ABSTRACT
Background
Autosomal dominant polycystic kidney disease (ADPKD) is prone to multiple complications, including cyst infection (CyI). 2-Deoxy-2-[18F]fluoro-d-glucose positron emission tomography/computed tomography ([18F]-FDG PET/CT) imaging has proved useful in the diagnosis of renal and hepatic CyI. A 4-point scale comparing the uptake of [18F]-FDG in the suspected infected cyst versus the hepatic physiological background has been recently proposed. We performed an independent validation of this semi-quantitative scoring system.
Methods
All ADPKD patients hospitalized between January 2009 and November 2019 who underwent an [18F]-FDG PET/CT for suspected CyI were retrospectively identified using computer-based databases. Medical files were reviewed. CyI was conventionally defined by the combination of fever (≥38°C), abdominal pain, increased plasma C-reactive protein levels (≥70 mg/L), absence of any other cause of inflammation and favourable outcome after ≥21 days of antibiotics. [18F]-FDG uptake of the suspected CyI was evaluated using a 4-point scale comparing the uptake of [18F]-FDG around the infected cysts with the uptake in the hepatic parenchyma. Statistics were performed using SAS version 9.4.
Results
Fifty-one [18F]-FDG PET/CT scans in 51 patients were included, of which 11 were cases of CyI. The agreement between the 4-point scale and the gold-standard criteria of CyI was significant [odds ratio of 6.03 for CyI in case of a score ≥3 (P = .014)]. The corresponding sensitivity, specificity, and positive and negative predictive values of [18F]-FDG PET/CT using the 4-point scale were 64% [Clopper–Pearson 95% confidence interval (CI) 30%–89%], 78% (95% CI 62%–89%), 44% (95% CI 20%–70%) and 89% (95% CI 73%–97%), respectively.
Conclusions
Our independent validation cohort confirms the use of a semi-quantitative 4-point scoring system of [18F]-FDG PET/CT imaging in the diagnosis of CyI in patients with ADPKD. Considering its performance metrics with high specificity and negative predictive value, the scoring system is particularly useful to distinguish other causes of clinical inflammation than CyI and as such avoid unnecessarily long antibiotic treatment.
Keywords: ADPKD, cyst infection, [18F]-FDG PET/CT; standardized scoring system
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is a common hereditary kidney disease with an estimated prevalence of 1:400 to 1:1000 people. ADPKD is primarily characterized by the development of renal cysts and often leads to end-stage renal disease. However, ADPKD is a systemic disorder and has many extra-renal manifestations, such as hepatic and pancreatic cysts, colonic diverticulosis, intracranial aneurysms and cardiac valvular abnormalities. Cysts are prone to multiple complications, such as haemorrhage and infection (CyI) [1–4].
Hepatic and renal CyI have an incidence of 0.01 episode per year per patient and result in 11% of all hospitalizations of patients with ADPKD, according to an 11-year follow-up retrospective study [1]. It is a severe complication that can lead to abscess formation, sepsis and death [5]. A fast and correct diagnosis is essential to optimize patient management [6]. However, diagnosing a CyI remains a difficult task given the unspecific clinical and biological parameters [7]. The diagnosis of a ‘definite’ CyI may only be made when a cyst aspiration is performed and shows signs of infection, including neutrophils and/or pathogens. Since cyst aspiration is rarely performed, one often speaks of a ‘probable’ CyI. The diagnosis of a ‘probable’ CyI is based on clinical, biochemical, microbiological and imaging findings [7, 8]. Pijl et al. [9] suggested that a CyI is retrospectively ‘likely’ when the following five conditions are concurrent: fever (≥38°C for >3 days), abdominal pain, increased plasma C-reactive protein (CRP) levels (CRP ≥50 mg/L), the absence of any other cause of inflammation and a favourable outcome after ≥21 days of antibiotics. This definition proposed by Pijl et al. [9] has added the retrospective criteria of the effect of 21 days of antibiotics to the initial criteria proposed by Sallée et al. [1]. Since CyI are routinely treated with long courses (up to 6 weeks) of antibiotics with concomitant psychological burden for the patients, potential side-effects, drug resistance and major impact on hospitalization costs, excluding other causes of infection or inflammation is as important as confirming the diagnosis of CyI.
The use of classic imaging techniques is of limited value in diagnosing or excluding CyI. Ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI) often fail to detect an infected cyst. More recently, the use of 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography/CT ([18F]-FDG PET/CT) has been explored in the detection of CyI [10]. The detection of a CyI relies on the increased metabolic rate of the inflammatory cells [2, 5, 8]. Multiple studies found promising results with a good diagnostic performance of [18F]-FDG PET/CT in detection of CyI when compared with the clinical definition of CyI [1, 7, 9, 11, 12].
In a recent retrospective study, Neuville et al. [13] further explored the role of [18F]-FDG PET/CT in the diagnosis of CyI in patients with ADPKD and clinical suspicion of CyI. They proposed a 4-point scoring scale that compares the signal intensity of the cyst with the signal of the liver and bloodpool. The aim of this scoring scale was to build a more standardized and objective tool for [18F]-FDG PET/CT evaluation. The authors found that, with this 4-point scale, [18F]-FDG PET/CT was able to identify a CyI in 58% of the cases of probable CyI and was positive in all cases where a cyst aspiration had been performed and proved positive for CyI. When using the 4-point scale, a score ≥3 (i.e. cyst uptake > liver uptake) resulted in an odds ratio (OR) of 13.1 for CyI, with an improvement of the specificity from 70.1% (solely based on the subjective assessment) to 85.3% [13]. However, further external validation was required. With the current study, we aimed to validate the diagnostic yield of the 4-point scoring scale [13] in an independent external validation cohort of ADPKD patients with suspected CyI.
MATERIALS AND METHODS
Study design
Analogous to the protocol used in the study by Neuville et al. [13], all ADPKD patients hospitalized between January 2009 and November 2019 at the University Hospital of Leuven (UZ Leuven), Belgium (single centre) and who underwent an [18F]-FDG PET/CT scan for evaluation of suspected CyI within a 14-day period prior to or following their admission were considered eligible for evaluation. The medical files were reviewed, and clinical and biological data were systematically extracted. Our study was approved by the Ethics Committee Research UZ/KU Leuven (s64739).
[18F]-FDG PET/CT acquisition and analysis
[18F]-FDG PET/CT scans were performed on a Biograph TruePoint 40 or Biograph 16 HiRez PET/CT (Siemens Healthcare, Erlangen, Germany) or a Discovery MI4 PET/CT system (GE Healthcare, Chicago, IL, USA). Patients fasted for at least 6 h, were given 20 mg of propranolol (a non-specific beta-blocker) and had glucose levels <180 mg/dL at the time of intravenous [18F]-FDG administration. Patients were injected with a weight-adjusted [18F]-FDG activity: initially activity (MBq) = 4 * [weight (kg)] + 20 MBq; from 11 December 2014 onwards, activity (MBq) = 4.25 * weight (kg). After 60 min, acquisition was performed including a diagnostic quality, dose-modulated whole-body CT scan (120 kVp; 85 mAs) from skull to mid-thigh, enhanced with oral [12 mL Telebrix® Gastro (ioxithalamate; 0.3 g iodine/mL) in 900 mL water] and intravenous [120 mL Ultravist®370 (iopromide)] contrast agents. Transverse CT images were reconstructed with 5 mm thickness and 3 mm increment. Next, a whole-body PET emission scan of the same region was performed, with acquisition time per bed position (typically 6 to 7) dependent on the body mass index (BMI): BMI <30 kg/m2: 130 s/bed, 30 ≤ BMI < 40 kg/m2: 160 s/bed, BMI ≥40 kg/m2: 180 s/bed on Biograph systems; and BMI <40 kg/m2: 80 s/bed, BMI ≥40 kg/m2: 120 s/bed on Discovery MI system. The PET emission images were corrected for scatter and attenuation based on the CT information using a scanner specific commercial ordered subset iterative reconstruction algorithm using three iterations with 21 subsets per iteration on Biograph systems and two iterations with 34 subsets per iteration on Discovery system with use of point spread function and without post-smoothing filtering.
All [18F]-FDG PET/CT scans were independently reviewed by two board-certified physician in nuclear medicine (K.G. and S.J.) who were unaware of the clinico-biological parameters. The visual 4-point scale designed by Neuville et al. [13] was used to score the suspected cyst. An accumulation of [18F]-FDG around the cyst ≤ mediastinal bloodpool was scored as 1. If uptake was >bloodpool, but ≤liver, it was scored as 2. If it was slightly >liver, it was scored as 3. If it was largely >liver, it was scored as 4. All [18F]-FDG PET/CT images not suggestive of CyI were scored as 1. The liver uptake reference was sampled at distance from potential suspect cysts and the blood pool reference was sampled in the mediastinum. All extra-cystic sites of pathological [18F]-FDG accumulation were documented.
Gold standard definition of CyI
The gold standard definition of CyI was based on conventionally accepted clinical criteria [1, 9, 13]. CyI was ‘definite’ if confirmed by pus drainage. CyI was ‘probable’ in the presence of all of the following five concomitant criteria: (i) fever ≥38°C; (ii) abdominal pain; (iii) peak (i.e. within 3 days of admission) plasma CRP levels ≥70 mg/L; (iv) absence of other causes of inflammation after a classical diagnostic work-up; and (v) favourable outcome after ≥21 days of antibiotics.
Statistics
The results are presented as medians and interquartile range for continuous variables and as frequency tables for qualitative variables. To assess the performance of [18F]-FDG PET/CT in cyst diagnosis, sensitivity, specificity, efficacy, and positive and negative predictive values were calculated between the [18F]-FDG PET/CT and clinical criteria. Logistic regression was used to assess the risk of CyI on the parameters, ORs and 95% confidence intervals (CI) were provided. In case of mathematical issue, the Firth's correction was applied. The agreement between observers was evaluated using Cohen's kappa coefficient and its 95% CI. Some parameters have undergone a logarithmic (log) transformation to normalize their distribution. The results are considered significant at the 5% uncertainty level (P < .05). The calculations were performed using SAS version 9.4.
RESULTS
Characteristics of ADPKD patients with suspected CyI
We identified 51 [18F]-FDG PET/CT scans performed for suspected CyI between January 2009 and November 2019 (51 individual patients; 30 men; median age of 57 years). Thirty-two patients (63%) had already undergone a kidney transplantation and 11 (22%) were dialysis-dependent at the time of the suspected CyI. Seventeen patients (34%) had a uni- or bilateral nephrectomy in the past. We categorized the cohort into two groups according to our gold standard definition of CyI: 11 cases positive for CyI, including 4 ‘definite’ CyI, and 40 cases negative for CyI. These groups are compared in Table 1. In the group of patients positive for CyI, we found four (36%) positive urine cultures and in five cases (45%) the haemocultures were positive. In no case were both haemocultures and urine cultures positive.
Table 1:
Clinical and biological characteristics of the cohort.
| Parameters | All patients (n = 51) |
CyI (+) (n = 11) |
CyI (–) (n = 40) |
|---|---|---|---|
| Age, years | 57.0 (18.0) | 54.0 (17.0) | 59.0 (16.0) |
| Gender, M/F | 30/21 | 6/5 | 24/16 |
| BMI, kg/m² | 23.7 (4.1) | 24.0 (5.8) | 23.7 (3.9) |
| Dialysis, N (%) | 11 (22.0) | 3 (27.3) | 8 (20.5) |
| Kidney transplant recipients, N (%) | 32 (64.0) | 6 (54.5) | 26 (66.7) |
| Nephrectomy, N (%) | 17 (34.0) | 3 (27.3) | 14 (35.9) |
| Duration of hospital stay* (P = .025), days | 12.5 (17.0) | 21 (29) | 9 (13) |
| Use of antibiotics, N (%) | 37 (72.5) | 11 (100.0) | 26 (65.0) |
| Duration of antibiotics, days | 24 (28) | 42 (89) | 19.5 (28) |
| Time between antibiotic initiation and PET-CT, days | 9 (12) | 7 (8) | 10 (11) |
| Average serum glucose (n = 48), mg/dL | 101 (23) | 101 (34) | 100 (20) |
| Temperature above 38°C, N (%) | 33 (64.7) | 11 (100.0) | 22 (55.0) |
| Abdominal pain* (P = .0059) , N (%) | 21 (42.0) | 11 (100.0) | 10 (25.6) |
| CRP admission* (P = .045), mg/L | 60.1 (106) | 198(234) | 52.9 (77.6) |
| CRP peak* (P = .0071), mg/L | 128 (141) | 258 (224) | 98.2 (118) |
Result expressed in N, number of patients and (%), percentage of the considered group, or median (interquartile range).
M/F, number of male/number of female; time between antibiotic initiation and PET-CT, number of days between antibiotic initiation and PET-CT imaging; average serum glucose, glycemia measured just before injection the [18F]-FDG; CyI (+), patient fulfilling the five criteria for cyst infection; CyI (−), patients not fulfilling the five criteria for cyst infection.
*P < .05.
Diagnostic performance of [18F]-FDG PET-CT in ADPKD patients with suspected CyI
On the basis of the 4-point scale, 17/51 cases were considered as positive for CyI using [18F]-FDG PET/CT. [18F]-FDG PET/CT was positive in all cases in which cyst aspiration was performed and positive for CyI. Of the 17 positive cases, 11 showed a CyI in the liver and 6 in the kidney. In 16 cases, a non-cystic cause of clinical symptoms was diagnosed using [18F]-FDG PET/CT scan. The scan was negative in 18 cases (35.3%) of our cohort (Table 2). When correlated with the clinical findings, 7 positive scans were true positives and 31 negative scans were true negatives. We found nine cases that were falsely positive and four cases that were falsely negative.
Table 2:
[18F]-FDG PET/CT findings.
| N (%) | |
|---|---|
| PET (+): cystic fixation | 17 (33.3) |
| Liver | 11 (21.6) |
| Kidneys | 6 (11.8) |
| PET (+): non-cystic fixation | 16 (31.4) |
| Pancreatitis | 2 |
| Lung | 7 |
| Cholangitis | 2 |
| Peritonitis | 1 |
| Tumour adrenal gland | 1 |
| Abscess of transplant kidney | 1 |
| Infection of liver hilus | 1 |
| Infected hematoma | 1 |
| PET (–) | 18 (35.3) |
CyI (+), patient fulfilling the five criteria for cyst infection; CyI (−), patients not fulfilling the five criteria for cyst infection.
Considering all cases, there was a significant agreement between the clinical criteria and the results of the 4-point scale (visual score 1–2 vs 3–4). A score of ≥3 was associated with a significantly higher risk of CyI compared with scores 1 and 2 (OR = 6.03, P = .014) (Table 3; Figure 1). The resulting sensitivity and specificity of the 4-point scale were 63.6% (Clopper–Pearson 95% CI 30%–89%) and 77.5% (95% CI 62%–89%), respectively. The positive and negative predictive values were 43.8% (95% CI 20%–70%) and 88.6% (95% CI 73%–97%), respectively (Table 4).
Table 3:
Distribution of the 4-grade scoring system.
| Visual scale | CyI (+) (n = 11), N (%) | CyI (–) (n = 40), N (%) | OR (95% CI) | P-value |
|---|---|---|---|---|
| 1 | 3 (27.3) | 31 (77.5) | 1.00 | .055 |
| 2 | 1 (9.1) | 0 (0.0) | 24.0 (0.26–>999) | |
| 3 | 2 (18.2) | 3 (7.5) | 6.43 (0.749–52.6) | |
| 4 | 5 (45.5) | 6 (15.0) | 7.62 (1.49–38.9) | |
| 1 + 2 | 4 (36.4) | 31 (77.5) | 1.00 | .014 |
| 3 + 4 | 7 (63.6) | 9 (22.5) | 6.03 (1.44–25.3) |
CyI (+), patient fulfilling the five criteria for cyst infection; CyI (−), patients not fulfilling the five criteria for cyst infection.
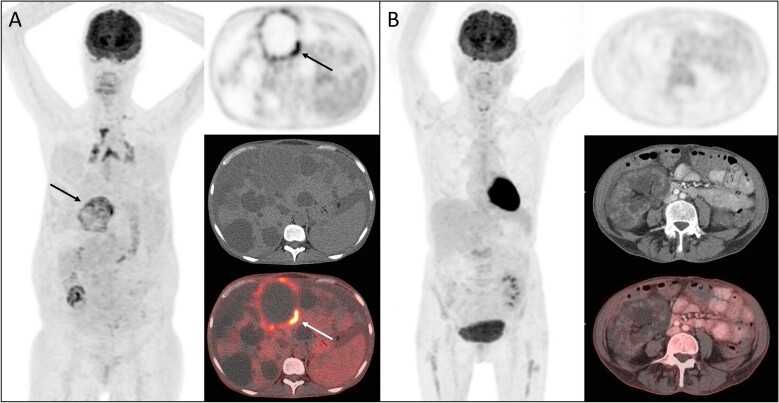
Figure 1:
Representative [18F]-FDG-PET/CT images of CyI according to the visual 4-point scoring system. (A) Maximal intensity projection (MIP) image, transversal PET image of the abdomen, the corresponding transversal CT image and the fusion PET/CT image in a patient with suspected liver CyI, score 4. The arrowhead shows the infected cyst considered for the scoring. (B) MIP image, transversal PET image of the abdomen, the corresponding transversal CT image and the fusion PET/CT image in a patient without suspicion of CyI, score 1.
Table 4:
Diagnostic performance of the 4-grade scoring system (visual score 1–2 vs 3–4).
| Clopper–Pearson 95% CI | ||
|---|---|---|
| Sensitivity | 63.6% | 30%–89% |
| Specificity | 77.5% | 62%–89% |
| PPV | 43.8% | 20%–70% |
| NPV | 88.6% | 73%–97% |
PPV, positive predictive value; NPV, negative predictive value.
Concerning the inter-observer agreement, a similar proportion of [18F]FDG-positive cysts was found (33.3% vs 43.1%, P = .23). Using the 4-point scale, the proportion of each score among the 51 [18F]FDG PET-CT scans similarly categorized by both observers showed significant agreement (kappa = 0.30; 0.13–0.46). When grouping scores of [1 + 2] vs [3 + 4], the inter-rater reliability was high (kappa = 0.66; 0.45–0.87).
DISCUSSION
In this study, we aimed to validate the diagnostic performance of the 4-point scoring scale [13] in a retrospective independent cohort of ADPKD patients with suspected CyI. A score of ≥3 (i.e. cyst uptake > liver) was associated with an OR of 6.03 for CyI. The resulting sensitivity and specificity of the 4-point scale were 64% (Clopper–Pearson 95% CI 30%–89%) and 78% (95% CI 62%–89%), respectively. The positive and negative predictive values were 44% (95% CI 20%–70%) and 89% (95% CI 73%–97%), respectively. There was good inter-reader agreement when evaluating a score [3 + 4] vs [1 + 2]. This suggests that using a 4-point scale when evaluating [18F]-FDG PET/CT scans may help in the diagnosis of CyI in patients with ADPKD. Note that, given its high specificity and negative predictive value, the semi-quantitative scoring scale seems especially useful in the exclusion of CyI.
CyI is a life-threatening complication in patients with ADPKD, since it can lead to serious consequences such as abscess formation and sepsis [1, 5]. An early diagnosis of CyI is therefore important to prevent further aggravation of the condition. A diagnosis using only clinical and biochemical findings has the limitation of being not specific for CyI [12]. This is important since CyI needs long courses of antibiotic treatment, while many other causes of inflammation or infection need to be treated otherwise. In order to avoid overtreatment, antibiotic side-effects and long hospital stays, it is important to properly distinguish CyI from other aetiologies of inflammation. In previous studies, the use of [18F]-FDG PET/CT to identify CyI has already been explored and showed great promise. In a study by Pijl et al. [9], which included 30 [18F]-FDG PET/CT scans from patients with ADPKD, a sensitivity of 88.9% and a specificity of 75.0% was found. This resulted in a positive predictive value of 84.2% and a negative predictive value of 81.8%. In a study by Bobot et al. [14] [18F]-FDG PET/CT was positive in 14 of 18 CyI out of a total of 32 cases. The false negatives could be explained by a long treatment of antibiotics before performing the [18F]-FDG PET/CT (median of 17 days, compared with 5.5 days in the positive scans). It provided a positive predictive value of 100% and a sensitivity of 77%. Another retrospective study by Balbo et al. [15] compared the diagnostic performance of [18F]-FDG PET/CT versus CT and MRI. They concluded a superior performance of [18F]-FDG PET/CT with a sensitivity of 95% compared with 25% for CT and 71.4% for MRI. In a more recent study by Neuville et al. [13] a 4-point scale, based on the [18F]-FDG uptake of the suspected cyst, was introduced to evaluate [18F]-FDG PET/CT scans. When evaluating 60 [18F]-FDG PET/CT scans, they found a sensitivity of 73.1% and specificity of 85.3%, and the positive and negative predictive values were, respectively, 65.5% and 77.4%.
While Neuville et al. [13] found an OR for scores ≥3 of 13.4, our validation cohort showed an OR of 6.03. While sensitivity and positive predictive value were lower in our cohort, specificity and negative predictive value were higher. Overall, even though the results differ in the size, a significant effect of the 4-point scale was demonstrated in both cohorts.
Several potential explanations for the size effect difference between our cohort and the one described in Neuville et al. [8] can be considered. First, in both studies the patients were included solely on the availability of a [18F]-FDG PET/CT scan in patients diagnosed with ADPKD. However, the fraction of positive cases was lower in our cohort compared with the study by Neuville et al. [13] (21.5% vs 43%). From this, we must acknowledge that a power issue may be an explanation for a difference in results. Second, our cohort had a higher fraction of liver CyI within the group of PET-positive cases (64.7% vs 34.5%). Since the scoring scale compares the uptake in the cyst with the uptake of the liver parenchyma, theoretically an infection of the liver cysts may change the ‘background’ to which the cyst was compared. However, upon reviewing, the value for uptake as ‘background’ was the same in patients with liver and kidney CyI, so that this cannot explain the difference in results between the two cohorts. Another finding worth mentioning is the relatively high number of false positive cases in our cohort. Upon reviewing, seven out of the nine ‘false positive’ cases were only negative for the clinical criteria because of the lack of abdominal pain, while all other clinical criteria were positive. Since this is a subjective criterium and considering the retrospective nature of our study, we should consider potential bias following an underreporting in the medical files. A post hoc analysis of the data where abdominal pain was removed from the clinical diagnostic gold-standard was performed. This resulted in an increase in the number of clinical positive cases from 11 to 21. The resulting sensitivity and specificity of PET/CT were 63.6% and 77.5%, respectively, with positive and negative predictive values of 76.5% and 76.5%, respectively. The agreement between the PET data and the clinical data remained significant (kappa = 0.50; 95% CI 0.26–0.74). As we expected, excluding a clinical criteria negatively impacts on the specificity. However, the sensitivity and positive predictive value increased by this alteration. This post hoc analysis highlights the need of a combination of both clinical and PET criteria for an optimal diagnostic approach. A prospective trial based on an invasive gold-standard confirming CyI by pus drainage may help to better define both the PET criteria and the clinical criteria of CyI. Such a trial could also assist with the development of a diagnostic scale combining clinical and imaging criteria.
Apart from the above-mentioned limitations, another limitation of our study, which again is inherent to the retrospective non-standardized clinical observation of our study and may have influenced the diagnostic performance of [18F]-FDG PET/CT, is the variable delay between the initiation of antibiotic therapy and the scan. Indeed, Piccoli et al. [16] stated that after 3–6 weeks of antibiotic therapy there was a reduction in [18F]-FDG uptake and there was no residual uptake after 9 weeks. In our cohort, the longest delay between the initiation of antibiotic therapy and the [18F]-FDG PET/CT was 14 days in the group of falsely negative cases. Overall, the mean delay was 9 days, which is similar to the study by Neuville et al. [13].
Of note, from a more global point of view, [18F]-FDG PET/CT was helpful in the diagnostic work-up of ADPKD patients with febrile abdominal pain in 33/51 cases (64.7%), including 16 non-cystic inflammations. It must be stressed, however, that specificity and negative predictive values of the scoring system were high. This again was a confirmation in the validation cohort of the previous findings by Neuville et al.
CONCLUSION
Our independent retrospective validation cohort confirms that the use of a semi-quantitative 4-point scoring system for [18F]-FDG PET/CT provides an additional tool in the difficult diagnostic approach to ADPKD patients with a suspected CyI, especially to exclude CyI when [18F]-FDG PET/CT is negative. Considering its performance metrics with high specificity and negative predictive value, the scoring system is particularly useful to distinguish other causes of clinical inflammation than CyI in ADPKD patients, and, as such, avoid unnecessarily long antibiotic treatment and its potential drawbacks.
ACKNOWLEDGEMENTS
All authors have participated sufficiently in the work to take public responsibility for the content: conception or design, or analysis and interpretation of data, or both: S.D., P.V., L.S., S.J., F.J., B.B., K.G.; drafting the article or revising it: S.D., P.V., L.S., S.J., D.M., F.J., B.B., K.G.; providing intellectual content of critical importance to the work described: S.D., P.V., L.S., S.J., D.M., F.J., B.B., K.G.; final approval of the version to be published: S.D., P.V., L.S., S.J., D.M., F.J., B.B., K.G.
Contributor Information
Selina Demuynck, Department of Nephrology, Dialysis and Renal Transplantation, University Hospitals Leuven, Leuven, Belgium.
Pierre Lovinfosse, Division of Nuclear Medicine and Oncological Imaging, Department of Medical Physics, University of Liège Hospital, Liège, Belgium.
Laurence Seidel, Biostatistics and Research Method Center (B-STAT), University of Liège Hospital (ULiège CHU), Liège, Belgium.
Sander Jentjens, Nuclear Medicine, UZ Leuven, Leuven, Belgium; Nuclear, Medicine & Molecular Imaging, KU Leuven, Leuven, Belgium.
Djalila Mekahli, PKD Research Group, Department of Cellular and Molecular Medicine, KU Leuven, Leuven, Belgium; Department of Pediatric Nephrology, University Hospitals Leuven, Leuven, Belgium.
François Jouret, Division of Nephrology, Department of Internal Medicine, University of Liège Academic Hospital (ULiège CHU), Liège, Belgium; Laboratory of Translational Research in Nephrology (LTRN), GIGA Cardiovascular Sciences, ULiège, Liège, Belgium.
Bert Bammens, Department of Nephrology, Dialysis and Renal Transplantation, University Hospitals Leuven, Leuven, Belgium; Department of Microbiology, Immunology and Transplantation, Nephrology & Renal Transplantation Research Group, KU Leuven, Leuven, Belgium.
Karolien Goffin, Nuclear Medicine, UZ Leuven, Leuven, Belgium; Nuclear, Medicine & Molecular Imaging, KU Leuven, Leuven, Belgium.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Sallée M, Rafat C, Zahar JR et al. Cyst infections in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2009;4:1183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grantham JJ, Mulamalla S, Swenson-Fields KI. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2011;7:556–66. 10.1038/nrneph.2011.109 [DOI] [PubMed] [Google Scholar]
- 3. Gabow P. Autosomal dominant polycystic kidney disease. N Engl J Med 1993;329:332–42. 10.1056/NEJM199307293290508 [DOI] [PubMed] [Google Scholar]
- 4. Drenth J, Barten T, Hartog H et al. EASL Clinical Practice Guidelines on the management of cystic liver diseases. J Hepatol 2022;77:1083–108. 10.1016/j.jhep.2022.06.002 [DOI] [PubMed] [Google Scholar]
- 5. Sklar AH, Caruana RJ, Lammers JE et al. Renal infections in autosomal dominant polycystic kidney disease. Am J Kidney Dis 1987;10:81–8. 10.1016/S0272-6386(87)80036-7 [DOI] [PubMed] [Google Scholar]
- 6. Jouret F, Hogan MC, Chebib FT. A practical guide for the management of acute abdominal pain with fever in patients with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 2022;37:1426–8. [DOI] [PubMed] [Google Scholar]
- 7. Lantinga MA, Drenth JPH, Gevers TJG. Diagnostic criteria in renal and hepatic cyst infection. Nephrol Dial Transplant 2015;30:744–51. 10.1093/ndt/gfu227 [DOI] [PubMed] [Google Scholar]
- 8. Neuville M, Hustinx R, Jacques J et al. Diagnostic algorithm in the management of acute febrile abdomen in patients with autosomal dominant polycystic kidney disease. PLoS One 2016;11:e0161277. 10.1371/journal.pone.0161277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pijl JP, Glaudemans AWJM, Slart RHJA et al. 18F-FDG PET/CT in autosomal dominant polycystic kidney disease patients with suspected cyst infection. J Nucl Med 2018;59:1734–41. 10.2967/jnumed.117.199448 [DOI] [PubMed] [Google Scholar]
- 10. Jouret F, Lhommel R, Beguin C et al. Positron-emission computed tomography in cyst infection diagnosis in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2011;6:1644–50. 10.2215/CJN.06900810 [DOI] [PubMed] [Google Scholar]
- 11. Bleeker-Rovers C, de Sévaux R, van Hamersvelt H et al. Diagnosis of renal and hepatic cyst infections by 18-F-fluorodeoxyglucose positron emission tomography in autosomal dominant polycystic kidney disease. Am J Kidney Dis 2003;41:E18–21. [DOI] [PubMed] [Google Scholar]
- 12. Jouret F, Lhommel R, Devuyst O et al. Diagnosis of cyst infection in patients with autosomal dominant polycystic kidney disease: attributes and limitations of the current modalities. Nephrol Dial Transplant 2012;27:3746–51. 10.1093/ndt/gfs352 [DOI] [PubMed] [Google Scholar]
- 13. Neuville MF, Lovinfosse P, Jadoul A et al. The use of a visual 4-point scoring scale improves the yield of 18F-FDG PET-CT imaging in the diagnosis of renal and hepatic cyst infection in patients with autosomal dominant polycystic kidney disease. Eur J Nucl Med Mol Imaging 2021;48:254–9. 10.1007/s00259-020-04903-x [DOI] [PubMed] [Google Scholar]
- 14. Bobot M, Ghez C, Gondouin B et al. Diagnostic performance of [18F]fluorodeoxyglucose positron emission tomography-computed tomography in cyst infection in patients with autosomal dominant polycystic kidney disease. Clin Microbiol Infect 2016;22:71–7. 10.1016/j.cmi.2015.09.024 [DOI] [PubMed] [Google Scholar]
- 15. Balbo BEP, Sapienza MT, Ono CR et al. Cyst infection in hospital-admitted autosomal dominant polycystic kidney disease patients is predominantly multifocal and associated with kidney and liver volume. Braz J Med Biol Res 2014;47:584–93. 10.1590/1414-431X20143584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Piccoli GB, Arena V, Consiglio V et al. Positron emission tomography in the diagnostic pathway for intracystic infection in adpkd and “cystic” kidneys. A case series. BMC Nephrol 2011;12:48. 10.1186/1471-2369-12-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.