ABSTRACT
Research from the last 20 years has provided important insights into the molecular pathogenesis of craniopharyngiomas (CPs). Besides the well-known clinical and histological differences between the subtypes of CPs, adamantinomatous (ACP) and papillary (PCP) craniopharyngiomas, other molecular differences have been identified, further elucidating pathways related to the origin and development of such tumors. The present minireview assesses current knowledge on embryogenesis and the genetic, epigenetic, transcriptomic, and signaling pathways involved in the ACP and PCP subtypes, revealing the similarities and differences in their profiles. ACP and PCP subtypes can be identified by the presence of mutations in CTNNB1 and BRAF genes, with prevalence around 60% and 90%, respectively. Therefore, β-catenin accumulates in the nucleus-cytoplasm of cell clusters in ACPs and, in PCPs, cell immunostaining with specific antibody against the V600E-mutated protein can be seen. Distinct patterns of DNA methylation further differentiate ACPs and PCPs. In addition, research on genetic and epigenetic changes and tumor microenvironment specificities have further clarified the development and progression of the disease. No relevant transcriptional differences in ACPs have emerged between children and adults. In conclusion, ACPs and PCPs present diverse genetic signatures and each subtype is associated with specific signaling pathways. A better understanding of the pathways related to the growth of such tumors is paramount for the development of novel targeted therapeutic agents.
Keywords: Craniopharyngioma, adamantinomatous, papillary, CTNNB1, BRAF V600E, molecular pathogenesis
INTRODUCTION
Craniopharyngiomas (CPs) are intracranial neoplasms located mainly in the sellar and supra-sellar regions, along the anatomical developmental pathway of the craniopharyngeal duct. CPs’ incidence is around 0.16-2 cases per million persons per year ( Table 1 ), accounting for 2% to 5% of all primary intracranial neoplasms and for 5.6% to 13% of intracranial tumors in children ( 1 - 6 ). The majority of studies show no sex asymmetry; however, more recently, Feng and cols. (2019) observed that men were more commonly affected than women were, specifically in a sample of patients of Chinese origin ( 2 , 5 - 7 ).
Table 1. Classification of craniopharyngiomas.
| Adamantinomatous | Papillary | |
|---|---|---|
| Incidence | 0.16–2 cases per million persons/ year | |
| Frequence (%) | 90% | 10% |
| Age (years) | 5–14 and 55–74 | 40–53 |
| Clinical presentation | Predominant inicial symptoms: increased intracranial pressure; visual field defects; hypopituitarism; cognitive impairment; overweight | |
| Macroscopy | Multicystic tumor (dark motor-oil’ content) with or without solid components | Purely or predominantly solid (if cystic: viscous yellow content) |
| Microscopy | Multicystic, “stellate reticulum”, “wet keratin", occasional calcification, finger-like protrusions into brain bordered by palisading cells, chronic inflammation in peritumoral brain | Papillary growth pattern with mature nonkeratinizing squamous epithelium; no wet keratin; no calcification; well circumscribed neoplastic epithelium, adjacent brain tissue infiltration usually absent |
| Molecular pathogenesis markers | CTNNB1 mutation | BRAF V600E mutation |
CPs were first described in 1857 by Friedrich Albert von Zenker. In 1904, Jakob Ederheim described CPs’ histopathological characteristics, suggesting that these tumors arose from ectodermal embryonic remnants of the primitive mouth or stomodeum ( 8 , 9 ). In 1932, Harvey Cushing described his experience with the management of CPs and characterized those as “the most baffling problem which confronts the Neurosurgeon” ( 10 , 11 ).
CPs are classified as histologically benign grade I tumors by the World Health Organization (WHO) ( 12 ). However, CPs are challenging tumors to treat due to their location and close relationship to neurovascular structures, including the optic apparatus, third ventricle and hypothalamus, pituitary stalk, and internal carotid artery and its branches, which may preclude gross surgical removal to avoid new postoperative neurological deficits ( 13 ). Subtotal resection of CPs is associated with higher recurrence rates and adjuvant radiation treatment is usually recommended ( 13 ). Due to their location and relationship with important brain structures, their pattern of recurrence and need for multimodality treatment, CPs are often associated with significant morbidity, including hypopituitarism, hypothalamic impairment, and visual, neurological, and cognitive deficits. In addition to these disabling complications, obesity due to hypothalamic disorders has been highlighted as a critical adverse complication in patients with CPs ( 10 , 14 , 15 ). The exact mechanisms responsible for the development of hypothalamic obesity are not yet fully understood. As the hypothalamus integrates peripheral neural and hormonal afferent signals of satiety and energy reserve and acts directly on efferent signals that affect energy supply and expenditure, damage to the hypothalamic control system can result in weight gain, as demonstrated by the presence of obesity or overweight in 51.4% of the patients at diagnosis, which increased to 86.5% after surgical treatment ( 16 - 19 ). In view of these serious chronic morbidities and increased mortality during long-term follow-up, CPs have been associated with the lowest quality of life (QoL) among the different types of pediatric brain tumors ( 20 - 23 ). The impact on QoL has also been observed in adults presenting CPs ( 24 ).
Two theories have been considered regarding the genesis of CPs. The first theory suggests that CPs result from metaplasia of adenohypophyseal cells in the pituitary stalk or gland. The basis for this theory is CPs’ putative origin in squamous cell nests with intracellular tonofilaments, desmosome-associated tonofilaments, and kerato-hyaline granules, which could be evidence of squamous differentiation. In addition, the histochemical analyses also indicate keratinization in CPs’ epithelial cells ( 25 - 27 ). The second theory postulates that CPs arise from pituitary progenitor or stem cells representing embryonal remnants of Rathke's pouch epithelium ( 28 - 33 ). Studies in mouse models have provided insights into the importance of stem cells in the tumorigenesis of craniopharyngiomas ( 34 ).
CPs are currently divided into two main subtypes, adamantinomatous (ACP) and papillary (PCP). The subtypes share a few similarities, such as anatomic location, adult pituitary stem cell markers, glial reaction proteins, and cytokeratin expression. However, ACP and PCP present distinct morphological and histological features as well as different epidemiological and biological behavior ( 10 , 25 , 35 ). In addition, recent studies have demonstrated the differentiation of ACP and PCP subtypes according to epigenetic and molecular profiles, indicating that these tumors represent different entities ( 34 , 36 - 43 ).
The purpose of this minireview is to assess the current state of knowledge on embryogenesis and the genetic, epigenetic, transcriptomic and signaling pathways involved in the ACP and PCP subtypes, thereby revealing the similarities and differences in their profiles.
Papillary craniopharyngioma (PCP)
PCPs have been almost exclusively described in adults, aging 40 to 53 years ( Table 1 ) with a mean age of 44.7 years ( 5 , 44 ). They often present as large tumors located in the suprasellar area and within the third ventricle ( 45 ).
Macroscopically, PCPs are mostly solid mass or mixed mass with viscous yellow cysts and solid components, but calcifications are rare ( 4 , 46 ). These tumors are well-circumscribed neoplastic epithelium, and adjacent brain tissue infiltration is usually absent ( 47 ). At the microscopic level, PCPs’ histological features resemble those of the oropharyngeal mucosa, but PCPs do not express enamel proteins, amelogenin, or enamel proteinase, as observed in ACPs, suggesting a different origin of these CP subtypes ( 4 , 46 ). PCPs are composed of mature squamous-epithelium-forming pseudopapillae and an anastomosing fibrovascular stroma with thin capillary blood vessels and scattered immune cells including macrophages and neutrophils ( 48 ). There are no peripheral palisading or stellate reticulum cells, with no wet keratin and, only occasionally, small collagenous whorls ( 42 , 47 ). PCPs are often difficult to distinguish from other suprasellar and infundibulotuberal masses, such as non-neoplastic Rathke's cleft cysts ( 42 ).
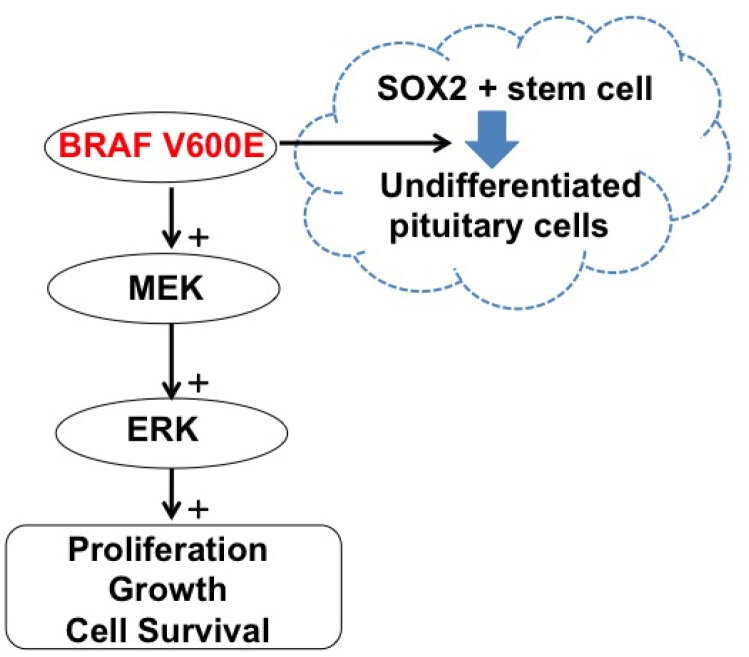
Understanding of the genetic and epigenetic profile of PCPs has significantly evolved in the last 10 years. Brastianos and cols. (2014) identified the BRAF V600E mutation via exome sequencing in three human PCP samples ( 41 ). The same mutation was subsequently observed in 36 out of 39 PCPs ( 48 ). BRAF participates in the signaling cascade of mitogen-dependent kinases (MAPK/ERK). It is a well-established oncogene that has been shown to constitutively activate serine-threonine kinase through ligands such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF) ( 49 ). The increased MAPK/ERK signaling leads to increased proliferative capacity of the SOX2 + cells, preventing the pituitary from differentiating into hormone-producing pituitary cells and resulting in cell transformation and tumorigenesis, as schematically represented in Figure 1 ( 41 , 43 , 49 , 50 ).
Figure 1. Schematic representation of the main molecular events underlying the tumorigenesis of papillary craniopharyngiomas (PCPs).
The BRAF V600E mutation constitutively activates the signaling cascade of mitogen-dependent kinases (MAPK/ERK, also known as the Ras-Raf-MEK-ERK pathway). This increases the proliferative capacity of the SOX2 + cells, preventing the pituitary's differentiation into hormone-producing pituitary cells and resulting in cell transformation and tumorigenesis.
To assess the role of the MAPK/ERK pathway during the development of PCPs, Haston and cols. (2017) crossed the Hesx1Cre −/+ mice with animals Braf V600E /+ or Kras G12D/+. Genotyping the postnatal mice from birth to 3 weeks, these authors failed to identify any viable animals ( 49 ). Histological examination at 18.5 days post conception (dpc) revealed the presence of changes in the airways in both Braf V600E/+ or Kras G12D /+ mouse models, suggesting that abnormal lung development was the cause of the observed perinatal deaths. Anterior pituitary hyperplasia was observed at 12.5 dpc and was pronounced at 14.5 dpc. At 18.5 dpc, a penetrating phenotype of severe anterior pituitary hyperplasia with branched cleft was observed in all analyzed embryos. In addition, MAPK/ERK pathway expression was temporarily upregulated at 10.5 to 18.5 dpc. Moreover, most cells that presented upregulation of the MAPK/ERK pathway were located in the cleft epithelium, an area enriched by undifferentiated Sox2 + embryonic precursors as well as by other stem cells. Therefore, the expression of the Braf V600E mutation in the mice pituitary developing seems to lead to the expansion of Sox2 + stem cells.
The presence of BRAF V600E mutation and the expression of BRAFV600E protein were confirmed in five samples of human PCP. The staining of pERK1/2 was more restricted and focused on the areas around the fibrovascular nuclei. Double immunostaining revealed that the components of the squamous epithelial tumor also significantly expressed SOX2 . The analyzes showed that 16% of these SOX2 + cells expressed Ki67, suggesting that the proliferation of SOX2 + cells may be responsible for the growth of PCPs ( 49 ).
BRAF V600E mutation, which prevalence ranges from 81%-100%, occurs almost exclusively in PCPs ( 41 , 43 ). In this way, a hallmark of the PCPs is the positive staining for a BRAF V600E mutation-specific antibody (VE1) while β-catenin has been located on cell membranes in PCPs ( 42 ). As the BRAF V600E mutation has also been described in melanomas and the treatment with MEK and BRAF inhibitors has drastically changed the evolution of that disease, MEK and BRAF inhibitors have also been considered for treatment of selected PCPs. Indeed, the first report of MEK/BRAF inhibitors for treatment of refractory PCP described a tumor reduction of 85% and 81% of the solid and cystic parts of the tumor, respectively. These findings were subsequently confirmed ( 42 , 48 ). More recently, additional studies have further reported the results of target therapy for PCPs harboring BRAF V600E mutations. In a series of 6 patients treated with dabrafenib, trametinib and vemurafenib, isolated or in association, there was 80% to 91% regression of the cystic and solid parts in all PCP cases, which allowed surgical and/or radiotherapy after initial medical treatment ( 51 ). Currently, phase 2 of an ongoing multicentric study is assessing the role of adjuvant MEK/BRAF inhibitors in the treatment of BRAF V600E -mutation-positive PCPs (NCT03224767).
The role of inflammatory pathways has also been a topic of the study of PCPs and ACPs. Liu and cols. (2016) observed that PCPs and squamous cells from ACPs have hyperexpression of triggering receptors expressed on myeloid cells-1 (TREM-1), suggesting this as a potential marker of squamous metaplasia via inflammatory pathways ( 52 ). Chen and cols. (2018) identified a dense neutrophilic inflammation in PCPs but rarely in ACPs. In fact, neutrophils may be correlated with antitumor immunity ( 53 , 54 ). Interestingly, PCPs treated with BRAF/MEK inhibitors developed a prominent inflammatory infiltrate that has been associated with significant radiologic reduction in tumor volume ( 48 , 54 ).
Adamantinomatous craniopharyngioma
ACPs represent 90% of all craniopharyngiomas and can occur at all ages but show a bimodal distribution with peaks from 5 to 14 years old and from 55 to 74 years old ( Table 1 ) ( 6 , 55 ). The median age at ACP diagnosis in children younger than 15 years old is 8.8 ( 10 , 14 ). Hölsken and cols. (2016) demonstrated that pediatric and adult ACPs seem to have no differences either in the epigenomic or in transcriptional and methylation levels ( 43 ). These data were recently confirmed by Prince and cols. (2020), who analyzed potential age-related transcriptional differences of ACPs and found no relevant distinction between pediatric and adult ACPs ( 56 ).
Müller and cols. (2019) described the rule of 90%, whereby ∼90% of tumors are predominantly cystic, ∼90% show typically prominent calcifications, and ∼90% take up contrast media in the cyst walls, which may contain dark, greenish-brown, turbid, cholesterol-rich liquid resembling “motor oil” ( 21 , 57 ). The margins of ACPs are sharp and irregular, composed of a palisaded basal layer of cells, that may infiltrate finger-like structures which can contain whorl-like cell clusters surrounded by an intense gliosis (an inflammatory reaction in the adjacent brain), often making identification of the surgical planes difficult ( 58 ). The heterogeneous tumor epithelium is adjacent to a layer of stellate cells (known as reticulum stellate) and nodules of wet keratin formed by nuclear squamous cells or ghost cells, frequently associated with a regressive change such as cholesterol clefts with a foreign-body giant cell reaction, as are calcification and hemosiderin deposits due to chronic hemorrhage. These traits are specific features of ACPs ( 10 ). The ACP reticulum stellate is similar to the one inside the enamel organ of an embryological tooth, as in odontogenic tumors, such as adamantinoma of the mandible ( 58 ). The expression of enamel proteins and LEF1 in ACPs suggests not only their morphological, but also their functional similarities with odontogenic epithelium ( 37 , 59 ).
The pathogenesis of ACPs has been characterized by activating somatic β-catenin 1 gene ( CTNNB1 ) mutations. Under normal conditions, the Wnt pathway regulates essential physiological processes including growth, reproduction, metabolism, and stress response, and a low level of β-catenin expression is limited to the cell membrane ( 60 ). In addition, β-catenin is also part of the adherent complex preserving cytoskeletal architecture that includes E-cadherin protein ( 61 , 62 ). Activating somatic CTNNB1 mutations were first identified by Sekine and cols. (2002), with a prevalence ranging from 16% to 100% in the ACPs analyzed ( 37 , 38 , 43 , 63 ). This variation may be secondary to the use of variable sequencing approaches such as Sanger or next generation sequencing (NGS) and/or due to the low proportion of tumor tissue within these samples ( 21 , 42 , 64 , 65 ).
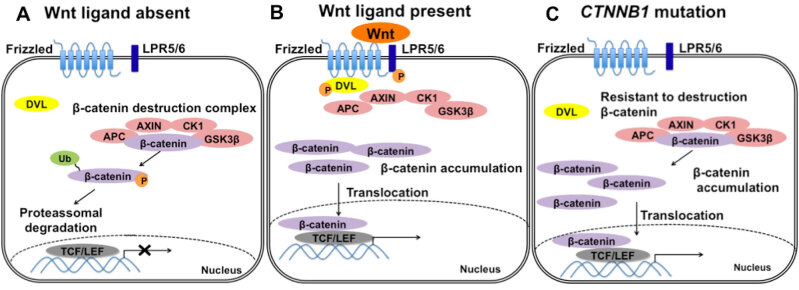
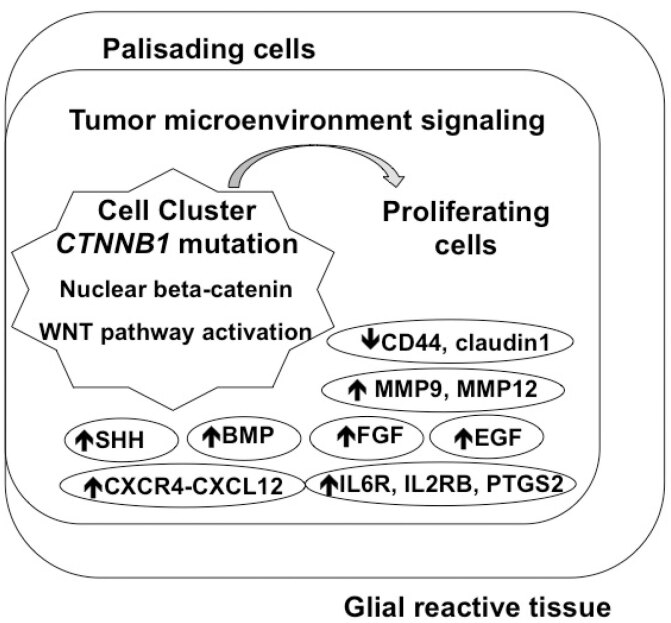
CTNNB1 mutations observed in ACPs affect the exon 3, which encodes the degradation targeting box of β-catenin protein, driving its instability and aberrant nucleo-cytoplasmic accumulation that occurs in almost 96% of ACPs ( 43 ). β-catenin nucleo-cytoplasmic accumulation leads to overactivation of the Wnt pathway, which is involved in control of cellular proliferation and pituitary embryogenesis, as evidenced by the expression of downstream pathway targets such as AXIN2, LEF1 , and BMP4 ( Figure 2 ) ( 39 , 40 , 66 , 67 ). Interestingly, in ACPs, the nucleo-cytoplasmic β-catenin accumulation is found only in small clusters of cells, with epithelial whorl-like structures, or in a few cells near the infiltrating edge of the tumor. These areas have shown to be critical signaling centers for determining the proliferation and differentiation of ACP cells through the paracrine effect of secreted factors ( Figure 3 ) ( 40 , 68 ). This aberrant pattern of β-catenin distribution has been found in several other tumors of epithelial origin, such as tumors containing nodules of wet keratin, as pilomatricoma and calcifying odontogenic cysts ( 69 ). However, hyperactivation of the Wnt pathway and aberrant nucleo-cytoplasmic β-catenin cell clusters are hallmarks of human ACPs and such characteristics are not observed in other sellar region tumors, including the PCPs ( 37 , 43 , 48 ). Similarly, CTNNB1 mutations appear to also be specific to ACPs and do not occur in other types of pituitary tumors or in PCPs ( 41 , 43 , 70 ). However, the coexistence of CTNNB1 and BRAF mutations has been described in a small number of ACPs presenting with mixed adamantinomatous and papillary histologic features ( 46 , 65 ). In addition to pathogenesis, activation of the Wnt pathway also appears to have a prognostic role in ACPs. A higher aberrant nucleo-cytoplasmic β-catenin ratio has been associated with more aggressive disease, and CTNNB1 mutations have also been related to worse overall survival rates ( 71 - 73 ). Apps and cols. (2018) identified the activation of the MAPK/ERK pathway in compartments of ACPs. The expression of several ligands, such as FGFs, epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and ERK1/2, were co-localized with the proliferation marker Ki67 within the palisading epithelium around the clusters and neighboring reactive tissue ( 74 ). Ex vivo culture experiments using small pieces of human ACPs grown with and without trametinib, an inhibitor of the MAPK/ERK pathway, revealed a reduction in the immunofluorescence of pERK1/2 in ACPs treated with trametinib compared to vehicle-treated controls that was associated with a dose-dependent increase on apoptosis and significant reduction proliferation, suggesting the downstream activation of the MAPK/ERK pathway ( 74 ).
Figure 2. Schematic representation of the canonical Wnt/β-catenin-signaling pathway. (A) In the absence of a Wnt ligand, β-catenin binds to the destruction complex (APC, AXIN, CK1 and GSK3β) and is phosphorylated by CK1 and GSK3β, then ubiquitinated and degraded by the proteasome, preventing the transcription of β-catenin target genes. (B) In the presence of a Wnt ligand, the ligand binds to its cellular receptors (Frizzled and LRP5/6), resulting in the recruitment of DVL to the membrane, which inactivates the β-catenin destruction complex, leading to accumulation of β-catenin. β-catenin translocates into the nucleus and activates target gene transcription by interacting with TCF/LEF transcription factors. (C) In the presence of CTNNB1 exon 3 mutation, β-catenin becomes resistant to degradation and accumulates, activating the Wnt pathway even in the absence of a Wnt ligand.
Figure 3. Schematic representation of the cellular compartments of the adamantonomatous craniopharyngioma (ACP) and the tumor microenvironment. The ACPs are composed of β-catenin positive cell clusters, adjacent to stellate cells known as reticulum stellate, surrounded by a palisaded basal layer of cells and intense gliosis and inflammatory reaction in the adjacent brain. These clusters could act as a paracrine tumor-signaling center by activating cells with a secretory phenotype, which may then secrete growth factors, cytokines, chemokines, and proteases, thereby changing the tumor microenvironment. Besides Wnt-pathway activation driven by the CTNNB1 mutations, different pathways and proteins have been shown to be overexpressed in ACPs as Sonic Hedgehog (SHH), epidermal growth factor (EGF), fibroblast growth factor (FGF), bone morphogenetic protein (BMP), matrix-metallopeptidases (MMP), pro-inflammatory factors as interleukins and chemokines and their receptors (IL6R, IL2RB, PTGS2, CKCR4, CXCL12), while adhesion molecules seem to be underexpressed (CD44, claudin-1).
As observed with Wnt activation, strong Sonig Hedgehog (SHH) pathway activation has also been observed, not only in cell clusters, but also in the basal layer of cells in palisades, which present cells with positive Ki67 staining ( 39 , 75 ). The SHH signaling pathway has been related to pituitary embryogenesis and seems to be involved in the maintenance of tumor stem cells. In the face of this evidence, researchers have hypothesized that the SHH pathway promotes tumor growth, infiltration, and angiogenesis by the activation of transcription factors in palisade cells by autocrine and paracrine actions ( 74 - 77 ). However, Carreno and cols. (2019), paradoxically, showed that SHH pathway inhibition in human ACPs led to a significant increase in tumor cell proliferation ( 78 ).
Many studies in ACPs have demonstrated, besides Wnt and SHH pathways, a pattern of expression of fibroblast growth factor (FGF), bone morphogenetic proteins (BMPs), and transforming growth factor β (TGFβ) families ( 40 , 74 ). Activation of the epidermal growth factor receptor (EGFR) pathway also led to β-catenin stabilization in tumor cell clusters co-expressing fascin, a member of the actin cross-linking family of proteins, which has been associated with matrix adhesion, cell migration, invasion by filopodia formation, and reorganization of the actin cytoskeleton. These findings have been demonstrated in a variety of tumors, including ACPs ( 77 , 79 - 81 ). Furthermore, fascin was associated with invasive growth behavior and consequently an unfavorable prognosis for ACP patients ( 80 , 82 ).
Cytokeratin markers (KL-1, CK5/6, CK7, CK19), which predominate along the edges of the palisade cells of ACPs, are related to invasive growth behavior ( 83 ). Of note, CK8 and CK18 were increased in positive β-catenin cell clusters, as observed in squamous carcinomas, and are indicators of loss of differentiation and tumor progression. In addition, claudin-1 (CLDN1), a component of tight junctions, has been downregulated in positive β-catenin cell clusters, in finger-like protrusions, and in tumoral cells bordering brain tissue, suggesting a role in the invasiveness pattern of ACPs. Furthermore, claudins can also influence the morphology of ACPs through the formation of cysts caused by the accumulation of fluid through leakage of the endothelium, since they have an important role in cell polarity and cell-cell adhesion ( 84 ). Claudins can also increase the typical inflammation observed in ACPs and can induce morphological changes in the glial cells (Rosenthal fibers) ( 79 , 84 ). These changes are typically present in the peritumoral brain area around the finger-like protrusions and are characterized by overexpression of Tenascin-C (TN-C), nestin, vimentin, microtubule associated protein 2 (MAP2), and glial fibrillary acid protein (GFAP). In the glial reactive tissue, the presence of cholesterol crystals leads to secretion of interleukin-1B (IL-1B), which in turn acts on the local immune effector cells to drive an inflammatory response ( 74 , 85 ).
Tumorigenic Microenvironment in ACP
In an elegant study, Gaston-Massuet and cols. (2011) developed a mutant mouse model resistant to β-catenin degradation in immature Rathke's pouch progenitor cells and provided evidence suggesting that the development of ACP is related to β-catenin activation in pituitary progenitor stem cells expressing SOX2, SOX9 , and p27KIP ( 39 ). Using laser capture microdissection to assess gene expression within the different cellular compartments of the ACPs, the authors observed the enrichment of Wnt signaling expression in palisade epithelium clusters, not glial reactive tissue. In addition, the researchers observed that these progenitor cells accumulated β-catenin and formed clusters and that surviving mice developed tumors similar to human ACPs. It is important to mention, however, that Ki67 staining was not present within the positive β-catenin cluster, suggesting that the mutated β-catenin cells may not be directly responsible for ACPs’ proliferation and growth ( 39 , 76 ).
Another mouse model characterized by overexpression of mutated β-catenin in stem cells ( Sox2 positive cells) of the adult pituitary gland also resulted in the appearance of tumors similar to human ACPs ( 86 ). It was observed a process of transient cell proliferation, the interruption of cell division and formation of clusters, which secrete (in a paracrine way) signals to neighboring cells that induce the transformation and growth of the surrounding cells, derived neither from the stem cell nor from positive β-catenin cell clusters. These clusters, therefore, may function as a tumor signaling center, leading to the hypothesis that stem cells could have a paracrine role in tumorigenesis. These cells would activate cells with a secretory phenotype associated with tumor senescence, which includes secretion of growth factors, cytokines, chemokines, proteases, and components of the extracellular compartments ( 79 ). Thus, senescent cell clusters activate a secretory phenotype that results in changes in the cellular microenvironment, characterized by inflammatory changes and immune response, which have a critical role in the pathogenesis of ACPs ( 74 , 82 , 87 ).
Inflammation seems to be closely correlated with the development of CPs. Pro-inflammatory mediators such as some interleukins (IL), including IL-6, IL-8, tumor necrosis factor (TNF), and other CXC and CC chemokines, interact with positive β-catenin cluster and their surrounding cells ( 40 , 74 , 82 ). CXCL12 and CXCR4 expressions correlate with the risk of recurrence and poor survival after resection in pediatric ACPs. Metalloproteinase-9 (MMP-9), collagen Col IV, and vascular endothelial growth factor (VEGF) may also be specific biomarkers related to the ACPs’ recurrence ( 88 , 89 ). Donson and cols. (2017) also found high levels of cytokines and chemokines, especially IL-6, CXCL1, IL-8, IL-10 and their receptors, in ACP cyst fluid and tumor tissue ( 90 ).
MMPs, regulated by β-catenin/TCF by VEGFs, are hyperexpressed in stromal capillaries and in the epithelial components of both ACPs and PCPs ( 91 , 92 ). MMPs are capable of inducing the expression of the anti-apoptotic protein B-cell leukemia/lymphoma 2 (Bcl-2) that participates in the regulation of tumor cell growth and, therefore, promotes CP growth and recurrence in an autocrine-paracrine manner ( 89 , 91 ). Using a monoclonal antibody that binds to VEGF with IL-6 receptor antagonist, Grob and cols. (2019) observed a significant cyst regression in pediatric ACPs ( 93 ). Additionally, therapy with anti-IL6 (tocilizumab) is currently being investigated in ACPs ( 94 ). The hyperexpression of IL-10 and IDO-1, immunosuppressive factors, has also been implicated as part of the pathological inflammatory microenvironment of CPs ( 90 ). In the face of all this evidence, Martinez-Barbera (2015) stated that ACPs may be an inflammation-driven tumor ( 68 ).
In conclusion, CPs can be subdivided into two groups: PCPs and ACPs. These groups differ in age distribution, clinical course, location and degree of attachment to surrounding neurovascular structures, histomorphology, and developmental pathways. The majority of ACPs and PCPs harbor single exclusive CTNNB1 or BRAF V600E clonal driver mutations, indicating a different molecular origin. Furthermore, target genes from Wnt ( LEF1 and AXIN2 ) and SHH ( GLI2, PTCH1 and SHH ) signaling pathways were up-regulated in ACPs, supporting the hypothesis that both variants of CP are different molecular entities. Additionally, protein expression and methylation profiles differ between subtypes, with hyperexpression of stem cell markers (CD133, TN-C, and MAP2) and down-regulation of CD44 and CLDN1 in ACPs, but not in PCPs. However, PCPs and ACPs also share some similarities, such as the profile of adult pituitary stem cell markers such as SOX2, OCT4 , KLF4 , and SOX9 , the expression of cytokeratins, and the expression of glial reaction proteins such as GFAP, nestin, and vimentin. Recent molecular studies of CPs have unraveled patterns of biological behavior to improve the current therapies and patients’ quality of life. Further data on genetic and epigenetic targets are still needed to better illuminate the pathways involved in the development and progression of CPs and to advance the development of additional therapeutic modalities.
REFERENCES
- 1.Martins C, Silva JISM, Machado HR, de Castro M. In: Neuroendocrinologia – SBEM . Garmes HM, Boguszewski CL, editors. São Paulo: Clannad; 2020. Craniofaringioma: Epidemiologia, classificação e bases moleculares; pp. 141–148. [Google Scholar]
- 2.Nielsen EH, Feldt-Rasmussen U, Poulsgaard L, Kristensen L, Astrup J, Jørgensen JO, et al. Incidence of craniopharyngioma in Denmark (n = 189) and estimated world incidence of craniopharyngioma in children and adults. J Neurooncol . 2011;104(3):755–763. doi: 10.1007/s11060-011-0540-6. [DOI] [PubMed] [Google Scholar]
- 3.Weiner HL, Wisoff JH, Rosenberg ME, Kupersmith MJ, Cohen, Henry MPH, et al. Craniopharyngiomas: a clinicopathological analysis of factors predictive of recurrence and functional outcome. Neurosurgery . 1994;35(6):1001–1011. doi: 10.1227/00006123-199412000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Karavitaki N, Wass JAH. Craniopharyngiomas. Endocrinol Metab Clin North Am . 2008;37(1):173–193. doi: 10.1016/j.ecl.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Feng Y, Ni M, Wang YG, Zhong LY. Comparison of neuroendocrine dysfunction in patients with adamantinomatous and papillary craniopharyngiomas. Exp Ther Med . 2019;17(1):51–56. doi: 10.3892/etm.2018.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Momin A, Recinos M, Cioffi G, Patil N, Soni P, Almeida J, et al. Descriptive epidemiology of craniopharyngiomas in the United States. Pituitary . 2021;24(4):517–522. doi: 10.1007/s11102-021-01127-6. [DOI] [PubMed] [Google Scholar]
- 7.Iglesias P, Nocete I, Moure Rodríguez MD, Venegas-Moreno E, Ares J, Biagetti B, et al. Craniopharyngioma in the elderly: a multicenter and nationwide study in Spain. Neuroendocrinology . 2021;111(10):925–936. doi: 10.1159/000512161. [DOI] [PubMed] [Google Scholar]
- 8.Lindholm J, Nielsen EH. Craniopharyngioma: historical notes. Pituitary . 2009;12(4):352–359. doi: 10.1007/s11102-008-0165-8. [DOI] [PubMed] [Google Scholar]
- 9.Raimondi AJ, Rougerie J. A critical review of personal experiences with craniopharyngioma: clinical history, surgical technique and operative results. Pediatr Neurosurg . 1994;21(2):134–154. doi: 10.1159/000120827. [DOI] [PubMed] [Google Scholar]
- 10.Karavitaki N, Cudlip S, Adams CBT, Wass JAH. Craniopharyngiomas. Endocr Rev . 2006;27(4):371–397. doi: 10.1210/er.2006-0002. [DOI] [PubMed] [Google Scholar]
- 11.Cushing H. In: Intracranial Tumours . Cushing H, editor. London: Bailliere, Tindall & Cox; 1932. The craniopharyngioma; pp. 93–98. [Google Scholar]
- 12.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol . 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 13.Almeida JP, Kalyvas A, Mohan N, Oswari S, Takami H, Velasquez C, et al. Current results of surgical treatment of raniopharyngiomas: the impact of endoscopic endonasal approaches. World Neurosurg . 2020;142:582–592. doi: 10.1016/j.wneu.2020.05.174. [DOI] [PubMed] [Google Scholar]
- 14.Müller HL. Craniopharyngioma. Endocr Rev . 2014;35(3):513–543. doi: 10.1210/er.2013-1115. [DOI] [PubMed] [Google Scholar]
- 15.Müller HL. The diagnosis and treatment of craniopharyngioma. Neuroendocrinology . 2020;110(9-10):753–766. doi: 10.1159/000504512. [DOI] [PubMed] [Google Scholar]
- 16.Müller HL. Management of hypothalamic obesity. Endocrinol Metab Clin North Am . 2020;49(3):533–552. doi: 10.1016/j.ecl.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Nogueira MC, Berbel AS, Júnior, Koenigkam-Santos M, Moreira AC, Nonino CB, de Castro M. Nutritional and endocrinologic evaluation of patients with craniopharyngioma. Clin Nutr ESPEN . 2015;10(6):e213–e218. doi: 10.1016/j.clnesp.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Spoudeas HA, Saran F, Pizer Z. A multimodality approach to the treatment of craniopharyngiomas avoiding hypothalamic morbidity: a UK perspective. J Pediatr Endocrinol Metab . 2006;19(Suppl 1):447–451. doi: 10.1515/jpem.2006.19.4.447. [DOI] [PubMed] [Google Scholar]
- 19.Kalina MA, Skala-Zamorowska E, Kalina-Faska B, Malecka-Tendera E, Mandera M. Practical approach to childhood craniopharyngioma: a role of an endocrinologist and a general paediatrician. Child's Nerv Syst . 2009;25(9):1053–1060. doi: 10.1007/s00381-009-0931-6. [DOI] [PubMed] [Google Scholar]
- 20.Müller HL. In: Adult Craniopharyngiomas: Differences and Lessons from Paediatrics . Jouanneau E, Raverot G, editors. Cham: Springer; 2020. Disease and treatment-related hypothalamic alterations in craniopharyngioma: clinical presentation, prognostic impact, and implications for treatment strategies; pp. 157–186. [Google Scholar]
- 21.Müller HL, Merchant TE, Warmuth-Metz M, Martinez-Barbera JP, Puget S. Craniopharyngioma. Nat Rev Dis Prim . 2019;5(1):1–19. doi: 10.1038/s41572-019-0125-9. [DOI] [PubMed] [Google Scholar]
- 22.Foreman NK, Faestel PM, Pearson J, Disabato J, Poole M, Wilkening G, et al. Health status in 52 long-term survivors of pediatric brain tumors. J Neurooncol . 1999;41(1):47–53. doi: 10.1023/a:1006145724500. [DOI] [PubMed] [Google Scholar]
- 23.Gump JM, Donson AM, Birks DK, Amani VM, Rao KK, Griesinger AM, et al. Identification of targets for rational pharmacological therapy in childhood craniopharyngioma. Acta Neuropathol Commun . 2015;3(1):1–12. doi: 10.1186/s40478-015-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mende KC, Kellner T, Petersenn S, Honegger J, Evangelista-Zamora R, Droste M, et al. Report from the German craniopharyngioma registry. J Clin Endocrinol Metab . 2020;105(1):252–265. doi: 10.1210/clinem/dgz043. [DOI] [PubMed] [Google Scholar]
- 25.Hunter IJ. Squamous metaplasia of cells of the anterior pituitary gland. J Pathol Bacteriol . 1955;69(1-2):141–145. doi: 10.1002/path.1700690120. [DOI] [PubMed] [Google Scholar]
- 26.Asa SL, Kovacs K, Bilbao JM, Penz G. Immunohistochemical localization of keratin in craniopharyngiomas and squamous cell nests of the human pituitary. Acta Neuropathol . 1981;54(3):257–260. doi: 10.1007/BF00687750. [DOI] [PubMed] [Google Scholar]
- 27.Asa SL, Kovacs K, Bilbao JM. The pars tuberalis of the human pituitary. Virchows Arch A Pathol Anat Histopathol . 1983;399(1):49–59. doi: 10.1007/BF00666218. [DOI] [PubMed] [Google Scholar]
- 28.Hermesz E, Mackem S, Mahon KA. Rpx: a novel anterior-restricted homeobox gene progressively activated in the prechordal plate, anterior neural plate and Rathke's pouch of the mouse embryo. Development . 1996;122(1):41–52. doi: 10.1242/dev.122.1.41. [DOI] [PubMed] [Google Scholar]
- 29.Gage PJ, Brinkmeier ML, Scarlett LM, Knapp LT, Camper SA, Mahon KA. The Ames dwarf gene, df, is required early in pituitary ontogeny for the extinction of Rpx transcription and initiation of lineage-specific cell proliferation. Mol Endocrinol . 1996;10(12):1570–1581. doi: 10.1210/mend.10.12.8961267. [DOI] [PubMed] [Google Scholar]
- 30.Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature . 1996;384(6607):327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- 31.Watkins-Chow DE, Camper SA. How many homeobox genes does it take to make a pituitary gland? Trends Genet . 1998;14(7):284–290. doi: 10.1016/s0168-9525(98)01476-0. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Barbera JP, Rodriguez TA, Beddington RSP. The homeobox gene Hesx1 is required in the anterior neural ectoderm for normal forebrain formation. Dev Biol . 2000;223(2):422–430. doi: 10.1006/dbio.2000.9757. [DOI] [PubMed] [Google Scholar]
- 33.De Almeida JPC, Sherman JH, Salvatori R, Quiñones-Hinojosa A. Pituitary stem cells: review of the literature and current understanding. Neurosurgery . 2010;67(3):770–780. doi: 10.1227/01.NEU.0000373013.75994.CD. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Barbera JP, Andoniadou CL. Biological behaviour of craniopharyngiomas. Neuroendocrinology . 2020;110(9-10):797–804. doi: 10.1159/000506904. [DOI] [PubMed] [Google Scholar]
- 35.Banna M. Craniopharyngioma: based on 160 cases. Br J Radiol . 1976;49(579):206–223. doi: 10.1259/0007-1285-49-579-206. [DOI] [PubMed] [Google Scholar]
- 36.Kato K, Nakatani Y, Kanno H, Inayama Y, Ijiri R, Nagahara N, et al. Possible linkage between specific histological structures and aberrant reactivation of the Wnt pathway in adamantinomatous craniopharyngioma. J Pathol . 2004;203(3):814–821. doi: 10.1002/path.1562. [DOI] [PubMed] [Google Scholar]
- 37.Buslei R, Nolde M, Hofmann B, Meissner S, Eyupoglu IY, Siebzehnrübl F, et al. Common mutations of β-catenin in adamantinomatous craniopharyngiomas but not in other tumours originating from the sellar region. Acta Neuropathol . 2005;109(6):589–597. doi: 10.1007/s00401-005-1004-x. [DOI] [PubMed] [Google Scholar]
- 38.Campanini ML, Colli LM, Paixao BMC, Cabral TPF, Amaral FC, Machado HR, et al. CTNNB1 gene mutations, pituitary transcription factors, and microRNA expression involvement in the pathogenesis of adamantinomatous craniopharyngiomas. Horm Cancer . 2010;1(4):187–196. doi: 10.1007/s12672-010-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaston-Massuet C, Andoniadou CL, Signore M, Jayakody SA, Charolidi N, Kyeyune R, et al. Increased Wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proc Natl Acad Sci U S A . 2011;108(28):11482–11487. doi: 10.1073/pnas.1101553108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andoniadou CL, Gaston-Massuet C, Reddy R, Schneider RP, Blasco MA, Le Tissier P, et al. Identification of novel pathways involved in the pathogenesis of human adamantinomatous craniopharyngioma. Acta Neuropathol . 2012;124(2):259–271. doi: 10.1007/s00401-012-0957-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brastianos PK, Taylor-Weiner A, Manley PE, Jones RT, Dias-Santagata D, Thorner AR, et al. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Genet . 2014;46(2):161–165. doi: 10.1038/ng.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brastianos PK, Santagata S. BRAF V600E mutations in papillary craniopharyngioma. Eur J Endocrinol . 2016;174(4):R139–R144. doi: 10.1530/EJE-15-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hölsken A, Sill M, Merkle J, Schweizer L, Buchfelder M, Flitsch J, et al. Adamantinomatous and papillary craniopharyngiomas are characterized by distinct epigenomic as well as mutational and transcriptomic profiles. Acta Neuropathol Commun . 2016;4:20–20. doi: 10.1186/s40478-016-0287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crotty TB, Scheithauer BW, Young WF, Davis DH, Shaw EG, Miller GM, et al. Papillary craniopharyngioma: a clinicopathological study of 48 cases. J Neurosurg . 1995;83(2):206–214. doi: 10.3171/jns.1995.83.2.0206. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen EH, Jørgensen JO, Bjerre P, Andersen M, Andersen C, Feldt-Rasmussen U, et al. Acute presentation of craniopharyngioma in children and adults in a Danish national cohort. Pituitary . 2013;16(4):528–535. doi: 10.1007/s11102-012-0451-3. [DOI] [PubMed] [Google Scholar]
- 46.Larkin SJ, Ansorge O. Pathology and pathogenesis of craniopharyngiomas. Pituitary . 2013;16(1):9–17. doi: 10.1007/s11102-012-0418-4. [DOI] [PubMed] [Google Scholar]
- 47.Larkin S, Karavitaki N. Recent advances in molecular pathology of craniopharyngioma. F1000 Res . 2017;6:1–9. doi: 10.12688/f1000research.11549.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brastianos PK, Shankar GM, Gill CM, Taylor-Weiner A, Nayyar N, Panka DJ, et al. Dramatic response of BRAF V600E mutant papillary craniopharyngioma to targeted therapy. J Natl Cancer Inst . 2016;108(2):1–5. doi: 10.1093/jnci/djv310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haston S, Pozzi S, Carreno G, Manshaei S, Panousopoulos L, Gonzalez-Meljem JM, et al. MAPK pathway control of stem cell proliferation and differentiation in the embryonic pituitary provides insights into the pathogenesis of papillary craniopharyngioma. Development . 2017;144(12):2141–2152. doi: 10.1242/dev.150490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Apps JR, Martinez-Barbera JP. In: Adult Craniopharyngiomas: Differences and Lessons from Paediatrics . Jouanneau E, Raverot G, editors. Cham: Springer International Publishing; 2020. Mouse models of craniopharyngioma; pp. 19–33. [Google Scholar]
- 51.Juratli TA, Jones PS, Wang N, Subramanian M, Aylwin SJB, Odia Y, et al. Targeted treatment of papillary craniopharyngiomas harboring BRAFV600E mutations. Cancer . 2019;125(17):2910–2910. doi: 10.1002/cncr.32197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Wang CH, Li DL, Zhang SC, Peng YP, Peng J, et al. TREM-1 expression in craniopharyngioma and Rathke's cleft cyst: its possible implication for controversial pathology. Oncotarget . 2016;7(31):50564–50574. doi: 10.18632/oncotarget.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen M, Zheng SH, Yang M, Chen ZH, Li ST. The diagnostic value of preoperative inflammatory markers in craniopharyngioma: a multicenter cohort study. J Neurooncol . 2018;138(1):113–122. doi: 10.1007/s11060-018-2776-x. [DOI] [PubMed] [Google Scholar]
- 54.Coy S, Rashid R, Lin JR, Du Z, Donson AM, Hankinson TC, et al. Multiplexed immunofluorescence reveals potential PD-1/PD-L1 pathway vulnerabilities in craniopharyngioma. Neuro Oncol . 2018;20(8):1101–1112. doi: 10.1093/neuonc/noy035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bunin GR, Surawicz TS, Witman PA, Preston-Martin S, Davis F, Bruner JM. The descriptive epidemiology of craniopharyngioma. J Neurosurg . 1998;89(4):547–551. doi: 10.3171/jns.1998.89.4.0547. [DOI] [PubMed] [Google Scholar]
- 56.Prince E, Whelan R, Donson A, Staulcup S, Hengartner A, Vijmasi T, et al. Transcriptional analyses of adult and pediatric adamantinomatous craniopharyngioma reveals similar expression signatures regarding potential therapeutic targets. Acta Neuropathol Commun . 2020;8(1):1–10. doi: 10.1186/s40478-020-00939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petito CK, DeGirolami U, Earle KM. Craniopharyngiomas: a clinical and pathological review. Cancer . 1976;37(4):1944–1952. doi: 10.1002/1097-0142(197604)37:4<1944::aid-cncr2820370446>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 58.Prabhu VC, Brown HG. The pathogenesis of craniopharyngiomas. Child's Nerv Syst . 2005;21(8-9):622–627. doi: 10.1007/s00381-005-1190-9. [DOI] [PubMed] [Google Scholar]
- 59.Sekine S, Takata T, Shibata T, Mori M, Morishita Y, Noguchi M, et al. Expression of enamel proteins and LEF1 in adamantinomatous craniopharyngioma: evidence for its odontogenic epithelial differentiation. Histopathology . 2004;45(6):573–579. doi: 10.1111/j.1365-2559.2004.02029.x. [DOI] [PubMed] [Google Scholar]
- 60.Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, et al. Homeodomain-mediated β-catenin-dependent switching events dictate cell-lineage determination. Cell . 2006;125(3):593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 61.Huber AH, Weis WI. The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell . 2001;105(3):391–402. doi: 10.1016/s0092-8674(01)00330-0. [DOI] [PubMed] [Google Scholar]
- 62.Preda V, Larkin SJ, Karavitaki N, Ansorge O, Grossman AB. The Wnt signalling cascade and the adherens junction complex in craniopharyngioma tumorigenesis. Endocr Pathol . 2015;26(1):1–8. doi: 10.1007/s12022-014-9341-8. [DOI] [PubMed] [Google Scholar]
- 63.Sekine S, Shibata T, Kokubu A, Morishita Y, Noguchi M, Nakanishi Y, et al. Craniopharyngiomas of adamantinomatous type harbor β-catenin gene mutations. Am J Pathol . 2002;161(6):1997–2001. doi: 10.1016/s0002-9440(10)64477-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Apps JR, Stache C, Gonzalez-Meljem JM, Gutteridge A, Chalker J, Jacques TS, et al. CTNNB1 mutations are clonal in adamantinomatous craniopharyngioma. Neuropathol Appl Neurobiol . 2020;46(5):510–514. doi: 10.1111/nan.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prieto R, Pascual JM. Can tissue biomarkers reliably predict the biological behavior of craniopharyngiomas? A comprehensive overview. Pituitary . 2018;21(4):431–442. doi: 10.1007/s11102-018-0890-6. [DOI] [PubMed] [Google Scholar]
- 66.Kato K, Nakatani Y, Kanno H, Inayama Y, Ijiri R, Nagahara N, et al. Possible linkage between specific histological structures and aberrant reactivation of the Wnt pathway in adamantinomatous craniopharyngioma. J Pathol . 2004;203(3):814–821. doi: 10.1002/path.1562. [DOI] [PubMed] [Google Scholar]
- 67.Hölsken A, Kreutzer J, Hofmann BM, Hans V, Oppel F, Buchfelder M, et al. Target gene activation of the Wnt signaling pathway in nuclear β-catenin accumulating cells of adamantinomatous craniopharyngiomas. Brain Pathol . 2009;19(3):357–364. doi: 10.1111/j.1750-3639.2008.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez-Barbera JP. Molecular and cellular pathogenesis of adamantinomatous craniopharyngioma. Neuropathol Appl Neurobiol . 2015;41(6):721–732. doi: 10.1111/nan.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hassanein AM, Glanz SM, Kessler HP, Eskin TA, Liu C. β-catenin is expressed aberrantly in tumors expressing shadow cells: pilomatricoma, craniopharyngioma, and calcifying odontogenic cyst. Am J Clin Pathol . 2003;120(5):732–736. doi: 10.1309/EALE-G7LD-6W71-67PX. [DOI] [PubMed] [Google Scholar]
- 70.Colli LM, Saggioro F, Serafini LN, Camargo RC, Machado HR, Moreira AC, et al. Components of the canonical and non-canonical Wnt pathways are not mis-expressed in pituitary tumors. PLoS One . 2013;8(4):1–7. doi: 10.1371/journal.pone.0062424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Z, Xu J, Huang S, You C. Aberrant membranous expression of β-catenin predicts poor prognosis in patients with craniopharyngioma. Ann Diagn Pathol . 2015;19(6):403–408. doi: 10.1016/j.anndiagpath.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 72.Jucá CEB, Colli LM, Martins CS, Campanini ML, Paixão B, Jucá RV, et al. Impact of the canonical Wnt pathway activation on the pathogenesis and prognosis of adamantinomatous craniopharyngiomas. Horm Metab Res . 2018;50(7):575–581. doi: 10.1055/a-0593-5956. [DOI] [PubMed] [Google Scholar]
- 73.Gao C, Wang Y, Broaddus R, Sun L, Xue F, Zhang W. Exon 3 mutations of CTNNB1 drive tumorigenesis: a review. Oncotarget . 2018;9(4):5492–5508. doi: 10.18632/oncotarget.23695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Apps JR, Carreno G, Gonzalez-Meljem JM, Haston S, Guiho R, Cooper JE, et al. Tumour compartment transcriptomics demonstrates the activation of inflammatory and odontogenic programmes in human adamantinomatous craniopharyngioma and identifies the MAPK/ERK pathway as a novel therapeutic target. Acta Neuropathol . 2018;135(5):757–777. doi: 10.1007/s00401-018-1830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gomes DC, Jamra SA, Leal LF, Colli LM, Campanini ML, Oliveira RS, et al. Sonic Hedgehog pathway is upregulated in adamantinomatous craniopharyngiomas. Eur J Endocrinol . 2015;172(5):603–608. doi: 10.1530/EJE-14-0934. [DOI] [PubMed] [Google Scholar]
- 76.Garcia-Lavandeira M, Saez C, Diaz-Rodriguez E, Perez-Romero S, Senra A, Dieguez C, et al. Craniopharyngiomas express embryonic stem cell markers (SOX2, OCT4, KLF4, and SOX9) as pituitary stem cells but do not coexpress RET/GFRA3 receptors. J Clin Endocrinol Metab . 2012;97(1):80–87. doi: 10.1210/jc.2011-2187. [DOI] [PubMed] [Google Scholar]
- 77.Martinez-Barbera JP, Buslei R. Adamantinomatous craniopharyngioma: pathology, molecular genetics and mouse models. J Pediatr Endocrinol Metab . 2015;28(1-2):7–17. doi: 10.1515/jpem-2014-0442. [DOI] [PubMed] [Google Scholar]
- 78.Carreno G, Boult JKR, Apps J, Gonzalez-Meljem JM, Haston S, Guiho R, et al. SHH pathway inhibition is protumourigenic in adamantinomatous craniopharyngioma. Endocr Relat Cancer . 2019;26(3):355–366. doi: 10.1530/ERC-18-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hölsken A. In: Basic Research and Clinical Aspects of Adamantinomatous Craniopharyngioma . Martinez-Barbera JP, Andoniadou CL, editors. Cham: Springer International Publishing AG; 2017. Pathogenesis of human ACP; pp. 1–26. [Google Scholar]
- 80.Hölsken A, Gebhardt M, Buchfelder M, Fahlbusch R, Blümcke I, Buslei R. EGFR signaling regulates tumor cell migration in craniopharyngiomas. Clin Cancer Res . 2011;17(13):4367–4377. doi: 10.1158/1078-0432.CCR-10-2811. [DOI] [PubMed] [Google Scholar]
- 81.Bogusz A, Müller HL. Childhood-onset craniopharyngioma: latest insights into pathology, diagnostics, treatment, and follow-up. Expert Rev Neurother . 2018;18(10):793–806. doi: 10.1080/14737175.2018.1528874. [DOI] [PubMed] [Google Scholar]
- 82.Whelan R, Hengartner A, Folzenlogen Z, Prince E, Hankinson TC. Adamantinomatous craniopharyngioma in the molecular age and the potential of targeted therapies: a review. Child's Nerv Syst . 2020;36(8):1635–1642. doi: 10.1007/s00381-020-04677-5. [DOI] [PubMed] [Google Scholar]
- 83.Buslei R, Hölsken A, Hofmann B, Kreutzer J, Siebzehnrubl F, Hans V, et al. Nuclear β-catenin accumulation associates with epithelial morphogenesis in craniopharyngiomas. Acta Neuropathol . 2007;113(5):585–590. doi: 10.1007/s00401-006-0184-3. [DOI] [PubMed] [Google Scholar]
- 84.Stache C, Hölsken A, Fahlbusch R, Flitsch J, Schlaffer SM, Buchfelder M, et al. Tight junction protein claudin-1 is differentially expressed in craniopharyngioma subtypes and indicates invasive tumor growth. Neuro Oncol . 2014;16(2):256–264. doi: 10.1093/neuonc/not195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burghaus S, Hölsken A, Buchfelder M, Fahlbusch R, Riederer BM, Hans V, et al. A tumor-specific cellular environment at the brain invasion border of adamantinomatous craniopharyngiomas. Virchows Arch . 2010;456(3):287–300. doi: 10.1007/s00428-009-0873-0. [DOI] [PubMed] [Google Scholar]
- 86.Andoniadou CL, Matsushima D, Mousavy Gharavy SN, Signore M, Mackintosh AI, Schaeffer M, et al. Sox2+ stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell . 2013;13(4):433–445. doi: 10.1016/j.stem.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 87.Gonzalez-Meljem JM, Haston S, Carreno G, Apps JR, Pozzi S, Stache C, et al. Stem cell senescence drives age-attenuated induction of pituitary tumours in mouse models of paediatric craniopharyngioma. Nat Commun . 2017;8(1):1–14. doi: 10.1038/s41467-017-01992-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gong J, Zhang H, Xing S, Li C, Ma Z, Jia G, et al. High expression levels of CXCL12 and CXCR4 predict recurrence of adamanti-nomatous craniopharyngiomas in children. Cancer Biomarkers . 2014;14(4):241–251. doi: 10.3233/CBM-140397. [DOI] [PubMed] [Google Scholar]
- 89.Xia Z, Liu W, Li S, Jia G, Zhang Y, Li C, et al. Expression of matrix metalloproteinase-9, type IV collagen and vascular endothelial growth factor in adamantinous craniopharyngioma. Neurochem Res . 2011;36(12):2346–2351. doi: 10.1007/s11064-011-0560-9. [DOI] [PubMed] [Google Scholar]
- 90.Donson AM, Apps J, Griesinger AM, Amani V, Witt DA, Anderson RCE, et al. Molecular analyses reveal inflammatory mediators in the solid component and cyst fluid of human adamantinomatous craniopharyngioma. J Neuropathol Exp Neurol . 2017;76(9):779–788. doi: 10.1093/jnen/nlx061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vidal S, Kovacs K, Lloyd RV, Meyer FB, Scheithauer BW. Angiogenesis in patients with craniopharyngiomas: correlation with treatment and outcome. Cancer . 2002;94(3):738–745. doi: 10.1002/cncr.10281. [DOI] [PubMed] [Google Scholar]
- 92.Olsen JJ, Pohl SÖ, Deshmukh A, Visweswaran M, Ward NC, Arfuso F, et al. The role of Wnt signalling in angiogenesis. Clin Biochem Rev . 2017;38(3):131–142. [PMC free article] [PubMed] [Google Scholar]
- 93.Grob S, Mirsky DM, Donson AM, Dahl N, Foreman NK, Hoffman LM, et al. Targeting IL-6 is a potential treatment for primary cystic craniopharyngioma. Front Oncol . 2019;9:1–6. doi: 10.3389/fonc.2019.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hengartner AC, Prince E, Vijmasi T, Hankinson TC. Adamantinomatous craniopharyngioma: moving toward targeted therapies. Neurosurg Focus . 2019;48(1):1–9. doi: 10.3171/2019.10.FOCUS19705. [DOI] [PubMed] [Google Scholar]