Abstract
Most cancer cells utilize glucose at a high rate to produce energy and precursors for the biosynthesis of macromolecules such as lipids, proteins, and nucleic acids. This phenomenon is called the Warburg effect or aerobic glycolysis— this distinct characteristic is an attractive target for developing anticancer drugs. Here, we found that Phosphofructokinase-1 (PFK-1) is a substrate of the Protein Phosphatase 4 catalytic subunit (PP4C)/PP4 regulatory subunit 1 (PP4R1) complex by using immunoprecipitation and in vitro assay. While manipulation of PP4C/PP4R1 does not have a critical impact on PFK-1 expression, the absence of the PP4C/PP4R1 complex increases PFK-1 activity. Although PP4C depletion or overexpression does not cause a dramatic change in the overall glycolytic rate, PP4R1 depletion induces a considerable increase in both basal and compensatory glycolytic rates, as well as the oxygen consumption rate, indicating oxidative phosphorylation. Collectively, the PP4C/PP4R1 complex regulates PFK-1 activity by reversing its phosphorylation and is a promising candidate for treating glycolytic disorders and cancers. Targeting PP4R1 could be a more efficient and safer strategy to avoid pleiotropic effects than targeting PP4C directly.
Keywords: Glycolysis, Phosphofructokinase-1, Protein phosphatase 4, Protein phosphatase 4 regulatory subunit 1, Warburg effect
INTRODUCTION
Cells generate energy molecules, such as ATP, through glycolysis and oxidative phosphorylation (OXPHOS) (1). In normal or nonmalignant cells, glycolysis produces only a small portion of ATP and pyruvate, which is processed in mitochondria to produce more ATP. Although the energy yield of glycolysis is low, most cancer cells utilize glycolysis more frequently than OXPHOS (2, 3). This phenomenon is called the Warburg effect or aerobic glycolysis. Although glycolysis seems disadvantageous for cancer cells, energy production is much faster by glycolysis than OXPHOS, and metabolic intermediates from glycolysis are used as precursors for the biosynthesis of macromolecules, such as lipids, proteins, DNA, and RNA (3). Reversing the glycolytic phenotype to OXPHOS or its activation in some cancer cells can lead to apoptosis and cell death, indicating that a higher rate of glycolysis is necessary for cancer survival instead of facilitating OXPHOS (3, 4). Thus, enhanced glycolysis is an attractive target for cancer treatment, and many anticancer drugs that target glycolysis and glucose transporter have been developed (2, 3, 5, 6).
Phosphofructokinase-1 (PFK-1) catalyzes the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate and is a rate-limiting enzyme in glycolysis together with hexokinase (2, 7). PFK-1 has three isoforms: PFKL (liver), PFKM (muscle), and PFKP (platelets), and functions as a homo- or hetero-tetramer, depending on the cell or tissue type (8, 9). PFKL is expressed abundantly in the liver and kidney; PFKM is expressed in the muscle, whereas platelets only express PFKP. However, many isoform combinations of PFK-1 are present in other tissues, including the brain. All PFK-1 isoforms can be modified by O-linked β-N-acetylglucosamine, which suppresses PFK-1 activity in response to hypoxia (10). Also, insulin-induced phosphorylation of PFKL at serine 775 increases its activity (11). Whereas AKT phosphorylates PFKP at serine 386 and increases its stability, phosphorylation of PFKP on tyrosine 64 by epidermal growth factor receptor (EGFR) indirectly increases fructose-2,6-bisphosphate (F-2,6-BP) — the activator of PFK-1 — through AKT-dependent PFK-2 activation, leading to PFK-1 activation (12, 13). However, ULK1-mediated PFKM phosphorylation at serine 74 and 762 suppresses its activity (14). While there are a few cases showing PFK-1 phosphorylation, studies on dephosphorylation of PFK-1 have not been reported yet.
Protein phosphatase 4 (PP4) is a serine/threonine phosphatase that has important roles in cellular pathways, including DNA damage response, immune response, stem cell development, and diabetes (15). Most known functions of PP4 are related to the DNA damage response, but PP4 can also affect metabolic pathways by dephosphorylating AMP-activated protein kinase (AMPK) and acetyl-CoA carboxylase 1 (ACC1) (16, 17). PP4 comprises of one catalytic subunit (PP4C) and five regulatory subunits (PP4R1, PP4R2, PP4R3α, PP4R3β, and PP4R4) and forms heterodimers (PP4C/PP4R1 or PP4C/PP4R4) or heterotrimers (PP4C/PP4R2/PP4R3α or PP4C/PP4R2/PP4R3β) (15, 18, 19). Its specificity for substrates is determined depending on the regulatory subunits, and not the catalytic subunit (20).
In this study, we found that the PP4C/PP4R1 complex interacts with and dephosphorylates PFK-1, especially PFKM and PFKP. PP4C/PP4R1 regulates the enzymatic activity, and not the expression and stability, of PFK-1. Furthermore, in contrast to PP4C alteration, PP4R1 depletion increases not only both basal and compensatory glycolysis rate, but also oxygen consumption rate (OCR), indicating OXPHOS levels. Altogether, these data indicate that the PP4C/PP4R1 complex controls PFK-1 activity by altering the phosphorylation status of PFK-1, and PP4R1 is a promising drug target for glycolysis-related diseases.
RESULTS
PFK-1 is a substrate of PP4
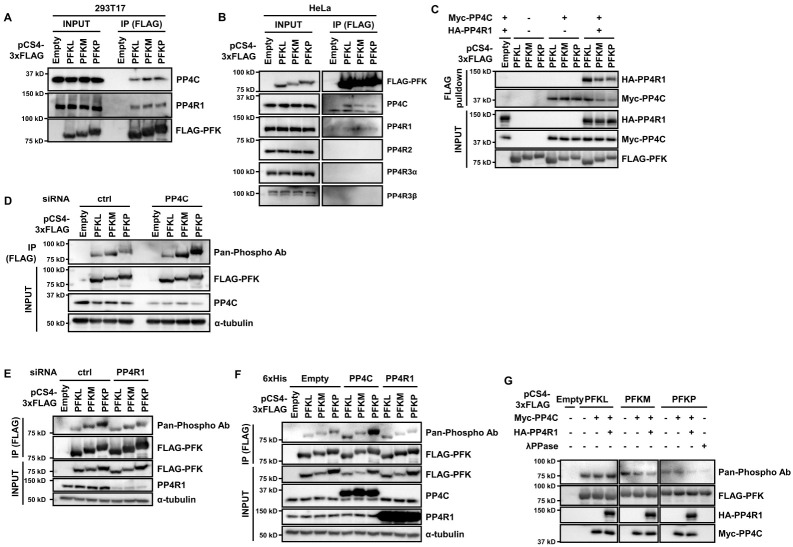
In a study, we performed tandem affinity purification-mass spectrometry (TAP-MS) using HeLa S3 cells stably expressing FLAG, HA (FH)-tagged PP4C to examine the role of PP4 (21). The data revealed three isoforms of PFK-1 (PFKL, PFKM, and PFKP) as candidate interacting partners of PP4C (21). To validate the interaction between PFK-1 and PP4, we transfected plasmids encoding FLAG-PFK-1 isoforms and performed immunoprecipitation. All isoforms of FLAG-PFK-1 interacted with endogenous PP4C and PP4R1, and not PP4R2, PP4R3α, and PP4R3β (Fig. 1A, B). Interaction between PFK-1 and PP4C/PP4R1 was confirmed again through in vitro binding assay using immunoprecipitated PFK-1 and PP4C/PP4R1 (Fig. 1C). In conclusion, the PP4C/PP4R1 complex interacts with PFK-1.
Fig. 1.
PFK-1 is a substrate of PP4. (A, B) FLAG-PFK-1 isoforms interact with endogenous PP4C and PP4R1. Plasmids encoding FLAG-PFK-1 were transfected into 293T17 (A) or HeLa (B) cells. The cells were immunoprecipitated with ANTI-FLAG M2-magnetic beads, followed by immunoblotting with antibodies against FLAG, PP4C, PP4R1, PP4R2, PP4R3α, and PP4R3β. (C) In vitro binding assay between PFK-1 and PP4C/PP4R1. FLAG-PFK-1, Myc-PP4C, and HA-PP4R1 were immunoprecipitated, mixed, and incubated for 1 h at 30°C. After wash, the mixtures were subjected to immunoblotting with antibodies against FLAG, HA, and Myc. (D, E) Phosphorylation of PFKM and PFKP increases upon PP4C/PP4R1 depletion. 293T17 cells were transfected with PP4C (D) or PP4R1 (E) siRNA and plasmids for FLAG-PFK-1, lysed, immunoprecipitated, and immunoblotted with antibodies against FLAG, PP4C, α-tubulin, and pan-phosphorylation. (F) PP4C overexpression increases PFKL and PFKP phosphorylation. 293T17 cells were cotransfected with plasmids encoding FLAG-PFK-1 and His-PP4C or PP4R1, immunoprecipitated, and immunoblotted with antibodies against FLAG, PP4C, PP4R1, α-tubulin, and pan-phosphorylation. (G) In vitro dephosphorylation assay. Immunoprecipitated FLAG-PFK-1, Myc-PP4C, and HA-PP4R1 were mixed and incubated for 1 h at 30°C, followed by immunoblotting. Lambda phosphatase (λPPase) was used as a positive control. Representative data are from experiments performed in triplicate.
To elucidate whether PFK-1 is a substrate of PP4, we analyzed PFK-1 phosphorylation in PP4C-depleted 293T17 cells. PP4C depletion increased the phosphorylation levels of PFKM and PKFP; however, the PP4-PFKL interaction did not alter PFKL phosphorylation (Fig. 1D). Unexpectedly, PP4R1 knockdown decreased the phosphorylation of PFKM and PFKP, but not PFKL (Fig. 1E). In addition, PP4C overexpression increased phosphorylation of PFKL and PFKP, but PP4R1 overexpression did not affect PFK-1 phosphorylation (Fig. 1F). Because PP4 has multifaceted functions in various pathways and may regulate PFK-1 phosphorylation status directly or indirectly, the total PFK-1 phosphorylation after manipulating PP4 may vary due to pleiotropic effects. Thus, simply detecting overall PFK-1 phosphorylation does not adequately reflect the specific effects of PP4. To solve this problem, we performed in vitro dephosphorylation assay by incubating immunoprecipitated PFK-1 with PP4C and PP4R1. Purified PP4C decreased phosphorylation of PFKM and PFKP, but not PFKL, and addition of PP4R1 further decreased the phosphorylation level (Fig. 1G). This means that PP4R1 is required for PP4C to dephosphorylate PFKM and PFKP efficiently. Collectively, PFKM and PFKP are the substrates of PP4C/PP4R1 complex. Based on these findings, we carried out the following experiments with PP4C/PP4R1 complex.
PP4C/PP4R1 does not regulate PFK-1 expression
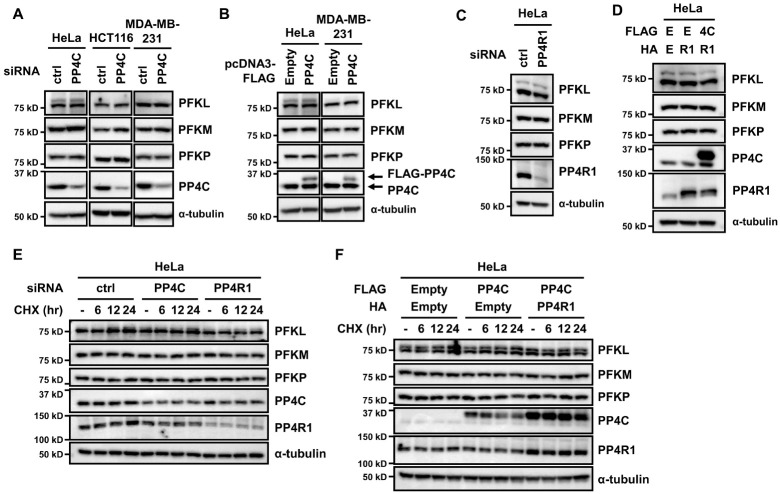
Phosphorylation can change protein expression by regulating protein stability (22). Generally, protein stability correlates with protein expression. Thus, we checked whether the expression of PFK-1 could be altered by PP4C knockdown. In all tested cell lines, the expression of PFK-1 isoforms did not change upon PP4C depletion (Fig. 2A). In addition, all PFK-1 isoforms were expressed constantly irrespective of PP4C overexpression (Fig. 2B). We further explored the potential role of PP4R1 in modulating PFK-1 expression. Similar to PP4C manipulation, PP4R1 depletion or overexpression, as well as simultaneous overexpression with PP4C, did not change the expression level of PFK-1 isoforms (Fig. 2C, D).
Fig. 2.
PP4C/PP4R1 does not regulate PFK-1 expression. (A) HeLa, HCT116, and MDA-MB-231 cells were transfected with PP4C siRNA, and the cell lysates were probed with antibodies against PFKL, PFKM, PFKP, PP4C, and α-tubulin. (B) In parallel, HeLa and MDA-MB-231 cells transiently transfected with FLAG-PP4C plasmid were lysed for immunoblotting. (C) HeLa cells were transfected with PP4R1 siRNA and subjected to immunoblotting. (D) HeLa cells were cotransfected with FLAG-PP4C and HA-PP4R1 and analyzed by western blot. E, Empty; 4C, PP4C; R1, PP4R1. (E, F) HeLa cells transfected with PP4C or PP4R1 siRNA (E) or a plasmid encoding PP4C or PP4R1 (F) were treated with 100 μg/ml cycloheximide (CHX) for 6, 12, or 24 h and subjected to immunoblotting. Representative data are from experiments performed in triplicate.
The phosphorylation of PFKP by AKT increases its expression and stability (12). To investigate whether the PP4C/PP4R1 complex affects the stability of PFK-1 isoforms, we treated cells in which PP4C/PP4R1 was manipulated with cycloheximide and assessed the expression level of PFK-1 isoforms. Contrary to the previous study, both depletion and overexpression of PP4C and PP4R1 did not change PFK-1 expression even in the presence of cycloheximide (Fig. 2E, F). Altogether, the PP4C/PP4R1 complex is not critical for PFK-1 expression and stability.
Dephosphorylation of PFK-1 by PP4C/PP4R1 downregulates PFK-1 activity
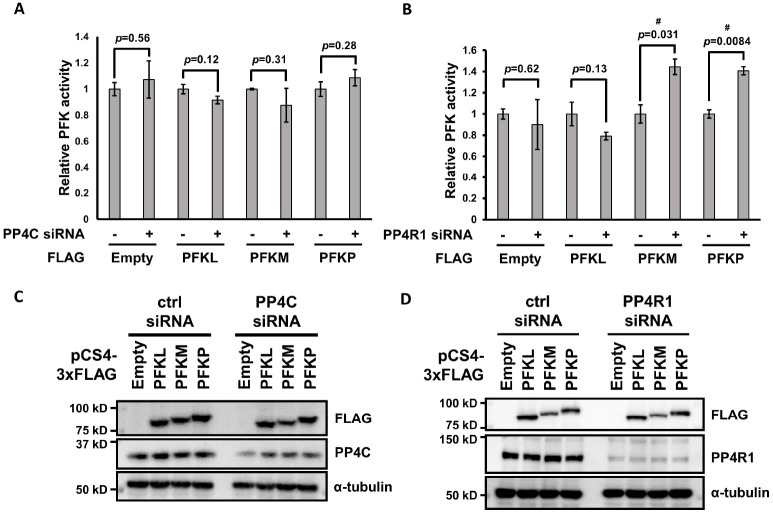
Enzymatic activity can be regulated through post-translational modifications, such as phosphorylation (23). To examine whether PFK-1 activity changes in response to its phosphorylation status, we measured PFK-1 activity using a commercially available assay kit, as described (12, 13). Unexpectedly, PP4C downregulation did not affect PFK-1 activity (Fig. 3A, C). However, PP4R1 depletion upregulated the activities of PFKM and PFKP, but not PFKL (Fig. 3B, D). This is in line with results that PP4C/PP4R1 dephosphorylates PFKM and PFKP (Fig. 1C). Altogether, the PP4C/PP4R1 complex can regulate PFK-1 activity.
Fig. 3.
The PP4C/PP4R1 complex inhibits PFK-1 activity. (A, B) HeLa cells were transfected with PP4C (A) or PP4R1 (B) siRNA in combination with plasmids encoding FLAG-PFK-1 isoforms and cells were collected to analyze PFK activity. Experiments were performed in triplicate and data are expressed as mean ± standard deviation (SD). Statistical analysis was performed using an unpaired, two-tailed Student’s t-test. P-values are described on the graph. # indicates statistical significance (P < 0.05). (C, D) Confirmation of expression level for the PFK activity assay. The cell lysates used for PFK activity assay were subjected to immunoblotting with antibodies against PP4C, PP4R1, FLAG, and α-tubulin. Representative blots are shown.
Depletion of the PP4C/PP4R1 complex stimulates glycolysis and oxygen consumption
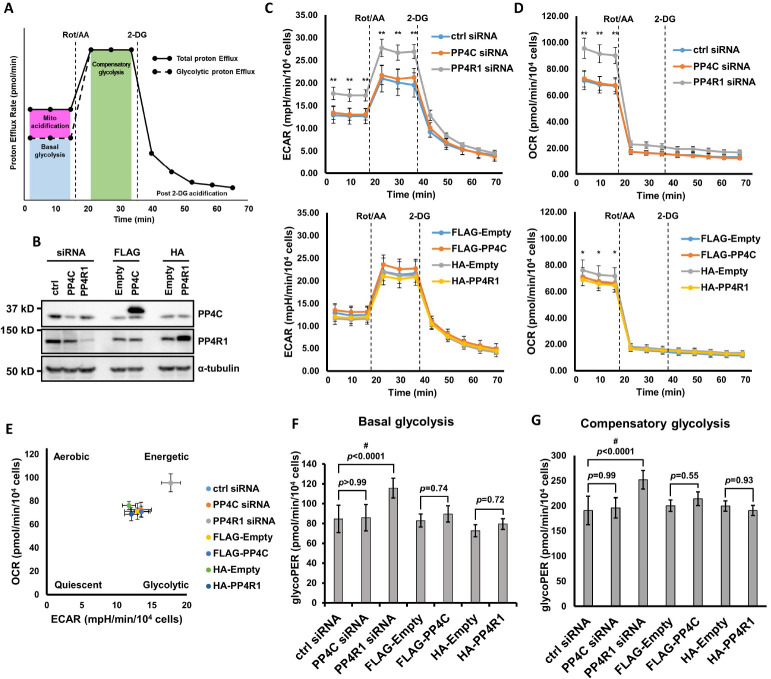
To check whether PP4C/PP4R1-mediated PFK-1 regulation could affect glycolysis efficiency, we measured glycolytic rate and OCR in cells where the expression of PP4C or PP4R1 was manipulated using Seahorse real-time cell metabolic analyzer (Fig. 4A). Consistent with the results of the PFK-1 activity assay, both PP4C depletion and overexpression did not affect metabolic efficiency (Fig. 4B-G). By contrast, PP4R1 depletion considerably enhanced the glycolytic rates as well as OCR (Fig. 4B-G). The energy map representing basal ECAR vs. basal OCR showed that PP4R1-depleted cells were more energetic than other cells (Fig. 4E). In addition, PP4R1 overexpression decreased OCR (Fig. 4D). The difference was very slight, but statistically significant. Thus, PP4R1 regulation could be more efficient to control PFK-1 activity, and consequently, glycolysis than PP4C regulation.
Fig. 4.
PP4R1 depletion boosts glycolytic rate and OCR. (A) Agilent Seahorse XF Glycolytic rate assay profile. This figure was adapted from the manufacturer’s manual. Measured PER contains both glycolysis and mitochondria-derived acidification. Basal glycoPER can be calculated by subtracting mitochondrial acidification from total PER. Rotenone/antimycin A (Rot/AA; inhibitors of mitochondrial electron transport chain) inhibit OXPHOS and induce compensatory changes to meet the energy demands. 2-DG confirms that PER measured is attributed primarily to glycolysis. (B) Confirmation of expression level for Seahorse analysis. HeLa cells were transfected with either siRNA or plasmids to manipulate PP4C or PP4R1 expression. Altered expression was analyzed by western blot. Representative blots are shown. (C, D) Measurement of ECAR (C) and OCR (D) levels in PP4C/PP4R1-depleted (upper panel) or overexpressing (lower panel) HeLa cells. OCR and ECAR levels were measured under basal conditions and after injecting Rot/AA and 2-DG, in that order. Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparison test. * means P < 0.05 for HA-Empty vs. HA-PP4R1, ** means P < 0.001 for ctrl siRNA vs. PP4R1 siRNA. (E) Energy map representing OCR vs ECAR. Values measured first under basal conditions were used to create the graph. (F, G) Basal (F) and compensatory (G) glycolytic rate in HeLa cells with altered expression of PP4C and PP4R1. Statistical analysis was performed using one-way ANOVA with Tukey’s test. # indicates statistical significance (P < 0.05). Experiments were performed in triplicate and representative results are shown. Data are expressed as mean ± standard deviation (SD). P-values are described on the graph.
DISCUSSION
Cancer cells use glycolysis preferentially even in the presence of available oxygen. This unusual phenotype can be an attractive target for developing anticancer drugs. PFK-1 is a rate-limiting enzyme in the glycolytic pathway and indispensable for glycolysis. Only a few studies have elucidated PFK-1 phosphorylation (11-14). However, to our knowledge, studies on PFK-1 dephosphorylation are lacking. In a previous study, we identified PFK-1 isoforms as interaction partners of PP4C through TAP-MS (21). Coimmunoprecipitation confirmed an interaction between PFK-1 and PP4C/PP4R1. Although all PFK-1 isoforms can be phosphorylated, only PFKM and PFKP can be dephosphorylated by PP4. However, PFKL can still interact with PP4, likely indirectly through other isoforms, as PFK-1 forms homo- or hetero-tetramers. To analyze the interaction between PFK-1 and PP4 specifically, in vitro binding assay or coimmunoprecipitation from cells expressing only one type of isoform must be performed.
Previously, it was shown that PFK-1 phosphorylation alters its activity directly or indirectly. Insulin treatment induces PFKL phosphorylation at serine 775 and its activation (11). But, ULK1-mediated PFKM phosphorylation at serine 74 and 762 suppresses its activity (14). On the other hand, phosphorylation of PFKP by AKT was reported to inhibit PFKP degradation, promoting glycolysis through increased PFKP levels and not alteration of enzymatic activity (12). In addition, phosphorylation of PFKP on tyrosine 64 by EGFR boosts its activity indirectly (13). While some phosphorylation sites of PFK-1 are known, the sites reversed by PP4 remain to be elusive. Although PP4C/PP4R1 manipulation did not consistently regulate total PFK-1 phosphorylation, in vitro study demonstrated that PP4C/PP4R1 dephosphorylated PFKM and PFKP. This suggests that PP4 could affect PFK-1 status directly or indirectly, potentially explaining why PFK-1 activity was not altered upon PP4C depletion. To assess the role of direct PFK-1 dephosphorylation catalyzed by PP4, identification and mutation of the specific sites are required. The strategies such as mass spectrometry after in vitro phosphorylation or stable isotope labeling using amino acids in cell culture (SILAC) could help identify the phosphorylation site of PFK-1 reversed by PP4.
Analysis using the Seahorse metabolic analyzer revealed that only PP4R1 depletion significantly increased glycolytic rates and OCR, indicative of OXPHOS levels. This result is consistent with data showing that PP4R1, and not PP4C, depletion upregulates PFK-1 activity. As PFK-1 is a crucial enzyme in glycolysis, it is plausible that PFK-1 activation upon PP4R1 depletion induces increased glycolysis rate. Conversely, PP4R1 overexpression led to a slight decrease in OCR, but not glycolysis. Alterations in PP4C expression did not affect glycolysis or OXPHOS. The substrate specificity of the PP4 complex is determined by its regulatory subunits, and it plays many roles in various cellular pathways. PP4C depletion disrupts all types of PP4 complexes, leading to complicated and unexpected results (Supplementary Fig. 1). For example, alteration of PP4C expression induces opposite phenotypes depending on its expression level and cell lines studied (15, 24-28). However, PP4R1 depletion disrupts only PP4C/PP4R1, keeping pathways regulated by the other PP4 complexes intact. The absence of the PP4C/PP4R1 complex may thus increase PFK-1 activity and glycolytic rate. These data underline the importance of targeting PP4 regulatory subunits. Nevertheless, many studies on PP4 have focused on its catalytic subunit (PP4C), and research on the role of its regulatory subunits is limited (15, 18, 20, 21, 29-37). Thus, the effects of the regulatory subunits of PP4 and the underlying mechanisms must be evaluated. Targeting its regulatory subunits or specific interactions between PP4 and its substrates could be a more efficient and safer, avoiding pleiotropic effects.
Each type of PP4 complex is located at a different subcellular location, depending on its regulatory subunits (Supplementary Fig. 1). PP4R2, PP4R3α, and PP4R3β are present in nuclei, while PP4R1 and PP4R4 are located in the cytoplasm (18, 19, 38-40). PP4C is expressed in both the nucleus and cytoplasm (18, 19, 38). Consistent with their localization, PP4C/ PP4R2/PP4R3α and PP4C/PP4R2/PP4R3β complexes play crucial roles in genomic stability (18, 21, 29, 30, 32). By contrast, the PP4C/PP4R1 complex regulates meiosis, HDAC3, insulin resistance, and inhibition of NF-κB signaling (35, 39, 41). The function of PP4R4 in cells has not been reported (19). Considering that glycolytic enzymes are present in the cytoplasm, it is reasonable to assume that the PP4C/PP4R1 complex may regulate glycolytic enzymes. Our data support this hypothesis. Consistently, miR-338-3p-mediated PP4R1 suppression has been shown to induce insulin sensitivity (35). Thus, development of drugs that inhibit the PP4C/PP4R1 complex or specifically regulate the PFK-1-PP4R1 interaction can contribute to treating glycolytic disorders and cancers.
MATERIALS AND METHODS
Materials and methods are available in the supplementary material.
ACKNOWLEDGEMENTS
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1I1A3059916).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Ashton TM, McKenna WG, Kunz-Schughart LA, Higgins GS. Oxidative phosphorylation as an emerging target in cancer therapy. Clin Cancer Res. 2018;24:2482–2490. doi: 10.1158/1078-0432.CCR-17-3070. [DOI] [PubMed] [Google Scholar]
- 2.Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 3.Ganapathy-Kanniappan S, Geschwind JFH. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152. doi: 10.1186/1476-4598-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnet S, Archer SL, Allalunis-Turner J, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 6.Chen XS, Li LY, Guan YD, Yang JM, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the Warburg effect. Acta Pharmacol Sin. 2016;37:1013–1019. doi: 10.1038/aps.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hitosugi T, Chen J. Post-translational modifications and the Warburg effect. Oncogene. 2014;33:4279–4285. doi: 10.1038/onc.2013.406. [DOI] [PubMed] [Google Scholar]
- 8.Mor I, Cheung EC, Vousden KH. Control of glycolysis through regulation of PFK1: old friends and recent additions. Cold Spring Harb Symp Quant Biol. 2011;76:211–216. doi: 10.1101/sqb.2011.76.010868. [DOI] [PubMed] [Google Scholar]
- 9.Wang G, Xu Z, Wang C, et al. Differential phosphofructokinase‑1 isoenzyme patterns associated with glycolytic efficiency in human breast cancer and paracancer tissues. Oncol Lett. 2013;6:1701–1706. doi: 10.3892/ol.2013.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi W, Clark PM, Mason DE, et al. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–980. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yugi K, Kubota H, Toyoshima Y, et al. Reconstruction of insulin signal flow from phosphoproteome and metabolome data. Cell Rep. 2014;8:1171–1183. doi: 10.1016/j.celrep.2014.07.021.5aeb92b1cd164234a5216b8ad109b25a [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Liu R, Li J, et al. Stabilization of phosphofructokinase 1 platelet isoform by AKT promotes tumorigenesis. Nat Commun. 2017;8:949. doi: 10.1038/s41467-017-00906-9.bda913409b324c509ae124cfd1dbb096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JH, Liu R, Li J, et al. EGFR-phosphorylated platelet isoform of phosphofructokinase 1 promotes PI3K activation. Mol Cell. 2018;70:197–210. doi: 10.1016/j.molcel.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Terytty Y, Sun Y, Liang Y, et al. ULK1/2 constitute a bifurcate node controlling glucose metabolic fluxes in addition to autophagy. Mol Cell. 2016;62:359–370. doi: 10.1016/j.molcel.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Park J, Lee DH. Functional roles of protein phosphatase 4 in multiple aspects of cellular physiology: a friend and a foe. BMB Rep. 2020;53:181–190. doi: 10.5483/BMBRep.2020.53.4.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng XY, Li M, Guo J, et al. Protein phosphatase 4 promotes hepatic lipogenesis through dephosphorylating acetyl-CoA carboxylase 1 on serine 79. Mol Med Rep. 2014;10:1959–1963. doi: 10.3892/mmr.2014.2397. [DOI] [PubMed] [Google Scholar]
- 17.Tomar D, Jana F, Dong Z, et al. Blockade of MCU-mediated Ca(2+) uptake perturbs lipid metabolism via PP4-dependent AMPK dephosphorylation. Cell Rep. 2019;26:3709–3725. doi: 10.1016/j.celrep.2019.02.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdhury D, Xu X, Zhong X, et al. A PP4-phosphatase complex dephosphorylates gamma-H2AX generated during DNA replication. Mol Cell. 2008;31:33–46. doi: 10.1016/j.molcel.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen GI, Tisayakorn S, Jorgensen C, D'Ambrosio LM, Goudreault M, Gingras AC. PP4R4/KIAA1622 forms a novel stable cytosolic complex with phosphoprotein phosphatase 4. J Biol Chem. 2008;283:29273–29284. doi: 10.1074/jbc.M803443200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueki Y, Kruse T, Weisser MB, et al. A consensus binding motif for the PP4 protein phosphatase. Mol Cell. 2019;76:953–964. doi: 10.1016/j.molcel.2019.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J, Adelmant G, Marto JA, Lee DH. Dephosphorylation of DBC1 by protein phosphatase 4 is important for p53-mediated cellular functions. Mol Cells. 2015;38:697–704. doi: 10.14348/molcells.2015.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zecha J, Gabriel W, Spallek R, et al. Linking post-translational modifications and protein turnover by site-resolved protein turnover profiling. Nat Commun. 2022;13:165. doi: 10.1038/s41467-021-27639-0.629f5a02a56949d3a37bcf1ab0caac97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller MM. Post-translational modifications of protein backbones: unique functions, mechanisms, and challenges. Biochemistry. 2018;57:177–185. doi: 10.1021/acs.biochem.7b00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mourtada-Maarabouni M, Williams GT. Protein phosphatase 4 regulates apoptosis, proliferation and mutation rate of human cells. Biochim Biophys Acta. 2008;1783:1490–1502. doi: 10.1016/j.bbamcr.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Zhao A, Sun L, et al. Protein phosphatase PP4 is overexpressed in human breast and lung tumors. Cell Res. 2008;18:974–977. doi: 10.1038/cr.2008.274. [DOI] [PubMed] [Google Scholar]
- 26.Weng S, Wang H, Chen W, et al. Overexpression of protein phosphatase 4 correlates with poor prognosis in patients with stage II pancreatic ductal adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2012;21:1336–1343. doi: 10.1158/1055-9965.EPI-12-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Liang L, Huang L, Ma X, Li D, Cai S. High expression of protein phosphatase 4 is associated with the aggressive malignant behavior of colorectal carcinoma. Mol Cancer. 2015;14:95. doi: 10.1186/s12943-015-0356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M, Li X, Xu S, et al. Protein phosphatase 4 catalytic subunit is overexpressed in glioma and promotes glioma cell proliferation and invasion. Tumour Biol. 2016;37:11893–11901. doi: 10.1007/s13277-016-5054-6. [DOI] [PubMed] [Google Scholar]
- 29.Lee DH, Pan Y, Kanner S, Sung P, Borowiec JA, Chowdhury D. A PP4 phosphatase complex dephosphorylates RPA2 to facilitate DNA repair via homologous recombination. Nat Struct Mol Biol. 2010;17:365–372. doi: 10.1038/nsmb.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee DH, Goodarzi AA, Adelmant GO, et al. Phosphoproteomic analysis reveals that PP4 dephosphorylates KAP-1 impacting the DNA damage response. EMBO J. 2012;31:2403–2415. doi: 10.1038/emboj.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hadweh P, Habelhah H, Kieff E, Mosialos G, Hatzivassiliou E. The PP4R1 subunit of protein phosphatase PP4 targets TRAF2 and TRAF6 to mediate inhibition of NF-kappaB activation. Cell Signal. 2014;26:2730–2737. doi: 10.1016/j.cellsig.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Lee DH, Acharya Sanket S, Kwon M, et al. Dephosphorylation enables the recruitment of 53BP1 to double-strand DNA breaks. Mol Cell. 2014;54:512–525. doi: 10.1016/j.molcel.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Lee DH. Leucine methylation of protein phosphatase PP4C at C-terminal is critical for its cellular functions. Biochem Biophys Res Commun. 2014;452:42–47. doi: 10.1016/j.bbrc.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 34.Wu G, Ma Z, Qian J, Liu B. PP4R1 accelerates cell growth and proliferation in HepG2 hepatocellular carcinoma. Onco Targets Ther. 2015;8:2067–2074. doi: 10.2147/OTT.S77709. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Dou L, Wang S, Sun L, et al. Mir-338-3p mediates Tnf-A-induced hepatic insulin resistance by targeting PP4r1 to regulate PP4 expression. Cell Physiol Biochem. 2017;41:2419–2431. doi: 10.1159/000475912.f961c670e4d64c07ad3ae213d7b48ab9 [DOI] [PubMed] [Google Scholar]
- 36.Herzig JK, Bullinger L, Tasdogan A, et al. Protein phosphatase 4 regulatory subunit 2 (PPP4R2) is recurrently deleted in acute myeloid leukemia and required for efficient DNA double strand break repair. Oncotarget. 2017;8:95038–95053. doi: 10.18632/oncotarget.21119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park J, Lee J, Lee DH. Identification of protein phosphatase 4 inhibitory protein that plays an indispensable role in DNA damage response. Mol Cells. 2019;42:546–556. doi: 10.14348/molcells.2019.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carnegie GK, Sleeman JE, Morrice N, et al. Protein phosphatase 4 interacts with the survival of motor neurons complex and enhances the temporal localisation of snRNPs. J Cell Sci. 2003;116:1905–1913. doi: 10.1242/jcs.00409. [DOI] [PubMed] [Google Scholar]
- 39.Brechmann M, Mock T, Nickles D, et al. A PP4 holoenzyme balances physiological and oncogenic nuclear factor-kappa B signaling in T lymphocytes. Immunity. 2012;37:697–708. doi: 10.1016/j.immuni.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Chang WH, Choi SH, Moon BS, et al. Smek1/2 is a nuclear chaperone and cofactor for cleaved Wnt receptor Ryk, regulating cortical neurogenesis. Proc Natl Acad Sci U S A. 2017;114:E10717–E10725. doi: 10.1073/pnas.1715772114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Ozawa Y, Lee H, et al. Histone deacetylase 3 (HDAC3) activity is regulated by interaction with protein serine/threonine phosphatase 4. Genes Dev. 2005;19:827–839. doi: 10.1101/gad.1286005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.