Abstract
Arthritis has significant adverse consequences on musculoskeletal tissues and often other organs of the body. Current methods for clinical evaluation of arthritis are suboptimal, and biomarkers that are objective and measurable indicators for monitoring of arthritis disease activity are in critical demand. Recently, total-body positron emission tomography (PET) has been developed that can collect imaging signals synchronously from the entire body at ultra-low doses and reduced scan times. These scanners have increased signal collection efficiency that overcomes several limitations of standard PET scanners in the evaluation of arthritis, and they may potentially provide biomarkers to assess local and systemic impact of the arthritis disease process. This article reviews current results from using total-body PET in the assessment of common arthritic conditions, and it outlines future opportunities and challenges.
Keywords: total-body positron emission tomography, rheumatoid arthritis, psoriatic arthritis, osteoarthritis, molecular imaging
Arthritis impacts an estimated one in four to five adults worldwide.1,2 Common arthritic conditions include rheumatoid arthritis (RA), psoriatic arthritis (PsA), and osteoarthritis (OA). Arthritis commonly results in severe functional limitations and significant societal costs (e.g., direct, indirect, intangible, and comorbid).3 Having arthritis also increases the risk of other health conditions4 and causes considerable psychosocial burden and detriment to quality of life.5 Furthermore, arthritis appears to have a significantly higher prevalence in individuals with other common health conditions such as heart disease, diabetes, and obesity,1 and some arthritic conditions such as RA and OA disproportionately affect women.6,7
Assessment of arthritis disease burden and severity and, consequently, rapid intervention with optimized treatment, are critical if clinical remission or at least low or minimal disease activity is to be achieved. However, current clinical evaluation primarily relies on a physical examination that is subjective and lacks sensitivity.8,9 Therefore, there is critical demand for biomarkers that are objective and measurable indicators of disease activity and aid in (1) identifying subtypes of arthritis and guiding treatment decisions based on individual patient characteristics10,11; (2) early detection and prediction of disease progression, allowing for timely intervention and personalized treatment strategies12; and (3) serving as surrogate end points in clinical trials, facilitating the evaluation of treatment efficacy and the development of new therapies.13
Imaging plays an important role in the assessment of arthritis. Conventional radiography remains the most commonly used imaging technique, but known limitations include projection imaging (therefore superposition of structures of interest) and the lack of soft tissue contrast. Magnetic resonance imaging (MRI) and ultrasonography (US) have demonstrated usefulness in overcoming these limitations and providing the ability to image both bone findings and soft tissue abnormalities including synovitis.14 MRI visualizes bone lesions and has also shown promise for early detection of articular cartilage damage.15 Both MRI and US are also able to assess vascular pathology16 that may be associated with angiogenesis. However, these modalities have notable limitations. US performance is operator dependent, may vary with anatomical location, and its sensitivity may be limited to assess axial involvement or deeply located tissues.17 Evaluation of multiple joints and associated musculoskeletal (MSK) features may be subjective and time consuming.18 Standard MRI is limited to the selected field of view (FOV), and it may be useful if local joint tissues are of interest, such as in OA; however, multiple scans may be needed to evaluate autoimmune arthritis, which is typically systemic, or to assess contralateral joints, for example in the context of asymmetric arthritis.19 Whole-body MRI methods have been implemented to overcome this limitation, but their spatial resolution, especially for distal peripheral sites, is low, and reliability is limited.20,21 MRI may be challenging in individuals with prostheses. Moreover, US and MRI have limited ability for evaluating the molecular targets along the pathogenesis of arthritis (e.g., the proliferative-inflammatory cascade in RA or PsA).22,23
Positron emission tomography (PET) uses targeted radiotracers and can interrogate molecular interactions and pathways of significance to arthritis.24-34 Commonly available PET scanners, typically with an axial FOV < 25 cm, can assess local PET signals, such as for the knees. However, images of the entire body, of importance to assess systemic arthritis disease activity, can only be acquired with serial imaging at multiple bed positions. In this configuration the signal collection efficiency is very low; only ~ 1% of the photons emitted from a human body are actually detected.35 This results in the need for a higher injected dose and/or longer scan times. Furthermore, sizable information available from characterizing the temporal biodistribution of the radiotracer (e.g., dynamic imaging and radiotracer kinetic parameters) is limited to only one region of the body at a time.
Recently, long axial FOV PET scanners (axial FOV: 106–194 cm), called total-body PET scanners, have been constructed and evaluated. The first of these systems is the uEXPLORER PET/computed tomography (CT) scanner36 constructed by University of California, Davis, investigators in collaboration with United Imaging Healthcare. The PET subsystem has a 194-cm axial FOV and can scan the whole adult body at once.37 A second system, PennPET EXPLORER, was built by University of Pennsylvania investigators in collaboration with Philips Healthcare, with an axial length of 140 cm.38 A third system, Biograph Vision Quadra, was built by Siemens Healthcare and has a 106-cm axial FOV.39 These systems have a spatial resolution of 3 to 4mm. Several human imaging studies that use these systems have been recently published,36,40-46 and two of these systems (uEXPLORER and Biograph Vision Quadra) are being utilized for clinical imaging.
In December 2019 our group initiated a prospective observational study with research grant funding from the National Institutes of Health to assess the utility of total-body PET (using the uEXPLORER system) in the context of RA. Subsequently, the National Psoriasis Foundation provided us grant support to extend these studies to imaging of PsA and OA. Across these studies we used an ultra-low-dose protocol consisting of a ~ 78 MBq (2.1 mCi) injection of the 18F-fluorodeoxyglucose (18F-FDG) radiotracer (an approximately four to five times lower injected dose compared with that utilized in routine clinical PET imaging) and ultra-low-dose CT (~ 1 mSv effective dose). In this review we discuss our initial findings from total-body PET/CT imaging of these arthritides and outline challenges and future opportunities.
Total-body Positron Emission Tomography of Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a common systemic autoimmune disorder that leads to joint destruction, functional disability, and impaired health status. Immune-mediated joint inflammation is considered the hallmark of RA, and synovitis is a bellwether for downstream joint destruction, pain, and disability.47 Consequently, a vast number of existing and new therapeutics target RA synovitis.48 Methods that provide a sensitive and objective assessment of systemic synovitis burden therefore would naturally be appropriate to quantify RA disease activity and treatment response. 18F-FDG PET has shown the ability to assess inflammatory activity of RA synovitis33,34,49 and, using standard scanners, the utility of 18F-FDG-based PET/CT for RA evaluation and therapeutic monitoring has been demonstrated.31,50-57 The limitations of these studies are the higher ionizing radiation exposure, limited spatial resolution for small joints, and inability to perform kinetic analysis across tissues of interest across the body.
Total-body PET images in RA participants typically showed characteristic symmetrical involvement of the large (e.g., shoulder and knee) and small (e.g., hands) joints (Fig. 1). Zoomed-in images of joints of interest, such as those of the wrist and hand, can be extracted directly from the same total-body scans (Fig. 1). Erosive changes and associated synovial pathology can also be visualized (Fig. 2). Concurrent rheumatologic assessment in our studies included the Disease Activity Score-28 (DAS28), a composite clinical metric that incorporates swelling and/or tenderness on clinical evaluation, C-reactive protein assessments, and a participant’s assessment of their disease activity and pain.58 On a joint-by-joint basis (28 joints per participant), ~ 70% of the joints showed concordant findings between DAS28 and PET assessments. However, an additional 20% joints were negative on DAS28 evaluation but were positive on PET. This finding suggests the ability of PET to assess subclinical inflammation that may fall below the sensitivity of the DAS28 exam. About 10% of joints were positive on the DAS28 examination but negative on PET. Most of these joints (>90%) were tender joints. These finding are consistent with those from other imaging modalities and support the observation that inclusion of tender joints in clinical scoring may contribute to misleading information about RA inflammatory activity.59-61 We evaluated three consolidated PET measures over the 28 joints, the total positive joint count, average PET positivity scores, and average maximum standardized uptake value (SUVmax).62 The Spearman rank correlations of these scores with the DAS28 were in the moderate category, ranging from 0.40 to 0.60 (p<0.05). We conducted dynamic PET scans in a subset of study participants, where we captured imaging data over 60 minutes following 18F-FDG injection. A comparison between images in a late static time window versus one demonstrating the 18F-FDG influx rate via kinetic modeling is shown in Fig. 3. Additionally, total-body PET images provided means to visualize other pathologies beyond joints, such as arterial wall alterations (Fig. 4), a known consequence of RA.
Fig. 1.
Total-body positron emission tomography (PET) with the fluorine-18 fluorodeoxyglucose (18F-FDG) radiotracer in a 49-year-old woman with rheumatoid arthritis (RA) at one fifth of the standard PET injected dose. (a) Maximum intensity projection (MIP) through the PET scan of the entire body imaged in a single shot, showing areas of increased, rather symmetrical, radiotracer uptake (elevated glucose metabolism) in multiple bilateral joints (arrows). (b) Zoomed-in PET/computed tomography of the right hand extracted from the same scan in (a), showing involvement of the wrist, metacarpophalangeal, and proximal interphalangeal joints, classic in RA. The coronal image is a MIP; the sagittal and axial images are cross-sectional images along the axes indicated in the coronal MIP. The axial slice shows the classic ring-like pattern of RA synovitis.
Fig. 2.
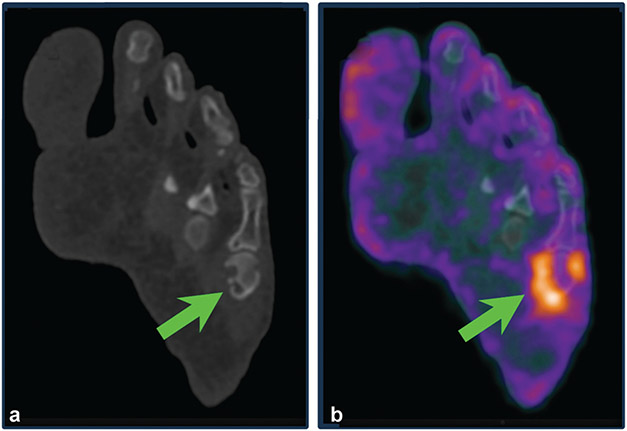
Erosive changes in small joints were visualized by total-body positron emission tomography (PET) in a 51-year-old man with an established diagnosis of rheumatoid arthritis. PET radiotracer uptake (consistent with synovitis) was noted around the left fifth metatarsophalangeal joint (b), co-localized with erosive changes on computed tomography (arrows) (a).
Fig. 3.
Kinetic parameters of fluorine-18 fluorodeoxyglucose (18F-FDG) from total-body positron emission tomography (PET). (a) Standardized uptake value image of the body and hand (extracted from 40- to 60-minute static frame data). (b) Map of kinetic parameter K1, indicating transport of 18F-FDG from plasma to tissue estimated via compartmental modeling. Some lesions showed high K1 (suggestive of active angiogenesis; red arrowheads); others showed high glucose metabolism but low K1 (likely local perfusion alteration; blue arrowhead).
Fig. 4.
Assessment of likely arterial wall pathology in rheumatoid arthritis (RA) using total-body positron emission tomography (PET). Axial slice showing (a) computed tomography (CT), (b) zoomed-in overlay of PET (color), on computed tomography (gray scale), and (c) PET alone. Images indicate areas of focal activity in the posterolateral aspect of the descending thoracic aorta (arrow) and in the ascending aorta (arrowheads).
Our findings to date support the following: (1) total-body PET was able to ascertain the symmetrical distribution of joint involvement in RA; (2) an ultra-low-dose protocol provided adequate image quality to visualize alterations in glucose metabolism, hence inflammation, from the total body down to a single small joint; (3) there is initial evidence to support the capability of total-body PET to assess subclinical joint activity that may contribute to improved disease staging and therapeutic selection; (4) there is opportunity to assess PET signals in other organ systems concurrently with joint involvement to better understand systemic burden of RA; and (5) total-body dynamics of the radiotracer can be tracked in participants with RA to derive additional measures (kinetic parameters) that may provide insights into RA disease activity.
Total-body Positron Emission Tomography of Psoriatic Arthritis
PsA is another systemic autoimmune form of inflammatory arthritis.63 It is a highly heterogeneous disease that may lead to MSK outcomes that are at least as severe if not more detrimental than RA. Six clinical domains are frequently involved in PsA that must be evaluated: arthritis of the large and small joints, enthesitis, dactylitis, axial arthritis, and nail and skin pathology.64,65 Access to diverse anatomical sites and the ability to palpate sites physically may vary significantly; therefore, physical examination by a rheumatologist/dermatologist is suboptimal.66 As a consequence, PsA is often underdiagnosed or misclassified as other forms of arthritis and treated ineffectively.67-70 PET imaging has been used to assess joint pathology in PsA.71,72 However, much like RA, limitations due to ionizing radiation exposure, inability to derive detailed radiotracer kinetic parameters, and spatial resolution have been noted. Many of the clinical domains encompass small structures, further substantiating the need for improved sensitivity and spatial resolution.
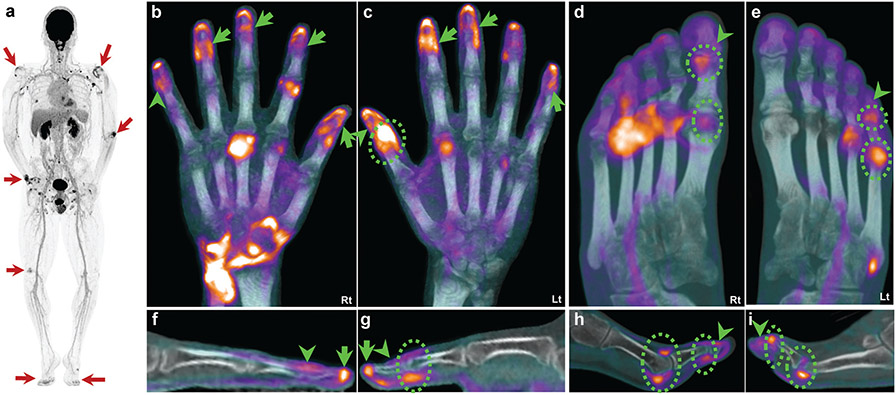
Total-body PET images from participants with PsA in our studies demonstrated the ability to scan all MSK tissues of the body in a single snapshot (Fig. 5). Images showed characteristic asymmetry of joint involvement, significant joint uptake, and generalized systemic enthesitis (Fig. 5), consistent with multi-domain PsA pathology.63 Other features visualized from total-body PET included nail matrix pathology, spinal involvement, active sacroiliitis, and dactylitis.
Fig. 5.
Systemic pathologies in a 33-year-old man with psoriatic arthritis. (a) Total-body positron emission tomography (PET) maximum intensity projection (MIP) showing scattered areas of rather asymmetric increased radiotracer uptake (elevated glucose metabolism) across multiple joints (arrows). (b–e) Fused PET/computed tomography MIP images of the hands (b, c) and feet (d, e). (f–i) Single sagittal slice of a representative digit. Abnormal fluorine-18 fluorodeoxyglucose (18F-FDG) uptake across the terminal extensor tendon and nail matrix of the right fifth finger (b, arrowhead) that is better demonstrated in sagittal view (f, arrowhead). The left thumb demonstrates an active interphalangeal (IP) joint (c and g, dashed circles) in addition to extensor tendon activity extending beyond the IP joint (arrowheads). Additional nail activity is seen across multiple other fingers (b and c, arrows). The right foot demonstrates active first metatarsophalangeal (MTP) and IP joints (d and h, dashed circles) with activity distal to the IP joint (arrowheads, d and h). Similarly, the left fifth toe has active MTP and proximal IP/distal IP (dashed circles, e and i) joint activity. Less prominent 18F-FDG uptake is seen distally, likely representing extensor insertion and nail matrix involvement (arrowheads, e and i).
In comparing PET signals with standardized clinical outcome measures of joint (Disease Activity in Psoriatic Arthritis [DAPSA]), entheseal (Leeds Enthesitis Index [LEI]) and nail pathology (Nail Psoriasis Severity Index [NAPSI]), the concordance was 72 to 78%. Total-body PET was positive for an additional 15% of joints, entheses, or nails that were negative on the standardized assessments. Much like RA, this is suggestive of subclinical inflammation that may fall below the sensitivity of the clinical examination. Most joints (> 90%) that exhibited clinical positivity but were PET-negative showed tenderness without any swelling. Again, as in RA, these results are consistent with those from other modalities73 and support the premise that tenderness may be influenced by factors other than local inflammation.
Total-body Positron Emission Tomography of Other Arthritic Conditions
OA is a prevalent condition, particularly in older individuals, and similar to RA and PsA, biomarkers are needed to aid in its diagnosis and prognosis.74 There is fairly mature published literature on utilizing MRI-based approaches for OA assessment.74,75 PET studies in OA patients have primarily used 18F-FDG to assess glucose metabolism and acute-phase cellular response in joint tissues or 18F-sodium fluoride (18F-NaF) for evaluating regional subchondral bone remodeling.76 A compelling case for OA assessment can be made for combining molecular information from PET with structural information from MRI.76,77
In most cases, however, OA impact is limited to local joint structures. Therefore, although total-body PET can visualize OA disease activity (Fig. 6), so can standard PET scanners, and the motivation for using total-body PET is not that strong. Exceptions perhaps could be made when utilizing radiotracers requiring kinetic modeling that may benefit from having large vessels in the FOV alongside MSK tissue, or when other organ systems must be studied in addition to the OA joint. Arthritic conditions such as ankylosing spondylitis, systemic lupus erythematosus, axial spondyloarthritis, and reactive arthritis with a possible systemic component are perhaps better suited to capitalize much more on the benefits of total-body PET.
Fig. 6.
Total-body positron emission tomography (PET) in osteoarthritis. (a) Maximum intensity projection (MIP) through total-body PET. (b) Fused coronal views of PET and computed tomography across the knees showing joint space narrowing of the medial compartment bilaterally with increased fluorine-18 fluorodeoxyglucose PET activity at the anatomical site of the cruciate ligament(s) of the right knee.
From the experience of nuclear medicine physicians at our institution when assessing total-body PET scans of clinical oncology patients, 18F-FDG patterns consistent with OA are frequently encountered. Anecdotally, we have also seen elevated uptake in joints presenting with anatomical anomalies on CT consistent with OA when using the radiotracer 18F-fluciclovine,78 used primarily for prostate cancer imaging. However, dedicated clinical diagnosis or evaluation for OA was not available in these cases, and a prospective study is needed to establish the significance of the imaging measures.
Challenges and Opportunities
Total-body PET represents a potential paradigm shift in PET imaging technology toward lowering dose and scan time, and enabling synchronous signal collection from the entire body (in the same phase of radiotracer uptake). In the context of RA and PsA, our initial results demonstrate the feasibility of assessing systemic alterations in glucose metabolism, hence sites of inflammation along with inflammatory burden, across tissues and organ systems, and the potential to differentiate between the arthritides (e.g., compared with RA, the dominant pathology in PsA appears to be enthesitis) and identify subclinical involvement. Studies to track response to autoimmune arthritis therapy are ongoing with total-body PET. To date, our observations support the need for evaluating total-body PET in larger studies for assessing the role of the modality for diagnosis, staging to optimize therapeutic selection, and monitoring of therapy.
The performance of total-body PET systems has yet not been compared with that of US or MRI for arthritis imaging. A growing number of long axial FOV PET systems are now being installed, and studies comparing imaging modalities will be necessary to better define the future role and strengths and limitations of each modality for arthritis evaluation. In our studies, we compared PET measures across standardized rheumatologic outcome measures such as DAS28, DAPSA, LEI, and NAPSI. It is noteworthy that although these scoring systems are used for research studies, their use is not uniform across clinical practice. For example, a 68-joint evaluation needed for DAPSA takes a significant amount of physician time over standard targeted evaluation. That makes comparison with imaging challenging. Furthermore, physician evaluation is currently conducted in the clinic, whereas an imaging examination may be a separate appointment for the patient and could add delays to a clinical workup. In this regard, the onus will be on radiologists and imaging specialists to demonstrate the value of imaging, and in the context of this review, total-body PET, over and above what can be gleaned from the physical examination.
The focus of our initial studies was on using the widely available 18F-FDG radiotracer. Published literature has shown the usefulness of PET imaging of macrophage activity (11C-(R)-PK1119525, 18F-fluoro-PEG-folate26), choline metabolism (11C-Choline24), cyclooxygenase-2 (COX-2) upregulation,27 integrin expression,28,29 vascular adhesion protein-1 activity,30 and bone turnover (18F-NaF31,32), especially for RA assessment. Several other targets along the proliferative-inflammatory cascade underlying autoimmune arthritis have been radiolabeled.79 Another promising area is using PET for assessing pain.80 In these areas, total-body PET may provide platform technology for performing more comprehensive studies to better guide the utility of PET radiotracers in arthritis assessment.
The optimal trade-off between injected dose and acquisition time for total-body PET scanning in arthritis is yet to be determined. On the one hand, dose reduction is considered critical in the paradigm of monitoring chronic arthritides such as RA and PsA, and using total-body PET as an adjunct to current diagnostic tools or for assessing response to therapy. On the other hand, to maintain signal-to-noise ratio, the scan time may need to be extended, which may not be well tolerated by arthritis patients. Then there is a question of scan start times. Early dynamic data from 18F-FDG may add information over and above that from late scans. However, long scan times (e.g., 1- to 2-hour dynamic PET acquisition with 18F-FDG) may be challenging for patients, especially those with arthritis. Furthermore, the uptake and acquisition time selected for optimal joint assessment may be unfavorable for other regions of interest in the body, such as for visualizing arterial wall pathology.
Imaging of small structures, such as joints and tendons critical to assessment of some of the arthritides, on total-body PET may need improvements. The spatial resolution of total-body PET scanners is in the 3- to 4-mm range and may be suboptimal for quantifying PET signal in small tendons of the digits or at the nail bed; the contrast recovery coefficient for a 10-mm sphere with 4-to-1 source-to-background ratio and using standard manufacturer-provided reconstruction method on the uEXPLORER is just ~ 50%.37 It is possible that advanced image reconstruction methods for total-body PET81 may address this challenge. Furthermore, virtual pinhole PET technology that improves the local spatial resolution by incorporating high-resolution PET detectors that can operate inside total-body PET scanners82 may offer a potential solution. Another challenge for imaging small structures is intra-scan motion that often can cause image blurring and artifacts. Impact of motion could be mitigated by hardware-driven immobilization62 but may not be well tolerated in some patients such as those with significant joint deformities. Shortening image acquisition time would be helpful, but maintaining the signal-to-noise ratio may require increase in the injected dose. Retrospective temporal binning of the data into shorter frames and either software-driven motion correction or choosing frames with the least intra-scan motion83 may be options but need to be evaluated carefully for the arthritis imaging application.
A concern with total-body PET technology is its accessibility, partly driven by cost. There is therefore strong motivation to better understand how knowledge from total-body PET-based evaluation may become useful to design protocols for standard PET scanners. In some arthritides, tissues of interest for evaluation may be anatomically clustered (such as joints of the hands or foot), and that would motivate focused protocols utilizing standard PET scanners. Collection of dynamic PET data on total-body PET could help create a library of input functions and delay corrections84 that could enable more sophisticated analyses on standard PET scanners. This would definitely be an area for future research.
Several arthritic conditions have an adverse impact on other systems of the body. For example, autoimmune arthritis consequences may involve vascular, hepatic, and neurologic alterations.85,86 Even though the arthritic conditions themselves may not be systemic, such as OA, they may impact or be influenced by abnormalities in other organ systems. Total-body PET systems are able to measure signals synchronously from multiple organs. The data sets acquired, especially with dynamic total-body PET, may provide novel means to understand the interconnection between organ systems and support a holistic approach to better understand arthritis. At this time, however, we have barely scratched the surface regarding processing the vast amounts of data produced by these scanners. We strongly believe that important insights could be provided into the arthritis disease process via total-body molecular and metabolic imaging.
Summary
Initial results from total-body PET of arthritis clearly demonstrate the potential utility of the technology to assess arthritis disease activity in MSK tissues and concurrent pathology in other organ systems. The ability to use ultra-low-dose protocols, short scan times, and dynamic total-body imaging may especially benefit arthritis patients. The total-body PET technology is in its early stages of implementation; therefore, several challenges must be addressed to capitalize opportunistically on the vast amount of data produced by the scanners. Larger, carefully planned studies in collaboration with rheumatologists and orthopaedists are needed to inform how best to utilize the technology for improving arthritis clinical decision-making.
Acknowledgments
We thank Drs. Ramsey D. Badawi, Simon R. Cherry, Guobao Wang, Fatma Sen, Cameron Foster, Benjamin Spencer, and also Dario Mazza from the University of California, Davis, for their contributions to the studies described in this article.
Footnotes
Conflict of interest
University of California, Davis, has a research agreement and a sales-based revenue-sharing agreement with United Imaging Healthcare, the manufacturer of the uEXPLORER scanner used for the studies described in this article. The work is supported in part by the National Institutes of Health (R01 AR076088, R61 AT012187, and R01 CA206187) and the National Psoriasis Foundation.
References
- 1.Barbour KE, Helmick CG, Boring M, Brady TJ. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2013-2015. MMWR Morb Mortal Wkly Rep 2017;66(09):246–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan-Olsen SL, Cook S, Leech MT, et al. Prevalence of arthritis according to age, sex and socioeconomic status in six low and middle income countries: analysis of data from the World Health Organization study on global AGEing and adult health (SAGE) Wave 1. BMC Musculoskelet Disord 2017;18(01):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Bone and Joint Initiative The Burden of Musculoskeletal Diseases in the United States (BMUS). 4th ed. 2020. Available at: http://www.boneandjointburden.orgexternal Accessed July 29, 2023 [Google Scholar]
- 4.Luque Ramos A, Redeker I, Hoffmann F, Callhoff J, Zink A, Albrecht K. Comorbidities in patients with rheumatoid arthritis and their association with patient-reported outcomes: results of claims data linked to questionnaire survey. J Rheumatol 2019;46(06):564–571 [DOI] [PubMed] [Google Scholar]
- 5.Husni ME, Merola JF, Davin S. The psychosocial burden of psoriatic arthritis. Semin Arthritis Rheum 2017;47(03):351–360 [DOI] [PubMed] [Google Scholar]
- 6.Favalli EG, Biggioggero M, Crotti C, Becciolini A, Raimondo MG, Meroni PL. Sex and management of rheumatoid arthritis. Clin Rev Allergy Immunol 2019;56(03):333–345 [DOI] [PubMed] [Google Scholar]
- 7.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage 2005;13(09):769–781 [DOI] [PubMed] [Google Scholar]
- 8.Iragorri N, Hazlewood G, Manns B, Danthurebandara V, Spackman E. Psoriatic arthritis screening: a systematic review and meta-analysis. Rheumatology (Oxford) 2019;58(04):692–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388(10055):2023–2038 [DOI] [PubMed] [Google Scholar]
- 10.Rida MA, Chandran V. Challenges in the clinical diagnosis of psoriatic arthritis. Clin Immunol 2020;214:108390. [DOI] [PubMed] [Google Scholar]
- 11.Zhang R, Yang X, Wang J, et al. Identification of potential biomarkers for differential diagnosis between rheumatoid arthritis and osteoarthritis via integrative genome-wide gene expression profiling analysis. Mol Med Rep 2019;19(01):30–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harnden K, Pease C, Jackson A. Rheumatoid arthritis. BMJ 2016;352:i387. [DOI] [PubMed] [Google Scholar]
- 13.Verweij CL. Predicting the future of anti-tumor necrosis factor therapy. Arthritis Res Ther 2009;11(03):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evangelisto A, Wakefield R, Emery P. Imaging in early arthritis. Best Pract Res Clin Rheumatol 2004;18(06):927–943 [DOI] [PubMed] [Google Scholar]
- 15.Roemer FW, Guermazi A, Trattnig S, et al. Whole joint MRI assessment of surgical cartilage repair of the knee: Cartilage Repair OsteoArthritis Knee Score (CROAKS). Osteoarthritis Cartilage 2014;22(06):779–799 [DOI] [PubMed] [Google Scholar]
- 16.Takase K, Ohno S, Takeno M, et al. Simultaneous evaluation of long-lasting knee synovitis in patients undergoing arthroplasty by power Doppler ultrasonography and contrast-enhanced MRI in comparison with histopathology. Clin Exp Rheumatol 2012;30(01):85–92 [PubMed] [Google Scholar]
- 17.Michelsen B, Diamantopoulos AP, Soldal DM, Hammer HB, Kavanaugh A, Haugeberg G. Achilles enthesitis defined by ultrasound is not associated with clinical enthesitis in patients with psoriatic arthritis. RMD Open 2017;3(02):e000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singla S, Eder L, Kaeley G, Aydin SZ. The use and availability of musculoskeletal ultrasonography for psoriatic disease among group for research and assessment of psoriasis and psoriatic arthritis members and the unmet needs. Clin Ther 2023:S0149-2918(23)00199-6 [DOI] [PubMed] [Google Scholar]
- 19.Coates LC, Hodgson R, Conaghan PG, Freeston JE. MRI and ultrasonography for diagnosis and monitoring of psoriatic arthritis. Best Pract Res Clin Rheumatol 2012;26(06):805–822 [DOI] [PubMed] [Google Scholar]
- 20.Poggenborg RP, Pedersen SJ, Eshed I, et al. Head-to-toe whole-body MRI in psoriatic arthritis, axial spondyloarthritis and healthy subjects: first steps towards global inflammation and damage scores of peripheral and axial joints. Rheumatology (Oxford) 2015;54(06):1039–1049 [DOI] [PubMed] [Google Scholar]
- 21.Krabbe S, Eshed I, Gandjbakhch F, et al. ; OMERACT MRI in Arthritis Working Group. Development and Validation of an OMERACT MRI Whole-Body Score for Inflammation in Peripheral Joints and Entheses in Inflammatory Arthritis (MRI-WIPE). J Rheumatol 2019;46(09):1215–1221 [DOI] [PubMed] [Google Scholar]
- 22.Husic R, Gretler J, Felber A, et al. Disparity between ultrasound and clinical findings in psoriatic arthritis. Ann Rheum Dis 2014;73(08):1529–1536 [DOI] [PubMed] [Google Scholar]
- 23.Fassio A, Matzneller P, Idolazzi L. Recent advances in imaging for diagnosis, monitoring, and prognosis of psoriatic arthritis. Front Med (Lausanne) 2020;7:551684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roivainen A, Parkkola R, Yli-Kerttula T, et al. Use of positron emission tomography with methyl-11C-choline and 2-18F-fluoro-2-deoxy-D-glucose in comparison with magnetic resonance imaging for the assessment of inflammatory proliferation of synovium. Arthritis Rheum 2003;48(11):3077–3084 [DOI] [PubMed] [Google Scholar]
- 25.van der Laken CJ, Elzinga EH, Kropholler MA, et al. Noninvasive imaging of macrophages in rheumatoid synovitis using 11C-(R)-PK11195 and positron emission tomography. Arthritis Rheum 2008;58(11):3350–3355 [DOI] [PubMed] [Google Scholar]
- 26.Verweij NJF, Yaqub M, Bruijnen STG, et al. First in man study of [18F]fluoro-PEG-folate PET: a novel macrophage imaging technique to visualize rheumatoid arthritis. Sci Rep 2020;10(01):1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shrestha S, Kim MJ, Eldridge M, et al. PET measurement of cyclooxygenase-2 using a novel radioligand: upregulation in primate neuroinflammation and first-in-human study. J Neuroinflammation 2020;17(01):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dietz M, Nicod Lalonde M, Omoumi P, Testart Dardel N, Hügle T, Prior JO. Imaging of ανβ3 integrin expression in rheumatoid arthritis with [68Ga]Ga-NODAGA-RGDyk PET/CT in comparison to [18F]FDG PET/CT. Med Nucl (Paris) 2021;45(5–6):293–295 [Google Scholar]
- 29.Zhu Z, Yin Y, Zheng K, et al. Evaluation of synovial angiogenesis in patients with rheumatoid arthritis using 68Ga-PRGD2 PET/CT: a prospective proof-of-concept cohort study. Ann Rheum Dis 2014;73(06):1269–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viitanen R, Moisio O, Lankinen P, et al. First-in-humans study of 68Ga-DOTA-Siglec-9, a PET ligand targeting vascular adhesion protein 1. J Nucl Med 2021;62(04):577–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe T, Takase-Minegishi K, Ihata A, et al. (18)F-FDG and (18)F-NaF PET/CT demonstrate coupling of inflammation and accelerated bone turnover in rheumatoid arthritis. Mod Rheumatol 2016;26(02):180–187 [DOI] [PubMed] [Google Scholar]
- 32.Park HJ, Chang SH, Lee JW, Lee SM. Clinical utility of F-18 sodium fluoride PET/CT for estimating disease activity in patients with rheumatoid arthritis. Quant Imaging Med Surg 2021;11(04):1156–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narayan N, Owen DR, Taylor PC. Advances in positron emission tomography for the imaging of rheumatoid arthritis. Rheumatology (Oxford) 2017;56(11):1837–1846 [DOI] [PubMed] [Google Scholar]
- 34.Mountz JM, Alavi A, Mountz JD. Emerging optical and nuclear medicine imaging methods in rheumatoid arthritis. Nat Rev Rheumatol 2012;8(12):719–728 [DOI] [PubMed] [Google Scholar]
- 35.Cherry SR, Badawi RD, Karp JS, Moses WW, Price P, Jones T. Total-body imaging: transforming the role of positron emission tomography. Sci Transl Med 2017;9(381):eaaf6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badawi RD, Shi H, Hu P, et al. First human imaging studies with the EXPLORER total-body pet scanner. J Nucl Med 2019;60(03):299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spencer BA, Berg E, Schmall JP, et al. Performance evaluation of the uEXPLORER total-body PET/CT scanner Based on NEMA NU 2-2018 with additional tests to characterize PET scanners with a long axial field of view. J Nucl Med 2021;62(06):861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karp J, Schmall J, Geagan M, et al. Imaging performance of the PennPET Explorer scanner. J Nucl Med 2018;59(Suppl 1):222–222 [Google Scholar]
- 39.Prenosil GA, Sari H, Fürstner M, et al. Performance characteristics of the Biograph Vision Quadra PET/CT System with a long axial field of view using the NEMA NU 2-2018 standard. J Nucl Med 2022;63(03):476–484 [DOI] [PubMed] [Google Scholar]
- 40.Zhao YM, Li YH, Chen T, et al. Image quality and lesion detectability in low-dose pediatric 18F-FDG scans using total-body PET/CT. Eur J Nucl Med Mol Imaging 2021;48(11):3378–3385 [DOI] [PubMed] [Google Scholar]
- 41.Zhang YQ, Hu PC, Wu RZ, et al. The image quality, lesion detectability, and acquisition time of 18F-FDG total-body PET/CT in oncological patients. Eur J Nucl Med Mol Imaging 2020;47(11):2507–2515 [DOI] [PubMed] [Google Scholar]
- 42.Hu P, Zhang Y, Yu H, et al. Total-body 18F-FDG PET/CT scan in oncology patients: how fast could it be? Eur J Nucl Med Mol Imaging 2021;48(08):2384–2394 [DOI] [PubMed] [Google Scholar]
- 43.Pantel AR, Viswanath V, Daube-Witherspoon ME, et al. PennPET Explorer: human imaging on a whole-body imager. J Nucl Med 2020;61(01):144–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alberts I, Hünermund JN, Prenosil G, et al. Clinical performance of long axial field of view PET/CT: a head-to-head intra-individual comparison of the Biograph Vision Quadra with the Biograph Vision PET/CT. Eur J Nucl Med Mol Imaging 2021;48(08):2395–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Sluis J, van Snick JH, Brouwers AH, et al. Shortened duration whole body 18F-FDG PET Patlak imaging on the Biograph Vision Quadra PET/CT using a population-averaged input function. EJNMMI Phys 2022;9(01):1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mannheim JG, Rausch I, Conti M, la Fougère C, Schmidt FP. Characterization of the partial volume effect along the axial field-of-view of the Biograph Vision Quadra total-body PET/CT system for multiple isotopes. EJNMMI Phys 2023;10(01):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365(23):2205–2219 [DOI] [PubMed] [Google Scholar]
- 48.Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA 2018;320(13):1360–1372 [DOI] [PubMed] [Google Scholar]
- 49.Gu JT, Nguyen L, Chaudhari AJ, MacKenzie JD. Molecular characterization of rheumatoid arthritis with magnetic resonance imaging. Top Magn Reson Imaging 2011;22(02):61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polisson RP, Schoenberg OI, Fischman A, et al. Use of magnetic resonance imaging and positron emission tomography in the assessment of synovial volume and glucose metabolism in patients with rheumatoid arthritis. Arthritis Rheum 1995;38(06):819–825 [DOI] [PubMed] [Google Scholar]
- 51.Beckers C, Ribbens C, André B, et al. Assessment of disease activity in rheumatoid arthritis with (18)F-FDG PET. J Nucl Med 2004;45(06):956–964 [PubMed] [Google Scholar]
- 52.Chaudhari AJ, Ferrero A, Godinez F, et al. High-resolution (18)F-FDG PET/CT for assessing disease activity in rheumatoid and psoriatic arthritis: findings of a prospective pilot study. Br J Radiol 2016;89(1063):20160138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaudhari AJ, Bowen SL, Burkett GW, et al. High-resolution (18)F-FDG PET with MRI for monitoring response to treatment in rheumatoid arthritis. Eur J Nucl Med Mol Imaging 2010;37(05):1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kubota K, Ito K, Morooka M, et al. FDG PET for rheumatoid arthritis: basic considerations and whole-body PET/CT. Ann N Y Acad Sci 2011;1228:29–38 [DOI] [PubMed] [Google Scholar]
- 55.Yamashita H, Kubota K, Mimori A. Clinical value of whole-body PET/CT in patients with active rheumatic diseases. Arthritis Res Ther 2014;16(05):423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kubota K, Yamashita H, Mimori A. Clinical value of FDG-PET/CT for the evaluation of rheumatic diseases: rheumatoid arthritis, polymyalgia rheumatica, and relapsing polychondritis. Semin Nucl Med 2017;47(04):408–424 [DOI] [PubMed] [Google Scholar]
- 57.Fosse P, Kaiser MJ, Namur G, de Seny D, Malaise MG, Hustinx R. 18F-FDG PET/CT joint assessment of early therapeutic response in rheumatoid arthritis patients treated with rituximab. Eur J Hybrid Imaging 2018;2(01):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prevoo ML, van ’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38(01):44–48 [DOI] [PubMed] [Google Scholar]
- 59.Hammer HB, Michelsen B, Provan SA, et al. Tender joint count and inflammatory activity in patients with established rheumatoid arthritis: results from a longitudinal study. Arthritis Care Res (Hoboken) 2020;72(01):27–35 [DOI] [PubMed] [Google Scholar]
- 60.Hammer HB, Michelsen B, Sexton J, et al. Swollen, but not tender joints, are independently associated with ultrasound synovitis: results from a longitudinal observational study of patients with established rheumatoid arthritis. Ann Rheum Dis 2019;78(09):1179–1185 [DOI] [PubMed] [Google Scholar]
- 61.Hammer HB, Jensen Hansen IM, Järvinen P, et al. Rheumatoid arthritis patients with predominantly tender joints rarely achieve clinical remission despite being in ultrasound remission. Rheumatol Adv Pract 2021;5(02):rkab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abdelhafez Y, Raychaudhuri SP, Mazza D, et al. Total-body 18F-FDG PET/CT in autoimmune inflammatory arthritis at ultra-low dose: initial observations. J Nucl Med 2022;63(10):1579–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376(10):957–970 [DOI] [PubMed] [Google Scholar]
- 64.Gladman DD. Clinical features and diagnostic considerations in psoriatic arthritis. Rheum Dis Clin North Am 2015;41(04):569–579 [DOI] [PubMed] [Google Scholar]
- 65.Raychaudhuri SP, Wilken R, Sukhov AC, Raychaudhuri SK, Maverakis E. Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun 2017;76:21–37 [DOI] [PubMed] [Google Scholar]
- 66.Wakefield RJ, Green MJ, Marzo-Ortega H, et al. Should oligoarthritis be reclassified? Ultrasound reveals a high prevalence of subclinical disease. Ann Rheum Dis 2004;63(04):382–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol 2013;69(05):729–735 [DOI] [PubMed] [Google Scholar]
- 68.Chandran V, Abji F, Perruccio AV, et al. Serum-based soluble markers differentiate psoriatic arthritis from osteoarthritis. Ann Rheum Dis 2019;78(06):796–801 [DOI] [PubMed] [Google Scholar]
- 69.Van Hal TW, Mulder MLM, Wenink MH, et al. Discovery of Psoriatic Arthritis in Psoriasis Patients for Early Rheumatological Referral (DAPPER) Study: a prospective observational cohort. Acta Derm Venereol 2022;102:adv00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McGonagle D, Hermann KG, Tan AL. Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era. Rheumatology (Oxford) 2015;54(01):29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wahl RL, Dilsizian V, Palestro CJ. At last, 18F-FDG for inflammation and infection!. J Nucl Med 2021;62(08):1048–1049 [DOI] [PubMed] [Google Scholar]
- 72.Basu S, Zhuang H, Torigian DA, Rosenbaum J, Chen W, Alavi A. Functional imaging of inflammatory diseases using nuclear medicine techniques. Semin Nucl Med 2009;39(02):124–145 [DOI] [PubMed] [Google Scholar]
- 73.Felbo SK, Wiell C, Østergaard M, et al. Do tender joints in active psoriatic arthritis reflect inflammation assessed by ultrasound and magnetic resonance imaging? Rheumatology (Oxford) 2022;61(02):723–733 [DOI] [PubMed] [Google Scholar]
- 74.Hunter DJ, Collins JE, Deveza L, Hoffmann SC, Kraus VB. Biomarkers in osteoarthritis: current status and outlook - the FNIH Biomarkers Consortium PROGRESS OA study. Skeletal Radiol 2023. January 24 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Collins JE, Losina E, Nevitt MC, et al. Semiquantitative imaging biomarkers of knee osteoarthritis progression: data from the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol 2016;68(10):2422–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jarraya M, Roemer FW, Bäuerle T, Kogan F, Guermazi A. PET imaging in osteoarthritis. PET Clin 2023;18(01):21–29 [DOI] [PubMed] [Google Scholar]
- 77.Jena A, Taneja S, Rana P, et al. Emerging role of integrated PET-MRI in osteoarthritis. Skeletal Radiol 2021;50(12):2349–2363 [DOI] [PubMed] [Google Scholar]
- 78.Abdelhafez YG, Nardo L, Cherry SR, Badawi RD, Chaudhari AJ 18F-Fluciclovine PET uptake in thumb carpometacarpal joint: initial observations. Paper presented at: Orthopaedics Research Society Annual Meeting; February 2–5, 2019; Austin, TX [Google Scholar]
- 79.Wu C, Li F, Niu G, Chen X. PET imaging of inflammation biomarkers. Theranostics 2013;3(07):448–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoon D, Kogan F, Gold GE, Biswal S. Identifying musculoskeletal pain generators using clinical PET. Semin Musculoskelet Radiol 2020;24(04):441–450 [DOI] [PubMed] [Google Scholar]
- 81.Qi J, Matej S, Wang G, Zhang X. 3D/4D Reconstruction and quantitative total body imaging. PET Clin 2021;16(01):41–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang J, Hua J, Wang H, et al. A virtual-pinhole PET device for improving contrast recovery and enhancing lesion detectability of a one-meter-long PET scanner: a simulation study. Phys Med Biol 2023;68(14): [DOI] [PubMed] [Google Scholar]
- 83.Shiyam Sundar LK, Lassen ML, Gutschmayer S, et al. Fully automated, fast motion correction of dynamic whole-body and total-body PET/CT imaging studies. J Nucl Med 2023;64(07):1145–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li EJ, Spencer BA, Schmall JP, et al. Efficient delay correction for total-body PET kinetic modeling using pulse timing methods. J Nucl Med 2022;63(08):1266–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Richards JS, Dowell SM, Quinones ME, Kerr GS. How to use biologic agents in patients with rheumatoid arthritis who have comorbid disease. BMJ 2015;351:h3658. [DOI] [PubMed] [Google Scholar]
- 86.Humphreys J, Hyrich K, Symmons D. What is the impact of biologic therapies on common co-morbidities in patients with rheumatoid arthritis? Arthritis Res Ther 2016;18(01):282. [DOI] [PMC free article] [PubMed] [Google Scholar]