1. Case Presentation and Introduction
1.1. Case Presentation
A 36-year-old male is brought into Emergency Department (ED) via helicopter after a motor vehicle accident. He was an unrestrained driver whose car was struck by a truck and was extricated from the scene within 20 minutes of first-responder arrival. Due to a Glasgow Coma Score (GCS) of 3, he was intubated with inline stabilization of the neck and a C-collar was placed. During intubation, there was blood seen in the posterior oropharynx. Large bore intravenous access was obtained and 2 liters of normal saline was delivered en route. The patient was hypotensive during transport and a phenylephrine infusion was initiated.
In the ED, the patient remained unresponsive with equal, but sluggishly, reactive pupils. His vital signs were BP 70/46, HR 125 (normal rhythm), and SpO2 of 90% on 100% FiO2 and initial labs revealed a hemoglobin (Hgb) of 6.5 g/dL and a hematocrit (Hct) of 19. A chest radiograph revealed a widened mediastinum and left hemopneumothorax and bedside ultrasound exhibited the “bar code sign” and a “lung point” on the left chest. A left-sided chest tube was placed with 500ml fresh blood evacuated in 10 minutes. Massive transfusion protocol (MTP) was initiated and the patient received 4 units of packed red blood cells (pRBC) and 3 units of fresh frozen plasma (FFP).
With transfusion ongoing, the patient was rushed to the operating room (OR) for a thoracotomy to determine the source of bleeding where arterial and central access was obtained. One-lung ventilation was achieved with a right-sided bronchial blocker to isolate the left lung for surgery exposure. While the surgery team was attempting to achieve control of ongoing patient bleeding, a thromboelastogram (TEG) revealed a prolonged R-time, decreased α-angle, and decreased maximum amplitude (MA). Six additional units of pRBC, 6 units of FFP, and 1 unit of platelets were transfused.
After surgical control of bleeding, a follow-up arterial blood gas (ABG) revealed a pH of 7.15, PaCO2 of 30, and PaO2 of 68 on 100% FiO2 one lung ventilation. TEG revealed improved R-time, α-angle, and MA, but significant fibrinolysis with an increased LY30. Tranexamic acid 1gm IV and 2 pools of cryoprecipitate were infused.
After surgery, the patient was transported to the intensive care unit (ICU) and continued on invasive mechanical ventilation. His TEG and coagulation status (per laboratory analysis) were near normal; however, he received active warming to treat a core temperature of 35° C. The patient was extubated three days after ICU admission and discharged to a rehabilitation facility two weeks later.
1.2. Introduction
In the case presentation, a young man suffered significant trauma in a motor vehicle accident. His injuries were severe and required emergent surgery, massive transfusion of blood products, advanced airway management, and treatment of coagulopathy among many other clinical decisions. Trauma management remains heterogeneous due to variable etiology, patient variables, including age, sex, co-morbidities, etc., and a host of other factors. This can necessitate a variety of imaging modalities to evaluate damage, labs to evaluate patient status, and strategies for surgical repair and patient resuscitation. The most common types of trauma and their respective management necessities are listed in Table I. In the following sections, we provide a comprehensive review of evaluation, surgical objectives, and resuscitation strategies for the critically injured patient. Additionally, we discuss the use of whole blood versus component therapy for blood product resuscitation and use of coagulation monitoring techniques. We believe these sections will provide anesthesiologists with the necessary information to make informed clinical decisions regarding the evaluation and resuscitation of injured patients.
Table I. Trauma type, clinical considerations and management options.
Listed are common types of injury and the appropriate clinical considerations and management options. Abbreviations: Chest radiograph (CXR); ultrasound (US); computed tomography (CT); focused assessment with sonography for trauma (FAST).
| TYPE OF TRAUMA | Considerations and possibilities | Management options |
|---|---|---|
| Chest | Possibilities of Pneumo/hemothorax, Rib fractures, Aortic or cardiac injury | CXR, US, Chest CT, CT angiogram, Possible surgery |
| Abdomen | Spleen, liver, kidney and intestinal/ omental injury. Also aortic injury can occur, hemoperitoneum or perforation, | FAST, CT/A, surgery or careful observation with serial CBC and lactate checks |
| Long bones | Any long bones, pelvic or OMF fractures, possible fat emoboli | Consider early stabilization and look out for blood loss |
| Retroperitoneum | Large vessels can bleed into a large potential space | CT scan early and CBC check, possible IR intervention |
| Neuro/spine | Fractures, contusions, bleeding and increased ICP. Ligamental spine injuries equally dangerous, potential for neurogenic shock. | Stabilization, CT scan immediately, observation, surgery |
| Soft tissue | Possible rhabdomyolysis | Low threshold for suspicion, check CK and blood gases. Follow renal function closely. |
| Blunt | Motor vehicle injuries, Falls and crush injuries | May present hemodynamic collapse late, consider close observation |
| Penetrating | Gunshot injuries or knife trauma | Early intervention usually required |
| Blast injuries | Widespread soft tissue destruction, increased chances of neuro, myocardial injuries. | Widespread damage and can be late presentations. Often multi trauma with severe trauma. |
2. Initial Evaluation and Damage Control Resuscitation
2.1. Initial Assessment
2.1.1. Assessment of Injuries (Primary Survey)
The initial assessment of traumatically injured patients encompasses a structured approach (primary survey) to identify life-threatening conditions by adhering to the ABCDE sequence: airway maintenance with restriction of cervical spine motion; breathing and ventilation; circulation with hemorrhage control; disability and assessment of neurologic status; exposure/environmental control1. The primary survey is based on the patient’s injuries and associated mechanisms, is always performed with simultaneous resuscitation of vital functions, and should be repeated frequently and whenever a patient’s status changes.
2.1.2. Trauma Airway Management (Difficult Airway Algorithm)
Upon initial evaluation, the airway is rapidly assessed to ascertain patency, including signs of airway obstruction by the ability of the patient to communicate verbally. However, particular attention and repeated assessment are necessary to recognize progressive airway loss and ensure early intervention. Although the jaw-thrust maneuver and naso- or oropharyngeal airways can be helpful temporarily, a definitive airway should be established in patients with non-purposeful motor responses, altered level of consciousness (GCS of ≤8), or if there is any doubt about the patient’s ability to maintain airway integrity. A cervical collar must be used to restrict cervical spine motion, yet when airway management is necessary, the cervical collar is opened and a team member should manually protect the cervical spine from excessive mobility. When endotracheal intubation is contraindicated or cannot be accomplished, the threshold to perform a surgical airway must be low.
Despite the recent definition of “difficult airway” by the American Society of Anesthesiologists (ASA), reflecting the traditional perceptions on airway evaluation and management2,3, increasing evidence suggest that physiological derangements are associated with increased peri-intubation complications, and patients should be also evaluated for a physiologically difficult airway with the aim of preventing cardiovascular decompensation and other complications4. Physiologic derangements can be due to traumatic injuries, pre-existing disease, the effects of anesthetic agents, and positive pressure ventilation. In these patients, improving peri-intubation oxygenation and hemodynamic stability is essential, especially before the first intubation attempt5,6.
2.1.3. Intravenous Access
Establishing intravenous access during the primary survey is a prerequisite for the replacement of intravascular volume. In most patients, two large-bore peripheral venous catheters are necessary for obtaining blood samples and administering fluids, blood products, and other agents. When peripheral sites cannot be accessed, central venous/intraosseous access, or venous cutdown may be used1. In addition to venous samples, ABGs provide important information concerning the adequacy of ventilation, oxygenation, and acid-base status.
2.1.4. Imaging / Ultrasound
Radiography can reveal potentially life-threatening injuries that require treatment or further investigation and can provide information to guide resuscitation, especially in patients with blunt trauma. Imaging can be performed in the resuscitation area to avoid delays, even in pregnant patients when necessary, but should not interrupt the resuscitation process.
The implementation of point of care ultrasound (POCUS) has significantly impacted management of trauma patients. FAST or extended FAST (eFAST) are rapid bedside ultrasound screening techniques for quick detection of intraabdominal or intrathoracic injuries7,8. The presence of pathologic fluid on FAST indicates the need for surgical intervention in hemodynamically unstable patients. Nevertheless, the quality of images depends on clinician skill and experience, and can be compromised in obese patients, in those with intraluminal bowel gas, and those with renal and pancreatic injuries9,10.
CT is important for the timely diagnosis of traumatic injuries and to determine the most suitable treatment. Currently, however, there is no agreement on an optimal CT trauma protocol. Although the Advanced Trauma Life Support (ATLS) guidelines recommend CT only if indicated, not by default, and on a selective basis, i.e., for specific body regions1, the European Society of Emergency Radiology (ESER) guidelines distinguish patients in categories and differentiate the approaches11. Nevertheless, in patients without a definitive diagnosis or in patients with severe multi-trauma, the ESER guidelines recommend a whole-body CT after initial resuscitation12,13.
2.1.5. Hemodynamic Goals
Cardiovascular reserves vary with age and/or comorbidities. Although most hypotensive patients present with tachycardia, others, especially the elderly, may not exhibit this sign because of limited cardiac response to catecholamine stimulation, adrenal insufficiency, and hypoglycemia14,15. Therefore, an individualized approach should be used to correct inadequate perfusion and increase tissue oxygenation. Maintaining venous return and cardiac output is crucial for recovery from shock and vasopressor administration should be based on the patient’s hemodynamic status. Taking into consideration the multiple causes of post-traumatic shock, a physiology-guided strategy to maintain a balance between circulatory volume and vascular tone seems prudent16,17.
2.1.6. ABC Score
The need for MTP can be assessed during the primary survey with the use of the assessment of blood consumption (ABC) score18. The ABC is a well-validated score that minimizes delays in MTP initiation and focuses on four immediately available variables: penetrating mechanism; positive FAST; arrival systolic blood pressure (SBP) ≤ 90 mmHg; and arrival heart rate ≥120. The ABC score is recommended by the American College of Surgeons Trauma Quality Improvement Program Massive Transfusion in Trauma Guidelines as a required trigger for MTP activation18.
2.1.7. Baseline Labs
Laboratory tests that may be useful include hemoglobin or complete blood count, ABG, central venous oxygen saturation, lactate, glucose, serum electrolytes, creatine phosphokinase, coagulation profile including prothrombin time/activated partial thromboplastin time, fibrinogen, urine examination for blood, beta human chorionic gonadotropin to rule out pregnancy, viscoelastic hemostatic assays, and toxicology screening19,20.
2.2. Massive Transfusion Protocol
Patients with severe injuries may develop refractory hemorrhage due to dilution of clotting factors, hypothermia, and acidosis. Citrate in blood products may also lead to hypocalcemia-induced coagulopathy. This subset of patients will require aggressive early hemostatic resuscitation and MTP. The latter includes administration of pRBCs, FFP, and platelets in a balanced ratio21.
2.2.1. Indications and Initiation/Timing
‘Massive Transfusion’ refers to transfusion of >10 units of pRBCs within the first 24 hours of admission. ‘Ultramassive transfusion’ has been defined as the administration of ≥20 U of pRBCs in 24 hours22. Identification of patients who may benefit from MTP can be challenging and several scores have been developed to assist in decision making. Currently, the American College of Surgeons Trauma Quality Improvement Program Massive Transfusion in Trauma Guidelines recommend that triggers for the activation of MTP should include an ABC score ≥2, blood transfusion in the trauma bay, persistent hemodynamic instability, or active bleeding requiring intervention23. As both Hgb and blood pressure take time to decrease after the onset of bleeding, initiation of a MTP protocol will be ultimately based on clinical judgement, especially in patients with worsening hypotension and/or vasopressor requirement. Appropriate ratios and types of blood products are described in Section 4.
2.3. Tenets of DCR
Damage control resuscitation (DCR) aims for early hemorrhage control, minimizing operative time, and delaying definitive repair until the patient’s physiologic status has normalized. The principles of DCR include rapid recognition of trauma-induced coagulopathy and shock; permissive hypotension; rapid surgical control of bleeding; prevention and treatment of hypothermia, acidosis, and hypocalcemia; avoidance of hemodilution by minimizing use of crystalloids; and transfusion of pRBC, FFP, and platelets with early use of coagulation factor concentrates24. Transfusion strategies are discussed in Section 4.
2.3.1. Rapid Anatomical Control
Competence in massive hemorrhage control is an essential skill for all staff involved in providing trauma care. Massive hemorrhage may be compressible or non-compressible. DCR includes three distinct phases: 1) a surgery limited to control of the lesions and control hemostasis; 2) temporary closure to limit the risk of abdominal compartment syndrome; and 3) pre-programmed re-operation within 24–48 hours. Damage control surgery should be applied only to the most seriously traumatized patients along with ongoing resuscitation. When correctly applied, damage control surgery can significantly improve patient survival rates.
2.3.3. Permissive Hypotension and Minimization of Crystalloids
Permissive hypotension is the restriction of crystalloids and vasopressors to maintain a lower blood pressure (SBP 80-90 mmHg) until bleeding is controlled to prevent dislodgement of clots by high pressure of circulating volume and improve outcomes. Nevertheless, permissive hypotension may worsen outcomes due to tissue hypoperfusion and is a temporary measure that must be used until bleeding is controlled. In patients with severe traumatic brain injury (TBI), a mean arterial pressure > 80 mm Hg is recommended to maintain cerebral perfusion pressure16.
An increasing amount of evidence suggests that excessive administration of crystalloids increases the risk of dilutional coagulopathy, acute lung injury/acute respiratory distress syndrome, hypothermia, infections, intraabdominal hypertension/abdominal compartment syndrome, multiple organ failure, and death25,26. Indeed, the latest ATLS guidelines include less stringent suggestions for crystalloid administration, recommending fluid resuscitation with up to 1 L of warm saline in patients with class I or II hemorrhage, while early resuscitation with blood/blood products only is advised in patients with evidence of class II or greater hemorrhage27. This is further supported by an analysis of 10-year trends in crystalloid resuscitation reporting that the decrease in high-volume crystalloid resuscitation parallels reduced mortality28.
2.4. Reversal of Anti-Coagulants
Prothrombin complex concentrates (PCC), primarily 4-factor-PCCs, are recommended for reversal of vitamin K antagonist (VKA), while several guidelines support use of FFP for VKA reversal in life-threatening hemorrhage only if PCCs are unavailable16. Of note, the majority of guidelines also recommend co-administration of vitamin K. Due to their thrombogenic profile, activated PCCs and recombinant activated coagulation factor VII (rFVIIa) are not indicated for VKA reversal, even in emergency bleeding situations29,30.
In patients under direct-acting oral anticoagulants (DOACs), most guidelines consider the administration (off-label) of aPCC or 3-/4F-PCC administration in cases of serious or life-threatening bleeding when specific reversal agents are not available31. Although some guidelines recommend the administration of rFVIIa for DOAC reversal, the associated risk of thrombosis limits its use in patients when other hemostatic measures have been ineffective32. The use of FFP is not recommended for reversal as the volume of FFP required for the inhibition of thrombin or Factor Xa would result in significant delays and can cause adverse effects, such as fluid overload33. Furthermore, most guidelines recommend the use of idarucizumab and andexanet alfa in patients with severe or life-threatening bleeding events, or prior to emergency surgery34,35. However, andexanet alfa is not indicated for edoxaban reversal and PCC is still recommended for this purpose36,37.
2.5. Acute Traumatic Coagulopathy
The recognition of acute traumatic coagulopathy (ATC) is extremely important for early diagnosis and management. In many patients, coagulopathy is present even before the onset of resuscitation and correlates with severity of trauma. Despite the lack of a universally accepted definition, acute traumatic coagulopathy is indicated by prolongation of the prothrombin time. Several mechanisms have been proposed to explain the development of trauma-induced coagulopathy. Hyperfibrinolysis and coagulopathy seen in ATC are mainly due to the effects of shock and direct injury on the endothelium38. Endothelial thrombomodulin and tissue plasminogen activator are upregulated in response to tissue hypoperfusion, which together with thrombin generated by tissue trauma, accelerate protein C activation and hyperfibrinolysis39,40. These, together with glycocalyx degradation and platelet and fibrinogen dysfunction eventually result in massive hemorrhage and increased mortality40-43.
3. Choice of Blood Product
3.1. Whole Blood Resuscitation
3.1.1. History
Fresh whole blood transfusion was first widely adopted by the military during World War I. It remained the mainstay of treatment during the war until blood banks developed an improved fractionation process in the 1970s44. With the advancement of blood bank capabilities, there was a shift to component therapy as the preferred method of transfusion based on components' longer shelf life45. This change in transfusion strategy occurred without evidence to compare the efficacy and risks of whole blood compared to component therapy in active hemorrhage and trauma patients46. Apart from war, component therapy also took hold in the civilian population, with specialties now able to give specific components to correct patient pathology. The Red Cross began streamlining component production and storage, thus focusing more on functional blood components, which accelerated the abandonment of whole blood in the civilian population47. During the U.S. wars in Iraq and Afghanistan, whole blood again made a resurgence as a treatment on the battlefield and pre-hospital resuscitation. With exhausted supplies of components, the military turned to "walking blood banks" and whole blood to help treat massive trauma during wartime. Reviews of over 8,000 units of whole blood administered during that period demonstrated equivalent, if not improved, outcomes in the treatment of massive hemorrhage47. With increasing evidence demonstrating that balanced resuscitation (1:1:1) via component therapy is associated with better patient outcomes in the trauma population, there is a renewed interest in using whole blood alone for hemorrhagic resuscitation45,48.
3.1.2. Whole Blood Storage
Whole blood has taken many shapes and is delivered successfully, both warm and cold. The American Association of Blood Banks endorses the use of low titer Type O Whole Blood as a universal donor. However, a variety of whole blood is studied, from fresh warm blood used in military settings to cold-stored blood O- or O+ of various titers used in civilian data44,45. Whole blood is collected in citrate-phosphate-dextrose-adenine solution and stored between 1-6° C for 14-21 days45. A single unit of whole blood contains 500cc and has a hematocrit of 38-50%, platelet count of 100-400k, 100% coagulation factors, and 1000mg of fibrinogen45. The use of whole blood reduces the volume of additives needed and reduces the overall volume transfused. Type O whole blood has universal red cell compatibility, but does not protect against donor plasma anti-A or anti-B antibodies47. Therefore, varying degrees of whole blood with low anti-A and anti-B IgM titers have been used with many institutions defining low titer as less than 1:256. Subsequent studies on whole blood with different titer levels did not show any significant transfusion-related reactions or increased levels of hemolysis at 24 hours45,47. Strandenes et al. utilized rotational thromboelastometry (ROTEM) to demonstrate that cold storage of whole blood had preserved platelet-dependent coagulation function at two weeks and fibrinogen-dependent coagulation function at 3.5 weeks49. Most centers reserve group O Rhesus (Rh) negative whole blood for women of childbearing age and utilize group O Rh-positive with low titers for all males and females over 5047. Given concerns for donor plasma ABO incompatibility and Rh alloimmunization, most centers restrict whole blood transfusions to 2-4 units, with more recent data indicating safety up to six units47.
3.1.3. Whole Blood Advantages
Intraoperatively, whole blood offers several advantages during massive resuscitation. It provides all components through a warmed rapid transfuser utilizing the same intravenous access and improves the speed of delivery by immediate arrival to the OR without a need for product thawing. Whole blood presents a simplified product that allows clinicians to give a single unit rather than focus on balancing pRBC, FFP, and platelets while checking each unit against the patient blood type44. This simplification and handling of fewer blood products should lead to a reduction in administrative error.
3.1.4. Whole Blood Limitations
Despite its benefits, several limitations limit the widespread use and acceptance of whole blood in civilian hospitals. The initiation of a whole blood program to an existing blood bank can be very expensive and, on average, adds an extra $170,000 in annual costs45. While the military has streamlined the process, many civilian facilities face logistical constraints regarding shipping, handling, and storing conditions45. Most centers only validate the use of whole blood for 14 days, and very few centers have adopted measures to centrifuge the unit on day 15 to salvage its components45. While all blood banks were affected by the COVID-19 pandemic, it was more challenging to find suitable donors of whole blood given the combination of extensive donation limitations and the need for the ideal candidate for O− whole blood making up less than 5% of the general population47. However, with research to further assess the risk of hemolytic transfusion reactions and alloimmunization to the Rh antibody, the pool of available donors may drastically increase.
3.2. Component Therapy Resuscitation
3.2.1. pRBC Definition and Storage
In contrast to whole blood therapy, component therapy allows blood to be divided into separate components via differential centrifugation and, subsequently, transfused individually. The three primary components are pRBC, FFP, and platelets.
pRBC are the most used blood component in the United States. One unit is typically 350cc in volume and comprised of red blood cells (250cc), plasma (<50cc), white blood cells, and CPDA-1 anticoagulant50. One unit of pRBC is stored at 1-6° C and maintains a hematocrit of 60-80%51. Many centers in the U. S. leukoreduce each unit of pRBC to reduce leukocytes to less than 5 x106 per unit to reduce the risk of alloimmunization and transfusion reactions52. One unit of pRBC will raise the Hct by 3% and the Hgb by 1g/dL, but must be compatible with the patients' plasma ABO antibodies50.
3.2.2. FFP Definition and Storage
Utilized initially as a volume expander, FFP is a mainstay for hemorrhage management and preventing coagulation abnormalities. FFP is derived from a single unit of whole blood into a citrate-containing anticoagulant solution and frozen within 8 hours. FFP is stored at temperatures between −18 and −30° C and remains viable for up to one year. FFP must be ABO compatible, with AB being the universal donor as it lacks both anti-A and anti-B antibodies. Each unit is approximately 250cc and contains all the necessary clotting factors, including up to 700 mg of fibrinogen per unit53. FFP must be used within 4 hours once thawed or factors V and VIII begin to decline53. Providers give FFP in a balanced resuscitation for bleeding or a surgical setting with an INR >1.6 or PTT >55. The initial dosing recommendations are 10 to 15cc/kg53.
3.2.3. Platelet Definition and Storage
Platelet concentrates are prepared from a unit of whole blood or via apheresis. Once collected, platelets require storage at approximately 22° C, which facilitates the possible growth of bacterial contaminants. The volume of one apheresed unit of platelets is approximately 200-400cc, whereas 4 to 6 units of platelets from whole blood are pooled to equal the same amount50. Platelets must be used within five days, which frequently causes system-wide shortages. Dosing is 10-15cc per kg, raising the platelet count by 30,000-50,000. Platelets must be ABO and Rh compatible with the recipient.
3.2.4. Cryoprecipitate Definition and Storage
Cryoprecipitate, formulated to treat hemophilia A in the 1950s, is now used to replenish fibrinogen in coagulopathic patients, particularly in the trauma and obstetric setting54. Prepared from FFP, cryoprecipitate contains high concentrations of factor VIII, factor XIII, and fibrinogen54. Cryoprecipitate is prepared from a small pool of donors rather than administered as single units as each unit is approximately 10-15 cc54. Ten pooled units should replenish the fibrinogen level by 65 to 70 mg/dL. Although hospital-specific, cryoprecipitate is typically indicated with a fibrinogen level <150 mg/dL in the setting of acute bleeding or surgery.
3.2.5. Anti-Fibrinolysis Therapy
Aminocaproic acid and tranexamic acid (TXA) target the fibrinolysis pathway, reducing the bleeding during trauma. There is an acute imbalance between the coagulation cascade and fibrinolytic pathway in trauma patients, often leading to an overall hyper-fibrinolytic state55. Aminocaproic acid and TXA act to block plasminogen’s lysine binding site irreversibly. Plasminogen is then unable to be activated to plasmin, thus stopping fibrinolysis55. Data has not indicated a detrimental prothrombic effect in the trauma population. TXA in the trauma population was studied explicitly in the CRASH-2 study and demonstrated significantly reduced bleeding rates with no increase in thromboembolic events55. Today, many institutions administer TXA in the trauma population within three hours of injury and encourage continued monitoring of the fibrinolysis pathway via TEG.
3.2.5. Balanced Transfusion Therapy
In trying to maintain a balanced resuscitation via component therapy, many questions have arisen as to the appropriate balance of components during the management of massive trauma. The Pragmatic, Randomized, Optimal Platelet and Plasma Ratios trial (PROPPR) offers the best evidence of massive transfusion component ratio. They compared the ratios of 1:1:1 and 1:1:2 in pRBCs: FFP: platelets. While unable to find a statistically significant difference in mortality at 24 hours and 30 days, Holcomb et al. did demonstrate that the 1:1:1 ratio achieved faster hemostasis and fewer deaths due to exsanguination at 24 hours21.
3.2.6. Other Individual Component Strategies
In Europe, there has been a push for individual administration of clotting factors to assist in massive hemorrhage. While beneficial in specific coagulation disorders, there may be limited use in the trauma population as many patients in the initial hemorrhage have a global coagulopathy that is not factor specific. PCC may be the exception as it can provide a mixture of vitamin K-dependent coagulation factors (factor II, VII, IX, and X)53. Derived from FFP, the concentrated nature of PCC results in 25 times higher clotting potential than plasma alone. While there is good rationale for the addition of PCC to trauma resuscitation, more data are required for its inclusion in component therapy in addition to FFP.
3.3. Early Identification of Blood Type
Early identification of blood type is essential to hemostatic resuscitation, but significant delays may occur in administrating recommended treatment. Initiating DCR as early as possible after severe trauma is pivotal for survival, but identification of trauma patients who need MTP is a real challenge56. Healthcare systems must notify the blood bank early to avoid delays in delivery of blood products. However, in a patient without unexpected antibodies, crossmatched erythrocytes can be available in about an hour after the blood bank receives the sample, which can be lengthier in patients with a positive antibody screen57. In patients with severe shock, the risk of administering non-crossmatched erythrocytes is low and outweighs the risks of waiting for crossmatched pRBCs, and thus, transfusion of the former can be lifesaving58.
3.4. Transfusion End Points
Targets of resuscitation must be individualized in the setting of MTP. In general, end-points include MAP 60-65 mmHg (or higher in TBI); hemoglobin 7-9 g/dL; INR <1.5; fibrinogen >1.5-2 g/L; PLTs > 50 K/μL; pH 7.35-7.45; core temperature >35 °C1,16.
3.5. Component Therapy Disadvantages
The delivery of blood components inherently comes with the risks of hemolytic reactions, transfusion-related lung injury, bacterial contamination, virus transmission, and blood group mismatch59. While extensive measures are in place to minimize these risks, there are more common intraoperative sequelae that require close monitoring during a massive transfusion. Such abnormalities include citrate toxicity, hypothermia, acid-base balance, and potassium derangements59. Citrate comprises all forms of anticoagulant preparations for blood component storage. Citrate's primary purpose is to prolong viability for each unit, but when transfused will bind calcium in vivo leading to hypocalcemia59. While physiologically unavoidable, close attention must be paid to calcium depletion during massive transfusion, particularly when transfusing more than one unit every five minutes. Citrate undergoes hepatic metabolism, and thus in hepatic failure or dysfunction, close attention should be paid to calcium levels. With massive transfusion, all efforts to warm blood products and fluids should be made prior to administration. Hypothermia from massive transfusion can lead to worsening acidosis and leave the patient susceptible to malignant arrhythmias59. Stored blood products are acidotic prior to administration due to citrate and accumulation of metabolites51. During massive resuscitation, a well perfused liver will appropriately metabolize both citrate and lactate to bicarbonate, leading to a compensatory metabolic alkalosis. Furthermore, close monitoring of a patient's potassium levels is critical during transfusion as extracellular potassium will accumulate in stored blood products. The rate of product transfusion is positively correlated with risk of hyperkalemia so close monitoring is key during massive transfusion.
As with whole blood therapy, component therapy has several restrictions and limitations, many of which are institution-dependent. As described, each component has a slightly different storage modality which leads to the need for more storage space and different protocols. With each unit separately transfused, there is a logistical burden placed on in-room providers to check each unit with the patient's blood type and appropriately verify the unit. pRBCs and FFP are administered through a rapid infuser that likely will have a warming mechanism, but the AABB recommends against the infusion of platelets and cryoprecipitate through a warmer59. Thus, at least two access points will need to be available for blood product delivery. Moreover, there can be a delay for the proper thawing of FFP and cryoprecipitate to be readily available for transfusion, which may prolong administration time.
3.6. Institutional Variation
Significant differences in mortality have been demonstrated between Level I trauma centers, which may be accounted for by transfusion practices60. Significant variability in the reporting of quality indicators has also been demonstrated, reflecting the lack of international consensus and benchmarks. In order to understand trends in MTPs and institutional variation, a standard and universally accepted definition of massive transfusion is imperative61.
4. Determination of Coagulopathy
4.1. Point of Care Viscoelastic Tests of Coagulation
4.1.1. Viscoelastic Test Introduction
As our understanding of trauma-induced coagulopathy (TIC) has improved, the need for a targeted, precise approach to trauma resuscitation has become more apparent62. Viscoelastic coagulation tests, such as TEG and ROTEM provide real-time feedback to guide transfusion during massive trauma. These point of care tests offer a visual representation of coagulation through three distinct phases of the clot life-cycle: propagation, stabilization, and dissolution63. Given the challenging logistics of utilizing conventional coagulation assays (CCA) in a suitable timeframe, TEG and ROTEM not only provide a rapid alternative assessment of coagulation, but also improve survival when compared to CCA in severely injured patients receiving massive transfusion64. Both TEG and ROTEM have been increasingly incorporated into algorithms to diagnose and treat bleeding in a variety of clinical applications, including massive trauma63.
4.1.2. Viscoelastic Test Introduction
Assessment of clot formation/dissolution kinetics and strength for both ROTEM and TEG are measured via rotational force. Specifically, a continuously applied rotational force is transmitted to an electromechanical transduction system which produces a real-time display of clot dynamics63. A similar cylindrical cup containing 340 μL of whole blood is employed for each system. For TEG, the cylindrical cup rotates 4° 45’ every five seconds through a pin on a torsion wire. As viscoelastic clot strength increases, more rotation is transmitted to the torsion wire and detected by the electromagnetic transducer. For ROTEM, the cylindrical cup remains fixed while a ball-bearing suspended pin rotates 4° 75’ every six seconds through the application of a constant force. As viscoelastic clot strength increases, pin rotation is impeded. The impedance is detected via a charge-coupled device image sensor system. The operating characteristics of TEG and ROTEM are summarized in Table II. Notably, a TEG device is capable of analyzing two samples simultaneously, while a ROTEM device can analyze four samples at once. Both devices are sensitive to vibration and must be maintained on a stable surface.
Table II. ROTEM and TEG Operating Characteristics.
Summary of operational considerations for each analytic method.
| ROTEM | TEG | |
|---|---|---|
| Cup Motion | Fixed | Rotates |
| Pin Motion | Rotates | Fixed |
| Angle of Rotation | 4° 75’ / 6 sec | 4° 45’ / 5 sec |
| Detection Method | Rotation Impedance | Pin Transduction |
| Temperaure Regulation | Heated Metal Block | Heated Cup |
| Temperature Control | 30-40°C | 24-40°C |
| Cup Interior | Ridged (0.6-0.9mm) | Smooth |
4.1.3. Viscoelastic Test Process
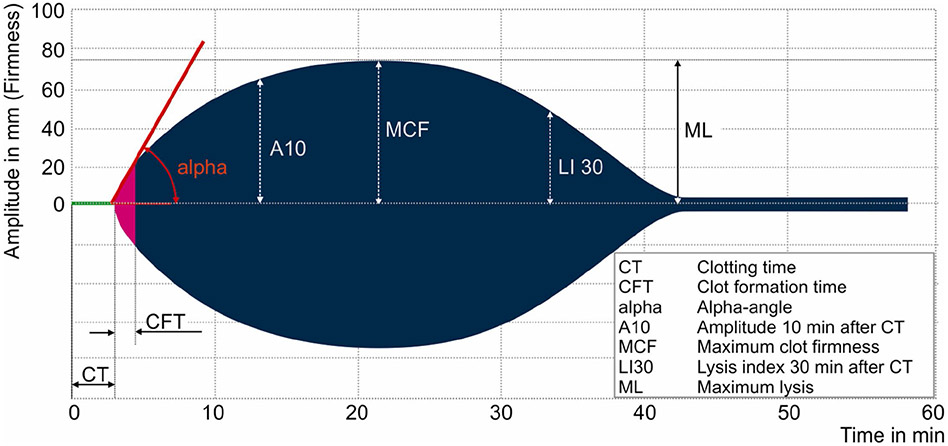
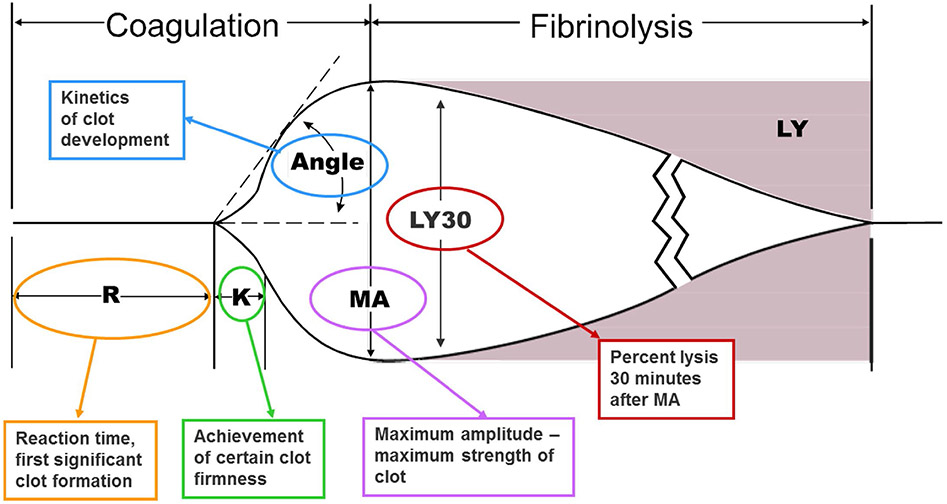
Information regarding clot formation kinetics, strength, and dissolution are very similar between ROTEM and TEG. However, the two tests use different nomenclature to describe the same parameters (Figures 1 and 2)65. The time in minutes required for the tracing to reach an amplitude of 2 mm is defined as the clotting time (CT) for ROTEM and reaction rate (R) for TEG. Similarly, the time necessary for clot amplitude to increase from 2 to 20 mm is defined as clot formation time (CFT) for ROTEM and kinetics time (K) for TEG. Angle (α) is measured by creating a tangent line from the point of clot initiation (CT or R) to the slope of the developing curve. Peak amplitude of the clot, a surrogate for clot strength, is defined as the maximum clot firmness (MCF) for ROTEM and maximum amplitude (MA) for TEG. TEG assesses clot lysis at 30 minutes (LY30) by measuring the percent reductions in the area under the curve that occurs 30 minutes after MA is achieved. However, ROTEM assesses clot lysis index at 30 minutes (LI30) by measuring the percent reduction in MCF when amplitude is measured 30 minutes after CT is detected.
Figure 1. Components of Rotational Thromboelastometry (ROTEM).
Representation of normal ROTEM evaluation of blood. Different analytic times, angles, and components are listed with abbreviations defined in the figure. Previously published materials that are unchanged from the source: “From Anderson L, Quasim I, Steven M, et al. Interoperator and intraoperator variability of whole blood coagulation assays: a comparison of thromboelastography and rotational thromboelastometry. J Cardiothorac Vasc Anesth. Dec 2014;28(6):1550-7. doi:10.1053/j.jvca.2014.05.023; with permission”.
Figure 2. Components of Thromboelastography (TEG).
Representation of normal TEG evaluation of blood. Different analytic times, angles, and components are listed with abbreviations defined in the figure. Previously published materials that are unchanged from the source: “From Anderson L, Quasim I, Steven M, et al. Interoperator and intraoperator variability of whole blood coagulation assays: a comparison of thromboelastography and rotational thromboelastometry. J Cardiothorac Vasc Anesth. Dec 2014;28(6):1550-7. doi:10.1053/j.jvca.2014.05.023; with permission”.
4.1.4. Viscoelastic Test Transfusion Guidelines
In massive trauma, ROTEM and TEG have become key elements of resuscitation algorithms to treat hemorrhage and mitigate TIC. Transfusion of red blood cells can therefore be augmented with targeted transfusion of additional component blood products by assessing the viscoelastic tracing produced in real-time by ROTEM and TEG64. Recently published algorithms are available to guide transfusion of individual components .62 FFP should be considered when R>10 min (TEG) or InTEM CT > 200 seconds (ROTEM). Fibrinogen (in the form of cryoprecipitate or fibrinogen concentrate) should be administered for an α-angle<55° or K>3 min, or MA<20 mm (TEG); or an ExTEM α-angle<65°, ExTEM CFT>140 seconds, or a FibTEM MCF<10 mm (ROTEM). Platelets should be transfused when MA<50 mm (TEG) or when ExTEM MCF<50 mm and FibTEM >10mm (ROTEM). In cases of hyperfibrinolysis (LY30>8% for TEG and ExTEM LI30<94% for ROTEM), tranexamic acid (TXA) should be considered based on results from the landmark CRASH-2 trial66.
4.1.5. Viscoelastic Tests in Clinical Practice
While we encourage the use of ROTEM and TEG in clinical practice, we acknowledge they are not perfect assays. When compared head-to-head against CCA such as partial thromboplastin time (PTT) and international normalized ratio (INR) in trauma patients, viscoelastic assessments of coagulation are sometimes inaccurate67. Clinicians utilizing ROTEM or TEG—versus CCA—may transfuse blood products excessively or when no longer indicated, increasing the risk of adverse reactions68. However, these pitfalls are largely outweighed by the advantages for viscoelastic assays. ROTEM and TEG-guided massive transfusion improves survival compared to CCA-guided resuscitation64. Therefore, the precise accuracy of viscoelastic tests may be immaterial to the bleeding patient. In fact, patients who received TEG-guided resuscitation received fewer plasma and platelet transfusions in the early phase of resuscitation64. TEG and ROTEM are point-of care tests which are usually available for clinical interpretation much earlier than CCA. Ease of use, rapidity of results, speed of interpretation, and improved survival are all advantages for viscoelastic assessments compared to CCA. We therefore strongly encourage either TEG or ROTEM guided resuscitation be employed during massive transfusion in trauma patients.
5. Whole Blood or Component Products – Which to Choose?
5.1. Historical Context
Transfusion with balanced blood components remains the standard of care within the United States for patients with traumatic hemorrhagic shock21,69. The transfusion of whole blood, as an alternative to component therapy, has been utilized widely during military conflicts since at least the World War I48. Due to logistical, standardization, and mechanistic concerns, whole blood was rarely used for civilian trauma patients until the last decade70. However, whole blood transfusion has recently become more widespread during early trauma resuscitation in civilian urban trauma populations70. Recent retrospective studies have determined that transfusion of whole blood is both safe and feasible; however prospective trials comparing whole blood directly to component therapy are lacking71.
5.2. Practice Heterogeneity
Currently, heterogeneity reigns supreme regarding the transfusion of whole blood. A recent meta-analysis identified significant variations in both the storage of whole blood and the definition of what constitutes whole blood transfusion44. Some military settings employ fresh warm blood, while civilian settings tend to utilize cold-stored O+ or O- blood. Some whole blood units are leuko-reduced; others are not. For research purposes, whole blood transfusion has been defined as patients exclusively receiving whole blood or those who received mixtures of whole blood with other components. Ultimately, no significant difference in 24-hour or in-hospital mortality was reported in the meta-analysis44.
5.3. Whole Blood Advantages
Despite these findings, whole blood does offer some distinct advantages to component therapy. The preparation and storage of whole blood may be more cost-efficient than similar amounts of component products72. Whole blood ensures a balanced transfusion strategy, similar to the often-cited 1:1:1 ratio for component therapy21. Furthermore, banking and handling fewer blood products may lead to fewer administrative errors. In resource-limited settings, transfusion of whole blood in hemorrhagic shock leads to fewer overall transfusions and preservation of component products73. These advantages have spurred calls for the adoption of whole blood transfusion into massive transfusion protocol algorithms in the United States and Europe.
5.4. Who Benefits?
Optimizing the patient populations most likely to benefit from whole blood transfusion remains a topic of ongoing research. The European Society of Intensive Care Medicine (ESICM) 2021 clinical practice guidelines for transfusion strategies in bleeding critically ill adults74 acknowledges ongoing investigations regarding whole blood but did not feel sufficient evidence was available to make a recommendation. This gap appears to be closing soon. More than 1,300 clinical trials investigating whole blood transfusion in trauma are currently recruiting patients.
5.5. Timing Considerations
The timing of whole blood transfusion appears to be more uniform across clinical settings. Whole blood is typically transfused early in the resuscitation process, as soon as massive bleeding or hemorrhagic shock are identified. One or two units of whole blood are transfused, followed by standard component therapy with red blood cells, FFP, and platelets75. However, massive transfusion of whole blood (greater than two units) is also feasible and appears to be safe76. The optimal time to switch from whole blood to a component strategy, therefore, remains unknown.
5.6. Future Questions
While the transfusion of whole blood may prove beneficial for bleeding trauma patients, further studies are required prior to changing clinical practice. Centers using whole blood regularly are well equipped to answer several lingering questions. Who should receive whole blood? How many units are appropriate? What is the optimal time to switch to a component-based strategy? Finally, will whole blood decrease transfusion requirements and/or improve patient-centered clinical outcomes? Rigorous clinical trials are required to address these questions and provide clarity on the use of whole blood in trauma resuscitation.
7. Conclusions
In conclusion, we have provided a comprehensive examination of trauma, hemorrhage, patient evaluation modalities, resuscitation strategies, and specific considerations for products and tests available for appropriate patient resuscitation. Initial evaluation should be focused on the best use of resources for to best care for the patient. Thereafter, any surgical intervention should be dedicated to control of bleeding and resuscitation should support end-organ function with prevention and/or treatment of acute blood loss anemia and coagulopathy. As to appropriate blood product resuscitation, it is clear that use of whole blood is returning in the military and some larger civilian medical centers. While whole blood removes some problems with subsequent coagulopathy, it is a resource-intensive process that is not achievable for every institution. Component therapy is a viable option for the patient that produces good clinical outcomes with appropriate transfusion ratios and use of viscoelastic techniques to guide therapy. Finally, our examination of this process leads to many aforementioned questions that should be the focus of intense investigation for the anesthesiologist including, but not limited to: which resuscitation strategy is best for the patient in the short- and long-term? Will different resuscitation strategies cost more societal resources than produce useful outcomes? Is starting with whole blood and converting to component therapy beneficial to the patient?
Synopsis.
Massive trauma remains the leading cause of mortality among people <45 years-old. Therapy has significantly improved in the last few decades and there are new, or renewed, therapeutic strategies for these patients. In this review, we discuss the initial care and diagnosis of trauma patients followed by a comparison of resuscitation strategies. We discuss various strategies including use of whole blood and component therapy, examine viscoelastic techniques for management of coagulopathy, and consider the benefits and limitations of the resuscitation strategies and consider a series of questions that will be important for researchers to answer to provide the best and most cost-effective therapy for severely injured patients.
Key Points.
Care of the trauma patient should focus on early detection, cessation of surgical bleeding, and appropriate resuscitation.
Whole blood and component therapy transfusion are viable techniques to support end-organ function.
Viscoelastic techniques are useful tools to prevent and/or treat coagulopathy.
While there are advantages and disadvantages to both whole blood and component therapy, there is no clear consensus on the use of each therapy, societal costs and patient benefit with either technique.
Statement of Disclosure:
None of the authors have any conflicts to disclose. Funding for this manuscript was provided by R01GM127584 to B. M. W.
References Cited
- 1.James D, Pennardt AM. Trauma Care Principles. StatPearls. 2022. [PubMed] [Google Scholar]
- 2.Detsky ME, Jivraj N, Adhikari NK, et al. Will This Patient Be Difficult to Intubate?: The Rational Clinical Examination Systematic Review. JAMA. Feb 5 2019;321(5):493–503. doi: 10.1001/jama.2018.21413 [DOI] [PubMed] [Google Scholar]
- 3.Apfelbaum JL, Hagberg CA, Connis RT, et al. 2022 American Society of Anesthesiologists Practice Guidelines for Management of the Difficult Airway. Anesthesiology. Jan 1 2022;136(1):31–81. doi: 10.1097/ALN.0000000000004002 [DOI] [PubMed] [Google Scholar]
- 4.Russotto V, Myatra SN, Laffey JG, et al. Intubation Practices and Adverse Peri-intubation Events in Critically Ill Patients From 29 Countries. JAMA. Mar 23 2021;325(12):1164–1172. doi: 10.1001/jama.2021.1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karamchandani K, Wheelwright J, Yang AL, Westphal ND, Khanna AK, Myatra SN. Emergency Airway Management Outside the Operating Room: Current Evidence and Management Strategies. Anesth Analg. Sep 1 2021;133(3):648–662. doi: 10.1213/ANE.0000000000005644 [DOI] [PubMed] [Google Scholar]
- 6.Kornas RL, Owyang CG, Sakles JC, Foley LJ, Mosier JM, Society for Airway Management's Special Projects C. Evaluation and Management of the Physiologically Difficult Airway: Consensus Recommendations From Society for Airway Management. Anesth Analg. Feb 1 2021;132(2):395–405. doi: 10.1213/ANE.0000000000005233 [DOI] [PubMed] [Google Scholar]
- 7.American Institute of Ultrasound in M, American College of Emergency P. AIUM practice guideline for the performance of the focused assessment with sonography for trauma (FAST) examination. J Ultrasound Med. Nov 2014;33(11):2047–56. doi: 10.7863/ultra.33.11.2047 [DOI] [PubMed] [Google Scholar]
- 8.Grunherz L, Jensen KO, Neuhaus V, et al. Early computed tomography or focused assessment with sonography in abdominal trauma: what are the leading opinions? Eur J Trauma Emerg Surg. Feb 2018;44(1):3–8. doi: 10.1007/s00068-017-0816-4 [DOI] [PubMed] [Google Scholar]
- 9.Tsui CL, Fung HT, Chung KL, Kam CW. Focused abdominal sonography for trauma in the emergency department for blunt abdominal trauma. Int J Emerg Med. Sep 2008;1(3):183–7. doi: 10.1007/s12245-008-0050-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato M, Yoshii H. Reevaluation of ultrasonography for solid-organ injury in blunt abdominal trauma. J Ultrasound Med. Dec 2004;23(12):1583–96. doi: 10.7863/jum.2004.23.12.1583 [DOI] [PubMed] [Google Scholar]
- 11.Wirth S, Hebebrand J, Basilico R, et al. European Society of Emergency Radiology: guideline on radiological polytrauma imaging and service (short version). Insights Imaging. Dec 10 2020;11(1):135. doi: 10.1186/s13244-020-00947-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stengel D, Mutze S, Guthoff C, et al. Association of Low-Dose Whole-Body Computed Tomography With Missed Injury Diagnoses and Radiation Exposure in Patients With Blunt Multiple Trauma. JAMA Surg. Mar 1 2020;155(3):224–232. doi: 10.1001/jamasurg.2019.5468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoong S, Kothari R, Brooks A. Assessment of sensitivity of whole body CT for major trauma. Eur J Trauma Emerg Surg. Jun 2019;45(3):489–492. doi: 10.1007/s00068-018-0926-7 [DOI] [PubMed] [Google Scholar]
- 14.Stein DM, Jessie EM, Crane S, et al. Hyperacute adrenal insufficiency after hemorrhagic shock exists and is associated with poor outcomes. J Trauma Acute Care Surg. Feb 2013;74(2):363–70; discussion 370. doi: 10.1097/TA.0b013e31827e2aaf [DOI] [PubMed] [Google Scholar]
- 15.Rushworth RL, Torpy DJ, Falhammar H. Adrenal Crisis. N Engl J Med. Aug 29 2019;381(9):852–861. doi: 10.1056/NEJMra1807486 [DOI] [PubMed] [Google Scholar]
- 16.Spahn DR, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care. Mar 27 2019;23(1):98. doi: 10.1186/s13054-019-2347-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelman S, Bigatello L. The physiologic basis for goal-directed hemodynamic and fluid therapy: the pivotal role of the venous circulation. Can J Anaesth. Mar 2018;65(3):294–308. Les fondements physiologiques de la therapie hemodynamique et liquidienne ciblee: le role fondamental de la circulation veineuse. doi: 10.1007/s12630-017-1045-3 [DOI] [PubMed] [Google Scholar]
- 18.Nunez TC, Voskresensky IV, Dossett LA, Shinall R, Dutton WD, Cotton BA. Early prediction of massive transfusion in trauma: simple as ABC (assessment of blood consumption)? J Trauma. Feb 2009;66(2):346–52. doi: 10.1097/TA.0b013e3181961c35 [DOI] [PubMed] [Google Scholar]
- 19.Bhandarkar P, Pal R, Munivenkatappa A, Roy N, Kumar V, Agrawal A. Distribution of Laboratory Parameters in Trauma Population. J Emerg Trauma Shock. Jan-Mar 2018;11(1):10–14. doi: 10.4103/JETS.JETS_70_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volod O, Bunch CM, Zackariya N, et al. Viscoelastic Hemostatic Assays: A Primer on Legacy and New Generation Devices. J Clin Med. Feb 7 2022;11(3)doi: 10.3390/jcm11030860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. Feb 3 2015;313(5):471–82. doi: 10.1001/jama.2015.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthay ZA, Hellmann ZJ, Callcut RA, et al. Outcomes after ultramassive transfusion in the modern era: An Eastern Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg. Jul 1 2021;91(1):24–33. doi: 10.1097/TA.0000000000003121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cotton BA, Dossett LA, Haut ER, et al. Multicenter validation of a simplified score to predict massive transfusion in trauma. J Trauma. Jul 2010;69 Suppl 1:S33–9. doi: 10.1097/TA.0b013e3181e42411 [DOI] [PubMed] [Google Scholar]
- 24.Camazine MN, Hemmila MR, Leonard JC, et al. Massive transfusion policies at trauma centers participating in the American College of Surgeons Trauma Quality Improvement Program. J Trauma Acute Care Surg. Jun 2015;78(6 Suppl 1):S48–53. doi: 10.1097/TA.0000000000000641 [DOI] [PubMed] [Google Scholar]
- 25.O'Mara MS, Slater H, Goldfarb IW, Caushaj PF. A prospective, randomized evaluation of intra-abdominal pressures with crystalloid and colloid resuscitation in burn patients. J Trauma. May 2005;58(5):1011–8. doi: 10.1097/01.ta.0000162732.39083.15 [DOI] [PubMed] [Google Scholar]
- 26.Duchesne JC, Kaplan LJ, Balogh ZJ, Malbrain ML. Role of permissive hypotension, hypertonic resuscitation and the global increased permeability syndrome in patients with severe hemorrhage: adjuncts to damage control resuscitation to prevent intra-abdominal hypertension. Anaesthesiol Intensive Ther. 2015;47(2):143–55. doi: 10.5603/AIT.a2014.0052 [DOI] [PubMed] [Google Scholar]
- 27.Galvagno SM Jr., Nahmias JT, Young DA. Advanced Trauma Life Support((R)) Update 2019: Management and Applications for Adults and Special Populations. Anesthesiol Clin. Mar 2019;37(1):13–32. doi: 10.1016/j.anclin.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 28.Harada MY, Ko A, Barmparas G, et al. 10-Year trend in crystalloid resuscitation: Reduced volume and lower mortality. Int J Surg. Feb 2017;38:78–82. doi: 10.1016/j.ijsu.2016.12.073 [DOI] [PubMed] [Google Scholar]
- 29.Kozek-Langenecker SA, Ahmed AB, Afshari A, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: First update 2016. Eur J Anaesthesiol. Jun 2017;34(6):332–395. doi: 10.1097/EJA.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 30.Association of Anaesthetists of Great B, Ireland, Thomas D, et al. Blood transfusion and the anaesthetist: management of massive haemorrhage. Anaesthesia. Nov 2010;65(11):1153–61. doi: 10.1111/j.1365-2044.2010.06538.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milling TJ, Pollack CV. A review of guidelines on anticoagulation reversal across different clinical scenarios - Is there a general consensus? Am J Emerg Med. Sep 2020;38(9):1890–1903. doi: 10.1016/j.ajem.2020.05.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frontera JA, Lewin JJ 3rd, Rabinstein AA, et al. Guideline for Reversal of Antithrombotics in Intracranial Hemorrhage: A Statement for Healthcare Professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit Care. Feb 2016;24(1):6–46. doi: 10.1007/s12028-015-0222-x [DOI] [PubMed] [Google Scholar]
- 33.Burnett AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. Jan 2016;41(1):206–32. doi: 10.1007/s11239-015-1310-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollack CV Jr., Reilly PA, van Ryn J, et al. Idarucizumab for Dabigatran Reversal - Full Cohort Analysis. N Engl J Med. Aug 3 2017;377(5):431–441. doi: 10.1056/NEJMoa1707278 [DOI] [PubMed] [Google Scholar]
- 35.Frontera JA, Bhatt P, Lalchan R, et al. Cost comparison of andexanet versus prothrombin complex concentrates for direct factor Xa inhibitor reversal after hemorrhage. J Thromb Thrombolysis. Jan 2020;49(1):121–131. doi: 10.1007/s11239-019-01973-z [DOI] [PubMed] [Google Scholar]
- 36.Cuker A, Burnett A, Triller D, et al. Reversal of direct oral anticoagulants: Guidance from the Anticoagulation Forum. Am J Hematol. Jun 2019;94(6):697–709. doi: 10.1002/ajh.25475 [DOI] [PubMed] [Google Scholar]
- 37.Raval AN, Cigarroa JE, Chung MK, et al. Management of Patients on Non-Vitamin K Antagonist Oral Anticoagulants in the Acute Care and Periprocedural Setting: A Scientific Statement From the American Heart Association. Circulation. Mar 7 2017;135(10):e604–e633. doi: 10.1161/CIR.0000000000000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brohi K, Cohen MJ, Ganter MT, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. May 2008;64(5):1211–7; discussion 1217. doi: 10.1097/TA.0b013e318169cd3c [DOI] [PubMed] [Google Scholar]
- 39.Cap A, Hunt BJ. The pathogenesis of traumatic coagulopathy. Anaesthesia. Jan 2015;70 Suppl 1:96–101, e32-4. doi: 10.1111/anae.12914 [DOI] [PubMed] [Google Scholar]
- 40.Duque P, Mora L, Levy JH, Schochl H. Pathophysiological Response to Trauma-Induced Coagulopathy: A Comprehensive Review. Anesth Analg. Mar 2020;130(3):654–664. doi: 10.1213/ANE.0000000000004478 [DOI] [PubMed] [Google Scholar]
- 41.Meizoso JP, Karcutskie CA, Ray JJ, Namias N, Schulman CI, Proctor KG. Persistent Fibrinolysis Shutdown Is Associated with Increased Mortality in Severely Injured Trauma Patients. J Am Coll Surg. Apr 2017;224(4):575–582. doi: 10.1016/j.jamcollsurg.2016.12.018 [DOI] [PubMed] [Google Scholar]
- 42.Hagemo JS, Christiaans SC, Stanworth SJ, et al. Detection of acute traumatic coagulopathy and massive transfusion requirements by means of rotational thromboelastometry: an international prospective validation study. Crit Care. Mar 23 2015;19:97. doi: 10.1186/s13054-015-0823-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gall LS, Vulliamy P, Gillespie S, et al. The S100A10 Pathway Mediates an Occult Hyperfibrinolytic Subtype in Trauma Patients. Ann Surg. Jun 2019;269(6):1184–1191. doi: 10.1097/SLA.0000000000002733 [DOI] [PubMed] [Google Scholar]
- 44.Crowe E, DeSantis SM, Bonnette A, et al. Whole blood transfusion versus component therapy in trauma resuscitation: a systematic review and meta-analysis. J Am Coll Emerg Physicians Open. Aug 2020;1(4):633–641. doi: 10.1002/emp2.12089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanna M, Knittel J, Gillihan J. The Use of Whole Blood Transfusion in Trauma. Curr Anesthesiol Rep. Jan 17 2022:1–6. doi: 10.1007/s40140-021-00514-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avery P, Morton S, Tucker H, Green L, Weaver A, Davenport R. Whole blood transfusion versus component therapy in adult trauma patients with acute major haemorrhage. Emerg Med J. Jun 2020;37(6):370–378. doi: 10.1136/emermed-2019-209040 [DOI] [PubMed] [Google Scholar]
- 47.McCoy CC, Brenner M, Duchesne J, et al. Back to the Future: Whole Blood Resuscitation of the Severely Injured Trauma Patient. Shock. Dec 1 2021;56(1S):9–15. doi: 10.1097/SHK.0000000000001685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Black JA, Pierce VS, Kerby JD, Holcomb JB. The Evolution of Blood Transfusion in the Trauma Patient: Whole Blood Has Come Full Circle. Semin Thromb Hemost. Mar 2020;46(2):215–220. doi: 10.1055/s-0039-3402426 [DOI] [PubMed] [Google Scholar]
- 49.Strandenes G, Austlid I, Apelseth TO, et al. Coagulation function of stored whole blood is preserved for 14 days in austere conditions: A ROTEM feasibility study during a Norwegian antipiracy mission and comparison to equal ratio reconstituted blood. J Trauma Acute Care Surg. Jun 2015;78(6 Suppl 1):S31–8. doi: 10.1097/TA.0000000000000628 [DOI] [PubMed] [Google Scholar]
- 50.Osterman JL, Arora S. Blood Product Transfusions and Reactions. Hematol Oncol Clin North Am. Dec 2017;31(6):1159–1170. doi: 10.1016/j.hoc.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 51.Yoshida T, Prudent M, D'Alessandro A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus. Jan 2019;17(1):27–52. doi: 10.2450/2019.0217-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fadeyi EA, Saha AK, Naal T, et al. A comparison between leukocyte reduced low titer whole blood vs non-leukocyte reduced low titer whole blood for massive transfusion activation. Transfusion. Dec 2020;60(12):2834–2840. doi: 10.1111/trf.16066 [DOI] [PubMed] [Google Scholar]
- 53.Nordmann GR, Obal D. Is Fresh Frozen Plasma Still Necessary for Management of Acute Traumatic Coagulopathy? Curr Anesthesiol Rep. 2020;10:297–307. [Google Scholar]
- 54.Nascimento B, Goodnough LT, Levy JH. Cryoprecipitate therapy. Br J Anaesth. Dec 2014;113(6):922–34. doi: 10.1093/bja/aeu158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ortmann E, Besser MW, Klein AA. Antifibrinolytic agents in current anaesthetic practice. Br J Anaesth. Oct 2013;111(4):549–63. doi: 10.1093/bja/aet154 [DOI] [PubMed] [Google Scholar]
- 56.Spahn DR, Bouillon B, Cerny V, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. Apr 19 2013;17(2):R76. doi: 10.1186/cc12685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tormey CA, Fisk J, Stack G. Red blood cell alloantibody frequency, specificity, and properties in a population of male military veterans. Transfusion. Oct 2008;48(10):2069–76. doi: 10.1111/j.1537-2995.2008.01815.x [DOI] [PubMed] [Google Scholar]
- 58.Yazer MH, Waters JH, Spinella PC, Aabb /Trauma HORNWP. Use of Uncrossmatched Erythrocytes in Emergency Bleeding Situations. Anesthesiology. Mar 2018;128(3):650–656. doi: 10.1097/ALN.0000000000002037 [DOI] [PubMed] [Google Scholar]
- 59.Sihler KC, Napolitano LM. Complications of massive transfusion. Chest. Jan 2010;137(1):209–20. doi: 10.1378/chest.09-0252 [DOI] [PubMed] [Google Scholar]
- 60.Wade CE, del Junco DJ, Holcomb JB, et al. Variations between level I trauma centers in 24-hour mortality in severely injured patients requiring a massive transfusion. J Trauma. Aug 2011;71(2 Suppl 3):S389–93. doi: 10.1097/TA.0b013e318227f307 [DOI] [PubMed] [Google Scholar]
- 61.McQuilten ZK, Flint AW, Green L, Sanderson B, Winearls J, Wood EM. Epidemiology of Massive Transfusion - A Common Intervention in Need of a Definition. Transfus Med Rev. Oct 2021;35(4):73–79. doi: 10.1016/j.tmrv.2021.08.006 [DOI] [PubMed] [Google Scholar]
- 62.Baksaas-Aasen K, Van Dieren S, Balvers K, et al. Data-driven Development of ROTEM and TEG Algorithms for the Management of Trauma Hemorrhage: A Prospective Observational Multicenter Study. Annals of Surgery. 2019;270(6) [DOI] [PubMed] [Google Scholar]
- 63.Whiting D, DiNardo JA. TEG and ROTEM: Technology and clinical applications. American Journal of Hematology. 2014;89(2):228–232. doi: 10.1002/ajh.23599 [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez E, Moore EE, Moore HB, et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Ann Surg. Jun 2016;263(6):1051–9. doi: 10.1097/SLA.0000000000001608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anderson L, Quasim I, Steven M, et al. Interoperator and intraoperator variability of whole blood coagulation assays: a comparison of thromboelastography and rotational thromboelastometry. J Cardiothorac Vasc Anesth. Dec 2014;28(6):1550–7. doi: 10.1053/j.jvca.2014.05.023 [DOI] [PubMed] [Google Scholar]
- 66.CRASH-2 Trial Collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. The Lancet. 2010;376(9734):23–32. doi: 10.1016/s0140-6736(10)60835-5 [DOI] [PubMed] [Google Scholar]
- 67.Hunt H, Stanworth S, Curry N, et al. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma-induced coagulopathy in adult trauma patients with bleeding. Cochrane Database of Systematic Reviews. 2015;(2)doi: 10.1002/14651858.CD010438.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fraga GP, Bansal V, Coimbra R. Transfusion of Blood Products in Trauma: An Update. The Journal of Emergency Medicine. 2010/August/01/ 2010;39(2):253–260. doi: 10.1016/j.jemermed.2009.02.034 [DOI] [PubMed] [Google Scholar]
- 69.Cannon JW, Khan MA, Raja AS, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. Mar 2017;82(3):605–617. doi: 10.1097/ta.0000000000001333 [DOI] [PubMed] [Google Scholar]
- 70.Walsh M, Fries D, Moore E, et al. Whole Blood for Civilian Urban Trauma Resuscitation: Historical, Present, and Future Considerations. Semin Thromb Hemost. Mar 2020;46(2):221–234. doi: 10.1055/s-0040-1702174 [DOI] [PubMed] [Google Scholar]
- 71.Jackson B, Murphy C, Fontaine MJ. Current state of whole blood transfusion for civilian trauma resuscitation. Transfusion. Jun 2020;60 Suppl 3:S45–s52. doi: 10.1111/trf.15703 [DOI] [PubMed] [Google Scholar]
- 72.Holcomb JB, Jenkins DH. Get ready: whole blood is back and it's good for patients. Transfusion. Aug 2018;58(8):1821–1823. doi: 10.1111/trf.14818 [DOI] [PubMed] [Google Scholar]
- 73.George EC, Uyoga S, M'Baya B, et al. Whole blood versus red cell concentrates for children with severe anaemia: a secondary analysis of the Transfusion and Treatment of African Children (TRACT) trial. The Lancet Global Health. 2022/March/01/ 2022;10(3):e360–e368. doi: 10.1016/S2214-109X(21)00565-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vlaar APJ, Dionne JC, de Bruin S, et al. Transfusion strategies in bleeding critically ill adults: a clinical practice guideline from the European Society of Intensive Care Medicine. Intensive Care Med. Dec 2021;47(12):1368–1392. doi: 10.1007/s00134-021-06531-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cotton BA, Podbielski J, Camp E, et al. A randomized controlled pilot trial of modified whole blood versus component therapy in severely injured patients requiring large volume transfusions. Ann Surg. Oct 2013;258(4):527–32. doi: 10.1097/SLA.0b013e3182a4ffa0 [DOI] [PubMed] [Google Scholar]
- 76.Gallaher JR, Dixon A, Cockcroft A, et al. Large volume transfusion with whole blood is safe compared with component therapy. J Trauma Acute Care Surg. Jul 2020;89(1):238–245. doi: 10.1097/ta.0000000000002687 [DOI] [PubMed] [Google Scholar]