ABSTRACT
People living with chronic kidney disease (CKD) frequently suffer from mild cognitive impairment and/or other neurocognitive disorders. This review in two parts will focus on adverse drug reactions resulting in cognitive impairment as a potentially modifiable risk factor in CKD patients. Many patients with CKD have a substantial burden of comorbidities leading to polypharmacy. A recent study found that patients seen by nephrologists were the most complex to treat because of their high number of comorbidities and medications. Due to polypharmacy, these patients may experience a wide range of adverse drug reactions. Along with CKD progression, the accumulation of uremic toxins may lead to blood–brain barrier (BBB) disruption and pharmacokinetic alterations, increasing the risk of adverse reactions affecting the central nervous system (CNS). In patients on dialysis, the excretion of drugs that depend on kidney function is severely reduced such that adverse and toxic levels of a drug or its metabolites may be reached at relatively low doses, unless dosing is adjusted. This first review will discuss how CKD represents a risk factor for adverse drug reactions affecting the CNS via (i) BBB disruption associated with CKD and (ii) the impact of reduced kidney function and dialysis itself on drug pharmacokinetics.
Keywords: adverse drug reactions, chronic kidney disease, cognitive impairment, drug prescription
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Neurocognitive disorders in the general population are associated with several risk factors including cardiovascular disease, inflammation and history of head injury. Cognitive impairment is the deterioration of cognitive function beyond what might be expected from normal aging. Deterioration can affect a single one or several of the following cognitive domains: attention span, planning, memory, reasoning, decision making, language and executive functioning. Neurocognitive disorder (NCD) is defined as primarily cognitive impairment, acquired and declining. The patient's cognitive pattern together with clinical examination, brain imaging and biology, allows the cause of the NCD to be determined (e.g. Alzheimer's disease, vascular NCD, frontotemporal degeneration, etc.), even though cognitive functions are interconnected and interdependent. Cognitively impaired CKD patients often exhibit executive dysfunction that would be expected in vascular neurocognitive disorder; however, CKD patients are also at higher risk of Alzheimer's disease compared with the general population [1, 2].
Chronic kidney disease (CKD) is an independent risk factor for the development of cognitive impairment [3]. Some general factors associated with cognitive performance in the general population are also linked to cognitive function in CKD such as demographic and psychosocial factors or depression, although specific data on CKD patients are limited [4]. A number of specific CKD-related factors are also associated with impaired cognitive performance such as anemia, hyperparathyroidism, uremic toxins and albuminuria [4–6]. The high proportion of cardiovascular risk factors in CKD patients makes it difficult to disentangle the effect of kidney function on cognitive function from the effect of coincident vascular damage [7].
CKD patients have a substantial comorbidity burden, which often results in polypharmacy [8]. A recent study found that the patients seen by nephrologists were the most complex to treat because of their high number of comorbidities and the high number of prescription medications taken [9]. Along with comorbidities and renal complications, the drug burden increases sharply as CKD progresses. Indeed, CKD patients not only have many comorbidities, but they also experience particular complications (such as anemia, hyperkalemia and bone mineral disorders) requiring specific medications. Various studies have found high levels of polypharmacy (from 10 to 12 drugs per day per individual, on average) in CKD patients [10–13]. Due to this polypharmacy, patients with CKD have also a high risk of developing adverse drug reactions (ADRs). In a large cohort of patients with moderate to advanced CKD, Laville et al. showed that ADRs are common, often serious and potentially preventable, and that the incidence was higher when CKD was severe [14]. Among ADRs, cognitive adverse effects are frequent in CKD patients and could be a modifiable factor targeting the different classes associated with cognitive impairment. Indeed, several medications frequently included in the treatment regimen of CKD patients have been linked to negative effects on cognition such as drugs with anticholinergic properties, opioids agents, psychotropic agents, antibacterials, antiviral drugs and immunosuppressive drugs [15].
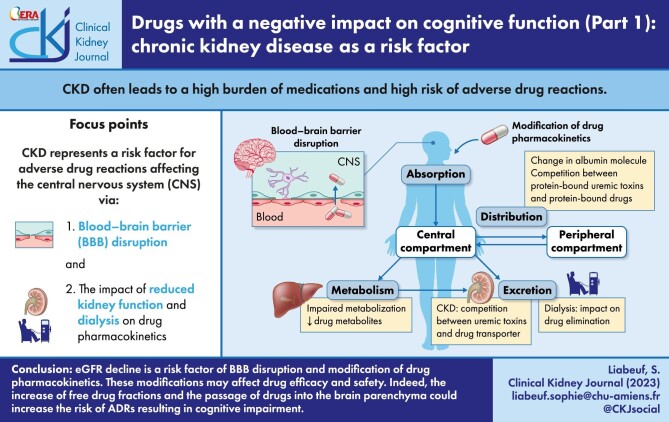
In addition, CKD is a very common clinical problem in older patients, thus older age is another important risk factor for ADRs, in particular drug-induced cognitive disorders [16]. In parallel with CKD progression, the accumulation of uremic toxins could lead to blood–brain barrier (BBB) disruption [17]. Alteration of the BBB could furthermore modify the efficacy of some drugs by increasing their penetration of the brain parenchyma and induce major central nervous system (CNS)-related ADRs. Prescribing to patients with CKD can be complex, because kidney disease and function has multiple effects on pharmacokinetics and these effects are dependent on both the drug and the clinical context [8]. In CKD patients, drug accumulation could lead to clinical symptoms augmenting the risk of ADRs affecting the CNS. Hence, dose adjustments may be required for selected drugs with pharmacokinetics that change significantly in kidney disease, especially for patients with end-stage kidney disease (ESKD) on dialysis.
In this narrative review, we will discuss how CKD represents a risk factor for ADRs affecting the CNS via (i) BBB disruption associated with CKD and (ii) the impact of reduced kidney function and dialysis on drug pharmacokinetics.
ALTERATION OF THE BBB ASSOCIATED WITH KIDNEY DISEASE
The BBB is vital for maintaining brain homeostasis by permitting fine control of the exchange of compounds and fluid between the blood and the brain parenchyma; moreover, the BBB prevents unwanted toxins, pathogens and drugs from entering the brain. The properties of the BBB are primarily determined by endothelial junctional complexes consisting of tight-junctions and adherens junctions, as well as by pericytes and astrocyte foot processes [18]. It is generally accepted that tight-junctions seal the interendothelial cleft forming a continuous blood vessel, while the adherens junctions are important for initiating and maintaining endothelial cell-to-cell contact. These junctions are composed of transmembrane proteins and cytoplasmic plaque proteins. Transmembrane proteins of the tight-junctions include occludin, claudins (claudin-5 being dominant in BBB endothelial cells), zonula occludens (ZO) proteins and junctional adhesion molecules (JAMs). Occludin has been shown to be increased in the peripheral circulation of animal models of cerebral ischemia [19], while JAM-1, located at the epithelial and endothelial tight-junctions, has been shown to be involved in regulation of endothelial cell migration. Adjacent cells also play a major role in the integrity of the BBB. Pericytes cover the outer surface of the capillaries along the basal lamina and play an important role in the integrity of the BBB by regulating the polarization of astrocytic foot processes in the BBB and endothelial transcytosis. In mice, knockout of the PDGF-β gene leads to a rarefaction of pericytes and a significantly increased permeability of the BBB [20]. Astrocytes are part of the glial cells of the CNS and have many functions that contribute to the protection of neurons. Astrocytes have long foot process–like extensions that allow connections to neurons and other astrocytes, but also to cerebral endothelial cells, and moreover participate in the architecture of the BBB and in the regulation of endothelial permeability and vascular tone [21]. After brain injury, astrocytes may hypertrophy and be recruited locally to form a barrier limiting the diffusion of leukocytes into the brain parenchyma [22].
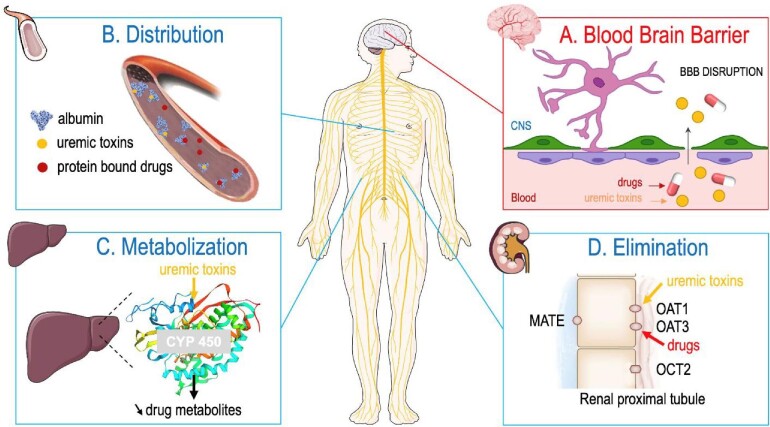
The BBB breaks down with age and further disruption is a hallmark of many age-related disorders such as Alzheimer's disease, which is also associated with pericyte dysfunction [23], and other chronic systemic diseases with a cerebral involvement. Thus, BBB disruption seems to be an important mechanism in diseases associated with cognitive impairment. In addition, certain pathophysiological factors like CKD may induce an impairment in BBB function (Fig. 1). This may lead to increased vascular permeability allowing entry of toxic substances into the CNS, resulting in damage that may manifest as cognitive impairments. There is accumulating evidence that CKD is associated with BBB disruption that may lead to cognitive impairment [17, 24]. One of the vascular mechanisms that may underly cognitive impairment in CKD patients involves “uremic toxins” causing BBB damage and dysfunction, since many of these toxins, including indoxyl sulfate, are known to induce endothelial dysfunction in CKD [25]. However, the acquisition of human brain tissues and subsequent isolation of brain micro-vessels is associated with complexities and there is limited research access to human brain endothelial cells. Therefore, studies aimed at modeling the BBB [26] have had limited success due to the complexity of its histological features. On the other hand, it was noted that small-vessel disease in the brain is mirrored by small-vessel pathologies in other organs [27]. Hernandez et al. [28] analyzed BBB dysfunction under uremic conditions assessing BBB permeability using serum CNS biomarkers and tight-junction integrity. They demonstrated that CNS biomarker neuron-specific enolase (NSE) was increased in hemodialysis patients, while brain-derived neurotrophic factor (BDNF) was reduced in the serum of hemodialysis patients compared with controls [29]. The expression of the tight-junction protein claudin-5, occludin and JAM-1 was lower in kidney transplant patients compared with controls. This study demonstrated that CKD is associated with biomarkers of BBB dysfunction, suggesting weakened tight-junction integrity.
Figure 1:
Impact of CKD on drug pharmacokinetics and the BBB. (A) BBB disruption. CKD is associated with alterations of the BBB leading to high number of CNS ADRs. (B) Distribution. A subject with late-stage CKD presented with low albumin levels, conformational changes in albumin molecule (such as carbamylation), competition between protein-associated uremic toxins and protein-bound drugs for albumin binding resulting in elevated free drugs fractions. (C) Metabolization. Severe CKD will result in decrease of expression and activity of drug-metabolizing enzymes such as CYP450 leading to unpredictable pharmacokinetics. (D) Elimination. CKD is associated with alteration of drug transporters. Example of competition between toxins and drugs as substrates of the OAT in renal proximal tubule leading to potential accumulation of drugs.
In a rodent model of CKD induced by a high adenine diet, several neurological abnormalities (akinesia, catalepsy) as well as anxiety- and depressive-like behavior were demonstrated. In this model BBB disruption was shown by Evans Blue dye extravasation into brain parenchyma [24]. Furthermore, in three rodent models of CKD the BBB permeability was assessed by single-photon emission computerized tomography (SPECT-CT) imaging, demonstrating that accumulation of the uremic toxin indoxyl sulfate in the serum activates aryl hydrocarbon receptors (AhR) which leads to disruption of the BBB and consequent cognitive impairment, while AhR-knockout mice were protected from indoxyl sulfate–induced BBB disruption [17].
The endothelial tight-junction markers in the brain cortex were analyzed in Sprague–Dawly rats with CKD induced by 5/6 nephrectomy. There was a significant reduction of ZO-1 protein, occludin and JAM-1 levels, while there was no significant change in claudin-5 protein expression [30]. Using the same CKD model, Lau et al. [31] showed that elevated blood urea induced actin cytoskeleton derangements and decreased claudin-5 expression, resulting in BBB dysfunction.
Finally, when analyzing BBB function in patients with CKD, associated comorbidities must be considered, particularly those associated with systemic inflammation. Diabetes, dyslipidemia and arterial hypertension are all associated with reduced cerebral blood flow and could contribute to BBB dysfunction. Indeed, BBB disruption has been described in patients with diabetes and hypertension [32, 33]. Alteration of BBB could modify CNS drug efficacy by increasing the passage of drugs into the brain parenchyma and induce ADRs resulting in cognitive impairment (Fig. 1A).
In addition to alteration of BBB, reduced kidney function could have an impact on drug pharmacokinetics inducing drug accumulation, leading to increased CNS ADRs.
REDUCED KIDNEY FUNCTION AND IMPACT ON DRUG PHARMACOKINETICS
Although most health professionals know that patients with a reduced estimated glomerular filtration rate (eGFR) have impaired clearance of drugs excreted primarily by the kidneys, the effect of CKD on non-kidney drug clearance is underappreciated. Indeed, there is evidence indicating that the drug distribution, the function of drug-metabolizing enzymes and transporters, which collectively determine net non-kidney drug clearance, is altered in CKD. Drug pharmacokinetics can be simply categorized as absorption, distribution, metabolization and elimination of the drug of interest. Pharmacokinetic properties are important determinants of the amount of drug reaching its site of action. In parallel with CKD progression, accumulation of uremic toxins could lead to drug pharmacokinetic alterations [34], especially distribution, metabolization and elimination phases (Fig. 1B–D). In clinical practice, eGFR equal to 40 mL/min/1.73 m2 corresponds to the threshold where the blood concentrations of uremic toxins increase.
Indeed, various CKD-associated metabolic disturbances might alter drug distribution and potentially trigger a CKD stage decrease in albumin drug binding [35]. Hence, (i) hypoalbuminemia might reduce the number of drug-binding dependent sites, (ii) conformational changes in the albumin molecule (such as carbamylation induced by urea accumulation) might have a similar effect, and (iii) the accumulation of endogenous substances (named uremic toxins) might competitively displace drugs from their albumin binding sites [35], resulting in an elevated free drug fraction.
Besides other factors such as genetics, animal studies suggest that expression and activity of drug-metabolizing enzymes such as hepatic cytochrome P450 (CYP450) are decreased in moderate and severe CKD. Hence, drug metabolism is significantly decreased in CKD which implies that patients with moderate to late CKD may be subject to unpredictable pharmacokinetics [36].
In addition to decreased renal elimination of drugs, CKD is associated with alterations in various drug transporters. Moreover, several uremic toxins can interact with membrane transporters (mediating their entry or exit from the cell) and may cause deleterious biological effects. Membrane transporters such as organic anion transporters (OAT) are particularly important for renal clearance and elimination by other routes of drugs and uremic toxins. Hence, there may be competition between toxins and drugs as substrates of the transporter or even inhibition of their activities. Consequently, tissue disposition and the elimination of drugs as well as uremic toxins may be affected [34].
In summary, CKD changes the pharmacokinetics of many drugs, both those with renal and those with non-renal clearance, leading to the need for dose adjustments. As a result both supra- and subtherapeutic dosing can occur in a CKD setting unless appropriate dose adjustments are made, and both have negative effects on patient outcomes (ADRs, hospital admissions and mortality). The risk of supratherapeutic exposure from drugs (or their toxic or active metabolites) that rely on renal elimination is amplified when the drug has a narrow therapeutic index, e.g. lithium or digoxin. Subtherapeutic dosing increases the risk of treatment failure that may be life-threatening (e.g. with some antibiotics) or organ threatening (e.g. with immunosuppressive drugs).
Patients with ESKD on dialysis are subject to extracorporeal clearance of small molecules, including many drugs. This aspect will be discussed in the next section.
CONSEQUENCES OF DIALYSIS ON DRUGS PHARMACOKINETICS
In patients with ESKD on dialysis, excretion of drugs that depend on kidney function is severely impaired. Hence, adverse and toxic levels of a drug or its metabolites may be reached at even low doses unless the dose is adjusted but also that a drug's concentration may be affected by dialysis itself.
In general, “dialyzability” through hemodialysis relates to a drug's excretion via the kidneys and its magnitude depends on a patient's preserved or residual kidney function. There are several key factors affecting the dialyzability of drugs [37] (Table 1). The extent to which hemodialysis removes a particular drug from plasma is dependent on its molecular weight, water solubility, protein binding capacity and volume of distribution. In addition, drug dialyzability is affected by the characteristics of modality of dialysis such as blood flow rate, dialysate solution, dialysis membrane, transmembrane pressure and duration/frequency of dialysis.
Table 1:
Factors affecting dialysability of drugs [37].
| Physicochemical and pharmacokinetic properties of the drug favoring dialyzability | |
| Water solubility | • Insoluble or fat-soluble drugs are not dialyzed—e.g. diazepam which is water insoluble |
| Low protein binding | • Tightly bound drugs are not dialyzed because dialysis is a passive process of diffusion—e.g. propranolol is 94% bound |
| Low MW | • Molecules with MW of <500 Da are easily dialyzed—e.g. vancomycin is poorly dialyzed via diffusion and has a MW of 1450 Da |
| Small Vd | • Widely distributed drugs are dialyzed more slowly because the rate limiting factor is the volume of blood entering the machine—e.g. digoxin, Vd = 250–300 L |
| • Drugs concentrated in the tissues are usually difficult to remove by dialysis | |
| Characteristics of the dialysis modality favoring dialyzability | |
| Blood flow rate | • Higher blood flows give higher clearance rates |
| Dialysate | • Higher dialysate flows give higher clearance rates |
| Dialysis membrane | • Higher permeability characteristics and surface area give higher clearance rates |
| Transmembrane pressure | • Ultrafiltration increases with transmembrane pressure increase |
| • Higher substitutive solution flows give higher clearance rates | |
| Duration and frequency of dialysis | • Longer duration and more frequent dialysis sessions give higher clearance rates |
MW: molecular weight; Vd: volume of distribution.
In peritoneal dialysis, where the convective and diffusive processes are continuous and slower, it may not be very significant [38].
Drug clearance by dialysis must be considered for appropriate timing of administration and dosing. For those drugs in which a substantial fraction is removed by hemodialysis they should be administered after dialysis to avoid drug removal and loss of efficacy. On the other hand, for drugs that are not significantly removed by dialysis, their administration does not need to be related to the timing of dialysis.
Figure 2 represents two examples of blood drug concentration after starting hemodialysis in drugs that are removed by convective or diffusive processes. Figure 2A represents general kinetics of dialyzable solute in blood during and immediately after the dialysis session. After the start of dialysis, the solute concentration in blood decreases during a dialysis session and recovers to some extent after the cessation of dialysis. This happens due to solute redistribution which takes place after the dialysis session has stopped. Figure 2B represents different large molecule (vancomycin) pharmacokinetics depending on different permeabilities of membranes. Non-dialyzable drug through low-flux membrane becomes dialyzable, when a high-flux membrane is used.
Figure 2:
The effect of hemodialysis on blood concentrations of drugs that are removable by convective or diffusive processes [38, 40]. (A) Example of small solute removal during dialysis and (B) removal of vancomycin (high molecular weight) through low-flux (red) vs high-flux (green) membranes.
In a clinical setting, it is important to know whether the drug is dialyzable or not. Variations in blood concentration of drugs may influence the effect of prescribed treatments that may have CNS effects and alter cognitive function. Table 2 represents common drugs with potential negative impact on the CNS and their relationship to dialysis [37–39].
Table 2:
Common drugs and their relation to dialysis [41–47].
| Drug | Dialyzablea | Recommended dose adjustmentb |
|---|---|---|
| Anticholinergic drugs | ||
| Antiepileptics | ||
| Carbamazepine | Controversial (dialyzable or Not) | NDA (75%–100% dose as in normal renal function + supplementary dose as in GFR <15 mL/min |
| Oxcarbazepine | Unknown | NDA + supplementary dose as for GFR <10 mL/min |
| Antiparkinsonians | ||
| Amantadine | Not dialyzable | No additional dose, dose as in GFR <15 mL/min |
| Trihexyphenidyl | Unknown | No known effects but should be used with caution |
| Anxiolytic | ||
| Hydroxyzine | Not dialyzable | NDA, supplement for IHD dose as in GFR <10 mL/min, consider risk of M(+), cetirizine |
| Antidepressants | ||
| Tricyclic | ||
| Amitriptyline | Not dialyzable | NDA, monitor ADRs, especially anticholinergic and QT interval prolonging ones, potentially due to accumulation of glucuronide metabolites |
| Amoxapine | Unknown | No dose recommendations; avoid use, due to risk of overdosage of parent and M(+) with antidopaminergic ADRs |
| Clomipramine | Not dialyzable | NDA, monitor for amitriptyline-like ADRs |
| Doxepin | Not dialyzable | NDA, monitor for amitriptyline-like ADRs |
| Imipramine | Not dialyzable | NDA, monitor for amitriptyline-like ADRs |
| Nortriptyline | Not dialyzable | As for amitriptyline |
| Trimipramine | Not dialyzable | NDA, monitor for amitriptyline-like ADR |
| SSRI | ||
| Citalopram | Not dialyzable | NDA, note high QT-prolonging potential vs lower potential SSRIs |
| Escitalopram | Not dialyzable | As for citalopram |
| Fluoxetine | Not dialyzable | NDA |
| Fluvoxamine | Not dialyzable | NDA |
| Paroxetine | Not dialyzable | NDA |
| Sertraline | Not dialyzable | NDA |
| Other serotonin and norepinephrine acting | ||
| Duloxetine (SNRI) | Not dialyzable | NDA, consider regulatory contraindication if GFR <30 mL/min |
| Mianserine (tetracyclic, NE-MM) | Not dialyzable | NDA, consider risks stemming from variable PK and ADRs from M(+) |
| Mirtazapine (tetracyclic, SN-Ran) | Unlikely to be dialyzable | NDA, based on high PB and large Vd, consider risks of ADRs from M(+) |
| Moclobemide (RIMA—SND-RevEI) | Likely to be dialyzable | Dose as in normal renal function |
| Venlafaxine (SNRI) | Not dialyzable? | NDA, consider risks stemming from variable PK ADRs from M(+) |
| Antihistaminics | ||
| Brompheniramine | Unlikely | Dose as in normal renal function, no supplementary dose required |
| Chlorpheniramine | Not dialyzable | Dose as in normal renal function, no supplementary dose required |
| Cyproheptadine | Unknown | Add 50%–100% dose supplement for IHD, consider overdosage risk |
| Diphenhydramine | Unlikely | Dose as in normal renal function, no supplementary dose required |
| Promethazine | Unlikely | Some experts recommend supplementary dose |
| Drugs related to cardiovascular system | ||
| Alverine | Unknown | Unknown |
| Atropine | Not dialyzable | No specific recommendations are available, but the need for dose adjustment is unlikely |
| Dimenhydrinate | Unknown | NDA + supplementary dose as in GFR <10 mL/min |
| Parasympatholytics | ||
| Scopolamine (hyoscine) | Unknown | Unknown |
| Urinary antispasmodics | ||
| Flavoxate | Unknown | Risk of overdosage due to renal excretion (57%) and presence of M(+), if dose is not adjusted, thus avoid use |
| Oxybutynin | Unlikely | Dose as in normal renal function, consider risk of QT prolongation and M(+) accumulation, transdermal use is less risky |
| Tolterodine | Unlikely | NDA, consider risk of QT prolongation and M(+) accumulation, not recommended use of extended formulations |
| Opioids | ||
| Buprenorphine | Yes | NDA; transdermal: dose as in normal renal function |
| Codeine | Not dialyzed | Avoid its use in ESKD and dialysis |
| Dihydrocodeine | Unknown | ND |
| Fentanyl | Not dialyzed | NDA |
| Hydrocodone | Unknown | Use alternative medicines |
| Meperidine/pethidine | Not dialyzed | NDA, risk for CNS and respiratory depression due to M(+) ADRs |
| Methadone | Poorly dialyzed | NDA |
| Hydromorphone | Unknown | NDA, metabolites may cause neuroexcitation and cognitive impairment |
| Morphine | Yes | NDA, some centres avoid use slow release preparations due to M(+) |
| Oxycodone | Unknown | NDA, limited accumulation of metabolites in renal failure compared with morphine |
| Oxymorphone | Unknown | Use alternative medicines |
| Propoxyphene | Poorly dialyzed | NDA |
| Tapentadol | Unknown | Not recommended in ESKD |
| Tramadol | Yes | NDA |
| Benzodiazepines and similar agents | ||
| Alprazolam | Not dialyzable | NDA |
| Bromazepam | Unlikely | NDA, risk of overdosage should be considered during long-term use due to several M(+) |
| Clobazam | Not dialyzable | NDA, effect is less predictable due to M(+) |
| Diazepam | Not dialyzable | NDA, effect is less predictable due to presence of several M(+)—nordazepam, oxazepam and temazepam |
| Estazolam | Not dialyzable | NDA |
| Lorazepam | Not dialyzable | NDA, caution should be exercised for repeated i.v. formulation use due to risk of propylene glycol toxicity; monitor osmol gap closely |
| Lormetazepam | Not dialyzable | NDA |
| Midazolam | Not dialyzable | NDA (oral), use with caution and monitor closely for excessive sedation; consider longer dosing intervals for intermittent dosing and slower titration of continuous infusions |
| Nitrazepam | Not dialyzable | NDA |
| Nordazepam | Unknown | Unknown |
| Oxazepam | Not dialyzable | NDA, risk of overdosage should be monitored |
| Prazepam | Not dialyzable | NDA, effect is less predictable due to presence of several M(+)—nordazepam and oxazepam |
| Z-Drugs | ||
| Zolpidem | Not dialyzable | NDA: option of dosage reductions is advised by some experts due to increased protein binding |
| Zopiclone | Controversial data | NDA, despite moderate PB (45%) |
| Psycholeptics (neuroleptics) | ||
| Aripiprazole | Unlikely | NDA, based on high PB of parent and M(+) |
| Clozapine | Not dialyzable | NDA, based on high PB and complete metabolism with limited or no activity metabolites, but consider regulatory contraindication to use in case of severe renal disorder |
| Chlorpromazine | Not dialyzable | NDA, caution should be exercised due to unknown level of M(+) |
| Levomepromazine (methotrimeprazine) | Unknown | NDA, although caution should be exercised due to unknown presence or absence of activity of metabolites |
| Loxapine | Controversial data | NDA, based on high PB and complete metabolism with limited or no activity metabolites |
| Olanzapine | Not dialyzable | NDA, based on high PB, large Vd and complete metabolism to M(–) |
| Perphenazine | Unknown | Unknown, caution should be exercised due to M(+) |
| Pimozide | Unknown | NDA, although caution should be exercised due to unknown presence or absence of M(+) and its PK |
| Quetiapine | Unlikely | NDA, based on relatively high PB (83%) and large Vd, although caution should be exercised due to M(+) and non-negligible renal elimination |
| Risperidone | Not significantly dialyzed | Oral: use with caution, doses up to 2 mg have been tolerated, i.m. or s.c.: avoid use; risk due to extensive hepatic metabolism to M(+); both risperidone and M(+) are mainly excreted by the kidney and may increase risk of ADRs (e.g. orthostatic hypotension, QT prolongation) |
| Other antipsychotics | ||
| Lithium | Dialyzable (80%) | Avoid use when possible, consider risk of lithium induced kidney damage in patients with significant residual kidney function; if necessary, initiate therapy at 300 mg 3 times weekly after dialysis; gradually titrate based on clinical response, tolerability, and serum lithium levels |
| Antibiotics | ||
| Penicillins | ||
| Amoxicillin and amoxicillin/clavulanate | Dialyzable | NDA, dose after IHD |
| Ampicillin and ampicillin/sulbactam | Dialyzable | NDA, dose after IHD |
| Benzylpenicillin | Dialyzable | NDA, dose after IHD |
| Cloxacillin | Not dialyzable | NDA |
| Oxacillin | Poorly dialyzable | Due to risk of neurotoxicity, the maximum daily dose of 8 g/day may be considered, otherwise—NDA |
| Phenoxymethylpenicillin | Dialyzable | NDA |
| Piperacillin | Dialyzable | 2 g every 8 h + 1 g after IHD |
| Piperacillin and tazobactam | Dialyzable | 2.25 g less frequently (every 8–12 h) + 0.75 g after IHD |
| Sultamicillin | Dialyzable | NDA, dose after IHD |
| Temocillin | Dialyzable | NDA, dose after IHD |
| Ticarcillin | Dialyzable | NDA, dose after IHD |
| Cephalosporines | ||
| Cefadroxil | Dialyzable | 1 g first dose; 500–1000 mg 3 times a week, or every 36 h, dose after IHD |
| Cefalexin | Dialyzable | NDA, dose after IHD |
| Cefazolin | Dialyzable | NDA, dose after IHD |
| Cefepime | Dialyzed | 1 g on day 1 followed by 0.5–1 g every 24 h, dose after IHD |
| Cefixime | Not dialyzable | NDA |
| Cefotaxime | Dialyzable | NDA, dose after IHD |
| Cefoxitin | Dialyzable | NDA, dose after IHD |
| Cefuroxime | Dialyzable | NDA, dose after IHD |
| Cefpodoxime | Dialyzable | 100–200 mg every 24 h, dose after IHD |
| Ceftazidime | Dialyzable | NDA, dose after IHD |
| Ceftazidime/avibactam | Dialyzable | 0.94 g every 24 h, dose after IHD |
| Ceftozolane/tazobactam | Dialyzable | 0.75–2.25 g loading dose, followed by 1250–450 mg every 8 h, dose after IHD |
| Ceftriaxone | Poorly dialyzable | NDA |
| Astreonam | Dialyzable | NDA, dose after IHD |
| Carbapenem | ||
| Ertapenem | Dialyzable | NDA, dose after IHD |
| Imipenem/cilastatin | Dialyzable | Due to neurotoxicity, consider alternative therapy; otherwise—NDA, dose after IHD |
| Meropenem | Dialyzable | NDA, dose after IHD |
| Fluoroquinolones | ||
| Ciprofloxacin | Minimally dialyzable (<10%) | NDA, dose after IHD |
| Levofloxacin | Dialyzable (up to 21% in HF) | NDA, dose after IHD |
| Moxifloxacin | Poorly dialyzable | NDA, dose after IHD |
| Norfloxacin | Not dialyzable | NDA, dose after IHD |
| Ofloxacin | Dialyzable | NDA, dose after IHD |
| Sulfonamides | ||
| Sulfadiazine | Dialyzable | In some countries the administration is contraindicated in case of severe renal insufficiency; if used NDA, dose after IHD |
| Sulfamethoxazole | Dialyzable | In some countries the administration is contraindicated in case of severe renal insufficiency where repeated plasma measurements cannot be performed; if used NDA, dose after IHD |
| Trimethoprim | Dialyzable | NDA, dose after IHD |
| Macrolides | ||
| Azithromycin | Not dialyzable | NDA |
| Clarithromycin | Not dialyzable | In ESKD: for IR formulation—prolong interval for the same single dose (from twice daily to once daily), for ER formulation—50% dose |
| Erythromycin | Not dialyzable | NDA, consider limiting dose to 2 g/day due to risk of ototoxicity |
| Josamycin (rovamycin) | Unlikely to be dialyzable | Consider use alternative macrolide |
| Spiramycin | Unlikely to be dialyzable | Consider use alternative macrolide |
| Roxithromycin | Unlikely to be dialyzable | Consider use alternative macrolide |
| Aminoglycosides | ||
| Amikacin | Dialyzable | I.v. (administered after IHD): 5–12.5 mg/kg/dose 3 times weekly; according to levels; postdialysis concentrations should be drawn ≥2 and up to 4 h after hemodialysis to allow for redistribution |
| Gentamicin | Dialyzable | I.v. (administered after IHD): after reduced loading dose (e.g. 2–3 mg/kg), in case of conventional dosage regimen—1 mg/kg/dose 3 times weekly; in case of consolidated dosage regimen—2–3 mg/kg/dose 3 times weekly; according to levels; postdialysis concentrations should be drawn ≥2 and up to 4 h after hemodialysis to allow for redistribution |
| Tobramycin | Dialyzable | I.v. (administered after IHD): after loading dose (e.g. 2–3 mg/kg), 1–2 mg/kg/dose 3 times weekly; according to levels; postdialysis concentrations should be drawn ≥2 and up to 4 h after hemodialysis to allow for redistribution |
| Other antibiotics | ||
| Metronidazole | Dialyzable in IHD, 10% removal in PD | Metabolized in the liver; M(+) have long half-life in renal impairment; NDA, dose after IHD |
| Vancomycin | Not dialyzable, but adsorbable in case of high-flux membrane | Oral: NDA; i.v. (administered after IHD): after normal loading dose (e.g. 25 mg/kg), in case of low-flux membrane—7.5 mg/kg after 48 h and every 48 h; in case of high-flux membrane—10 mg/kg after 48 h and every 48 h |
| Linezolid | Dialyzable | NDA, dose after dialysis |
| Polymyxins (Colistin) | Dialyzable | Increased dose: after normal loading dose (e.g. 9 MIU), 4.3 MIU per day in 2 divided doses on nondialysis days, adding 1.2 MIU after 3 h of IHD or 1.6 MIU after 4 h IHD |
| Isoniazid | Dialyzable | Dose as in normal renal function, dose after dialysis |
| Antivirals | ||
| Acyclovir | Dialyzable | NDA, dose after dialysis |
| Darunavir | Unlikely | Dose as in normal renal function |
| Famciclovir | Dialyzable | NDA, dose after dialysis |
| Foscarnet | Dialyzable | NDA, dose after dialysis |
| Ganciclovir | Dialyzable | Oral: 500 mg 3 weekly post IHD; i.v.: NDA, dose after dialysis; for IHD, the fraction of ganciclovir removed in a single dialysis session varied from 50% to 63% |
| Indinavir | Not dialyzable | Dose as in normal renal function |
| Ritonavir | Not dialyzable | Dose as in normal renal function |
| Nirmatrelvir | NA | Nirmatrelvir is contraindicated in case of severe renal impairment |
| Tenofovir | Dialyzable | Avoid use; if no alternative therapy is available—NDA, dose after dialysis |
| Valaciclovir | Dialyzable | NDA, dose after dialysis |
Based on intermittent haemodialysis; not dialyzable category includes limited (0% to 5%) dialyzability.
The dosing recommendations are based upon the dosage level of CKD 5 (not on dialysis) with CrCL <10 mL/min (if not otherwise stated), best available evidence and clinical expertise, but not considering obesity, cachexia and/or other risk factors for PK.
ER: extended release (formulation); HF: high-flux (dialyzer); ID: insufficient data; IHD: intermittent haemodialysis; i.m.: intramuscular; IR: immediate release (formulation); i.v.: intravenous; NA: not applicable; NDA: no dose adjustments in case of IHD of CKD patients, dose as in case of GFR <10 mL/min; MIU: million international units; M(+): active metabolites; M(–): inactive metabolites; NE-MM: norepinephrine multimodal; PB: protein binding; PD: peritoneal dialysis; PK: pharmacokinetic; RIMA: reversible inhibitor of monamino oxidase A; s.c.: subcutaneous; SN-Ran: serotonin, norepinephrine receptor antagonist; SND-RevEI: serotonin, norepinephrine, dopamine reversible enzyme inhibitor; SNRI: serotonin norepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; Vd: volume of distribution.
CONCLUSION
This narrative review presents eGFR decline as a risk factor of BBB disruption and modification of drug pharmacokinetics. These modifications may affect drug efficacy and safety. Indeed, the increase of free drug fractions and the passage of drugs into the brain parenchyma could increase the risk of ADRs, resulting in cognitive impairment. In an associated review [15] we describe drugs that are commonly recognized as a risk for causing cognitive impairment.
ACKNOWLEDGEMENTS
The authors would like to thank Cognitive Decline in Nephro-Neurology: European Cooperative Target (CONNECT) Action and members of COST Action for their support.
APPENDIX
The CONNECT collaborators are: Giovambattista Capasso, Alexandre Andrade, Maie Bachmann, Inga Bumblyte, Adrian Constantin Covic, Pilar Delgado, Nicole Endlich, Andreas Engvig, Denis Fouque, Casper Franssen, Sebastian Frische, Liliana Garneata, Loreto Gesualdo, Konstantinos Giannakou, Dimitrios Goumenos, Ayşe Tuğba Kartal, Sophie Liabeuf, Laila-Yasmin Mani, Hans-Peter Marti, Christopher Mayer, Rikke Nielsen, Vesna Pešić, Merita Rroji (Molla), Giorgos Sakkas, Goce Spasovski, Kate Stevens, Evgueniy Vazelov, Davide Viggiano, Lefteris Zacharia, Ana Carina Ferreira, Jolanta Malyszko, Ewout Hoorn, Andreja Figurek, Robert Unwin, Carsten Wagner, Christoph Wanner, Annette Bruchfeld, Marion Pepin, Andrzej Wiecek, Dorothea Nitsch, Ivo Fridolin, Gaye Hafez, Maria José Soler Romeo, Michelangela Barbieri, Bojan Batinić, Laura Carrasco, Sol Carriazo, Ron Gansevoort, Gianvito Martino, Francesco Mattace Raso, Ionut Nistor, Alberto Ortiz, Giuseppe Paolisso, Daiva Rastenytė, Gabriel Stefan, Gioacchino Tedeschi, Ziad Massy, Boris Bikbov, Karl Hans Endlich, Olivier Godefroy, Anastassia Kossioni, Justina Kurganaite, Norberto Perico, Giuseppe Remuzzi, Tomasz Grodzicki, Francesco Trepiccione, Carmine Zoccali, Mustafa Arici, Peter Blankestijn, Kai-Uwe Eckardt, Danilo Fliser, Eugenio Gutiérrez Jiménez, Maximilian Konig, Ivan Rychlik, Michela Deleidi, George Reusz, Michele Farisco, Norberto Perico, Pedro Imenez Silva, Mickaël Bobot, Aleksandra Golenia, Alessandra Perna, Alma Idrizi, Brian Hansen and Mariadelina Simeoni.
Notes
See Appendix for CONNECT Action collaborators.
Contributor Information
Sophie Liabeuf, Pharmacoepidemiology Unit, Department of Clinical Pharmacology, Amiens University Medical Center, Amiens, France; MP3CV Laboratory, EA7517, Jules Verne University of Picardie, Amiens, France.
Vesna Pešić, Faculty of Pharmacy, University of Belgrade, Belgrade, Serbia.
Goce Spasovski, Department of Nephrology, Clinical Centre “Mother Theresa”, Saints Cyril and Methodius University, Skopje, North Macedonia.
Romaldas Maciulaitis, Department of Nephrology, Lithuanian University of Health Sciences, Kaunas, Lithuania; Institute of Physiology and Pharmacology, Faculty of Medicines, Lithuanian University of Health Sciences, Kaunas, Lithuania.
Mickaël Bobot, Aix-Marseille University, Department of Nephrology, AP-HM, La Conception Hospital, Marseille, France; C2VN Laboratory, Inserm 1263, INRAE 1260, Aix-Marseille University, Marseille, France.
Ana Farinha, Department of Nephrology, Hospital de Vila Franca de Xira, Lisbon, Portugal.
Carsten A Wagner, Institute of Physiology, University of Zürich, Zurich, Switzerland.
Robert J Unwin, Department of Renal Medicine, Royal Free Hospital, University College London, London, UK.
Giovambattista Capasso, Department of Translantional Medical Sciences, University of Campania Luigi Vanvitelli , Naples, Italy; Biogem Research Institute , Ariano Irpino, Italy.
Inga Arune Bumblyte, Department of Nephrology, Lithuanian University of Health Sciences, Kaunas, Lithuania.
Gaye Hafez, Department of Pharmacology, Faculty of Pharmacy, Altinbas University, Istanbul, Turkey.
CONNECT Action (Cognitive Decline in Nephro-Neurology European Cooperative Target):
Giovambattista Capasso, Alexandre Andrade, Maie Bachmann, Inga Bumblyte, Adrian Constantin Covic, Pilar Delgado, Nicole Endlich, Andreas Engvig, Denis Fouque, Casper Franssen, Sebastian Frische, Liliana Garneata, Loreto Gesualdo, Konstantinos Giannakou, Dimitrios Goumenos, Ayşe Tuğba Kartal, Sophie Liabeuf, Laila-Yasmin Mani, Hans-Peter Marti, Christopher Mayer, Rikke Nielsen, Vesna Pešić, Merita Rroji (Molla), Giorgos Sakkas, Goce Spasovski, Kate Stevens, Evgueniy Vazelov, Davide Viggiano, Lefteris Zacharia, Ana Carina Ferreira, Jolanta Malyszko, Ewout Hoorn, Andreja Figurek, Robert Unwin, Carsten Wagner, Christoph Wanner, Annette Bruchfeld, Marion Pepin, Andrzej Wiecek, Dorothea Nitsch, Ivo Fridolin, Gaye Hafez, Maria José Soler Romeo, Michelangela Barbieri, Bojan Batinić, Laura Carrasco, Sol Carriazo, Ron Gansevoort, Gianvito Martino, Francesco Mattace Raso, Ionut Nistor, Alberto Ortiz, Giuseppe Paolisso, Daiva Rastenytė, Gabriel Stefan, Gioacchino Tedeschi, Ziad Massy, Boris Bikbov, Karl Hans Endlich, Olivier Godefroy, Anastassia Kossioni, Justina Kurganaite, Norberto Perico, Giuseppe Remuzzi, Tomasz Grodzicki, Francesco Trepiccione, Carmine Zoccali, Mustafa Arici, Peter Blankestijn, Kai-Uwe Eckardt, Danilo Fliser, Eugenio Gutiérrez Jiménez, Maximilian Konig, Ivan Rychlik, Michela Deleidi, George Reusz, Michele Farisco, Norberto Perico, Pedro Imenez Silva, Mickaël Bobot, Aleksandra Golenia, Alessandra Perna, Alma Idrizi, Brian Hansen, and Mariadelina Simeoni
FUNDING
This article is published as financially supported by the Horizon EU COST Action CA19127-Cognitive Decline in Nephro-Neurology: European Cooperative Target (CONNECT).
AUTHORS’ CONTRIBUTIONS
S.L. and G.H. were responsible for the research idea and supervision of review writing. V.P., G.C., R.M., M.B., A.F. and I.A.B. contributed to writing parts of the manuscript. C.A.W., R.J.U. and G.C. critically revised the manuscript. All authors reviewed and approved the manuscript for publication.
DATA AVAILABILITY STATEMENT
Not applicable.
CONFLICT OF INTEREST STATEMENT
S.L., A.F., R.M., G.C., V.P. and G.H. declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article. C.A.W. reports honoraria from Kyowa Kirin and Mecice. R.J.U. is currently employed by AstraZeneca BioPharmaceuticals R&D, Cambridge, UK. I.A.B. reports honoraria from Amgen and AstraZeneca. M.B. reports congress invitations from Otsuka, Vifor and Sanofi.
REFERENCES
- 1. Richards M. The power of birth cohorts to study risk factors for cognitive impairment. Curr Neurol Neurosci Rep 2022;22:847–54. 10.1007/s11910-022-01244-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med 2014;30:421–42. 10.1016/j.cger.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berger I, Wu S, Masson P et al. Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Med 2016;14:206. 10.1186/s12916-016-0745-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pepin M, Ferreira AC, Arici M et al. Cognitive disorders in patients with chronic kidney disease: specificities of clinical assessment. Nephrol Dial Transplant 2021;37(Suppl 2):ii23–32. 10.1093/ndt/gfab262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zoccali C, Ortiz A, Blumbyte IA et al. Neuropeptide Y as a risk factor for cardiorenal disease and cognitive dysfunction in chronic kidney disease: translational opportunities and challenges. Nephrol Dial Transplant 2021;37(Suppl 2):ii14–23. 10.1093/ndt/gfab284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liabeuf S, Pepin M, Franssen CFM et al. Chronic kidney disease and neurological disorders: are uraemic toxins the missing piece of the puzzle? Nephrol Dial Transplant 2021;37(Suppl 2):ii33–44. 10.1093/ndt/gfab223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pépin M, Levassort H, Boucquemont J et al. Cognitive performance is associated with glomerular filtration rate in patients with chronic kidney disease: results from the CKD-REIN cohort. J Neurol Neurosurg Psychiatry 2023;94:457–66. [DOI] [PubMed] [Google Scholar]
- 8. Liabeuf S, Laville M. Drug prescription in patients with chronic kidney disease: a true challenge. Nephrol Dial Transplant 2021;36:385–6. 10.1093/ndt/gfaa164 [DOI] [PubMed] [Google Scholar]
- 9. Tonelli M, Wiebe N, Manns BJ et al. Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Netw Open 2018;1:e184852. 10.1001/jamanetworkopen.2018.4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laville SM, Metzger M, Stengel B et al. Evaluation of the adequacy of drug prescriptions in patients with chronic kidney disease: results from the CKD-REIN cohort. Brit J Clinical Pharma 2018;84:2811–23. 10.1111/bcp.13738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kimura H, Tanaka K, Saito H et al. Association of polypharmacy with kidney disease progression in adults with CKD. Clin J Am Soc Nephrol 2021;16:1797–804. 10.2215/CJN.03940321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmidt IM, Hübner S, Nadal J et al. Patterns of medication use and the burden of polypharmacy in patients with chronic kidney disease: the German Chronic Kidney Disease study. Clin Kidney J 2019;12:663–72. 10.1093/ckj/sfz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marienne J, Laville SM, Caillard P et al. Evaluation of changes over time in the drug burden and medication regimen complexity in ESRD patients before and after renal transplantation. Kidney Int Rep 2021;6:128–37. https://www.kireports.org/article/S2468-0249(20)31649-1/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laville SM, Gras-Champel V, Moragny J et al. Adverse drug reactions in patients with CKD. Clin J Am Soc Nephrol 2020;15:1090–102. 10.2215/CJN.01030120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hafez G, Malyszko J, Golenia A et al. Drugs with a negative impact on cognitive functions (Part 2): drug classes to consider while prescribing in CKD patients. Clin Kidney J 2023;sfad239. 10.1093/ckj/sfad239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gray SL, Lai KV, Larson EB. Drug-induced cognition disorders in the elderly: incidence, prevention and management. Drug Saf 1999;21:101–22. 10.2165/00002018-199921020-00004 [DOI] [PubMed] [Google Scholar]
- 17. Bobot M, Thomas L, Moyon A et al. Uremic toxic blood-brain barrier disruption mediated by AhR activation leads to cognitive impairment during experimental renal dysfunction. J Am Soc Nephrol 2020;31:1509–21. 10.1681/ASN.2019070728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stamatovic SM, Keep RF, Andjelkovic AV. Brain endothelial cell-cell junctions: how to “open” the blood brain barrier. Curr Neuropharmacol 2008;6:179–92. 10.2174/157015908785777210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pan R, Yu K, Weatherwax T et al. Blood occludin level as a potential biomarker for early blood brain barrier damage following ischemic stroke. Sci Rep 2017;7:40331. 10.1038/srep40331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Armulik A, Genové G, Mäe M et al. Pericytes regulate the blood-brain barrier. Nature 2010;468:557–61. 10.1038/nature09522 [DOI] [PubMed] [Google Scholar]
- 21. Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006;7:41–53. 10.1038/nrn1824 [DOI] [PubMed] [Google Scholar]
- 22. Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci 2015;16:249–63. 10.1038/nrn3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nation DA, Sweeney MD, Montagne A et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 2019;25:270–6. 10.1038/s41591-018-0297-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mazumder MK, Giri A, Kumar S et al. A highly reproducible mice model of chronic kidney disease: evidences of behavioural abnormalities and blood-brain barrier disruption. Life Sci 2016;161:27–36. 10.1016/j.lfs.2016.07.020 [DOI] [PubMed] [Google Scholar]
- 25. Lano G, Burtey S, Sallée M. Indoxyl sulfate, a uremic endotheliotoxin. Toxins 2020;12:229. 10.3390/toxins12040229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hajal C, Le Roi B, Kamm RD et al. Biology and models of the blood-brain barrier. Annu Rev Biomed Eng 2021;23:359–84. 10.1146/annurev-bioeng-082120-042814 [DOI] [PubMed] [Google Scholar]
- 27. O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005;46:200–4. 10.1161/01.HYP.0000168052.00426.65 [DOI] [PubMed] [Google Scholar]
- 28. Hernandez L, Ward LJ, Arefin S et al. Blood-brain barrier and gut barrier dysfunction in chronic kidney disease with a focus on circulating biomarkers and tight junction proteins. Sci Rep 2022;12:4414. 10.1038/s41598-022-08387-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kurajoh M, Kadoya M, Morimoto A et al. Plasma brain-derived neurotrophic factor concentration is a predictor of chronic kidney disease in patients with cardiovascular risk factors—Hyogo Sleep Cardio-Autonomic Atherosclerosis study. PLoS One 2017;12:e0178686. 10.1371/journal.pone.0178686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jing W, Jabbari B, Vaziri ND. Uremia induces upregulation of cerebral tissue oxidative/inflammatory cascade, down-regulation of Nrf2 pathway and disruption of blood brain barrier. Am J Transl Res 2018;10:2137–47. [PMC free article] [PubMed] [Google Scholar]
- 31. Lau WL, Nunes ACF, Vasilevko V et al. Chronic kidney disease increases cerebral microbleeds in mouse and man. Transl Stroke Res 2020;11:122–34. 10.1007/s12975-019-00698-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoffman WH, Stamatovic SM, Andjelkovic AV. Inflammatory mediators and blood brain barrier disruption in fatal brain edema of diabetic ketoacidosis. Brain Res 2009;1254:138–48. 10.1016/j.brainres.2008.11.100 [DOI] [PubMed] [Google Scholar]
- 33. Katsi V, Marketou M, Maragkoudakis S et al. Blood-brain barrier dysfunction: the undervalued frontier of hypertension. J Hum Hypertens 2020;34:682–91. 10.1038/s41371-020-0352-2 [DOI] [PubMed] [Google Scholar]
- 34. da Cunha RS, Azevedo CAB, Falconi CA et al. The interplay between uremic toxins and albumin, membrane transporters and drug interaction. Toxins 2022;14:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klammt S, Wojak H-J, Mitzner A et al. Albumin-binding capacity (ABiC) is reduced in patients with chronic kidney disease along with an accumulation of protein-bound uraemic toxins. Nephrol Dial Transplant 2012;27:2377–83. 10.1093/ndt/gfr616 [DOI] [PubMed] [Google Scholar]
- 36. Velenosi TJ, Fu AYN, Luo S et al. Down-regulation of hepatic CYP3A and CYP2C mediated metabolism in rats with moderate chronic kidney disease. Drug Metab Dispos 2012;40:1508–14. 10.1124/dmd.112.045245 [DOI] [PubMed] [Google Scholar]
- 37. Hon Y. Dose adjustment in renal and hepatic disease. In: Ducharme MP, Shargel L, Yu ABC. (eds), Applied Biopharmaceutics & Pharmacokinetics, 8th edn. McGraw Hill; 2022. https://accesspharmacy.mhmedical.com/content.aspx?bookid=3127§ionid=264444107(25 September 2023, last date accessed). [Google Scholar]
- 38. Maher JF. Principles of dialysis and dialysis of drugs. Am J Med 1977;62:475–81. 10.1016/0002-9343(77)90400-4 [DOI] [PubMed] [Google Scholar]
- 39. National Institutes of Health . Dunleavy A. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov (12 July 2023, last date accessed). [PubMed] [Google Scholar]
- 40. DeSoi CA, Sahm DF, Umans JG. Vancomycin elimination during high-flux hemodialysis: kinetic model and comparison of four membranes. Am J Kidney Dis 1992;20:354–60. 10.1016/S0272-6386(12)70298-6 [DOI] [PubMed] [Google Scholar]
- 41. Ashley C, Dunleavy A. The Renal Drug Handbook—The Ultimate Prescribing Guide for Renal Practitioners, 5th edn. CRC Press. 2019. 10.1201/9780429460418 [DOI] [Google Scholar]
- 42. University of Louisville, Louisville, KY, USA. Kidney Disease Program [Internet] [cited 19 May 2023]. Available from: https://kdpnet.kdp.louisville.edu/drugbook/adult/ (12 July 2023, last date accessed) [Google Scholar]
- 43. Lexicomp: evidence-based drug referential content [Internet] [cited 19 May 2023]. Available from: https://www.wolterskluwer.com/en/solutions/lexicomp (12 July 2023, last date accessed) [Google Scholar]
- 44. Micromedex Products. Available from: https://www.micromedexsolutions.com/home/dispatch (12 July 2023, last date accessed) [Google Scholar]
- 45. Nevado-Holgado AJ, Kim C-H, Winchester L et al. Commonly prescribed drugs associate with cognitive function: a cross-sectional study in UK Biobank. BMJ Open 2016;6:e012177. 10.1136/bmjopen-2016-012177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stahl SM. Prescriber's Guide: Stahl's Essential Psychopharmacology [Internet] [cited May 19 2023]. 7th edn. Cambridge: Cambridge University Press, 2020. Available from: https://www.cambridge.org/core/books/prescribers-guide/ (12 July 2023, last date accessed) [Google Scholar]
- 47. Martindale: the complete drug reference [Internet] [cited 23 May 2019]. MedicinesComplete. Available from: https://about.medicinescomplete.com/publication/martindale-the-complete-drug-reference/ (12 July 2023, last date accessed) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.