Abstract
Background and Objectives
Amyotrophic lateral sclerosis (ALS) is a rare neurodegenerative disorder. Familial (fALS) cases are usually reported to constitute 5%–10% of all ALS cases; however, no recent literature review or meta-analysis of this proportion (referred to throughout as “proportion fALS”) has been conducted. Our objective was to estimate the proportion fALS by geographic region and to assess the effect of study characteristics on the estimates.
Methods
A comprehensive literature review was performed to identify all original studies reporting the number of fALS cases in an ALS cohort. The results were stratified by geographic region, study design (case series or population-based), and decade of study publication. Subgroup analyses were conducted according to family history criteria used to define fALS. We report pooled estimates of the proportion fALS from random-effects meta-analyses when >2 studies are available and I2 is < 90%; weighted averages and ranges are otherwise presented.
Results
The overall pooled proportion fALS based on a total 165 studies was 8% (0%, 71%). The proportion fALS was 9% (0%, 71%) among 107 case series and 5% (4%, 6%) among 58 population-based studies. Among population-based studies, proportion fALS by geographic region was 6% (5%, 7%; N = 37) for Europe, 5% (3%, 7%; N = 5) for Latin America, and 5% (4%, 7%; N = 12) for North America. Criteria used to define fALS were reported by 21 population-based studies (36%), and proportion fALS was 5% (4%, 5%; N = 9) for first-degree relative, 7% (4%, 11%; N = 4) for first or second-degree relative, and 11% (N = 1) for more distant ALS family history. Population-based studies published in the 2000s or earlier generated a lower pooled proportion fALS than studies published in the 2010s or later.
Discussion
The results suggest that variability in the reported proportion fALS in the literature may be, in part, due to the differences in geography, study design, fALS definition, and decade of case ascertainment. Few studies outside of European ancestral populations were available. The proportion fALS was marginally higher among case series compared with population-based studies, likely because of referral bias. Criteria used to define fALS were largely unreported. Consensus criteria for fALS and additional population-based studies in non-European ancestral populations are needed.
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder characterized by the progressive deterioration of motor neurons, which affects voluntary movement. Based on summary meta-analyses across the literature, ALS is estimated to be newly diagnosed in 2.02 (95% confidence interval [CI] 1.76–2.31) cases per 100,000 person-years.1 The incidence rate (per 100,000 person-years) varies substantially according to region: 2.35 (95% CI 1.75–3.15) for North America, 2.31 (95% CI 2.08–2.55) for Europe, 1.25 (95% CI 0.54–2.89) for Latin America, and 0.93 (95% CI 0.57–1.51) for Asia.1
Most ALS cases (90%–95%) are believed to occur sporadically (sporadic ALS [sALS]) while 5%–10% report known family history of the disease (familial ALS [fALS]).2 Extensive heterogeneity in study designs across the literature has lessened the ability to confidently reach conclusions about the proportion fALS among patients with ALS. A 2011 meta-analysis of 34 studies found the pooled proportion fALS to be 4.6% (95% CI 3.9–5.5).3 Despite important variation in ALS incidence according to geographic region, pooled estimates of fALS proportion were not presented according to geographic region. In the decade since the publication of this meta-analysis, studies have reported higher proportions of fALS (7%–17%), emphasizing the need for further research.4-11 Understanding the true proportion of ALS cases with reported family histories, and the reasons for variation in such estimates reported in the literature, may contribute to an improved understanding of the genetic etiology of ALS and the usefulness of the fALS vs sALS classification system. Distinguishing fALS vs sALS is not possible based on clinical presentation alone and instead relies on detailed family history. Even with this information, false reporting can occur for several reasons, including misdiagnosis and early death of relatives who would have developed ALS.12-14 Moreover, the binary classification of fALS vs sALS ignores the complexities of ALS genetics.15 For example, low gene penetrance and recessive transmission that may occur in some ALS-associated mutations can result in the apparent lack of family history.12,13,15,16 Furthermore, the probability of a mutation-carrying family having only one or none of its members affected, given less than complete penetrance, is dependent on family size.17 These situations may result in erroneous labeling of patients as sporadic, when family inheritance occurred. Alternatively, classification of cases as familial in the absence of genetic inheritance can occur if multiple family members are affected because of random chance or shared environmental risk factors (e.g., heavy metals), although this evidence remains inconclusive.18-20 For this reason, it has been proposed that “definite fALS” should be considered with at least 2 affected family members or clear evidence of genetic inheritance, reserving “probable fALS” for those with one affected first or second-degree relative.12 “Possible fALS” allows for ALS family history in more distant relatives.12 Notwithstanding, there is currently no consensus regarding a preferred definition of fALS for use in epidemiologic studies.21 As a result, studies may define fALS based on family history of ALS only or may allow for family history of alternative neurodegenerative disorders or incorporate genetic testing, introducing difficulties for synthesizing the literature. Moreover, it has been reported that less than 10% of studies reporting the proportion fALS provide a definition of the criteria used to differentiate fALS and sALS.3 It is vital to consider whether studies provide clear definitions of fALS and what those definitions are, to better understand the true population fALS proportion.
In addition to discrepancies in how fALS is defined, the estimated fALS proportions in the literature may also vary because of study design features. The proportion fALS from meta-analytic estimates has been reported to be higher among studies with recruitment based on a sequential series of cases (5.1%, 95% CI 3.4–7.1) compared with population-based studies (4.5%, 95% CI 3.8–5.3).3 It has been suggested that cohorts of patients with ALS drawn from hospital databases may be subject to referral bias because patients in these settings are more likely to have affected family members than the general population.22 Consideration of patient recruitment methods is essential for understanding heterogeneity in estimates of proportion fALS.
There may also be a temporal trend in the estimated fALS proportions in the literature, for example, due to improvements in ALS case ascertainment, diagnostic criteria, and fALS classification over time.14,23-25 In addition, distributions of population age and environmental risk factors and average family size may have changed over time, which are expected to affect observed fALS proportion.17,23-25 These considerations suggest the importance of considering the period that a study's fALS proportion represents.
The objective of this comprehensive literature review and meta-analysis was to estimate the proportion of ALS cases that are familial (henceforth referred to as “proportion fALS”) by geographic region. We explore variation in the proportion fALS because of study design (population-based registry or case series), fALS definition (family history of ALS in a first-degree, second-degree, or more distant relative), and publication decade.
Methods
Literature Review
We initiated our sample by including all studies identified in the 2011 meta-analysis.3 These studies were identified through a MEDLINE search from 1966 to October 2009 with the following MeSH terms: ‘ALS,’ ‘amyotrophic lateral sclerosis,’ ‘fALS,’ ‘familial amyotrophic lateral sclerosis,’ ‘familial motor neuron(e) disease,’ ‘motor neuron(e) disease,’ ‘MND,’ ‘incidence,’ ‘prevalence,’ and ‘mortality.’ To update this study list, PubMed and EMBASE were searched from January 1990 to August 2021 by a medical librarian using identical search terms. Additional sources within the same scope were sought from references of the identified articles.
Studies eligible for inclusion were those presenting original data on a defined ALS cohort that adequately described enrollment methods and included enough information, published in the main text or supplemental, to calculate the proportion fALS at the individual level (i.e., studies presenting only counts of fALS pedigrees in a population were excluded). Estimation of the proportion fALS did not necessarily need to be the objective of the study. Study-level ALS diagnostic criteria may have included other motor neuron diseases (e.g., spinal muscular atrophy, progressive muscular atrophy, and primary lateral sclerosis); sensitivity analyses in which these studies were excluded are described in more detail below. Abstracts and unpublished studies were not included. Articles published in languages other than English were translated. Contact with corresponding authors was attempted for articles with ambiguity in the proportion fALS that were otherwise eligible for inclusion.
Studies were excluded for the following reasons: (1) They lacked sufficient description of participant enrollment to allow for determination of whether enrollment was population-based or plausibly based on a series of clinic or hospital-based cases, (2) they based enrollment on selection of a special group of patients with ALS (e.g., patients with fALS, juvenile ALS, and genetic mutations), (3) they followed separate procedures to recruit fALS and sALS cases and would not be expected to represent an accurate ratio of fALS to sALS in an underlying source population, and (4) they obtained data on ALS cases from a biobank because it is expected that the fALS distribution may not be representative of the population-level distribution.
Analytic Approach
Details regarding case ascertainment, diagnostic criteria, region, and fALS definition were extracted by a single researcher and independently reviewed by a second researcher; discrepancies were resolved by consensus. In each study, the proportion fALS is reported as the number of fALS cases among all ALS cases. Studies were grouped according to region to produce pooled estimates of the proportion fALS. We report a summary for each region detailing the included studies with their countries of origin, total number of ALS cases, and total number of fALS cases in eTable 1 (links.lww.com/NXG/A646).
If a region included 3 or more eligible studies reporting the proportion fALS, a meta-analytic fALS summary proportion and the corresponding 95% confidence interval were calculated. Meta-analyses were conducted according to random-effects models, which were fit using the R package “metafor” with restricted maximum-likelihood estimators. Random-effect meta-analytic estimates are presented for regions for which the I2 heterogeneity statistic value was found to be <90%; otherwise, weighted averages and ranges are presented as a descriptive summary because of substantial heterogeneity.26 If a region included 2 eligible studies reporting the proportion fALS in a defined cohort, a weighted average of the estimates was calculated for the pooled estimate and the range is presented as the interval. Otherwise, if a given region only had a single eligible study, the single study's estimate is presented without an interval.
Because we suspected that important study characteristics might affect the estimated proportion fALS, we stratified our results based on study design (population-based or case series), family history criteria used to define fALS, and publication decade. A study was considered population-based if the procedures were expected to capture all ALS cases over a specified period in a defined population. Studies that otherwise recruited a collection of cases, such as a clinic or hospital-based series of cases, were classified as case series. Studies that did not explicitly report the criteria used to define fALS, including those that stated a requirement for “family history” without any further detail regarding the degree of relatives considered, were considered not clear and were excluded from the family history–defined subgroup analysis. The remaining studies were grouped according to whether the fALS definition was based on family history of ALS only, allowed for family history of alternative neurodegenerative disorders (e.g., frontotemporal dementia), or incorporated confirmed genetic diagnoses. Studies that operationalized fALS based on family history of ALS alone were further divided based on the degree of ALS-affected relatives: (1) first-degree, (2) first or second-degree, or (3) first or second-degree or more distant. First-degree relatives include parents, full siblings, and children. Second-degree relatives include grandparents, grandchildren, uncles, aunts, nephews, nieces, and half siblings. Subgroup pooled estimates are only presented for the definitions based on degree of family history of ALS, partially because of substantial variability in estimates among the other subgroups. Publication decade was used as a proxy for the time of case diagnosis. Studies published before 1990 were included in a single group because of the small number of studies from each prior decade.
Meta-regression
To further explore the potential sources of heterogeneity described above, we conducted meta-regression analyses. Meta-regression applies regression techniques to study-level data to discern whether a linear relationship exists between the reported outcome measure and the covariate(s) of interest. Meta-regressions were conducted using generalized linear mixed-effects models, using logit transformation to model proportion fALS with a random intercept for each study. The models were fit using the R package “lme4.” Analyses were conducted among all studies and then repeated among population-based studies. Univariate models separately included study type, region, family history criteria used to define fALS, and publication decade as fixed effects. We additionally explored average family size as a fixed predictor, which was estimated based on average fertility rate (number of children per woman) in the country of publication 20 years before the end of study data collection.27 Multivariate models included multiple fixed effects. We quantitatively described the variance explained by the fixed predictor(s) by calculating the percent change in tau2 estimate of between-study variance when adding the predictor(s) to the model with no covariates.
Sensitivity Analysis
In the main analyses, ALS diagnostic criteria may include other motor neuron diseases (e.g., spinal muscular atrophy, progressive muscular atrophy, primary lateral sclerosis, and progressive supranuclear palsy), cases were required to be reported as either fALS or sALS, and fALS classification criteria may include confirmed ALS-associated genetic variants. We conducted a sensitivity analysis in which more stringent criteria to define ALS were applied, such that analyses were restricted to studies only including “pure ALS” cases (i.e., no diagnoses for alternative motor neuron diseases). A second sensitivity analysis was conducted in which study-level estimates were altered such that all cases of ALS without reported fALS or sALS status were included as sALS cases. A third sensitivity analysis was conducted in which we excluded studies allowing patients with confirmed genetic mutations to be categorized as fALS given the shared genetic architecture of fALS and sALS.28,29
Data Availability
Data not provided in the article because of space limitations may be shared at the request of any qualified investigator for purposes of replicating procedures and results.
Results
Literature Review
Byrne et al. described 34 studies eligible for inclusion and published between 1966 and October 2009.3 We included 31 of these studies in our analytic sample. The 3 remaining studies were excluded because of lack of information regarding family history or overlap with data from a more recent study.30-32
Our comprehensive literature search identified 6,816 articles. An additional 150 sources were identified from review of identified articles for a total of 6,966 studies. Those deemed ineligible based on a review of titles and abstracts were excluded (N = 6,450). Exclusions at this stage included review articles, studies not involving human subjects and/or ALS populations, studies restricted to either fALS or sALS cases, studies not reporting data for fALS, and case studies. The remaining 366 articles underwent in-depth full-text review. The final analytic sample included 165 study estimates (from 160 articles), 58 (35%) of which identified cases based on population-based methods and 107 (65%) based on case series. Details of the included studies are included in eTable 1.
Of the 56 studies that reported a clear definition of their criteria for fALS, 13 (23%) restricted family history of ALS to first-degree relatives only, 14 (25%) restricted to first or second-degree relatives, and 8 (14%) allowed for more distant generations. In addition, 8 studies (14%) allowed patients with confirmed genetic mutations to be categorized as fALS and 1 study (2%) required family history of ALS in multiple relatives. Finally, 12 studies (21%) also considered family history of other neurologic diseases in the categorization of fALS.
Meta-analysis
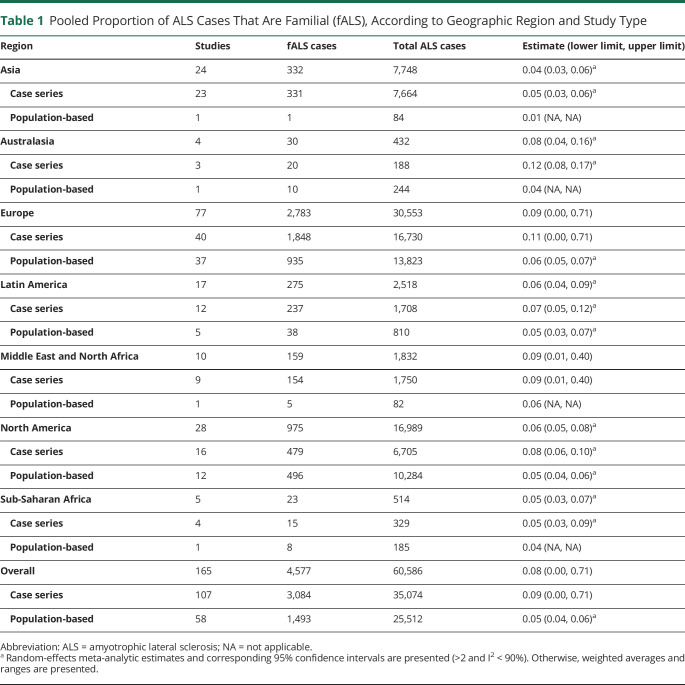
The details regarding number of studies by region and the pooled estimate with its corresponding interval (lower limit and upper limit from random-effects meta-analysis when >2 studies are available and I2 < 90%; otherwise minimum and maximum) are provided in Table 1. All meta-analytic estimates and I2 values are reported in eTable 2 (links.lww.com/NXG/A646), and forest plots are available in eFigure 1 (case series) and eFigure 2 (population-based). The overall summary proportion fALS across all 165 studies was 0.08 (interval 0.00, 0.71). The proportion fALS varied according to region. Europe had the highest proportion of fALS (0.09, interval 0.00, 0.71), followed by the Middle East and North Africa (0.09, interval 0.01, 0.40) and Australasia (0.08, interval 0.04, 0.16). The lowest proportion fALS was observed in Asia (0.04, interval 0.03, 0.06) and Sub-Saharan Africa (0.05, interval 0.03, 0.07). Substantial variability in estimates of proportion fALS remained at the regional level, with most I2 values being >90%.
Table 1.
Pooled Proportion of ALS Cases That Are Familial (fALS), According to Geographic Region and Study Type
| Region | Studies | fALS cases | Total ALS cases | Estimate (lower limit, upper limit) |
| Asia | 24 | 332 | 7,748 | 0.04 (0.03, 0.06)a |
| Case series | 23 | 331 | 7,664 | 0.05 (0.03, 0.06)a |
| Population-based | 1 | 1 | 84 | 0.01 (NA, NA) |
| Australasia | 4 | 30 | 432 | 0.08 (0.04, 0.16)a |
| Case series | 3 | 20 | 188 | 0.12 (0.08, 0.17)a |
| Population-based | 1 | 10 | 244 | 0.04 (NA, NA) |
| Europe | 77 | 2,783 | 30,553 | 0.09 (0.00, 0.71) |
| Case series | 40 | 1,848 | 16,730 | 0.11 (0.00, 0.71) |
| Population-based | 37 | 935 | 13,823 | 0.06 (0.05, 0.07)a |
| Latin America | 17 | 275 | 2,518 | 0.06 (0.04, 0.09)a |
| Case series | 12 | 237 | 1,708 | 0.07 (0.05, 0.12)a |
| Population-based | 5 | 38 | 810 | 0.05 (0.03, 0.07)a |
| Middle East and North Africa | 10 | 159 | 1,832 | 0.09 (0.01, 0.40) |
| Case series | 9 | 154 | 1,750 | 0.09 (0.01, 0.40) |
| Population-based | 1 | 5 | 82 | 0.06 (NA, NA) |
| North America | 28 | 975 | 16,989 | 0.06 (0.05, 0.08)a |
| Case series | 16 | 479 | 6,705 | 0.08 (0.06, 0.10)a |
| Population-based | 12 | 496 | 10,284 | 0.05 (0.04, 0.06)a |
| Sub-Saharan Africa | 5 | 23 | 514 | 0.05 (0.03, 0.07)a |
| Case series | 4 | 15 | 329 | 0.05 (0.03, 0.09)a |
| Population-based | 1 | 8 | 185 | 0.04 (NA, NA) |
| Overall | 165 | 4,577 | 60,586 | 0.08 (0.00, 0.71) |
| Case series | 107 | 3,084 | 35,074 | 0.09 (0.00, 0.71) |
| Population-based | 58 | 1,493 | 25,512 | 0.05 (0.04, 0.06)a |
Abbreviation: ALS = amyotrophic lateral sclerosis; NA = not applicable.
Random-effects meta-analytic estimates and corresponding 95% confidence intervals are presented (>2 and I2 < 90%). Otherwise, weighted averages and ranges are presented.
When stratified by study design, the proportion fALS was higher among case series (0.09, interval 0.00, 0.71) compared with population-based studies (0.05, interval 0.04, 0.06). This observation was consistent at the regional level (Table 1). For most regions, a meaningful difference in the I2 value from case series vs population-based studies was not observed. However, substantially greater variability was observed in case series vs population-based studies from North America (88% vs 74%) and from Latin America (88% vs 18%).
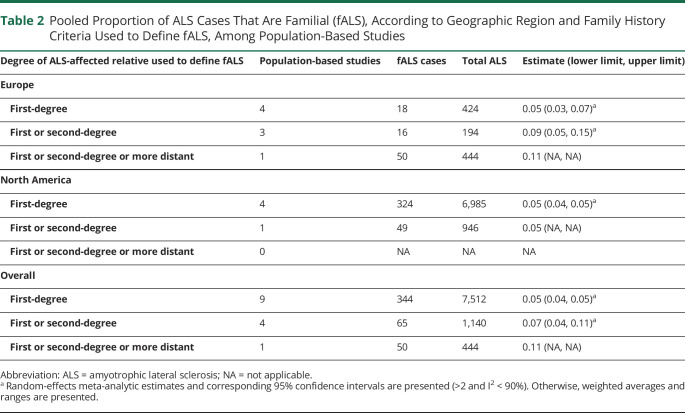
Owing to the observed variability in the proportion fALS according to study design, subgroup estimates according to family history criteria used to define fALS were only computed among population-based studies (Table 2). As expected, population-based studies defining fALS according to first or second-degree or more distant family history of ALS generated a higher pooled proportion fALS (0.11, based on a single study) compared with studies restricting to family history within the first or second degree (0.07, interval 0.04, 0.11) or only the first degree (0.05, interval 0.04, 0.05). We present regional-level fALS proportions according to family history criteria used to define fALS for Europe and North America only because these were the only regions with population-based studies in the 3 categories of degree of family history. Grouping studies by family history criteria used to define fALS resulted in substantially reduced heterogeneity, as evidenced by all I2 values being <50%.
Table 2.
Pooled Proportion of ALS Cases That Are Familial (fALS), According to Geographic Region and Family History Criteria Used to Define fALS, Among Population-Based Studies
| Degree of ALS-affected relative used to define fALS | Population-based studies | fALS cases | Total ALS | Estimate (lower limit, upper limit) |
| Europe | ||||
| First-degree | 4 | 18 | 424 | 0.05 (0.03, 0.07)a |
| First or second-degree | 3 | 16 | 194 | 0.09 (0.05, 0.15)a |
| First or second-degree or more distant | 1 | 50 | 444 | 0.11 (NA, NA) |
| North America | ||||
| First-degree | 4 | 324 | 6,985 | 0.05 (0.04, 0.05)a |
| First or second-degree | 1 | 49 | 946 | 0.05 (NA, NA) |
| First or second-degree or more distant | 0 | NA | NA | NA |
| Overall | ||||
| First-degree | 9 | 344 | 7,512 | 0.05 (0.04, 0.05)a |
| First or second-degree | 4 | 65 | 1,140 | 0.07 (0.04, 0.11)a |
| First or second-degree or more distant | 1 | 50 | 444 | 0.11 (NA, NA) |
Abbreviation: ALS = amyotrophic lateral sclerosis; NA = not applicable.
Random-effects meta-analytic estimates and corresponding 95% confidence intervals are presented (>2 and I2 < 90%). Otherwise, weighted averages and ranges are presented.
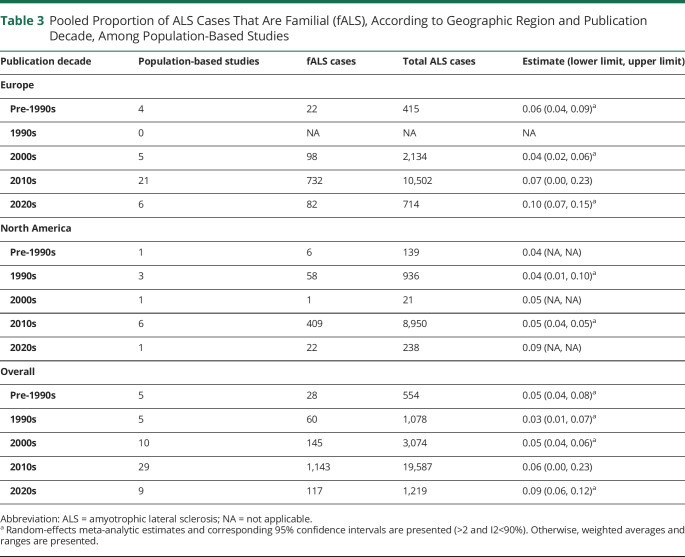
Subgroup estimates according to publication decade were only computed among population-based studies because of observed variability in proportion fALS according to study design (Table 3). Population-based studies published in the 2000s or earlier generated a lower pooled proportion fALS than studies published in the 2010s or later (1990s: 0.03, interval 0.01, 0.07; 2020s: 0.09, interval 0.06, 0.12). We present regional-level fALS proportions according to publication decade for Europe and North America only because these were the only regions with population-based studies in all decades. Heterogeneity was substantially reduced when studies were grouped by publication decade, as evidenced by most I2 values being <65%. Heterogeneity remained considerable among studies published in the 2010s, which was the decade during which most population-based studies (50%) were published.
Table 3.
Pooled Proportion of ALS Cases That Are Familial (fALS), According to Geographic Region and Publication Decade, Among Population-Based Studies
| Publication decade | Population-based studies | fALS cases | Total ALS cases | Estimate (lower limit, upper limit) |
| Europe | ||||
| Pre-1990s | 4 | 22 | 415 | 0.06 (0.04, 0.09)a |
| 1990s | 0 | NA | NA | NA |
| 2000s | 5 | 98 | 2,134 | 0.04 (0.02, 0.06)a |
| 2010s | 21 | 732 | 10,502 | 0.07 (0.00, 0.23) |
| 2020s | 6 | 82 | 714 | 0.10 (0.07, 0.15)a |
| North America | ||||
| Pre-1990s | 1 | 6 | 139 | 0.04 (NA, NA) |
| 1990s | 3 | 58 | 936 | 0.04 (0.01, 0.10)a |
| 2000s | 1 | 1 | 21 | 0.05 (NA, NA) |
| 2010s | 6 | 409 | 8,950 | 0.05 (0.04, 0.05)a |
| 2020s | 1 | 22 | 238 | 0.09 (NA, NA) |
| Overall | ||||
| Pre-1990s | 5 | 28 | 554 | 0.05 (0.04, 0.08)a |
| 1990s | 5 | 60 | 1,078 | 0.03 (0.01, 0.07)a |
| 2000s | 10 | 145 | 3,074 | 0.05 (0.04, 0.06)a |
| 2010s | 29 | 1,143 | 19,587 | 0.06 (0.00, 0.23) |
| 2020s | 9 | 117 | 1,219 | 0.09 (0.06, 0.12)a |
Abbreviation: ALS = amyotrophic lateral sclerosis; NA = not applicable.
Random-effects meta-analytic estimates and corresponding 95% confidence intervals are presented (>2 and I2<90%). Otherwise, weighted averages and ranges are presented.
Meta-regression
Detailed results of the meta-regression analyses are presented in eTable 3 (links.lww.com/NXG/A646) (all studies) and eTable 4 (population-based). Study type, region, family history definition, publication decade, and average family size each explained <10% of between-study heterogeneity among all studies. Among population-based studies, family history definition and publication decade together explained substantial between-study heterogeneity, in that the tau2 was reduced by 24%.
Sensitivity Analysis
In the main analysis, ALS cases with missing/unknown family history information were excluded from 10 studies. Results from a sensitivity analysis in which these cases were reincluded as sALS were consistent with the main analysis. Similarly, a sensitivity analysis that excluded studies in which ALS diagnostic criteria allowed for diagnosis of alternative motor neuron diseases also produced results consistent with the main analysis. Finally, a sensitivity analysis in which 8 studies allowing patients with confirmed genetic mutations to be classified as fALS were excluded produced slightly lower overall proportion fALS estimates among all studies (0.07 vs 0.08) and case series (0.08 vs 0.09), driven by the change in European studies (0.08 vs 0.09 for all studies; 0.10 vs 0.11 for case series); heterogeneity remained >90%. Population-based and other regional results remained unchanged.
Discussion
The proportion of ALS cases that are of familial, rather than sporadic, origin is commonly cited as 5%–10%. Our analysis suggests that observed variability in the reported proportion fALS in the literature may be, in part, due to differences in region, study design, definition of fALS, and decade of case ascertainment. The pooled proportion fALS among ALS cases was observed to be 9% according to studies in which participant recruitment was based on a clinic or hospital-based series of cases, but was only 5% according to population-based studies. Notably, these overall pooled results are driven by the large number of publications (47%) with data derived from Europe. Notwithstanding, when examining pooled estimates at the regional level, a higher proportion fALS among case series vs population-based studies was consistently observed. Meaningful difference in meta-analytic estimates of the proportion fALS according to study design has previously been described, although the reported difference (5.1% for case series vs 4.5% for population-based) was not as pronounced as we found.3 Population-based studies (which make up the minority of this literature) are expected to more accurately capture the true proportion of ALS cases that are familial, given that a series of cases from clinics or hospitals may be inadvertently enriched by fALS cases.22 Evidence from population-based registers, however, should not be used without careful consideration of potential biases (e.g., shifts in demography, increased awareness, “startup bias” in newly established registers, and “information creep” in registers of longer duration).33,34
Our study also explores global geographic variability in the proportion of fALS. Substantial variability across regions was observed, providing support for potential differences in underlying genetic structure, distribution of environmental factors, clinical practices related to fALS assessment, and average family size in these populations.17,35,36 Among population-based studies, the proportion fALS was highest for Europe (6%) and the Middle East and North Africa (6%), followed by North America (5%) and Latin America (5%). It is important to note that our population-based estimate for Europe is based on more available literature (37 studies) than Latin American (5 studies) and the Middle East and North Africa (1 studies). Furthermore, the Latin American estimates may be affected by founder effects, which have been described in the literature in this region for various neurodegenerative disorders.37-39 The lowest pooled, population-based proportion fALS was from Asia (1%), although only one study was available. Lower incidence and prevalence rates of ALS in Asia compared with Europe and North America have previously been reported, which may affect ALS incidence within families.1,40-42 Furthermore, because the proportion fALS is dependent on family size, it is worth noting that this population-based estimate is derived from China and expected to be affected by China's historic one-child policy.17
Our analysis also demonstrates that variation in the reported proportion fALS is partly attributable to study-level differences in the operational definition of fALS. There is currently no consensus regarding a preferred definition of fALS among clinicians, although it has been suggested that the optimal classification system should reserve naming a “definite” fALS case based on the presence of at least 2 affected family members or clear evidence of genetic inheritance.12,21 “Possible fALS” may also incorporate cases with a first-degree relative with frontotemporal dementia because of the overlap in phenotype and genotype of these disorders.12,43-45 Additional neuropsychiatric disorders (e.g., all-type dementia and schizophrenia) are also genetically linked to ALS, suggesting that incorporation of these disorders in an extended fALS definition may be important for capturing familial aggregation related to ALS.14,46 Moreover, it has been suggested that the binary classification of ALS cases as fALS vs sALS is an “oversimplification” because of the complexities of genetic pleiotropy, as well as oligogenic and polygenic inheritance patterns that have been documented in ALS, including in apparently sporadic cases.47 Even in cases with familial inheritance, incomplete gene penetrance and recessive transmission may result in the apparent lack of family history.12,13,16 We observed that the minority (34%) of studies provided a clear fALS. Approximately 60% of these studies based their definition on family history of ALS within an explicitly stated number of generations while the remaining allowed for family history of other neurologic diseases or confirmed genetic mutations. To our knowledge, this is the first study to comprehensively examine the proportion fALS according to a gradient of family history criteria used to define fALS in the literature. Although it may have been preferable to examine the proportion fALS according to the “definite,” “probable,” or “possible” categorizations described above, the level of detail provided in the literature did not allow for this analysis. We observed, as expected, that the pooled proportion of fALS among population-based studies using the most stringent family history criteria was substantially lower compared with those using more lenient family history criteria. We observed, importantly, that I2 heterogeneity statistic values were substantially reduced for subgroups based on the family history criteria compared with all population-based studies, suggesting this as a potentially important source of heterogeneity.
Our analysis also demonstrates variation in the proportion fALS in the literature according to publication decade, which has not previously been described. We observed a positive temporal trend in proportion fALS, likely due to changes in ALS case ascertainment and diagnostic criteria, enhanced disease understanding, fALS classification (including changes the classification criteria themselves, as well as changes in incidence and recognition of family histories of ALS-related phenotypes), distribution of population age and environmental risk factors, and average family size over time14,17,23-25,34 Collapsing across several decades of published studies on fALS proportion to create a single summary estimate may, therefore, not be appropriate.14 It is important to note that publication decade was used as a proxy for period of case ascertainment because many studies did not provide these details. For studies that did provide information on case ascertainment years, case diagnosis often spanned multiple decades, but fALS proportion information was not presented with the level of granularity that would allow stratification by time.
This analysis has several limitations. We observed meaningful variability within regions, even after accounting for study design methods, as evidenced by many I2 values being greater than 90%. Random-effects meta-analyses and weighted averages were calculated as central values, causing us to collapse across potentially meaningful within-region variability. It is important to note that, in the presence of substantial between-study heterogeneity, weighted averages are presented as a descriptive summary of our findings and do not necessarily represent any population-based measure. Further variations in study features, beyond study design and fALS definition features examined here, may have contributed to observed variation in the proportion fALS. Unfortunately, we were limited by the shortcomings of the literature, which included a lack of detailed reporting on additional features that may have affected proportion fALS variability. For example, diagnostic criteria varied widely across studies, such that some studies defined ALS cases according to the El Escorial criteria, to varying degrees (i.e., definite only; definite or probable; definite, probable, or suspect), while others used the Awaji criteria or their own institution-defined criteria.48-50 In addition, we observed inconsistency in whether investigators excluded all, or some subset of, other neuromuscular disorders. To investigate the effect of including patients with other neuromuscular disorders in the cohort on the proportion fALS, we conducted a sensitivity analysis in which we restricted our analysis to studies that explicitly reported the exclusion of all other diagnoses from the ALS case group. Although we did not observe a meaningful difference in the results, we recognize that detailed information on ALS diagnostic criteria was not always reported by authors, and therefore, discrimination of studies based on this feature was not always possible.
We also observed a lack of clarity regarding inclusion of multiple cases from a given fALS pedigree. In some studies, the authors referred to fALS cases as being “unrelated,” but it was not necessarily clear whether this was an intentional feature of recruitment or an incidental occurrence. Similarly, some studies referred to cases as “index cases,” a term typically used in genetics literature to refer to the first affected case in a family, but it is unclear whether use of the term was consistent with this meaning. We found that most studies (60%) did not comment on whether fALS cases were related. We acknowledge this as a potentially meaningful source of variability in the literature and expect that those studies that restrict to one individual with fALS per family would underestimate the true, individual-level population proportion fALS.
In addition to these concerns, additional unrecognized study flaws could have biased or affected representativeness of study-level estimates of the proportion fALS, which may have affected our summary estimates. For the purposes of this study, we included all studies that did not explicitly report any method that would affect representativeness relative to the total population of patients with ALS. It is possible, however, that some studies may have failed to disclose certain recruitment features that hinder representativeness. It is also important to note that current understanding of fALS is dependent on current practices of reporting and genetic testing, which may change over time.
Despite these recognized limitations, this study contributes to an improved understanding of factors that affect variability in reports of the proportion of ALS cases that are of familial vs sporadic origin in the epidemiologic literature, namely geographic region, study design, operational definition of fALS, and publication decade. Future identification of ALS cases, especially fALS cases, with an underlying genetic etiology may benefit from increased genetic testing to address limitations in estimating proportion fALS based on unstandardized family history information alone.
Glossary
- ALS
amyotrophic lateral sclerosis
- fALS
familial amyotrophic lateral sclerosis
Appendix. Authors
| Name | Location | Contribution |
| Julie Barberio, PhD | Epidemiologic Research and Methods LLC; Rollins School of Public Health, Emory University, Atlanta, GA | Major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Cathy Lally, MSPH | Epidemiologic Research and Methods LLC, Atlanta, GA | Study concept or design; analysis or interpretation of data |
| Varant Kupelian, PhD | Biogen, Cambridge, MA | Study concept or design; analysis or interpretation of data |
| Orla Hardiman, MD | Trinity Biomedical Sciences Institute, Dublin, Ireland | Drafting/revision of the manuscript for content, including medical writing for content |
| W. Dana Flanders, MD, DSc | Epidemiologic Research and Methods LLC; Rollins School of Public Health, Emory University, Atlanta, GA | Study concept or design; analysis or interpretation of data |
Study Funding
Biogen.
Disclosure
J. Barberio: employee of Epidemiologic Research & Methods, LLC, doctoral stipend and tuition are supported by an award to Emory University from Amgen Inc; C. Lally: employee of Epidemiologic Research and Methods; V. Kuplian: employee of and holds stock/stock options in Biogen; O. Hardiman: Science Foundation Ireland grants SFI 16/RC/3948 and 20/SP/8953, consulting fees from Cytokinetics, Wave Pharmaceuticals, Orion, Biogen, Denali, Novartis and Accelsior, Editor-in-Chief of the journal ALS and Frontotemporal Degeneration; W.D. Flanders: employee of Epidemiologic Research & Methods, LLC, employee of Rollins School of Public Health. Go to Neurology.org/NG for full disclosures.
References
- 1.Brown CA, Lally C, Kupelian V, Flanders WD. Estimated prevalence and incidence of amyotrophic lateral sclerosis and SOD1 and C9orf72 genetic variants. Neuroepidemiology. 2021;55(5):342-353. doi: 10.1159/000516752 [DOI] [PubMed] [Google Scholar]
- 2.Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942-955. doi: 10.1016/s0140-6736(10)61156-7 [DOI] [PubMed] [Google Scholar]
- 3.Byrne S, Walsh C, Lynch C, et al. Rate of familial amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82(6):623-627. doi: 10.1136/jnnp.2010.224501 [DOI] [PubMed] [Google Scholar]
- 4.Andrew AS, Pioro EP, Li MF, et al. The incidence of amyotrophic lateral sclerosis in Ohio 2016-2018: the Ohio population-based ALS registry. Neuroepidemiology. 2021;55(3):196-205. doi: 10.1159/000515103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marjanović IV, Selak-Djokić B, Perić S, et al. Comparison of the clinical and cognitive features of genetically positive ALS patients from the largest tertiary center in Serbia. J Neurol. 2017;264(6):1091-1098. doi: 10.1007/s00415-017-8495-y [DOI] [PubMed] [Google Scholar]
- 6.Bartoletti-Stella A, Vacchiano V, De Pasqua S, et al. Targeted sequencing panels in Italian ALS patients support different etiologies in the ALS/FTD continuum. J Neurol. 2021;268(10):3766-3776. doi: 10.1007/s00415-021-10521-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jericó I, Elizalde-Beiras I, Pagola I, et al. Clinical features and incidence trends of amyotrophic lateral sclerosis in Navarre, Spain, 2007-2018: a population-based study. Amyotroph Lateral Scler Frontotemporal Degener. 2021;22(5-6):401-409. doi: 10.1080/21678421.2021.1891249 [DOI] [PubMed] [Google Scholar]
- 8.Cady J, Allred P, Bali T, et al. Amyotrophic lateral sclerosis onset is influenced by the burden of rare variants in known amyotrophic lateral sclerosis genes. Ann Neurol. 2015;77(1):100-113. doi: 10.1002/ana.24306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borg R, Farrugia Wismayer M, Bonavia K, et al. Genetic analysis of ALS cases in the isolated island population of Malta. Eur J Hum Genet. 2021;29(4):604-614. doi: 10.1038/s41431-020-00767-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scialò C, Novi G, Bandettini di Poggio M, et al. Clinical epidemiology of amyotrophic lateral sclerosis in Liguria, Italy: an update of LIGALS register. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17(7-8):535-542. doi: 10.1080/21678421.2016.1197942 [DOI] [PubMed] [Google Scholar]
- 11.Tarlarini C, Lunetta C, Mosca L, et al. Novel FUS mutations identified through molecular screening in a large cohort of familial and sporadic amyotrophic lateral sclerosis. Eur J Neurol. 2015;22(11):1474-1481. doi: 10.1111/ene.12772 [DOI] [PubMed] [Google Scholar]
- 12.Byrne S, Bede P, Elamin M, et al. Proposed criteria for familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2011;12(3):157-159. doi: 10.3109/17482968.2010.545420 [DOI] [PubMed] [Google Scholar]
- 13.Belzil VV, Rouleau GA. Familial ALS: less common than we think? J Neurol Neurosurg Psychiatry. 2012;83(12):1133-1133. doi: 10.1136/jnnp-2012-303127 [DOI] [PubMed] [Google Scholar]
- 14.Ryan M, Heverin M, Doherty MA, et al. Determining the incidence of familiality in ALS: a study of temporal trends in Ireland from 1994 to 2016. Neurol Genet. 2018;4(3):e239. doi: 10.1212/nxg.0000000000000239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goutman SA, Hardiman O, Al-Chalabi A, et al. Emerging insights into the complex genetics and pathophysiology of amyotrophic lateral sclerosis. Lancet Neurol. 2022;21(5):465-479. doi: 10.1016/s1474-4422(21)00414-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camu W, Khoris J, Moulard B, et al. Genetics of familial ALS and consequences for diagnosis. French ALS Research Group. J Neurol Sci. 1999;165(suppl 1):S21-S26. doi: 10.1016/S0022-510X(99)00022-2 [DOI] [PubMed] [Google Scholar]
- 17.Al-Chalabi A, Lewis CM. Modelling the effects of penetrance and family size on rates of sporadic and familial disease. Hum Hered. 2011;71(4):281-288. doi: 10.1159/000330167 [DOI] [PubMed] [Google Scholar]
- 18.Belbasis L, Bellou V, Evangelou E. Environmental risk factors and amyotrophic lateral sclerosis: an umbrella review and critical assessment of current evidence from systematic reviews and meta-analyses of observational studies. Neuroepidemiology. 2016;46(2):96-105. doi: 10.1159/000443146 [DOI] [PubMed] [Google Scholar]
- 19.Farace C, Fenu G, Lintas S, et al. Amyotrophic lateral sclerosis and lead: a systematic update. Neurotoxicology. 2020;81:80-88. doi: 10.1016/j.neuro.2020.09.003 [DOI] [PubMed] [Google Scholar]
- 20.Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013;9(11):617-628. doi: 10.1038/nrneurol.2013.203 [DOI] [PubMed] [Google Scholar]
- 21.Byrne S, Elamin M, Bede P, Hardiman O. Absence of consensus in diagnostic criteria for familial neurodegenerative diseases. J Neurol Neurosurg Psychiatry. 2012;83(4):365-367. doi: 10.1136/jnnp-2011-301530 [DOI] [PubMed] [Google Scholar]
- 22.Logroscino G, Marin B, Piccininni M, et al. Referral bias in ALS epidemiological studies. PLoS One. 2018;13(4):e0195821. doi: 10.1371/journal.pone.0195821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arthur KC, Calvo A, Price TR, Geiger JT, Chiò A, Traynor BJ. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat Commun. 2016;7(1):12408. doi: 10.1038/ncomms12408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontana A, Marin B, Luna J, et al. Time-trend evolution and determinants of sex ratio in Amyotrophic Lateral Sclerosis: a dose–response meta-analysis. J Neurol. 2021;268(8):2973-2984. doi: 10.1007/s00415-021-10464-2 [DOI] [PubMed] [Google Scholar]
- 25.Tobin K, Gilthorpe MS, Rooney J, et al. Age-period-cohort analysis of trends in amyotrophic lateral sclerosis incidence. J Neurol. 2016;263(10):1919-1926. doi: 10.1007/s00415-016-8215-z [DOI] [PubMed] [Google Scholar]
- 26.Deeks JJ, Higgins JP, Altman DG. 9.5.2 Identifying and measuring heterogeneity. In: Cochrane Handbook Syst Rev Interventions Version 2011;5. [Google Scholar]
- 27.World Bank. World Development Indicators, Fertility rate, total (births per woman). Accessed January 15, 2022. data.worldbank.org/indicator/SP.DYN.TFRT.IN.
- 28.Renton AE, Chiò A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci. 2014;17(1):17-23. doi: 10.1038/nn.3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chia R, Chiò A, Traynor BJ. Novel genes associated with amyotrophic lateral sclerosis: diagnostic and clinical implications. Lancet Neurol. 2018;17(1):94-102. doi: 10.1016/s1474-4422(17)30401-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGuire V, Longstreth W, Koepsell TD, van Belle G. Incidence of amyotrophic lateral sclerosis in three counties in western Washington state. Neurology. 1996;47(2):571-573. doi: 10.1212/wnl.47.2.571 [DOI] [PubMed] [Google Scholar]
- 31.Chiò A, Mora G, Calvo A, Mazzini L, Bottacchi E, Mutani R, PARALS. Epidemiology of ALS in Italy: a 10-year prospective population-based study. Neurology. 2009;72(8):725-731. doi: 10.1212/01.wnl.0000343008.26874.d1 [DOI] [PubMed] [Google Scholar]
- 32.Del Aguila M, Longstreth W, McGuire V, Koepsell T, Van Belle G. Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology. 2003;60(5):813-819. doi: 10.1212/01.wnl.0000049472.47709.3b [DOI] [PubMed] [Google Scholar]
- 33.Hardiman O, Al-Chalabi A, Brayne C, et al. The changing picture of amyotrophic lateral sclerosis: lessons from European registers. J Neurol Neurosurg Psychiatry. 2017;88(7):557-563. doi: 10.1136/jnnp-2016-314495 [DOI] [PubMed] [Google Scholar]
- 34.Rooney JPK, Brayne C, Tobin K, Logroscino G, Glymour MM, Hardiman O. Benefits, pitfalls, and future design of population-based registers in neurodegenerative disease. Neurology. 2017;88(24):2321-2329. doi: 10.1212/wnl.0000000000004038 [DOI] [PubMed] [Google Scholar]
- 35.Vajda A, McLaughlin RL, Heverin M, et al. Genetic testing in ALS: a survey of current practices. Neurology. 2017;88(10):991-999. doi: 10.1212/wnl.0000000000003686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan M, Zaldívar Vaillant T, McLaughlin RL, et al. Comparison of the clinical and genetic features of amyotrophic lateral sclerosis across Cuban, Uruguayan and Irish clinic-based populations. J Neurol Neurosurg Psychiatry. 2019;90(6):659-665. doi: 10.1136/jnnp-2018-319838 [DOI] [PubMed] [Google Scholar]
- 37.Pineda-Trujillo N, Apergi M, Moreno S, et al. A genetic cluster of early onset Parkinson's disease in a Colombian population. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(8):885-889. doi: 10.1002/ajmg.b.30375 [DOI] [PubMed] [Google Scholar]
- 38.Acosta-Uribe J, Aguillón D, Cochran JN, et al. A neurodegenerative disease landscape of rare mutations in Colombia due to founder effects. Genome Med. 2022;14(1):27. doi: 10.1186/s13073-022-01035-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodríguez-Labrada R, Martins AC, Magaña JJ, et al. Founder effects of Spinocerebellar Ataxias in the American continents and the Caribbean. Cerebellum. 2020;19(3):446-458. doi: 10.1007/s12311-020-01109-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiò A, Logroscino G, Traynor BJ, et al. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology. 2013;41(2):118-130. doi: 10.1159/000351153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marin B, Boumédiene F, Logroscino G, et al. Variation in worldwide incidence of amyotrophic lateral sclerosis: a meta-analysis. Int J Epidemiol 2017;46(1):57-74. doi: 10.1093/ije/dyw061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.GBD 2016 Motor Neuron Disease Collaborators. Global, regional, and national burden of motor neuron diseases 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(12):1083-1097. doi: 10.1016/s1474-4422(18)30404-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mackenzie IR, Rademakers R, Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 2010;9(10):995-1007. doi: 10.1016/s1474-4422(10)70195-2 [DOI] [PubMed] [Google Scholar]
- 44.Vance C, Al-Chalabi A, Ruddy D, et al. Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2-21.3. Brain. 2006;129(Pt 4):868-876. doi: 10.1093/brain/awl030 [DOI] [PubMed] [Google Scholar]
- 45.Renton AE, Majounie E, Waite A, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257-268. doi: 10.1016/j.neuron.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McLaughlin RL, Schijven D, van Rheenen W, et al. ; Project MinE GWAS Consortium, Schizophrenia Working Group of the Psychiatric Genomics Consortium. Genetic correlation between amyotrophic lateral sclerosis and schizophrenia. Nat Commun. 2017;8:14774. doi: 10.1038/ncomms14774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hardiman O, Al-Chalabi A, Chio A, et al. Amyotrophic lateral sclerosis. Nat Rev Dis Primers. 2017;3:17071. doi: 10.1038/nrdp.2017.71 [DOI] [PubMed] [Google Scholar]
- 48.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial "Clinical limits of amyotrophic lateral sclerosis" workshop contributors. J Neurol Sci. 1994;124(Suppl):96-107. doi: 10.1016/0022-510x(94)90191-0 [DOI] [PubMed] [Google Scholar]
- 49.Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Mot Neuron Disord. 2000;1(5):293-299. doi: 10.1080/146608200300079536 [DOI] [PubMed] [Google Scholar]
- 50.Costa J, Swash M, de Carvalho M. Awaji criteria for the diagnosis of amyotrophic lateral sclerosis:a systematic review. Arch Neurol. 2012;69(11):1410-1416. doi: 10.1001/archneurol.2012.254 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not provided in the article because of space limitations may be shared at the request of any qualified investigator for purposes of replicating procedures and results.