Abstract
UDP-N-acetylglucosamine-3-O-acyltransferase (UDP-GlcNAc acyltransferase) catalyzes the first step of lipid A biosynthesis (M. S. Anderson and C. R. H. Raetz, J. Biol. Chem. 262:5159–5169, 1987). We here report the isolation of the lpxA gene of Pseudomonas aeruginosa from a library of Pseudomonas strain PAO1 expressed in Escherichia coli LE392 (J. Lightfoot and J. S. Lam, J. Bacteriol. 173:5624–5630, 1991). Pseudomonas lpxA encodes a 10-carbon-specific UDP-GlcNAc acyltransferase, whereas the E. coli transferase is selective for a 14-carbon acyl chain. Recombinant cosmid 1137 enabled production of a 3-hydroxydecanoyl-specific UDP-GlcNAc acyltransferase in E. coli. It was identified by assaying lysozyme-EDTA lysates of individual members of the library with 3-hydroxydecanoyl-acyl carrier protein (ACP) as the substrate. Cosmid 1137 contained a 20-kb insert of P. aeruginosa DNA. The lpxA gene region was localized to a 1.3-kb SalI-PstI fragment. Sequencing revealed that it contains one complete open reading frame (777 bp) encoding a new lpxA homolog. The predicted Pseudomonas LpxA is 258 amino acids long and contains 21 complete hexapeptide repeating units, spaced in approximately the same manner as the 24 repeats of E. coli LpxA. The P. aeruginosa UDP-GlcNAc acyltransferase is 54% identical and 67% similar to the E. coli enzyme. A plasmid (pGD3) containing the 1.3-kb SalI-PstI fragment complemented E. coli RO138, a temperature-sensitive mutant harboring lpxA2. LpxA assays of extracts of this construct indicated that it is >1,000-fold more selective for 3-hydroxydecanoyl-ACP than for 3-hydroxymyristoyl-ACP. Mass spectrometry of lipid A isolated from this strain by hydrolysis at pH 4.5 revealed [M-H]− 1,684.5 (versus 1,796.5 for wild-type lipid A), consistent with 3-hydroxydecanoate rather than 3-hydroxymyristate at positions 3 and 3′.
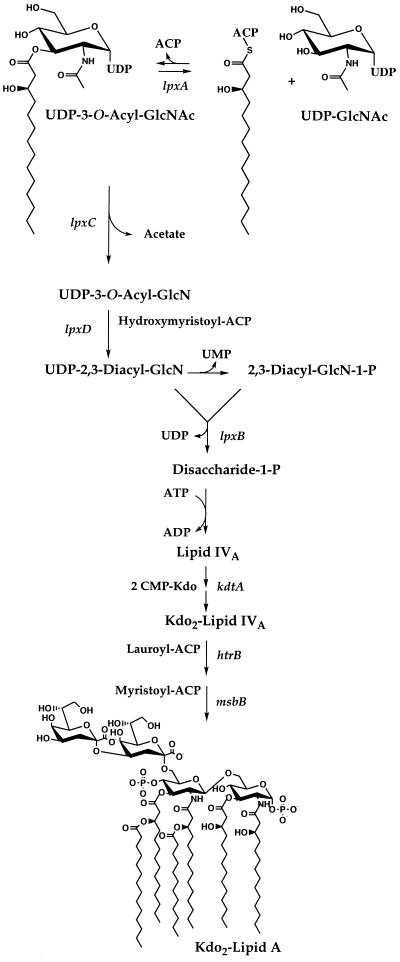
Genetic (13, 15, 30) or pharmacological (22) inhibition of enzymes catalyzing the early steps of lipid A biosynthesis in gram-negative bacteria usually causes cell death. The lipid A pathway is therefore a target for the development of novel antibacterial agents (25). The lpxA gene product, UDP-N-acetylglucosamine-3-O-acyltransferase (UDP-GlcNAc acyltransferase) (2, 3, 10, 11), catalyzes the first reaction of lipid A biosynthesis (Fig. 1). The enzyme transfers an R-3-hydroxyacyl group from hydroxyacyl-acyl carrier protein (ACP) to the glucosamine 3-OH of UDP-GlcNAc (Fig. 1) (2, 3). This hydroxyacyl chain eventually resides at both the 3 and 3′ positions of the glucosamine disaccharide of mature lipid A (Fig. 1) (24–26).
FIG. 1.
Role of LpxA in lipid A biosynthesis in E. coli. LpxA catalyzes the first step of lipid A biosynthesis (24, 25). The transfer of an R-3-hydroxyacyl group from R-3-hydroxyacyl–ACP to UDP-GlcNAc is very selective for R-3-hydroxymyristoyl–ACP in the case of E. coli LpxA (3, 33). UDP-GlcNAc acyltransferases from other gram-negative bacteria have specificity for ACP thioesters of different acyl chain lengths (33). Biosynthetic intermediates and known genes encoding the enzymes of the rest of the E. coli pathway are shown (24, 25). Chemical hydrolysis of lipopolysaccharide or of whole cells at pH 4.5 in the presence of sodium dodecyl sulfate cleaves the 3-deoxy-d-manno-octulosonic acid (Kdo)-lipid A linkage without disturbing the phosphates (7, 8). The released lipid product is designated lipid A throughout this paper. The 10-carbon-specific Pseudomonas LpxA can replace the 14-carbon-specific E. coli enzyme in living cells without interfering with the functioning of the other enzymes of the pathway.
The fatty acid compositions and structures of lipid A’s have been determined for many bacterial species (26, 29). Williamson et al. have shown that the UDP-GlcNAc acyltransferases from several common gram-negative bacteria display high degrees of specificity in measuring the chain lengths of the hydroxy fatty acyl moieties found at the 3 and 3′ positions in the lipid A’s of these bacteria (33).
To date, only the UDP-GlcNAc acyltransferase from Escherichia coli, in which enzymatic specificity is primarily for the transfer of R-3-hydroxymyristate, has been isolated and characterized in depth (2, 3). The X-ray crystal structure of the E. coli UDP-GlcNAc acyltransferase has been determined to a 2.6-Å resolution (28). The enzyme is composed of an unusual homotrimer (28). Each monomer of 262 amino acids contains 24 complete, mostly contiguous hexad repeats that fold into a novel secondary structure, termed a left-handed parallel β-helix (28). The crystal structure reveals that several additional hexad-like segments also contribute to the β-helix (28). The location of the active site is not known, but comparison of available LpxA sequences reveals clustering of conserved residues in a basic cleft between the subunits. How the E. coli transferase achieves its extraordinary selectivity for 14-carbon hydroxyacyl chains is unclear.
The sequence of a 10-carbon-specific UDP-GlcNAc acyltransferase has not been reported previously. We here present (i) the identification of a 3-hydroxydecanoyl-specific UDP- GlcNAc acyltransferase gene by assaying crude extracts of individual members of a Pseudomonas aeruginosa library expressed in E. coli LE392, (ii) the subcloning and sequencing of the P. aeruginosa lpxA gene, and (iii) the functional complementation of the temperature-sensitive E. coli mutant RO138 (lpxA2) by P. aeruginosa lpxA. Research on this class of enzymes should provide new opportunities for structure-based drug design and might facilitate the development of novel antibiotics directed against lipid A biosynthesis.
MATERIALS AND METHODS
Materials.
UDP-N-acetylglucosamine, glucosamine-6-phosphate, inorganic pyrophosphatase, UDP-glucose pyrophosphorylase, ACP, (−)-ephedrine, RS-3-hydroxymyristic acid, RS-3-hydroxylauric acid, decanoic acid, buffers, and salts were obtained from Sigma. Yeast extract and tryptone were obtained from Difco. Polyethyleneimine-cellulose thin-layer plates and Silica Gel 60 thin-layer plates (thickness, 0.25 mm) were purchased from E. Merck (Darmstadt, Germany). Restriction enzymes were from New England Biolabs, and DNA ligase was from Boehringer Mannheim. A Sequenase version 2.0 DNA sequencing kit and shrimp alkaline phosphatase were from United States Biochemical. t-Butylacetate, lithium diisopropylamide, octyl aldehyde, and trifluoroacetic acid (TFA) were obtained from Aldrich Chemical Co. (Milwaukee, Wis.). Anhydrous ethyl ether, chloroform, and methanol were obtained from Mallinckrodt, and glacial acetic acid was from EM Science. [α-32P]UTP was purchased from DuPont, NEN. α-35S–dATP was obtained from Amersham. [α-32P]UDP-GlcNAc was prepared and purified as described previously (15). PhosphorImager screens were from Molecular Dynamics, Inc.
Bacterial strains and plasmids.
Strains used in this study and their genotypes are listed in Table 1. LCH109/pLCH5/pGP1-2, a T7 promoter-driven overproducer of acyl-ACP synthetase, was obtained from C. O. Rock (St. Jude Hospital) (14). Cultures were grown in Luria broth (LB), consisting of 5 g of NaCl, 5 g of yeast extract, and 10 g of tryptone/liter (4). Antibiotics were added, when required, at 50 μg/ml for ampicillin, 12 μg/ml for tetracycline, and 30 μg/ml for streptomycin.
TABLE 1.
Plasmids and bacterial strains used in this study
| Bacterial strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| P. aeruginosa PAO1 | Wild type | G. Pier, Harvard University |
| E. coli | ||
| LE392 | Wild type | 17 |
| SURE | lacIqZΔM15, Tcr | Stratagene |
| RO138 | lpxA temperature-sensitive mutant, recA | 21 |
| Plasmids | ||
| Cosmid 1137 | lpxA+ cosmid clone in pCP13, Tcr | 17, this study |
| pBluescript KS II | lacZ, Ampr | Stratagene |
| pGD2 | 3.0-kb PstI fragment from cosmid 1137 cloned into pBluescript, lpxA+ Ampr | This study |
| pGD3 | 1.7-kb SalI deletion from pGD2, lpxA+ Ampr | This study |
Recombinant DNA techniques.
Plasmid DNAs were isolated with a Wizard Plus Miniprep kit (Promega) or a BIGGERprep kit (5 Prime-3 Prime, Inc.). All other DNA manipulations and cell transformations were done as described previously (4). DNA sequencing was performed by the dideoxy method with a Sequenase version 2.0 DNA sequencing kit with 7-deaza-dGTP (United States Biochemical) and α-35S–dATP.
Synthesis of RS-3-hydroxydecanoic acid.
The acetate-aldol condensation reaction was used to synthesize RS-3-hydroxydecanoic acid in gram quantities. To a solution of t-butylacetate (3 ml; 22 mmol) in anhydrous ethyl ether (80 ml) at −78°C, stirred under nitrogen, was added slowly over 5 min 1.1 equivalents of lithium diisopropylamide (12.1 ml; 24.2 mmol). The reaction mixture was stirred for 20 min to allow complete enolization. To this, 1.0 equivalent of octyl aldehyde (3.5 ml; 22 mmol) in 10 ml of anhydrous ethyl ether was added slowly over 10 min. The reaction mixture was stirred for 2 h and then concentrated by rotary evaporation. The t-butyl ester was then removed by treating the mixture with TFA (excess) at room temperature for 1 h. The TFA was removed by rotary evaporation, facilitated by three 30-ml additions of chloroform. The residue was redissolved in chloroform, and it was then extracted with 30 ml of 5% aqueous NaOH. The alkaline aqueous layer was acidified by the addition of 1 M HCl until the pH was less than 4, as judged by a reaction on pH paper, and then extracted with 30 ml of chloroform. The chloroform layer was dried over anhydrous sodium sulfate and then concentrated to give a near quantitative yield of RS-3-hydroxydecanoic acid (4.1 g). 1H nuclear magnetic resonance (500 MHz, CDCl3) δ 4.03 (m, 1H), 2.57 (dd, 1H), 2.48 (dd, 1H), 1.6 to 1.2 (m, 12H), 0.89 (t, 3H).
Synthesis of RS-3-hydroxydecanoate (−)-ephedrine salt.
RS-3-hydroxydecanoic acid (4.1 g) was suspended in anhydrous ethyl ether (230 ml), and (−)-ephedrine (4.47 g) was added. The mixture was warmed slightly by swirling it in a 50°C water bath, and acetonitrile was added until the solution was clear. The water bath was allowed to cool gradually to room temperature, at which time the RS-3-hydroxydecanoate ephedrine salt crystallized. Two subsequent recrystallizations resulted in 4.2 g of RS-3-hydroxydecanoate ephedrine salt (54% yield).
Preparation of acyl-ACP analogs.
The substrate RS-3-hydroxydecanoyl–ACP was prepared from purified ACP (Sigma) and synthetic RS-3-hydroxydecanoate, with Triton X-100-solubilized LCH109/pLCH5/pGP1-2 membranes being used as the source of acyl-ACP synthetase (9, 14). The enzymatic acylation of ACP with RS-3-hydroxydecanoate was carried out as follows. ACP (1 mg) and 8.6 mM dithiothreitol were incubated in 500 μl of 40 mM Tris-HCl (pH 8.0) in a sealed tube at 37°C for 1 h. Next, a 320-μl solution consisting of 0.7 M LiCl, 40 mM MgCl2, 20 mM ATP (pH 8.0), 750 μM RS-3-hydroxydecanoate ephedrine salt, 0.26% Triton X-100, and 540 mM Tris-HCl (pH 8.0) was added to the tube with the ACP. Last, 400 μl of 1.25 mg of solubilized LCH109/pLCH5/pGP1-2 membranes per ml, which had been on ice for 15 min in the presence of 2.2 mM 3-decynoyl-N-acetylcysteamine (18), was added, and the acylation reaction was allowed to proceed at room temperature for 2 h. The extent of acylation was determined by analyzing 5-μl portions of the reaction mixture on a urea-polyacrylamide gel (23). The other acyl-ACPs used in this study were made with the appropriate fatty acids as described above with the following modifications: 3-decynoyl-N-acetylcysteamine was omitted in the synthesis of RS-3-hydroxymyristoyl– and decanoyl-ACP, and a 10-fold-lower concentration of solubilized membranes of LCH109/pLCH5/pGP1-2 was used in the RS-3-hydroxymyristoyl–ACP preparation.
To isolate the acyl-ACP product, each reaction mixture was diluted 10-fold with water and loaded onto a 1-ml column of DEAE-Sepharose equilibrated with 10 mM bis-Tris (pH 6.0). The column was washed with 5 bed volumes of 10 mM bis-Tris (pH 6.0), 5 volumes of 10 mM bis-Tris (pH 6.0) containing 50% isopropyl alcohol, and 5 volumes of 10 mM bis-Tris (pH 6.0). The column was eluted with 3 volumes of 10 mM bis-Tris (pH 6.0) containing 0.2 M LiCl and 3 volumes of 10 mM bis-Tris (pH 6.0) containing 0.6 M LiCl. Fractions of 1 ml were collected. The acyl-ACP eluted in the second fraction of 0.6 M LiCl. This fraction was desalted on a P-2 column (10 ml) equilibrated in 50 mM Tris-HCl (pH 7.4) and lyophilized. The lyophilized protein was dissolved in distilled H2O (1 ml). The acyl-ACPs were ∼90% pure, as judged by electrophoresis in the urea-polyacrylamide gel system and staining with Coomassie blue. The concentrations of acyl-ACPs were determined as described previously (3).
Screening of a P. aeruginosa PAO1 library for 3-hydroxydecanoyl transferase activity.
The P. aeruginosa PAO1 library, provided generously by Joseph Lam (University of Guelph), consisted of 10 glycerol stocks, each containing 100 recombinant cosmid clones (17). In a typical round of screening, 100-μl portions of a 10,000-fold dilution of two glycerol stocks were plated onto LB-tetracycline plates and incubated at 37°C. From each agar plate, representing one glycerol stock, 192 colonies were picked to inoculate two 96-well microtiter dishes (150 μl of LB with tetracycline per well), and the microtiter dishes were incubated at 37°C with shaking overnight. From these overnight cultures, fresh microtiter dishes (200 μl of LB-tetracycline per well) were inoculated with a sterile 96-prong transferable solid phase (Nalge Nunc International). These dishes were grown for 6 h at 37°C and were then centrifuged at 3,600 × g for 20 min at 4°C. The supernatants were decanted, and the pellets were resuspended on ice in 25 μl of 33 mM Tris-HCl, pH 8.0. To the resuspended cells was added 25 μl of 33 mM Tris-HCl (pH 8.0) containing 0.2 mg of lysozyme per ml and 5 mM EDTA, and the cells were left on ice for 5 min. The cells were lysed by freezing at −80°C for at least 15 min, followed by thawing at 25°C in a water bath for 3 to 5 min. Portions of the lysates (15 μl from each well) from four microtiter plates (representative of two of the original glycerol stocks) were combined into 96 pools (four lysates per pool) in the wells of a fresh microtiter plate (60 μl per well).
The pooled lysates were assayed for 3-hydroxydecanoyl-specific UDP-GlcNAc acyltransferase activity as follows. A 96-well assay plate was prepared with each well containing 40 mM HEPES (pH 8.0), [α-32P]UDP-GlcNAc (0.25 μCi at a concentration of about 0.1 μM), 20 μM 3-hydroxydecanoyl–ACP, 10 mM MgCl2, and 6 μl of lysate (added last to give a final volume of 10 μl). The plate was incubated at 30°C for 15 min, and a portion of each reaction mixture (3 μl) was spotted onto silica gel thin-layer chromatography plates. The plates were developed in chloroform-methanol-water-acetic acid (25:15:4:2, vol/vol), and the extent of acylation was determined by PhosphorImager analysis.
Preparation of RO138/pGD3 cell extracts.
A single colony of RO138/pGD3 was used to inoculate 5 ml of LB and grown overnight at 42°C. A larger volume of LB (500 ml) was inoculated by 100-fold dilution of the overnight culture and incubated at 42°C with vigorous shaking (240 rpm) until the A600 reached 0.8. The cells were harvested by centrifugation (5,000 × g) at 2°C and washed once with 20 ml of 10 mM sodium phosphate buffer, pH 7.2. The washed cell pellet was resuspended in 10 mM sodium phosphate buffer, pH 7.2 (5 ml per g of cell pellet), and the cells were disrupted by passage through an ice-cold French pressure cell (SLM Instruments, Urbana, Ill.) at 20,000 lb/in2. The broken cell suspension was centrifuged at 100,000 × g for 75 min to prepare a membrane-free cytosolic extract. The latter was stored in 200-μl aliquots at −80°C. Protein concentrations were determined by using the Bio-Rad (Richmond, Calif.) protein assay with bovine serum albumin as the standard.
UDP-GlcNAc acyltransferase assay.
UDP-GlcNAc acyltransferase assays were performed as described previously (2, 3) with some minor modifications. Assay mixtures contained 100 μM [α-32P]UDP-GlcNAc (103 cpm/nmol), 20 μM RS-3-hydroxydecanoyl–ACP, 10 mM MgCl2, 40 mM HEPES (pH 8.0), and 10 to 100 μg of cell extract (added last) per ml in a final volume of 20 μl. The reaction mixture was incubated at 30°C, and portions (3 μl) were removed and spotted after 2 and 5 min onto silica gel thin-layer chromatography plates. The plates were developed in chloroform-methanol-water-acetic acid (25:15:4:2, vol/vol), and the extent of acylation was determined by PhosphorImager analysis.
Preparation of lipid A from E. coli RO138/pGD3.
Lipid A (containing both the 1 and 4′ phosphates) was prepared as previously described (21). Briefly, E. coli RO138/pGD3 cells, obtained from a 250-ml culture grown on LB in the presence of ampicillin at 42°C to saturation (A600 = 1.6), were suspended in 20 ml of 10 mM phosphate buffer, pH 7.2. To the cell suspension was added 300 ml of chloroform-methanol (1:2, vol/vol), and the resulting mixture was allowed to remain at room temperature for 60 min before it was centrifuged (4,000 × g for 15 min). The pellet was washed with 250 ml of chloroform-methanol-water (1:2:0.8, vol/vol), and the suspension was centrifuged as described above. The washed pellet was resuspended, with sonic irradiation, in an aqueous solution (80 ml) consisting of 12 mM sodium acetate (pH 4.5) and 1% sodium dodecyl sulfate, and this suspension was then placed in a boiling water bath for 30 min (7, 8). The insoluble material was once again removed by centrifugation (4,000 × g for 20 min) and discarded. The resulting supernatant (80 ml) was mixed with 178 ml of chloroform-methanol (1:1, vol/vol), and the emulsion was centrifuged at 4,000 × g for 10 min to separate the two phases. The lower phase was saved, and the solvent was removed by rotary evaporation.
The dried material was dissolved in 10 ml of chloroform-methanol-water (2:3:1, vol/vol), and then half of the material (5 ml) was loaded onto a 1-ml DEAE cellulose (Whatman DE52) column, equilibrated as the acetate form in the same solvent. The column was washed with 4 ml of chloroform-methanol-water (2:3:1, vol/vol), and the lipid A was eluted by the stepwise inclusion of increasing amounts of ammonium acetate (60, 120, 240, and 480 mM) as the aqueous component of the solvent. Each step of the elution consisted of four column volumes. The lipid A emerged with the 240 mM ammonium acetate step. The 4 ml of the 240 mM ammonium acetate eluate was mixed with 0.67 ml of chloroform and 1.13 ml of water, and the phases were allowed to separate. The lower phase was removed and dried under nitrogen to yield ∼0.5 mg of lipid A.
Mass spectrometry.
Negative-ion liquid secondary-ion mass spectra were acquired with a Concept IH (Kratos Analytical, Manchester, United Kingdom) two-sector mass spectrometer at a resolution of 1,000. A 1-μl portion of sample solution in methanol-chloroform (1:2, vol/vol) was mixed with mono-thioglycerol or triethanolamine (Aldrich Chemical Co.) on the tip of the probe. Analyte ions were desorbed from the matrix by an 8 keV Cs+ primary ion beam. Mass spectra were acquired by scanning the magnet in the 100- to 2,500-amu range at a scan rate of 10 s/decade. Typically 10 to 20 scans were signal averaged for each spectrum.
RESULTS
Screening of a P. aeruginosa PAO1 library for a 3-hydroxydecanoyl-specific UDP-GlcNAc acyltransferase.
Multiple attempts to clone the Pseudomonas lpxA gene by PCR to amplify conserved sequences present in known lpxA genes or to detect Pseudomonas DNA restriction fragments by low-stringency hybridization with E. coli lpxA as the probe were unsuccessful. An expression cloning strategy was therefore developed. Lysozyme-EDTA lysates were made from a total of 1,152 individual clones of E. coli LE392 bearing P. aeruginosa PAO1 DNA inserts. The 1,152 lysates were assayed in 288 pools of four lysates for their ability to transfer a 3-hydroxydecanoyl group to [α-32P]UDP-GlcNAc. Interfering background activity attributable to the E. coli chromosomal UDP-GlcNAc acyltransferase is nonexistent because of the selectivity of UDP-GlcNAc acyltransferase for the 3-hydroxymyristoyl rather than the 3-hydroxydecanoyl moiety (33).
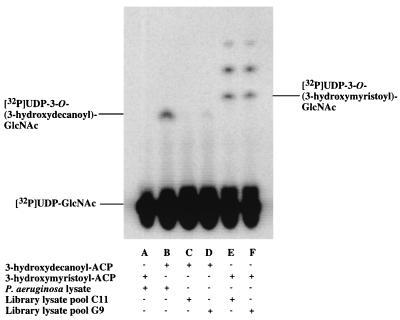
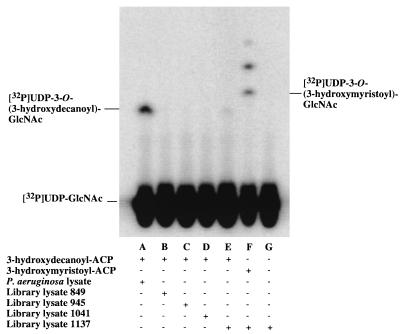
Of the 288 pools assayed, only one was positive for the expression of a 3-hydroxydecanoyl–ACP-dependent UDP-GlcNAc acyltransferase (Fig. 2). This pool contained lysates from clones 849, 945, 1041, and 1137. These lysates were assayed individually. Only the one derived from clone 1137 displayed the desired activity (Fig. 3). The cosmid from clone 1137 was isolated and found to contain an insert of about 20 kb by restriction enzyme analysis (data not shown).
FIG. 2.
Expression cloning of a 3-hydroxydecanoyl-specific UDP-GlcNAc acyltransferase from a P. aeruginosa PAO1 DNA library in E. coli LE392 assayed in pools of four. When [α-32P]UDP-GlcNAc is acylated, it migrates off the origin in this thin-layer system, as indicated. Acylation with 3-hydroxymyristate results in a product that migrates slightly faster than does the product of acylation with 3-hydroxydecanoate. Extracts of wild-type P. aeruginosa acylate [α-32P]UDP-GlcNAc efficiently in the presence of 3-hydroxydecanoyl–ACP (lane B) but not at all in the presence of 3-hydroxymyristoyl–ACP (lane A). Conversely, strains of E. coli that do not harbor the P. aeruginosa lpxA gene acylate [α-32P]UDP-GlcNAc in the presence of 3-hydroxymyristoyl–ACP (lane E) but not in the presence of 3-hydroxydecanoyl–ACP (lane C). The more rapidly migrating compounds in lanes E and F represent further metabolites of the lipid A pathway that are derived in E. coli extracts from acylated-UDP-GlcNAc (1, 3). Lanes C to F show the assay results obtained with pools of four lysates (prepared as described in Materials and Methods), each derived from distinct E. coli colonies harboring different P. aeruginosa DNA inserts. With 3-hydroxydecanoyl–ACP as the substrate, only lysate pool G9 (of the 288 pools assayed) catalyzed measurable acylation of [α-32P]UDP-GlcNAc (lane D). With 3-hydroxymyristoyl–ACP as the substrate (lane F), pool G9 was comparable in activity to all other pools in the collection (such as C11 in lane E), indicating that the protein concentrations of G9 and C11 were about the same.
FIG. 3.
Identification of a single P. aeruginosa library clone in E. coli LE392 expressing a 3-hydroxydecanoyl-specific UDP-GlcNAc acyltransferase. Assays of lysozyme-EDTA lysates of the individual colonies that were used to make pool G9 were carried out as described for Fig. 2. Only extracts of the E. coli strain harboring P. aeruginosa cosmid 1137 catalyzed 3-hydroxydecanoyl–ACP-dependent acylation of [α-32P]UDP-GlcNAc.
Subcloning of cosmid 1137.
Cosmid 1137, isolated from library clone 1137, was digested in two separate incubations with either ClaI or PstI. Fragments from each digest were ligated into pBluescript KS II and used to transform E. coli SURE cells. The transformed bacteria were plated on LB agar containing ampicillin, IPTG (isopropyl-β-d-thiogalactopyranoside), and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (for selection of blue- or white-stained colonies) and grown overnight at 37°C. White colonies, 36 from the ClaI-derived transformants and 60 from the PstI-derived transformants, were used to inoculate a microtiter plate containing 150 μl of LB with ampicillin per well. The cells were grown to late log phase at 37°C, and cell lysates were assayed for 3-hydroxydecanoyl–ACP-dependent UDP-GlcNAc acyltransferase activity, as described in Materials and Methods for the initial screening of the library. None of the ClaI-derived transformants expressed the desired activity, but there was one PstI-derived transformant that displayed robust 3-hydroxydecanoyl transferase activity (data not shown). The plasmid DNA (pGD2) isolated from this clone contained a 3-kb PstI insert. pGD2 was further digested with SalI and religated to yield pGD3. When it was used to transform E. coli SURE cells, pGD3 directed the expression of 3-hydroxydecanoyl–ACP-dependent UDP-GlcNAc acyltransferase activity (see below). The P. aeruginosa-derived SalI-PstI DNA fragment in pGD3 was 1.3 kb long, as judged by agarose gel electrophoresis.
DNA sequence analysis of pGD3.
The DNA sequence of the SalI-PstI insert contained in pGD3 is shown in Fig. 4. The insert is 1,352 bp in length, and it contains one complete open reading frame of 777 bp (nucleotides 54 to 830). This open reading frame is flanked at its 5′ end by 53 bp and at its 3′ end by 522 bp. The single complete open reading frame encodes a protein of 258 amino acids with a molecular mass of approximately 28 kDa. The predicted protein is 54% identical and 67% similar to the E. coli UDP-GlcNAc acyltransferase (LpxA). As has been observed in other UDP-GlcNAc acyltransferases (28, 32), the N-terminal two-thirds of the P. aeruginosa LpxA contains more than 20 mostly contiguous hexapeptide repeats (Fig. 5). Hexapeptide repeats are indicative of an unusual protein secondary structure, known as a left-handed parallel β-helix (28). The hexapeptide repeats of Pseudomonas LpxA are spaced in approximately the same manner as those of E. coli, with the exceptions that the first repeat (starting at E. coli residue 2) is truncated in Pseudomonas and that what would be the last complete repeat of E. coli (starting at E. coli residue 171) begins with serine in Pseudomonas, rather than with valine, the typical aliphatic residue at the beginning of a hexad (Fig. 5).
FIG. 4.
DNA sequence of the P. aeruginosa lpxA gene and its flanking regions. The predicted protein sequences of LpxA and of the N-terminal part of LpxB are indicated.
FIG. 5.
Comparison of the amino acid sequences of P. aeruginosa and E. coli LpxA. The overall sequence identity is 54%, and the sequence similarity is 67%. The aliphatic residues that denote the beginning of each hexapeptide repeat are in bold print. The sequence has been arranged to indicate the stacking of the hexads on top of each other in the formation of the left-handed β-helix seen in the E. coli LpxA crystal structure (28). The positions of two loops (residues 72 to 83 and 102 to 108) that can be recognized as interruptions of the contiguous hexad repeats in the primary sequences are located in the same places in both enzymes. One hexad is truncated at the N terminus of Pseudomonas LpxA, and valine 171 of the last complete hexad of E. coli is replaced by serine (italicized) in Pseudomonas LpxA. The significance of these differences to the overall protein fold and acyl chain selectivity of the Pseudomonas enzyme remains to be established. PSEUD, P. aeruginosa; ECOLI, E. coli.
Other genes present on pGD2 and pGD3.
The downstream partial open reading frame present on the Pseudomonas insert of pGD3 (nucleotides 834 to 1352) has a high degree of homology to the lpxB gene (Fig. 1) (12). In E. coli both lpxA and lpxB are in a complex operon that begins with fabZ, which in turn is downstream of lpxD (Fig. 1) (15, 19). Since pGD2 contains 1.7 kb of additional P. aeruginosa DNA upstream of lpxA, we determined its sequence to establish the presence or absence of fabZ and lpxD. Both these genes are indeed present on pGD2 (not shown). The full sequences of Pseudomonas lpxD and fabZ will be reported elsewhere. The termination codon of the P. aeruginosa fabZ gene overlaps the initiation codon of lpxA (Fig. 4).
Complementation of E. coli RO138 with Pseudomonas lpxA.
E. coli RO138 is a recA mutant strain with a point mutation in the UDP-GlcNAc acyltransferase gene (lpxA2) (13) that imparts a temperature-sensitive growth phenotype and causes inhibition of lipid A biosynthesis at 42°C. In order to determine whether Pseudomonas lpxA can correct for this growth phenotype, RO138 was transformed by electroporation with pGD3. A separate derivative of RO138 harboring pBluescript was constructed as a control. Transformation with pGD3, but not with pBluescript, was able to correct the temperature sensitivity of RO138, as judged by its ability to grow and form single colonies at 42°C on LB-ampicillin agar (data not shown).
Substrate specificity of Pseudomonas UDP-GlcNAc acyltransferase expressed in RO138/pGD3.
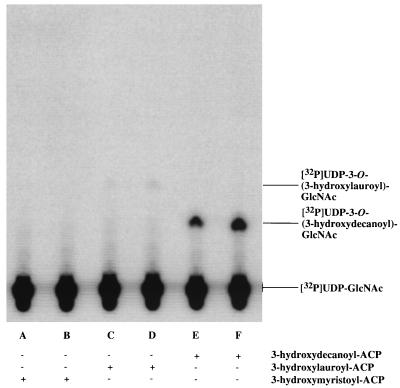
Extracts prepared from RO138 contain almost no measurable UDP-GlcNAc acyltransferase activity at any assay temperature (13, 21). The substrate specificity of Pseudomonas UDP-GlcNAc acyltransferase was therefore determined with a cytosolic fraction isolated by centrifugation at 100,000 × g from RO138/pGD3 grown at 42°C. Figure 6 and Table 2 show the results obtained by assaying this fraction with [α-32P]UDP-GlcNAc and either 3-hydroxydecanoyl–, 3-hydroxylauroyl–, or 3-hydroxymyristoyl–ACP as the acyl donor. The Pseudomonas enzyme expressed in E. coli RO138 exhibited a 370-fold preference for 3-hydroxydecanoyl- over 3-hydroxylauroyl–ACP, while no detectable activity was observed with 3-hydroxymyristoyl–ACP. The acyl chain specificity of the cloned Pseudomonas LpxA (Table 2) is the opposite of that observed with E. coli LpxA, which is over 100-fold more selective for 3-hydroxymyristoyl–ACP than for 3-hydroxydecanoyl–ACP (33).
FIG. 6.
Acyl-ACP specificity of Pseudomonas UDP-GlcNAc acyltransferase determined in cytosolic extracts of RO138/pGD3. Cells were grown to late log phase at 42°C in LB in the presence of ampicillin, and assays of supernatants centrifuged at 100,000 × g were performed at 30°C, as described in the Materials and Methods. The extract concentration was 0.1 mg/ml. Results at the 2- and 5-min time points are shown for each reaction.
TABLE 2.
Acyl-ACP specificity of P. aeruginosa UDP-GlcNAc acyltransferase expressed in E. coli RO138
| Substrate | Sp act (nmol/min/mg) | % Maximum activity |
|---|---|---|
| 3-Hydroxydecanoyl–ACP | 11.12 | 100 |
| 3-Hydroxylauroyl–ACP | 0.032 | 0.29 |
| 3-Hydroxymyristoyl–ACP | None detected | None detected |
| Decanoyl-ACP | 0.015 | 0.13 |
Composition of lipid A isolated from cells of E. coli RO138/pGD3 grown at 42°C.
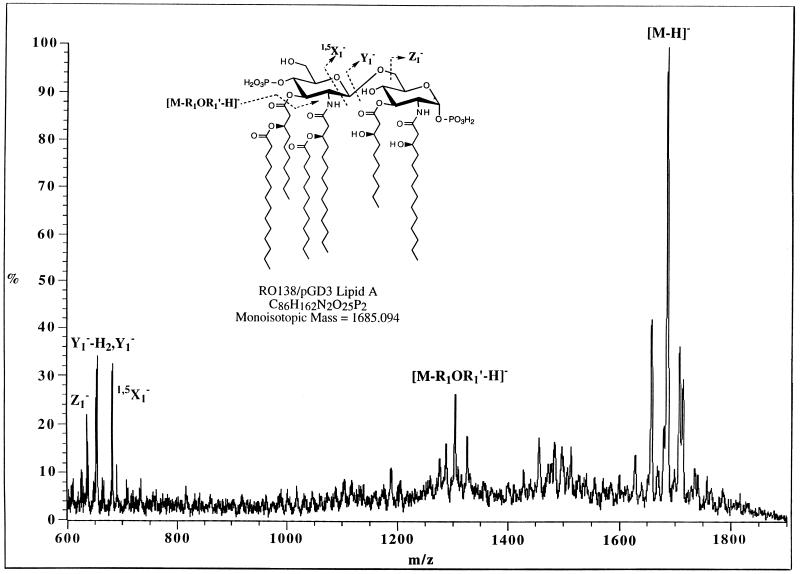
Given the in vitro specificity of Pseudomonas LpxA for the transfer of the 3-hydroxydecanoyl moiety (Table 2 and Fig. 6), lipid A (containing both the 1 and the 4′ phosphates) from RO138/pGD3 was isolated by pH 4.5 hydrolysis in the presence of sodium dodecyl sulfate and analyzed by mass spectrometry. Figure 7 shows the mass spectrum obtained by liquid secondary-ion mass spectrometry of the isolated lipid A taken in the negative-ion mode. The spectrum clearly shows the major molecular ion peak [M-H]− at m/z 1,684.5, which corresponds exactly to the mass predicted from replacement of the two hydroxymyristoyl moieties in wild-type E. coli lipid A (21) with two hydroxydecanoyl groups in RO138/pGD3. The fragmentation peak at m/z 1,303.9 arises from the loss of an acyloxyacyl group from the 3′-hydroxyl position of the glucosamine disaccharide, and it is also consistent with the presence of a hydroxydecanoyl rather than a hydroxymyristoyl group within the lost acyloxyacyl fragment. Fragmentation of the glycosidic bond (fragment ion Y1− in Fig. 7) gives rise to a peak at m/z 654.1, corresponding to the diacylated glucosamine bearing the anomeric phosphate group. The observed mass is 56 mass units less than that expected for the cleavage of lipid A of wild-type E. coli (21) and corresponds to a difference of four CH2 groups. Consequently, the mass spectrum of RO138/pGD3 lipid A is exactly what is expected if R-3-hydroxydecanoate moieties are incorporated at positions 3 and 3′ of lipid A by Pseudomonas LpxA. The origin of the small peak at m/z 1,456.2 is uncertain, but it might arise by the loss of a myristoyl side chain from lipid A, with the subsequent loss of one water molecule.
FIG. 7.
Mass spectrum of lipid A isolated from strain RO138 complemented at 42°C with pGD3. There is no peak at m/z 1,796 in the lipid A isolated from RO138/pGD3, demonstrating the absence of residual E. coli LpxA function in living cells under these conditions.
DISCUSSION
Using an expression cloning approach, we have identified Pseudomonas lpxA, the structural gene for the first enzyme of lipid A biosynthesis (24, 25, 27). Although several other lpxA genes, like that of E. coli (10), have been sequenced previously, Pseudomonas lpxA represents the first example of a homolog encoding a 10-carbon-specific UDP-GlcNAc acyltransferase (33). A gene encoding a 12-carbon-selective UDP-GlcNAc acyltransferase from Neisseria meningitidis has also been reported recently (21), but all other known LpxA sequences are thought to represent 14-carbon-specific acyltransferases. Sixteen- or 18-carbon-specific UDP-GlcNAc acyltransferases probably remain to be discovered, given the compositions of lipid A variants from strains of Rhizobium (6) and Helicobacter (20).
The sequence of Pseudomonas LpxA displays 54% identity and 67% similarity to its E. coli counterpart (Fig. 5). Of particular interest is the presence of the hexapeptide repeat motifs, which extend over the N-terminal two-thirds of both UDP-GlcNAc acyltransferases. This motif has been shown to specify a unique left-handed parallel β-helix domain in the X-ray crystal structures of the E. coli UDP-GlcNAc acyltransferase (28) and of two other enzymes that possess such hexad repeats (5, 16). Many of the enzymes that contain hexapeptide repeat motifs are hydroxyacyl-, succinyl-, or acetyltransferases that utilize either ACP or coenzyme A thioester as a substrate. The E. coli genome contains as many as 15 hexapeptide-repeat proteins.
Although it is very similar in sequence to E. coli LpxA, the P. aeruginosa LpxA, expressed in RO138, has a greater than 1,000-fold preference for 3-hydroxydecanoyl-ACP over 3-hydroxymyristoyl-ACP (Fig. 6; Table 2). How fatty acyl chain length is recognized and measured by these acyltransferases is not known. The X-ray crystal structure of the E. coli enzyme, which has recently been solved at a 2.6-Å resolution (28), provides a unique opportunity to investigate the chain length issue. To date, no other acyltransferase has had its crystal structure determined.
The active site of E. coli LpxA has not been identified conclusively. The available model of the native LpxA homotrimer (28) suggests that the acyl-ACP substrate may dock in a large basic cleft situated between the subunits. Without the structures of both the E. coli and the Pseudomonas enzymes in the presence of an acyl-ACP, it will be difficult to determine exactly how the acyl chain measuring process works. Given the related sequences and the nearly identical spacings of the hexad repeats (Fig. 5), we expect that the overall folds of the E. coli and the Pseudomonas enzymes will be quite similar. Only two hexads are missing or are significantly altered in Pseudomonas LpxA (Fig. 5). These are the N-terminal hexad (which is not likely to be near the active site) and the hexad that begins at residue 171 in E. coli LpxA. In Pseudomonas LpxA, this residue is serine, not one of the expected aliphatic amino acids (Fig. 5). Interestingly, N. meningitidis LpxA has a threonine at this location (31). It is tempting to speculate that a hydroxylated side chain at this site might alter the protein fold sufficiently to account for the short chain selectivities of the Pseudomonas and N. meningitidis LpxA enzymes (21, 33).
Even without additional crystal structures, it may be possible to obtain information about key residues involved in determining acyl chain selectivity. One could begin by constructing E. coli-Pseudomonas LpxA hybrids. A short peptide segment or even a single key side chain that plays a role in establishing selectivity might be identified in this manner. Probing chain length specificity by alteration of small peptide segments has been successful with plant acyl-ACP thioesterase. With these thioesterases, it has been shown that two or three amino acid substitutions can alter the chain length specificity from 12 to 14 carbons (34). However, X-ray structures have not been reported for these thioesterases.
In addition to providing insights into the molecular mechanisms of acyltransferases, the LpxA system offers a new opportunity to modify the structure of lipid A in living cells (Fig. 7) and to study the effects of such modifications on cell growth and outer membrane assembly. Although RO138 can grow at 42°C with Pseudomonas LpxA complementing the chromosomal lpxA2 mutation, the cells are likely to be antibiotic hypersensitive (21). A search for ancillary phenotypes associated with modifications of the lipid A structure is worthwhile, as new insights into the biological functions of lipid A might emerge.
ACKNOWLEDGMENTS
We thank Joseph Lam for providing us with the Pseudomonas DNA library and John Cronan and V. Jo Davidson for providing the 3-decynoyl-N-acetylcysteamine.
This research was supported by NIH grants GM-51310 to C. R. H. Raetz and GM-33967 to R. J. Cotter.
REFERENCES
- 1.Anderson M S, Bulawa C E, Raetz C R H. The biosynthesis of gram-negative endotoxin: formation of lipid A precursors from UDP-GlcNAc in extracts of Escherichia coli. J Biol Chem. 1985;260:15536–15541. [PubMed] [Google Scholar]
- 2.Anderson M S, Bull H S, Galloway S M, Kelly T M, Mohan S, Radika K, Raetz C R H. UDP-N-acetylglucosamine acyltransferase of Escherichia coli: the first step of endotoxin biosynthesis is thermodynamically unfavorable. J Biol Chem. 1993;268:19858–19865. [PubMed] [Google Scholar]
- 3.Anderson M S, Raetz C R H. Biosynthesis of lipid A precursors in Escherichia coli: a cytoplasmic acyltransferase that converts UDP-N-acetylglucosamine to UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine. J Biol Chem. 1987;262:5159–5169. [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1989. [Google Scholar]
- 5.Beaman T W, Binder D A, Blanchard J S, Roderick S L. Three-dimensional structure of tetrahydrodipicolinate N-succinyltransferase. Biochemistry. 1997;36:489–494. doi: 10.1021/bi962522q. [DOI] [PubMed] [Google Scholar]
- 6.Bhat U R, Forsberg L S, Carlson R W. The structure of the lipid A component of Rhizobium leguminosarum bv. phaseoli lipopolysaccharide. A unique, non-phosphorylated lipid A containing 2-amino-2-deoxy-gluconate, galacturonate, and glucosamine. J Biol Chem. 1994;269:14402–14410. [PubMed] [Google Scholar]
- 7.Caroff M, Deprun C, Karibian D, Szabó L. Analysis of unmodified endotoxin preparations by 252Cf plasma desorption mass spectrometry. J Biol Chem. 1991;266:18543–18549. [PubMed] [Google Scholar]
- 8.Caroff M, Tacken A, Szabó L. Detergent-accelerated hydrolysis of bacterial endotoxins and determination of the anomeric configuration of the glycosyl phosphate present in the “isolated lipid A” fragment of the B. pertussis endotoxin. Carbohydr Res. 1988;175:273–282. doi: 10.1016/0008-6215(88)84149-1. [DOI] [PubMed] [Google Scholar]
- 9.Clementz T, Bednarski J J, Raetz C R H. Function of the htrB high temperature requirement gene of Escherichia coli in the acylation of lipid A. HtrB catalyzed incorporation of laurate. J Biol Chem. 1996;271:12095–12102. doi: 10.1074/jbc.271.20.12095. [DOI] [PubMed] [Google Scholar]
- 10.Coleman J, Raetz C R H. First committed step of lipid A biosynthesis in Escherichia coli: sequence of the lpxA gene. J Bacteriol. 1988;170:1268–1274. doi: 10.1128/jb.170.3.1268-1274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowell D N, Anderson M S, Raetz C R H. Molecular cloning of the genes for lipid A disaccharide synthase and UDP-N-acetylglucosamine acyltransferase in Escherichia coli. J Bacteriol. 1986;168:152–159. doi: 10.1128/jb.168.1.152-159.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowell D N, Reznikoff W S, Raetz C R H. Nucleotide sequence of the Escherichia coli gene for lipid A disaccharide synthase. J Bacteriol. 1987;169:5727–5734. doi: 10.1128/jb.169.12.5727-5734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galloway S M, Raetz C R H. A mutant of Escherichia coli defective in the first step of endotoxin biosynthesis. J Biol Chem. 1990;265:6394–6402. [PubMed] [Google Scholar]
- 14.Jackowski S, Jackson P D, Rock C O. Sequence and function of the aas gene in Escherichia coli. J Biol Chem. 1994;269:2921–2928. [PubMed] [Google Scholar]
- 15.Kelly T M, Stachula S A, Raetz C R H, Anderson M S. The firA gene of Escherichia coli encodes UDP-3-O-(R-3-hydroxymyristoyl)-α-d-glucosamine N-acyltransferase: the third step of endotoxin biosynthesis. J Biol Chem. 1993;268:19866–19874. [PubMed] [Google Scholar]
- 16.Kisker C, Schindelin H, Alber B E, Ferry J G, Rees D C. A left-hand beta-helix revealed by the crystal structure of a carbonic anhydrase from the archaeon Methanosarcina thermophila. EMBO J. 1996;15:2323–2330. [PMC free article] [PubMed] [Google Scholar]
- 17.Lightfoot J, Lam J S. Molecular cloning of genes involved with expression of A-band lipopolysaccharide, an antigenically conserved form, in Pseudomonas aeruginosa. J Bacteriol. 1991;173:5624–5630. doi: 10.1128/jb.173.18.5624-5630.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnuson K, Jackowski S, Rock C O, Cronan J E., Jr Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol Rev. 1993;57:522–542. doi: 10.1128/mr.57.3.522-542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohan S, Kelly T M, Eveland S S, Raetz C R H, Anderson M S. An Escherichia coli gene (fabZ) encoding R-3-hydroxymyristoyl acyl carrier protein dehydrase. Relation to fabA and suppression of mutations in lipid A biosynthesis. J Biol Chem. 1994;269:32896–32903. [PubMed] [Google Scholar]
- 20.Moran A P, Helander I M, Kosunen T U. Compositional analysis of Helicobacter pylori rough-form lipopolysaccharides. J Bacteriol. 1992;174:1370–1377. doi: 10.1128/jb.174.4.1370-1377.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odegaard T J, Kaltashov I A, Cotter R J, Steeghs L, van der Ley P, Khan S, Maskell D J, Raetz C R H. Shortened hydroxyacyl chains on lipid A of Escherichia coli cells expressing a foreign UDP-N-acetylglucosamine O-acyltransferase. J Biol Chem. 1997;272:19688–19696. doi: 10.1074/jbc.272.32.19688. [DOI] [PubMed] [Google Scholar]
- 22.Onishi H R, Pelak B A, Gerckens L S, Silver L L, Kahan F M, Chen M H, Patchett A A, Galloway S M, Hyland S A, Anderson M S, Raetz C R H. Antibacterial agents that inhibit lipid A biosynthesis. Science. 1996;274:980–982. doi: 10.1126/science.274.5289.980. [DOI] [PubMed] [Google Scholar]
- 23.Post-Beittenmiller D, Jaworski J G, Ohlrogge J B. The in vivo pools of free and acylated acyl carrier proteins in spinach. Evidence of sites of regulation of fatty acid biosynthesis. J Biol Chem. 1991;266:1858–1865. [PubMed] [Google Scholar]
- 24.Raetz C R H. Bacterial endotoxins: extraordinary lipids that activate eucaryotic signal transduction. J Bacteriol. 1993;175:5745–5753. doi: 10.1128/jb.175.18.5745-5753.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raetz C R H. Bacterial lipopolysaccharides: a remarkable family of bioactive macroamphiphiles. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C: American Society for Microbiology; 1996. pp. 1035–1063. [Google Scholar]
- 26.Raetz C R H. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- 27.Raetz C R H, Dowhan W. Biosynthesis and function of phospholipids in Escherichia coli. J Biol Chem. 1990;265:1235–1238. [PubMed] [Google Scholar]
- 28.Raetz C R H, Roderick S L. A left-handed parallel β helix in the structure of UDP-N-acetylglucosamine acyltransferase. Science. 1995;270:997–1000. doi: 10.1126/science.270.5238.997. [DOI] [PubMed] [Google Scholar]
- 29.Rietschel E T, Brade L, Lindner B, Zähringer U. Biochemistry of lipopolysaccharides. In: Morrison D C, Ryan J L, editors. Bacterial endotoxic lipopolysaccharides. I. Molecular biochemistry and cellular biology. Boca Raton, Fla: CRC Press; 1992. pp. 3–41. [Google Scholar]
- 30.Sorensen P G, Lutkenhaus J, Young K, Eveland S S, Anderson M S, Raetz C R H. Regulation of UDP-3-O-[R-3-hydroxymyristoyl]-N-acetyl-glucosamine deacetylase in Escherichia coli. The second enzymatic step of lipid A biosynthesis. J Biol Chem. 1996;271:25898–25905. doi: 10.1074/jbc.271.42.25898. [DOI] [PubMed] [Google Scholar]
- 31.Steeghs L, Jennings M P, Poolman J T, van der Ley P. Isolation and characterization of the Neisseria meningitidis lpxD-fabZ-lpxA gene cluster involved in lipid A biosynthesis. Gene. 1997;190:263–270. doi: 10.1016/s0378-1119(97)00005-x. [DOI] [PubMed] [Google Scholar]
- 32.Vaara M. Eight bacterial proteins, including UDP-N-acetylglucosamine acyltransferase (LpxA) and three other transferases of Escherichia coli, consist of a six-residue periodicity theme. FEMS Microbiol Lett. 1992;97:249–254. doi: 10.1016/0378-1097(92)90344-n. [DOI] [PubMed] [Google Scholar]
- 33.Williamson J M, Anderson M S, Raetz C R H. Acyl-acyl carrier protein specificity of UDP-GlcNAc acyltransferases from gram-negative bacteria: relationship to lipid A structure. J Bacteriol. 1991;173:3591–3596. doi: 10.1128/jb.173.11.3591-3596.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan L, Voelker T A, Hawkins D J. Modification of the substrate specificity of an acyl-acyl carrier protein thioesterase by protein engineering. Proc Natl Acad Sci USA. 1995;92:10639–10643. doi: 10.1073/pnas.92.23.10639. [DOI] [PMC free article] [PubMed] [Google Scholar]