Abstract
Introduction
Studies have shown that pregnant women with COVID-19 have a higher risk of intensive care unit admission and invasive mechanical ventilation support than non-pregnant women. Pregnancy-associated physiological changes in respiratory function may contribute to the elevated risk. Alteration in lung volumes and capacities are attributed to the mechanical impediment caused by the growing fetus. Multiple pregnancies may therefore compromise functional lung capacity earlier than singleton pregnancies and contribute to severe respiratory symptoms of COVID-19.
Materials and Methods
A total of 5514 women with a symptomatic SARS-CoV-2 infection during pregnancy registered in the COVID-19 Related Obstetric and Neonatal Outcome Study were included. The COVID-19-related adverse maternal outcomes were compared in 165 multiple versus 5349 singleton pregnancies. Combined adverse maternal outcome was defined as presence of COVID-19-related hospitalization and/or pneumonia and/or oxygen administration and/or transfer to ICU and/or death. Multivariate logistic regression was used to estimate the odds ratios and 95% confidence intervals were calculated.
Results
The frequency of dyspnea, likelihood of developing dyspnea in a defined pregnancy week and duration of the symptomatic phase of the COVID-19 infection did not differ between the two groups. On average, COVID-19-related combined adverse outcome occurred earlier during pregnancy in women expecting more than one child than in singleton pregnancies. The overall incidence of singular and combined COVID-19-associated adverse maternal outcomes was not significantly different between groups. However, regression analysis revealed that multiple gestation, preconceptional BMI > 30 kg/m 2 and gestational age correlated significantly with an increased risk of combined adverse maternal outcome. Conversely, maternal age and medically assisted reproduction were not significant risk factors for combined adverse maternal outcome.
Conclusion
Our data show that multiple gestation alone is a risk factor for COVID-19-associated combined adverse maternal outcome. Moreover, severe courses of COVID-19 in women expecting more than one child are observed earlier in pregnancy than in singleton pregnancies.
Keywords: COVID-19, SARS-CoV-2 infection, multiple pregnancy, maternal outcome, CRONOS
Zusammenfassung
Einleitung
Studien haben gezeigt, dass schwangere Frauen mit COVID-19 ein höheres Risiko für eine Aufnahme in die Intensivstation und invasive mechanische Beatmungsunterstützung haben, verglichen mit nicht schwangeren Frauen. Schwangerschaftsbedingte physiologische Änderungen der Atemfunktion tragen möglicherweise zu diesem höheren Risiko bei. Änderungen des Lungenvolumens und der Lungenkapazität werden der durch das fötale Wachstum verursachten mechanischen Behinderung zugeschrieben. Es ist daher vorstellbar, dass Mehrlingsschwangerschaften die funktionelle Lungenkapazität früher beeinträchtigen als Einlingsschwangerschaften und dass sie schwerwiegende Atemwegsbeschwerden bei COVID-19 verschärfen.
Material und Methoden
Insgesamt wurden 5514 Frauen mit symptomatischer SARS-CoV-2-Infektion während der Schwangerschaft, die in der COVID-19 Related Obstetric and Neonatal Outcome Study (CRONOS) registriert waren, in die Studie eingeschlossen. Es wurden die COVID-19-bedingten ungünstigen mütterlichen Outcomes bei 165 Mehrlings- und 5349 Einlingsschwangerschaften verglichen. Ein kombiniertes ungünstiges mütterliches Outcome wurde definiert als eine COVID-19-bedingte Krankenhausaufnahme und/oder eine Lungenentzündung und/oder eine Sauerstoffgabe und/oder eine Verlegung auf die Intensivstation und/oder Tod. Multivariate logistische Regression wurde zur Schätzung des Odds Ratios eingesetzt, und die 95%-Konfidenzintervalle wurden berechnet.
Ergebnisse
Es gab keine Unterschiede zwischen den 2 Gruppen hinsichtlich der Häufigkeit von Dyspnoe, der Wahrscheinlichkeit, Dyspnoe in einer bestimmten Schwangerschaftswoche zu entwickeln, oder der Dauer der symptomatischen Phase der COVID-19-Infektion. Im Schnitt trat ein COVID-19-bedingtes kombiniertes ungünstiges Outcome zu einem früheren Zeitpunkt der Schwangerschaft ein bei Frauen, die mehr als ein Kind erwarteten, verglichen mit Einlingsschwangerschaften. Insgesamt unterschied sich die Häufigkeit von einzelnen und kombinierten COVID-19-bedingten ungünstigen mütterlichen Outcomes nicht signifikant zwischen den Gruppen. Die Regressionsanalyse zeigte aber, dass Mehrlingsschwangerschaft, ein präkonzeptioneller BMI > 30 kg/m 2 und das Schwangerschaftsalter signifikant mit einem erhöhten Risiko für kombinierte ungünstige mütterliche Outcomes korrelierten. Dagegen waren mütterliches Alter und medizinisch unterstützte Fortpflanzung keine signifikanten Risikofaktoren für kombinierte ungünstige mütterliche Outcomes.
Schlussfolgerung
Unsere Daten zeigen, dass eine Mehrlingsschwangerschaft an sich bereits einen Risikofaktor für COVID-19-bedingte kombinierte ungünstige mütterliche Outcomes darstellt. Dazu kommt noch, dass ein schwerer Verlauf der COVID-19-Erkrankung früher in der Schwangerschaft auftritt bei Frauen, die mehr als ein Kind erwarten, als bei Einlingsschwangerschaften.
Schlüsselwörter: COVID-19, SARS-CoV-2-Infektion, Mehrlingsschwangerschaft, mütterliches Outcome, CRONOS
Introduction
During the COVID-19 pandemic, concerns arose as to whether infections with SARS-CoV-2 adversely affect pregnancy outcomes. Indeed, observational cohort studies report that pregnant women infected with SARS-CoV-2 are more likely to be admitted to an intensive care unit (ICU) or need invasive ventilation than non-pregnant women 1 . To reduce risk of infection it has been suggested to wear face masks and to avoid crowds 2 3 4 . Data from the COVID-19 Related Obstetric and Neonatal Outcome Study (CRONOS) registry in Germany, which prospectively enrolled women with confirmed SARS-CoV-2 infection during their pregnancy, confirmed a high rate of severe COVID-19 cases requiring intensive care, mainly for respiratory support. The incidence correlated positively with increased gestational age at time of infection: amongst symptomatic women the risk of transfer to an ICU increased from < 0.3 % in the first trimester to approximately 7% in the early third trimester, with the highest risk at about 30 weeks of gestation 5 6 .
Physiological respiratory system changes during pregnancy occur on every level and include
mucosal hyperemia and edema in nasopharynx, larynx, trachea and bronchi,
lower carbon dioxide response threshold of the respiratory center,
displacement of the diaphragm by the enlarged uterus and
expansion of the ribcage and increase of the subcostal angle, resulting in a decrease of functional residual capacity (FRC) by 20% to 30% 4 .
The total lung capacity (TLC) is maintained to meet the increased demand for oxygen (by 20%) with an unchanged (or only slightly increased) respiratory rate, a higher tidal volume, and a steep increase in minute ventilation already in the first trimester 7 . Existing literature shows that mouth occlusion pressure increases progressively during pregnancy and falls significantly after delivery, indicating that effective respiratory impedance increases during pregnancy 8 . Staub et al. have shown that the symphysis-fundal height in twin pregnancies is substantially greater than in singleton pregnancies 9 . Therefore, multiple pregnancy could be a risk factor for early manifestation of adverse maternal outcome after SARS-CoV-2 infection in pregnancy.
In order to better counsel pregnant women in situations of increased risk for respiratory viral infection or COVID-19, we aimed to analyze COVID-19-specific outcome in women with multiple pregnancies. We hypothesized that women with multiple pregnancies are susceptible for severe COVID-19 earlier in gestation than singleton pregnancies, due to the earlier and more evident displacement of the diaphragm during pregnancy.
Material and Methods
Study design and setting
CRONOS is a multicentric, prospective, monitored observational study established by the German Society of Perinatal Medicine (DGPM) in April 2020 to rapidly provide data to counsel women with SARS-CoV-2 infection during pregnancy. The method is in detail described previously 10 . Information on the study is available at www.dgpm-online.org and the German Clinical Trials Register (DRKS00021208). Methodology and study results have been published recently 6 11 12 13 14 15 16 17 . Approval of the Institutional Ethics Board was obtained for the study (University Hospital Schleswig-Holstein in Kiel, file number D 451/20) and for each study site separately. Women with confirmed SARS-CoV-2 infection any time during their pregnancy were eligible for inclusion in the CRONOS registry.
All German maternity hospitals were invited to participate in the CRONOS registry. The registry collected data between April 3 rd , 2020 and February 10 th , 2023 with 130 of 686 (18.9%) German obstetric hospitals and the Kepler University Hospital in Linz, Austria, actively participating. These maternity units attended approximately 250000 deliveries per year, accounting for ⅓ of the births in Germany. CRONOS aimed at a complete recruitment of all eligible women.
Data capture and study variables
A reporting form was developed for collecting data, initially using the cloud-based electronic data capture platform of the service provider castoredc.com (Amsterdam, Netherlands). The database was updated in December 2020 to include further data, e.g. vaccination status. Informed consent was either obtained in the antepartum period or waived in the postpartum period if COVID-19 had occurred in the current pregnancy. After the patients had given informed consent, information on the demographic characteristics, comorbidities, previous and current pregnancy characteristics, SARS-CoV-2-specific symptoms and treatments, pregnancy- and birth-specific events and neonatal outcomes were entered by each participating hospital in the data capture platform 6 . Primary endpoint of the study was the COVID-19-related combined adverse maternal outcome, which was defined as presence of COVID-19-related hospitalization and/or pneumonia and/or oxygen administration and/or transfer to ICU and/or death. The above-mentioned adverse outcomes were also assessed separately as secondary endpoints. Furthermore, prevalence of COVID-associated dyspnea among women with a symptomatic SARS-CoV-2 infection was assessed in the two groups, as a manifest symptom associated with respiratory tract infections.
Statistical analysis
A multi-step analysis was performed in order to evaluate whether multiple pregnancy constituted a greater risk for COVID-19-related adverse maternal outcomes than singleton pregnancy. First, baseline data were analyzed to identify statistically significant differences in baseline characteristics between the two groups ( Table 1 ). Categorical variables were presented as absolute and relative frequencies, continuous baseline variables are shown as mean and standard deviation for each group. Statistical significance was tested for categorical baseline variables using the chi-square test, for continuous variables with Pearson’s chi-squared test.
Table 1 Baseline and pregnancy characteristics of the study participants.
| Parameter | Multiple pregnancies (n = 165) | Singleton pregnancies (n = 5349) | P value |
| ART = assisted reproductive technology; BMI = body mass index Data are shown as mean ± standard deviation or absolute/relative frequencies (percentage). tt2 = Welch’s two-sample t-test; chi2 = Pearson’s chi-squared test | |||
| Maternal age (years) | 32 ± 4.6 | 31 ± 5.3 | 0.005 tt2 |
| Smoking (during pregnancy) | 5/161 (3%) | 209/5244 (4%) | 0.57 chi2 |
| BMI (kg/m 2 ) | 26 ± 5.3 (n = 155) | 26 ± 5.6 (n = 5014) | 0.05 tt2 |
| ART in this pregnancy | 47/143 (43%) | 174/4861 (4%) | |
| Maternal comorbidities (any) | 65 (39%) | 1919 (36%) | < 0.001 chi2 |
|
6/163 (4%) | 218/5295 (4%) | 0.35 chi2 |
|
0/163 (0%) | 54/5295 (1%) | 0.78 chi2 |
|
5/163 (3%) | 181/5295 (3%) | 0.20 chi2 |
| Coagulation disorder or state after thrombosis | 1/163 (1%) | 126/5295 (2%) | 0.81 chi2 |
| Vaccinated against SARS-CoV-2 | 59/154 (38%) | 1550/5030 (31%) | 0.14 chi2 |
| Gestational age (week) at onset of COVID-19 symptoms | 25 ± 8.8 | 27 ± 9.7 | |
|
18 (11%) | 545 (10%) | 0.05 chi2 |
|
77 (47%) | 1933 (36%) | 0.002 tt2 |
|
70 (42%) | 2871 (54%) | 0.01 chi2 |
In a second step, it was evaluated whether COVID-19-associated symptoms and COVID-associated maternal outcome differed significantly between singleton and multiple pregnancies ( Table 2 , Supplementary Table S2 ). Statistical significance for categorical variables was tested using Pearson’s chi-squared test. In addition, P values, risk estimators (odds ratios [OR]) and corresponding 95% confidence intervals (95% Cl) were calculated for the comparison of maternal outcomes between the two groups.
Table 2 COVID-19-associated maternal outcome in singleton and multiple pregnancies.
| COVID-19-associated outcomes | Multiple pregnancies (n = 165) |
Singleton pregnancies (n = 5349) |
OR | 95% CI | P value |
| Data are shown as absolute/relative frequencies (percentage). chi2 = Pearson’s chi-squared test; ICU = intensive care unit * The combined endpoint is composed of the following: COVID-19-associated need for inpatient treatment and/or pneumonia and/or oxygen supplementation and/or ICU admission and/or mortality. | |||||
| COVID-19-associated need for inpatient treatment | 31 (19%) | 748 (14%) | 1.42 | [0.96; 2.12] | 0.08 chi2 |
| Pneumonia | 8/161 (5%) | 260/5294 (5%) | 1.01 | [0.49; 2.08] | 0.97 chi2 |
| Oxygen supplementation | 8/164 (5%) | 328/5339 (6%) | 0.79 | [0.38; 1.60] | 0.51 chi2 |
| ICU admission | 8/164 (5%) | 195/5336 (4%) | 1.36 | [0.65; 2.79] | 0.41 chi2 |
| Mortality | 1/164 (1%) | 9/5332 (0%) | 3.62 | [0.46; 28.81] | 0.19 chi2 |
| Combined outcome* | 31 (19%) | 748 (14%) | 1.42 | [0.96; 2.12] | 0.08 chi2 |
| COVID-19-associated indication for caesarean section | 4/151 (3%) | 140/4733 (3%) | 0.80 | [0.33; 2.44] | 0.83 chi2 |
Thirdly, a multivariate regression analysis was conducted for COVID-19-associated combined adverse maternal outcome ( Table 3 ). The baseline variables which differed significantly between groups (maternal age, IVF, gestational age) or are known as potential risk factors for adverse outcome (BMI > 30 kg/m 2 , maternal comorbidities, multiple gestation, smoking during pregnancy) were included into the model. SARS-CoV-2-vaccination status was not integrated in the logistic regression, due to great variability within the vaccinated population (vaccination before or during pregnancy, number and type of vaccinations, time since last vaccination). Virus variants were assumed in each case from date of infection and postal code, based on the given dominant virus variant according to the national institute for disease control, the Robert Koch-Institute (RKI). However, this variable was also not integrated in the logistic regression due to partially small numbers within the subgroups (see Supplementary Table S1 ). Statistical analysis was performed using the R software version 4.2.2. All inferential statistics are intended to be exploratory (hypothesis generating), not confirmatory and are interpreted accordingly. Therefore, no power calculation was performed. The p values were considered to be statistically significant if p < 0.05.
Table 3 Multivariate logistic regression analysis results for combined maternal outcome of women with COVID-19 during pregnancy.
| Combined Outcome | Level | OR | 95% CI | P value |
| ART = assisted reproductive technology; BMI = body mass index; CI = confidence interval; OR = odds ratio | ||||
| Multiple gestation | present | 1.75 | [1.05; 2.80] | 0.02 |
| BMI > 30 (before pregnancy) | present | 1.52 | [1.23; 1.88] | < 0.001 |
| Gestational age in weeks | 1.05 | [1.03; 1.06] | < 0.001 | |
| Maternal age > 35 | present | 0.89 | [0.72; 1.09] | 0.26 |
| Maternal comorbidities | present | 0.98 | [0.81; 1.18] | 0.83 |
| Mode of conception | ART | 0.96 | [0.61; 1.46] | 0.86 |
| Smoking (during pregnancy) | present | 0.57 | [0.32; 0.94] | 0.04 |
Results
Study population
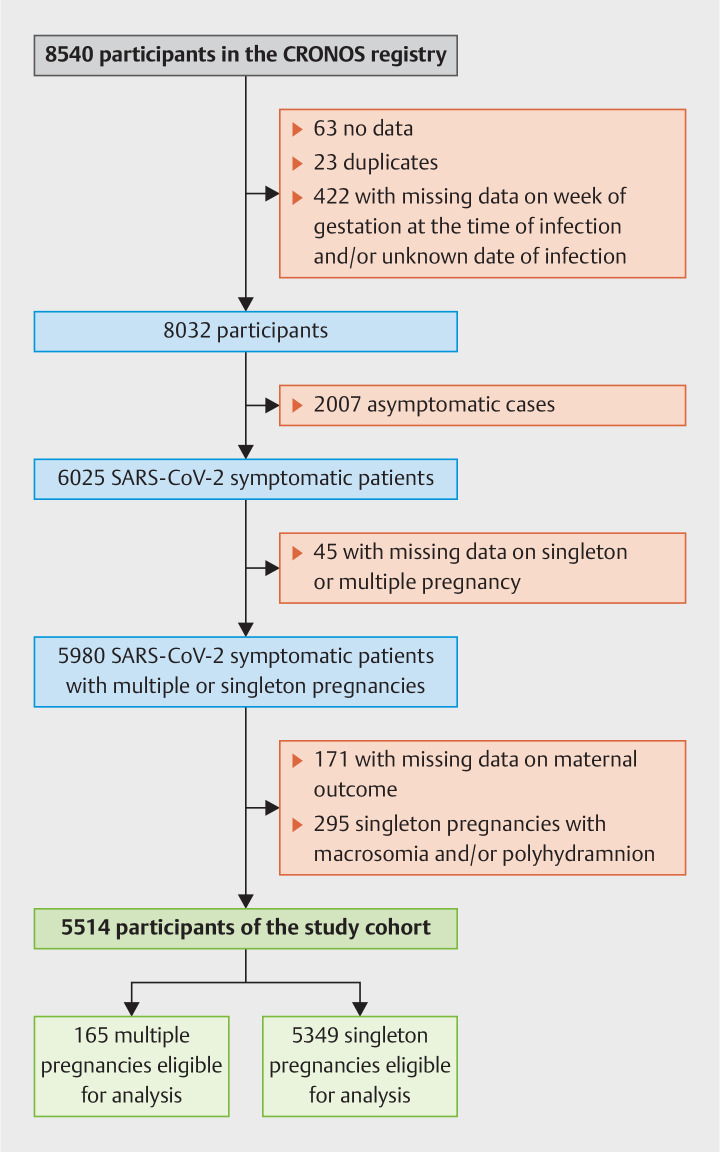
By February 2023 in total 8540 women were registered. During the review of the registry and the plausibility check a total of 508 cases were excluded, consisting of 63 cases with no data, 23 confirmed duplicates, and 422 cases in which mandatory information was missing, either the week of gestation at the time of infection and/or the date of infection.
Of the remaining 8032 women with confirmed SARS-CoV-2 infection during pregnancy 6025 (75%) were symptomatic and 2007 (25%) asymptomatic. We excluded asymptomatic cases, cases in which data on singleton or multiple pregnancy was not provided (n = 45) and cases missing the date of COVID-related maternal outcome (n = 171). Singleton pregnancies with increased intra-abdominal pressure due to macrosomia (fetal weight greater than or equal to the 90th percentile for gestational age) and/or polyhydramnios (n = 295) were also excluded ( Fig. 1 ).
Fig. 1.
Flow diagram describing patient inclusion.
The final study cohort consisted of 5514 patients with symptomatic SARS-CoV-2 infection, valid information on whether or not the patient was expecting multiple pregnancy, and on COVID-related maternal outcome. SARS-CoV-2 infection was confirmed via viral RNA detection with polymerase chain reaction testing in 4842 cases (88%), detection of maternal SARS-CoV-2 antibodies in 38 cases (1%) and antigen testing in 293 cases (5%). In 341 cases (6%) no information on the diagnostic test used to confirm SARS-CoV-2 infection was available.
The baseline maternal demographic and clinical characteristics of the final cohort of 5514 women with symptomatic SARS-CoV-2 infection during pregnancy, consisting of 165 women with multiple (162 twin and 3 triplet pregnancies) and 5349 with singleton pregnancies are presented in Table 1 . Overall, women expecting more than one child were significantly older, achieved pregnancy more often after assisted reproductive technology (ART) and were infected earlier in pregnancy (p < 0.05). Around a quarter of women with a symptomatic COVID-19 infection developed dyspnea, the difference between multiple and singleton pregnancies was not significant (n = 38/157, 24% vs. n = 1248/5043, 25%, respectively, p = 0.877). The duration of the symptomatic phase, reported by the patient, did not differ between the two groups (p = 0.594) and the vast majority (85% and 84%, respectively) had symptoms for less than 14 days ( Supplementary Table S2 ).
COVID-19-associated symptoms and adverse maternal outcomes in multiple vs. singleton pregnancies
The COVID-19-associated adverse maternal outcomes, including need for hospitalization, pneumonia, oxygen supplementation, transfer to intensive care unit and death, were not significantly different among the two groups ( Table 3 ). Also, the incidence of combined adverse maternal outcome was comparable between the two groups (n = 31, 19% vs. n = 748, 14%, p = 0.081, Table 3 ). Interestingly, although planned or emergency cesarean sections were more common in multiple pregnancies (70% vs. 34%, p < 0.001), the frequency of COVID-19 as indication for a cesarean section was identical, with 3% in either group (p = 0.825, OR 0.89 [0.33–2.44]).
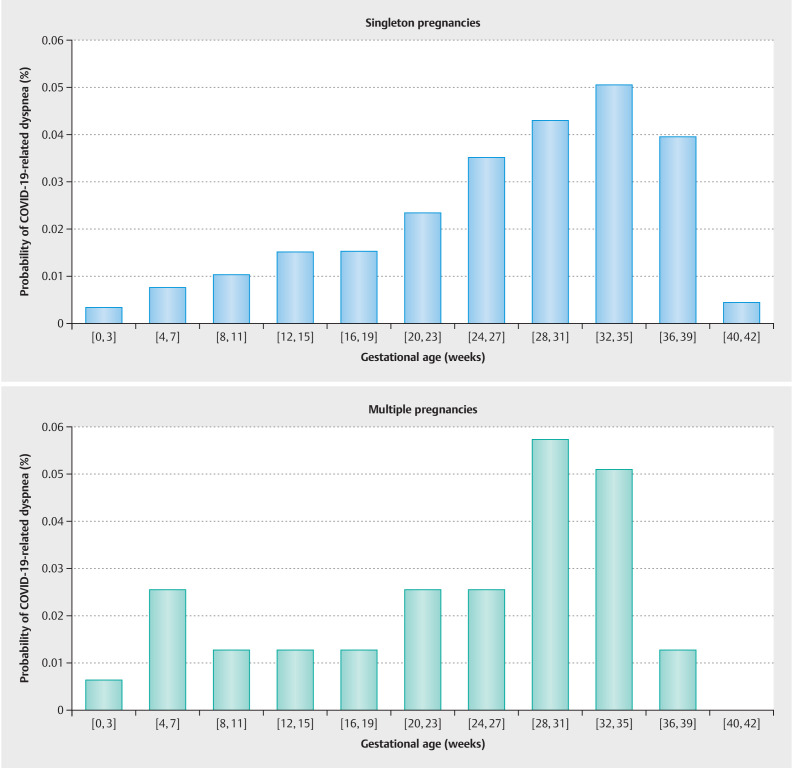
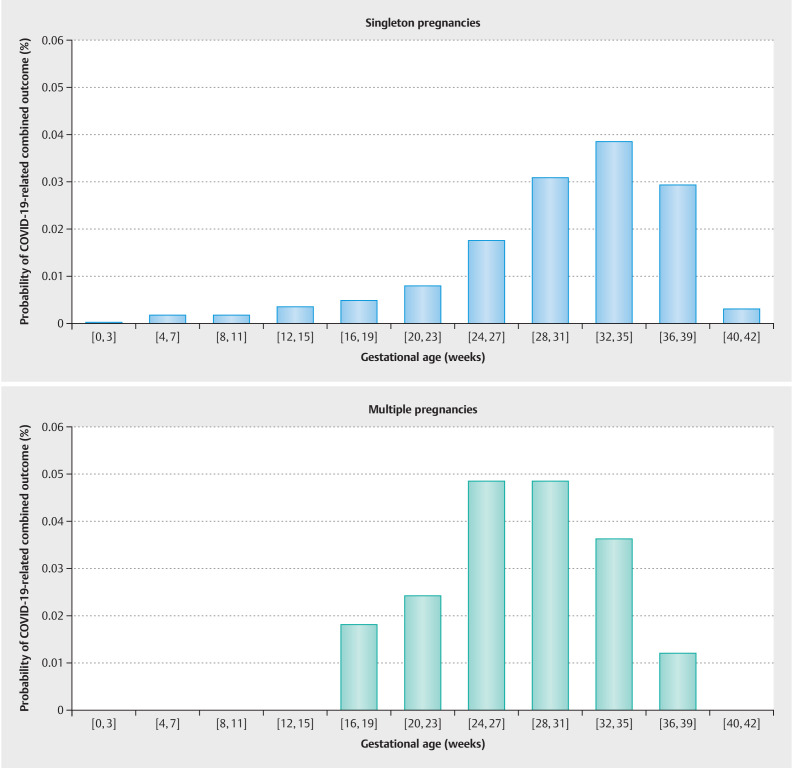
A case was considered as symptomatic, if the patient reported experiencing one or more of the following symptoms in association to the COVID-19 infection: fever > 38 °C, cough, diarrhea, dyspnea, myalgia, fatigue, sore throat, general feeling of illness/chills, nasal respiratory obstruction, thoracic pain/chest pain, headache, dizziness or lightheadedness, altered sense of taste or smell, productive ejection/mucus, nausea or vomiting, or other (with the option to specify symptoms in free text). Patients were explicitly asked to report symptoms in temporal relation to their COVID-19 infection, in order to distinguish between dyspnea related to COVID-19 and breathing discomfort associated with pregnancy. The probability of COVID-19-related dyspnea or combined adverse maternal outcome among women with a symptomatic SARS-CoV-2 infection in a defined pregnancy week is depicted in Fig. 2 and Fig. 3 . Although there was no difference in the timing or frequency of dyspnea (mean gestational age 24.1 vs. 26.8 weeks, p = 0.12, Fig. 2 ), COVID-related combined adverse maternal outcome occurred significantly earlier in women with multiple pregnancies (mean gestational age 24.1 vs. 30.2 weeks, p = 0.001, Fig. 3 ).
Fig. 2.
Probability of COVID-19-related dyspnea when infected with SARS-CoV-2 in a defined pregnancy week. There was no difference in the timing of occurrence of dyspnea between multiple and singleton pregnancies (Welch Two Sample t-test, mean gestational age singleton 26.8 vs. 24.1 multiple pregnancy, p value = 0.12).
Fig. 3.
Probability of COVID-19-related combined adverse maternal outcome when infected with SARS-CoV-2 in a defined pregnancy week. COVID-19 related combined outcome occurred on average significantly earlier during pregnancy in women expecting more than one child in comparison to singleton pregnancies (Welch Two Sample t-test, mean gestational age singleton 30.2 vs. 24.1 multiple pregnancy, p value = 0.001).
Although the incidence of combined adverse maternal outcome was not significantly different in the two groups, multivariate logistic regression analysis showed that multiple gestation contributes significantly to the increased risk for combined adverse maternal outcome (OR 1.75; 95% CI 1.05–2.80; p = 0.02). Preconceptional BMI > 30 kg/m 2 (OR 1.52; 95% CI 1.23–1.88; p < 0.001) and gestational age (OR 1.05; 95% CI 1.03–1.06; p < 0.001) were also shown to be statistically significant predictors of combined adverse outcome. On the other hand, maternal comorbidities, maternal age > 35 years and ART were not significant risk factors ( Table 3 ). Multivariate logistic regression analysis was also conducted separately for the two groups, confirming preconceptional BMI > 30 kg/m 2 and higher gestational age as significant predictors for combined adverse maternal outcome in singleton pregnancies ( Supplementary Table S3 ). However, none of these factors were shown to be significantly predictive in multiple pregnancies ( Supplementary Table S3 ).
Discussion
COVID-19-associated maternal outcomes (need for inpatient treatment, pneumonia, oxygen supplementation, ICU admission and maternal death), combined adverse maternal outcomes, prevalence of dyspnea and duration of the symptomatic phase of COVID-19 infection were descriptively comparable between multiple and singleton pregnancies. However, on average the combined adverse maternal outcome occurred earlier in multiple than in singleton pregnancies, possibly because pronounced uterine distension and elevation of the diaphragm result in superficial ventilation of the lungs. This may be observed especially at the end of the second trimester with increase of uterine volume and elevation of the uterine fundus beyond the level of the navel. Existing literature shows that respiratory impedance increases during pregnancy and that the symphysis-fundal height in twin pregnancies is substantially greater than in singleton pregnancies 8 9 . Multivariate logistic regression revealed that multiple pregnancy, preconceptional BMI > 30 kg/m 2 and higher gestational age at time of infection are risk factors for combined adverse maternal COVID-19-specific outcome. Within the group of multiple pregnancies, no single variable was shown to be predictive.
Several cohort studies report an association between COVID-19 in pregnancy and substantially increased maternal and neonatal morbidity and mortality, compared to pregnant women without a diagnosis of COVID-19 18 . Physiological pregnancy-associated changes, such as an altered immune response, an angiotensin-converting enzyme 2 (ACE2) receptor expression in the placenta and physiological changes in the respiratory and cardiovascular system during pregnancy are aspects that may potentially lead to a more severe course of SARS-CoV-2 infection in pregnant than in non-pregnant women 19 . However, insight into the maternal outcome of multiple pregnancies after COVID-19 is lacking. Changes in the respiratory system during pregnancy include the displacement of the diaphragm by the enlarged uterus. We hypothesized that alteration in lung volumes and capacities attributed to the greater mechanical impediment caused by the growth of multiple fetuses can lead to an earlier onset and/or to a worse maternal outcome after SARS-CoV-2 infection in pregnancy. Our findings support this hypothesis, as multiple gestation was proven to be a risk factor for combined adverse maternal outcome, and women expecting more than one child experienced COVID-19-related combined outcome on average 6 weeks earlier than singleton pregnancies. In this study, the probability of dyspnea and of combined adverse maternal outcome continuously increased during gestation, especially between the 20th and 36th week of pregnancy, following the fundal height with its maximum at around the 36th week of gestation. The sparse literature examining the differences in respiratory function in multiple and singleton pregnancies report that despite its higher physiological demands, multiple pregnancy does not appear to further compromise the respiratory system in healthy women 20 21 . However, this may not be the case in pregnant women experiencing a symptomatic SARS-CoV-2 infection with clinical signs of lung involvement, such as dyspnea, pneumonia, admission to ICU, oxygen supplementation or death. In these cases, even slight differences in respiratory function may influence the outcome. In accordance with data already published, we confirmed that BMI > 30 kg/m 2 is a risk factor for COVID-19-related adverse maternal outcome in symptomatic women 16 22 while ART is not 16 . Also, in conformance with existing literature, higher gestational age was a significant risk factor for adverse outcome 23 . Maternal age has previously been reported as a significant risk factor for worse maternal outcome in women infected with SARS-CoV-2 during pregnancy 16 23 . This is in contrast to our observation, which is however based on a larger sample size (5514 vs. 1485 women). Women of reproductive age can generally be considered young and minor age differences within the cohort may therefore not affect the outcome regarding COVID-19 24 . Although several studies report an association of smoking or smoking history and COVID-19 22 25 , in our study smoking during pregnancy was shown to be a protective factor. This paradox association can most likely be attributed to a selection bias, as women who smoke were more likely to receive obstetric care for associated complications, such as preterm delivery and fetal growth restriction. This bias led to a higher rate of smokers with mild symptoms who were identified by SARS-CoV-2 screening measures upon hospitalization for other reasons. In this context, we conclude that multiple gestation, gestational age and BMI > 30 kg/m 2 were the main predictors of combined adverse maternal outcome after SARS-CoV-2 infection in pregnancy.
Although planned or emergency cesarean sections were significantly more common in multiple pregnancies, the frequency of COVID-19 as indication for a cesarean section was identical between the two groups. COVID-19-associated reasons for a cesarean section included cases where delivery was deemed necessary for further maternal treatment due to severe COVID-19. These were distinguished from cases categorized as obstetrically indicated termination of pregnancy or maternal request/no medical indication for pregnancy termination. However, it is possible that COVID-19 may have influenced obstetric complications leading to pregnancy termination (e.g. pathological cardiotocography, preterm contractions). Although this potential overlap is likely to affect both singleton and multiple pregnancies, the degree of uniformity across these groups remains undetermined.
The strengths of this work include high data quality as analyzed data were collected from a well-supervised prospective registry study using a standardized electronic clinical report form. Hospitals participating in CRONOS cover approximately 33% of all deliveries in Germany. Multiple pregnancies in our cohort were adequately represented (3.0% of analyzed cases), as the proportion of multiple births in Germany and of multiple pregnancies after spontaneous conception (miscarriage not excluded) is 3.7% and 4% respectively 26 . Accordingly, the present data set is to be considered representative of the national collective.
As asymptomatic cases of SARS-CoV-2 infection are very likely documented due to clinical consultation for pregnancy-related complications, e.g. spontaneous preterm delivery, fetal growth restriction, or preeclampsia, they were considered as “incidental SARS-CoV-2 infection” and excluded from further analysis. However, women expecting more than one child visit the hospital more often and earlier during pregnancy due to higher risk of obstetric complications. This may have potentially led to earlier detection of mild symptomatic SARS-CoV-2 infection in multiple pregnancies than in singleton pregnancies. As this affects the denominator, the relative frequency of severe COVID-19-related outcomes may be underestimated in multiple pregnancies. Moreover, there were less cases attributed to the delta variant amongst unvaccinated women with multiple pregnancies compared to unvaccinated women with singleton pregnancies. As the delta variant is mainly responsible for severe courses of SARS-CoV-2 infection, this could theoretically mask multiple gestation as a risk factor for adverse maternal outcome. Despite this, multiple pregnancy was proven to be a risk factor in our cohort.
Our study has a variety of limitations, including the difference in cohort sizes. This factor makes it difficult to reliably show statistical correlations. Moreover, as immunity could only be assumed based on vaccination history and no antibody status was determined, the role of vaccination status was not thoroughly addressed in our work. Another conflicting point was the limited documentation of naturally acquired immunity due to previous infection in the registry. However, according to the national center of disease control RKI, the seroprevalence of SARS-CoV-2 antibodies at the time of national recommendation of vaccination in pregnancy (September 2021) was below 10% 27 . Therefore, the effect on these results can be neglected. While the vaccination status was not included into our statistical models, it did not differ significantly between singletons and multiple pregnancies in our cohort (31% vs. 38%).
Virus variants were not separately addressed due to the relatively small sample size in the subgroups ( Supplementary Table S3 ). Furthermore, while additional information on multiple pregnancy was requested, neonatal outcome data were available for only one of the newborns. Per definition, outcome data of the neonate with the worse outcome (for example neonate with lower birth weight, lower umbilical artery pH or neonatal death) was requested. Therefore, neonatal outcome cannot be meaningfully assessed and compared between multiple and singleton pregnancies in this study. Lastly, potential limitations associated with subjective reporting coming from patients or physicians, which cannot be objectively verified afterwards, pose a challenge in observational studies.
Conclusion
In conclusion, our data shows that multiple gestation was associated with a substantially higher risk for combined adverse maternal outcome after onset of COVID-19, which was also observed earlier in pregnancy than in singleton pregnancies. This could result due to a more evident displacement of the diaphragm by the enlarged uterus in comparison to singleton pregnancies. These findings are of clinical importance during the ongoing pandemic, but also for similar respiratory viral infections. They can serve physicians when advising couples expecting more than one child or considering multiple embryo transfer after ART. Further research is needed to assess and quantify the respiratory stress in women with SARS-CoV-2 infection during multiple pregnancy and to evaluate whether a different therapeutic management than in singleton pregnancies is required, particularly if ventilatory support is needed.
Supplementary Material
Supplementary Table S1: Frequency of different virus variants for unvaccinated and vaccinated women with singleton or multiple pregnancy.
Supplementary Table S2: Dyspnea due to COVID-19 infection and duration of symptomatic phase according to mother.
Supplementary Table S3: Multivariate logistic regression analysis results for combined maternal outcome in women with COVID-19 in multiple and singleton pregnancies.
Acknowledgement
The authors wish to thank all the participants and participating hospitals for providing clinical information to the CRONOS database. This research would not have been possible without their efforts.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Supporting information
References
- 1.PregCOV-19 Living Systematic Review Consortium Allotey J, Stallings E, Bonet Met al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis BMJfor2020370m3320. 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pecks U, Agel L, Doubek K et al. SARS-CoV-2 in Schwangerschaft, Geburt und Wochenbett. Leitlinie der DGGG und DGPM (S2k-Level, AWMF-Registernummer 015/092, März 2022) Geburtshilfe Frauenheilkd. 2023;83:517–546. doi: 10.1055/a-2003-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stumpfe FM, Schneider MO, Hein A et al. Limited Effects of SARS-CoV-2 Pandemic-related Lockdowns and Reduced Population Mobility on Preterm Birth Rates: A Secondary Analysis of Bavarian Obstetric Quality Parameters from 2010 to 2020. Geburtshilfe Frauenheilkd. 2022;82:842–851. doi: 10.1055/a-1857-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hein A, Kehl S, Häberle L et al. Prevalence of SARS-CoV-2 in Pregnant Women Assessed by RT-PCR in Franconia, Germany: First Results of the SCENARIO Study (SARS-CoV-2 prEvalence in pregNAncy and at biRth In FrancOnia) Geburtshilfe Frauenheilkd. 2022;82:226–234. doi: 10.1055/a-1727-9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takla A, Matysiak-Klose D, Bogdan C et al. Empfehlung und Begründung der STIKO zur Impfung gegen COVID-19 von Schwangeren und Stillenden. Epidemiol Bull. 2021;38:10–29. [Google Scholar]
- 6.Pecks U, Kuschel B, Mense L et al. Pregnancy and SARS-CoV-2 infection in Germany – the CRONOS Registry. Dtsch Arztebl Int. 2020;117:841–842. doi: 10.3238/arztebl.2020.0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LoMauro A, Aliverti A. Respiratory physiology of pregnancy. Breathe. 2015;11:297–301. doi: 10.1183/20734735.008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contreras G, Gutiérrez M, Beroíza T et al. Ventilatory drive and respiratory muscle function in pregnancy. Am Rev Respir Dis. 1991;144:837–841. doi: 10.1164/ajrccm/144.4.837. [DOI] [PubMed] [Google Scholar]
- 9.Staub D, Harpes P, Zimmermann R et al. Reference curves of symphysis-fundus height in twin pregnancies. Eur J Obstet Gynecol Reprod Biol. 2006;128:236–242. doi: 10.1016/j.ejogrb.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Enengl S, Pecks U, Oppelt P et al. Antibody Response and Maternofetal Antibody Transfer in SARS-CoV-2-Positive Pregnant Women: A Multicenter Observational Study. Geburtshilfe Frauenheilkd. 2022;82:501–509. doi: 10.1055/a-1768-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleinwechter HJ, Weber KS, Mingers N et al. COVID-19-Related Obstetric and Neonatal Outcome Study (CRONOS) Network. Gestational diabetes mellitus and COVID-19: results from the COVID-19-Related Obstetric and Neonatal Outcome Study (CRONOS) Am J Obstet Gynecol. 2022;227:6310–6.310000000000001E21. doi: 10.1016/j.ajog.2022.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sitter M, Pecks U, Rudiger M et al. Pregnant and Postpartum Women Requiring Intensive Care Treatment for COVID-19-First Data from the CRONOS-Registry. J Clin Med. 2022;11:701. doi: 10.3390/jcm11030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zöllkau J, Bohlmann M, Mingers N et al. SARS-CoV-2/COVID-19 and Hypertensive Pregnancy Disorders: Evaluation of the CRONOS National Registry. Z Geburtshilfe Neonatol. 2023;227:120–126. doi: 10.1055/a-1962-6964. [DOI] [PubMed] [Google Scholar]
- 14.Mand N, Iannaccone A, Longardt AC et al. Neonatal outcome following maternal infection with SARS-CoV-2 in Germany: COVID-19-Related Obstetric and Neonatal Outcome Study (CRONOS) Arch Dis Child Fetal Neonatal Ed. 2022;107:454–456. doi: 10.1136/archdischild-2021-322100. [DOI] [PubMed] [Google Scholar]
- 15.Cronos-Network Weschenfelder F, Zöllkau J, Schohe Aet al. Obesity during Pregnancy and SARS-CoV-2/COVID-19-Case Series of the Registry Study “COVID-19 Related Obstetric and Neonatal Outcome Study” (CRONOS-Network) J Clin Medon Behalf Of2023122089. 10.3390/jcm12062089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.COVID-19-Related Obstetric and Neonatal Outcome Study (CRONOS) Network . Ziert Y, Abou-Dakn M, Backes C et al. Maternal and neonatal outcomes of pregnancies with COVID-19 after medically assisted reproduction: results from the prospective COVID-19-Related Obstetrical and Neonatal Outcome Study. Am J Obstet Gynecol. 2022;227:4950–4.95E13. doi: 10.1016/j.ajog.2022.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sourouni M, Braun J, Oelmeier K et al. Assessment of Neonatal Cord Blood SARS-CoV-2 Antibodies after COVID-19 Vaccination in Pregnancy: A Prospective Cohort Study. Geburtshilfe Frauenheilkd. 2022;82:510–516. doi: 10.1055/a-1721-4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villar J, Ariff S, Gunier RB et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021;175:817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Celewicz A, Celewicz M, Michalczyk M et al. Pregnancy as a Risk Factor of Severe COVID-19. J Clin Med. 2021;10:5458. doi: 10.3390/jcm10225458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAuliffe F, Kametas N, Costello J et al. Respiratory function in singleton and twin pregnancy. BJOG. 2002;109:765–769. doi: 10.1111/j.1471-0528.2002.01515.x. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui AH, Tauheed N, Ahmad A et al. Pulmonary function in advanced uncomplicated singleton and twin pregnancy. J Bras Pneumol. 2014;40:244–249. doi: 10.1590/s1806-37132014000300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahamat-Saleh Y, Fiolet T, Rebeaud ME et al. Diabetes, hypertension, body mass index, smoking and COVID-19-related mortality: a systematic review and meta-analysis of observational studies. BMJ Open. 2021;11:e052777. doi: 10.1136/bmjopen-2021-052777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.for the CRONOS Network . Pecks U, Mand N, Kolben T et al. SARS-CoV-2 infection during pregnancy—an analysis of clinical data from Germany and Austria from the CRONOS Registry. Dtsch Arztebl Int. 2022;119:588–594. doi: 10.3238/arztebl.m2022.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers of Disease and Control Prevention . Risk for COVID-19 Infection, Hospitalization, and death by age group. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html
- 25.Patanavanich R, Glantz SA. Smoking Is Associated With COVID-19 Progression: A Meta-analysis. Nicotine Tob Res. 2020;22:1653–1656. doi: 10.1093/ntr/ntaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Statistisches Bundesamt . 14400 Mehrlingsgeburten im Jahr 2019. https://www.destatis.de/DE/Presse/Pressemitteilungen/Zahl-der-Woche/2020/PD20_47_p002.html https://www.destatis.de/DE/Presse/Pressemitteilungen/Zahl-der-Woche/2020/PD20_47_p002.html
- 27.Robert Koch Institut . Ergebnisse zur SARS-Cov-2 Seroprävalenz in der Allgemeinbevölkerung-Aktualisierung September 2022. https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/AK-Studien/Ergebnisse.html?nn=13490888#doc16719120bodyText4 https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/AK-Studien/Ergebnisse.html?nn=13490888#doc16719120bodyText4
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.