Abstract
Group III capsular polysaccharides (e.g., K54) of extraintestinal isolates of Escherichia coli, similar to group II capsules (e.g., K1), are important virulence traits that confer resistance to selected host defense components in vitro and potentiate systemic infection in vivo. The genomic organization of group II capsule gene clusters has been established as a serotype-specific region 2 flanked by regions 1 and 3, which contain transport genes that are highly homologous between serotypes. In contrast, the organization of group III capsule gene clusters is not well understood. However, they are defined in part by an absence of genes with significant nucleotide homology to group II capsule transport genes in regions 1 and 3. Evaluation of isogenic, TnphoA-generated, group III capsule-minus derivatives of a clinical blood isolate (CP9, O4/K54/H5) has led to the identification of homologs of the group II capsule transport genes kpsDMTE. These genes and their surrounding regions were sequenced and analyzed. The genomic organization of these genes is distinctly different from that of their group II counterparts. Although kpsK54DMTE are significantly divergent from their group II homologs at both the DNA and protein levels phoA fusions and computer-assisted analyses suggest that their structures and functions are similar. The putative proteins KpsK54M and KpsK54T appear to be the integral membrane component and the peripheral ATP-binding component of the ABC-2 transporter family, respectively. The putative KpsK54E possesses features similar to those of the membrane fusion protein family that facilitates the passage of large molecules across the periplasm. At one boundary of the capsule gene cluster, a truncated kpsM (kpsMtruncated) and its 5′ noncoding regulatory sequence were identified. In contrast to the complete kpsK54M, this region was highly homologous to the group II kpsM. Fifty-three base pairs 3′ from the end of kpsMtruncated was a sequence 75% homologous to the 39-bp inverted repeat in the IS110 insertion element from Streptomyces coelicolor. Southern analysis established that two copies of this element are present in CP9. These findings are consistent with the hypothesis that CP9 previously possessed group II capsule genes and acquired group III capsule genes via IS110-mediated horizontal transfer.
Over 80 serologically and chemically unique capsular polysaccharides can be produced by Escherichia coli (22, 31). Initially, these polysaccharides were divided into group I and group II based on chemical, physical, genetic, and microbiological distinctions (20, 21). Subsequently, the division of the group II capsules into groups II and III (formerly I and II) has been proposed (36).
Group I capsules are chemically and physically characterized by a high molecular weight (>100,000), an acidic component usually consisting of hexuronic acid or pyruvate, a low charge density and electrophoretic mobility, and stability at pH 5 to 6 at 100°C. Group I capsules may protect against desiccation and may contribute to adherence in enteric disease-producing isolates of E. coli (17, 27, 30). However, a role in the pathogenesis of extraintestinal E. coli infection has not been demonstrated (45).
In contrast, epidemiologic and experimental evidence supports a role for group II and group III capsules as virulence factors for extraintestinal infection (10, 43, 44), and these capsules possess multiple similarities with the capsules of pathogenic strains of Neisseria meningitidis and Haemophilus influenza (26). The group II capsules are characterized by a molecular weight of <50,000; hexuronic acids; N-acetyl neuraminic acid, phosphate, or 2-keto-3-deoxyoctonic acid (KDO) as acidic components; a higher charge density and electrophoretic mobility; and a general lack of stability at pH 5 to 6 at 100°C. Several group II capsules are linked to KDO-phosphatidic acid, which may serve both as a recognition signal for transport across the cytoplasmic membrane and as a membrane anchor (6, 7). The genes that code for these capsules have been mapped near serA (32, 33, 53), and these capsules are coexpressed with a large number of O antigens. It was originally believed that a given E. coli strain possessed only genes for either a group I or a group II capsule. However, it has been subsequently shown that three group II or III capsule (K1, K5, and K54)-producing strains were also capable of producing the group I capsule colanic acid (25, 46). This finding suggests the possibility that many if not all strains of E. coli have the capability to produce a group I capsule, whereas only a subset can produce a group II or group III capsule. The gene clusters coding for the group II capsules K1, K4, K5, K7, K12, and K92 have been cloned and extensively studied (particularly K1 and K5) and have a common organization of three functional regions (38–40, 48). Region 2, which is unique for a given capsular antigen, codes for genes whose products are responsible for the synthesis of the K-specific serotype. This region is flanked by regions 1 and 3, which are highly conserved among the group II capsule gene clusters evaluated to date. In fact, a DNA probe generated from region 1 in the K1 capsule gene cluster was used to identify the K4, K5, K7, K12, and K92 capsule gene clusters (13, 38). Region 1 contains six genes (kpsFEDUCS), and region 3 contains two genes (kpsMT); each region is organized in a single transcriptional unit and is temperature regulated. These gene products are needed for transport of the capsular polysaccharide across the cytoplasmic membrane and assembly onto the cell’s surface (4).
Group III capsules were originally categorized as group II capsular polysaccharides. Although these groups have similar biochemical and physical characteristics (31), map to the same location on the chromosome (32, 33, 53), confer resistance to selected host defense components in vitro, and potentiate systemic infection in vivo (10, 43, 44), differences exist between them. Group III serotypes K2, K3, K10, K11, K19, and K54 do not show temperature regulation of capsule expression, a characteristic which correlates with constitutive levels of CMP-KDO activity (14), whereas group II capsules have increased capsule expression and CMP-KDO activity at 37°C. Further, only the K2 capsule gene cluster, but not those from K3, K10, K11, K19, and K54, possesses DNA sequences homologous to group II capsule gene cluster regions 1 and 3 on the basis of Southern analysis (12, 36). In a recent study that described the cloning of a K10 and a different K54 (E. coli A12b) capsule gene cluster, Southern analysis and complementation studies were used to elucidate group III capsule gene organization (36). The preliminary results of these analyses suggested that a central serotype-specific region was flanked by two regions in which there was homology between the K10 and K54 gene clusters. Further, complementation studies demonstrated that group II kpsK5D and kpsK5E mutations, but not kpsK5M or kpsK5T mutations, were complemented by subclones from the K10 and K54 capsule gene clusters. Therefore, this finding suggested that, despite a lack of DNA homology, functional homology exists, at least in part, between proteins involved in the export of group II and group III capsular polysaccharides. The combination of these findings has resulted in the designation of serotypes K3, K10, K11, K19, and K54 (with or without K2) as group III capsules (36) and has suggested that these gene clusters are phylogenetically divergent from those of group II. In support of this concept, a clonal group of clinical E. coli isolates was recently identified from multiple geographic regions (23, 24). These strains were characterized in part by possession of the papGJ96 (class I) and prsGJ96 (class III) genes, the O4-specific antigen moiety of lipopolysaccharide, the H5 flagellar antigen, the F13 fimbrial antigen, and a group III capsule (K3, K10, and K54/96).
Researchers in our laboratory have been studying a clinical bacteremic isolate of E. coli (CP9, O4/K54/H5) as a model pathogen for extraintestinal infection (42). Its group III K54 capsular polysaccharide has been shown to be important for serum resistance in vitro (44) and systemic infection in vivo (43) but not for resistance to bactericidal permeability-increasing protein in vitro (45) or urinary tract infection in vivo (41). Previously, we reported the construction and initial characterization of TnphoA-generated, isogenic K54-minus derivatives of CP9 that were used in these studies (42). In this study, we describe the genomic location and novel organization of a portion of the K54 capsule gene cluster, the DNA sequences of kpsK54DMTE, and an analysis of these genes.
MATERIALS AND METHODS
Strains.
The strains used for this study are listed in Table 1. The wild-type strain (CP9, O4/K54/H5), a clinical blood isolate, and its K54 capsule-minus isogenic derivatives have been previously described in part (23, 42).
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Genotype or other relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| CP9 | O4/K54/H5 clinical blood isolate, serum resistant | 42 |
| CP9.29 | cl1.29::TnphoA active TnphoA fusion, serum sensitive | 42 |
| CP9.108 | kpsK54M::TnphoA (formerly cl1.108:TnphoA) active TnphoA fusion, serum sensitive | 42 |
| CP9.137 | kpsK54D::TnphoA (formerly cl1.137:TnphoA) active TnphoA fusion, serum sensitive | 42 |
| CP9.171 | kpsK54E::TnphoA (formerly cl1.171:TnphoA) active TnphoA fusion, serum sensitive | 42 |
| CP9.C56 | cl1::TnphoA inactive TnphoA fusion, serum sensitive | 42 |
| CP9.C54 | cl1::TnphoA inactive TnphoA fusion, serum sensitive | 42 |
| CP9.C43 | cl2::TnphoA inactive TnphoA fusion, serum sensitive | 42 |
| NM554 | recA13 F−araD139 Δ(ara-leu)7696 galE15 galK16 Δ(lac)X74 rpsL(Strr) hsdR2 (rK− mK+) mcrA mcrB1 | Lab strain |
| XL1 Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac (F+proAB lacIqZΔM15 Tn10) | Stratagene |
| Plasmids | ||
| p29.1 | 14.0-kb BamHI/ApaI fragment from CP9.29 containing the leftward 5.0 kb of TnphoA (active fusion) and capsule genes cloned into pBS II SK (−); capsule gene transcription is in the opposite orientation to the pBS II SK lac promoter | This study |
| p29.2 | The above 14.0-kb fragment cloned into pBS II KS (−); capsule gene transcription is in the same orientation as the pBS II SK lac promoter | This study |
| p108.1 | 8.5-kb BamHI/XbaI fragment from CP9.108 containing the leftward 5.0 kb of TnphoA (active fusion) and capsule genes cloned into pBS II SK (−); capsule gene transcription is in the same orientation as the pBS II SK lac promoter | This study |
| p108.2 | The above 8.5-kb fragment cloned into pBS II KS (−); capsule gene transcription is in the opposite orientation to the pBS II SK lac promoter | This study |
| p108.3 | 9.7-kb ClaI fragment from CP9.108 containing the rightward 6.7 kb of TnphoA (nonfusion) and capsule genes cloned into pBS II SK (−) | This study |
| p137.1 | 7.5-kb BamHI/XbaI fragment from CP9.137 containing the leftward 5.0 kb of TnphoA (active fusion) and capsule genes cloned into pBS II SK (−); capsule gene transcription is in the same orientation as the pBS II SK lac promoter | This study |
| p137.2 | The above 7.5-kb fragment cloned into pBS II KS (−); capsule gene transcription is in the opposite orientation to the pBS II SK lac promoter | This study |
| p171.1 | 6.4-kb BamHI fragment from CP9.171 containing the leftward 5.0 kb of TnphoA (active fusion) and capsule genes cloned into pBS II SK (−); capsule gene transcription is in the same orientation as the pBS II SK lac promoter | This study |
| p171.2 | The above 6.4-kb fragment cloned into pBS II SK (−); capsule gene transcription is in the opposite orientation to the pBS II SK lac promoter | This study |
| p171.3 | 8.7-kb ClaI fragment from CP9.171 containing the rightward 6.7 kb of TnphoA (nonfusion) and capsule genes cloned into pBS II SK (−) | This study |
| pC56.1 | 5.25 kb BamHI/XbaI fragment from CP9.C56 containing the leftward 5.0 kb of TnphoA (inactive fusion) and capsule genes cloned into pBS II SK (−) | This study |
| pcos9a | An approximately 40-kb fragment that contains capsule genes cloned into the BamHI site of the cosmid pWE15 | This study |
Construction of capsule gene subclones.
Subclones of the K54 capsule gene locus 5′ to the TnphoA insertions in CP9.29, CP9.108, CP9.137, CP9.171, CP9.C43, CP9.C54, and CP9.C56 were obtained by restricting whole-cell DNA with BamHI, which recognizes a site located 3′ to the kanamycin resistance gene in TnphoA with or without XbaI (CP9.108, 137, 171, C43, C54, and C56) or ApaI (CP9.29), neither of which restricts within TnphoA. Ligations of these restrictions into pBSII SK−, electroporation into XL1 Blue (Stratagene, La Jolla, Calif.), and selection of ampicillin (100 μg/ml)- and kanamycin (40 μg/ml)-resistant transformants resulted in the identification of the subclones p29.1, p108.1, p137.1, p171.1, pC43.1, pC54.1, and pC56.1. To construct a second set of subclones, in which the active phoA fusion was in the opposite orientation, p108.1, p137.1, and p171.1 were restricted with BamHI and XbaI, p29.1 was restricted with BamHI and ApaI, and the inserts were purified by electroelution and ligated into pBSII KS−. This set of constructs has been designated p29.2, p108.2, p137.2, and p171.2. These plasmids are described in detail in Table 1.
Subclones of the K54 capsule gene locus 3′ to the TnphoA insertions in CP9.108 and CP9.171 were obtained by restricting whole-cell DNA with ClaI, which recognizes a site 5′ to the kanamycin resistance gene in TnphoA. Ligations of these restrictions into pBSII SK−, electroporation into XL1 Blue (Stratagene), and selection of ampicillin- and kanamycin-resistant transformants resulted in the identification of the subclones p108.3 and p171.3. Each contains the right 6.7 kb of TnphoA and either 3.0 kb (p108.3) or 2.0 kb (p171.3) of chromosomal DNA 3′ to the respective TnphoA insertions.
Identification of a cosmid clone containing capsule genes.
Whole-cell DNA was purified from CP9 as described previously (42), and DNA fragments (30 to 50 kb) were ligated into the unique BamHI site of the 8.8-kb cosmid cloning vector pWE15 (Clontech Laboratories, Palo Alto, Calif.). The ligation mix was packaged into lambda phage in vitro and transduced into E. coli NM554, and the resultant CP9-derived DNA library was amplified once. The amplified library was screened for clones containing capsule genes via colony filter hybridization as described previously (16). The probe used for detection was generated by digesting p171.1 with PvuI/XbaI, purifying the 1.3-kb restriction product via electroelution, and subsequent radioactive labelling with [α-32P]dCTP by random oligonucleotide priming. Approximately 1,000 colonies of NM554 containing the CP9 DNA library were screened, and a cosmid clone (cos9a) was detected. Cos9a was confirmed to contain capsule gene DNA via Southern analysis (42), with the p171.1 PvuI/XbaI 1.3-kb fragment as the probe.
DNA sequencing, determination of TnphoA insertion sites, and analysis of capsule genes.
DNA sequence was determined by the dideoxy chain termination method of Sanger et al. (47) with the capsule gene subclones (p29.1, p108.1, p108.3, p137.1, p171.1, p171.3, and pC56.1) and cos9a as the DNA templates. DNA sequencing of the capsule gene subclones p29.1, p108.1, p137.1, p171.1, and pC56.1 was initially with a TnphoA′ fusion joint primer (5′ AATATCGCCCTGAGC 3′), which established the location for a given TnphoA insertion. Sequencing of capsule gene subclones p108.3 and p171.3 was initially with the TnphoA primer (5′ CATGTTAGGAGGTCACAT 3′). Subsequent DNA sequence was determined with primers derived from the deduced sequences of the capsule gene subclones or the cosmid cos9a. A consensus sequence was generated by assembling and editing the DNA sequence obtained from 76 overlapping but independent sequencing reactions with AssemblyLIGN 1.0.2 (Oxford Molecular Group, Beaverton, Oreg.). Both strands of the capsule gene sequence submitted in this report were sequenced. The organization of the assembled subclone sequences and that of the cosmid cos9a sequence were in agreement. Sequence analysis, comparisons, and CLUSTAL alignments were performed, in part with MacVector (version 6.0; Oxford Molecular Group). Comparisons were also performed via BLAST analysis of the nonredundant GenBank, EMBL, DDBJ, and PDB sequences. Percentages of similarity and identity were determined by the GAP program of the Wisconsin Sequence Analysis Packages (Genetics Computer Group, Madison, Wis.). The PROSITE database was used for motif searches (2). SignalP V1.1 was used for identification of signal sequences (28). A terminator sequence search was performed by the method of Brendel and Trifonov adapted for the Wisconsin Sequence Analysis Packages (TERMINATOR) (5).
Capsule loci based on XbaI DNA fragments.
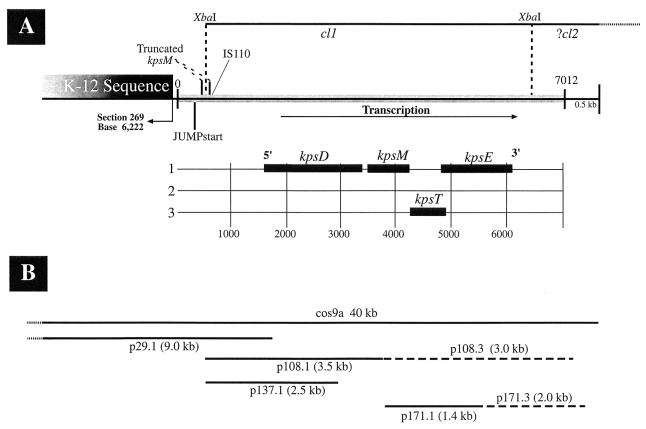
We previously reported, based on Southern analysis of pulsed-field gel electrophoretically separated DNA from CP9 and TnphoA-generated isogenic capsule-minus derivatives, that the K54 capsule genes were located on at least three different XbaI DNA fragments (capsule loci [cl] 1, 2, and 3). These fragments were linked within the transducing range of bacteriophage T4 (100 to 150 kb) (42). cl1 was estimated to be a 10.3-kb fragment, and strains CP9.29, CP9.108, CP9.137, CP9.171, and CP9.C56 had insertions within this locus. Sequence analysis from the present study confirmed this finding and indicated that cl1 was approximately 6.0 kb (Fig. 1). The fragment identified as cl3 and containing the TnphoA insertion in CP9.C54 was proven by sequence analysis to also be cl1. CP9.C54 has subsequently been shown to contain a truncated form of TnphoA, a finding which led to the incorrect interpretation that cl1 and cl3 were separate loci. cl2 was an estimated 18.5 kb, and strain CP9.C43 had a TnphoA insertion within this locus. Sequence analysis has demonstrated that the TnphoA insertion, responsible for the K54− phenotype in CP9.C43, is in a novel DNA sequence which has no identifiable homology with any known capsule gene. This sequence is not part of the K54 capsule gene cluster reported here (Fig. 1). This data, in conjunction with the information described below, suggests that the TnphoA insertion in CP9.C43 and cl2 are located 3′ to the end of kpsK54E (bp 6132).
FIG. 1.
(A) Schematic diagram of the K54 group III capsule gene sequence described in this study. From left to right are (i) the sequence homologous with the K-12 genome and its intersection (section 269, bp 6222) with a sequence unique to CP9 (90° arrow); (ii) bp 0 to 345, which are 85 to 90% homologous to the 5′ noncoding region of kpsK1,5M (including the JUMPstart site as marked); (iii) a truncated kpsM (bp 346 to 476 and 501 to 526 are designated kpsMtruncated) that is 85 to 90% homologous to the corresponding region of kpsK1,5M; (iv) an IS110 element (bp 581 to 626) 53 bp from the 3′ end of kpsMtruncated; and (v) the shaded region marked from 0 to 7012, representing the capsule gene sequence submitted in this report. The region from bp 627 to 1644 is unidentified but is probably capsule gene sequence. This region is followed by kpsK54DMTE, with their respective ORFs and reading frames depicted below. The 0.9 kb 3′ to kpsK54E (bp 6133 to 7012) plus 0.5 kb is unidentified K54 capsule gene sequence. Prior to sequence analysis, the K54 cl were defined as XbaI fragments (44). The defined location of cl1 (6.0 kb) and the presumed location of cl2 are marked above. (B) The lines represent various inserts of subclones used for sequence analysis and promoter localization. Insert sizes are as marked. Length is proportional, and location corresponds to the schematic diagram above. The insert in p29 consists of the first 154 bp of kpsK54D and an 8.9-kb region 5′ to the start of kpsK54D. The insert in p108 contains the first half of kpsK54M, all of kpsK54D, and 1.2 kb 5′ to kpsK54D (bp 452 to 3878). The insert in p137 consists of two-thirds of kpsK54D and the 1.2 kb 5′ to it (bp 452 to 2801). The insert in p171 (bp 3894 to 5288) covers the first half of kpsK54E, all of kpsK54T, and the last half of kpsK54M. The dotted lines at the leftward boundaries of cos9a and p29.1 represent extension into K-12 homologous sequence beyond what is depicted above.
Southern analysis.
Whole-cell DNA was prepared as described previously (42) and restricted with AccI as suggested by the manufacturer (New England Biolabs, Beverly, Mass.). Southern hybridization was performed as described previously (42) with the following modifications. A Robbins Scientific model 1000 hybridization oven was used. Salmon sperm DNA (180 μl of 150 μg/ml stock) was added to 10 ml of prehybridization solution, which was removed and replaced with 10 ml of hybridization solution (5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]-1% sodium dodecyl sulfate). An oligonucleotide was made from the ISCP9110 sequence (bp 581 to 626), labelled with [γ-32P]dATP with T4 polynucleotide kinase according to the manufacturer’s instructions (Gibco BRL, Gaithersburg, Md.), and used as a probe. After hybridization at 65°C for 18 h, the blot was washed once at 65°C with 1× SSC-0.1% sodium dodecyl sulfate for 3 min, followed by five washes at 25°C with 6× SSC-1% Sarkosyl for 5 min.
Alkaline phosphatase assays.
Alkaline phosphatase assays were performed as previously described except that a Beckman DU 640B spectrophotometer was used to record the hydrolysis rates of p-nitrophenyl phosphate (46). The baseline activity of CP9 is negligible and therefore was not accounted for in this calculation. PhoA activity from each of the measured constructs represents the mean of five independent evaluations.
Nucleotide sequence accession number.
The accession no. of the nucleotide sequence shown in Fig. 2 is AF007777.
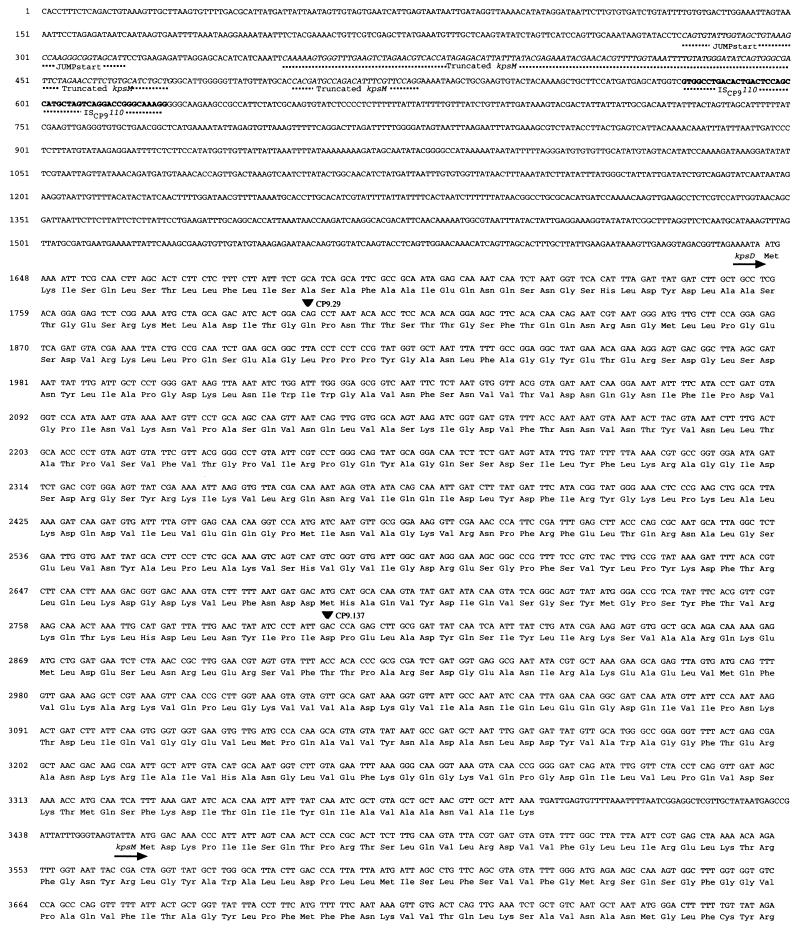
FIG. 2.
Nucleotide sequence and deduced amino acid sequence of kpsK54D, kpsK54M, kpsK54T, and kpsK54E. Arrows identify putative transcriptional start sites, solid triangles identify the insertion site of active TnphoA fusions, the open triangle identifies the insertion site of an inactive TnphoA fusion, and the underlined regions identify the inverted repeats of a strong theoretical rho-independent RNA polymerase terminator. The JUMPstart site, the truncated kpsM (kpsMtruncated), and ISCP9110 are marked and identified by the dotted lines.
RESULTS AND DISCUSSION
Location, organization, and analysis of the K54 capsule gene locus.
K54-deficient strains generated by TnphoA insertion mutagenesis (42) were used to identify clones carrying the group III capsule genes (see Materials and Methods), which were in turn used to identify a cosmid (pcos9a) carrying the wild-type genes. Sequencing of the region and identification of the sites of TnphoA insertion were carried out. The K54 group III capsule gene locus is depicted in Fig. 1. As expected, since group II or III capsule gene sequences have not been detected in E. coli K-12, a search of GenBank did not identify any DNA or protein homology of this capsule gene locus with the deposited E. coli K-12 sequence. Novel loci of unique DNA not present in laboratory strains of E. coli have been termed “pathogenicity islands,” and this sequence likely represents a portion of such a locus. One of the two boundaries of this novel CP9 DNA sequence with E. coli sequence from the K-12 genome was established (Fig. 1). The boundary was contiguous with the third base (bp 6222, section 269, accession no. AE000379) of a 178-amino-acid open reading frame (ORF) (bp 6220 to 6756) of unknown identity from the complete E. coli K-12 genome. Interestingly, this novel CP9 sequence is 150 bp 3′ to the phenylalanine tRNA (bp 5996 to 6071). The points of insertion of several pathogenicity islands are within various tRNAs (15). This location was consistent with the genetic linkage of the K54 capsule genes with serA (32), and its point of insertion is identical with that of the K5 capsule gene cluster.
Four homologs of group II capsule transport genes were recognized in the K54 capsule gene locus described in this study (Fig. 1). kpsK54D (bp 1645 to 3387, Fig. 2), kpsK54M (bp 3457 to 4254, Fig. 2), kpsK54T (bp 4269 to 4916, Fig. 2), and kpsK54E (bp 4888 to 6132, Fig. 2) were identified. However, while in group II capsule gene loci, kpsD and kpsE are in region 1 along with four other genes (kpsFEDUCS), and kpsMT are in region 3, in the CP9 (K54) capsule gene locus, these four genes are grouped together (kpsDMTE). These findings demonstrate that the organization of the K54 capsule transport genes in CP9 is unequivocally different from that of the corresponding regions in strains with group II capsule genes (39). Further, this data confirms the prediction, from complementation studies, that functional homologs of kpsK5D and kpsK5E existed in the K10 and K54 capsule gene clusters (36).
The putative molecular weights, estimated pIs, guanosine-plus-cytosine content, and presence or absence of an identifiable Shine-Dalgarno or signal sequence of kpsK54DMTE and their comparison with kpsK1,5DMTE are summarized in Table 2 (9, 34, 35, 49, 54). The guanosine-plus-cytosine content of kpsK54DMTE ranged from 37 to 43%, compared to the 51% observed for E. coli K-12, and suggested that these genes were acquired by horizontal transfer from an unknown species. A nucleic acid subsequence analysis program and a manual search failed to identify any highly conserved Shine-Dalgarno sequences. The reason for this is unknown. However, since these genes are in essence “foreign DNA,” perhaps their mRNAs possess sequence elements with complementarity to parts of the 16S rRNA that are distinct from those recognized by Shine-Dalgarno sequences, which in turn serve as translational enhancers.
TABLE 2.
Comparisons of putative molecular weight, pI, and GC content and the presence or absence of Shine-Dalgarno and signal sequences between kpsK54DMTE and kpsK1,5DMTE
| Gene and region | Mol wt (in thousands) | Estimated pI | % GC | Presence or absence of sequencea
|
|
|---|---|---|---|---|---|
| Shine-Dalgarno | Signal | ||||
| kpsK54D | 63.9 | 6.29 | 40 | − | + |
| kpsK1D | 60 | 7.33 | 51 | + | + |
| kpsK5D | 60 | 8.37 | 52 | + | + |
| kpsK54M | 30.2 | 10.04 | 38 | − | − |
| kpsK1M | 29.6 | 9.22 | 43 | + | − |
| kpsK5M | 29.5 | 9.06 | 45 | + | − |
| kpsK54T | 24.5 | 9.36 | 37 | − | − |
| kpsK1T | 24.9 | 9.29 | 35 | + | − |
| kpsK5T | 25.5 | 8.73 | 39 | + | − |
| kpsK54E | 47.3 | 8.64 | 43 | − | − |
| kpsK1E | 39.0 | 5.0 | 50 | + | − |
| kpsK5E | 43.0 | 6.02 | 50 | + | − |
+, present; −, absent. The presence or absence of Shine-Dalgarno and signal sequences was determined by MacVector version 6.0 and PSORT, respectively.
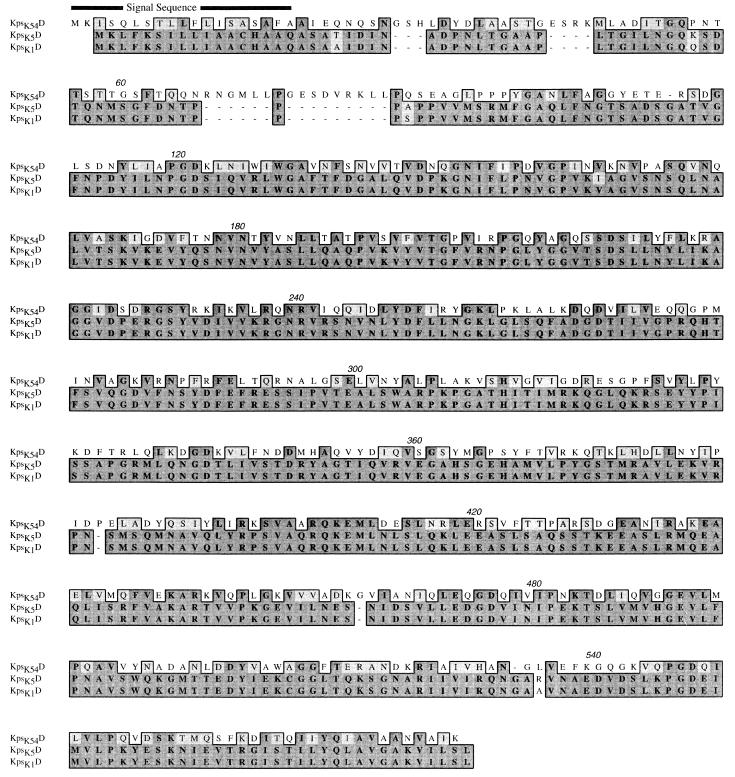
The ORF (ORF1) from bp 1645 to 3387 encoded kpsK54D. It is 50% homologous at the nucleotide level and has 33% identity and 54% similarity at the predicted protein level with the group II genes kpsK1,5D. The limited homology with GumB (16% identity, 32% similarity) and OtnA (17% identity, 32% similarity) may represent common functional regions involved with transport (4). The identification of a putative signal sequence (Fig. 3), the presence of active TnphoA fusions within kpsK54D, a hydrophilic hydropathy profile, and secondary-structure predictions similar to those of KpsK1,5D (data not shown) suggest that KpsD has a periplasmic location and a function similar to that of its group II counterpart, despite sequence divergence.
FIG. 3.
CLUSTAL alignment of the predicted amino acid sequences of E. coli kpsK54D (this study), kpsK1D, and kpsK5D. The boxed sequence identifies amino acid residues that are functionally similar (lighter shading) or identical (darker shading). Numbers above the sequences are residue numbers. The predicted signal sequence of KpsK54D is identified.
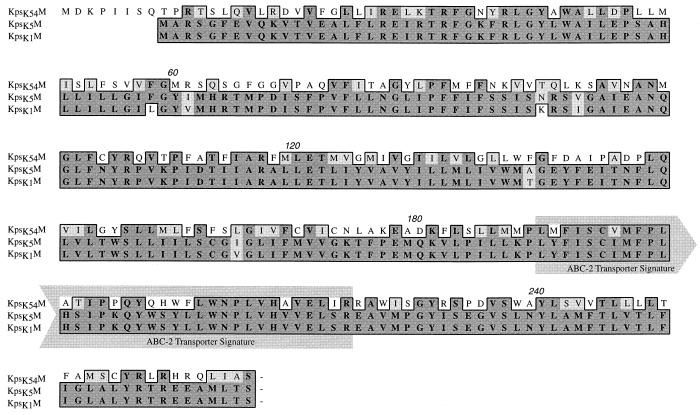
ORF2 (bp 3457 to 4254) encoded kpsK54M. No significant homology was detected at the nucleotide level; however, it has 39% identity and 52% similarity at the predicted protein level with the group II genes kpsK1,5M. Other homologs in Actinobacillus pleuropneumoniae (CpxB), N. meningitidis (CtrC), H. influenzae (BexB), and Salmonella typhi (VexB) revealed amino acid identities from 23 to 24% and similarities from 37 to 34%. All of these homologs have been implicated as the integral membrane component of the ABC-2 transporters of capsular polysaccharide across the cytoplasmic membrane (1, 18, 37). In kpsK54M, the identification of the ABC-2 transporter system integral membrane protein signature (Fig. 4), a similar hydropathy profile (hydrophobic protein with six transmembrane regions), and secondary-structure predictions similar to those of KpsK1,5M (data not shown) support the notion that KpsK54M is also a member of this family.
FIG. 4.
CLUSTAL alignment of the predicted amino acid sequences of E. coli kpsK54M (this study), kpsK5M, and kpsK1M. The boxed sequence identifies amino acid residues that are functionally similar (lighter shading) or identical (darker shading). The ABC-2 transporter system integral membrane protein signature is marked and corresponds to amino acid residues 190 to 224 (1, 18). Numbers above the sequences are residue numbers.
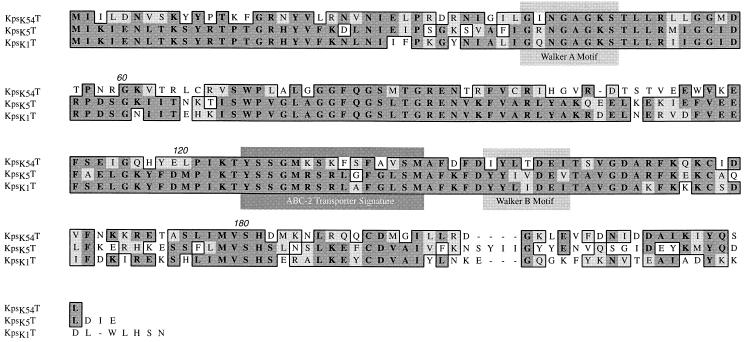
ORF3 (bp 4269 to 4916) encoded kpsK54T. It is 65 to 62% homologous at the nucleotide level and has 51 to 45% identity and 66 to 62% similarity at the predicted protein level with the group II genes kpsK1,5T. Other homologs in A. pleuropneumoniae (CpxA), N. meningitidis (CtrD), H. influenzae (BexA), and S. typhi (VexC) showed amino acid identities from 47 to 24% and similarities of 59 to 36%. These proteins are the peripheral ATP-binding components of the ABC-2 transporter protein family. The identification of Walker motifs (Fig. 5), an ABC transporter signature sequence, a similar hydrophilic hydropathy profile, and secondary-structure predictions similar to those of KpsK1,5T support the notion that KpsK54T is also a member of the ABC-2 transporter family, and its structure seems conserved in comparison with that of KpsK1,5T.
FIG. 5.
CLUSTAL alignment of the predicted amino acid sequences of E. coli kpsK54T (this study), kpsK5T, and kpsK1T. The boxed sequence identifies amino acid residues that are functionally similar (lighter shading) or identical (darker shading). The ATP-binding domain Walker A (residues 38 to 45), and Walker B (residues 145 to 151) motifs (34) and the ABC-2 transporter signature sequence (residues 125 to 139) are marked (1, 18). Numbers above the sequences are residue numbers.
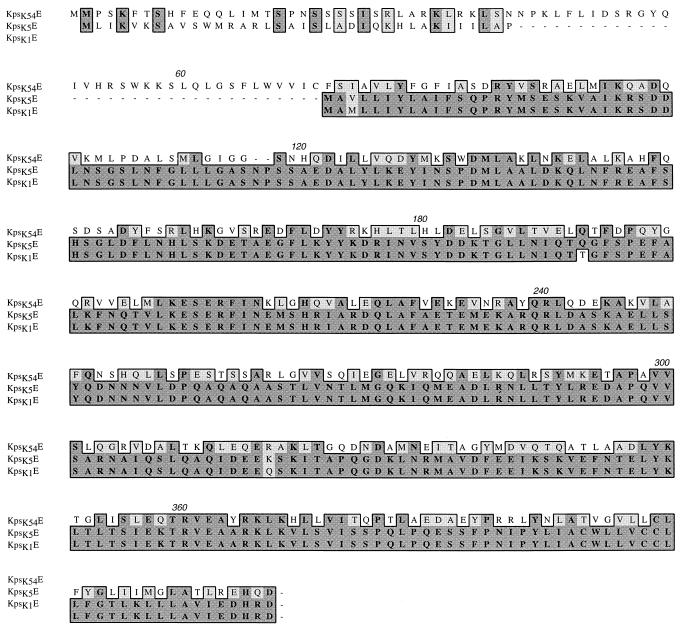
ORF4 (bp 4888 to 6132) encoded kpsK54E. No significant homology was detected at the nucleotide level; however, it has 31% identity and 46% similarity at the predicted protein level with the group II genes kpsK1,5E. Other homologs in A. pleuropneumoniae (CpxC), N. meningitidis (CtrB), H. influenzae (BexC), and S. typhi (VexD) showed amino acid identities from 27 to 20% and similarities from 40 to 32%. Analysis of the putative KpsK54E protein via hydropathy profiles and secondary-structure predictions suggests that this protein is similar to the membrane fusion protein family (4, 11). These proteins are believed to interact with ABC-type transport proteins (and others) and perhaps outer membrane proteins to facilitate substrate transport of large molecules. KpsK54E has a number of features of this family, including (i) a hydrophilic amino terminus located in the cytoplasm (amino acids 1 to 60 of KpsK54E with an excess of basic over acidic residues of a net +11), (ii) a hydrophobic amino terminus region that may both span and anchor the protein in the cytoplasmic membrane, (iii) a hydrophilic, largely alpha-helical periplasmic region (supported by the presence of active TnphoA fusions at amino acid residue 136), and (iv) a hydrophobic carboxy terminus. However, the KpsK54E hydrophobic carboxy terminus is significantly smaller than in the membrane fusion protein family, and the conservation of residues in this region is absent. KpsK1,5E possess a similar predicted structure (4) except that KpsK1E has a deletion of amino acid residues 1 to 71 and KpsK5E has a deletion of residues 37 to 71 (Fig. 6).
FIG. 6.
CLUSTAL alignment of the predicted amino acid sequences of E. coli kpsK54E (this study), kpsK1E, and kpsK5E. The boxed sequence identifies amino acid residues that are functionally similar (lighter shading) or identical (darker shading). Numbers above the sequences are residue numbers.
Based on DNA and protein homologies, the sequence from bp 1645 to 6132 clearly comprised genes involved in K54 transport. Located 5′ to the end of this cluster is 1.0 kb (bp 626 to 1644) of sequence which has no identifiable DNA or protein homology. No TnphoA insertions have been mapped to this region. A 369-bp ORF was identified from bp 1247 to 1615. Whether this ORF or another sequence in this 1.0-kb region codes for products involved with capsule transport or synthesis is unclear.
However, 5′ to this region, a sequence homologous to the initial 20% of kpsM and its entire 5′ noncoding regulatory region (0.35 kb) was identified. The sequence homologous to the 5′ coding region of kpsM (bp 346 to 476 and 501 to 526) has been designated kpsMtruncated (Fig. 1 and 2). Over the entire kpsMtruncated sequence and its 5′ noncoding regulatory region, an 85 to 90% DNA sequence homology to its K1 and K5 counterparts was observed. Previous investigators, using Southern analysis, did not identify any regions of homology with a variety of strains that contained group III capsule gene clusters (including a different K54 serotype) when probes containing kpsK5M were used (12, 36). The reasons for this discrepancy are unclear. However, the degree of homology of kpsMtruncated with kpsK1,5M was notable, since the complete kpsK54M possessed no significant homology at the nucleotide level to kpsK1,5M. This finding suggested the possibility that CP9 originally possessed a group II capsule gene locus and that the K54 capsule genes were subsequently acquired by horizontal transfer. Therefore, the DNA sequence immediately 3′ to kpsMtruncated was analyzed for potential insight into the evolution of this group III capsule gene locus. A 51-bp region (bp 581 to 626) identified 53 bp 3′ to the end of kpsMtruncated (Fig. 1 and 2) was 75% homologous with the IS110 insertion sequence identified from Streptomyces coelicolor. The area of homology coincided with a 39-bp inverted repeat that has been identified within this element (8) and the 12 bp 5′ to the repeat (bp 1073 to 1122, accession no. Y00434). Runs of cytosines appear to be the target site for this element, and the 11-bp region 5′ to this element contained two runs of cytosines (5′ CCCGTTTCCCCC 3′). These findings were consistent with the hypothesis that CP9 previously possessed group II capsule genes and acquired group III capsule genes via IS110-mediated horizontal transfer. A second prediction of this model would be the presence of a second ISCP9110 element at the 3′ end of the transferred segment. Southern analysis of AccI-restricted chromosomal DNA isolated from CP9 and the closely related strain J96 (23) was performed under high-stringency conditions, with the ISCP9110 element used as an oligonucleotide probe. Two copies of the ISCP9110 element were detected in the chromosomes of CP9 and J96 on identically sized restriction fragments (Fig. 7). One of the fragments was the size predicted from the known sequence of the K54 capsule gene cluster containing ISCP9110 (1.4 kb). Insertion elements are well-known mediators of the evolution of genomic organization, and in other studies researchers have shown their role in the horizontal transfer of genes involved in polysaccharide synthesis (3, 55). The origin of the group III genes, however, remains speculative.
FIG. 7.
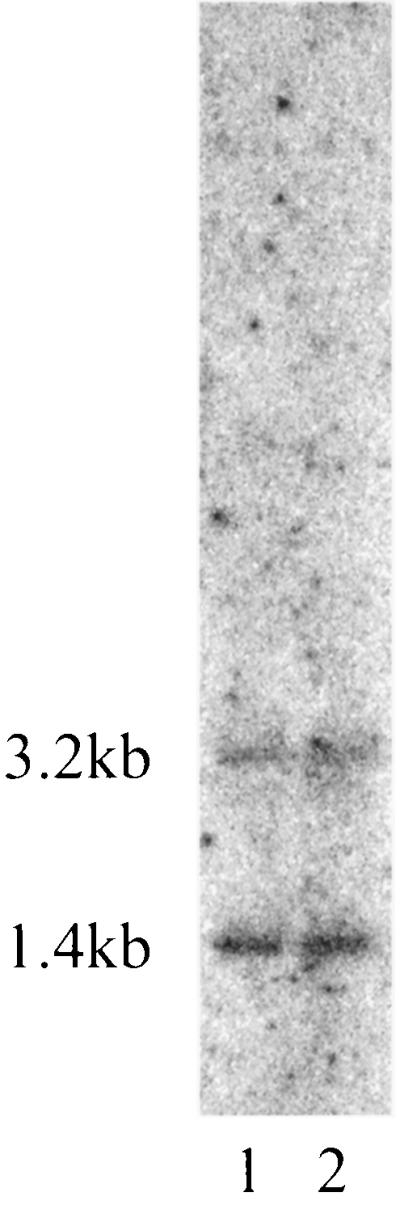
Southern analysis of DNA from CP9 and J96 to detect the copy number of ISCP9110 element. AccI-restricted whole-cell DNA from CP9 and J96 was separated by conventional electrophoresis, blotted onto nylon, and subjected to Southern analysis under high-stringency conditions as described in Materials and Methods, with the ISCP9110 element as the probe. Lane 1, J96; lane 2, CP9.
The 1.3 kb downstream from bp 6132 (bp 6133 to 7012 plus 0.5 kb 3′ to the submitted sequence) appeared, at least in part, to code for genes involved in capsule synthesis or transport. Although no homology to any known capsule genes was identified in this region, the transposon insertion in the K54 capsule-deficient strain CP9.C56 was located at bp 6179, which is 3′ to kpsK54E. Further evidence for the presence of these additional genes is suggested by the isolation of the capsule-negative strain CP9.C43 (42), which contains a TnphoA insertion in an 18.5-kb XbaI fragment, cl2 (Fig. 1). cl2 and the cl1 fragment sequenced here are cotransducible by phage T4, suggesting that the gene defined by CP9.C43 insertion and cl2 are located 3′ to the end of kpsK54E.
Transcriptional organization of the K54 capsule gene locus.
CP9.29, CP9.108, CP9.137, and CP9.171 possessed active phoA fusions with genes in the K54 capsule gene locus. The transposon insertion in CP9.29 (bp 1799) and that in CP9.137 (bp 2801) were within kpsK54D, the transposon insertion in CP9.108 (bp 3878) was within kpsK54M, and the transposon insertion in CP9.171 (bp 5288) was within kpsK54E (Fig. 1 and 2). These active fusions confirm the direction of transcription. kpsK54D and kpsK54M are separated by 69 bp, kpsK54M and kpsK54T are separated by 14 bp, and kpsK54T and kpsK54E overlap by 29 bp. No predicted promoter regions were identified. Bp 6135 to 6157, located just 3 bp 3′ to kpsK54E, were consistent with a strong theoretical rho-independent RNA polymerase terminator. These base pairs formed a hairpin structure which was followed by a 6-bp poly(T) sequence (bp 6163 to 6169). The free energy of the stem-loop (ΔG [25°C]) was calculated to be −10.2 kcal (52). The K1 K5 capsule gene cluster regions 1 and 3 are organized as a single transcription unit. The organization of these regions for the K54 genes is not yet known.
Alkaline phosphatase assays on the subclones containing active TnphoA insertions in either of two orientations were performed. In constructs in which the capsule gene insert was in the opposite orientation to the vector lacZ promoter (p29.1, p108.2, p137.2, and p171.2), PhoA activity likely reflects transcription from an insert promoter; however, the possibility of read-through transcription from a cryptic promoter cannot be excluded. In constructs with the insert in the same orientation as the lacZ promoter, PhoA activity reflects transcription from the insert and potentially the vector promoter. The similar degrees of PhoA activity seen with p29.1 (15.2 ± 1.6) (mean ± standard error) and p29.2 (17.7 ± 2.1) suggested that this insert, not the vector, contained a promoter that is responsible for its phoA gene fusion activity. The inserts in p108 and p137 do not appear to contain promoters, based on the low level of PhoA activity produced by p108.2 (0.59 ± 0.2) and p137.2 (0.61 ± 0.1). p171 may possess a region with promoter activity, since p171.2 produced 1.93 ± 0.1 U of PhoA activity. The extent of these inserts (Fig. 1) and their activity or lack of activity suggest that an essential promoter element is upstream of bp 452, which is located within kpsMtruncated. Although the insert in p29 contains about 8 kb of DNA upstream of bp 452, it seems likely that the promoter for these genes lies within the 741 bp 5′ to the initiation site for kpsMtruncated (bp 345), since the K5 region 3 promoter has been mapped to this location (50). No stem-loop terminators were identified between this region and the start of kpsK54D (bp 1645). Also present in this region is the JUMPstart sequence (bp 279 to 317), which has been implicated in the regulation of a variety of polysaccharide genes and genes encoding secreted products (19, 29). This sequence is present 5′ to region 3 in both the K1 and K5 capsule gene sequences, in the promoter-operator region of the cps genes coding for the group I colanic acid capsule (51), and in the 5′ noncoding region of several lipopolysaccharide gene clusters (19) and the hly and tra genes (29). It has been shown that this site is required for the up-regulation of hly genes and kpsK5 region 2 genes by RfaH via antitermination (29, 50). The effect of RfaH on K54 capsule gene activity has not yet been evaluated. However, in previous studies we have demonstrated that RcsA is a negative regulator of group III capsule genes, which we have now identified as kpsK54DME (46). Although the precise promoter-operator region for these group III capsule genes has not been definitively established, it is intriguing that the JUMPstart site is present within both the insert in p29 and the promoter-operator region of the cps genes encoding for the group I colanic acid capsule. Further, RcsA is a positive regulator of group I capsule production and interacts with the promoter-operator region of the cps genes (51). Since RcsA divergently regulates the group 1 colanic acid and the K54 capsular polysaccharides, it is tempting to speculate that this regulation is mediated by RcsA either via direct interaction with the JUMPstart site or by interaction with protein complexes assembled there.
In summary, the genomic location, the novel organization of a portion of the group III, K54 capsule gene cluster, and the DNA sequences of kpsK54DMTE have been determined. Despite a divergence of these genes at the nucleotide and protein levels, analysis suggests that their function is similar to that of described homologs. Further, this study lends insight into the phylogenetic evolution of a group III capsule gene cluster. Findings support the hypothesis that CP9 previously possessed group II capsule genes and acquired group III capsule genes via IS110-mediated horizontal transfer. Future studies will be focused on the remaining genes in the K54 capsule gene cluster, in particular those that do not possess homology to reported capsule genes. The products of these genes may function differently from those responsible for group II capsule expression.
ACKNOWLEDGMENTS
This work was supported by Research for Health in Erie County (T.A.R.) and the Office of Research and Development, Department of Veterans Affairs (A.J.L.).
We appreciate the continued support of Tim Murphy.
REFERENCES
- 1.Ames G F-L, Mimura C S, Holbrook S R, Shyamala V. Traffic ATPases: a superfamily of transport proteins operating from Escherichia coli to humans. Adv Enzymol. 1992;65:1–47. doi: 10.1002/9780470123119.ch1. [DOI] [PubMed] [Google Scholar]
- 2.Bairoch A, Bucher P, Hofmann K. The PROSITE database, its status in 1995. Nucleic Acids Res. 1995;24:189–196. doi: 10.1093/nar/24.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bik E M, Bunschoten A E, Gouw R D, Mooi F. Genesis of the novel epidemic Vibrio cholerae O139 strain: evidence for horizontal transfer of genes involved in polysaccharide synthesis. EMBO J. 1995;14:209–216. doi: 10.1002/j.1460-2075.1995.tb06993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliss J M, Silver R P. Coating the surface: a model for expression of capsular polysialic acid in Escherichia coli K1. Mol Microbiol. 1996;21:221–231. doi: 10.1046/j.1365-2958.1996.6461357.x. [DOI] [PubMed] [Google Scholar]
- 5.Brendel V, Trifonov E N. A computer algorithm for testing potential prokaryotic terminators. Nucleic Acids Res. 1984;12:4411–4427. doi: 10.1093/nar/12.10.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronner D, Sieberth V, Pazzani C, Roberts I S, Boulnois G J, Jann B, Jann F. Expression of the capsular K5 polysaccharide of Escherichia coli: biochemical and electron microscopic analyses of mutants with defects in region 1 of the K5 gene cluster. J Bacteriol. 1993;175:5984–5992. doi: 10.1128/jb.175.18.5984-5992.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bronner D, Sieberth V, Pazzani C, Smith A N, Boulnois G J, Roberts I S, Jann B, Jann F. Synthesis of the K5 (group II) capsular polysaccharide in transport-deficient recombinant Escherichia coli. FEMS Microbiol Lett. 1993;113:279–284. doi: 10.1111/j.1574-6968.1993.tb06527.x. [DOI] [PubMed] [Google Scholar]
- 8.Bruton C J, Chater K F. Nucleotide sequence of IS110, an insertion sequence of Streptomyces coelicolor A3(2) Nucleic Acids Res. 1987;15:7053–7065. doi: 10.1093/nar/15.17.7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cieslewicz M J, Steenbergen S M, Vimr E R. Cloning, sequencing, expression, and complementation analysis of the Escherichia coli K1 kps region 1 gene, kpsE, and identification of an upstream open reading frame encoding a protein with homology to GutQ. J Bacteriol. 1993;175:8018–8023. doi: 10.1128/jb.175.24.8018-8023.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross A S. The biologic significance of bacterial encapsulation. Curr Top Microbiol Immunol. 1990;150:87–95. doi: 10.1007/978-3-642-74694-9_5. [DOI] [PubMed] [Google Scholar]
- 11.Dinh T, Paulsen I T, Saier M H., Jr A family of extracytoplasmic proteins that allow transport of large molecules across the outer membranes of gram-negative bacteria. J Bacteriol. 1994;176:3825–3831. doi: 10.1128/jb.176.13.3825-3831.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drake C R, Boulnois G J, Roberts I S. The Escherichia coli ser-A-linked capsule locus and its flanking sequences are polymorphic, genetic evidence of more than two groups of capsule gene clusters. J Gen Microbiol. 1993;139:1707–1714. doi: 10.1099/00221287-139-8-1707. [DOI] [PubMed] [Google Scholar]
- 13.Drake C R, Roberts I S, Jann B, Jann K, Boulnois G J. Molecular cloning and expression of the genes encoding the Escherichia coli K4 capsular polysaccharide, a fructose-substituted chondroitin. FEMS Microbiol Lett. 1990;66:227–230. doi: 10.1016/0378-1097(90)90287-z. [DOI] [PubMed] [Google Scholar]
- 14.Finke A, Jann B, Jann K. CMP-KDO synthetase activity in Escherichia coli expressing capsular polysaccharide. FEMS Microbiol Lett. 1990;69:129–134. doi: 10.1016/0378-1097(90)90426-q. [DOI] [PubMed] [Google Scholar]
- 15.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godoy V G, Dallas M M, Russo T A, Malamy M H. A role for Bacteroides fragilis neuraminidase in bacterial growth in two model systems. Infect Immun. 1993;61:4415–4426. doi: 10.1128/iai.61.10.4415-4426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadad J J, Gyles C L. The role of K antigens of enteropathogenic Escherichia coli in colonization of the small intestine of calves. Can J Comp Med. 1982;46:21–26. [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 19.Hobbs M, Reeves P. The JUMPstart sequence: a 39 bp element common to several polysaccharide gene clusters. Mol Microbiol. 1994;12:855–856. doi: 10.1111/j.1365-2958.1994.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 20.Jann B, Jann K. Structure and biosynthesis of the capsular antigens of Escherichia coli. Curr Top Microbiol Immunol. 1990;150:19–42. doi: 10.1007/978-3-642-74694-9_2. [DOI] [PubMed] [Google Scholar]
- 21.Jann K, Jann B. Polysaccharide antigens of Escherichia coli. Rev Infect Dis. 1987;9:S517–S526. doi: 10.1093/clinids/9.supplement_5.s517. [DOI] [PubMed] [Google Scholar]
- 22.Jann K, Jann B. Capsules of Escherichia coli, expression and biologic significance. Can J Microbiol. 1992;38:705–710. doi: 10.1139/m92-116. [DOI] [PubMed] [Google Scholar]
- 23.Johnson J R, et al. Discovery of a disseminated J96-like clone of uropathogenic Escherichia coli O4:H5 containing both papGJ96 (“Class I”) and prsGJ96 (“Class III”) Gal(alpha1-4)Gal-binding adhesion sequences. J Infect Dis. 1997;175:983–988. doi: 10.1086/514006. [DOI] [PubMed] [Google Scholar]
- 24.Johnson J R, Stapleton A E, Russo T A, Scheutz F, Brown J J, Maslow J N. Characteristics and prevalence within serogroup O4 of a J96-like clonal group of uropathogenic Escherichia coli O4:H5 containing the class I and class III alleles of papG. Infect Immun. 1997;65:2153–2159. doi: 10.1128/iai.65.6.2153-2159.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keenleyside W J, Jayaratne P, MacLachlan P R, Whitfield C. The rcsA gene of Escherichia coli O9:K30:H12 is involved in the expression of the serotype-specific group I K (capsular) antigen. J Bacteriol. 1992;174:8–16. doi: 10.1128/jb.174.1.8-16.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moxon E R, Kroll J S. The role of bacterial polysaccharide capsules as virulence factors. Curr Top Microbiol Immunol. 1990;150:65–85. doi: 10.1007/978-3-642-74694-9_4. [DOI] [PubMed] [Google Scholar]
- 27.Nagy B, Moon H W, Isaacson R E. Colonization of porcine small intestine by Escherichia coli: ileal colonization and adhesion by pig enteropathogens that lack K88 antigen and by some acapsular mutants. Infect Immun. 1976;13:1214–1220. doi: 10.1128/iai.13.4.1214-1220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of procaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Nieto J M, Bailey M J A, Hughes C, Koronakis V. Suppression of transcription polarity in the Escherichia coli haemolysin operon by a short upstream element shared by polysaccharide and transfer determinants. Mol Microbiol. 1996;19:705–713. doi: 10.1046/j.1365-2958.1996.446951.x. [DOI] [PubMed] [Google Scholar]
- 30.Opir T, Gutnick D L. A role for exopolysaccharides in the protection of microorganisms from desiccation. Appl Environ Microbiol. 1994;60:740–745. doi: 10.1128/aem.60.2.740-745.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orskov F, Orskov I, Jann B, Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977;41:667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orskov I, Nyman K. Genetic mapping of the antigenic determinants of two polysaccharide K antigens, K10 and K54, in Escherichia coli. J Bacteriol. 1974;120:43–51. doi: 10.1128/jb.120.1.43-51.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orskov I, Sharina V, Orskov F. Genetic mapping of the K1 and K4 antigens (L) of Escherichia coli. Acta Pathol Microbiol Scand Sect B. 1976;84:125–131. [PubMed] [Google Scholar]
- 34.Pavelka M S, Wright L F, Silver R P. Identification of two genes, kpsM and kpsT, in region 3 of the polysialic acid gene cluster of Escherichia coli K1. J Bacteriol. 1991;173:4603–4610. doi: 10.1128/jb.173.15.4603-4610.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pazzani C, Rosenow C, Boulnois G J, Bronner D, Jann K, Roberts I S. Molecular analysis of region 1 of the Escherichia coli K5 antigen gene cluster: a region encoding proteins involved in cell surface expression of capsular polysaccharide. J Bacteriol. 1993;175:5978–5983. doi: 10.1128/jb.175.18.5978-5983.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearce R, Roberts I S. Cloning and analysis of gene clusters for production of the Escherichia coli K10 and K54 antigens: identification of a new group of serA-linked capsule gene clusters. J Bacteriol. 1995;177:3992–3997. doi: 10.1128/jb.177.14.3992-3997.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pigeon R P, Silver R P. Topological and mutational analysis of KpsM, the hydrophobic component of the ABC-transporter involved in the export of polysialic acid in Escherichia coli K1. Mol Microbiol. 1994;14:871–881. doi: 10.1111/j.1365-2958.1994.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 38.Roberts I, Mountford R, High N, Bitter-Suermann D, Jann K, Timmis K, Boulnois G. Molecular cloning and analysis of genes for production of K5, K7, K12, and K92 capsular polysaccharides in Escherichia coli. J Bacteriol. 1986;168:1228–1233. doi: 10.1128/jb.168.3.1228-1233.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts I S. The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu Rev Microbiol. 1996;50:285–315. doi: 10.1146/annurev.micro.50.1.285. [DOI] [PubMed] [Google Scholar]
- 40.Roberts I S, Mountford R, Hodge R, Jann K B, Boulnois G J. Common organization of gene clusters for production of different capsular polysaccharides (K antigens) in Escherichia coli. J Bacteriol. 1988;170:1305–1310. doi: 10.1128/jb.170.3.1305-1310.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo T A, Brown J J, Jodush S T, Johnson J R. The O4 specific antigen moiety of lipopolysaccharide but not the K54 group 2 capsule is important for urovirulence of an extraintestinal isolate of Escherichia coli. Infect Immun. 1996;64:2343–2348. doi: 10.1128/iai.64.6.2343-2348.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russo T A, Guenther J E, Wenderoth S, Frank M M. Generation of isogenic K54 capsule-deficient Escherichia coli strains through TnphoA-mediated gene disruption. Mol Microbiol. 1993;9:357–364. doi: 10.1111/j.1365-2958.1993.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 43.Russo T A, Liang Y, Cross A S. The presence of K54 capsular polysaccharide increases the pathogenicity of Escherichia coli in vivo. J Infect Dis. 1994;169:112–118. doi: 10.1093/infdis/169.1.112. [DOI] [PubMed] [Google Scholar]
- 44.Russo T A, Moffitt M C, Hammer C H, Frank M M. TnphoA-mediated disruption of K54 capsular polysaccharide genes in Escherichia coli confers serum sensitivity. Infect Immun. 1993;61:3578–3582. doi: 10.1128/iai.61.8.3578-3582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russo T A, Sharma G, Weiss J, Brown C. The construction and characterization of colanic acid deficient mutants in an extraintestinal isolate of Escherichia coli (O4/K54/H5) Microb Pathog. 1995;18:269–278. doi: 10.1016/s0882-4010(05)80003-8. [DOI] [PubMed] [Google Scholar]
- 46.Russo T A, Singh G. An extraintestinal, pathogenic isolate of Escherichia coli (O4/K54/H5) can produce a group 1 capsule which is divergently regulated from its constitutively produced group 2, K54 capsular polysaccharide. J Bacteriol. 1993;175:7617–7623. doi: 10.1128/jb.175.23.7617-7623.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silver R P, Finn C W, Vann W F, Aaronson W, Schneerson R, Kretschmer P J, Garon C F. Molecular cloning of the K1 capsular polysaccharide genes of E. coli. Nature. 1981;289:696–698. doi: 10.1038/289696b0. [DOI] [PubMed] [Google Scholar]
- 49.Smith A N, Boulnois G J, Roberts I S. Molecular analysis of the Escherichia coli K5 kps locus: identification and characterization of an inner-membrane capsular polysaccharide transport system. Mol Microbiol. 1990;4:1863–1869. doi: 10.1111/j.1365-2958.1990.tb02035.x. [DOI] [PubMed] [Google Scholar]
- 50.Stevens M P, Clarke B R, Roberts I S. Regulation of the Escherichia coli K5 capsule gene cluster by transcription antitermination. Mol Microbiol. 1997;24:1001–1012. doi: 10.1046/j.1365-2958.1997.4241780.x. [DOI] [PubMed] [Google Scholar]
- 51.Stout V. Identification of the promoter region for the colanic acid polysaccharide biosynthetic genes in Escherichia coli K-12. J Bacteriol. 1996;178:4273–4280. doi: 10.1128/jb.178.14.4273-4280.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tinoco I, Borer P N, Dengler B, Levine M D, Uhlenbeck O C, Crothers D M, Gralla J. Improved estimation of secondary structure in ribonucleic acids. Nature. 1973;246:40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- 53.Vimr E R. Map position and genome organization of the kps cluster for polysialic acid biosynthesis in Escherichia coli K1. J Bacteriol. 1991;173:1335–1338. doi: 10.1128/jb.173.3.1335-1338.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wunder D E, Aaronson W, Hayes S F, Bliss J M, Silver R P. Nucleotide sequence and mutational analysis of the gene encoding KpsD, a periplasmic protein involved in transport of polysialic acid in Escherichia coli K1. J Bacteriol. 1994;176:4025–4033. doi: 10.1128/jb.176.13.4025-4033.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao S, Sandt C H, Feulner G, Vlazny D A, Gray J A, Hill C W. Rhs elements of Escherichia coli K-12: complex composites of shared and unique components that have different evolutionary histories. J Bacteriol. 1993;175:2799–2808. doi: 10.1128/jb.175.10.2799-2808.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]