Abstract
Background
Helicobacter pylori treatment failure remains a challenging problem. This study aimed to identify predictive factors for successful eradication in patients following treatment failures.
Methods
This was a retrospective cohort study. This study included 1,050 dyspeptic patients diagnosed with H. pylori infection at tertiary care center in Thailand between March 2014 and October 2021. Patients’ demographic data, endoscopic findings, H. pylori culture, antimicrobial susceptibility testing (AST), treatment regimens and outcomes were analysed.
Results
Of 1,050 patients with H. pylori infections, 302 (28.7%) experienced treatment failure (mean age 58.4 years; 44.7% males). AST was performed in 192. Resistance was observed for metronidazole (43.2%), levofloxacin (33.9%), clarithromycin (24%), and amoxicillin (2.1%). There was no tetracycline resistance. Multidrug-resistance (MDR) was significantly more common following treatment failure (45.5% vs. 15.7%, p<0.001). Baseline characteristics were similar between treatment successes and failures. Eradication rates after first-line and second-line regimens were 71.2% and 54.5%, respectively. Medication nonadherence [OR 36.6 (95%CI 8.65–155.03, p<0.001)] and MDR [OR 4.49 (95%CI 2.29–8.81, p<0.001)] were associated with treatment failure. Over time, resistance increased for metronidazole, levofloxacin, and clarithromycin, while eradication rates with triple therapy declined. Tailored antibiotic therapy [OR 4.92 (95%CI 1.61–14.99, p = 0.005)] and a regimen including 4-times-daily dosing of amoxicillin (2 grams/day) [OR 3.05 (95%CI 1.10–8.41, p = 0.032)] were significantly associated with treatment success after first-line failure. Eradication rates when using tailored therapy and 4-times-daily dosing of amoxicillin (2 grams/day) were 91.1% and 89.4%, respectively. Performing AST before first-line therapy resulted in the highest cure rates. AST performed after multiple treatment failures was also associated with higher eradication rates compared with the group without AST (94.4% vs. 50%,p = 0.008).
Conclusions
AST either before or after treatment failure correlated with a higher proportion of successful eradication. Nonadherence and the MDR infections predicted treatment failure. Tailored therapy and 4-times-daily dosing of amoxicillin after treatment failure were likely to be successful.
Introduction
Helicobacter pylori (H. pylori) is a small curved Gram-negative, rod-shaped bacterium with flagella that colonizes the gastric mucous layer, triggers a host inflammatory response, and results in chronic gastritis, peptic ulcer disease, gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric cancer [1]. Gastric cancer is the fourth leading cause of cancer mortality worldwide [2]. H. pylori has been designated as group I human carcinogen and eradication is recommendation to prevent gastric cancer [3, 4]. Recommended first-line treatment regimens differ depending on antimicrobial resistance patterns [5]. However, increasing resistance is the major hindrance preventing successful eradication [6].
H. pylori treatment failure or unsuccessful eradication after completing one or more course(s) of standard H. pylori treatment is increasing [7]. The most common causes of eradication failure are antibiotic resistance and poor adherence to medical therapy [7]. Increasing antibiotic resistance has been observed worldwide, especially in Southeast Asia which exhibited a significant increase in resistance to all antibiotics [8]. Thailand, located in mainland Southeast Asia, has also experienced rising antibiotic resistance. The 2015 Thai guideline recommends 14-day triple therapy, 10-day concomitant therapy, and 10-day sequential therapy as first-line treatment options, while bismuth quadruple therapy is recommended for first-line treatment in patients with penicillin allergy [9]. First-line regimens containing clarithromycin might not be effective due to higher clarithromycin resistance and the guideline needs to be revised in the near future.
As antibiotic resistance increases, more patients experience refractory H. pylori infections. After failure of second-line treatment, antimicrobial susceptibility testing is recommended to be performed [9]. However, there have been limited data about antibiotic resistance and eradication rates after failure of H. pylori treatment in Thailand. This study aimed to determine predictive factors for successful eradication in patients with H. treatment failures as well as to gather data on current antibiotic resistance pattern in Thailand.
Methods
Study design
This was a retrospective cohort study conducted at tertiary care center in Thailand. The study was conducted from February 22, 2022 to February 19, 2023. Patients’ data were retrospectively collected by chart review between March 6, 2014, and October 31, 2021. The inclusion criteria were patients older than 15 years old with H. pylori infection defined as positive rapid urease test, histopathology, or culture. Each patient underwent gastroscopy and had gastric biopsies performed. Patients’ demographic data, comorbidities, endoscopic findings, H. pylori culture and antimicrobial susceptibility testing, and treatment regimens were extracted from the medical database. Regimen composition of each first-line and second-line therapy was defined as in our previous review [10]. First-line regimen selection for each patient was decided by his physician depending on history of drug allergy. Each follow-up visit for H. pylori treatment was recorded. Patients who did not have post-treatment testing to confirm eradication were excluded from this study.
The primary aim of this study was to determine predictive factors for successful eradication in patients experiencing H. pylori treatment failure. The secondary outcomes were to determine current antibiotic resistance patterns as well as trends in eradication rates of first-line regimens in Thailand.
Diagnosis of H. pylori infection
During upper GI endoscopy, at least four biopsies from antrum and body of stomach were performed and sent for rapid urease test and histological examination. H. pylori culture was performed in some treatment-naïve patients and patients with treatment failure. H. pylori infection was defined as a positive result to either one of these three diagnostic methods.
H. pylori culture and antimicrobial susceptibility testing
H. pylori culture and antimicrobial susceptibility testing (AST) was previously recommended after treatment failure [11]. However, the current guideline recommends performing susceptibility tests before first-line treatment to provide higher cure rates and promote responsible antibiotic use [3]. Two antral biopsies were manually ground in broth and streaked on a Mueller Hinton II agar medium using an inoculating loop. After incubation at 37°C in a microaerophilic atmosphere for 3 to 5 days, H. pylori colonies appear on the agar plate [12]. AST was then performed to determine the minimum inhibitory concentrations (MICs) of antibiotics using the Epsilometer test (E-test). The MIC, which was the lowest concentration of antibiotics that could prevent visible bacterial growth, was determined at the intersection point between the inhibition ellipse and the scale on the E-test strip [13]. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) defined resistant strains by MIC values of >0.125 mg/L for amoxicillin (AMX), >0.5 mg/L for clarithromycin (CLR), >8 mg/L for metronidazole (MTZ), >1 mg/L for levofloxacin (LVX), and >1 mg/L for tetracycline (TET) [14].
Definitions
H. pylori treatment failure was defined as persistent H. pylori infection after completing an eradication regimen. Persistent H. pylori infection was diagnosed by a positive rapid urease test, histopathology, or culture.
Treatment success after first-line eradication failure was defined as a negative urea breath test or stool antigen test after completing a treatment regimen after least one failed eradication attempt.
Multidrug-resistant H. pylori strains (MDR) was defined as strains which were resistant to two or more classes of antibiotics.
Tailored therapy was defined as H. pylori treatment by using susceptibility-guided antibiotics. Patients who did not have AST performed were considered as not using tailored therapy.
Nonadherence was defined as taking less than 80% of prescribed medication for H. pylori eradication (e.g., <11 days of total medication for 14-day regimen) [15].
Standard-dose proton-pump inhibitor (PPI) is as follows: omeprazole 20 mg, esomeprazole 20 mg, rabeprazole 20 mg, lansoprazole 30 mg, dexlansoprazole 30 mg, or pantoprazole 40 mg [7].
High-dose PPI is defined as double dose of a standard dose mentioned above [7].
Potassium-competitive acid blocker (P-CAB) in this study is vonoprazan (VPZ) 20 mg given b.i.d.
Statistical analysis
All data were analysed by using SPSS version 22 (SPSS Inc., Chicago, IL, USA). Categorical variables were analysed by Chi-square test, or Fisher’s exact test where appropriate. Continuous variables were analysed by using Student’s t-test and reported as mean ± standard deviation (SD). Univariate analysis was performed to identify predictive factors associated with successful eradication after H. pylori treatment failure. All variables with p-values <0.05 by univariate analysis entered into multivariate analysis. Multivariate analysis was then performed to determine an adjusted p-value for each strategy in order to reach the same endpoint: treatment success after first-line eradication failure. All tests were two-sided and p values of less than 0.05 were considered as statistical significance.
Ethics statements
The study obtained ethical approval by the Human Research Ethics Committee of Thammasat University, Thailand and was conducted according to the good clinical practice guideline, as well as the Declaration of Helsinki. All data had been fully anonymized before they were accessed. Waiver of documentation of informed consent was issued by the Ethics Committee because of no more than minimal risk to study subjects. Authors did not have access to information that could identify individual participants during or after data collection.
Results
Demographic data of patients with H. pylori infection
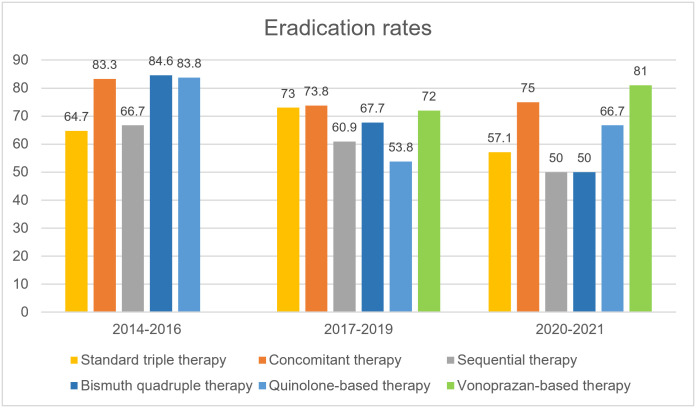
During the study period, 1,840 patients underwent upper gastrointestinal endoscopy at tertiary care center in Thailand. Of 1,840 patients, there were 1,050 with H. pylori infections [471 men and 579 women; mean age 59 ± 13 (range 17–91) years] included in this study. After first-line treatment, 302 patients experienced treatment failure (28.7%); mean age = 58.4 years; 44.7% males. The baseline characteristics were comparable between treatment success and treatment failure groups (Table 1). The most common underlying diseases were hypertension (37.5%), dyslipidemia (35%), and diabetes mellitus (21%). Triple therapy (a PPI, clarithromycin, and amoxicillin for 14 days) was the most commonly prescribed first-line regimen for H. pylori eradication (39.7%), followed by bismuth quadruple therapy (14%), concomitant regimen (13.9%), levofloxacin triple therapy (12.8%), vonoprazan-containing regimens (12.8%; vonoprazan triple therapy 89/134, vonoprazan bismuth quadruple therapy 35/134, vonoprazan dual therapy 10/134), and sequential therapy (6.8%). All first-line regimens were equally distributed between treatment success and failure group except for sequential therapy which was more common in the treatment failure group. The most frequently used second-line regimens were levofloxacin triple therapy (44.9%) and bismuth quadruple therapy (27.9%). Eradication rates after first-line, second-line, and third-line regimens were 71.2% (95%CI 68.4%-74.0%), 54.5% (95%CI 48.7%-60.2%), and 44.9% (95%CI 36.1%-54.0%), respectively. Time trends demonstrated a declining eradication rate for standard triple therapy and more frequent use of vonoprazan-containing regimens since 2019 (Fig 1). Sequential therapy had an eradication rate of 50% as first-line treatment. Nonadherence to treatment and presence of MDR H. pylori strains were significantly associated with treatment failure with OR 36.6 (95%CI 8.65–155.03, p<0.001), and OR 4.49 (95%CI 2.29–8.81, p<0.001), respectively.
Table 1. Baseline characteristics and univariate analysis of predictors associated with treatment failure.
| Characteristics | Treatment success | Treatment failure | P-value | ||
|---|---|---|---|---|---|
| (N = 748) | (N = 302) | ||||
| Male | 336 | (44.9%) | 135 | (44.7%) | 0.949 |
| Age (years ± SD) | 59.2 ± 12.9 | 58.4 ± 13.2 | 0.338 | ||
| BMI (kg/m2 ± SD) | 24.2 ± 4.1 | 24.4 ± 5.1 | 0.699 | ||
| Underlying diseases | |||||
| None | 225 | (30.1%) | 83 | (27.5%) | 0.403 |
| Hypertension | 281 | (37.6%) | 113 | (37.4%) | 0.964 |
| Dyslipidemia | 260 | (34.8%) | 108 | (35.8%) | 0.758 |
| Diabetes mellitus | 167 | (22.3%) | 53 | (17.5%) | 0.085 |
| Cirrhosis and hepatitis | 134 | (17.9%) | 45 | (14.9%) | 0.240 |
| Chronic kidney disease | 44 | (5.9%) | 22 | (7.3%) | 0.397 |
| FH of gastric cancer | 6 | (0.8%) | 3 | (1.0%) | 0.722 |
| Smoking | 60 | (8.0%) | 23 | (7.6%) | 0.826 |
| Alcohol | 107 | (14.3%) | 36 | (11.9%) | 0.308 |
| First-line treatment | |||||
| Duration | 12.1 ± 2.7 | 12.2 ± 2.6 | 0.417 | ||
| Triple therapy | 287 | (38.4%) | 130 | (43.0%) | 0.161 |
| Concomitant therapy | 110 | (14.7%) | 36 | (11.9%) | 0.238 |
| Sequential therapy | 44 | (5.9%) | 28 | (9.3%) | 0.049 |
| Bismuth quadruple therapy | 100 | (13.4%) | 47 | (15.6%) | 0.354 |
| Quinolone-based therapy | 103 | (13.8%) | 31 | (10.3%) | 0.123 |
| VPZ-containing therapy | 104 | (13.9%) | 30 | (9.9%) | 0.081 |
| Nonadherence | 2 | (0.3%) | 27 | (8.9%) | <0.001 |
| MDR strains | 18/115 | (15.7%) | 35/77 | (45.5%) | <0.001 |
BMI = body mass index, FH = family history, VPZ = Vonoprazan, MDR = multidrug-resistant
Fig 1. Time trends of eradication rates of each first-line regimen during 2014–2021.
Predictive factors associated with treatment success of first-line failure
After first-line eradication failure, 301 patients received a second-line treatment. Levofloxacin triple therapy and bismuth quadruple therapy were the most commonly used empiric second-line regimens as recommended by the Thai guideline [9]. Various treatment strategies were implemented to eradicate H. pylori infection. Univariate and multivariate analyses of strategies associated with treatment success after first-line eradication failure are shown in Table 2. Tailored therapy based on AST (91.1% vs. 64.9%; OR 4.92, 95%CI 1.61–14.99, p = 0.005) and 4-times-daily dosing of amoxicillin (2 grams/day) (89.4% vs. 65.7%; OR 3.05, 95%CI 1.10–8.41, p = 0.032) were significantly associated with treatment success after first-line failure. Four-times-daily dosing of amoxicillin in successful eradication regimens were demonstrated in S1 Table. Treatment duration of 14 days was associated with treatment success after first-line failure in the univariate analysis but did not reach statistical significance in the multivariate analysis. Vonoprazan was used in 24 patients (8%) for treatment after first-line failure yielding an eradication rate of 70.8%. Vonoprazan-based regimens were demonstrated in S2 Table. High-dose PPI was used in remaining patients providing a similar cure rate (69.7%). Eradication rates between regimens with or without bismuth were not different (69.2% vs. 70.2%, p = 0.853). Furazolidone-containing regimen [(PPI or VPZ) + bismuth + (AMX or TET) + furazolidone] was used for salvage therapy after multiple treatment failures providing the eradication rate of 76.9%.
Table 2. Eradication rates, univariate and multivariate analyses of strategies associated with treatment success after first-line eradication failure.
| Strategies | Eradication rates | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | p-value | OR | (95% CI) | p-value | |||
| Non-tailored therapy | 159/245 | (64.9%) | 1 | |||||
| Tailored therapy based on AST | 51/56 | (91.1%) | 5.52 | (2.12–14.34) | <0.001 | 4.92 | (1.61–14.99) | 0.005 |
| Twice-daily dosing of AMX (2 g/day) | 113/172 | (65.7%) | 1 | - | ||||
| Four-times-daily dosing of AMX (2 g/day) | 42/47 | (89.4%) | 4.39 | (1.65–11.68) | 0.003 | 3.05 | (1.10–8.41) | 0.032 |
| High-dose PPI use | 193/277 | (69.7%) | 1 | - | ||||
| Vonoprazan use | 17/24 | (70.8%) | 1.06 | (0.42–2.64) | 0.906 | |||
| No bismuth in regimen | 127/181 | (70.2%) | 1 | |||||
| Addition of bismuth | 83/120 | (69.2%) | 0.95 | (0.58–1.58) | 0.853 | |||
| 7- or 10-day treatment duration | 41/69 | (59.4%) | 1 | |||||
| 14-day treatment duration | 169/232 | (72.8%) | 1.83 | (1.05–3.21) | 0.034 | 1.68 | (0.87–3.23) | 0.121 |
AMX = amoxicillin, PPI = proton pump inhibitor
Effects of antibiotic susceptibility testing on eradication rates
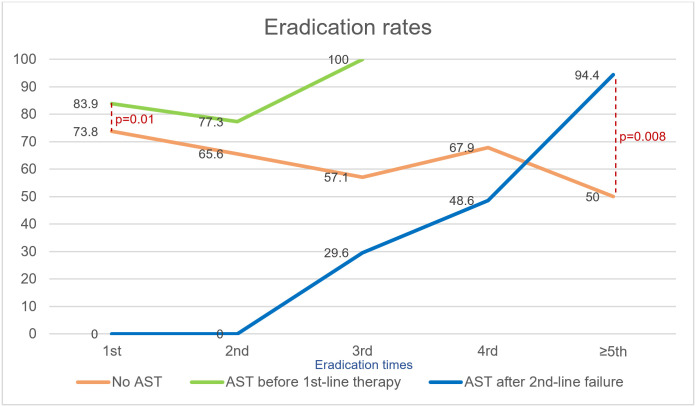
Patients with H. pylori infection were divided into 3 subgroups: 1) group without AST performed (n = 858); 2) group with AST performed before the first-line treatment (n = 137); 3) group with AST performed after failure of second-line therapy (n = 55). Those who had AST performed before either first therapy or second therapy had higher eradication rates (83.9% vs. 73.8%, p = 0.01) and (77.3% vs. 65.6%, p = 0.27) respectively than those without AST (Fig 2). All patients who failed second therapy who came for follow-up achieved successful eradication by the third eradication. Four patients (2.9%) in group 2 were lost to follow-up at the third eradication. The eradication rate of group 3 gradually increased after performing AST and became higher than that of group 1 after the fifth eradication (94.4% vs. 50%, p = 0.008), whereas eradication rates of group 1 decreased from 73.8% to 50% after fifth-line treatment.
Fig 2. Eradication rates of 3 subgroups according to the time of performed AST.
AST = Antibiotic susceptibility testing.
H. pylori antibiotic resistance
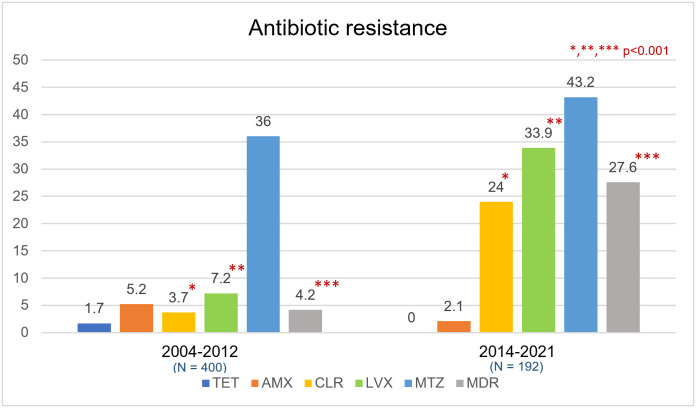
AST was performed for 192 strains (Table 3). The resistance rates to metronidazole, levofloxacin, clarithromycin, and amoxicillin were 43.2%, 33.9%, 24%, and 2.1%, respectively. There was no tetracycline resistance (n = 192). In subgroup of clarithromycin-resistant strains, almost all patients receiving triple therapy, concomitant therapy, and sequential therapy had first-line eradication failure (29/31, 93.5%). Seventy strains (36.5%) had no antibiotic resistance and 53 (27.6%) were MDR strains. MDR strains were significantly more common after treatment failure (45.5% vs. 15.7%, p<0.001). An eradication rate of first-line therapy for MDR strains was only 34%. Seventeen strains (8.9% of all strains) in treatment failure group were resistant to clarithromycin, metronidazole, and levofloxacin and were eradicated by amoxicillin plus tetracycline (5/17), amoxicillin or tetracycline plus furazolidone (5/17), amoxicillin or tetracycline plus sitafloxacin (3/17), amoxicillin or tetracycline plus metronidazole (2/17), and amoxicillin plus moxifloxacin (1/17). One patient with CLR, MTZ, and LVX resistance lost to follow-up after 4th-line treatment. Compared with 2004–2012, CLR, LVX, and multidrug resistance substantially increased during 2014–2021 (p<0.001) (Fig 3).
Table 3. Antibiotic resistance pattern of H. pylori strains in patients with treatment failure.
| Antibiotic resistance | Total patients | Treatment success | Treatment failure | P-value | |||
|---|---|---|---|---|---|---|---|
| (N = 192) | (N = 115) | (N = 77) | |||||
| No resistance | 70 | (36.5%) | 58 | (50.4%) | 12 | (15.6%) | <0.001 |
| Antibiotic resistance | 122 | (63.5%) | 57 | (49.6%) | 65 | (84.4%) | <0.001 |
| Metronidazole (MTZ) | 83 | (43.2%) | 42 | (36.5%) | 41 | (53.2%) | 0.022 |
| Levofloxacin (LVX) | 65 | (33.9%) | 23 | (20.0%) | 42 | (54.5%) | <0.001 |
| Clarithromycin (CLR) | 46 | (24.0%) | 11 | (9.6%) | 35 | (45.5%) | <0.001 |
| Amoxicillin (AMX) | 4 | (2.1%) | 1 | (0.9%) | 3 | (3.9%) | 0.304 |
| Tetracycline (TET) | 0 | (0%) | 0 | (0%) | 0 | (0%) | - |
| Single drug resistance | 69 | (35.9%) | 39 | (33.9%) | 30 | (39.0%) | 0.475 |
| MTZ | 38 | (19.8%) | 26 | (22.6%) | 12 | (15.6%) | 0.231 |
| LVX | 23 | (12.0%) | 9 | (7.8%) | 14 | (18.2%) | 0.030 |
| CLR | 8 | (4.2%) | 4 | (3.5%) | 4 | (5.2%) | 0.716 |
| Multidrug resistance | 53 | (27.6%) | 18 | (15.7%) | 35 | (45.5%) | <0.001 |
| MTZ and LVX | 14 | (7.3%) | 10 | (8.7%) | 4 | (5.2%) | 0.360 |
| MTZ and CLR | 9 | (4.7%) | 4 | (3.5%) | 5 | (6.5%) | 0.488 |
| CLR and LVX | 8 | (4.2%) | 2 | (1.7%) | 6 | (7.8%) | 0.062 |
| CLR, MTZ, and LVX | 18 | (9.4%) | 1 | (0.9%) | 17 | (22.1%) | <0.001 |
| AMX, CLR, and MTZ | 2 | (1.0%) | 0 | (0%) | 2 | (2.6%) | 0.160 |
| AMX, MTZ, and LVX | 1 | (0.5%) | 1 | (0.9%) | 0 | (0%) | 1.000 |
| AMX, CLR, MTZ, and LVX | 1 | (0.5%) | 0 | (0%) | 1 | (1.3%) | 1.000 |
Fig 3. Antibiotic resistance in Thailand during 2014–2021 compared with 2004–2012.
TET = Tetracycline, AMX = Amoxicillin, CLR = Clarithromycin, LVX = Levofloxacin, MTZ = Metronidazole, MDR = Multidrug-resistant strains.
Discussion
H. pylori treatment failure has become an important global issue. Traditionally, clarithromycin resistance has been low and patients in this study mainly received clarithromycin triple therapy as first-line treatment. Most of patients with clarithromycin resistant strains receiving clarithromycin-containing regimens for first-line therapy had eradication failure. This indicates that our national guideline should be revised according to recent antibiotic resistance pattern. The mostly used second-line regimens were levofloxacin triple therapy and bismuth quadruple therapy as suggested by the Thailand consensus on H. pylori treatment [9]. Sequential therapy, which was recommended as first-line treatment in the latest guideline also had a low eradication rate (50%). Only vonoprazan-containing and concomitant regimens provided acceptable eradication rates. This is in concordance with the latest meta-analysis comparing effectiveness of first-line regimens which demonstrated high eradication rates (91.4%) when using vonoprazan triple therapy [16]. Triple therapy is currently an ineffective first-line therapy in area with high clarithromycin resistance. Despite having a well-developed guideline for H. pylori treatment in Thailand, there was heterogeneity of real-world practice and we found that most of non-gastroenterologists used triple therapy as first-line regimen. Therefore, results of this study emphasize the importance of improving medical education and implementing guideline recommendations into clinical practice. After failed second-line therapy, the eradication rate decreased to below 50% signifying the importance of achieving successful eradication with the initial therapy.
Multiple factors influencing treatment failure including host factors such as age, medication adherence, smoking, and CYP2C19 phenotype [7]. This study confirmed that medication nonadherence was significantly associated with treatment failure. Complex regimens, high pill burden, multiple dosing, and high drug costs are common adherence barriers [17]. Apart from host factors, the primary microbial factor was the presence of MDR H. pylori which was associated with a >4 times higher risk for treatment failure. For MDR strains with resistance to MTZ and other antibiotics, bismuth quadruple therapy can still be used because MTZ resistance can be overcome by using at least 1500 mg/day of MTZ for 14 days [18]. Common resistance to three classes of antibiotics (CLR, MTZ, and LVX) caused difficulty in identifying effective treatment options. The most effective regimens were a combination of AMX and TET, or AMX or TET combined with furazolidone. Rising trend of MDR strains in Thailand aligned with increasing combined CLR and MTZ resistance in many world regions [8]. Although MDR strains have been increasing rapidly in Thailand [19], we were able to achieve eradication in 92.5% of patients with MDR with susceptibility data playing a pivotal role.
After treatment failure, several strategies were used to accomplish successful H. pylori eradication. Optimized regimens provided high cure rates when guided by AST. Currently, AST is recommended to be performed prior to first-line treatment to achieve high eradication rates [3]. However, if resources are limited, AST can be performed after an initial empirical treatment failure. Our study demonstrated that tailored therapy based on AST was significantly associated with treatment success after first-line failure and should be considered in management of multiple treatment failures in Asia. Subgroup analysis revealed that the group with AST performed before first-line treatment had the best eradication rates of between 80% and 100%. Eradication failure after first-line tailored therapy might be resulted from other factors such as medication nonadherence and CYP2C19 extensive metabolizer. Moreover, endosymbiosis between H. pylori and Candida species might be one of mechanisms that could facilitate intracellular protection of H. pylori. [20] Eradication rates of the group with AST performed after second-line failure increased and were eventually higher than the group without AST by fifth-line treatment. On the contrary, the group without AST experienced a gradual decline of eradication rate from 73.8% to 50% after multiple eradication attempts. It can be inferred that performing AST either before first-line therapy or after multiple treatment failure would shift trends toward more successful eradications. AST must be coupled with knowledge of which therapies are highly effective locally and with high adherence to be successful. Four-times-daily dosing of amoxicillin (500 mg every 6 hours) with high dose PPI was another factor possibly contributing to treatment success after first-line failure. In this study high dose was defined as double dose rather than use of dosing and PPI proven to provide high antisecretory effect. Frequent amoxicillin dosing schemes (≥3 times daily) can also provide longer time above minimum inhibitory concentration (MIC) [21]. CYP2C19 polymorphisms also have an impact on PPI metabolism contributing to change in intragastric pH, and affect H. pylori eradication when using pH-sensitive antibiotics [22]. All patients in this study used double dose PPI or vonoprazan to achieve adequate acid suppression [7]. Importantly, there was no difference of eradication rate whether using double dose PPI or vonoprazan. The addition of bismuth could also produce synergistic effect with antibiotics and improve eradication rates [23]. However, there was no difference between group with or without bismuth. Clearly, AST alone is insufficient to reliably achieve high cure rates as it must be coupled with locally proven effective antibiotic therapies.
Rising antibiotic-resistant H. pylori has posed a worrisome threat to public health [6, 8]. The majority of strains (63.5%) in this study were resistant to at least one class of antibiotic. The key to achieving high cure rate is prescribing a therapy which provides a local eradication rate of at least 90% [24]. CLR, LVX, and MTZ resistance in this study were all higher than 15%. Compared with other countries in the Association of Southeast Asian Nations (ASEAN), H. pylori strains in Thailand demonstrated relatively higher CLR, LVX, and MTZ resistance. ASEAN countries with high CLR, LVX, and MTZ resistance include Vietnam, Philippines, Cambodia, and Thailand [25–27]. MDR strains were most often found in Cambodia (76.4%). ASEAN countries had strains with low tetracycline and amoxicillin resistance except for Vietnam [28–32]. The prevalence of H. pylori antibiotic resistance in Southeast Asian countries is shown in Table 4. In countries which had high LVX and MTZ resistance, but low CLR resistance such as Malaysia, Indonesia, and Myanmar, CLR-containing empiric regimens might still be used. In contrast, susceptibility-based therapy would be required to be able to use CLR, MTZ, and LVX in Thailand, Philippines, Cambodia, and Vietnam. Either bismuth quadruple therapy or high dose PPI plus amoxicillin can still be used provided that it has been optimized for use locally.
Table 4. The prevalence of H. pylori antibiotic resistance in Southeast Asian countries.
| Countries, year | Number of patients | TET | AMX | CLR | LVX | MTZ | MDR | References |
|---|---|---|---|---|---|---|---|---|
| Vietnam, 2019 | 2,318 | 17.9 | 15.0 | 34.1 | 27.9 | 69.4 | 48.8 | Khien et al. [25] |
| Philippines, 2019 | 42 | 0 | 0 | 28.6 | 61.9 | 40.5 | N/A | Yumang et al. [26] |
| Cambodia, 2019 | 55 | 0 | 9.1 | 25.5 | 67.3 | 96.4 | 76.4 | Tuan et al. [27] |
| Thailand, 2022 | 192 | 0 | 2.1 | 24.0 | 33.9 | 43.2 | 27.6 | This study |
| Singapore, 2016 | 708 | 7.6 | 4.1 | 17.1 | 14.7 | 48.2 | 13.5 | Ang et al. [28] |
| Laos, 2016 | 31 | N/A | N/A | 12.6 | 13.4 | N/A | N/A | Vannarath et al. [29] |
| Malaysia, 2019 | 59 | 0 | 0 | 12.2 | 17.1 | 56.1 | 40.9 | Hanafiah et al. [30] |
| Indonesia, 2016 | 77 | 2.6 | 5.2 | 9.1 | 31.2 | 46.7 | 24.7 | Miftahussurur et al. [31] |
| Myanmar, 2022 | 65 | 0 | 4.6 | 7.7 | 33.8 | 80.0 | 33.8 | Subsomwong et al. [32] |
TET = Tetracycline, AMX = Amoxicillin, CLR = Clarithromycin, LVX = Levofloxacin, MTZ = Metronidazole, MDR = Multidrug-resistant strains, N/A = Not available
Our study has several strengths. This is a large population-based study demonstrating predictors for successful eradication in patients experiencing H. pylori treatment failure. Furthermore, this study suggests eradication strategies and raises awareness of appropriate antibiotic use by reporting the current situation of antibiotic-resistant H. pylori strains. However, our study also had some limitations. First, there was heterogeneity of real-world practice despite having a well-developed guideline for H. pylori treatment. Prescribed first-line regimens were highly varied depending on physicians’ preference. Second, this was a retrospective cohort study. Therefore, there were some missing data of culture and AST. We tried our best to perform culture and AST as much as possible and collect all positive culture results in our analysis.
In conclusion, H. pylori treatment failure has recently become a global challenging problem. Medication nonadherence and development of MDR were the primary factors predictive of treatment failure. Tailored therapy and 4-times-daily dosing of amoxicillin in treatment regimen should be considered in management of multiple treatment failures. Performing early AST after treatment failures provides higher eradication rates and should be suggested in future clinical practice, especially in the antibiotic resistance area.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
This study was supported by Thailand Science Research and Innovation Fundamental Fund, Bualuang ASEAN Chair Professorship at Thammasat University, Center of Excellence in Digestive Diseases, Thammasat University, Thailand, and Japan Agency for Medical Research and Development (AMED) [e-ASIA JRP] (YY and RV). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006; 19(3):449–90. doi: 10.1128/CMR.00054-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3.Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022; 71(9):1724–1762. doi: 10.1136/gutjnl-2022-327745 [DOI] [PubMed] [Google Scholar]
- 4.Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut. 2020; 69(12):2113–2121. doi: 10.1136/gutjnl-2020-320839 [DOI] [PubMed] [Google Scholar]
- 5.Mahachai V, Vilaichone RK, Pittayanon R, Rojborwonwitaya J, Leelakusolvong S, Maneerattanaporn M, et al. Helicobacter pylori management in ASEAN: The Bangkok consensus report. J Gastroenterol Hepatol. 2018; 33(1):37–56. doi: 10.1111/jgh.13911 [DOI] [PubMed] [Google Scholar]
- 6.Kuo Y-T, Liou J-M, El-Omar EM, Wu J-Y, Leow AHR, Goh KL, et al. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017; 2(10):707–715. doi: 10.1016/S2468-1253(17)30219-4 [DOI] [PubMed] [Google Scholar]
- 7.Shah SC, Iyer PG, Moss SF. AGA Clinical practice update on the management of refractory Helicobacter pylori Infection: expert review. Gastroenterology. 2021; 160(5):1831–1841. doi: 10.1053/j.gastro.2020.11.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 2018; 155(5):1372–1382 e17. doi: 10.1053/j.gastro.2018.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahachai V, Vilaichone RK, Pittayanon R, Rojborwonwitaya J, Leelakusolvong S, Kositchaiwat C, et al. Thailand consensus on Helicobacter pylori treatment 2015. Asian Pac J Cancer Prev. 2016; 17(5):2351–2360. doi: 10.7314/apjcp.2016.17.5.2351 [DOI] [PubMed] [Google Scholar]
- 10.Aumpan N, Mahachai V, Vilaichone RK. Management of Helicobacter pylori infection. JGH Open. 2023; 7(1):3–15. doi: 10.1002/jgh3.12843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017; 66(1):6–30. doi: 10.1136/gutjnl-2016-312288 [DOI] [PubMed] [Google Scholar]
- 12.Perez-Perez GI, Van TN, Thu Huong D, Zhan G, Nguyet Anh D, Nguyet NT, et al. Isolation and characterization of Helicobacter pylori recovered from gastric biopsies under anaerobic conditions. Diagn Microbiol Infect Dis. 2016; 86(2):136–40. doi: 10.1016/j.diagmicrobio.2016.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Megraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev. 2007; 20(2):280–322. doi: 10.1128/CMR.00033-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EUCAST. Breakpoint tables for interpretation of MICs and zone diameters, version 12.0. 2022; http://www.eucast.org. [Accessed 2 October 2022].
- 15.Kim SJ, Jee SR, Park MI, Jung K, Kim GH, Lee MW, et al. A randomized controlled trial to compare Helicobacter pylori eradication rates between the empirical concomitant therapy and tailored therapy based on 23S rRNA point mutations. Medicine (Baltimore). 2022; 101(33):e30069. doi: 10.1097/MD.0000000000030069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rokkas T, Gisbert JP, Malfertheiner P, Niv Y, Gasbarrini A, Leja M, et al. Comparative effectiveness of multiple different first-line treatment regimens for Helicobacter pylori Infection: a network meta-analysis. Gastroenterology. 2021; 161(2):495–507 e4. doi: 10.1053/j.gastro.2021.04.012 [DOI] [PubMed] [Google Scholar]
- 17.Argueta EA, Moss SF. How We Approach Difficult to Eradicate Helicobacter pylori. Gastroenterology. 2022; 162(1):32–37. doi: 10.1053/j.gastro.2021.10.048 [DOI] [PubMed] [Google Scholar]
- 18.Graham DY, Dore MP, Lu H. Understanding treatment guidelines with bismuth and non-bismuth quadruple Helicobacter pylori eradication therapies. Expert Rev Anti Infect Ther. 2018; 16(9):679–687. doi: 10.1080/14787210.2018.1511427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vilaichone RK, Gumnarai P, Ratanachu-Ek T, Mahachai V. Nationwide survey of Helicobacter pylori antibiotic resistance in Thailand. Diagn Microbiol Infect Dis. 2013; 77(4):346–349. doi: 10.1016/j.diagmicrobio.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 20.Bacic A, Milivojevic V, Petkovic I, Kekic D, Gajic I, Medic Brkic B, et al. In Search for Reasons behind Helicobacter pylori Eradication Failure-Assessment of the Antibiotics Resistance Rate and Co-Existence of Helicobacter pylori with Candida Species. J Fungi (Basel). 2023; 9(3). doi: 10.3390/jof9030328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furuta T, Sugimoto M, Yamade M, Uotani T, Sahara S, Ichikawa H, et al. Effect of dosing schemes of amoxicillin on eradication rates of Helicobacter pylori with amoxicillin-based triple therapy. J Clin Pharmacol. 2014; 54(3):258–66. doi: 10.1002/jcph.195 [DOI] [PubMed] [Google Scholar]
- 22.Kuo CH, Lu CY, Shih HY, Liu CJ, Wu MC, Hu HM, et al. CYP2C19 polymorphism influences Helicobacter pylori eradication. World J Gastroenterol. 2014; 20(43):16029–36. doi: 10.3748/wjg.v20.i43.16029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNicholl AG, Bordin DS, Lucendo A, Fadeenko G, Fernandez MC, Voynovan I, et al. Combination of bismuth and standard triple therapy eradicates Helicobacter pylori infection in more than 90% of patients. Clin Gastroenterol Hepatol. 2020; 18(1):89–98. doi: 10.1016/j.cgh.2019.03.048 [DOI] [PubMed] [Google Scholar]
- 24.Graham DY, Moss SF. Antimicrobial Susceptibility Testing for Helicobacter pylori Is Now Widely Available: When, How, Why. Am J Gastroenterol. 2022; 117(4):524–528. doi: 10.14309/ajg.0000000000001659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khien VV, Thang DM, Hai TM, Duat NQ, Khanh PH, Ha DT, et al. Management of Antibiotic-Resistant Helicobacter pylori Infection: Perspectives from Vietnam. Gut Liver. 2019; 13(5):483–497. doi: 10.5009/gnl18137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yumang Z, Bondoc E, Pangilinan JA, Cortes MC, Chua HG, Dujunco MM, et al. Incidence of helicobacter pylori antibiotic resistance a single center cross-sectional study. Gut. 2019; 68(Suppl 1):A39–A40. doi: 10.1136/gutjnl-2019-IDDFAbstracts.74 [DOI] [Google Scholar]
- 27.Tuan VP, Narith D, Tshibangu-Kabamba E, Dung HDQ, Viet PT, Sokomoth S, et al. A Next-Generation Sequencing-Based Approach to Identify Genetic Determinants of Antibiotic Resistance in Cambodian Helicobacter pylori Clinical Isolates. J Clin Med. 2019; 8(6). doi: 10.3390/jcm8060858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ang TL, Fock KM, Ang D, Kwek AB, Teo EK, Dhamodaran S. The Changing Profile of Helicobacter pylori Antibiotic Resistance in Singapore: A 15-Year Study. Helicobacter. 2016; 21(4):261–5. doi: 10.1111/hel.12291 [DOI] [PubMed] [Google Scholar]
- 29.Vannarath S, Vilaichone RK, Rasachak B, Mairiang P, Yamaoka Y, Mahachai V. Antibiotic Resistant Pattern of Helicobacter Pylori Infection Based on Molecular Tests in Laos. Asian Pac J Cancer Prev. 2016; 17(1):285–7. doi: 10.7314/apjcp.2016.17.1.285 [DOI] [PubMed] [Google Scholar]
- 30.Hanafiah A, Binmaeil H, Raja Ali RA, Mohamed Rose I, Lopes BS. Molecular characterization and prevalence of antibiotic resistance in Helicobacter pylori isolates in Kuala Lumpur, Malaysia. Infect Drug Resist. 2019; 12:3051–3061. doi: 10.2147/IDR.S219069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miftahussurur M, Syam AF, Nusi IA, Makmun D, Waskito LA, Zein LH, et al. Surveillance of Helicobacter pylori Antibiotic Susceptibility in Indonesia: Different Resistance Types among Regions and with Novel Genetic Mutations. PLoS One. 2016; 11(12):e0166199. doi: 10.1371/journal.pone.0166199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subsomwong P, Doohan D, Fauzia KA, Akada J, Matsumoto T, Yee TT, et al. Next-Generation Sequencing-Based Study of Helicobacter pylori Isolates from Myanmar and Their Susceptibility to Antibiotics. Microorganisms. 2022; 10(1). doi: 10.3390/microorganisms10010196 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.