ABSTRACT
Background
The prevalence of atrial fibrillation (AF) in end stage kidney disease (ESKD) patients undergoing dialysis is high, however, the high risk of bleeding often hampers with a correct anticoagulation in ESKD patients with AF, despite high thromboembolic risk. Left atrial appendage (LAA) occlusion is a anticoagulation (OAT) for thromboembolism prevention in AF populations with high hemorrhagic risk.
Methods and Results
The purpose of the study was to evaluate the efficacy and safety of LAA occlusion in a cohort of dialysis patients undergoing the procedure (LAA occlusion cohort, n = 106), in comparison with two other ESKD cohorts, one taking warfarin (Warfarin cohort, n = 114) and the other without anticoagulation therapy (No-OAT cohort, n = 148). After a median follow-up of 4 years, a Cox regression model, adjusted for possible confounding factors, showed that the hazard ratios (HRs) of thromboembolic events in the LAA occlusion cohort were 0.19 (95%CI 0.04–0.96; p = 0.045) and 0.16 (95%CI 0.04–0.66; p = 0.011) as compared with Warfarin and No-OAT cohorts, respectively. The HR of bleeding in the LAA occlusion cohort was 0.37 (95%CI 0.16–0.83; p = 0.017) compared to Warfarin cohort, while there were no significant differences between the LAA occlusion and the No-OAT cohort (HR 0.51; 95%CI 0.23–1.12; p = 0.094). Adjusted Cox regression models showed lower mortality in patients undergoing LAA occlusion as compared with both the Warfarin cohort (HR 0.60; 95%CI 0.38–0.94; p = 0.027) and no-OAT cohort (HR 0.52; 95%CI 0.34–0.78; p = 0.002). Thromboembolic events in the LAA occlusion cohort were lower than expected according to the CHA2DS2VASc score (1.7 [95%CI 0.3–3.0] vs 6.7 events per 100 person/years, p < 0.001).
Conclusion
In ESKD patients with AF, LAA occlusion is safe and effective and is associated with reduced mortality compared with OAT or no therapy.
Keywords: atrial fibrillation, bleeding, end stage kidney disease, left atrial appendage occlusion, thromboembolism
INTRODUCTION
The prevalence of atrial fibrillation (AF) in patients undergoing dialysis is high [1], and probably underestimated because of the high rate of asymptomatic, undiagnosed episodes [2].
Both thromboembolic and hemorrhagic risk is elevated in end-stage kidney disease (ESKD) because pro-thrombotic and bleeding promoting factors are simultaneously present [3]. Therefore, there is uncertainty in how to treat these patients. Randomized controlled trials (RCTs) demonstrating the efficacy of vitamin K antagonists (VKAs) for thromboembolic prevention are lacking and dialysis patients were excluded from RCTs that allowed direct oral anticoagulants (DOACs) to be included in the guidelines for thromboembolism prevention in AF [4–7]. Real-life studies on VKAs in hemodialysis (HD) patients showed inconclusive results on efficacy and negative results on safety [8] and real-life studies of DOACs failed to demonstrate an advantage on thromboembolic risk, either compared with warfarin or no oral anticoagulant therapy (OAT) [9, 10]. Therefore, cardiology and nephrology guidelines have been unable to provide clear recommendations for ESKD patients with AF [11, 12], and nephrologists often don't prescribe OAT in these patients or discontinue the drug after a hemorrhagic event [13].
Left atrial appendage (LAA) occlusion is an available alternative to OAT in patients with AF and high hemorrhagic risk. However, two RCTs aiming to evaluate the safety of LAA occlusion vs. OAT in patients with severe renal failure (Watch-AFIB and STOP-HARM) were stopped early because of under-recruitment [14]. A previous prospective cohort study demonstrated the feasibility of the procedure in a population of ESKD patients [15]. However, the follow-up was too short and the number of events too low to reach any conclusion regarding the efficacy and safety of LAA occlusion when compared with HD patients with AF taking warfarin or not taking OAT [16].
The main purposes of this study are (i) to assay the efficacy and safety of LAA occlusion in a large population of dialysis patients with a long follow-up (ii) to evaluate whether LAA occlusion leads to an advantage on thromboembolic and hemorrhagic risk compared to warfarin or no OAT. A secondary aim of the study is to compare the incidence of mortality and cardiovascular events of the LAA occlusion cohort compared with warfarin or no-OAT cohorts of ESKD patients.
MATERIALS AND METHODS
This multi-center, prospective, open label, observational study was performed according to STROBE guidelines. All ESKD patients requiring renal replacement therapy (hemodialysis or peritoneal dialysis), with documented AF (paroxysmal, persistent or permanent), CHA2DS2VASc score ≥1 if men and ≥2 if women and HASBLED score ≥3 or a contraindication for long-term OAT (e. g. previous life-threatening bleeding without a reversible cause) and who underwent LAA occlusion from May 2014 to January 2021 at one of the study participant centers that were included in the study. All patients recruited had to be age >18 years and have signed an informed consent to participate in the study.
Primary outcomes were cumulative incidence of thromboembolic and bleeding events (first event), secondary outcomes were mortality and cumulative incidence of cardiovascular events (first event) at the end of follow-up. For comparative purposes, a historical cohort of dialysis patients with AF, deriving from a prospective study previously performed running from October 2010 to December 2014 [17], was included in the study. Patients were selected from the historical cohort according to the same inclusion criteria as the LAA occlusion cohort, except for the procedure, and were divided according to anticoagulant therapy in two groups: one taking VKAs (Warfarin cohort) and the other not taking any anticoagulant therapy (No-OAT cohort).
Left atrial appendage occlusion
Left atrial appendage is the major source of thromboembolism in patients with AF. Percutaneous LAA occlusion is a minimally invasive transcatheter procedure involving a device implantation into the LAA to prevent thrombus formation and to reduce the risk of stroke. The procedure can be performed through an endocardial approach, with trans-esophageal ultrasound guidance and under general anesthesia to ensure patient immobility and minimize complications. The preferred access site for the procedure is the right femoral vein, but the left femoral vein can be used if the right access is unavailable. The trans-septal puncture, a critical step of the procedure, is preferably performed in the postero-inferior area of the interatrial septum, considering the typical orientation of the LAA.
Pre-procedural imaging obtained by trans-esophageal ultrasound or cardiac computerized axial tomography is crucial for LAA occlusion planning, allowing for the exclusion of LAA thrombi, detailed assessment of LAA anatomy, and measurement of LAA dimensions to select the appropriate device size and location for the trans-septal puncture. Heparin is administered before or during trans-septal puncture to prevent clot formation. Trans-esophageal ultrasound is used during the procedure to guide the trans-septal puncture, verify device position, and check for peri-device leaks.
Data collection
The following comorbidities and echocardiography parameters were collected: arterial hypertension, diabetes mellitus, dyslipidemia, peripheral arterial disease, ischemic heart disease, heart failure, chronic pulmonary disease, presence of left ventricular hypertrophy, left ventricular dysfunction and atrial dilation (see supplementary material for definitions).
Different types of AF were defined in agreement with the European Society of Cardiology [18]. In all patients, the thromboembolic (CHA2DS2VASc) and hemorrhagic (HASBLED) scores were determined to quantify patient-specific risk of thromboembolic and bleeding events [18].
Systemic thromboembolism was collected only if imaging-proven (computed tomographic scan or nuclear magnetic resonance) and major bleeding was defined as a fall in hemoglobin level of 2 g/dl or more or documented transfusion of at least two units of packed red blood cells, or an involvement of a critical anatomical site (intracranial, spinal, ocular, pericardial, articular, intramuscular with compartment syndrome, retroperitoneal) [19].
In patients taking warfarin, the International Normalized Ratio (INR) values were assessed at least once a month. To determine the achieved intensity of anticoagulation, the percentage time in the target INR range (target therapeutic range, TTR) was also calculated [20].
The rate of thromboembolic events observed was compared with the expected rate according to the mean CHA2DS2VASc score [21] and the rate of major bleedings observed was compared with the expected rate according to the mean HASBLED score [21].
The study protocol adhered to the 1975 Helsinki Declaration for Ethical Treatment of Human Subjects, with local ethics committee approval (Comitato Etico della Provincia di Monza e Brianza, study LAAO-DIA, 17 032 016). All involved subjects provided an informed consent to participate and for data publication.
Statistical methods (see supplementary material for extended statistical methods)
Baseline characteristics were summarized using descriptive statistics (median and range for continuous variables, and absolute and percentage frequencies for categorical variables). A logistic regression model was used to detect imbalances between baseline characteristics. Overall survival function was estimated by the Kaplan–Meier method. The cumulative incidence function was used to estimate the probability of thromboembolic, hemorrhagic and cardiovascular events in the presence of death as a competing event. The observed number of incidence events per 100 person/years was estimated as a weighted average. For each patient the expected number of thromboembolic and hemorragic incidence events per 100 person/years was retrieved from the reference [21] and it was weighted by the patient's survival time to obtain the expected number of thromboembolic and hemorragic incidence events per 100 person/years in each cohort. In order to evaluate the association between LAA occlusion and the four endpoints (thromboembolic events, hemorrhagic events, mortality and cardiovascular events) with respect to the historical cohort, we ran a multivariable Cox regression model adjusting for potential confounders. Survival analysis was performed using Stata software version 18.0 (StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX, USA: StataCorp LLC). The Stata command stcompet was used to generate cumulative incidence in the presence of competing events. R statistical software, version 4.2.1 (R Core Team 2022) was used to generate bar plots of expected and observed number of incidence events. Bar plots were generated using the ggplot2 package in R. Any other statistical analysis was performed using SAS software, version 9.4 of the SAS System for Linux. Copyright © 2012–2020, SAS Institute Inc., Cary, NC, USA.
Sensitivity analysis
As a sensitivity analysis, we evaluated the effect of the LAA occlusion by an inverse probability of treatment weight (IPTW) Cox model. We created a pseudo-population which mitigates the selection bias in treatment assignment at recruitment [22] on the propensity to undergo LAA occlusion that included all variables shown in Table 1 and several two and three-way interactions (see online supplementary material for details). In order to evaluate the balance induced by these weights, the confounders among patients under the three arms in this pseudo-population were compared by standardized differences and Chi-square test. The weighted Cox regression model with robust standard error was applied to the IPTW cohort to assess the effect of LAA occlusion on the different endpoints (first thromboembolic event, first bleeding event, overall mortality, first cardiovascular event). Variables with a maximum standardized difference among arms higher than 0.25 were included in the model. Results of the Cox models are expressed in terms of estimated hazard ratios (HR), 95% confidence intervals (95% CI) and P-values.
Table 1:
Clinical characteristics, and baseline comorbidities and anti-thrombotic therapy of the LAA occlusion population.
| N = 106 | ||
|---|---|---|
| Gender | ||
| Male | N (%) | 77 (72.6) |
| Age (yrs) | Median (min-max) | 74 (46–87) |
 75 yrs 75 yrs |
N (%) | 49 (46.2) |
| Dialytic age (yrs) | Median (min-max) | 2.21 (0.47–7.33) |
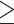 3 yrs 3 yrs |
N (%) | 42 (39.6) |
| BMI (kg/m2) | Median (Q1–Q3) | 25 (22–28) |
| Missing data (%) | 20 (18.9) | |
| Current smoking | ||
| Yes | N (%) | 10 (9.6) |
| Missing data (%) | 2 (1.9) | |
| CHA2DS2VASc | Median (Q1–Q3) | 4 (3–5) |
| HASBLED | Median (Q1–Q3) | 4 (4–5) |
| Atrial fibrillation | ||
| Paroxysmal | N (%) | 38 (35.8) |
| Persistent | N (%) | 18 (17.0) |
| Permanent | N (%) | 50 (47.2) |
| Comorbidities | ||
| Hypertension | N (%) | 95 (89.6) |
| Diabetes mellitus | N (%) | 40 (37.7) |
| Dyslipidemia | N (%) | 58 (54.7) |
| Peripheral artery disease | N (%) | 57 (53.8) |
| Ischaemic heart disease | N (%) | 46 (43.4) |
| Heart failure | N (%) | 36 (34.0) |
| Chronic pulmonary disease | N (%) | 22 (20.8) |
| Previous ischaemic stroke | N (%) | 11 (10.4) |
| Previous pulmonary thromboembolism | N (%) | 2 (1.9) |
| Previous major bleeding | N (%) | 66 (62.3) |
| Echocardiography | ||
| Atrium dilatation | N (%) | 88 (85.4) |
| N of missing data | 3 | |
| Left ventricular ejection fraction <50% | N (%) | 20 (19.0) |
| N of missing data | 1 | |
| Left ventricular hypertrophy | N (%) | 52 (57.8) |
| N of missing data (%) | 16 | |
| Baseline antithrombotic therapy | ||
| Antiplatelet | N (%) | 68 (64.2) |
| Heparin | N (%) | 33 (31.1) |
| Warfarin | N (%) | 31 (29.2) |
| Baseline antiarrhythmic therapy | ||
| Beta-blockers | N (%) | 59 (56.0) |
| Amiodarone | N (%) | 9 (8.5) |
| Sotalol | N (%) | 2 (1.9) |
| Other antiarrhythmic drugs | N (%) | 5 (4.7) |
BMI, body mass index; LAA, left atrial appendage.
RESULTS
One hundred and six consecutive dialysis patients who underwent LAA occlusion in 12 Italian centers between May 2014 and January 2021 were enrolled in the study.
The characteristics of the study population at baseline are shown in Table 1. The median values of the thromboembolic score (CHA2DS2VASc) and hemorrhagic score (HASBLED) were 4 (range 3–5) and 5 (range 4–5), respectively, and 64.2% of the patients had non-paroxysmal AF. A total of 12.3% of patients suffered a previous thromboembolic event and 62.3% had a major bleeding. About 90% of the patients were hypertensive. The other common comorbidities were diabetes mellitus (37.7%), dyslipidemia (54.7%), peripheral artery disease (53.8%), ischaemic heart disease (43.4%) and heart failure (34.0%).
The differences between the LAA occlusion cohort and the comparison cohorts (Warfarin and No-OAT) are shown in Table S1 (see online supplementary material). Left atrial appendage occlusion patients were more frequently male, with a higher prevalence of paroxysmal AF, dyslipidemia and previous major bleeding than other two cohorts. Dialysis age and prevalence of peripheral arterial disease were lower compared with Warfarin and No-OAT patients. Table S2 (see online supplementary material) shows the weighted clinical characteristics of the three cohorts.
Thromboembolic and hemorrhagic events
In the LAA occlusion cohort the median follow-up was 5 years (minimum 0.1, maximum 9.2 years).
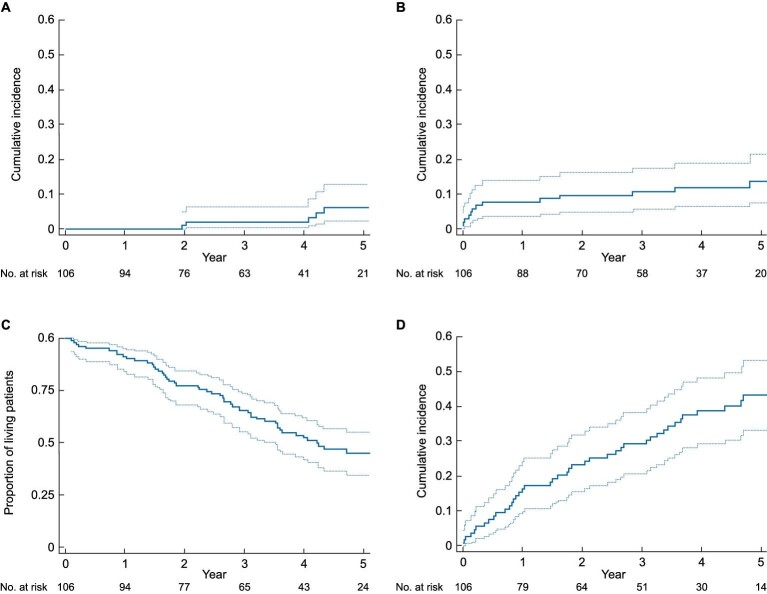
During follow-up, 6 (5.7%) thromboembolic events and 14 (13.2%) major bleeding events occurred. The cumulative incidence of thromboembolisms and bleedings is depicted in Fig. 1 (A and B). No thromboembolic event occurred in the first three months after the procedure, while at five years the percentage of thromboembolic events was 6.1% (95%CI 2.2–12.8%). The percentage of bleedings occurred in the first three months after the procedure was 6.8% (95%CI 3.0–12.7%), while at five years the result was 13.7% (95%CI 7.6–21.7%).
Figure 1:
Cumulative incidence of thromboembolisms (A), bleeding (B) and cardiovascular events (D) and survival curve (C) in the Left Atrial Appendage Occlusion cohort.
The incidence of thromboembolic events in the Warfarin and No-OAT groups (median follow-up 4 years, minimum 0.2, maximum 4.5 years) were 8/114 (5.4%) and 16/148 (10.8%), respectively. By the adjusted Cox regression model, the hazard ratio (HR) of thromboembolic events in the LAA occlusion cohort was 0.19 (95%CI 0.04–0.96; P = 0.045) and 0.16 (95%CI 0.04–0.66; P = 0.011) as compared with Warfarin and No-OAT cohorts, respectively (Table 2).
Table 2:
Cox regression model results on thromboembolic (Model 1) and hemorrhagic events (Model 2).
| Model 1: | Model 2: | |||
|---|---|---|---|---|
| N of patients = 368 | N of patients = 368 | |||
| N of thromboembolic events = 30 | N of hemorrhagic events = 65 | |||
| HR (95%CI) | P-value | HR (95%CI) | P-value | |
| LAA occlusion vs warfarin | 0.19 (0.04–0.96) | 0.045 | 0.37 (0.16–0.83) | 0.017 |
| LAA occlusion vs No-OAT | 0.16 (0.04–0.66) | 0.011 | 0.51 (0.23–1.12) | 0.094 |
| Gender (males) | 0.98 (0.43–2.24) | 0.954 | 1.2 (0.68–2.15) | 0.529 |
| Age (yrs) | 1.01 (0.96–1.07) | 0.634 | 1.02 (0.98–1.05) | 0.283 |
| Dialytic age (yrs) | 0.95 (0.88–1.04) | 0.261 | 1.03 (0.99–1.06) | 0.111 |
| CHA2DS2VASc (for each point) | 1.22 (0.88–1.7) | 0.236 | 0.97 (0.74–1.27) | 0.822 |
| HASBLED (for each point) | 1.26 (0.71–2.25) | 0.436 | 1.17 (0.76–1.8) | 0.466 |
| Persistent vs paroxysmal AF | 0.62 (0.25–1.55) | 0.309 | 1.24 (0.6–2.56) | 0.567 |
| Permanent vs paroxysmal AF | 0.56 (0.2–1.58) | 0.270 | 1.33 (0.64–2.77) | 0.442 |
| Dyslipidemia | 1.15 (0.52–2.54) | 0.723 | 0.72 (0.4–1.28) | 0.260 |
| Peripheral artery disease | 0.61 (0.24–1.55) | 0.299 | 0.95 (0.52–1.72) | 0.863 |
| Previous major bleedings | 0.94 (0.33–2.65) | 0.901 | 1.77 (0.93–3.37) | 0.085 |
| Antiplatelet therapy | 1.11 (0.46–2.65) | 0.813 | 0.74 (0.4–1.36) | 0.334 |
AF, atrial fibrillation; LAA, left atrial appendage; OAT, oral anticoagulant therapy.
Major bleedings were 27/114 (23.7%) in patients taking warfarin with an adjusted HR of 0.37 (95%CI 0.16–0.83; P = 0.017, Table 2) compared to LAA occlusion patients. There were no significant differences between the incidence of bleeding in the LAA occlusion cohort and the No-OAT cohort (24/148, 16.2%; HR 0.51; 95%CI 0.23–1.12; P = 0.094).
Sensitivity analyses performed after weighting confirmed these results (Table S3, see online supplementary material).
The INR range in patients taking warfarin was between 1.01 and 7.69, with a median of 2.2 (first-third quartile 1.68–2.59). During the study, the TRR in patients taking warfarin had a median of 53% (first-third quartile 42–64%).
Mortality and cardiovascular events
During follow-up, 59/106 (55.7%) patients in the LAA occlusion cohort died and 47 (44.3%) patients underwent a cardiovascular event. Nineteen out of 59 (32.2%) were cardiovascular deaths. Figure 1 shows the survival curve (C) and the cumulative incidence of cardiovascular events (D).
Deaths in the warfarin and no-OAT and cohorts were 67/114 (58.8%) and 94/148 (63.5%), respectively. Cardiovascular events in patients taking warfarin were 68/114 (59.6%) and those in patients not taking OAT 77/148 (52.1%).
A table comparing causes of death in the three cohorts is shown as supplementary material (Table S4, see online supplementary material). Death from cachexia was more frequent in patients who did not undergo the procedure than in the LAA occlusion cohort (P = 0.021).
Cox regression models showed lower mortality in patients undergoing LAA occlusion as compared with both the Warfarin cohort (HR 0.60; 95%CI 0.38–0.94; P = 0.027) and no-OAT cohort (HR 0.52; 95%CI 0.34–0.78; P = 0.002). Age, CHA2DS2VASc value, presence of permanent AF, and taking antiplatelet at baseline were all factors associated with reduced survival (Table 3, Model 1).
Table 3:
Cox regression on results on mortality (Model 1) and cardiovascular events (Model 2).
| Model 1: | Model 2: | |||
|---|---|---|---|---|
| N of patients = 368 | N of patients = 368 | |||
| N of deaths = 220 | N of cardiovascular events = 192 | |||
| HR (95%CI) | P-value | HR (95%CI) | P-value | |
| LAA occlusion vs warfarin | 0.60 (0.38–0.94) | 0.027 | 0.38 (0.23–0.62) | <0.001 |
| LAA occlusion vs No-OAT | 0.52 (0.34–0.78) | 0.002 | 0.45 (0.29–0.7) | <0.001 |
| Gender (males) | 1.06 (0.78–1.45) | 0.687 | 0.74 (0.53–1.03) | 0.071 |
| Age (yrs) | 1.02 (1.00–1.04) | 0.013 | 0.97 (0.95–0.99) | 0.004 |
| Dialytic age (yrs) | 1.01 (0.99–1.03) | 0.267 | 1.03 (1–1.05) | 0.022 |
| CHA2DS2VASc (for each point) | 1.23 (1.08–1.4) | 0.002 | 1.1 (0.95–1.29) | 0.197 |
| HASBLED (for each point) | 0.87 (0.69–1.1) | 0.245 | 1.4 (1.09–1.78) | 0.008 |
| Persistent vs paroxysmal AF | 1.02 (0.69–1.5) | 0.917 | 0.86 (0.58–1.27) | 0.451 |
| Permanent vs paroxysmal AF | 1.60 (1.09–2.33) | 0.016 | 0.88 (0.58–1.32) | 0.526 |
| Dyslipidemia | 0.94 (0.69–1.26) | 0.663 | 1.11 (0.81–1.54) | 0.510 |
| Peripheral artery disease | 0.96 (0.69–1.34) | 0.807 | 1.67 (1.15–2.43) | 0.007 |
| Previous major bleedings | 1.37 (0.93–2.02) | 0.108 | 1.43 (0.97–2.11) | 0.069 |
| Antiplatelet therapy | 1.43 (1.02–2.00) | 0.038 | 0.69 (0.48–0.98) | 0.038 |
AF, atrial fibrillation; LAA, left atrial appendage; OAT, oral anticoagulant therapy.
Cardiovascular events occurred less frequently in patients in the LAA occlusion cohort as compared with both patients taking warfarin (HR 0.38; 95%CI 0.23–0.62; P < 0.001) and those not taking OAT (HR 0.45; 95%CI 0.29–0.70; P < 0.001). Dialysis age, HASBLED value, and presence of peripheral artery at baseline were independently associated with cardiovascular events. There was an inverse correlation between cardiovascular events and age and antiplatelet assumption at baseline (Table 3, Model 2).
Sensitivity analysis performed after weighting confirmed the results (Table S5, see online supplementary material).
Post‑procedural antithrombotic therapy and bleeding
After the procedure, the percentage of patients who were prescribed two-drug therapy (two antiplatelet in the 93.5% of cases) was 77/106 patients (72.6%). The prescription of two-drug therapy ranged from 1 month (21%), to 3 months (40%), to 6 months (27%). Twenty-seven patients (25.5%) were prescribed one-drug therapy (one antiplatelet in 88.9% of cases) and 2 (1.9%) did not take antithrombotic therapy. Of the 14 episodes of major bleeding observed in the follow-up, five (36%) occurred in patients who were taking a two-drug therapy, nine (64%) in patients taking only one drug, and 0 (0%) in those not taking antithrombotic therapy. No patients developed device thrombosis during follow-up.
Thromboembolic and hemorrhagic events expected and observed
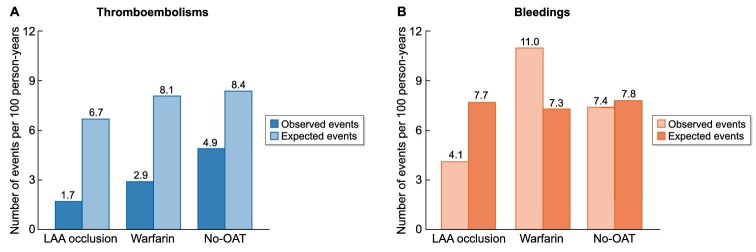
Figure 2 shows the thromboembolisms and major bleedings observed in the cohorts compared with the expected events according to the CHA2DS2VASC (A) and HASBLED (B) score value.
Figure 2:
Thromboembolisms and major bleedings observed in the three cohorts compared with the expected events according to the CHA2DS2VASC (A) and HASBLED (B) score value.
Thromboembolic events in the LAA occlusion cohort were lower than expected according to the CHA2DS2VASC score (1.7 (95%CI 0.3–3.0) vs 6.7 events per 100 person/years). The observed thromboembolic events were lower than those expected in both the Warfarin cohort and in the No-OAT cohort (2.9 (95% CI 0–24.7) vs 8.1, and 4.9 (95% CI 0–17.7) vs 8.4 events per 100 person/years, respectively) (Fig. 2A). However, the wide confidence intervals did not allow to observe a significant difference between observed and expected events except for the LAA occlusion cohort (P < 0.001).
Hemorrhagic events in the LAA occlusion cohort were lower than expected according to the HASBLED score [4.1 (95% CI 0–18.2) vs 7.7 events per 100 person/years], while they were higher in the Warfarin cohort [11.1 (95% CI 0–27.3) vs 7.3 events per 100 person/years] and similar to the expected ones in the No-OAT cohort [7.4 (95% CI 0–17.7) vs 7.8 events per 100 person/years] (Fig. 2B). The high confidence intervals did not allow to observe a significant difference between observed and expected events.
DISCUSSION
The study shows that LAA occlusion is associated with a long-term reduction in thromboembolic events in a population of dialysis patients with AF. This evidence comes both from comparing patients undergoing the procedure with two cohorts of dialysis patients with AF, one taking warfarin and the other not taking OAT, and from the analysis of actually observed and expected events according to the CHA2DS2VASc score.
The study results are relevant because to date there is a lack of clear evidence that OAT, either VKAs or DOACs, has an impact on the thromboembolic risk of ESKD patients with AF [8–10]. This is a nontrivial problem for clinicians dealing with a dialyzed patient presenting with AF, as this is a population at high thromboembolic risk with a lack of clear indications on therapeutic prescribing from cardiology and/or nephrology guidelines. Our study suggests that, after correction for possible confounding factors, over a long follow-up, LAA occlusion may have greater efficacy than medical therapy in reducing strokes and systemic thromboembolism. However, due to the relatively low TTR (53%), it is possible that some of the patients taking warfarin, not being in therapeutic range, were not fully protected from thromboembolic risk.
The incidence of major bleeding in patients included in the LAA occlusion cohort is significantly lower than that observed in patients taking warfarin. As expected, there were no significant differences between bleeding in patients who underwent the procedure and those who were not taking antithrombotic drugs.
Both total mortality and incidence of cardiovascular events are lower in the LAA occlusion cohort than in the other two cohorts. The finding is not easily explained, although it has already been reported in other LAA occlusion populations with preserved renal function [23]. The incidence of mortality is extremely high in our population, as is that of cardiovascular events. For each CHA2DS2VASc point, the risk of death increases by 23%, and the presence of permanent AF is significantly associated with reduced survival. Some previous studies showed that HD patients with AF treated with warfarin have longer survival than those not taking VKAs [17, 24, 25]. Our results suggest that LAA occlusion may provide an additional benefit in reducing the risk of death in this population. It is possible that nephrology patients who underwent LAA occlusion were more closely followed, particularly for their cardiovascular diseases. It is likely that they performed more frequent cardiology controls and that this interdisciplinary follow-up allowed more effective treatment of their comorbidities. This underscores the importance of close collaboration between nephrologists and cardiologists in the management of patients with both kidney and heart disease. Although all analyses were adjusted for observed potential confounding factors, we cannot rule out the possibility that the patients proposed by the treating physicians for LAA occlusion were less frail than those included in the other cohorts. This hypothesis is supported by the observation that patients in the LAA occlusion cohort die less frequently from cachexia than patients in Warfarin and No-OAT cohorts.
It is difficult to compare the results of our study with already published data. Prospective studies performed in dialysis patients who underwent LAA occlusion are very limited and almost all include too few patients (three studies recruited fewer than 10 patients and one recruited 25 patients) and events (no stroke or bleeding reported) to provide a comparison [26–28]. A Spanish group published a letter describing the experience of LAA occlusion in 51 HD patients followed for a median follow-up of 246 days. During follow-up, one stroke, five cases of bleeding, and 10 deaths were observed. The letter does not report further analysis of the population [29]. Other information regarding ESKD patients who underwent the procedure is derived from retrospective registry studies that included patients with various stages of renal failure, including very few dialysis patients. From these studies it would appear that the ESKD patients do not have a higher incidence of strokes and bleeding than patients without severe CKD [30, 31].
The number of major peri-procedural complications reported by us [15] and the other prospective studies [26–28] is extremely low. However, a large retrospective registry study described excess in-hospital mortality in ESKD patients undergoing LAA occlusion compared with those without CKD or with CKD not requiring dialysis [32]. Because of the high risk of complications due to invasive cardiology procedures, our strong suggestion is to entrust LAA occlusion in ESKD patients only to skilled cardiology teams.
In the LAA occlusion cohort, the actually observed thromboembolic events according to the CHA2DS2VASc score are higher than those expected (1.7 vs 6.7 events per 100 person/years). This is also the case in the cohort taking warfarin (2.9 vs 8.1 events per 100 person/years) and, to a lesser extent, in the cohort not taking OAT (4.9 vs 8.4 events per 100 person/years). One possible explanation could be that almost all of our patients (only two were peritoneal dialysis patients) were periodically treated with heparin, fractionated or low molecular weight, to prevent clot formation in the dialysis circuit. Therefore, these patients are at least partially uncoagulated three times a week, and this could reduce their risk of developing thrombi, even when they are not taking OAT. Frequent administration of heparin does not appear to expose patients in the cohort not taking OAT to excess bleeding (7.4 vs 7.8, observed vs expected events), while the risk of bleeding is higher than expected in HD patients who are taking warfarin (11.1 vs 7.3, observed vs expected events). Based on these observations, LAA occlusion could be a good option, as, without increased hemorrhagic risk, it would prevent intra LAA thrombus formation in patients who are already periodically taking an anticoagulant drug anyway, albeit off label for AF. However, it should be emphasized that the patient cohorts in which the CHA2DS2VASc and HASBLED scores were created and validated did not include ESKD patients. Our results raise the issue of the need to develop scores, also usable in dialysis patients with AF who have special and different characteristics from those of other AF populations.
The choice of the type of post-procedure therapy in this frail cohort of patients is very hard. There is evidence that in dialysis patients, antiplatelet is an important risk factor for bleeding [33]. Our data show a high number of bleeding cases, especially in the first three months after the procedure, when patients frequently took double antiplatelet therapy, but also in patients taking a single antiplatelet. Because we observed no device thrombosis in our population, perhaps we could consider prescribing only one antiplatelet and for a short period of time in dialysis patients undergoing LAA occlusion.
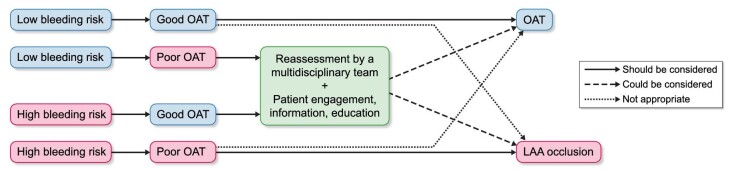
Although our results suggest that LAA occlusion is better than OAT for thromboembolic protection in HD patients, we do not think this evidence is enough to state that the procedure is indicated in all patients with ESKD and AF. In our opinion, the decision should be made taking into account the individual patient's clinical situation. Although RCTs demonstrating the efficacy of DOACs for the prevention of thromboembolic events in HD population are still lacking, it is important that further efforts be made in this direction. LAA occlusion remains an invasive procedure, and this is a factor that needs to be taken into account in a population as frail as the one we are talking about. A proposed algorithm for the treatment of HD patients with AF is shown in Fig. 3. An important limitation of our study is that this is not a RCT, so the comparison made between the LAA occlusion cohort and the Warfarin and No-OAT cohorts, even if performed with a rigorous statistical criterion (Cox model adjusted for possible confounders and IPTW analysis) may be biased, especially due to unobserved confounders. However, given that the only two RCTs designed to evaluate the effect of the procedure in HD patients, Watch-AFIB and STOP-HARM, were prematurely ended due to lack of recruitment (14), we think that our results may be useful in suggesting that ESKD patients with AF should not be precluded from accessing a non-therapeutic alternative for the prevention of thromboembolic events, when OAT has been shown to be ineffective and unsafe in this population. Further evidence is still needed to confirm that LAA occlusion could be an answer for the unsolved problem of antithrombotic treatment in ESKD patients.
Figure 3:
Proposed algorithm for thromboembolism prevention in hemodialysis patients with atrial fibrillation. Good OAT: patients taking VKA with TTR ≥65% or patients taking DOAC (off-label situation in Europe); Poor OAT: patients taking VKA with TTR <65% or patients not taking OAT; DOAC: direct oral anticoagulant; OAT: oral anticoagulant therapy; TTR: time in therapeutic range; VKA: vitamin K antagonist.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Dr Jacopo Oreglia, Dr Mario Gaggiotti, Dr Federica Ettori, Dr Roberto Palumbo, Prof. Francesca Viazzi, Dr Marco Breschi, Dr Paolo Orselli, Dr GianMaria Iadarola, Dr Consuela Mazzucchelli, Dr Marco Contarini and Dr Stefano Bianchi for helping in the data collection. We thank the Ethics Committee of the Province of Monza and Brianza for approving the study (LAAO-DIA, 17032016).
Contributor Information
Simonetta Genovesi, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy; Istituto Auxologico Italiano, IRCCS, Milan, Italy.
Luca Porcu, Cancer Research UK Cambridge Institute, University of Cambridge, Cambridge, UK.
Paola Rebora, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Giorgio Slaviero, Nephrology Unit, IRCCS Ospedale San Raffaele, Milano, Italy.
Gavino Casu, Cardiology Unit, Azienda Ospedaliera Universitaria di Sassari, Sassari, Italy.
Silvio Bertoli, Dialysis and Nephrology Unit-IRCCS-Multimedica, Sesto S.Giovanni, Italy.
Flavio Airoldi, Electrophysiology Unit-IRCCS-Multimedica, Sesto S.Giovanni, Italy.
Monique Buskermolen, Nephrology and Dialysis Unit, ASST Fatebenefratelli Sacco, Milano, Italy.
Maurizio Gallieni, Nephrology and Dialysis Unit, ASST Fatebenefratelli Sacco, Milano, Italy; Department of Biomedical and Clinical Sciences, University of Milano, Milano, Italy.
Federico Pieruzzi, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy; Istituto Auxologico Italiano, IRCCS, Milan, Italy.
Giovanni Rovaris, Interventional Electrophysiology Unit, San Gerardo Hospital, Monza, Italy.
Alberto Montoli, Nephrology Unit, Niguarda Hospital, Milano, Italy.
Emanuela Piccaluga, Interventional Cardiology Unit, Niguarda Hospital, Milano, Italy.
Giulio Molon, Cardiology Department, IRCCS Sacro Cuore Don Calabria Hospital, Negrar, Italy.
Federico Alberici, Nephrology Unit, ASST degli Spedali Civili di Brescia, Brescia, Italy.
Marianna Adamo, Cardiology Unit, ASST degli Spedali Civili di Brescia, Brescia, Italy.
Achille Gaspardone, Cardiology Unit, S.Eugenio Hospital, Roma, Italy.
Giuseppe D'Angelo, Cardiac Pacing Unit, IRCCS Ospedale San Raffaele, Milano, Italy.
Pierluigi Merella, Cardiology Unit, Azienda Ospedaliera Universitaria di Sassari, Sassari, Italy.
Giuseppe Vezzoli, Nephrology Unit, IRCCS Ospedale San Raffaele, Milano, Italy.
Barbara Trezzi, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Patrizio Mazzone, Cardiology 3, “A. De Gasperis” Cardio Center, ASST GOM Niguarda Ca' Granda, Milan, Italy.
FUNDING
This work was supported by Italian Ministry of Health—Ricerca Corrente.
AUTHORS’ CONTRIBUTIONS
Research idea and study design: S.G. and L.P.; data acquisition: G.S., G.C., S.B., F.A., M.B., M.G., F.P., G.R., A.M., E.P., G.M., F.A., M.A., A.G., G.DA., P.M., G.V.; Data analysis/interpretation: S.G., L.P., P.R. and P.M.; Statistical analysis: L.P. and P.R.; Supervision or mentorship: S.G. and P.M. Each author contributed important intellectual content during manuscript drafting and agrees to be personally accountable for the individual's own contributions.
DATA AVAILABILITY STATEMENT
S.G., L.P. and P.R. had full access to all of the data in the study and take responsability for the integrity of the data and the accuracy of the data analysis. Please contact the corresponding author regarding data requests.
CONFLICT OF INTEREST STATEMENT
(Honoraria for speaking at symposia or consultation). S.G.: Boston Scientific; G.C.: Abbott Structural Heart, Boston Scientific, Lifetech; G.R.: Boston Scientific, Abbott Structural Heart, Biosense, Medtronic, Biotronik, Hylomorph ; G.M.: Boston Scientific; M.A.: Abbott Structural Heart, Medtronic, Edwards Lifesciences; P.M.: Abbott Vascular, Boston Scientific. Other authors report no relationships that could be construed as a conflict of interest.
REFERENCES
- 1. Cardiovascular disease in patients with CKD. Am J Kidney Dis 73:3 Supplement 1S1-S772; US Renal Data System 2018 Annual Data Report. www.usrds.org (7 October 2023, last date accessed). [Google Scholar]
- 2. Buiten MS, de Bie MK, Rotmans JI et al. The dialysis procedure as a trigger for atrial fibrillation: new insights in the development of atrial fibrillation in dialysis patients. Heart 2014;100:685–90. 10.1136/heartjnl-2013-305417 [DOI] [PubMed] [Google Scholar]
- 3. Ng KP, Edwards NC, Lip GYH et al. Atrial fibrillation in CKD: balancing the risks and benefits of anticoagulation. Am J Kidney Dis 2013;62:615–32. 10.1053/j.ajkd.2013.02.381 [DOI] [PubMed] [Google Scholar]
- 4. Connolly SJ, Ezekowitz MD, Yusuf S et al. Dabigatran versus Warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 5. Patel MR, Mahaffey KW, Garg J et al. Rivaroxaban versus Warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 6. Granger CB, Alexander JH, McMurray JJV et al. Apixaban versus Warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 7. Giugliano RP, Ruff CT, Braunwald E et al. Edoxaban versus Warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
- 8. Dahal K, Kunwar S, Rijal J et al. Stroke, major bleeding and mortality outcomes in warfarin users with atrial fibrillation and chronic kidney disease: a meta-analysis of observational studies. Chest 2016;149:951–9. 10.1378/chest.15-1719 [DOI] [PubMed] [Google Scholar]
- 9. Kuno T, Takagi H, Ando T et al. Oral anticoagulation for patients with atrial fibrillation on long-term hemodialysis. J Am Coll Cardiol 2020;75:273–85. 10.1016/j.jacc.2019.10.059 [DOI] [PubMed] [Google Scholar]
- 10. Xiaole S, Bingjuan Y, Lihua W et al. Oral anticoagulant agents in patients with atrial fibrillation and CKD: a systematic review and pairwise network meta-analysis. Am J Kidney Dis 2021;78:678–89. 10.1053/j.ajkd.2021.02.328 [DOI] [PubMed] [Google Scholar]
- 11. Steffel J, Collins R, Antz M et al. 2021 European Heart Rhythm Association practical guide on the use of Non-Vitamin K Antagonist oral anticoagulants in patients with atrial fibrillation. Europace 2021;00:1–65. 10.1093/europace/euab065 [DOI] [PubMed] [Google Scholar]
- 12. Turakhia MP, Blankestijn PJ, Carrero JJ et al. Chronic kidney disease and arrhythmias: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Eur Heart J 2018;0:1–17. 10.1093/eurheartj/ehy060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Genovesi S, Rossi E, Gallieni M et al. Warfarin use, mortality, bleeding and stroke in haemodialysis patients with atrial fibrillation. Nephrol Dial Transplant 2015;30:491–8. 10.1093/ndt/gfu334 [DOI] [PubMed] [Google Scholar]
- 14. Vallurupalli S, Sharma T, Al'Aref S et al. Left atrial appendage closure: an alternative to anticoagulation for stroke prevention in patients with kidney disease. Kidney360 2021;3:396–402. 10.34067/KID.0004082021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Genovesi S, Slaviero G, Porcu L et al. Implant success and safety of left atrial appendage occlusion in end stage renal disease patients: peri-procedural outcomes from an Italian dialysis population. Int J Cardiol 2018;262:38–42. 10.1016/j.ijcard.2018.03.083 [DOI] [PubMed] [Google Scholar]
- 16. Genovesi S, Porcu L, Slaviero G et al. Outcomes on safety and efficacy of left atrial appendage occlusion in end stage renal disease patients undergoing dialysis. J Nephrol 2020;34:63–73. 10.1007/s40620-020-00774-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Genovesi S, Rebora P, Gallieni M et al. Effect of oral anticoagulant therapy on mortality in end-stage renal disease patients with atrial fibrillation: a prospective study. J Nephrol 2017;30:573–81. 10.1007/s40620-016-0364-8 [DOI] [PubMed] [Google Scholar]
- 18. Camm AJ, Lip GY, De Caterina R et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation—Developed with the special contribution of the European Heart Rhythm Association. Europace 2012;14:1385–413. 10.1093/europace/eus305 [DOI] [PubMed] [Google Scholar]
- 19. Schulman S, Kearon C Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemostasis 2005;3:692–4. 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 20. Rosendaal FR, Cannegieter SC, van der Meer FJM et al. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 1993;69:236–9. [PubMed] [Google Scholar]
- 21. Friberg L, Rosenqvist M, Lip GYH. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012;33:1500–10. 10.1093/eurheartj/ehr488 [DOI] [PubMed] [Google Scholar]
- 22. Imai K, Van Dyk DA. Causal inference with general treatment regimes: generalizing the propensity score. J Am Stat Assoc 2004;467:854–66. 10.1198/016214504000001187 [DOI] [Google Scholar]
- 23. Reddy VY, Doshi SK, Kar S et al. 5-Year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol 2017;70:2964–75. 10.1016/j.jacc.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 24. Shen JI, Montez-Rath ME, Lenihan CR et al. Outcomes after warfarin initiation in a cohort of hemodialysis patients with newly diagnosed atrial fibrillation. Am J Kidney Dis 2015;66:677–88. 10.1053/j.ajkd.2015.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brancaccio D, Neri L, Bellocchio F et al. Patients’ characteristics affect the survival benefit of Warfarin treatment for hemodialysis patients with atrial fibrillation. A historical cohort study. Am J Nephrol 2016;44:258–67. 10.1159/000448898 [DOI] [PubMed] [Google Scholar]
- 26. Xipell M, Flores-Umanzor E, Ojeda R et al. Percutaneous left atrial appendage closure, a safe alternative to anticoagulation for patients with nonvalvular atrial fibrillation and end-stage renal disease on hemodialysis: a single center experience. Artif Organs 2020;44:513–21. 10.1111/aor.13603 [DOI] [PubMed] [Google Scholar]
- 27. Torres-Saura F, Romero-Vazquianez M, Perez-Berbel P et al. New approach to the prevention of stroke in patients with non-valvular fibrillation in hemodialysis: percutaneous closure of left atrial appendage. Arch Cardiol Mex 2020;90:102–5. 10.24875/ACME.M19000055 [DOI] [PubMed] [Google Scholar]
- 28. Manes M, Radin E, Pellu V et al. Monocentric experience of left atrial appendage occlusion among patients with advanced chronic kidney disease and non-valvular atrial fibrillation. G Ital Nefrol 2020;37:1. [PubMed] [Google Scholar]
- 29. Benito-González T, Quirós A, Torres-Saura F et al. Design and interim results of a registry of left atrial appendage occlusion with the Watchman device in patients on hemodialysis: EPIC06-WATCH-HD. Rev Esp Cardiol (Engl Ed) 2022;75:179–80. 10.1016/j.recesp.2021.07.016 [DOI] [PubMed] [Google Scholar]
- 30. Kefer J, Tzikas A, Freixa X et al. Impact of chronic kidney disease on left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation. Int J Cardiol 2016;207:335–40. 10.1016/j.ijcard.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 31. Fastner C, Brachmann J, Lewalter T et al. Left atrial appendage closure in patients with chronic kidney disease: results from the German multicentre LAARGE registry. Clin Res Cardiol 2021;110:12–20. 10.1007/s00392-020-01638-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Munir MB, Khan MZ, Darden D et al. Association of chronic kidney disease and end-stage renal disease with procedural complications and in-hospital outcomes from left atrial appendage occlusion device implantation in patients with atrial fibrillation: insights from the national inpatient sample of 36,065 procedures. Heart Rhythm 02 2021;2:472–9. 10.1016/j.hroo.2021.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hiremath S, Holden RM, Fergusson D et al. Antiplatelet medications in hemodialysis patients: a systematic review of bleeding rates. Clin J Am Soc Nephrol 2009;4:1347–55. 10.2215/CJN.00810209 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
S.G., L.P. and P.R. had full access to all of the data in the study and take responsability for the integrity of the data and the accuracy of the data analysis. Please contact the corresponding author regarding data requests.