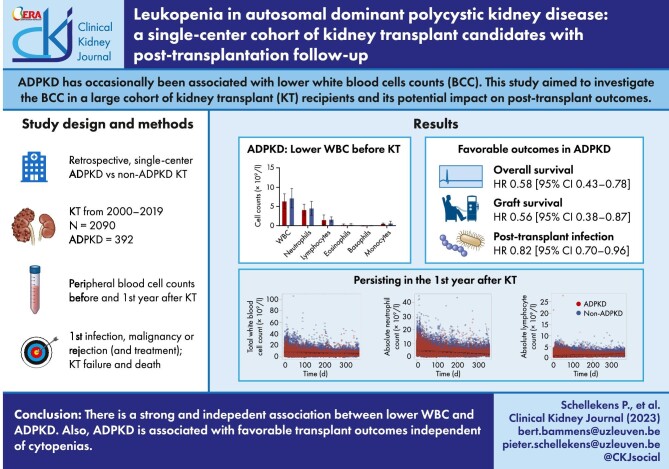
ABSTRACT
Background
Autosomal dominant polycystic kidney disease (ADPKD) has occasionally been associated with lower peripheral white blood cell (WBC) counts. This study aimed to investigate the peripheral blood cell counts in a large cohort of kidney transplant recipients before and after kidney transplantation and its potential impact on post-transplant outcomes.
Methods
This was a retrospective study with long-term follow-up data of 2090 patients who underwent a first kidney transplantation in the Leuven University Hospitals, of whom 392 had ADPKD.
Results
In total, 2090 patients who underwent a first kidney transplantation in the Leuven University Hospitals were included, of whom 392 had ADPKD. Both pre- and post-transplantation, ADPKD patients had significantly lower total WBC counts, and more specifically lower neutrophil, lymphocyte and eosinophil counts compared with the non-ADPKD patients. This observation was independent of potential confounders such as level of inflammation, smoking habit, vitamins and pre-transplant medication. Overall survival and kidney transplant survival were significantly better in ADPKD vs non-ADPKD transplant recipients and a longer time to first infection was observed. However, no association between blood cell counts and outcome differences was found.
Conclusions
In conclusion, this large single-center study reports a strong and independent association between ADPKD and lower peripheral WBC counts both before and after kidney transplantation. Considering the role of inflammation in disease progression, further investigation into the role of WBC in ADPKD is needed.
Keywords: ADPKD, inflammation, kidney transplantation, lymphopenia, white blood cells
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is the most common monogenic inherited kidney disease with an estimated prevalence of 1:400 to 1:1000, affecting 12.5 million people worldwide [1, 2]. Mutations in PKD1 and PKD2 account for approximately 78% and 15% of affected individuals, respectively [1, 2]. PKD1 and PKD2 respectively encode the proteins polycystin-1 (PC-1) and polycystin-2 (PC-2) [1, 2]. The remaining ∼7% of cases are genetically unresolved or are due to mutations in other recently identified genes, such as GANAB, DNAJB11 and ALG9, involved in the maturation and trafficking of PC-1 [1–4]. It is a systemic disorder primarily characterized by the development of renal cysts, but extra-renal manifestations also occur, including hepatic/pancreatic cysts, intra-cranial aneurysms, colon diverticulosis and cardiac valve lesions [1–4].
In the pathophysiology of ADPKD an important role has been attributed to the immune system [3, 4]. Generally, renal cyst growth is thought to involve two phases: first the initiation of cyst formation due to the unscheduled proliferation of tubular epithelial cells driven by the molecular PC defects [3–5]. Second, the microscopic injury to the renal environment is thought to provoke an injury response, which might contribute to cyst growth [3–5]. Recent studies showed that the tubular epithelial cells can also directly express cytokines, chemokines and complement encoding genes [3]. The expression of these key immunological regulators could as such trigger infiltration of immune cells even before the onset of severe cyst formation [3]. Thus, the interaction between tubular epithelial cells and the immune system seems to be important in defining the cystic phenotype of ADPKD [3]. On the other hand, some studies have shown that ADPKD patients with chronic kidney disease (CKD) and end-stage kidney disease (ESKD) have lower lymphocyte, monocyte, eosinophil, neutrophil and thrombocyte counts compared with patients with ESKD due to other causes [5, 6]. Van Laecke et al. reported that lymphopenia was twice as common in patients with ADPKD, and the lymphocyte counts correlated inversely with CKD stage [6]. Importantly, polycystins are expressed in B and T lymphocytes and a relation between the molecular PC defects and lymphocyte functioning in vitro has been demonstrated [7, 8]. Finally, sparse and conflicting data have suggested a tendency to greater vulnerability for cancer and infections in ADPKD renal transplant recipients, which may be associated with the above observations [9–14].
The aim of the present study was to investigate the association between ADPKD and lower peripheral white blood cell counts (WBC) in a well-defined and large cohort of ADPKD patients, immediately before and during the first year after kidney transplantation (KT), and to assess whether this has an impact on post-transplant outcomes.
MATERIALS AND METHODS
Data collection and definitions
This is a retrospective cohort study of all patients who received a first kidney or combined transplantation at University Hospitals Leuven between 1 January 2000 and 1 September 2019. Renal diagnosis was classified according the ERA classification. The diagnosis of ADPKD is based on the Ravine's clinical criteria and/or genetic diagnosis including pathogenic variants in the PKD1, PKD2, GANAB, DNAB11 or ALG9 genes. At baseline, which was set at 1 day before transplantation, we collected anthropometric data, demographic data, comorbidity, modality of dialysis, unilateral or bilateral nephrectomy pre-transplant, medication, renal diagnosis and biochemical analysis. Lymphopenia was defined as an absolute lymphocyte count of <1.2 × 109/L. Neutropenia was defined as an absolute neutrophil count <1.5 × 109/L. Thrombocytopenia was defined as a thrombocyte count <150.0 × 109/L. Anemia was defined as a hemoglobin level <14.0 g/dL in males and <12.0 g/dL in females. In addition to the baseline evaluation, all peripheral blood cell counts measured in the first year post-transplant were included. Outcome data included: date of first infection, date of first malignancy, time of death, time of transplant failure, date of first transplant rejection and treatment of rejection. Rejection was defined as all histopathological subtypes of rejection considered clinically relevant and for which anti-rejection treatment was installed.
Statistical analysis
We reported means and standard deviation for continuous variables, and frequencies and percentages for discrete variables, for ADPKD and non-ADPKD patients separately (Table 1, Supplementary data, Table S1). Means were compared with unpaired t-tests, while we compared percentages with the χ2 test. Univariable and multivariable linear regression analysis were performed to assess associations between ADPKD and cell counts. We preselected a set of clinical and biochemical variables that could influence blood cell counts. Univariable linear mixed models were used to detect differences in evolution over time in peripheral blood cells counts between ADPKD and non-ADPKD patients. Concerning the random effect, only random intercepts were included in the model. The time to first infection, time to first rejection, time to first malignancy and time to graft failure or recipient death were analyzed by the Kaplan–Meier method, including Log-Rank testing for group differences, and by univariable Cox proportional hazards analyses. All survival analyses started from the day of transplantation. For overall survival, patients were censored at the time of transplant failure or at the last clinical follow-up visit. For the analysis of transplant failure, patients were censored at death with functioning graft or at last follow-up. Time to first infection, rejection or malignancy, was censored at time of death with functioning graft, at transplant failure or at the end of follow-up. For the multivariable analysis, variables with P < .15 in univariable analyses were included in the final multivariable Cox model. The selected variables are summarized in Supplementary data, Table S2. An interaction term was used to evaluate the potential interaction between the presence of lymphopenia pre-transplant and ADPKD vs non-ADPKD on the different transplant outcomes. We used SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA) for the analyses, always performed two-sided hypothesis tests and considered P values <.05 as significant.
Table 1:
Baseline demographics immediately before KT, comparing ADPKD and non-ADPKD patients.
| ADPKD (n = 392) | Non-ADPKD (n = 1698) | P-value | |
|---|---|---|---|
| Demographics | |||
| Male, n (%) | 195 (49.7) | 1079 (63.6) | <.001 |
| Age at KT (years), mean (SD), median (min–max) |
56.5 (8.6), 56.50 (34.43–82.64) |
53.4 (13.8), 55.73 (15.74–81.55) |
<.001 |
| Weight (kg), mean (SD), median (min–max) |
75.0 (14.5), 73.25 (43.10–123.60) |
73.0 (15.1), 72.00 (31.90–133.00) |
.35 |
| Height (cm), mean (SD), median (min–max) |
171.9 (10.4), 171.00 (149.00–203.00) |
168.8 (9.7), 170.00 (126.00–194.00) |
<.001 |
| BMI (kg/m2), mean (SD), median (min–max) |
25.1 (4.0), 24.57 (16.94–40.69) |
24.9 (4.4), 24.39 (11.58–40.86) |
.07 |
| Active smoker, n (%) | 102 (6.1) | .68 | |
| Comorbidity , n (%) | |||
| Diabetes | 22 (5.6) | 410 (24.2) | <.001 |
| Hypertension | 229 (58.4) | 811 (47.8) | <.001 |
| Cancer | 65 (16.5) | 304 (17.9) | .62 |
| Cardiovascular disease | 93 (23.7) | 448 (26.4) | .66 |
| Dialysis | |||
| No dialysis, n (%) | 26 (6.6) | 80 (4.7) | .17 |
| Home hemodialysis, n (%) | 3 (0.8) | 9 (0.6) | .58 |
| Peritoneal dialysis, n (%) | 80 (20.5) | 350 (20.6) | .81 |
| In-center hemodialysis, n (%) | 283 (72.1) | 1259 (74.2) | .18 |
| Time in dialysis before KT (days), mean (SD) | 925.8 (692.3) | 1184.9 (1099.8) | <.001 |
| Biochemistry | |||
| CRP (mg/L), mean (SD), median (min–max) |
8.1 (15.2), 3.10 (0.3–184.4) |
7.8 (13.9), 3.50 (0.3–250.4) |
.02 |
| Ferritin (µg/L), mean (SD), median (min–max) |
240.7 (252.6), 163.0 (2.0–1386.0) |
258.2 (472.4), 140.5 (2.0–12351.0) |
<.001 |
| Vitamin B12 (ng/L), mean (SD), median (min–max) |
688.1 (458.5), 530.0 (139.0–2000.0) |
750.5 (449.1), 619.0 (75.0–2000.0) |
.62 |
| Folic acid (µg/L), mean (SD), median (min–max) |
10.9 (5.9), 9.0 (3.0–20.0) |
10.5 (5.8), 9.0 (1.40–20.0) |
.76 |
| Transplantation related | |||
| Combined transplantation, n (%) | 46 (11.7) | 166 (9.8) | .25 |
| Nephrectomy pre-transplant, n (%) | 102 (26.0) | 165 (9.7) | <.001 |
| Number of HLA mismatches, median (LQR–UQR) , n (%) | 3 (2–4) | 3 (2–4) | .09 |
| 0 HLA mismatch | 9 (2.3) | 42 (2.8) | .84 |
| 1 HLA mismatch | 39 (10.0) | 99 (5.8) | .003 |
| 2 HLA mismatches | 69 (17.6) | 285 (16.8) | .70 |
| 3 HLA mismatches | 107 (27.0) | 463 (27.3) | .93 |
| 4 HLA mismatches | 72 (18.4) | 391 (23.0) | .05 |
| 5 HLA mismatches | 25 (14.8) | 243 (14.3) | .74 |
| 6 HLA mismatches | 39 (10.0) | 178 (10.5) | .75 |
BMI, body mass index; CRP, C-reactive protein; HLA, human leukocyte antigen; LQR–UQR, lower quartile range—upper quartile range; SD, standard deviation.
Ethical statement
The study was approved by the local ethical board (Ethical Comity Research KU/UZ Leuven, s64953) and in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects or their legal guardian(s).
RESULTS
Population characteristics
We included 2090 patients with a first KT between January 2000 and September 2019, including 212 (10.14%) patients with a combined organ transplantation (Supplementary data, Fig. S1 and S2). The original kidney disease diagnosis was classified according to the ERA classification (Supplementary data, Fig. S3). The group of patients with familial/hereditary nephropathies (n = 435) consisted largely of ADPKD patients with only a small subgroup of non-ADPKD hereditary kidney diseases (n = 43, Supplementary data, Fig. S3). ADPKD was the original kidney disease in 392 patients (Table 1). As compared with the non-ADPKD cohort, the ADPKD cohort contained significantly more females (P < .001) and was significantly older (P < .001) (Table 1). All patients were adults (>18 years), except for five adolescent with age range from 15.74 to 17.74 years, none whom belonged to the ADPKD group (subgroup analysis in Supplementary data, Table S3). In terms of comorbidity, a higher prevalence of a pre-transplantation history of hypertension (P < .001) and a lower prevalence of diabetes (P < .001) was observed in the ADPKD patient cohort (Table 1). No overall differences in pre-transplantation history of malignancy, cardiovascular disease or body mass index were observed (Table 1). The distribution of dialysis modalities was not different between the two groups (Table 1). However, time on dialysis before KT was significantly shorter in the ADPKD patient cohort (P < .001) (Table 1). In terms of pre-transplant medication, a significantly lower usage of erythropoietin-stimulating agents (P = .003) and insulin (P < .001) was observed in ADPKD patients.
ADPKD patients had significant lower WBC counts and thrombocytes prior to KT
Immediately before KT, lower values were observed in the ADPKD patient cohort for total WBC (P < .001), absolute neutrophil (P < .001), absolute lymphocyte (P < .001), absolute eosinophil (P = .006) and thrombocyte (P = .001) counts (Table 2). No significant differences were observed for red blood cell counts (P = .53), hemoglobin (P = .83) and absolute basophil counts (P = .12) (Table 2). A higher prevalence of lymphopenia (P = .008) and thrombocytopenia (P = .02) in the ADPKD patient cohort was observed (Table 2). In contrast, a lower prevalence of anemia (P < .001) pre-transplant was detected, using the sex specific cutoff values for hemoglobin levels in non-CKD (Table 2).
Table 2:
Peripheral blood cell counts obtained prior to kidney transplantation and the prevalence of cytopenias, comparison between ADPKD and non-ADPKD patients.
| ADPKD (n = 392) | Non-ADPKD (n = 1698) | P-value | |
|---|---|---|---|
| Absolute blood cell counts | |||
| Total WBC counts (×109/L), mean (SD) | 6.38 (1.94) | 7.15 (2.52) | <.001 |
| Neutrophil (×109/L) | 4.12 (1.46) | 4.53 (1.82) | <.001 |
| Lymphocyte (×109/L) | 1.48 (1.50) | 1.62 (0.66) | <.001 |
| Eosinophil (×109/L) | 0.23 (0.21) | 0.26 (0.24) | .006 |
| Basophil (×109/L) | 0.02 (0.04) | 0.03 (0.04) | .12 |
| Monocyte (×109/L) | 0.50 (0.20) | 0.59 (0.58) | <.001 |
| Red blood cell count (×1012/L), mean (SD) | 4.12 (0.59) | 3.91 (3.89) | .53 |
| Hemoglobin (g/dL) | 12.69 (1.68) | 12.19 (1.67) | .83 |
| MCV (fL) | 94.97 (6.10) | 95.89 (6.55) | .08 |
| MCH (pg) | 30.87 (2.14) | 31.27 (31.16) | .27 |
| MCHC (g/dL) | 32.51 (31.41) | 32.62 (32.56) | .002 |
| RDW (%) | 14.47 (1.41) | 14.73 (14.68) | <.001 |
| Erythroblasts (/L) | 0.0009 (0.006) | 0.005 (0.0015) | <.001 |
| Erythroblasts (/100 WBC) | 0.02 (0.12) | 0.06 (0.82) | <.001 |
| Hematocrit (%) | 0.39 (0.05) | 0.37 (0.05) | .54 |
| Reticulocytes (×109/L) | 47.38 (25.44) | 53.28 (25.74) | .83 |
| Thrombocyte count (×109/L), mean (SD) | 208.00 (66.48) | 225.10 (221.4) | .001 |
| Cytopenias (according to standardized cutoffs) | |||
| Leukopenia, n (%) | 25 (6.4) | 84 (5.0) | .25 |
| Lymphopenia, n (%) | 125 (32.0) | 429 (25.3) | .008 |
| Neutropenia, n (%) | 31 (0.8) | 31 (1.8) | .16 |
| Thrombocytopenia, n (%) | 70 (17.9) | 222 (13.1) | .02 |
| Anemia, n (%) | 208 (53.1) | 1211.0 (71.3) | <.001 |
| Neutrophil/lymphocyte ratio, mean (SD) | 3.2 (1.6) | 3.3 (2.8) | <.001 |
MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red blood cell distribution width; SD, standard deviation.
ADPKD was independently associated with lower WBC
Multivariable linear regression analysis was used to detect independent associations of ADPKD with the different blood cell counts. After correction for age, sex, combined transplantation, kidney diagnosis, gender, smoking, level of inflammation, serum folic acid, serum vitamin B12, serum ferritin, time and type of dialysis pre-transplant, ADPKD remained significantly associated with total WBC (estimate, P-value: –0.69, P < .001), absolute neutrophil (–0.35, P = .02), absolute lymphocyte (–0.23, P < .001), absolute monocyte (–0.082, P < .001) and thrombocyte (–13.62, P = .03) counts prior to KT (Supplementary data, Table S4).
WBC counts remained significantly lower in the first year after KT in ADPKD patients
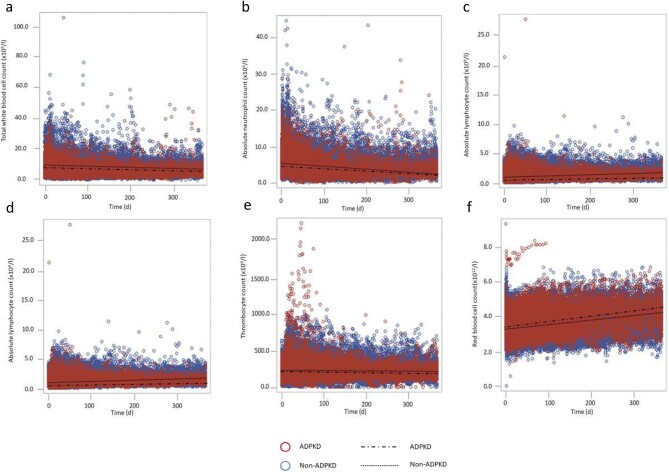
Based on all available peripheral blood cell counts (119 926 measurements) between baseline (Day 0, prior to KT) and 1 year post-transplantation, WBC (P < .001), absolute neutrophil (P < .001), absolute lymphocyte (P = .001), absolute eosinophil (P < .001) and thrombocyte (P = .01) counts were significantly lower in the ADPKD cohort compared with non-ADPKD patients (Fig. 1a–e). In contrast, red blood cell counts were significantly higher at baseline in ADPKD patients compared with non-ADPKD patients and the divergence increased in the first year after KT (Fig. 1f).
Figure 1:
The evolution of the different blood cell counts over time for all patients (N = 2090, 392 ADPKD and 1698 non-ADPKD patients). Time zero is the day of transplantation. Each dot represents one measurement (119 926 measurements). The blood cell counts of ADPKD patients are indicated in red, the cell counts for non-ADPKD patients in blue. The regression line from the linear mixed model is added for ADPKD and non-ADPKD patients separately. (a) The total WBC counts are significantly different over time between ADPKD and non-ADPKD patients (P < .001). Furthermore, the interaction between time and ADPKD is also significantly different (P = .001). (b) The absolute neutrophil counts are significantly different over time between ADPKD and non-ADPKD patients (P < .001), the slope is also significantly different (P < .001). (c) The absolute lymphocyte counts are significantly different between ADPKD and non-ADPKD (P = .001), the slope is also significantly different (P < .001). (d) Absolute eosinophil counts are significantly different over time (P < .001) with a significant interaction between time and ADPKD (P = .002). (e) Thrombocyte counts are significantly lower over time in the ADPKD group (P = .01) with significantly different slopes (P = .02). (f) Red blood cell counts are significantly higher in the ADPKD cohort with a significantly (P < .001) steeper increase over time (P < .001).
ADPKD was associated with favorable post-transplant outcomes—this association was not influenced by differences in blood cell counts
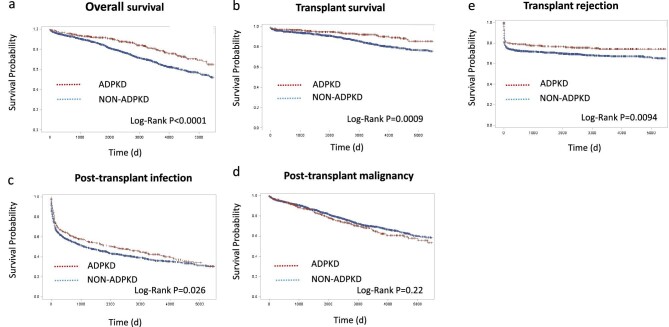
During the post-transplant follow-up [median 6.51 years (interquartile range 3.01–10.69 years)], significant differences in overall survival in favor of ADPKD transplant recipients were seen (Log-Rank P < .001) (Fig. 2a) with the Kaplan–Meier analysis. In univariable and multivariable Cox proportional regression analysis, ADPKD was significantly associated with overall survival [hazard ratio (HR) 0.583, (95% confidence interval 0.43; 0.78), P < .001] (Supplementary data, Table S5). Both absolute pre-transplant lymphocyte [HR 0.83 (0.70; 0.99), P = .04] and pre-transplant monocyte [HR 1.61 (1.06; 2.45), P = .03] counts were, independent of ADPKD, associated with overall survival (Supplementary data, Table S5). ADPKD was also associated with a better death-censored graft survival compared with non-ADPKD (Log-Rank P < .001) [adjusted HR 0.56 (0.38; 0.82), P = .004] (Fig. 2b, Supplementary data, Table S5), whereas pre-transplant WBC counts were not. Furthermore, ADPKD associated with a significantly later onset of the first post-transplant infection (Log-Rank P = .03) (Fig. 2c). In the multivariable analysis, both ADPKD [HR 0.82 (0.70; 0.96), P = .02] and pre-transplant monocyte counts [HR 1.36 (1.05; 1.76), P = .02] remained significantly associated with time to first infection (Supplementary data, Table S5). No significant differences between ADPKD and non-ADPKD patients were observed for time to first post-transplant malignancy [Log-Rank P = .22; HR 0.94 (0.74; 1.91), P = 0.61] (Fig. 2d, Supplementary data, Table S5). The time to develop a first transplant rejection was significantly longer in the ADPKD cohort (Log-Rank P = .009) (Fig. 2e). However, ADPKD was not significantly associated with rejection in the multivariable Cox model (Supplementary data, Table S5). Overall, we concluded that ADPKD associated with favorable transplant outcomes independent of pre-transplant blood cell counts. No significant interaction between ADPKD and the different pre-transplant blood cell counts was observed for overall survival, death-censored graft survival, time to first infection, malignancy or rejection. Findings were essentially the same when patients with a combined transplantation were excluded from the analyses (Supplementary data, Figs S4 and S5, and Tables S6–S8).
Figure 2:
Kaplan–Meier curves for overall survival (a), transplant survival cencored for death (b), time to first infection (c), time to first malignancy (d) and time to first rejection (e), between ADPKD (red) versus non-ADPKD (blue).
DISCUSSION
This single-center study of a large and well characterized cohort of 2090 patients who underwent their first KT demonstrated that pre-transplant blood counts of total WBC, neutrophils, lymphocytes, monocytes and thrombocytes were significantly lower in ADPKD patients as compared with their non-ADPKD counterparts. This association was independent of potential confounders as age, sex, smoking, time and type of dialysis pre-transplant, level of inflammation and vitamins. Furthermore, the association between ADPKD and lower WBCs and thrombocytes persisted after kidney transplantation.
There have been few reports on the phenomenon of altered blood cell counts in ADPKD, the existing evidence seems most convincing for cytopenias in the lymphocytic lineages [5, 6]. In 2002, Banerjee et al. [5] reported an increased prevalence of leukopenia and abnormalities of the differential WBC count in a group of patients (n = 360; 26 ADPKD vs 334 CKD5 of other etiologies) with ADPKD on hemodialysis [5]. Van Laecke et al. [6]. reported significantly lower lymphocyte, neutrophils, monocyte and thrombocyte counts in patients with CKD5 due to ADPKD (n = 126) vs non-ADPKD (n = 574) adjusting for potential confounders such as age, gender, estimated glomerular filtration rate and C-reactive protein [6]. Lymphopenia was 1.6 times more common in patients with ADPKD [6]. In a second cohort, they compared peripheral blood cell counts from 204 stable outpatients with ADPKD-related CKD with 204 age- and gender-matched controls across the same severity of CKD, with other kidney diagnoses [6]. Similar to the CKD 5 cohort, they found significantly lower lymphocyte, monocyte and thrombocyte counts in ADPKD patients, however without difference in neutrophil count [6]. Lymphopenia was twice as common in patients with ADPKD and the lymphocyte number correlated with CKD stage (lower numbers at more severe stage of CKD for all patients) [6]. After stratification for CKD category, lymphocyte counts remained significantly lower in the ADPKD group [6].
The pathophysiological mechanism behind the association of ADPKD and low blood cell counts is not fully elucidated [4–6]. One of the hypothetical mechanisms described earlier to explain this association is the sequestration of blood cells in cystic organs [4, 5, 15–17]. However, while this factor cannot be excluded based on our data, it is of note that ADPKD remained significantly associated with the above-mentioned blood cell counts after correction for nephrectomy pre-transplant. The sequestration of blood cells in other affected and cystic organs could not be excluded. Secondly, differences in inflammation levels could contribute to the observed differences in cell counts [4–6]. Disease progression in ADPKD is believed to be highly influenced by immune cells, cytokines and chemokines [3]. Kidney cysts are surrounded by a variety of immunological cells with both renoprotective as well as disease-promoting effects [3, 4]. The associated chronic inflammation could influence hematopoiesis through its effect on cytokines, chemokines and iron metabolism [4, 18]. As such, the differences in peripheral blood cell counts in ADPKD could be secondary to the chronic inflammation in the cystic organs [4, 18]. However, the association between lower WBC counts prior to KT and ADPKD remained significant after correction for serum C-reactive protein levels pre-transplant. As mentioned before, the observed differences in blood cell counts pre-transplant persisted during the entire first year after renal transplantation. This supports the hypothesis that ADPKD is intrinsically associated with lower peripheral WBC counts. Indeed, cytopenia could also be an unrecognized extra-renal manifestation of ADPKD, directly linked to its genetic basis [4–6]. Of note, polycystin expression has been described in B lymphoblastoid cells and T lymphocytes and might interfere with lymphocyte function in vitro [7, 8]. Unfortunately, only 11% (n = 45) of the ADPKD patients in our retrospective cohort the genotype was known. This precludes any relevant conclusion on a correlation between the genetic background and the presence of cytopenia in this cohort of patients. Abnormalities in WBC counts could also be a broader ciliopathy-related feature [4]. But again, our study does not allow sufficiently powered conclusions on a comparison of ADPKD or other ciliopathies, given the small number (n = 43) of non-ADPKD inherited nephropathies (Supplementary data, Tables S9 and S10). The lower prevalence of anemia in ADPKD patients as compared with patients with other etiologies of renal insufficiency has been repeatedly reported and is confirmed by this large monocentric study [19, 20]. Generally, the lower prevalence of anemia in ADPKD patients is thought to be secondary to elevated levels of endogenous erythropoietin [19, 20]. A local ischemic trigger, due to compression of renal parenchyma by cysts, has been postulated as main causal mechanism here [19, 20].
In terms of transplant outcomes, we observed a better overall survival and transplant survival in ADPKD patients as compared with non-ADPKD patients. ADPKD patients are generally considered as excellent candidates for KT, mainly due to fewer comorbidities, which may of course explain outcome differences [9–14]. However, even after adjusting for pretransplant comorbidities survival was better in ADPKD patients. Furthermore, a later onset of the first infection after transplantation in ADPKD vs non-ADPKD patients was observed. Combining the favorable outcomes of ADPKD and its association with lower blood cell counts, it is tempting to speculate that ADPKD influences outcome through a disease-related effect on blood cells. Divergent effects in the different subsets of lymphocytes, could provide an explanation for the observed findings. However, after correction for confounders, including correction for the different WBC counts, ADPKD remained significantly associated with a better overall survival, transplant survival and a longer time until the first infectious episode. Furthermore, it was not possible to prove a significant interaction between ADPKD and peripheral blood cell counts in the multivariate Cox proportional hazards model for the different outcome parameters. As such, based on our data, the association of ADPKD with favorable outcomes post-transplant cannot be explained by differences in blood cell counts. Furthermore, while the association of ADPKD with lower WBC counts was clearly significant and persistent over time, the absolute WBC count differences were rather subtle. The differences in WBC counts “disappear,” except for lymphopenia, when applying the standardized cutoffs (Table 2), which may explain why the impact of the putative ADPKD-associated leukopenia on post-transplant outcomes is limited. From this, the question whether the findings of lower WBC counts have clinical significance is still unanswered [21, 22].
The retrospective nature of our study precludes any conclusion on causality. It is a single-center study with a Caucasian population, which limits options for extrapolation to other populations. Furthermore, differences in immunosuppressive regimens and post-transplant infections (like cytomegalovirus) are important contributors to cytopenias post-transplant and may have impacted on post-transplantation outcomes [22]. However, these variables have no impact on pre-transplantation blood cell counts, which are the main focus of the present work.
However, considering the indispensable role of inflammation in progression of ADPKD, the findings merit further exploration and inspire both in vitro expression and functional studies, as well as prospective clinical cohorts, to further elucidate the role of WBC in ADPKD.
CONCLUSION
This large single-center study describes a strong association between ADPKD and lower peripheral WBCs immediately before and during the first year after KT. Even after correction for both clinical and biochemical factors that are known to influence blood cell counts, ADPKD remained significantly associated with lower WBC counts. Furthermore, ADPKD was also associated with differences in post-transplant outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
D.M. and B.B. conceived the idea for this study. P.S. retrieved and analyzed the data, and drafted the article. E.V.L., B.B., D.M., R.V., I.M., M.C. and D.K. critically revised the article. All authors provided final approval of the version to be published. The study was approved by the local ethical board (Ethical Comity Research KU/UZ Leuven).
Contributor Information
Pieter Schellekens, Department of Microbiology, Immunology and Transplantation, Nephrology and Renal Transplantation Research Group, KU Leuven, Leuven, Belgium; Department of Cellular and Molecular Medicine, PKD Research Group, KU Leuven, Leuven, Belgium; Department of Nephrology, Dialysis and Renal Transplantation, University Hospitals of Leuven, Leuven, Belgium.
Elisabet Van Loon, Department of Microbiology, Immunology and Transplantation, Nephrology and Renal Transplantation Research Group, KU Leuven, Leuven, Belgium; Department of Nephrology, Dialysis and Renal Transplantation, University Hospitals of Leuven, Leuven, Belgium.
Maarten Coemans, Department of Microbiology, Immunology and Transplantation, Nephrology and Renal Transplantation Research Group, KU Leuven, Leuven, Belgium.
Isabelle Meyts, Department of Microbiology, Immunology and Transplantation, Laboratory of Inborn Errors of Immunity, KU Leuven, Leuven, Belgium; Department of Pediatrics, Pediatric Immunology, University Hospitals of Leuven, Leuven, Belgium.
Rudi Vennekens, Department of Cellular and Molecular Medicine, VIB Centre for Brain and Disease Research, Laboratory of Ion Channel Research, KU Leuven, Leuven, Belgium.
Dirk Kuypers, Department of Microbiology, Immunology and Transplantation, Nephrology and Renal Transplantation Research Group, KU Leuven, Leuven, Belgium; Department of Nephrology, Dialysis and Renal Transplantation, University Hospitals of Leuven, Leuven, Belgium.
Djalila Mekahli, Department of Cellular and Molecular Medicine, PKD Research Group, KU Leuven, Leuven, Belgium; Department of Pediatric Nephrology, University Hospitals of Leuven, Leuven, Belgium.
Bert Bammens, Department of Microbiology, Immunology and Transplantation, Nephrology and Renal Transplantation Research Group, KU Leuven, Leuven, Belgium; Department of Nephrology, Dialysis and Renal Transplantation, University Hospitals of Leuven, Leuven, Belgium.
FUNDING
Fonds Wetenschappelijk Onderzoek—grant number 1 804 123 N.
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
The research activities described in this manuscript are supported by Otsuka and Sanofi. Pieter Schellekens is supported by the Research Foundation Flanders (F.W.O.). EVL, holds a fellowship grant (1143919N) from The Research Foundation Flanders (F.W.O). DM reports research grants from Otsuka and serves in advisory boards for Otsuka, Sanofi Genzyme and Reata, all outside the submitted work and all paid to her institutions UZ Leuven and KU Leuven, Belgium.
REFERENCES
- 1. Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 2007;369:1287–301. 10.1016/S0140-6736(07)60601-1 [DOI] [PubMed] [Google Scholar]
- 2. Douguet D, Patel A, Honoré E. Structure and function of polycystins: insights into polycystic kidney disease. Nat Rev Nephrol 2019;15:412–22. 10.1038/s41581-019-0143-6 [DOI] [PubMed] [Google Scholar]
- 3. Zimmerman KA, Hopp K, Mrug M. Role of chemokines, innate and adaptive immunity. Cell Signal 2020;73:1–15. 10.1016/j.cellsig.2020.109647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schellekens P, Roosens W, Meyts I et al. Cytopenia in autosomal dominant polycystic kidney disease (ADPKD): merely an association or a disease-related feature with prognostic implications? Pediatr Nephrol 2021;36:3505–14. 10.1007/s00467-021-04937-9 [DOI] [PubMed] [Google Scholar]
- 5. Banerjee A, Chandna S, Jayasena D et al. Leucopenia in adult polycystic kidney disease patients on haemodialysis. Nephron 2002;91:175–6. 10.1159/000057625 [DOI] [PubMed] [Google Scholar]
- 6. Van Laecke S, Kerre T, Nagler EV et al. Hereditary polycystic kidney disease is characterized by lymphopenia across all stages of kidney dysfunction: an observational study. Nephrol Dial Transplant 2018;33:489–96. 10.1093/ndt/gfx040 [DOI] [PubMed] [Google Scholar]
- 7. Aguiari G, Banzi M, Gessi S et al. Deficiency of polycystin-2 reduces Ca2+ channel activity and cell proliferation in ADPKD lymphoblastoid cells. FASEB J 2004;18:884–6. 10.1096/fj.03-0687fje [DOI] [PubMed] [Google Scholar]
- 8. Magistroni R, Mangolini A, Guzzo S et al. TRPP2 dysfynction decreases ATP evoked calcium, induces aggregation and stimulates proliferation in T lymfocytes. BMC Nephrol 2019;20:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bretagnol A, Halimi JM, Roland M et al. Autosomal dominant polycystic kidney disease: risk factor for nonmelanoma skin cancer following kidney transplantation. Transpl Int 2010;23:878–86. [DOI] [PubMed] [Google Scholar]
- 10. Nielsen LH, Jensen-Fangel S, Jespersen B et al. Risk and prognosis of hospitalization for pneumonia among individuals with and without functioning renal transplants in Denmark: a population-based study. Clin Infect Dis 2012;55:679–86. 10.1093/cid/cis488 [DOI] [PubMed] [Google Scholar]
- 11. Moua T, Zand L, Hartman RB et al. Radiologic and clinical bronchiectasis associated with autosomal dominant polycystic kidney disease. PLoS One 2014;9:e93674. 10.1371/journal.pone.0093674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mehrabi A, Golriz M, Maier J et al. Long-term follow-up of kidney transplant recipients with polycystic kidney disease. Exp Clin Transplant 2015;13:413–20. [PubMed] [Google Scholar]
- 13. Jacquet A, Pallet N, Kessler K et al. Outcomes of renal transplantation in patients with autosomal dominant polycystic kidney disease: a nationwide longitudinal study. Transpl Int 2011;24:582–7. 10.1111/j.1432-2277.2011.01237.x [DOI] [PubMed] [Google Scholar]
- 14. Singh T, Peery S, Astir BC et al. Cause of end-stage renal disease is not a risk factor for cytomegalovirus infection after kidney transplant. Transplant Proc 2019;51:1810–5. 10.1016/j.transproceed.2019.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bath P, Saggar-Malik A, MacDougall I et al. Increased platelet volume in patients with adult polycystic kidney disease. Platelets 1995;6:336–9. 10.3109/09537109509078468 [DOI] [PubMed] [Google Scholar]
- 16. Setyapranata S, Holt SG. Platelet counts in autosomal dominant polycystic kidney disease. Platelets 2015;27:262–3. 10.3109/09537104.2015.1071481 [DOI] [PubMed] [Google Scholar]
- 17. Yin X, Prince WK, Blumenfeld JD et al. Spleen phenotype in autosomal dominant polycystic kidney disease. Clin Radiol 2019;74:975e17–24. 10.1016/j.crad.2019.08.015 [DOI] [PubMed] [Google Scholar]
- 18. Cohen G, Hörl WH. Immune dysfunction in uremia-an update. Toxins 2012;4:962–90. 10.3390/toxins4110962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Verdalles U, Abad S, Vega A et al. Factors related to the absence of anemia in hemodialysis patients. Blood Purif 2011;32:69–74. 10.1159/000323095 [DOI] [PubMed] [Google Scholar]
- 20. Landau L, London L, Bandach I et al. The hypoxia inducible factor/erythropoietin (EPO)/EPO receptor pathway is disturbed in a rat model of chronic kidney disease related anemia. PLoS One 2018;13:1–13. 10.1371/journal.pone.0196684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Warny M, Helby J, Birgens H et al. Lymphopenia and risk of infection and infection-related death in 98,344 individuals from a prospective Danish population-based study. PLoS Med 2018;15:e1002685. 10.1371/journal.pmed.1002685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luque Y, Jamme M, Rabant M et al. Long-term CD4 lymphopenia is associated with accelerated decline of kidney allograft function. Nephrol Dial Transplant 2015;31:487–95. 10.1093/ndt/gfv362 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.