ABSTRACT
Background
Contrast-associated acute kidney injury (CA-AKI) has been associated with a higher risk of cardiovascular (CV) events. We studied the risk of CV events in chronic kidney disease (CKD) patients undergoing angiography and whether biomarkers can predict such events. We also explored whether CA-AKI mediates the association of pre-angiography estimated glomerular filtration rate (eGFR) on CV events.
Methods
We analysed participants from the Prevention of Serious Adverse Events following the Angiography (PRESERVE) trial. Urinary tissue inhibitor of matrix metalloproteinase [TIMP]-2 and insulin growth factor binding protein [IGFBP]-7, plasma brain-type natriuretic peptide (BNP), high sensitivity C-reactive protein (hs-CRP), and serum cardiac troponin-I (Tn-I) were assayed before and after angiography. We assessed the composite risk of CV events by day 90.
Results
Of the 922 participants, 119 (12.9%) developed CV events, and 73 (7.9%) developed CA-AKI. Most cases of CA-AKI (90%) were stage 1. There were no differences in urinary [TIMP-2]•[IGFBP7] concentrations or the proportion of patients with CA-AKI among those with and without CV events. Higher BNP, Tn-I, and hs-CRP were associated with CV events, but their discriminatory capacity was modest (AUROC <0.7). CA-AKI did not mediate the association of the pre-angiography eGFR on CV events.
Conclusions
Most episodes of CA-AKI are stage 1 AKI and are not associated with CV events. Less severe CA-AKI episodes also did not mediate the risk of pre-angiography eGFR on CV events. Our findings suggest that most CV events after contrast procedures are due to underlying CKD and CV risk factors rather than less severe CA-AKI episodes and should help enhance the utilization of clinically indicated contrast procedures among high-risk patients with CKD. Further research is required to examine whether moderate-to-severe CA-AKI episodes are associated with CV events.
Keywords: angiography, biomarker, cardiovascular events, chronic kidney disease, contrast-associated acute kidney injury
INTRODUCTION
Cardiovascular (CV) events frequently occur in patients undergoing angiography, especially in those developing contrast-associated acute kidney injury (CA-AKI). In one study, 36% of patients who developed CA-AKI after non-coronary angiography had a CV event within 1 year, compared to 12% of patients who did not develop CA-AKI [1]. In another study, 22% of patients who developed CA-AKI after coronary angiography had a CV event within 1 year, compared to 15.4% of patients who did not develop CA-AKI [2]. However, prior work in high-risk patients with chronic kidney disease (CKD) undergoing angiography shows that most CA-AKI episodes are stage 1 AKI and occur due to hemodynamic perturbation rather than intrinsic kidney tubular epithelial cell injury [3, 4]. Whether less severe CA-AKI episodes are associated with CV events and whether CA-AKI is a mediator or just a marker for subsequent CV events in such patients is unclear. Understanding the relative risk of CV events after less severe CA-AKI episodes with biomarker risk stratification may help clinicians make decisions regarding angiography in patients with CKD.
Urinary tissue inhibitor of metalloproteinase (TIMP)-2 and insulin growth factor binding protein (IGFBP7) are two kidney tubular epithelial G1 cell cycle arrest biomarkers. They are used for detecting severe AKI [5, 6] and are associated with major adverse kidney events in critically ill patients [7, 8]. However, a previous study showed that the predictive accuracy of urinary [TIMP-2]•[IGFBP7] for stage 1 CA-AKI cases is poor in high-risk patients with CKD undergoing angiography [9]. Nevertheless, the predictive accuracy of urinary [TIMP-2]•[IGFBP7] for CV events has not been examined in a large cohort of patients with CKD. Previous studies have also shown that cardiac biomarkers such as serum cardiac troponin-I (Tn-I), brain-type natriuretic peptide (BNP), and high-sensitivity C-reactive protein (hs-CRP) are elevated before and after angiography, and their concentrations are not altered by CA-AKI status [9]. Whether these cardiac biomarkers aid in the risk stratification of CV events is unclear.
In this study, we first examined whether CA-AKI is associated with CV events among high-risk patients with CKD undergoing angiography. Second, we examined the association and predictive accuracy of urinary [TIMP-2]•[IGFBP7], serum Tn-I, plasma BNP, and hs-CRP concentrations before and after angiography on the risk of CV events. Third, we explored whether CA-AKI was a mediator of the association of baseline estimated glomerular filtration rate (eGFR) with CV events. For these analyses, we used the data from a large randomized clinical trial entitled Prevention of Serious Adverse Events following Angiography (PRESERVE) and the biomarker sub-study among high-risk patients with CKD undergoing angiography [9, 10].
MATERIALS AND METHODS
Study design
The methods of the PRESERVE clinical trial have been described previously [10, 11]. Briefly, PRESERVE was a two-by-two factorial design randomized clinical trial comparing intravenous isotonic sodium bicarbonate with intravenous isotonic saline and oral N-acetyl cysteine with oral placebo in patients with non-dialysis dependent CKD (eGFR <45 mL/min/1.73 m2, or eGFR <60 mL/min/1.73 m2 and diabetes mellitus) who were undergoing coronary or non-coronary angiography across 53 medical centers in the United States (35 Veterans Affairs sites), Australia, Malaysia, and New Zealand. Participants (n = 5177) were recruited from February 2013 to March 2017 and were excluded for the following: receiving dialysis; eGFR<15 mL/min/1.73 m2; unstable baseline blood creatinine; decompensated congestive heart failure (CHF); emergent angiogram; having received iodinated contrast in the past 5 days; known allergy to acetylcysteine; known anaphylactic allergy to iodinated contrast; incarceration; age <18 years; pregnancy; unwillingness to comply with outcome assessment; or ongoing participation in a concurrent interventional trial [10].
CA-AKI was defined as an increase in serum creatinine level ≥25% and/or ≥0.5 mg/dL from the baseline at 96 hours after angiography as defined in the PRESERVE trial and several observational studies [10–14]. Baseline serum creatinine used to assess CA-AKI was the most recent measurement within 3 months before angiography [11]. AKI severity staging was performed using the Kidney Disease Improving Global Outcomes criteria [14]. The primary outcome was a composite of CV events within 90 days of angiography defined as either a primary or secondary diagnosis of the acute coronary syndrome, ST-segment elevation myocardial infarction (STEMI), non-ST segment elevation myocardial infarction (NSTEMI), unstable angina, CHF, cerebrovascular accident, or death. Information related to CV events was prospectively collected in the PRESERVE trial [10].
A total of 922 participants from 19 centers in the United States participated in this ancillary biomarker sub-study [3, 4, 9]. Ninety-six participants were missing either baseline serum creatinine (n = 13) or serum creatinine at 96 hours (n = 95) and were presumed not to have CA-AKI [3, 4]. A total of 742 subjects had urine samples, and 854 had plasma samples collected before and after angiography. The PRESERVE trial and the biomarker sub-study were approved by the Veteran's Affairs Central Institutional Review Board, and all appropriate study site ethics and regulatory committees and written informed consent was obtained from all study participants. This biomarker study, Biomarker Effectiveness Analysis in Contrast Nephropathy (BEACON), was also approved by the University of Pittsburgh's Human Research Protection Office (study no. 19 070 228) [9].
Sample collection and biomarker measurement
We collected plasma and urine samples 1–2 hours before and 2–4 hours after angiography [4]. Biomarkers measured were urinary [TIMP-2]•[IGFBP7], serum Tn-I, plasma BNP and hs-CRP and were chosen a priori based on the use of these markers in the clinical setting [15] and reliable assays [5, 16–19]. All samples were aliquoted and stored at −80°C until biomarker measurements. Aliquoted samples were stored in a central repository, and biomarker assays were performed without additional freeze-thaw cycles. Samples were collected and stored in a central repository, and all personnel measuring the biomarkers were blinded to clinical outcomes. Details of the biomarker assays and coefficient of variation for each biomarker have been previously described [9].
Statistical analyses
We first performed an outcome-stratified analysis comparing participant characteristics by CV events. We used t tests to compare normally distributed continuous variables and the Wilcoxon rank-sum test for variables without normal distribution. We used the Chi-squared test or Fisher's exact test for categorical variables. We used half the detection threshold for biomarker data that were censored below the detection threshold, and we assigned the maximum value for markers censored at the maximum detection threshold (Method S1, see online supplementary material) [20, 21]. We performed log transformation before analysis for skewed plasma BNP concentrations (Figure S1, see online supplementary material). Urine biomarker measurements were normalized for urine creatinine concentration to account for the hydration therapies tested in the clinical trial.
We fitted logistic regression to examine the association of pre- and post-angiography biomarker concentration on the risk of CV events after adjusting for eGFR and urine albumin- creatinine ratio (UACR). The adjusted odds ratios (aOR) were calculated for each unit increase in biomarker concentration for all biomarkers. For urine creatinine indexed [TIMP-2]•[IGFBP7], the aORs were calculated for each natural log-transformed unit increase in biomarker concentration. We did not adjust the alpha level for multiple comparisons because of a potential increase in Type 2 error [22–24]. To examine risk prediction of biomarkers on the risk of CV events, we generated area under the receiver operating characteristic curves (AUROCs).
We conducted mediation analyses to determine whether CA-AKI mediated the association between pre-angiography eGFR and CV events. We used the PROC CAUSALMED procedure for SAS software (SAS Institute, Cary, NC, USA), a regression-based approach using counterfactual framework [25, 26]. We included pre-angiography eGFR, UACR (categorical variable, <30, 30–300, and >300 mg/g), history of CHF as a predictor, and CA-AKI as a mediator of the CV events. Finally, we used bootstrap testing of 1000 randomly derived samples to examine the confidence interval (CI) surrounding the OR for the effect of CA-AKI on the association of pre-angiography eGFR with CV events.
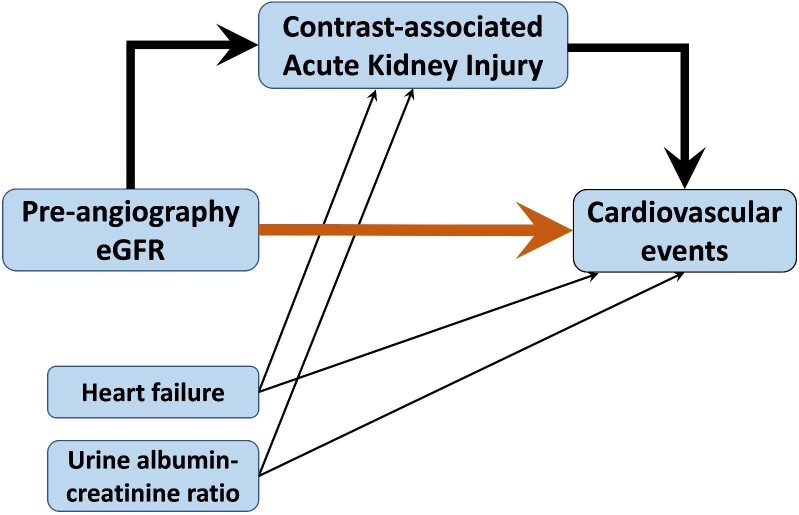
To gauge the magnitude of the effect, we also used the PROC CAUSALMED procedure in SAS software to estimate the proportion mediated, which was calculated as the indirect effect (i.e. the effect of pre-angiography eGFR on CV events related to CA-AKI) divided by the sum of the direct (i.e. direct effect of pre-angiography eGFR on CV events) and indirect effect (Fig. 1). We included UACR, history of CHF, and biomarkers in these models to determine the proportion of change in regression coefficients in CA-AKI. We considered a P-value of <.05 to denote statistical significance for all analyses. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Figure 1:
Mediation analysis. The orange arrow denotes the direct effect of pre-angiography estimated glomerular filtration rate (eGFR) on the risk of cardiovascular events by day 90. Thick black arrows denote the indirect effect of pre-angiography eGFR on cardiovascular events.
RESULTS
Participant population
Of 5177 participants in the parent PRESERVE study, 922 (17.8%) were enrolled in this biomarker study. The mean ±SD age was 70 ± 8 years, 97.2% were men, and 82% had a history of diabetes (Table 1). Overall, 7.9% (n = 73) of participants developed CA-AKI following angiography, and most patients (n = 66; 90.4%) developed stage 1 AKI; 12.9% (n = 119) of participants developed CV events by day 90. The baseline characteristics of patients developing CA-AKI and no CA-AKI are shown in Table S1 (see online supplementary material) and elsewhere [3, 9]. There was no difference in the proportion of patients developing CA-AKI among patients with and without CV events (11.7% vs. 7.3%; P = .10). Of patients developing CV events, 63% developed CHF, 16.8% developed NSTEMI, 9.2% developed cerebrovascular accident, 7.6% developed unstable angina, 1.7% developed STEMI, 3.4% developed acute coronary syndrome, and 23.5% died (Table 2).
Table 1:
Baseline: characteristics of study participants by cardiovascular events.
| No. (%) | |||
|---|---|---|---|
| Characteristics | Cardiovascular eventa (n = 119) | No cardiovascular event (n = 803) | P-value |
| Age, years, mean ± SD | 71.2 ± 8.4 | 69.9 ± 7.9 | .12 |
| Male sex | 117 (98.3) | 772 (96.1) | .38 |
| Race/ethnicity | |||
| White | 93 (78.1) | 619 (77.1) | .78 |
| Black | 17 (14.3) | 127 (15.8) | |
| Hispanic | 4 (3.4) | 27 (3.4) | |
| Other | 5 (4.2) | 21 (2.6) | |
| Weight, kilograms, mean ± SD | 98.3 ± 23.3 | 100.7 ± 21.8 | .14 |
| Baseline serum creatinine, mg/dL, median (IQR) | 1.59 (1.33–1.98) | 1.48 (1.28–1.73) | .002 |
| Baseline urine creatinine, mg/dL, median (IQR) | 76.80 (54.40–110.40) | 93.05 (65.10–128.05) | .006 |
| Baseline post-operative urine creatinine, mg/dL, median (IQR) | 56.10 (36.90–81.35) | 56.95 (38.90–79.50) | .58 |
| UACR categories, mg/g | |||
| <30 | 33 (27.7) | 335 (41.7) | .0006 |
| 30–300 | 35 (29.4) | 249 (31.0) | |
| >300 | 41 (34.4) | 163 (20.3) | |
| Baseline eGFR, mL/min/1.73m2 | |||
| 15–30 | 16 (13.4) | 52 (6.5) | .020 |
| 30–45 | 41 (34.4) | 271 (33.7) | |
| >45 | 59 (49.6) | 457 (56.9) | |
| Comorbid conditions | |||
| Heart failure | 67 (56.3) | 297 (36.9) | <.001 |
| Diabetes mellitus | 100 (84.0) | 652 (81.2) | .59 |
| Myocardial infarction | 47 (39.5) | 263 (32.7) | .16 |
| Peripheral vascular disease | 45 (37.8) | 246 (30.6) | .13 |
| Cerebrovascular disease | 25 (21.0) | 126 (15.7) | .15 |
| Chronic pulmonary disease | 37 (31.1) | 193 (24.2) | .11 |
| Hypertension | 113 (94.9) | 745 (92.8) | .59 |
| Angiographic procedure | |||
| Coronary | 110 (92.4) | 695 (86.5) | .13 |
| Carotid | 0 | 7 (0.9) | |
| Peripheral | 4 (3.3) | 75 (9.3) | |
| Mesenteric | 0 | 0 | |
| Aortic and/or iliac | 2 (1.7) | 10 (1.2) | |
| Pulmonary | 0 | 0 | |
| Renal | 1 (0.8) | 3 (0.4) | |
| Other | 1 (0.8) | 4 (0.5) | |
| Percutaneous intervention | 28 (23.5) | 224 (27.9) | .30 |
| LVEDP, mmHg, mean ± SD | 19.9 ± 8.5 | 19.3 ± 8.3 | .53 |
| Intervention arm | |||
| Saline + placebo | 22 (18.5) | 188 (23.4) | .29 |
| Saline + NAC | 37 (31.1) | 198 (24.5) | |
| Sodium bicarbonate + placebo | 34 (24.0) | 205 (25.5) | |
| Sodium bicarbonate + NAC | 26 (21.8) | 205 (25.5) | |
| Contrast type | |||
| Iodixanol | 52 (43.7) | 431 (53.7) | .03 |
| Low-osmolal agent | 66 (55.5) | 360 (44.8) | |
| Contrast volume, mL, mean ± SD | 106.8 ± 60.1 | 106.9 ± 67.8 | .52 |
| AKI stageb | |||
| No AKI | 114 (95.8) | 744 (92.7) | .10 |
| Stage 1 | 1 (0.8) | 12 (1.5) | |
| Stage 2 | 4 (3.4) | 26 (3.2) | |
| Stage 3 | 0 | 21 (2.6) | |
| CA-AKIc | 14 (11.7) | 59 (7.3) | .10 |
| MAKE or death by day 90d | 33 (27.7) | 26 (3.2) | <.001 |
| Death | 28 (23.5) | 0 (0) | |
| Need for dialysis | 5 (4.2) | 7 (0.9) | .01 |
| ≥50% in serum creatinine from baseline | 5 (4.2) | 58 (7.2) | .22 |
AKI, acute kidney injury; CA-AKI, contrast-associated acute kidney injury; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LVEDP, left ventricular end-diastolic pressure; MAKE, major adverse kidney events; NAC, N-acetylcysteine; SD, standard deviation; UACR, urine albumin-creatinine ratio.
Cardiovascular event(s) within 90 days is defined as a composite of either a primary or secondary diagnosis of the acute coronary syndrome, ST-elevation myocardial infarction, non-ST segment elevation myocardial infarction, unstable angina, congestive heart failure, cerebrovascular accident, or death.
Stages of AKI were defined by Kidney Disease: Improving Global Outcome criteria: stage 1: increase in serum creatinine to 1.5–1.9 times baseline or increase in serum creatinine by ≥0.3 mg/dl; stage 2: increase in serum creatinine to 2.0–2.9 times baseline; stage 3: increase in serum creatinine to 3.0 times baseline or increase in serum creatinine to ≥4.0 mg/dl or need for dialysis. Ninety-six participants were missing either (or both) the baseline or 3- to 5-day serum creatinine and are presumed to have no AKI.
Contrast-associated acute kidney injury was defined as an increase in serum creatinine level ≥25% or ≥0.5 mg/dL from the baseline 3 to 5 days after angiography.
Major adverse kidney events were defined as a composite of death, persistent kidney dysfunction, or dialysis dependence by day 90. A persistent decrease in kidney function was defined as a ≥50% increase in serum creatinine level at day 90 after angiography, confirmed by subsequent testing within 14 days of the initial measurement [10, 11].
Table 2:
No. and type of cardiovascular event.
| Cardiovascular eventa | No. (%)b |
|---|---|
| Any cardiovascular event | 119 (100) |
| Death | 28 (23.5) |
| Acute coronary syndrome | 4 (3.4) |
| STEMI | 2 (1.7) |
| NSTEMI | 20 (16.8) |
| Unstable angina | 9 (7.6) |
| CHF | 75 (63.0) |
| Cerebrovascular accident | 11 (9.2) |
CHF, congestive heart failure; NSTEMI, non-ST segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction.
Cardiovascular event(s) in 90 days defined as present if primary or secondary diagnosis of acute coronary syndrome, ST elevation myocardial infarction (STEMI), non-ST elevation myocardial infarction (NSTEMI), unstable angina, CHF, cerebrovascular accident, or death.
The no. of cardiovascular events is not mutually exclusive.
Participants developing CV events had higher baseline serum creatinine, lower eGFR, and higher UACR. There was a higher prevalence of a history of CHF (56.3% vs. 36.9%; P < .001) at baseline and a higher proportion of low-osmolal contrast use compared with iodixanol (53.7% vs. 43.7%; P = .03) use among patients developing CV events. There were no significant differences in other demographics, clinical, or procedural characteristics between participants who did and did not develop CV events. Patients with CV events were more likely to develop major adverse kidney events (MAKE) than patients without CV events (27.7% vs. 3.2%, P < .001). This difference was primarily due to death as both CV event and MAKE definitions include death, and the need for dialysis by day 90 (4.2% vs. 0.9%, P = .01). However, of patients with non-fatal CV events, there was no difference in the risk of MAKE ([7/91] 7.7% vs. [26/796] 3.3%; P = .07). There were no differences in participant characteristics between those enrolled in this biomarker sub-study and those in the PRESERVE trial (Table S2, see online supplementary material) [3, 4, 9, 10].
Biomarker concentration by cardiovascular events
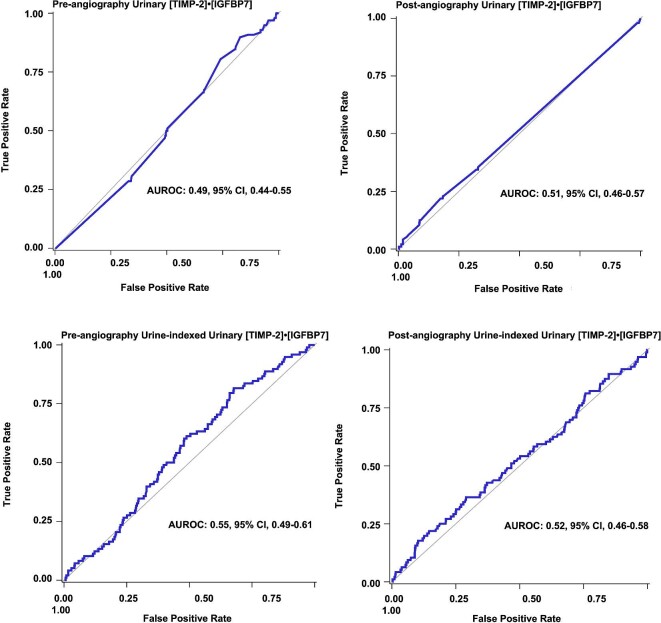
There were no differences in pre- and post-angiography median urinary [TIMP-2]•[IGFBP7] and urine creatinine-indexed urinary [TIMP-2]•[IGFBP7] concentrations among those with and without CV events (Table 3). Of 200 patients who had pre-angiography urinary [TIMP-2]•[IGFBP7] concentrations >0.3 ng/mL, only 10% (n = 20) developed CV events. There was no difference in the risk of CV events among those patients with urinary [TIMP-2]•[IGFBP7] concentrations of >0.3 ng/mL and ≤0.3 ng/mL (pre-angiography, 10.0% vs 13.6%, P = .2; post-angiography, 16.2% vs 12.2%, P = .4). However, pre- and post-angiography concentrations of plasma BNP; serum Tn-I; and plasma hs-CRP were significantly increased among those who developed CV events compared with those who did not (Table 3).
Table 3:
Biomarker: concentrations by cardiovascular event.
| Median (IQR) | ||||
|---|---|---|---|---|
| Biomarker | Cardiovascular event (n = 119) | No cardiovascular event ( n = 803) | Unadjusted P value | Adjusted P valuel |
| Pre-angiography | ||||
| Urinary [TIMP-2]•[IGFBP7], (ng/mL)2/1000a,b | 0.16 (0.05–0.30) |
0.17 (0.05–0.40) |
.99 | .41 |
| Urine creatinine-indexed urinary [TIMP-2]•[IGFBP7], (ng/mL)2/1000 mg/dLc,d,e | 0.002 (0.001–0.003) |
0.002 (0.0009–0.003) |
.10 | .55 |
| Plasma BNP, pg/mLf,g | 197.0 (72.0–334.0) |
66.0 (27.0–161.0) |
<.0001 | .01 |
| Serum troponin-I, ng/mLh,i | 0.025 (0.01–0.08) |
0.01 (0.005–0.04) |
<.0001 | .004 |
| Plasma hs-CRP, mg/Lj,k | 7.8 (3.1–10.4) |
3.4 (1.5–7.7) |
<.0001 | <.001 |
| Post-angiography | ||||
| Urinary [TIMP-2]•[IGFBP7], (ng/mL)2/1000a,b | 0.05 (0.05–0.10) |
0.05 (0.05–0.10) |
.60 | .70 |
| Urine creatinine-indexed urinary [TIMP-2]•[IGFBP7], (ng/mL)2/1000 mg/dLc,d,e | 0.001 (0.0009–0.002) |
0.001 (0.0009–0.002) |
.36 | .48 |
| Plasma BNP, pg/mLf,g | 190.5 (89.0–363.0) |
73.0 (31.0–171.0) |
<.0001 | .007 |
| Serum troponin-I, ng/mLh,i | 0.04 (0.01–0.13) |
0.02 (0.005–0.05) |
<.0001 | .01 |
| Plasma hs-CRP, mg/Lj,k | 7.0 (2.2–10.4) |
3.1 (1.3–6.9) |
<.0001 | .0004 |
BNP, brain-type natriuretic peptide; dL, deciliter; hs-CRP, high sensitivity C-reactive protein; IGFBP, insulin growth factor binding protein; IQR, interquartile range; ng, nanograms; L, liter; mL, milliliter; pg, picograms; TIMP, tissue inhibitor of matrix metalloproteinase.
Pre-angiography urinary [TIMP-2]•[IGFBP7] concentrations were assayed in 797 patients.
Post angiography urinary [TIMP-2]•[IGFBP7] concentrations were assayed in 842 patients.
Pre-angiography urine creatinine-indexed urinary [TIMP-2]•[IGFBP7] concentrations were assayed in 791 patients.
Post-angiography urine creatinine-indexed urinary [TIMP-2]•[IGFBP7] concentrations were assayed in 836 patients.
Normalized for urine creatinine.
Pre-angiography plasma BNP concentrations were assayed in 884 patients.
Post-angiography plasma BNP concentrations were assayed in 838 patients.
Pre-angiography serum troponin-I concentrations were assayed in 911 patients.
i Post-angiography serum troponin-I concentrations were assayed in 846 patients.
Pre-angiography plasma hs-CRP concentrations were assayed in 646 patients.
Post-angiography plasma hs-CRP concentrations were assayed in 592 patients.
Adjusted for differences in baseline eGFR and urine albumin-creatinine ratio (UACR).
Table 4:
Biomarker: association and risk prediction for cardiovascular events.
| Biomarker | Unadjusted OR (95%CI) |
P-value | Adjusted OR (95%CI)a |
P-value | AUROC (95%CI) |
|---|---|---|---|---|---|
| Pre-angiography | |||||
| Urinary [TIMP-2]•[IGFBP7], (ng/mL)2/1000 | 0.91 (0.65–1.27) |
.58 | 0.88 (0.63–1.24) |
.47 | 0.49 (0.44–0.55) |
| Log urine creatinine-indexed urinary [TIMP-2]•[IGFBP7], (ng/mL)2/1000 mg/dL | 1.16 (0.94–1.43) |
.17 | 1.10 (0.88–1.36) |
.39 | 0.55 (0.49–0.61) |
| Log plasma BNP, pg/mL | 1.85 (1.56–2.21) |
<.001 | 1.71 (1.42–2.06) |
<.001 | 0.69 (0.64–0.75) |
| Serum troponin-I, ng/mL | 1.07 (1.01–1.12) |
.01 | 1.06 (1.00–1.12) |
.03 | 0.63 (0.58–0.69) |
| Plasma hs-CRP, mg/L | 1.12 (1.07–1.18) |
<.001 | 1.11 (1.05–1.17) |
<.001 | 0.65 (0.58–0.72) |
| Post-angiography | |||||
| Urinary [TIMP-2]•[IGFBP7], (ng/mL)2/1000 | 1.11 (0.72–1.73) |
.62 | 1.00 (0.64–1.57) |
.99 | 0.51 (0.46–0.57) |
| Log urine creatinine-indexed urinary [TIMP-2]•[IGFBP7], (ng/mL)2/1000 mg/dL | 1.15 (0.88–1.51) |
.29 | 1.05 (0.79–1.40) |
.72 | 0.52 (0.46–0.58) |
| Log plasma BNP, pg/mL | 1.83 (1.53–2.20) |
<.001 | 1.69 (1.34–2.06) |
<.001 | 0.68 (0.63–0.74) |
| Serum troponin-I, ng/mL | 1.07 (1.00–1.15) |
.04 | 1.07 (1.00–1.14) |
.04 | 0.64 (0.58–0.70) |
| Plasma hs-CRP, mg/L | 1.12 (1.06–1.18) |
<.001 | 1.11 (1.05–1.18) |
<.001 | 0.66 (0.59–0.74) |
Adjusted for baseline eGFR and urine albumin-creatinine ratio.
Association of biomarker concentration with cardiovascular events
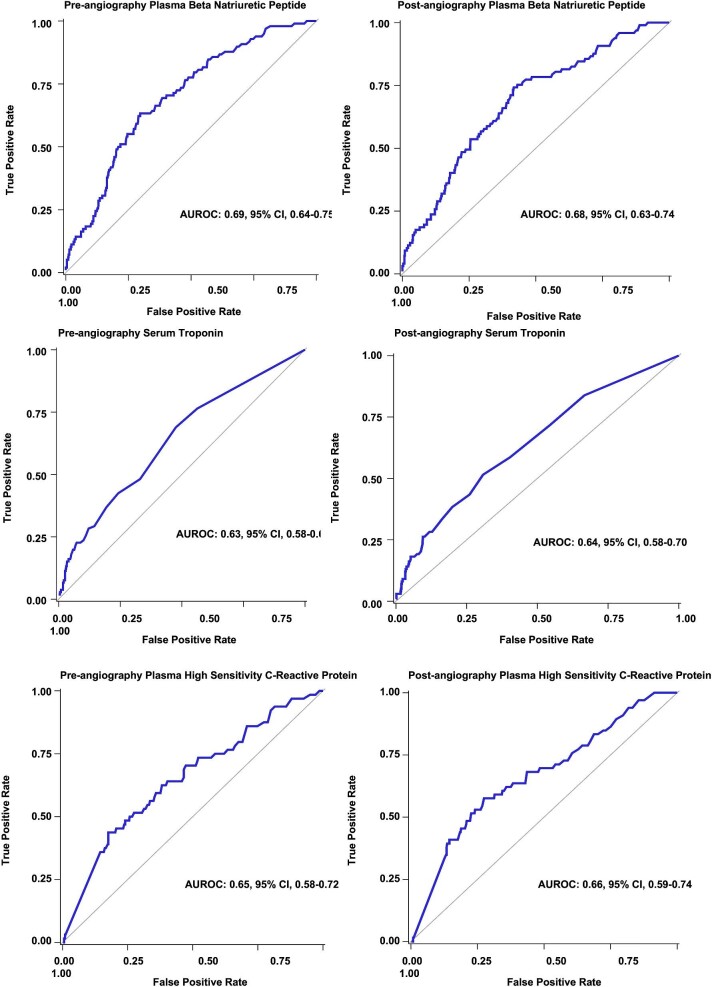
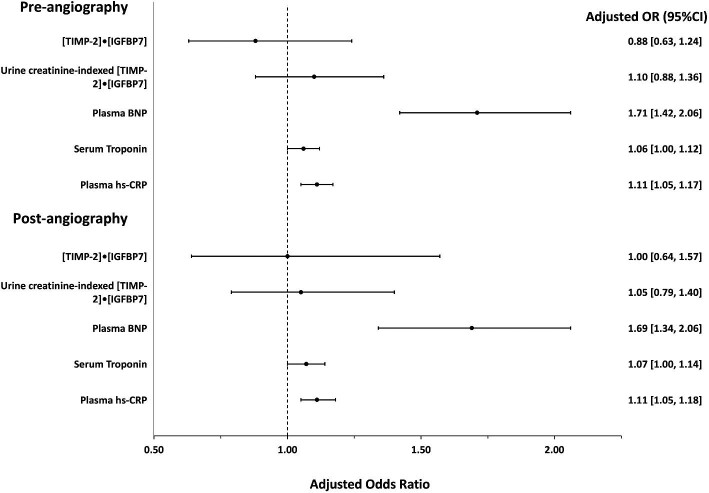
Pre-angiography urinary [TIMP-2]•[IGFBP7] and urine creatinine-indexed [TIMP-2]•[IGFBP7] were not associated with the risk of CV events, whereas higher median concentrations of pre-angiography plasma BNP (aOR, 1.71, 95%CI, 1.42–2.06; P < .001; Table 4) and post-angiography BNP (aOR 1.69, 95%CI, 1.34–2.06; P < .001) were associated with risk of CV events (Fig. 2). However, the predictive value was only modest (pre-angiography AUROC, 0.69, 95%CI, 0.64–0.75, and post-angiography AUROC, 0.68 95%CI, 0.63–0.75; Fig. 3; Table 4).
Figure 4:
Receiver operator characteristic curves for pre-angiography and post-angiography cardiac biomarkers for risk prediction of cardiovascular events.
Figure 2:
Forest plot showing the adjusted odds ratios for pre-angiography and post-angiography biomarkers on the risk of cardiovascular events.
Figure 3:
Receiver operator characteristic curves for pre-angiography and post-angiography kidney cell cycle arrest biomarkers for risk prediction of cardiovascular events.
Pre-angiography and post-angiography concentrations of serum Tn-I (pre, aOR, 1.06, 95%CI, 1.00–1.12, P = .03; post, aOR, 1.07, 95%CI, 1.00–1.16, P = .04) were associated with a higher risk of CV events (Fig. 2). However, they had only a modest predictive value for CV events (pre, AUROC, 0.63, 0.58–0.69; post, 0.64, 0.58–0.70). Higher concentrations of plasma hs-CRP (pre, aOR, 1.11, 95%CI, 1.05–1.17; P < .001; and post, aOR, 1.11, 95%CI, 1.05–1.18; P < .001) were associated with CV events though its predictive value was only modest (pre, AUROC, 0.65, 95%CI, 0.58–0.72; post, AUROC, 0.66, 95%CI, 0.59–0.74; Fig. 4).
Mediation analyses
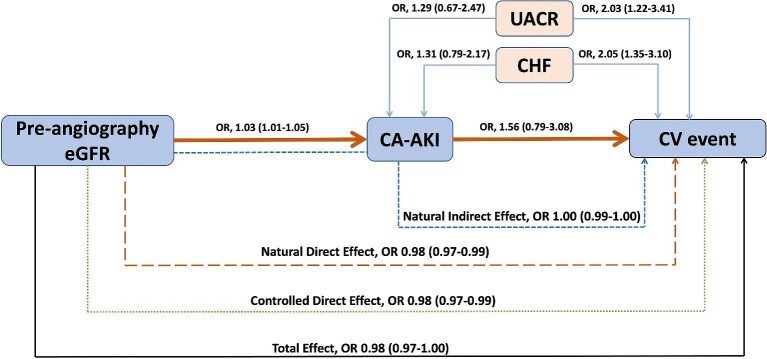
While lower pre-angiography eGFR was significantly associated with an increased risk of CA-AKI (aOR, 1.03, 95%CI, 1.01–1.05; P < .001), UACR (>300 vs. <30 mg/g) was not (aOR, 1.29, 95%CI, 0.67–2.47, P = .4; Table 5). However, both lower eGFR (aOR, 0.98, 95%CI, 0.97–0.99; P = .039) and higher UACR were independently associated with CV events (aOR, 2.03, 95%CI, 1.22–3.41, P = .006) after adjusting for history of CHF. In contrast, CA-AKI had no association with CV events (aOR, 1.56, 95%CI, 0.79–3.08; P = .2) even after excluding CHF from the CV events (aOR, 1.65, 95%CI, 0.67–4.03; P = .27). The indirect effect of pre-angiography eGFR on CV events mediated through CA-AKI was characterized by an OR of 1.00 with a 95%CI on bootstrap analyses of 0.99 to 1.00; P = .29 (Table 6; Fig. 5). The estimated proportion of the total effect of pre-angiography eGFR on CV events mediated by CA-AKI demonstrated no mediation (percentage mediated, 7.6%, 95%CI, −118.45%–3.18%; P = .30; Table 6). There was no significant change in mediation when biomarkers were added to the models (Tables S3 and S4, see online supplementary material).
Table 5:
Association of eGFR with CA-AKI and CV events from mediation analysis.
| CV Event (outcome) Model | CA-AKI (mediator) Model | |||
|---|---|---|---|---|
| Characteristic | adjusted odds ratio (95%CI) | P-value | adjusted odds ratio (95%CI) | P-value |
| eGFR | 0.98 (0.97–0.99) | .039 | 1.03 (1.01–1.05) | <.001 |
| CA-AKI | 1.56 (0.79–3.08) | .2 | ||
| UACR (>300 vs. <30, mg/g) | 2.03 (1.22–3.41) | .006 | 1.29 (0.67–2.47) | .4 |
| UACR (30-300 vs. <30, mg/g) | 1.25 (0.75–2.09) | .4 | 1.34 (0.76–2.38) | .3 |
| CHF | 2.05 (1.35–3.10) | <.001 | 1.31 (0.79–2.17) | .3 |
CA_AKI, contrast-associated acute kidney injury; CHF, congestive heart failure; eGFR, estimated glomerular filtration rate; UACR, urine albumin-creatinine ratio.
Table 6:
Summary of estimation of mediation effects.
| Effect | Estimate (95%CI) | Bootstrap bias corrected 95% CI | P-value |
|---|---|---|---|
| Total effect (TE) | 0.98 (0.97–1.00)a | 0.97–0.99 | .052 |
| Controlled direct effect (CDE) | 0.98 (0.97–0.99)a | 0.97–0.99 | .04 |
| Natural direct effect (NDE) | 0.98 (0.97–0.99)a | 0.97–0.99 | .04 |
| Natural indirect effect (NIE) | 1.00 (0.99–1.00)a | 0.99–1.00 | .29 |
| Percentage mediated (PM) | −7.65 (−24.06–8.76) | −118.45–3.18 | .36 |
| Percentage due to interaction (PDI) | 4.60 (−4.28–13.48) | −2.22–19.98 | .31 |
| Percentage eliminated (PE) | −3.17 (−13.23–6.88) | −154.95–2.23 | .53 |
Represents odds ratios adjusted for differences in urine albumin-creatinine ratio and history of congestive heart failure.
TE represents the association of eGFR with CV events after accounting for all confounders in the model. CDE represents the association of eGFR with CV events after controlling for CA-AKI (i.e. mediator). NDE represents the association of eGFR with CV events without controlling for CA-AKI. NIE is the association of eGFR with CV events mediated through CA-AKI (non-significant). PM represents the percentage of NIE in relation to TE. Only 7.6% of the association of eGFR with CV events is mediated by CA-AKI (non-significant). PDI represents the percentage of the TE due to the interaction between eGFR and CA-AKI, which is only 4.6% (non-significant). PE represents the percentage of the association of eGFR with CV events that could be eliminated by intervening on CA-AKI. Because PE is only −3.1%, even if CA-AKI is eliminated, we can only expect a 3.1% lowering in CV events (non-significant).
Figure 5:
Diagram depicting mediation analysis. Shown are odds ratios with 95% confidence intervals. The orange solid arrows represent the association of eGFR with CA-AKI (mediator), and the association of CA-AKI with CV event. The light blue arrows represent the confounding association of UACR and CHF with CA-AKI and CV event. The blue dotted arrow from eGFR to CV event via CA-AKI represents the natural indirect effect of eGFR on CV event mediated via CA-AKI. The orange dashed arrow from eGFR to CV event represents the natural direct effect of the association of eGFR with CV events without controlling for CA-AKI. The green dotted arrow from eGFR to CV event shows the controlled direct effect of eGFR on CV outcomes after controlling for CA-AKI only. The black solid arrow represents the total effect of the association of eGFR with CV event after controlling for UACR, CHF, and CA-AKI. CA-AKI, contrast-associated acute kidney injury; CHF, congestive heart failure; CV, cardiovascular, eGFR, estimated glomerular filtration rate; OR, odds ratio; UACR, urine albumin-creatinine ratio.
DISCUSSION
In this multicenter prospective observational study of high-risk patients with CKD undergoing angiography, most CA-AKI episodes were stage 1 AKI and were not associated with an increased risk of CV events. We also found no significant differences in pre- and post-angiography concentrations of urinary [TIMP-2]•[IGFBP7] in patients with and without CV events. However, as expected, cardiac biomarkers plasma BNP, hs-CRP, and serum Tn concentrations were higher among patients with CV events. Nevertheless, their discriminatory predictive capacity for the CV events was only modest. Lower pre-angiography eGFR levels and UACR were independently associated with a higher risk of CV events, and mediation analysis revealed that CA-AKI episodes were not a mediator of the baseline eGFR on the risk of CV events. These results suggest that the increased risk of CV events in high-risk patients with CKD undergoing angiography is due to underlying low eGFR and CV risk factors rather than less severe CA-AKI episodes.
Many clinical studies have shown that CA-AKI is associated with CV events [27–29]. However, establishing causality in observational studies is difficult, and the observed strong association between CA-AKI and CV events could be due to residual confounding factors that cause both CV events and AKI (e.g. baseline eGFR, CV risk factors, hemodynamic changes). As serum creatinine is currently the routinely measured clinical marker of kidney function, previous studies have typically used acute changes in creatinine to define CA-AKI. However, these prior studies generally used large registries and could not distinguish the reasons for the change in serum creatinine (prerenal/hemodynamic versus intrinsic CA-AKI due to contrast exposure). Thus, we used other kidney injury biomarkers to parse this relationship in the clinical setting. Our study suggests that minor and transient elevations in serum creatinine after angiography in high-risk patients are mostly due to hemodynamic changes rather than intrinsic tubular injury [3].
Our findings also support a previous mediation analysis that found CA-AKI was not a mediator of the association between pre-angiography eGFR and the risk of major adverse kidney events [30]. Our findings are relevant because several observational studies have documented lower utilization of angiography among patients with CKD, which may relate to physician concern for precipitating CA-AKI among those with CKD [31–40]. However, underutilizing potentially life-saving contrast-enhanced procedures in high-risk patients may be deleterious because poor outcomes exist even before contrast exposure, and contrast exposure does not significantly increase the risk of CA-AKI and CV events in most patients [30]. It is also well recognized that the risk for progressive CKD after CA-AKI is very low [10, 30, 41]. Thus, our findings further support the concept that undue concern for serious, adverse kidney or cardiovascular sequelae of CA-AKI should not routinely limit the performance of clinically indicated angiographic procedures.
High circulating pre-angiography concentrations of serum Tn-I, BNP, and hs-CRP among patients with CV events suggest that these markers are elevated even before contrast exposure among patients predisposed to CV events and these cardiac biomarkers did not modify the relationship of baseline eGFR and CA-AKI with CV events. These findings suggest that most CV events are attributable to underlying low baseline eGFR and pre-existing cardiovascular disease.
There are several strengths to our study. First, we systematically assessed pre-angiography kidney function, prospectively tracked the development of CA-AKI and CV events, and comprehensively adjusted for potential confounders in a clinical trial setting. Second, to our knowledge, our study is the largest to examine urinary [TIMP-2]•[IGFBP7] on the risk of CV events among patients undergoing angiography and at risk for CA-AKI. Third, sample procurement, marker assays, reagents, and freeze-thaw methods were standardized across sites.
Our study has several limitations. First, because most cases of CA-AKI were stage 1, we could not examine whether moderate-to-severe CA-AKI episodes mediate the association of baseline eGFR with CV events. Second, we did not follow patients beyond 90 days of contrast exposure and could not assess whether stage 1 CA-AKI episodes are associated with longer-term risk of CV events. Third, the number of patients who developed CA-AKI and CV events was small as only 17.8% of patients were enrolled in the biomarker sub-study; thus, our study may be underpowered to detect the smaller risk of CV events. Fourth, most participants received diagnostic angiography instead of a percutaneous coronary intervention, limiting the amount of contrast administered. Thus, our findings may not be generalizable to the most severe cases of CA-AKI or to settings where greater volume of contrast is used. Finally, PRESERVE trial participants were predominantly men, potentially limiting the generalizability of our findings to women.
CONCLUSIONS
In high-risk patients with CKD undergoing angiography, most CA-AKI episodes were stage 1 AKI and were not associated with an increased risk of CV events. Less severe CA-AKI episodes also did not mediate the association of low pre-angiography eGFR with CV events. However, low pre-angiography eGFR, high UACR, high plasma BNP, hs-CRP, and serum Tn-I levels were associated with an increased risk of CV events. These findings suggest that baseline renal impairment and cardiac risk factors dominate adverse events among patients undergoing angiography. Our study should help inform and enhance the use of clinically indicated contrast-enhanced procedures among patients at high risk of CV events. Further research is required to examine whether moderate-to-severe CA-AKI episodes mediate the association of low eGFR with CV events.
Supplementary Material
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health under Award Number R01DK106256. The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health. We thank the study participants of the Prevention of Serious Adverse Events Following Angiography (PRESERVE) clinical trial, the PRESERVE study investigators, and the Veterans Affairs (VA) for providing the study data, and the NIDDK for providing funding and specimens for this work. Specimens from the PRESERVE study reported here were supplied by the NIDDK Central Repository. We also thank the Biostatistics and Data Management Core (BDMC) of the Clinical Research, Investigation, Systems Modelling of Acute Illness (CRISMA) Center and the Veterans Affairs Office of Research and Development for data management. This manuscript was not prepared in collaboration with all PRESERVE study investigators and did not necessarily reflect the opinions or views of the PRESERVE study investigators, the US Department of Veterans Affairs, or the NIDDK.
Contributor Information
Raghavan Murugan, The Program for Critical Care Nephrology, Department of Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; The Clinical Research, Investigation, and Systems Modeling of Acute Illness (CRISMA) Center, Department of Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Monique Y Boudreaux-Kelly, Office of Research and Development, Veterans Affairs Pittsburgh Healthcare System, Pittsburgh, PA, USA.
John A Kellum, The Program for Critical Care Nephrology, Department of Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; The Clinical Research, Investigation, and Systems Modeling of Acute Illness (CRISMA) Center, Department of Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Paul M Palevsky, The Program for Critical Care Nephrology, Department of Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; Renal and Electrolyte Division, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; Kidney Medicine Section, Veterans Affairs Pittsburgh Healthcare System, Pittsburgh, PA, USA.
Steven Weisbord, Renal and Electrolyte Division, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; Kidney Medicine Section, Veterans Affairs Pittsburgh Healthcare System, Pittsburgh, PA, USA.
AUTHORS’ CONTRIBUTIONS
R.M. and M.Y.B.-K. take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: R.M. Acquisition of data: P.M.P., S.W., R.M. Analysis and interpretation of data: M.Y.B.-K., R.M., P.M.P., J.A.K., S.W. Drafting of the manuscript: R.M. Critical revision of the manuscript for important intellectual content: J.A.K., P.M.P., M.Y.B.-K., S.W. Statistical analysis: M.Y.B.-K. Administrative, technical, or material support: R.M., P.M.P., S.W. Study supervision: R.M., P.M.P, J.A.K., S.W.
FUNDING
This study was supported by the United States Department of Veterans Affairs Office of Research and Development grant VA CSP #578 PRESERVE Trial (Principal Investigators: S.W.; co-Principal Investigator: P.M.P.), National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK098214 Biomarker Collection and Analysis in the PRESERVE Trial Cohort (Multiple Principal Investigators: S.W., P.M.P., C.R. Parikh), and National Institute of Diabetes, and Digestive, and Kidney Diseases award 5R01DK106256 (Principal Investigator: R.M.).
DATA AVAILABILITY STATEMENT
The datasets used and/or analysed during the current study may be available from the US Department of Veterans Affairs upon reasonable request.
CONFLICT OF INTEREST STATEMENT
R.M. reported receiving grants from the National Institute of Diabetes and Digestive and Kidney Diseases and consulting fees from Baxter Inc unrelated to this study. P.M.P. received consulting fees and advisory committee fees from Durect, Health-Span Dx, and Novartis; served on a Data and Safety Monitoring Board for Baxter; served as a member of an endpoint adjudication committee for GE Healthcare; and reports receiving grants from BioPorto and Dascena outside the submitted work. S.W. has received consulting fees and advisory fees from Durect and Saghmos Therapeutics and reports receiving personal fees from Cytokinetics and Saghmos Therapeutics outside the submitted work. J.A.K. has received grant support and/or consulting fees from Astute Medical/BioMerieux, and GE Healthcare outside the submitted work. M. Kelly has nothing to disclose.
REFERENCES
- 1. Mitchell AM, Kline JA, Jones AE et al. Major adverse events one year after acute kidney injury after contrast-enhanced computed tomography. Ann Emerg Med 2015;66:267–274.e4. 10.1016/j.annemergmed.2015.04.028 [DOI] [PubMed] [Google Scholar]
- 2. Giacoppo D, Madhavan MV, Baber U et al. Impact of contrast-induced acute kidney injury after percutaneous coronary intervention on short- and long-term outcomes: pooled analysis from the HORIZONS-AMI and ACUITY trials. Circ Cardiovasc Interv 2015;8:e002475. 10.1161/CIRCINTERVENTIONS.114.002475 [DOI] [PubMed] [Google Scholar]
- 3. Liu C, Mor MK, Palevsky PM et al. Postangiography increases in serum creatinine and biomarkers of injury and repair. Clin J Am Soc Nephrol 2020;15:1240–50. 10.2215/CJN.15931219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parikh CR, Liu C, Mor MK et al. Kidney biomarkers of injury and repair as predictors of contrast-associated AKI: a substudy of the PRESERVE trial. Am J Kidney Dis 2020;75:187–94. 10.1053/j.ajkd.2019.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kashani K, Al-Khafaji A, Ardiles T et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013;17:R25. 10.1186/cc12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vijayan A, Faubel S, Askenazi DJ et al. Clinical use of the urine biomarker [TIMP-2] × [IGFBP7] for acute kidney injury risk assessment. Am J Kidney Dis 2016;68:19–28. 10.1053/j.ajkd.2015.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bihorac A, Chawla LS, Shaw AD et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 2014;189:932–9. 10.1164/rccm.201401-0077OC [DOI] [PubMed] [Google Scholar]
- 8. Koyner JL, Shaw AD, Chawla LS et al. Tissue inhibitor metalloproteinase-2 (TIMP-2)IGF-binding protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J Am Soc Nephrol 2015;26:1747–54. 10.1681/ASN.2014060556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murugan R, Boudreaux-Kelly MY, Kellum JA et al. Biomarker effectiveness analysis in contrast nephropathy study I; kidney cell cycle arrest and cardiac biomarkers and acute kidney injury following angiography: the prevention of serious adverse events following angiography (PRESERVE) study. Kidney Med 2023;5:100592. 10.1016/j.xkme.2022.100592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weisbord SD, Gallagher M, Jneid H et al. Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med 2018;378:603–14. 10.1056/NEJMoa1710933 [DOI] [PubMed] [Google Scholar]
- 11. Weisbord SD, Gallagher M, Kaufman J et al. Prevention of contrast-induced AKI: a review of published trials and the design of the prevention of serious adverse events following angiography (PRESERVE) trial. Clin J Am Soc Nephrol 2013;8:1618–31. 10.2215/CJN.11161012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mehran R, Dangas GD, Weisbord SD. Contrast-associated acute kidney injury. N Engl J Med 2019;380:2146–55. 10.1056/NEJMra1805256 [DOI] [PubMed] [Google Scholar]
- 13. Nijssen EC, Rennenberg RJ, Nelemans PJ et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non-inferiority trial. Lancet 2017;389:1312–22. 10.1016/S0140-6736(17)30057-0 [DOI] [PubMed] [Google Scholar]
- 14. Kidney disease improving global outcomes (KDIGO) workgroup: clinical practice guideline for acute Kidney injury. Kidney Int Suppl 2012;2:1–138. 10.1038/kisup.2012.7 [DOI] [Google Scholar]
- 15. Guzzi LM, Bergler T, Binnall B et al. Clinical use of [TIMP-2]*[IGFBP7] biomarker testing to assess risk of acute kidney injury in critical care: guidance from an expert panel. Crit Care 2019;23:225. 10.1186/s13054-019-2504-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao F, Zhou YJ, Zhu X et al. C-reactive protein and the risk of contrast-induced acute kidney injury in patients undergoing percutaneous coronary intervention. Am J Nephrol 2011;34:203–10. 10.1159/000329534 [DOI] [PubMed] [Google Scholar]
- 17. Guerchicoff A, Stone GW, Mehran R et al. Analysis of biomarkers for risk of acute kidney injury after primary angioplasty for acute ST-segment elevation myocardial infarction: results of the HORIZONS-AMI trial. Catheter Cardiovasc Interv 2015;85:335–42. 10.1002/ccd.25620 [DOI] [PubMed] [Google Scholar]
- 18. Watabe H, Sato A, Hoshi T et al. Association of contrast-induced acute kidney injury with long-term cardiovascular events in acute coronary syndrome patients with chronic kidney disease undergoing emergent percutaneous coronary intervention. Int J Cardiol 2014;174:57–63. 10.1016/j.ijcard.2014.03.146 [DOI] [PubMed] [Google Scholar]
- 19. Jarai R, Dangas G, Huber K et al. B-type natriuretic peptide and risk of contrast-induced acute kidney injury in acute ST-segment-elevation myocardial infarction: a substudy from the HORIZONS-AMI trial. Circ Cardiovasc Interv 2012;5:813–20. 10.1161/CIRCINTERVENTIONS.112.972356 [DOI] [PubMed] [Google Scholar]
- 20. Senn S, Holford N, Hockey H. The ghosts of departed quantities: approaches to dealing with observations below the limit of quantitation. Stat Med 2012;31:4280–95. 10.1002/sim.5515 [DOI] [PubMed] [Google Scholar]
- 21. Croghan C, Egeghy P.; Methods of Dealing with Values below the Limit of Detection Using SAS. 2003; US-EPA: Research Triangle Park 2016. [Google Scholar]
- 22. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–6. 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
- 23. Perneger TV. What's wrong with Bonferroni adjustments. BMJ 1998;316:1236–8. 10.1136/bmj.316.7139.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Method 2002;2:8. 10.1186/1471-2288-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. SAS Institute Inc. : User's Guide the CAUSALMED Procedure. Cary, NC, USA: AS Institute Inc, 2017. [Google Scholar]
- 26. Yung Y-F, Lamm M, Zhang W. Causal mediation analysis with the CAUSALMED procedure. In: Proceedings of the SAS Global Forum 2018 Conference. Cary, NC: SAS Institute Inc, 2018. [Google Scholar]
- 27. James MT, Samuel SM, Manning MA et al. Contrast-induced acute kidney injury and risk of adverse clinical outcomes after coronary angiography. Circ Cardiovasc Interv 2013;6:37–43. 10.1161/CIRCINTERVENTIONS.112.974493 [DOI] [PubMed] [Google Scholar]
- 28. Watabe H, Sato A, Hoshi T et al. Association of contrast-induced acute kidney injury with long-term cardiovascular events in acute coronary syndrome patients with chronic kidney disease undergoing emergent percutaneous coronary intervention. Int J Cardiol 2014;174:57–63. 10.1016/j.ijcard.2014.03.146 [DOI] [PubMed] [Google Scholar]
- 29. Ng AK, Ng PY, Ip A et al. Impact of contrast-induced acute kidney injury on long-term major adverse cardiovascular events and kidney function after percutaneous coronary intervention: insights from a territory-wide cohort study in Hong Kong. Clin Kidney J 2022;15:338–46. 10.1093/ckj/sfab212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weisbord SD, Palevsky PM, Kaufman JS et al. Contrast-associated acute kidney injury and serious adverse outcomes following angiography. J Am Coll Cardiol 2020;75:1311–20. 10.1016/j.jacc.2020.01.023 [DOI] [PubMed] [Google Scholar]
- 31. Fox CS, Muntner P, Chen AY et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation 2010;121:357–65. 10.1161/circulationaha.109.865352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Szummer K, Lundman P, Jacobson SH et al. Relation between renal function, presentation, use of therapies and in-hospital complications in acute coronary syndrome: data from the SWEDEHEART register. J Intern Med 2010;268:40–9. 10.1111/j.1365-2796.2009.02204.x [DOI] [PubMed] [Google Scholar]
- 33. Lau JK, Anastasius MO, Hyun KK et al. Evidence-based care in a population with chronic kidney disease and acute coronary syndrome. Findings from the Australian Cooperative National Registry of Acute Coronary Care, Guideline Adherence and Clinical Events (CONCORDANCE). Am Heart J 2015;170:566–72.e1. 10.1016/j.ahj.2015.06.025 [DOI] [PubMed] [Google Scholar]
- 34. Medi C, Chew DP, Amerena J et al. An invasive management strategy is associated with improved outcomes in high-risk acute coronary syndromes in patients with chronic kidney disease. Intern Med J 2011;41:743–50. 10.1111/j.1445-5994.2010.02361.x [DOI] [PubMed] [Google Scholar]
- 35. Cashion W, Weisbord SD. Radiographic contrast Media and the kidney. Clin J Am Soc Nephrol 2022;17:1234–42. 10.2215/CJN.16311221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rhee JW, Wiviott SD, Scirica BM et al. Clinical features, use of evidence-based therapies, and cardiovascular outcomes among patients with chronic kidney disease following non-ST-elevation acute coronary syndrome. Clin Cardiol 2014;37:350–6. 10.1002/clc.22253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saad M, Karam B, Faddoul G et al. Is kidney function affecting the management of myocardial infarction? A retrospective cohort study in patients with normal kidney function, chronic kidney disease stage III-V, and ESRD. Int J Nephrol Renovasc Dis 2016;9:5–10. 10.2147/IJNRD.S91567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chertow GM, Normand SL, McNeil BJ. “Renalism”: inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol 2004;15:2462–8. 10.1097/01.ASN.0000135969.33773.0B [DOI] [PubMed] [Google Scholar]
- 39. Bhatt DL, Roe MT, Peterson ED et al. Utilization of early invasive management strategies for high-risk patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. JAMA 2004;292:2096–104. 10.1001/jama.292.17.2096 [DOI] [PubMed] [Google Scholar]
- 40. Charytan DM, Setoguchi S, Solomon DH et al. Clinical presentation of myocardial infarction contributes to lower use of coronary angiography in patients with chronic kidney disease. Kidney Int 2007;71:938–45. 10.1038/sj.ki.5002159 [DOI] [PubMed] [Google Scholar]
- 41. Kooiman J, Pasha SM, Zondag W et al. Meta-analysis: serum creatinine changes following contrast enhanced CT imaging. Eur J Radiol 2012;81:2554–61. 10.1016/j.ejrad.2011.11.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study may be available from the US Department of Veterans Affairs upon reasonable request.