal thinning in prediabetes. The relationship between severity of retinopathy and changes in thickness as well as the effect of glycemic control on thickness changes are not well-understood [16]. A study by Choi et al. suggests that early inner retinal thinning is due to retinal nerve fiber layer loss in diabetes without diabetic retinopathy [17]. The Maastricht Study shows macular thinning in both prediabetes and T2DM without DR, which may indicate that structural neurodegeneration occurs in subclinical stages of glucose dysregulation [18]. Our work shows more thinning as subjects moved to diabetes and agrees in finding some changes in prediabetes. Though it is well known that macular thickness is thinner in females than in males, gender was accounted for in our model but was not a significant factor in the multivariate regression [19].
N1 and P1 implicit time were found to be delayed in individuals with prediabetes and T2DM in the current study. In the backwards regression model of diabetes status, N1 implicit time and age were consistently the strongest factors. Systolic blood pressure and P1 latency had individual associations with diabetes status but fell out of the multivariate model. Structural measures also strongly contributed to both univariate and multivariate models. In the multivariate model, diabetic status was best predicted by a combination of N1 implicit time, age, and foveal thickness. Though both diabetic status and HbA1c were assessed in the model, factors more strongly predicted diabetic status over HbA1c.
We found that retinal function is associated with diabetic status rather than HbA1c alone. This is surprising but likely from the fact that HbA1c may change with treatment (there was overlap in HbA1c between the prediabetes and diabetes groups) and our DM and PreDM groups were both on the lower end of the expected ranges. The multivariate model of HbA1c did not have the strong associations found in the model of DM status, even though the two are highly correlated. It may be that one time point of HbA1c alone is not enough to determine retinal health and more data is needed. The diabetes status encompasses a wider representation of glucose dysfunction. It is unknown whether these changes are lasting or may improve with treatment. As many of our prediabetes and even some of our diabetes subjects were unaware of their glucose dysfunction a model predicting status based on objective ocular testing may have widespread helpful implications for the overall health of patients.
The study has a few important limitations to note. Firstly, there is a significant difference in age between the control group as compared to the prediabetes and diabetes groups, which resulted in potential confounding and age remaining in both the univariate and multivariate models. Studies have shown that there are significant decreases in N1 implicit time in older populations as a natural consequence of the aging process. The average rate of change for implicit time is an increase of 0.02ms per decade for N1 latency and 0.03 ms per decade for P1 latency [20]. The control group was significantly younger than the pre-diabetes and diabetes groups, therefore age must factor into the delay in implicit time. Based on these expected changes, the difference in age for the subjects in the control group (43 years) versus the prediabetes group (51 years) would result in an expected increase in latency of 0.016 ms. However, the average difference between the two groups was greater than 1.0 ms. This difference is greater than what is expected due to normative aging, suggesting that despite the role age plays in the N1 implicit time delay, a large portion of the difference in implicit times is likely due to other causes. Delays in P1 implicit time are expected given the delays in N1 implicit time. Similar differences were found for P1 implicit time between the control and prediabetes groups, with an expected increase of 0.024 ms and an actual difference of 0.87 ms. These differences are attributed to the loss of retinal function that occurs in prediabetes. Similar losses of retinal function due to retinal neurodegeneration are found in patients with diabetes and diabetic retinopathy [21].
A second limitation of this study is the classification of subjects based primarily on HbA1c. This method of classification does not consider the impact of duration of chronic hyperglycemia on retinal function. One of the greatest risk factors for the development of diabetic retinopathy is the duration of diabetes, indicating that duration of chronic hyperglycemia plays a role in the neurovascular disease process. Furthermore, subjects in the diabetes group were not separated based on whether they were treated or untreated for diabetes, which may result in falsely elevated or decreased HbA1c levels. Though most diabetic subjects were treated, a few subjects (n = 2) were not diagnosed with diabetes at the time of their visit. HbA1c is only one measure by which diabetic status is classified; other measures of glycemic status must also be considered as potential factors in retinal structure and function changes. Included in this consideration is that many of the subjects recruited in the prediabetes group were not aware of their glycemic status until enrollment in the study. Therefore, there is no information regarding how long these subjects have been considered prediabetic based on HbA1c.
The last limitation of this study is that the retinal layers at the fovea were not segmented, therefore the exact location from which the foveal thinning occurs is undetermined. Though literature indicates that inner retinal changes result in overall retinal thinning, these inner retinal layers are different in the fovea with less contributions of the inner retina overall compared to the more peripheral retina. Further studies segmenting the foveal layers must be done to determine the location of the thinning. Further studies looking at changes in the choroid must also be done to determine if choroidal changes are associated with changes in photoreceptor function.
This study is a cross-sectional study of subjects in different states of glycemic control. A longitudinal study of subjects may show a more significant relationship between glycemic status and retinal structure/function. Our findings show a difference in N1 implicit time and average foveal thickness in prediabetic subjects. This study assumes that foveal thickness loss is primarily due to inner retinal layer damage. This knowledge, when combined with the functional delays of the cone photoreceptors, provides additive information regarding the effects of glucose dysregulation at the fovea. It must be noted that although not all individuals with prediabetes are guaranteed to progress to DM, it is predicted that up to 70% of all individuals with prediabetes will eventually develop T2DM [22]. Though there is limited knowledge of the effect of glycemic control on reversing retinal neurodegeneration, the early identification of prediabetes and early intervention may reduce the risk of progression to DM. Further investigation is warranted to determine if the reversal of hyperglycemia through lifestyle modification or medical treatment in individuals with prediabetes may restore retinal function. Overall, we believe this is the first study to evaluate foveal structure and function together in prediabetes. This population serves as a good starting place for future evaluation of this important group.
Acknowledgments
This work is part of the MS Thesis of Dr. Angelica Echiverri. The authors thank Drs Laura Frishman and Kaitlyn Sapoznik for their help with this paper. We thank Drs Jennyffer Smith and Vladmir Yevsey-senkov for assistance in data collection and Dr. Brittany Hillier for assistance in data organization.
Funding
This work was funded in part of NIH-EY T35 EY007088 to AE and University of Houston startup funds to WH.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
References
- [1].American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011;34(Suppl 1):S62–9. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sayin N, Kara N, Pekel G. Ocular complications of diabetes mellitus. World J Diabetes 2015;6(1):92–108. doi: 10.4239/wjd.v6.i1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wykoff C, Khurana R, Nguyen QD, et al. Risk of blindness among patients with diabetes and newly diagnosed diabetic retinopathy. Diabetes Care 2021;44(3):748–56. doi: 10.2337/dc20-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shukla UV, Tripathy K. Diabetic retinopathy. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. August 22. [Google Scholar]
- [5].Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights 2016;11:95–104. doi: 10.4137/BMI.S38440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Montesano G, Ometto G, Higgins BE, et al. Evidence for structural and functional damage of the inner retina in diabetes with no diabetic retinopathy. Invest Ophthalmol Vis Sci 2021;62(3):35. doi: 10.1167/iovs.62.3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fortune B, Schneck M, Adams A. Multifocal electroretinogram delays reveal local retinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci 1999;40(11):2638–51. [PubMed] [Google Scholar]
- [8].Hood DC, Frishman LJ, Saszik S, Viswanathan S. Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci 2002;43(5):1673–85. [PubMed] [Google Scholar]
- [9].Harrison WW, Bearse MA Jr, Ng JS, et al. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Invest Ophthalmol Vis Sci 2011;52(2):772–7 Published 2011 Feb 9. doi: 10.1167/iovs.10-5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Damian I, Roman G, Nicoarǎ SD. Analysis of the choroid and its relationship with the outer retina in patients with diabetes mellitus using binarization techniques based on spectral-domain optical coherence tomography. J Clin Med 2021;10(2):210. doi: 10.3390/jcm10020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brannick B, Dagogo-Jack S. Prediabetes and cardiovascular disease: pathophysiology and interventions for prevention and risk reduction. Endocrinol Metab Clin North Am 2018;47(1):33–50. doi: 10.1016/j.ecl.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hostalek U. Global epidemiology of prediabetes - present and future perspectives. Clin Diabetes Endocrinol 2019;5:5. doi: 10.1186/s40842-019-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kohner EM, Stratton IM, Aldington SJ, Turner RC, Matthews DR. Microaneurysms in the development of diabetic retinopathy (UKPDS 42). UK prospective diabetes study group. Diabetologia 1999;42(9):1107–12. doi: 10.1007/s001250051278. [DOI] [PubMed] [Google Scholar]
- [14].Ratra D, Nagarajan R, Dalan D, et al. Early structural and functional neurovascular changes in the retina in the prediabetic stage. Eye 2021;35(3):858–67 (Lond). doi: 10.1038/s41433-020-0984-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Karson N, Smith J, Jones, et al. , et al. Functional retinal outcomes in patients with prediabetes and type 2 diabetes. Ophthalmic Physiol Opt 2020;40:770–7. doi: 10.1111/opo.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pincelli NM, Lima VC, Pacheco MA, Unonius N, Gracitelli CP, Prata TS. Macular inner retinal layer thinning in diabetic patients without retinopathy measured by spectral domain optical coherence tomography. Med Hypothesis Discov Innov Ophthalmol 2018;7(3):133–9. [PMC free article] [PubMed] [Google Scholar]
- [17].Choi JA, Kim HW, Kwon JW, et al. Early inner retinal thinning and cardiovascular autonomic dysfunction in type 2 diabetes. PLoS One 2017;12(3):e0174377. doi: 10.1371/journal.pone.0174377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].De Clerck EEB, Schouten JSAG, Berendschot TTJM, et al. Macular thinning in prediabetes or type 2 diabetes without diabetic retinopathy: the Maastricht study. Acta Ophthalmol 2018;96(2):174–82. doi: 10.1111/aos.13570. [DOI] [PubMed] [Google Scholar]
- [19].Chen H, Xu G, Lai WKG, et al. Gender difference of macular thickness measured by stratus and cirrus optical coherence tomography in normal subjects. Invest Ophthalmol Vis Sci 2010;51(13):2300. -2300. [Google Scholar]
- [20].Seiple W, Vajaranant T, Szlyk J, et al. Multifocal electroretinography as a function of age: the importance of normative values for older adults. Invest Ophthalmol Vis Sci 2003;44(4):1783–92. doi: 10.1167/iovs.02-0518. [DOI] [PubMed] [Google Scholar]
- [21].Wang W, Lo ACY. Diabetic Retinopathy: pathophysiology and treatments. Int J Mol Sci 2018;19(6):1816. Published 2018 Jun 20. doi: 10.3390/ijms19061816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zeng Y, Cao D, Yu H, et al. Early retinal neurovascular impairment in patients with diabetes without clinically detectable retinopathy. Br J Ophthalmol 2019;103:1747–52. doi: 10.1136/bjophthalmol-2018-313582. [DOI] [PubMed] [Google Scholar]


