Abstract
Background:
Lipedema is a chronic inflammatory subcutaneous adipose-rich connective tissue disease affecting millions of women worldwide. Disproportionate fat accumulation on the extremities characterized by heaviness, tenderness, and swelling can affect function, mobility, and quality of life. Treatments include conservative measures and lipedema reduction surgery (LRS). Here, we report lipedema comorbidities and surgical techniques, outcomes measures, and complications after LRS.
Methods:
This is a single outpatient clinic retrospective chart review case series of comorbidities and complications in 189 women with lipedema. Bioelectrical impedance analyses, knee kinematics, gait, physical examinations, Patient-Reported Outcomes Measurement Information System, and RAND Short Form-36 questionnaires collected before and after LRS were analyzed for 66 of the 189 women. Hemoglobin levels were measured by transdermal hemoglobin monitor (Masimo noninvasive hemoglobin monitoring; Irvine, Calif.).
Results:
Common comorbidities in 189 women were hypermobile joints (50.5%), spider/varicose veins (48.6/24.5%), arthritis (29.1%), and hypothyroidism (25.9%). The most common complication in 5.5% of these women after LRS was lightheadedness with a 2-g reduction or more in hemoglobin. After conservative measures and LRS in 66 women, significant improvements (P ≤ 0.0009) were found for: (1) knee flexion (10 degrees); (2) gait; (3) Patient-Reported Outcomes Measurement Information System T-score (16%); (4) mobility questions: gait velocity, rising from a chair, stair ascent; (5) RAND Short Form-36 scores: physical functioning, energy/fatigue, emotional well-being, social function, general health; (6) and Bioelectrical impedance analyses total and segmental body fat mass.
Conclusion:
LRS provided significant improvements to women with lipedema using direct physical measurements and validated outcome measures, comparable to those seen after total knee replacement.
Takeaways
Question: How effective is lipedema reduction surgery (LRS)?
Findings: In this retrospective chart analysis, LRS improved knee mechanics, range of motion, and gait. Patient-Reported Outcomes showed decreased pain and improved walking speed, stair ascent, sit to rise functions, and emotional and social well-being. LRS resulted in tissue changes consistent with regression of stage in some patients.
Meaning: LRS is at least as effective in treating lipedema as total knee arthroscopy is for treating arthritis using the same outcome measures.
INTRODUCTION
Lipedema is a subcutaneous adipose-rich connective tissue (SAT) disease estimated to affect 6%–19% of women.1–3 Signs and symptoms of lipedema include limb heaviness and pain.4 Affected limbs can become bulky and heavy, which can make daily tasks such as walking, cleaning, or shopping difficult or impossible. Disproportionate accumulation of lipedema tissue on the inner leg causes valgus stresses (increasing the risk of meniscus damage and osteoarthritis),5 restricts knee flexion,6 and alters gait mechanics, which is a risk factor for falls.7
Lipedema tissue can grow and present in three stages: stage 1 is thickening and disproportionate accumulation of SAT in the extremities with smooth skin; stage 2 is increased fibrous SAT with skin indentations; stage 3 lipedema tissue forms lobules of skin and SAT. Women with stage 2–3 lipedema often have swelling on physical examination and most have evidence of impaired lymphatic function (Fig. 1).8–10
Fig. 1.
Characteristic lipedema phenotype. Photographs of a woman with lipedema type 3 stage 3 view from front, back, and side, showing characteristic skin changes. In the first frontal view (A), notice the mattress changes in the thighs; the red arrows are pointed at the wrist cuff and tissue overhanging the knees. In the second panel (B), arrow is pointed at arm lobule. In the third panel (C), the red arrows are pointed at lobules on the upper arms, hips, and inner knees.
There is no cure for lipedema. Treatment focuses on symptom management, improving patient outcomes, and prevention of complications. Two treatments are (1) nonsurgical conservative measures and (2) lipedema reduction surgery (LRS).
Women with lipedema have been treated with LRS, including suction lipectomy, in Europe for years.11,12 LRS is the only method for removing large amounts of fibrotic tissue with a single treatment, has extensive published data showing it slows the progression of lipedema, and can be performed before complications and disability from lipedema occur.13,14 Women with lipedema are at increased risk for secondary lymphedema.15 The principal superficial lymphatic collecting ducts have been well described and run in or adjacent to the great and small saphenous sheaths.17,18 There are no clinical trials that examine whether presurgical mapping of venous and lymphatic structures improves the safety of LRS. However, lymphatic vessels are dilated and tortuous in the legs of women with lipedema, even in early stages of the disease.16,17 Lymphedema is a comorbidity,18 and lymphatic injury can occur after liposuction.19–21 Up to 50% of women with lipedema also have venous disease.22–24 In this study, LRS uses suction lipectomy with techniques to protect lymphatic structures and can include manual extraction of fibrous tissues that remain after maximal debulking of tissue. Although both liposuction and LRS use cannulas attached to suction, they are distinctly different surgical procedures. Liposuction is one of the most common cosmetic surgical procedures performed,25 and focuses on fat removal for maximal aesthetic results. LRS focuses on pain relief and functional improvement. Lipedema patients on average have a higher body mass index (BMI) and disproportionately larger areas need treatment with longer procedure times to remove greater SAT volumes.
The purpose of this article is to describe the benefits experienced by women in the USA after LRS, using techniques to protect lymphatics.
MATERIALS AND METHODS
Subjects were sequential patients diagnosed with lipedema in an outpatient clinic seen by a single surgeon (TW) from July 2016 to December 2022 who presented for LRS. (See table, Supplemental Digital Content 1, which displays the physical assessment and diagnostic criteria. http://links.lww.com/PRSGO/C891.)
This study was considered exempt from institutional review board oversight because it is an analysis of data collected during usual medical and surgical treatment. Over the years, the data collected evolved. BMI, lobules (subcutaneous tissue extrusions extending beyond the normal contour), and complications after LRS have been documented since 2016. Outcomes questionnaires and knee mechanics (Fig. 2) were integrated into the evaluation in 2019 and added to postoperative assessments in 2021; gait was integrated into the evaluation in 2020 and added to the postoperative assessment in 2021; transdermal hemoglobin monitoring was added in 2020. Charts from 189 patients who underwent 507 surgical cases were reviewed. See Figure 3 for inclusion and exclusion criteria. The number of patients included in each outcome measure is listed in the text, figure legends or tables.
Fig. 2.
Demonstration of measuring knee joint ROM and angles. Photographs showing Q-angle, knee flexion, and valgus measurements with a long arm goniometer. A, Q angle or Quadricep angle from mid patella to inferior iliac spine. The Q-angle is measured by extending a line through the center of the patella to the anterior superior iliac spine and another line from the tibial tubercle through the center of the patella. B, Valgus measured in the anterior plane, the angle of knee joint alignment. This patient with lipedema demonstrates nearly 10 degrees of valgus angulation. Valgus/varus is the angle of deviation of the knee from normal in the sagittal plane while standing. A normal knee is neutral (no varus or valgus angulation). In lipedema, valgus deviation can be caused by bulky lipedema tissue on the medial leg and is associated with lateral tibiofemoral knee arthritis. C, Knee flexion with arms aligned over the femur and fibula and center of the goniometer over the center of the knee joint; the red arrow over the calf shows the measured flexion of 90 degrees in this patient with lipedema, and an additional arm was added for this demonstration and marked with a green arrow for the expected position of the calf with normal flexion of 140 degrees. The difference between measured knee flexion of about 90 degrees and the expected 140 degrees is caused by limitations imposed by the bulky lipedema tissue.
Fig. 3.
Enrollment flowchart. A flowchart showing the inclusion and exclusion criteria used.
LIPEDEMA REDUCTION SURGERY
LRS in this study was designed (by TW) to protect lymphatic (and venous) vessels and improve patient outcomes as follows: (1) Patients were asked to follow conservative lipedema management, as published (See table, Supplemental Digital Content 2, http://links.lww.com/PRSGO/C892).26 (2) Presurgical mapping of the principal superficial veins and lymphatic structures in surgical sites (arms or legs) was performed using vascular Doppler ultrasound (Terason 3000) with a 12-20 MHz linear probe16,17 or indocyanine green direct lymphography.27 (3)The patients were given 2 mg oral lorazepam 2 hours before arrival and upon arrival, oral narcotic pain medication, and additional lorazepam and nitrous oxide/oxygen gas mixture if needed; an intravenous line was started after preoperative oral sedation. (4) Tumescent anesthesia followed published recommendations.28 (5) Lidocaine dosage was limited to 35–55 mg per kg per procedure. 6) Each patient was risk stratified for DVT using Caprini scoring.29,30 Prophylactic oral Xa inhibitors were prescribed for patients with increased DVT risk. (See table, Supplemental Digital Content 2, which displays the compression and conservative treatment. A table showing pre- and postoperative conservative treatment protocol including compression garment type and strength used for each surgery, diet recommendations, lymphatic therapy, and exercise recommendations. These were ordered pre-LRS and recommended to be continued postsurgery. These nonsurgical recommendations and their benefits and limitations were published in 2022. http://links.lww.com/PRSGO/C892.)
LRS was performed with the patient initially in a supine position. The patient’s position was frequently adjusted to ensure longitudinal suction cannula strokes around superficial venous and lymphatic structures to reduce the risk of transection. Sterile prepping was repeated as the patient was repositioned.
Procedures were staged after distributions of lymphotomes, so extremities were paired with adjacent areas of the trunk: anterior/inner thighs in one surgery, lateral thighs/buttocks in a second surgery, calves/ankles in a third surgery, arms/midback (if needed) in a fourth surgery, and the abdomen/flanks were treated last in patients who had lipedema of the torso. The tumescent solution was infiltrated using a 16-gauge Monty tip infiltration cannula and Klein tumescent pump. Microaire Power Assisted Liposuction 650 system was used with 3-mm and 4-mm multi-hole cannulas. The cannula was directed to the plane of least resistance, not too superficial or deep, especially in mapped areas of lymph collectors to minimize the risk of injuring the ducts that lie on top of the deep subcutaneous fascia. Manual lipedema extraction was performed for lipedema nodules not removed during liposuction by massaging along the long axis of limbs to gently mobilize nodules. Nodules are typically removed through liposuction incisions; however, these may need to be enlarged or another incision may be needed to accommodate larger nodules. Before discharge, patients were able to ambulate to the bathroom, and they were discharged in the care of a responsible designate.
Patients were seen 2–4 days postoperatively with a postoperative hemoglobin (HgB) check and then at 6- to 8-week intervals. Most patients have four to five follow-up visits (range of ≥2–10) over 6 months to 3 years.
PROMIS
Patient-Reported Outcomes Measurement Information System (PROMIS) is a set of person-centered measures to evaluate and monitor physical, mental, and social health in adults. PROMIS raw scores were rescaled into a standardized T-score and then compared with a large sample of the US general population; the normalized mean is 50 with an SD of 10.31 Each PROMIS physical function (PF) question has been individually validated.
RAND Short Form (SF)-36 Medical Outcomes Study
The SF-36 was developed and validated for clinical practice and research to follow eight health domains, with each domain contributing to overall quality of life (QOL) and health.32
Statistics
Data are reported as total values, percentages, or average ± SD (GraphPad Prism version 10.0.0, Boston, Mass.). Shapiro-Wilk test for normality was performed before statistical analyses with nonparametric tests. Differences between stages were evaluated by nonparametric testing (Kruskal-Wallis test with multiple comparisons) due to large differences in numbers between groups: Paired t tests were used to evaluate individual changes before and after LRS. Values of P less than 0.05 were considered significant.
RESULTS
A total of 189 women who underwent a total of 507 procedures were evaluated for comorbidities, surgery volumes, times, and surgical complications (Tables 1 and 2).
Table 1.
Demographics and Comorbidities in 189 Women with Lipedema
| Demographic | Stage 1 | Stage 2 | Stage 3 | Total or Average ± SD | P | |
|---|---|---|---|---|---|---|
| No. patients | 7 | 80 | 102 | 189 | ||
| Age (y) | 48 ± 16.7 | 48 ± 16.7* | 52.4 ± 10.1 | 51.2 ± 11.3 | 0.223 | |
| BMI (kg/m2) | 23.7 ± 5.9** | 30.9 ± 5.2** | 38.1 ± 7.1 | 34.5 ± 7.5 | <0.0001 | |
| No. Subjects with Limbs of Different Stages and Types | ||||||
| Stage 1 | Stage 2 | Stage 3 | Total | |||
| Arms (type 4) | 44 | 83 | 47 | 174 | ||
| Leg type 1 | 0 | 0 | 0 | 0 | ||
| Leg type 2 | 0 | 27 | 5 | 32 | ||
| Leg type 3 | 7 | 52 | 95 | 154 | ||
| Leg type 5 | 0 | 1 | 1 | 2 | ||
| Comorbidity | Number Affected | Total Affected (%) | P | |||
| Stage 1 | Stage 2 | Stage 3 | ||||
| Flat feet | 0 | 13 | 20 | 17.7 | 0.36 | |
| Hypermobile joints | 3 | 44 | 47 | 50.5 | 0.56 | |
| Lymphedema | 3 | 58 | 78 | 74 | 0.13 | |
| Knee arthritis | 0 | 11 | 19 | 15.8 | 0.34 | |
| Arthritis | 0 | 20 | 35 | 29.1 | 0.56 | |
| Hypothyroid | 0 | 15 | 34* | 25.9 | 0.13 | |
| Asthma | 1 | 12 | 24 | 19.5 | 0.34 | |
| Anxiety | 0 | 9 | 14 | 12.1 | 0.089 | |
| Depression | 0 | 19 | 24 | 22.8 | 0.3 | |
| Diabetes | 0 | 2 | 8 | 5.3 | 0.2 | |
| Migraine | 1 | 11 | 4 | 8.4 | 0.5 | |
| Anemia | 0 | 51 | 6 | 11.1 | 0.34 | |
| Varicose veins (%) | 0 | 23.5 ± 43 | 25.7 ± 44 | 24.5 ± 43 | 0.2 | |
| Spider veins (%) | 42.9 ± 53 | 49.4 ± 50 | 48.5 ± 50 | 48.6 ± 50 | 0.05 | |
SD=standard deviation; Significance by Wilcoxon matched pairs signed rank test between
P<0.05 vs. Stage 3;
P=0.0007 vs. Stage 3
Table 2.
Locations, Average Fluid Volumes, and Times of Lipedema Reduction Surgery
| Locations | Total Fluid Volumes Removed (Liters ± SD) | Surgery Time | Tumescent Used/Infused (L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Aspirate* | Supernatant | Infranatant | Minimum† | Maximum† | Average | Minimum | Maximum | Average | Minimum | Maximum | |
| Inner/anterior thighs (n = 164) | 6.1 ± 2.1 | 4.7 ± 2 | 1.4 ± 0.6 | 1.8 | 13.8 | 5.5 ± 1.0 | 3.4 | 9.1 | 8.5 | 3.9 | 13 |
| Lateral thighs/buttocks (n = 125) | 5.6 ± 2.1 | 4.6 ± 2.0 | 0.93 ± 0.7 | 1.2 | 12.2 | 5.1 ± 0.9 | 3.32 | 8.0 | 8.3 | 4.3 | 14.9 |
| Calves (n = 121) | 3.2 ± 1.5 | 2.6 ± 1.3 | 0.74 ± 0.6 | 0.3 | 7.6 | 4.9 ± 1.0 | 3.5 | 7.13 | 5.7 | 2.2 | 14.0 |
| Arms (n = 60) | 3.5 ± 1.3 | 2.5 ± 1.0 | 1.0 ± 0.4 | 1.0 | 7.2 | 5.2 ± 0.9 | 3.4 | 8.0 | 7.1 | 3.7 | 9.8 |
| Abdomen/flanks (n = 61) | 5.2 ± 1.7 | 3.4 ± 1.5 | 1.8 ± 0.7 | 1.6 | 8.4 | 5.0 ± 0.8 | 3.88 | 6.67 | 8.2 | 5.1 | 10.5 |
Total aspirate is the volume collected during the entire surgical procedure (supranatant + infranatant). Fat and other tissue components are located within the supernatant, and the infranatant is primarily tumescent fluid with blood, blood products, and dissolved proteins.
Minimum and maximum refer to total aspirate. Data from 189 women with lipedema who underwent a total of 507 procedures with an average 2.7 procedures per patient.
Comorbidities
A common medical condition in our population was arthritis (29%). We also observed a high prevalence of generalized joint hypermobility in this study (50.5%) along with additional comorbidities (Table 1).
Lipoaspirate Volume and Times
The average LRS time was 5.2 hours, and average total aspirate was 5.0 L. The anterior thighs had the highest average total aspirate (7 L) [1.8–13.8 L]. The surgical area with the smallest total aspirate was the calves and ankles at 3.8 L [1.2–7.8 L] (Table 2; Figs. 4–6). The average amount of SAT removed increased with stage; combining all LRS volumes per patient, a maximum of 38.5 L was removed from a single patient over four procedures (Fig. 7).
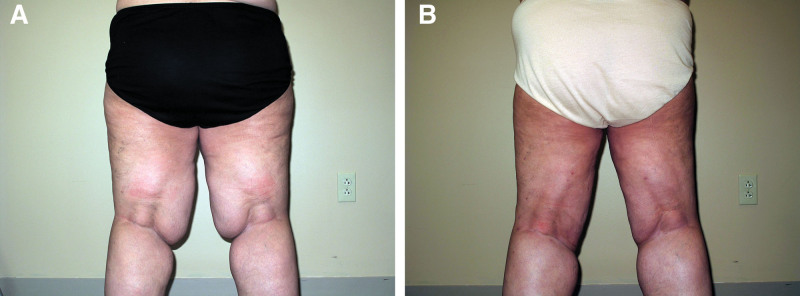
Fig. 4.
Before and after LRS on the thighs. Photographs showing a woman with lipedema type 3 stage 3 before (A) and after LRS (B), view from behind. Notice the lobules just above the knee have been reduced postoperatively and can be restaged to stage 2.
Fig. 6.
Before and after LRS on the full legs. Photographs showing a woman with lipedema type 3 stage 2 before (A) and after LRS (B), lateral view.
Fig. 7.
Suction aspirate totals by stage and average aspirate. The average suction aspirates are graphed by lipedema stage 1–3. Medians with interquartile ratios for total suction aspirate removed when all procedures for each woman with lipedema are combined (n = 189). The aspirate volume increased by stage. Significance was assessed by Kruskal-Wallis test with multiple comparisons.
Fig. 5.
Before and after LRS on the arms. Photographs showing a woman with lipedema type 4 stage 3 before (A) and after LRS (B) on arms, frontal view.
Complications
In 507 procedures (189 women), the following complications after LRS were identified: one patient had postoperative anemia that required overnight hospitalization and a blood transfusion of two units of packed RBCs, and one patient with lupus was noncompliant with prescribed DVT prophylaxis and subsequently experienced a DVT.
In the 401 procedures, the average preoperative hemoglobin was 14.64 g per dL and the average postoperative hemoglobin was 12.8 g per dL; 5.5% experienced lightheadedness with a greater than or equal to 2-g drop in hemoglobin from their preoperative measurement within 96 hours postoperatively. No other complications occurred.
Knee Kinematics and Mechanics
In 26 women, the average preoperative range of motion (ROM) of the left (115.8 ± 13 degrees) and right knee (114.5 ± 14 degrees) were below normal and significantly improved after surgery with a gain of flexion of 9 degrees in the right and 10 degrees in the left knee. Preoperative average valgus deviation in the left and right knees was 6 degrees, which reduced to 3 degrees bilaterally postoperatively. There was no significant postoperative change in the average Q-angle. Gait improved after LRS (Fig. 8).
Fig. 8.
Knee kinematics, knee ROM, knee joint angulation in degrees, quadriceps (Q) angle, and gait pre- and post-LRS. A, Median values with interquartile range for ROM, angulation, and Q-angle of the right and left knee pre- and post-LRS (N = 26). B, Normal (orange) and abnormal (blue) gait pre- and post-LRS (N = 19). Significance was assessed by Wilcoxon matched pairs sign rank test.
Bioelectrical Impedance Analysis
Average total body and segmental body fat mass (BFM) decreased significantly postoperatively (Fig. 9). There was also a decrease in percentage body fat (47% ± 7.5% to 43% ± 8.5%, respectively P < 0.0001).
Fig. 9.
Tissue bioimpedance. Median and interquartile ranges for bioelectrical impedance analysis values for BFM, visceral fat area, and extracellular water in the limbs pre- and post-LRS (N = 64). Significance was assessed by Wilcoxon matched pairs signed rank test.
PROMIS
The mean PROMIS T-score improved significantly FROM 40 ± 6 before LRS to 46 ± 9 after surgery (P < 0.0001; Fig. 10). There were improvements in all PF questions relating to mobility. Improvement in three areas correlates with future disability and mortality, including the ability to walk with normal gait, speed, and distance (P < 0.0001), ascend and descend stairs (P < 0.0001) and get up from a chair (P < 0.009; Table 3).
Fig. 10.
Change in median with interquartile ranges for PROMIS T-score pre- and post-LRS (N = 53). The average population mean is represented by a dotted line. Significance was assessed by Wilcoxon matched pairs
signed rank test.
Table 3.
Individual PROMIS Physical Function (PF) Question Scores ± SD Pre- and Post surgery
| PROMIS Item | Question | Score ± SD | P * | |
|---|---|---|---|---|
| Presurgery | Postsurgery | |||
| PFC38 | Are you able to walk at a normal speed? | 3.6 ± 0.9 | 4.4 ± 0.8 | P < 0.0001 |
| PFA15 | Are you able to stand up from an armless straight chair? | 4.1 ± 1 | 4.6 ± 0.7 | 0.0009 |
| PFA21 | Are you able to go up and down stairs at a normal pace? | 3.0 ± 1.0 | 3.9 ± 1.0 | <0.0001 |
| PFA23 | Are you able to go for a walk of at least 15 minutes? | 3.8 ± 1.0 | 4.4 ± 0.8 | 0.0002 |
| PFA31r1 | Are you able to get up from the floor from lying on your back without help? | 3.0 ± 1.2 | 3.8 ± 1.2 | <0.0001 |
| PFB9 | Are you able to jump up and down? | 2.9 ± 1.4 | 3.6 ± 1.2 | 0.0009 |
| PFB10 | Are you able to climb up five steps? | 4.1 ± 0.9 | 4.5 ± 0.8 | 0.0122 |
| PFB24 | Are you able to run a short distance, such as to catch a bus? | 2.5 ± 1.5 | 3.5 ± 1.4 | <0.0001 |
| PFB32 | Are you able to stand up unsupported for 10 minutes? | 4.1 ± 0.9 | 4.5 ± 0.8 | 0.0163 |
| PFA10 | Are you able to stand for one hour? | 3.1 ± 1.4 | 3.8 ± 1.3 | <0.0001 |
| PFB40 | Are you able to stand up on tiptoes? | 3.7 ± 1.2 | 4.2 ± 1.0 | 0.0088 |
| PFB42 | Are you able to stand unsupported for 30 minutes? | 3.7 ± 1.1 | 4.2 ± 1.1 | 0.0010 |
| PFC37 | Does your health now limit you in climbing one flight of stairs? | 3.8 ± 1.2 | 4.3 ± 0.8 | <0.0001 |
| PFB49 | Does your health now limit you in going for a short walk (less than 15 minutes)? | 4.1 ± 0.9 | 4.5 ± 0.8 | 0.0025 |
| PFC10 | Does your health now limit you in climbing several flights of stairs? | 2.8 ± 1.3 | 3.8 ± 1.1 | <0.0001 |
Significance by Wilcoxon matched pairs signed rank test. N = 53.
SF-36
There was a statistically significant improvement in all eight domains of the SF-36 questionnaire (Fig. 11; Table 4). The average of each domain started below the validated population mean and improved towards or surpassed it.
Fig. 11.
Outcomes data of SF-36 and PROMIS scores. SF-36 graphed by the eight component health domains. Median and interquartile ranges for SF-36 outcome scores pre- and post-LRS (n = 53). Significance was assessed by Wilcoxon matched pairs signed rank test.
Table 4.
SF-36 Data Pre- and Post-LRS (N = 53)
| Scale | Preoperative Score | Postoperative Score | P | Population Mean32 |
|---|---|---|---|---|
| Physical functioning | 57.4 | 72.9 | <0.0001 | 70.6 |
| Role limitations due to physical health | 39.4 | 61.3 | <0.0089 | 53 |
| Role limitations due to emotional problems | 56.6 | 76.7 | <0.0001 | 65.8 |
| Energy/fatigue | 35.9 | 53.9 | <0.0001 | 52.1 |
| Emotional well-being | 66.4 | 75.5 | <0.0001 | 70.4 |
| Social functioning | 62.7 | 77.4 | <0.0001 | 78.8 |
| Pain | 48.1 | 65.7 | <0.0001 | 70.8 |
| General health | 52.6 | 65.0 | <0.0001 | 57.0 |
RAND SF-36 is a Patient-reported medical outcome that measures eight principal domains of health and QOL.
Lobules
Lobules of tissue define stage 3 lipedema. After LRS, 69 of 101 (68%) stage 3 patients no longer had lobules (Fig. 12).
Fig. 12.
Lobules on legs and arms. Median and interquartile ranges for the percentage of women with lipedema stage 3 with lobules pre- and post-LRS (N = 101). Significance was assessed by Wilcoxon matched pairs signed rank test.
DISCUSSION
In the case series before and after LRS, we observed improvements in QOL and pain that mirror improvements after LRS in Germany, Spain, and the United Kingdom.33–36 In addition, our data show improvements in multiple outcomes after LRS for women with stage 1, 2, and 3 lipedema, including knee flexion, knee alignment, gait, body fat, and tissue lobules.
LRS is not aesthetic surgery; it is reconstructive surgery to improve function. Knee function is critical for ambulation and activities of daily living (ADL), including walking, climbing stairs, and getting in and out of a car or a chair. In the USA, total knee arthroplasty (TKA) is one of the most expensive surgical procedures in adults, with average total costs ranging from $31.6–37.4K.37,38 One published systematic review and meta-analysis found the complication rate of TKA was 3.3% at 30 days and 9.7% at 90 days,39 with an average cost of $39K ($4.8–104.8K).40 These data suggest preserving knee function with LRS could reduce operational and rehabilitation costs after TKA.
In the orthopedic literature, the average increase in knee flexion after TKA is 4.8 degrees, approximately half the 10 degrees improvements after LRS reported here.41 Improvements in knee kinematics allow women with lipedema to perform ADL that they could not before LRS due to bulky lipedema tissue on the thigh and calf (Tables 3 and 4; Figs. 8–10). The increased prevalence of knee arthritis before LRS could have blunted the improvement in knee flexion seen in this report. Direct physical observations of improved knee flexion after LRS were confirmed by improvements in responses to mobility questions on the PROMIS and SF-36 questionnaires (Tables 3 and 4).
In total, 16% of the subjects in this case series underwent TKA before LRS. TKA is the most frequent orthopedic joint surgery performed42 with estimates of 4.2%–5.2% of women in the general population undergoing this procedure;43 our observed rate is three to four times higher, likely related to mechanical factors affecting the knee in women with lipedema, including excess tissue on the legs.
Most women with lipedema have a valgus angulation at their knee caused in part by increased hip and thigh tissue. Even a slight degree of valgus misalignment can increase the risk of meniscus damage and osteoarthritis.5 Consideration should be given to performing LRS to reduce the progression of arthritis.
Gait abnormalities limit mobility and ADL and are a risk factor for falls.7 Gait improved in women with lipedema after LRS through clinical assessment and the PROMIS and SF-36 questionnaires. We are not aware of previous reports of gait changes measured in women with lipedema after LRS.
INDIVIDUAL PROMIS PF OUTCOMES
Individual PROMIS PF questions improved significantly after LRS, providing examples of functional improvements in ADL. For example, the average response to PFA-21 (are you able to go up and down stairs at a normal pace?) before LRS was “with some difficulty” and after was “with little difficulty.” PFA-21 has been correlated to timed stair ascent.44 Stair negotiation time was a significant predictor of functional decline.45 Improvements in PFB-24 (are you able to run a short distance, such as to catch a bus?) and PFA-15 (are you able to stand up from an armless straight chair?) also correlate with objectively measured physical performance tests.44
These outcomes also corroborate objectively measured improvements on the clinical examination. A change in rating measurements of two to three points is considered to be a minimally clinically important difference in health.46 Other publications report 1.5–5-point improvements in PF after TKA.47,48 Changes in PROMIS mobility observed in this study are at least of magnitude similar to PROMIS measures after TKA.
RAND SF 36 MOS
Overall health and QOL for women with lipedema significantly improved after LRS, as demonstrated by SF-36. Amitkumar reported similar improvement in SF-36 domains from spine surgery.49
Bioelectrical Impedance Analysis
Total and segmental BFM decreased after LRS, as expected.
Lobule Changes
Over 68% of women with stage 3 lipedema had a reduction in the presence of lobules, which likely contributed to an improved gait. The absence of lobules suggests that these women could be clinically diagnosed as having stage 2 lipedema. Regression in stage has been reported previously50 and is an important clinical improvement in this disease.
COMPLICATIONS
The most common complication of LRS was lightheadedness with a hemoglobin drop of greater than 2 g per day (5.5%). We recommend following hemoglobin levels post-LRS.
There are abnormalities and impairments in lymphatic function in lipedema, and lymphatic injury can occur during suction lipectomy.16 Using high-frequency ultrasound to identify venous and lymphatic structures is a straightforward and relatively inexpensive addition to LRS; once identified and mapped, it follows naturally that extra care would be taken not to transect these structures. Using bioelectrical impedance analyses to assess tissue fluid, and ultrasound to look for signs of lymphatic injury after LRS, no evidence of lymphatic injury was found in 507 procedures using LRS techniques described in this article. The availability and low risk of ultrasound guidance of vascular mapping is a safe and reasonable addition to LRS.
Comorbidities
Because arthritis was common in our population, frequent assessment of joints and symptoms of knee arthritis in women with lipedema may be warranted since earlier treatment with LRS could prevent further knee damage and possible TKA. Joint hypermobility prevalence was also increased in our population and should be assessed.
LIPOASPIRATE VOLUMES AND FUNCTIONAL GAINS
The average total aspirate for all LRS was over 5 L (2.8−13.8 L). Women with higher stages had larger volumes removed. LRS is considered a large-volume reconstructive surgery,51 distantly related to cosmetic suction lipectomy. The goal of LRS is to remove SAT, precipitated solids, and fibrous nodules between the skin and deep fascia to improve function. Adequate debulking of SAT results not only in pain relief but improvements in QOL and mechanical and mobility benefits.
The American Society of Plastic Surgery expressed concerns about large-volume liposuction and its derivatives. Several states in the United States have enacted limits of 5 L for these procedures. Chow et al showed no difference in major complications between the non–large-volume (≤5000 mL) and large-volume (>5000 mL) liposuction cohorts.52 Chow further showed lower complications in larger volume lipoaspirates in patients with higher BMI. We agree with Chow that there should not be an arbitrary aspirate volume limit, and rather, each patient’s health history, risk factors, and goals should be individually considered, and the surgical aspirate modified accordingly. In our study, large volumes were safely removed, allowing treatment of patients with advanced lipedema in fewer procedures.
There is currently no CPT code that accurately describes LRS. Moreover, the 15877-79 suction lipectomy CPT codes do not have a full description in the coding manual because they are placeholders for cosmetic procedures.
SURGERY TIME
The average time for LRS was ~5 hours, and the maximum time was 9 hours, much longer than for cosmetic liposuction. Increased time was the result of the preoperative mapping of venous and lymphatic structures/landmarks, larger volumes of aspirate removed, techniques to limit the risk of lymphatic injury, manual removal of fibrous tissue, super wet tumescent technique, use of small blunt cannulas, which are safe but remove tissue slowly, and frequent repositioning to allow for safer cannula strokes.
STRENGTHS AND LIMITATIONS
The following are the strengths of this study:
Improvements in knee mechanics, mobility, and QOL add new information about the benefits of LRS and corroborate with observed improvements in gait.
The study adds additional data on the prevalence of lipedema comorbidities.
LRS with conservative measures improved mobility, whereas conservative measures alone did not.26
Limitations of the study are as follows:
1) Data reported in this study are from a single site and surgeon (TW).
2) The number of patients is small to moderate.
3) Patient-reported outcome measures are subject to recall bias.
4) This is not a randomized placebo-controlled clinical trial, which would not be ethical.53
CONCLUSIONS
LRS using lymphatic sparing techniques for debulking of fibrotic SAT in women with lipedema resulted in significant improvements in mobility, knee ROM, gait, QOL, social function, and pain. Improvements in movement, knee mechanics, and QOL outcomes are equivalent to or better than published data for TKA. Objectively assessed PF improvements align with patient-reported outcome improvements, and importantly, should lower the risk of future functional decline and mortality in women living with lipedema.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online 30 November 2023.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
References
- 1.Schook CC, Mulliken JB, Fishman SJ, et al. Differential diagnosis of lower extremity enlargement in pediatric patients referred with a diagnosis of lymphedema. Plast Reconstr Surg. 2011;127:1571–1581. [DOI] [PubMed] [Google Scholar]
- 2.Herpertz U. Krankheitsspektrum des Lipödems an einer Lymphologischen Fachklinik—Erscheinungsformen, Mischbilder und Behandlungsmöglichkeiten. Vasomed. 1997;5:301–307. [Google Scholar]
- 3.Forner-Cordero I, Szolnoky G, Forner-Cordero A, et al. An overview of its clinical manifestations, diagnosis and treatment of the disproportional fatty deposition syndrome - systematic review. Clinical Obesity. 2012;2:86–95. [DOI] [PubMed] [Google Scholar]
- 4.Angst F, Lehmann S, Aeschlimann A, et al. Cross-sectional validity and specificity of comprehensive measurement in lymphedema and lipedema of the lower extremity: a comparison of five outcome instruments. Health Qual Life Outcomes. 2020;18:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felson DT, Niu J, Gross KD, et al. Valgus malalignment is a risk factor for lateral knee osteoarthritis incidence and progression: findings from the Multicenter Osteoarthritis Study and the Osteoarthritis Initiative. Arthritis Rheum. 2013;65:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowe PJ, Myles CM, Walker C, et al. Knee joint kinematics in gait and other functional activities measured using flexible electrogoniometry: how much knee motion is sufficient for normal daily life? Gait Posture. 2000;12:143–155. [DOI] [PubMed] [Google Scholar]
- 7.Kikkert LHJ, de Groot MH, van Campen JP, et al. Gait dynamics to optimize fall risk assessment in geriatric patients admitted to an outpatient diagnostic clinic. PLoS One. 2017;12:e0178615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crescenzi R, Donahue PMC, Hartley KG, et al. Lymphedema evaluation using noninvasive 3T MR lymphangiography. J Magn Reson Imaging. 2017;46:1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gould DJ, El-Sabawi B, Goel P, et al. Uncovering lymphatic transport abnormalities in patients with primary lipedema. J Reconstr Microsurg. 2019;36:136–141. [DOI] [PubMed] [Google Scholar]
- 10.van de Pas CB, Boonen RS, Stevens S, et al. Does tumescent liposuction damage the lymph vessels in lipoedema patients? Phlebology. 2020;35:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reich-Schupke S, Schmeller W, Brauer WJ, et al. S1 guidelines: lipedema. J Dtsch Dermatol Ges. 2017;15:758–768. [DOI] [PubMed] [Google Scholar]
- 12.Gensior M, Cornely M. Complications and their management in the surgical treatment of lipohyperplasia dolorosa. Dermatologie (Heidelb). 2023;74:114–120. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner A, Hueppe M, Schmeller W. Long-term benefit of liposuction in patients with lipoedema: a follow-up study after an average of 4 and 8 years. Br J Dermatol. 2015;174:1061–1067. [DOI] [PubMed] [Google Scholar]
- 14.Peled AW, Slavin SA, Brorson H. Long-term outcome after surgical treatment of lipedema. Ann Plast Surg. 2012;68:303–307. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen JC, Aldrich MB, Fife CE, et al. Lymphatic function and anatomy in early stages of lipedema. Obesity (Silver Spring, Md). 2022;30:1391–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Heumen S, Riksen JJM, Bramer WM, et al. Imaging of the lymphatic vessels for surgical planning: a systematic review. Ann Surg Oncol. 2023;30:462–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Heumen S, Riksen JJM, Bramer WM, et al. Imaging of the lymphatic vessels for surgical planning: a systematic review. Ann Surg Oncol. 2022;29:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herbst K, Mirkovskaya L, Bharhagava A, et al. Lipedema fat and signs and symptoms of illness, increase with advancing stage. Archives of Medicine. 2015;7:1–8. [Google Scholar]
- 19.Frick A, Hoffmann JN, Baumeister RG, et al. Liposuction technique and lymphatic lesions in lower legs: anatomic study to reduce risks. Plast Reconstr Surg. 1999;103:1868–73; discussion 1874. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann JN, Fertmann JP, Baumeister RG, et al. Tumescent and dry liposuction of lower extremities: differences in lymph vessel injury. Plast Reconstr Surg. 2004;113:718–24; discussion 725. [DOI] [PubMed] [Google Scholar]
- 21.Wright TF, Herbst KL. A case series of lymphatic injuries after suction lipectomy in women with lipedema. Am J Case Rep. 2022;23:e935016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dean SM, Valenti E, Hock K, et al. The clinical characteristics of lower extremity lymphedema in 440 patients. J Vasc Surg Venous Lymphat Disord. 2020;8:851–859. [DOI] [PubMed] [Google Scholar]
- 23.Child AH, Gordon KD, Sharpe P, et al. Lipedema: an inherited condition. Am J Med Genet A. 2010;152A:970–976. [DOI] [PubMed] [Google Scholar]
- 24.Wold LE, Hines EA, Jr, Allen EV. Lipedema of the legs: a syndrome characterized by fat legs and edema. Ann Intern Med. 1951;34:1243–1250. [DOI] [PubMed] [Google Scholar]
- 25.Wu S, Coombs DM, Gurunian RL. Liposuction: concepts, safety, and techniques in body-contouring surgery. Cleve Clin J Med. 2020;87:367–375. [DOI] [PubMed] [Google Scholar]
- 26.Wright T, Scarfino CD, O’Malley EM. Effect of pneumatic compression device and stocking use on symptoms and quality of life in women with lipedema: a proof-in-principle randomized trial. Phlebology. 2023;38:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campisi CC, Ryan M, Boccardo F, et al. Fibro-lipo-lymph-aspiration with a lymph vessel sparing procedure to treat advanced lymphedema after multiple lymphatic-venous anastomoses: the complete treatment protocol. Ann Plast Surg. 2017;78:184–190. [DOI] [PubMed] [Google Scholar]
- 28.Klein JA. Tumescent Technique. Tumescent Anesthesia and Microcannular Liposuction.: Mosby; 2000. [Google Scholar]
- 29.Cronin M, Dengler N, Krauss ES, et al. Completion of the Updated Caprini Risk Assessment Model (2013 version). Clin Appl Thromb Hemost. 2019;25:1076029619838052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golemi I, Salazar Adum JP, Tafur A, et al. Venous thromboembolism prophylaxis using the Caprini score. Dis Mon. 2019;65:249–298. [DOI] [PubMed] [Google Scholar]
- 31.PROMIS physical function scoring manual. Available at https://www.healthmeasures.net/administrator/components/com_instruments/uploads/PROMIS%20Physical%20Function%20Scoring%20Manual.pdf Published 2019. Accessed November 8, 2022.
- 32.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) I Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 33.Rapprich S, Dingler A, Podda M. Liposuction is an effective treatment for lipedema-results of a study with 25 patients. J Dtsch Dermatol Ges. 2011;9:33–40. [DOI] [PubMed] [Google Scholar]
- 34.Baumgartner A, Hueppe M, Meier-Vollrath I, et al. Improvements in patients with lipedema 4, 8 and 12 years after liposuction. Phlebology. 2021;36:152–159. [DOI] [PubMed] [Google Scholar]
- 35.Alcolea JM, Alonso Alvarez B, Arroyo Bielsa A, et al., ed. Documento de Consenso Lipedema 2018. In: 33rd National Congress of the Spanish Society of Aesthetic Medicine (SEME); 22–24 February 2018; Malaga/Barcelona, Spain. Barcelona, Spain: LITOGAMA S.L.; 2018. [Google Scholar]
- 36.Coppel T, Cunneen J, Fetzer S, et al. Best practice guidelines: the management of lipoedema. Wounds UK. 2017;13:1–36. [Google Scholar]
- 37.Nichols CI, Vose JG. Clinical outcomes and costs within 90 days of primary or revision total joint arthroplasty. J Arthroplasty. 2016;31:1400–1406.e3. [DOI] [PubMed] [Google Scholar]
- 38.Hua Y, Salcedo J. Cost-effectiveness analysis of robotic-arm assisted total knee arthroplasty. PLoS One. 2022;17:e0277980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramkumar PN, Chu CT, Harris JD, et al. Causes and rates of unplanned readmissions after elective primary total joint arthroplasty: a systematic review and meta-analysis. Am J Orthop (Belle Mead NJ). 2015;44:397–405. [PubMed] [Google Scholar]
- 40.Clair AJ, Evangelista PJ, Lajam CM, et al. Cost analysis of total joint arthroplasty readmissions in a bundled payment care improvement initiative. J Arthroplasty. 2016;31:1862–1865. [DOI] [PubMed] [Google Scholar]
- 41.Park KK, Chang CB, Kang YG, et al. Correlation of maximum flexion with clinical outcome after total knee replacement in Asian patients. J Bone Joint Surg Br. 2007;89:604–608. [DOI] [PubMed] [Google Scholar]
- 42.Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of Total Hip and Knee Replacement in the United States. J Bone Joint Surg Am. 2015;97:1386–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houck J, Jacobson R, Bass M, et al. Improving interpretation of the patient-reported outcomes measurement information system (PROMIS) physical function scale for specific tasks in community-dwelling older adults. J Geriatr Phys Ther. 2020;43:142–152. [DOI] [PubMed] [Google Scholar]
- 45.Oh-Park M, Wang C, Verghese J. Stair negotiation time in community-dwelling older adults: normative values and association with functional decline. Arch Phys Med Rehabil. 2011;92:2006–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khalil LS, Darrith B, Franovic S, et al. Patient-reported outcomes measurement information system (PROMIS) global health short forms demonstrate responsiveness in patients undergoing knee arthroplasty. J Arthroplasty. 2020;35:1540–1544. [DOI] [PubMed] [Google Scholar]
- 47.Klemt C, Tirumala V, Oganesyan R, et al. Single-stage revision of the infected total knee arthroplasty is associated with improved functional outcomes: a propensity score-matched cohort study. J Arthroplasty. 2021;36:298–304. [DOI] [PubMed] [Google Scholar]
- 48.Shim J, Hamilton DF. Comparative responsiveness of the PROMIS-10 Global Health and EQ-5D questionnaires in patients undergoing total knee arthroplasty. Bone Joint J. 2019;101-B:832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amitkumar M, Singh PK, Singh KJ, et al. Surgical outcome in spinal operation in patients aged 70 years and above. Neurol India. 2020;68:45–51. [DOI] [PubMed] [Google Scholar]
- 50.Herbst KL, Hansen EA, Cobos Salinas LM, et al. Survey outcomes of lipedema reduction surgery in the United States. PRS Global Open. 2021;9:e3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cárdenas-Camarena L, Tobar-Losada A, Lacouture AM. Large-volume circumferential liposuction with tumescent technique: a sure and viable procedure. Plast Reconstr Surg. 1999;104:1887–1899. [PubMed] [Google Scholar]
- 52.Chow I, Alghoul MS, Khavanin N, et al. Reply: is there a safe lipoaspirate volume? A risk assessment model of liposuction volume as a function of body mass index. Plast Reconstr Surg. 2016;137:756e–758e. [DOI] [PubMed] [Google Scholar]
- 53.Yeh RW, Valsdottir LR, Yeh MW, et al. ; PARACHUTE Investigators. Parachute use to prevent death and major trauma when jumping from aircraft: randomized controlled trial. BMJ. 2018;363:k5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.