ABSTRACT
Background
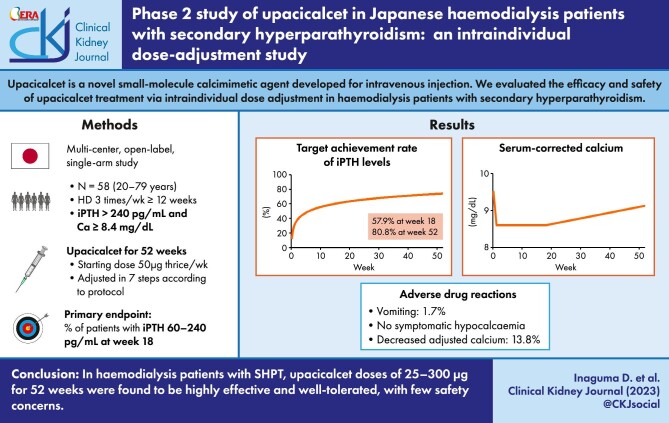
Upacicalcet is a novel small-molecule calcimimetic agent developed for intravenous injection. Here, we evaluated the long-term efficacy and safety of upacicalcet treatment via intraindividual dose adjustment in haemodialysis patients with secondary hyperparathyroidism (SHPT).
Methods
A phase 2, multicentre, open-label, single-arm study was conducted. Upacicalcet was administered for 52 weeks; the starting dose was 50 μg thrice a week, and then adjusted to 25, 50, 100, 150, 200, 250, or 300 μg, according to the dose-adjustment method set in the protocol. The primary endpoint was the percentage of patients with serum intact parathyroid hormone (iPTH) level achieving a target range of 60–240 pg/mL (target achievement rate) at week 18.
Results
A total of 58 patients were administered upacicalcet. The target achievement rate of serum iPTH level at week 18 was 57.9%, which increased to 80.8% at week 52. The serum-corrected calcium (cCa) level decreased immediately after upacicalcet administration, but no further decrease was observed. Adverse events were observed in 94.8% of patients, and adverse drug reactions (ADRs) occurred in 20.7% of patients. The most common ADR was decreased adjusted calcium in eight patients; dizziness occurred as a serious ADR in one patient. The serum cCa level of patients who interrupted upacicalcet treatment at a serum cCa level of <7.5 mg/dL recovered to ≥7.5 mg/dL immediately after the interruption.
Conclusions
In haemodialysis patients with SHPT, upacicalcet doses of 25–300 μg for 52 weeks were found to be highly effective and well-tolerated, with minor safety concerns.
Keywords: calcimimetics, haemodialysis patients, parathyroid hormone, secondary hyperparathyroidism, upacicalcet
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Secondary hyperparathyroidism (SHPT) is a common complication of end-stage kidney disease with dialysis [1]. In addition, SHPT has been reported to be associated with an increased mortality risk and fractures [2, 3]. Calcimimetics can lower serum levels of parathyroid hormone (PTH), calcium, and phosphate in dialysis patients [4–6]. However, calcimimetics have been reported to cause hypocalcaemia and upper gastrointestinal (GI) symptoms [6, 7]. Evocalcet, a novel oral calcimimetic agent, was reported to be non-inferior to cinacalcet in suppressing serum PTH level to the target range, with a lower incidence of GI symptoms [5]. In addition, it has been reported that etelcalcetide, an intravenous calcimimetic agent, lowers serum PTH level more effectively than cinacalcet but shows a similar incidence of GI symptoms to cinacalcet [6, 8].
Upacicalcet sodium hydrate is a novel, small-molecule, positive allosteric modulator of the calcium-sensing receptor that is developed for intravenous injection [9–12]. A phase 1/2 study of upacicalcet in haemodialysis patients with SHPT demonstrated that the effect of upacicalcet was dose-dependent, and over 80% of upacicalcet was removed by single haemodialysis. Furthermore, vomiting was observed as a mild adverse drug reaction (ADR) only in a group receiving ≥400 μg of a single intravenous administration [10]. Therefore, it was thought that upacicalcet at a dose of <400 μg might serve as an intravenous formulation with fewer GI adverse events (AEs).
The current study was a dose-finding and proof-of-concept phase 2 trial for upacicalcet. Here, we aimed to evaluate the efficacy and safety of upacicalcet using intraindividual dose adjustment for 52 weeks in Japanese haemodialysis patients with SHPT.
MATERIALS AND METHODS
Study design
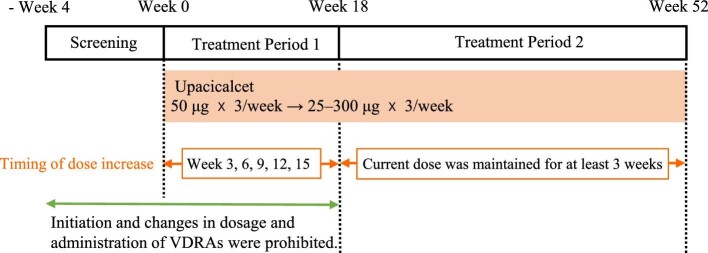
This phase 2 multicentre, open-label, single-arm study was conducted at 19 sites in Japan from June 2017 to September 2018 (Table S1, see online supplementary material). The treatment period was 52 weeks, including 18 weeks in Treatment Period 1 and 34 weeks in Treatment Period 2 (Fig. 1). In Treatment Period 1, the efficacy and safety of upacicalcet with intraindividual dose adjustments were evaluated. In Treatment Period 2, the long-term safety and efficacy of the treatment were assessed. This study was conducted in compliance with the Declaration of Helsinki, Good Clinical Practice, and the related laws and regulations. The study protocol was reviewed and approved by the Institutional Review Board of each study site. Approval numbers are presented in Table S1 (see online supplementary material). In addition, written informed consent was obtained from all patients. This study was registered at ClinicalTrials.gov (NCT03226171).
Figure 1:
Study design. VDRAs, Vitamin D receptor activators.
Patients
The inclusion criteria were as follows: Japanese patients with chronic kidney disease (CKD) aged 20–79 years receiving haemodialysis or haemodiafiltration three times a week for ≥12 weeks before the screening, serum intact PTH (iPTH) level of >240 pg/mL (average of measurements of two consecutive weeks), and serum corrected calcium (cCa) level of ≥8.4 mg/dL during the screening period. The exclusion criteria are listed in Table S2 (see online supplementary material).
Treatment
Upacicalcet was administered thrice a week into the venous side of the dialysis circuit during blood return at the end of dialysis for 52 weeks. The starting dose was 50 μg. The dose was then adjusted in seven steps (25, 50, 100, 150, 200, 250, or 300 μg) according to the dose-adjustment criteria of the protocol to maintain the serum iPTH level within 60–240 pg/mL, as recommended by the Japanese Society for Dialysis Therapy (JSDT) guidelines [13]. These doses were set based on the results of the phase 1/2 study [10].
In Treatment Period 1, the timing of the dose increase was weeks 3, 6, 9, 12, and 15. The dose was increased by one step if the present dose was maintained for more than three weeks, the serum iPTH level was >240 pg/mL, the serum cCa level was ≥8.4 mg/dL, and the investigator or sub-investigator judged that there were no safety concerns. The dose was reduced by one step if the iPTH serum level was <60 pg/mL for two consecutive weeks or if the investigator or sub-investigator deemed it necessary. Upacicalcet administration was interrupted if the serum cCa level was <7.5 mg/dL or if the investigator or sub-investigator deemed it necessary.
In Treatment Period 2, the dose could be increased by one step if the present dose was maintained for more than three weeks, the serum cCa level was ≥8.4 mg/dL, and the investigator or sub-investigator judged that there were no safety concerns. The dose was reduced by one step if the investigator or sub-investigator deemed it necessary. Upacicalcet administration was interrupted if the serum cCa level was <7.5 mg/dL or if the investigator or sub-investigator deemed it necessary.
Initiation of vitamin D receptor activator (VDRA) use and changes in both dosage and administration of VDRAs were prohibited from two weeks before the screening period until the end of Treatment Period 1.
Initiation of calcium preparations and phosphate binders and changes in their dosage and administration were prohibited from two weeks before the screening period until the end of the screening period.
Changes in dialysate calcium concentrations were prohibited from two weeks before the screening period until the end of Treatment Period 1 and prohibited in principle during Treatment Period 2.
Cinacalcet and etelcalcetide were prohibited from 2 and 12 weeks before the screening period, respectively, until the end of the study.
Efficacy
The primary endpoint was the percentage of patients who achieved the serum iPTH levels of 60–240 pg/mL (target achievement rate) at week 18.
The secondary endpoint was the target achievement rate of serum iPTH level at each time point. In addition, measurements of the serum levels of iPTH, cCa, phosphate (P), intact fibroblast growth factor 23 (iFGF23), bone alkaline phosphatase (BAP), total N-terminal propeptide of type I procollagen (P1NP; includes both trimeric and monomeric propeptides), and tartrate-resistant acid phosphatase-5b (TRACP-5b) at each time point were treated as secondary endpoints. All biochemical samples were collected before the first haemodialysis session of the week (three days after the previous haemodialysis).
All clinical laboratory samples were measured by SRL Medisearch Inc. (Tokyo, Japan). In addition, serum iPTH levels were measured using an electrochemiluminescence immunoassay (ECLusys PTH; Roche Diagnostics, Tokyo, Japan). The Payne's formula was used to calculate serum cCa levels in patients with serum albumin level of <4.0 g/dL [14].
Safety
Safety was evaluated based on AEs, ADRs, clinical laboratory measurements, vital signs, and 12-lead electrocardiograms. AEs and ADRs were coded using MedDRA/J (version 20.0), and the preferred terms and system organ classes were used for tabulation. If a relationship between an AE and upacicalcet could not be ruled out by the investigator, the AE was defined as an ADR. Upper GI symptoms, hypocalcaemia, and decreased adjusted calcium were considered as AEs of special symptoms. A symptomatic decrease in the calcium level was classified as hypocalcaemia. A decrease in the serum cCa level that was asymptomatic but judged by the investigator as an AE was counted as decreased adjusted calcium. The 12-lead electrocardiograms were analysed by Suzuken Co., Ltd (Nagoya, Japan).
Statistical analyses
A total of 60 patients were considered necessary to evaluate a 5% incidence of AEs. Therefore, the target number of patients in the phase 2 study was set at 60 to evaluate the long-term efficacy and safety of upacicalcet. A total of 58 patients, >95% of target number of patients, were enrolled; this was deemed feasible to evaluate the efficacy and safety of upacicalcet. The efficacy analysis population included all patients who received more than one dose of upacicalcet and were assessed for more than one efficacy endpoint after administration [Full Analysis Set (FAS)]. The safety analysis population consisted of all patients who received more than one dose of upacicalcet. All evaluations were conducted using a descriptive manner in this study. In general, continuous data are presented as mean ± standard deviation or median (interquartile range).
RESULTS
Patient flow and background
A total of 58 patients were administered upacicalcet. During Treatment Period 1, 57 patients completed the study with one patient discontinuing. In Treatment Period 2, 52 patients completed the study, whereas five patients did not complete the study (Fig. 2).
Figure 2:
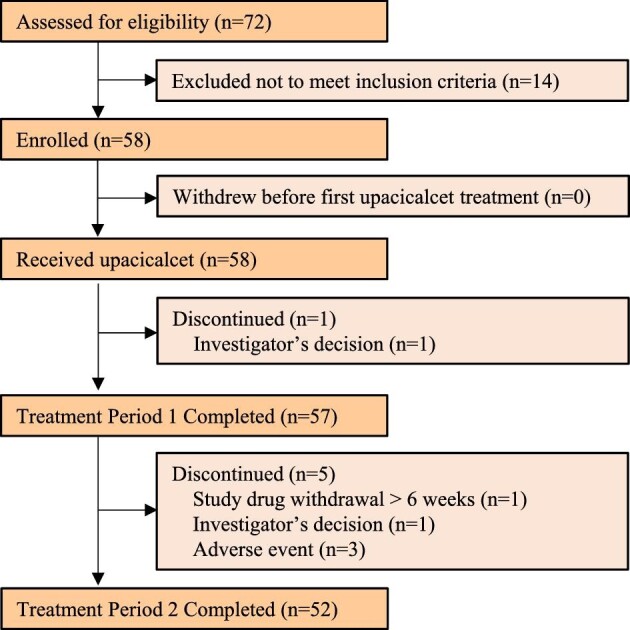
Patient flow.
A total of 58 patients were included in the FAS and safety analyses. Their baseline demographic characteristics are summarized in Table 1. Of the patients, 94.8% used phosphate binders and 79.3% used VDRAs. The baseline characteristics of patients with and without VDRAs are shown in Table S3 (see online supplementary material). Among the VDRAs, the usage rate and dose of alfacalcidol decreased, while the rate of maxacalcitol usage increased. The dose of calcium carbonate was increased (Table S4, see online supplementary material). Dialysate calcium concentration was changed from 1.5 mmol/L to 1.375 mmol/L in three patients during Treatment Period 2.
Table 1:
Demographic characteristics of subjected patients.
| Patient characteristics | Enrolled patient (N = 58) |
|---|---|
| Gender (male) | 43 (74.1) |
| Age (years) | 61.6 ± 9.1 |
| ≥65 | 25 (43.1) |
| Primary diseases | |
| Chronic glomerulonephritis | 20 (34.5) |
| Diabetic nephropathy | 16 (27.6) |
| Nephrosclerosis | 3 (5.2) |
| Polycystic kidney disease | 4 (6.9) |
| Other | 15 (25.9) |
| Dry weight (kg) | 61.94 ± 14.02 |
| Duration of dialysis (years) | 9.25 ± 6.47 |
| Duration of dialysis category (years) | |
| <5 | 17 (29.3) |
| 5 to <10 | 19 (32.8) |
| 10 to <20 | 17 (29.3) |
| ≥20 | 5 (8.6) |
| Dialysate Ca concentration | |
| 1.25 mmol/L [2.5 mEq/L] | 12 (20.7) |
| 1.375 mmol/L [2.75 mEq/L] | 16 (27.6) |
| 1.5 mmol/L [3.0 mEq/L] | 30 (51.7) |
| Vitamin D receptor activators | |
| With | 46 (79.3) |
| Without | 12 (20.7) |
| P binders | 55 (94.8) |
| Ca-based | 8 (13.8) |
| Non-Ca-based | 21 (36.2) |
| Both above | 26 (44.8) |
| Serum iPTH (pg/mL) | 322 (261, 532) |
| ≤240 | 11 (19.0) |
| 240 to <500 | 32 (55.2) |
| ≥500 | 15 (25.9) |
| Serum cCa (mg/dL) | 9.56 ± 0.68 |
| Serum P (mg/dL) | 5.33 ± 1.13 |
| cCa × P (mg2/dL2) | 51.095 ± 12.274 |
| Serum iFGF23 (pg/mL) | 6300 (1830, 16 900) |
| Serum BAP (µg/L) | 14.1 (10.8, 19.5) |
| Serum total P1NP (ng/mL) | 322 (212, 450) |
| Serum TRACP-5b (mU/dL) | 682 (469, 908) |
Number (%), mean ± standard deviation or median (25th percentile, 75th percentile)
BAP, bone alkaline phosphatase; Ca, calcium; cCa, corrected calcium; iFGF23, intact fibroblast growth factor 23; iPTH, intact parathyroid hormone; P, phosphate; P1NP, type I procollagen N-terminal propeptide; TRACP-5b, tartrate-resistant acid phosphatase-5b.
Efficacy
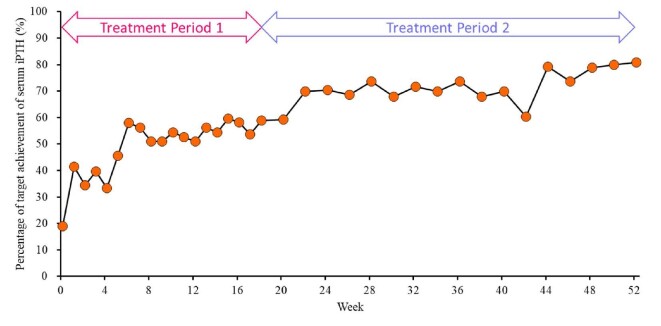
The target achievement rate of the serum iPTH level at week 18 (the primary endpoint) was 57.9% [95% confidence interval (CI): 44.1, 70.9]. The target achievement rate of the serum iPTH level at each time point gradually increased from 19.0% (95% CI: 9.9, 31.4) at baseline to 80.8% (95% CI: 67.5, 90.4) at week 52 (Fig. 3).
Figure 3:
Time course of changes in the percentage of patients achieving the serum iPTH target range (60–240 pg/mL, as recommended by the Japanese Society for Dialysis Therapy guidelines). iPTH, intact parathyroid hormone.
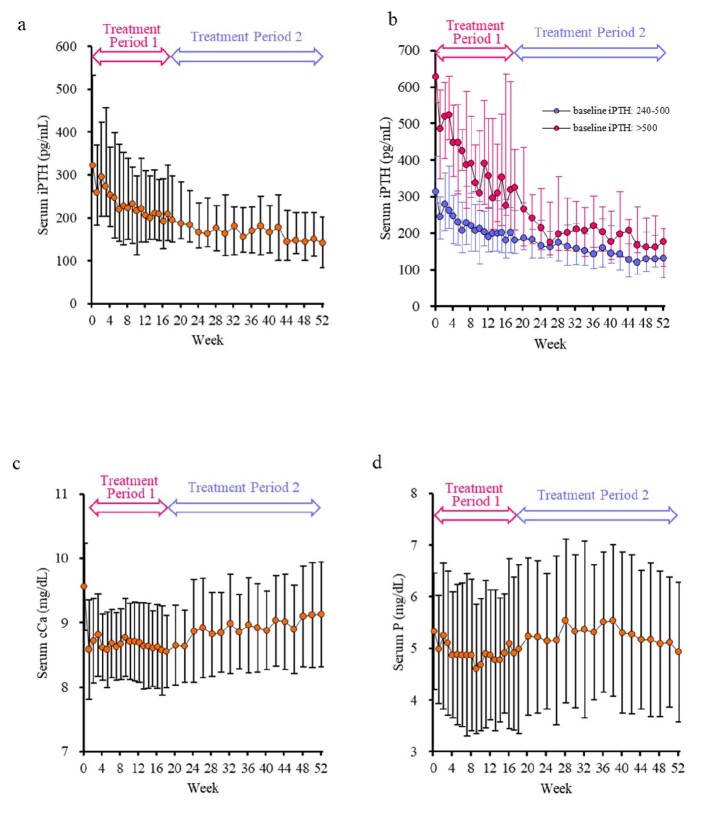
The serum iPTH level decreased gradually from 322 (261, 532) pg/mL at baseline to 140.5 (83.5, 201.5) pg/mL at week 52 (Fig. 4a). At all doses at week 52, the serum iPTH level at week 52 was 96–210 pg/mL, within the upper limit of the target range recommended by in the JSDT guidelines [13] (Table 2). Furthermore, even at the minimum dose of 25 μg, the median serum iPTH level did not drop below 60 pg/mL. The changes in serum iPTH levels were similar in patients treated with and without VDRAs (Table 3). There were no differences in serum iPTH levels at week 52 among the dialysate calcium concentration (Table 3). The changes and % changes in serum iPTH levels stratified by baseline serum iPTH levels of 240–500 pg/mL and >500 pg/mL decreased over time in both groups, and the serum iPTH levels at week 52 were within the target range recommended by the JSDT guidelines [13] (Fig. 4b and Fig S1, see online supplementary material). The serum cCa level decreased from 9.56 ± 0.68 mg/dL at baseline to 8.58 ± 0.77 mg/dL at week 1. Following that point, the serum cCa level was maintained without further decrease in Treatment Period 1 and gradually increased in Treatment Period 2, reaching 9.13 ± 0.81 mg/dL at week 52 (Fig. 4c). The serum cCa levels of patients not treated with VDRAs were lower than those treated with VDRAs during the entire study period (Table 3). The serum P level was 5.33 ± 1.13 mg/dL at baseline and 4.93 ± 1.35 mg/dL at week 52 (Fig. 4d).
Figure 4:
Time course of changes in the serum iPTH (a), iPTH levels stratified at baseline (b), cCa (c), and P (d). Data are shown as median with interquartile range for iPTH (a and b), and mean with standard deviation for cCa (c) and P (d). cCa, corrected calcium; iPTH, intact parathyroid hormone; P, phosphate.
Table 2:
Changes in serum iPTH levels stratified by upacicalcet dose at week 52.
| Upacicalcet dose at week 52 | Baseline | Week 18 | Week 52 |
|---|---|---|---|
25 μg  3/week 3/week | |||
| Number | 14 | 13 | 12 |
| Serum iPTH (mg/dL) | 301 (244.5, 339.5) | 151 (96, 230) | 108.5 (56.75, 193.5) |
| Percent changes in serum iPTH (%) | −42.7 ± 24.7 | −54.2 ± 34.0 | |
50 μg  3/week 3/week | |||
| Number | 14 | 13 | 12 |
| Serum iPTH (mg/dL) | 285 (212.25, 332) | 142 (98, 172.5) | 96 (64.5, 129) |
| Percent changes in serum iPTH (%) | −34.4 ± 64.2 | −54.1 ± 43.7 | |
100 μg  3/week 3/week | |||
| Number | 7 | 7 | 6 |
| Serum iPTH (mg/dL) | 262 (206, 532) | 155 (139, 247) | 97.5 (73.25, 186.5) |
| Percent changes in serum iPTH (%) | −30.9 ± 39.6 | −47.0 ± 55.0 | |
150 μg  3/week 3/week | |||
| Number | 5 | 5 | 5 |
| Serum iPTH (mg/dL) | 460 (337.5, 1033) | 188 (176.5, 299.5) | 191 (146.5, 222) |
| Percent changes in serum iPTH (%) | −55.8 ± 23.8 | −61.1 ± 24.5 | |
200 μg  3/week 3/week | |||
| Number | 4 | 4 | 4 |
| Serum iPTH (mg/dL) | 545 (336.5, 557) | 234.5 (168.5, 386.75) | 145 (109.5, 196.25) |
| Percent changes in serum iPTH (%) | −39.9 ± 32.2 | −63.1 ± 26.1 | |
250 μg  3/week 3/week | |||
| Number | 4 | 4 | 4 |
| Serum iPTH (mg/dL) | 638.5 (406.5, 653.75) | 371.5 (275.5, 493.75) | 150 (114, 171.75) |
| Percent changes in serum iPTH (%) | −26.8 ± 34.9 | −72.2 ± 11.5 | |
300 μg  3/week 3/week | |||
| Number | 10 | 10 | 9 |
| Serum iPTH (mg/dL) | 540 (397, 628) | 304.5 (257.25, 460.75) | 210 (177, 215.5) |
| Percent changes in serum iPTH (%) | −30.6 ± 25.2 | −58.7 ± 15.0 | |
Data are presented as mean ± standard deviation or median (25th percentile, 75th percentile).
iPTH, intact parathyroid hormone.
Table 3:
Changes in serum levels of iPTH and cCa stratified according to the administration of VDRAs and dialysate calcium concentration.
| Baseline | Week 18 | Week 52 | |
|---|---|---|---|
| Baseline VDRAs use | |||
| User of VDRAs | |||
| Number | 46 | 45 | 41 |
| Serum iPTH (pg/mL) | 322 (253, 536) | 199 (142, 280) | 137 (101, 201) |
| Serum cCa (mg/dL) | 9.68 ± 0.64 | 8.61 ± 0.60 | 9.25 ± 0.82 |
| Nonuser of VDRAs | |||
| Number | 12 | 11 | 11 |
| Serum iPTH (pg/mL) | 324 (288.5, 400) | 190 (145, 395) | 144 (72, 202) |
| Serum cCa (mg/dL) | 9.08 ± 0.64 | 8.30 ± 0.17 | 8.65 ± 0.55 |
| Baseline dialysate calcium concentration | |||
| 1.25 mmol/L [2.5 mEq/L] | |||
| Number | 12 | 11 | 11 |
| Serum iPTH (pg/mL) | 321.5 (278.5, 521.5) | 262 (132, 371) | 137 (62, 203) |
| Serum cCa (mg/dL) | 9.34 ± 0.72 | 8.19 ± 0.42 | 9.25 ± 1.14 |
| 1.375 mmol/L [2.75 mEq/L] | |||
| Number | 16 | 15 | 15 |
| Serum iPTH (pg/mL) | 276.5 (214.5, 310.5) | 188 (99, 311) | 131 (80, 229) |
| Serum cCa (mg/dL) | 9.28 ± 0.62 | 8.53 ± 0.67 | 8.82 ± 0.64 |
| 1.5 mmol/L [3.0 mEq/L] | |||
| Number | 30 | 30 | 26 |
| Serum iPTH (pg/mL) | 442.5 (301, 591) | 181.5 (150, 263) | 156 (94, 191) |
| Serum cCa (mg/dL) | 9.80 ± 0.64 | 8.69 ± 0.49 | 9.25 ± 0.70 |
Data are presented as median (25th percentile, 75th percentile) or mean ± standard deviation.
cCa, corrected calcium; iPTH, intact parathyroid hormone; VDRA, vitamin D receptor activator.
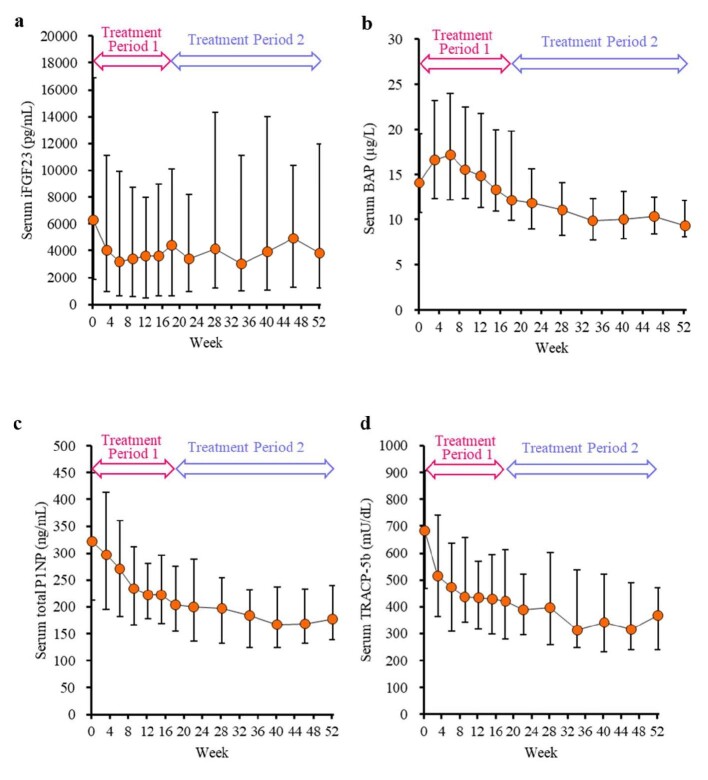
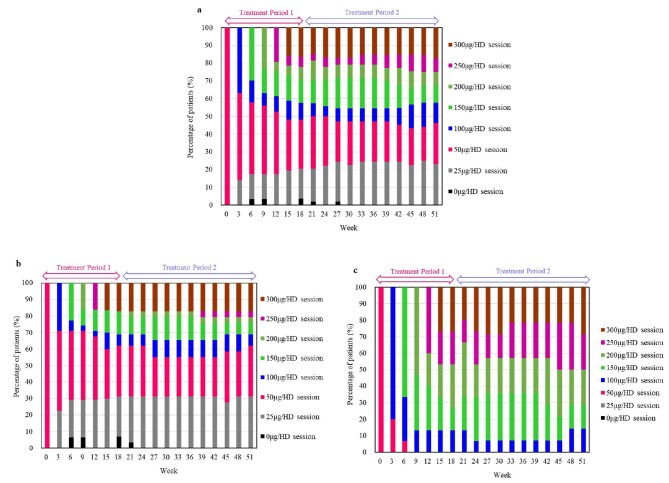
The serum iFGF23 level decreased (Fig. 5a). The serum BAP level once increased, but the level then decreased (Fig. 5b). The serum levels of total P1NP and TRACP-5b decreased (Fig. 5c and d). The upacicalcet dosage remained nearly steady after week 18 (Fig. 6). More patients with baseline serum iPTH >500 pg/mL were administered high-dose upacicalcet than those with baseline serum iPTH ranging from 240–500 pg/mL (Fig. 6b and c).
Figure 5:
Time course of changes in serum levels of iFGF23 (a), serum BAP (b), serum total P1NP (c), and serum TRACP-5b (d). Data are shown as median with interquartile range. BAP, bone alkaline phosphatase; iFGF23, intact fibroblast growth factor 23; P1NP, N-terminal propeptide of type I procollagen; TRACP-5b, tartrate-resistant acid phosphatase-5b.
Figure 6:
Time course of changes in upacicalcet dosing showing the percentage of all patients (a), those with baseline serum iPTH level ranging from 240–500 pg/mL (b), and those with baseline serum iPTH level >500 pg/mL (c). HD, haemodialysis; iPTH, intact parathyroid hormone.
Safety
AEs were recorded in 94.8% (55/58) of the participants and ADRs in 20.7% (12/58) (Table 4). ADRs were reported as decreased adjusted calcium in eight patients (13.8%), prolonged electrocardiogram QT interval in two patients (3.4%), and vomiting, dizziness, and Parkinson's disease in each one patient (1.7%). Dizziness was judged to be serious, whereas the other events were considered to be non-serious. ADRs that caused treatment discontinuation were vomiting and dizziness. Upper GI AEs were recorded in 18 (31.0%) patients. Of these, one patient was considered to have as an ADR (vomiting), as described above. The incidence of ADRs for decreased adjusted calcium was 10.9% (5/46) and 25.0% (3/12) for patients with and without VDRAs, respectively.
Table 4:
Adverse events and adverse drug reactions.
| AEs | ADRs | |
|---|---|---|
| Any AEs and ADRs | 55 (94.8) | 12 (20.7) |
| Serious AEs and ADRs | 10 (17.2) | 1 (1.7) |
| AEs and ADRs caused discontinuation of study | 3 (5.2) | 2 (3.4) |
| AEs and ADRs caused dosing reduction | 1 (1.7) | 1 (1.7) |
| AEs and ADRs caused interruption of administration | 7 (12.1) | 7 (12.1) |
| Any AEs and ADRs of special interest | ||
| Upper gastrointestinal symptoms | 18 (31.0) | 1 (1.7) |
| Nausea | 1 (1.7) | 0 (0.0) |
| Vomiting | 4 (6.9) | 1 (1.7) |
| Abdominal discomfort | 3 (5.2) | 0 (0.0) |
| Abdominal pain | 5 (8.6) | 0 (0.0) |
| Hypocalcaemia | 0 (0.0) | 0 (0.0) |
| Decreased adjusted calcium | 8 (13.8) | 8 (13.8) |
Number (%) of patients with adverse events
ADR, adverse drug reaction; AE, adverse event.
AEs were tabulated using the preferred terms specified in MedDRA v20.0.
The AEs and ADRs of special interest include upper gastrointestinal symptoms, hypocalcaemia, and decreased adjusted calcium.
Symptomatic hypocalcaemia was not reported. In nine patients, upacicalcet treatment was interrupted because their serum cCa level was <7.5 mg/dL. All of them had the serum cCa level <9.0 mg/dL at baseline. In seven of these patients, the treatment interruptions were within two weeks of starting treatment. After a one-week interruption, the serum cCa level elevated to ≥7.5 mg/dL in all of the patients. Eight patients had serum cCa level ≥8.4 mg/dL within three weeks after the interruption and resumed 25 μg of upacicalcet. Seven of these patients did not have a serum cCa level of <7.5 mg/dL again (Table 5). In all patients with baseline serum cCa level ≥9.0 mg/dL, no interruption due to decreases in the serum cCa level was observed, including in one patient who was judged as decreased adjusted calcium.
Table 5:
Summary of patients who had interrupted upacicalcet due to serum corrected calcium level of <7.5 mg/dL.
| Serum corrected calcium (mg/dL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient No. | Interruption week | Upacicalcet dose before interruption | Baseline | Interruption week | + 1-weeka | + 2-weeka | + 3-weeka | + 4-weeka | Resumed week (upacicalcet dose) |
| 1 | Week 1 | 50 μg | 8.9 | 7.3 | 8.4 | 8.5 | 8.0 | 7.7 | + 2-weeka, (25 μg) |
| 2 | Week 1 | 50 μg | 8.8 | 7.4 | 8.5 | 9.2 | 8.5 | 8.0 | + 2-weeka, (25 μg) |
| 3 | Week 1 | 50 μg | 8.9 | 7.4 | 8.4 | 8.6 | 8.0 | 7.8 | + 2-weeka, (25 μg) |
| 4 | Week 1 | 50 μg | 8.7 | 7.3 | 8.5 | 8.7 | 8.0 | 7.7 | + 2-weeka, (25 μg) |
| 5 | Week 1 | 50 μg | 8.4 | 7.3 | 8.7 | 8.6 | 7.8 | 7.9 | + 2-weeka, (25 μg) |
| 6 | Week 1 | 50 μg | 8.9 | 7.4 | 8.6 | 8.7 | 7.6 | 7.7 | + 2-weeka, (25 μg) |
| 7 | Week 2 | 50 μg | 8.4 | 7.2 | 7.9 | 8.3 | 8.2 | Discontinuation c | |
| 8 | Week 7 | 25 μg | 8.7 | 7.4 | 8.0 | 8.0 | 8.8 | 9.0 | + 3-weeka, (25 μg) |
| 9 | Week 5 | 50 μg | 8.4 | 7.4 | 8.1 | 8.6 | 8.1 | 8.0 | + 2-weeka, (25 μg) |
| Week 15 | 50 μg | 8.4 | 7.4 | 8.5 | 7.9 | 7.9 | –b | + 1-weeka, (25 μg) | |
Time points after interruption.
Not measured (for transition to Treatment Period 2).
Reason of discontinuation: investigator's decision.
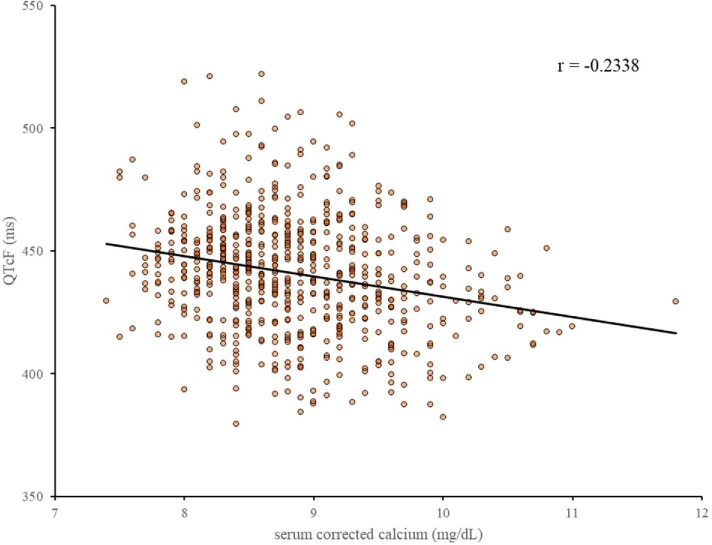
In the 12-lead electrocardiograms, the mean Fridericia's corrected QT (QTcF) was prolonged from 424.0 ± 20.7 at baseline to 439.1 ± 22.9 at week 52. Three AEs of prolonged electrocardiogram QT interval were reported, but all of them were non-serious. In addition, QTcF and serum cCa level showed a weak negative correlation (r, −0.2338) (Fig. 7).
Figure 7:
Correlation between QT interval corrected using the Fridericia method (QTcF) and serum cCa levels (Treatment Periods 1 and 2). cCa, corrected calcium.
DISCUSSION
This intraindividual dose-adjustment study showed that upacicalcet reduced the iPTH level to the target range and was safe and well-tolerated. The target achievement rate of serum iPTH level was as high as approximately 60% at week 18 and approximately 80% at week 52 (Fig. 3). These target achievement rates were similar to those in placebo-controlled and long-term studies on other calcimimetics in Japanese haemodialysis patients [15–17]. In addition, the iPTH level at week 52 was <240 pg/mL at all doses, even at 300 μg of upacicalcet (Table 2). In addition, 25 μg of upacicalcet lowered the iPTH level, but there were few interruptions due to an excessive decrease in PTH and cCa levels. Based on these results, it was considered appropriate to set the dose of upacicalcet at 25–300 μg.
In the present study, GI-related ADR was reported in only one patient. As expected [10], up to 300 μg of upacicalcet reduced the serum iPTH level without increasing the incidence of GI symptoms, previously observed as calcimimetics-related AEs [6–8, 18]. Therefore, upacicalcet may be beneficial for patients with GI symptoms using calcimimetics. In addition, the incidence of decreased adjusted calcium was 13.8% in the current study, similar to that observed with other calcimimetics [5, 19]. The incidence of decreased adjusted calcium in patients not treated with VDRAs was higher than in those treated with VDRAs. Therefore, it is considered that the combination of VDRAs with upacicalcet can prevent from the decreased serum calcium level. In patients who interrupted upacicalcet treatment due to a serum cCa level of <7.5 mg/dL, the serum cCa level recovered to ≥ 7.5 mg/dL after a one-week interruption (Table 5). Because upacicalcet, unlike etelcalcetide, is well removed by haemodialysis [10, 20, 21], the serum cCa level may have recovered after a one-week interruption. Mild and asymptomatic hypocalcaemia due to calcimimetics has been reported to be harmless [22, 23], whereas it was reported that severe hypocalcaemia is rare but can be fatal [24]. A position paper on CKD-MBD from the Italian Society of Nephrology reported that the best course of action in episodes of symptomatic hypocalcaemia might be dose adjustment of calcimimetics rather than calcium preparations or VDRAs [25]. Therefore, upacicalcet may be easy to be handed in episodes of severe hypocalcaemia although severe hypocalcaemia was not recognized in this study. All patients who interrupted upacicalcet due to a decrease in the cCa level had a baseline serum cCa level of <9.0 mg/dL. Furthermore, in most of these patients, the serum cCa level which decreased within two weeks of starting upacicalcet at 50 μg, recovered to >7.5 mg/dL after a one-week interruption, and did not drop below 7.5 mg/dL after resuming upacicalcet at 25 μg. In contrast, patients with a baseline serum cCa level of ≥9.0 mg/dL did not experience interruptions due to a decrease in the cCa level. Based on these results, we considered it appropriate to start 25 μg of upacicalcet if the serum cCa level was <9.0 mg/dL and 50 μg if the serum cCa level was ≥9.0 mg/dL. This starting dose may reduce the AE involving decreased adjusted calcium and prevent treatment interruption. Based on the above findings, comparative and long-term studies in phase 3 should be conducted at this starting dose with dose adjustments of 25–300 μg.
Upacicalcet decreased the serum TRACP-5b. In contrast, the serum level of BAP increased transiently and then decreased. The different responses of TRACP-5b and BAP are expected to increase bone mass [26]. This phenomenon is thought to contribute to improving bone metabolism in SHPT due to PTH reduction [27, 28]. Furthermore, calcimimetics have recently been reported to have a direct anabolic effect on bone [29]. Although the present study suggested that upacicalcet might improve in bone turnover, long-term studies are needed to determine if these responses result in increased bone mass.
This study has several limitations. This was a single-arm study involving a small number of patients. Furthermore, only Japanese patients were included. The target range of serum iPTH level in this study was 60–240 pg/mL, as recommended in the JSDT guidelines [13]. This target range is lower than that in other guidelines [22, 30]. In addition, AEs such as decreased adjusted calcium were judged by the investigator and not evaluated by a third party. Finally, the use of dialysate with a higher calcium concentration was more frequent in this study than in the United States, Europe, and other countries [31, 32]. Although we evaluated the long-term efficacy and safety of upacicalcet treatment, we need to compare it with a placebo and conduct a long-term study using a design that is more in line with clinical practice.
In conclusion, the present study showed that upacicalcet was effective for 18 weeks of treatment and displayed higher efficacy after 52 weeks in haemodialysis patients with SHPT. Throughout the study period, upacicalcet was well-tolerated, with few safety concerns. The decreased adjusted calcium due to upacicalcet administration was adequately managed by drug treatment interruption. Therefore, a starting dose of 25 or 50 μg of upacicalcet, depending on the serum cCa level was considered to be appropriate.
Supplementary Material
ACKNOWLEDGEMENTS
We appreciate the valuable contributions of the investigators, local study coordinators, and patients to this study. Sanwa Kagaku Kenkyusho Co., Ltd (SKK) was involved in collecting, managing, analysing, and interpreting the study data. We would like to thank Editage (www.editage.com) for English language editing.
Contributor Information
Daijo Inaguma, Department of Internal Medicine, Fujita Health University Bantane Hospital, Aichi, Japan.
Fumihiko Koiwa, Division of Nephrology, Department of Internal Medicine, Showa University Fujigaoka Hospital, Yokohama, Japan.
Masanori Tokumoto, Department of Nephrology, Japanese Red Cross Fukuoka Hospital, Fukuoka, Japan.
Masafumi Fukagawa, Division of Nephrology, Endocrinology, and Metabolism, Department of Internal Medicine, Tokai University School of Medicine, Kanagawa, Japan.
Shinji Yoneda, Medical Affairs Department, Sanwa Kagaku Kenkyusho Co., Ltd, Nagoya, Japan.
Hisami Yasuzawa, Medical Affairs Department, Sanwa Kagaku Kenkyusho Co., Ltd, Nagoya, Japan.
Kenji Asano, Clinical Development Department, Sanwa Kagaku Kenkyusho Co., Ltd, Nagoya, Japan.
Keiko Hagita, Clinical Development Department, Sanwa Kagaku Kenkyusho Co., Ltd, Nagoya, Japan.
Yosuke Inagaki, Clinical Development Department, Sanwa Kagaku Kenkyusho Co., Ltd, Nagoya, Japan.
Daisuke Honda, Project Management Department, Sanwa Kagaku Kenkyusho Co., Ltd, Nagoya, Japan.
Tadao Akizawa, Division of Nephrology, Department of Medicine, Showa University School of Medicine, Tokyo, Japan.
FUNDING
This study was funded by Sanwa Kagaku Kenkyusho Co., Ltd.
AUTHORS’ CONTRIBUTIONS
D.I., M.T., M.F., S.Y., H.Y., K.H., and Y.I. substantially contributed to the analysis and interpretation of the data. F.K., K.A., D.H., and T.A. substantially contributed to the study design and the acquisition and interpretation of the data. All authors critically revised the report, commented on drafts of the manuscript, and approved the final report.
DATA AVAILABILITY STATEMENT
The dataset from this study is not available in any open data repository.
CONFLICT OF INTEREST STATEMENT
D.I. has received personal fees from SKK, Kyowa Kirin, Kissei Pharmaceutical, and Ono Pharmaceutical. F.K. has received personal fees from SKK, Kyowa Kirin, and Ono Pharmaceutical. M.T. has received personal fees from SKK, Kyowa Kirin, AstraZeneca, Torii Pharmaceutical, Astellas Pharma, Kissei Pharmaceutical, Chugai Pharmaceutical, Mitsubishi Tanabe Pharma Corporation, Kowa, Bayer Yakuhin. M.F. has received personal fees from SKK, Kyowa Kirin, Bayer Japan, Kissei Pharmaceutical, Torii Pharmaceutical, Ono Pharmaceutical, and Astra Zeneca; grants from Kyowa Kirin and Ono Pharmaceutical. S.Y., H.Y., K.A., K.H., Y.I., and D.H. are employees of SKK. T.A. has received personal fees from SKK, Kyowa Kirin, Bayer, Astellas Pharma, GlaxoSmithKline, Japan Tobacco, Nipro, Torii Pharmaceutical, Kissei Pharmaceutical, Chugai Pharmaceutical, Mitsubishi Tanabe Pharma, Ono Pharmaceutical, and Fuso Pharmaceutical Industries.
REFERENCES
- 1. Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol 2011;6:913–21. 10.2215/CJN.06040710 [DOI] [PubMed] [Google Scholar]
- 2. Block GA, Klassen PS, Lazarus JM et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004;15:2208–18. 10.1097/01.ASN.0000133041.27682.A2 [DOI] [PubMed] [Google Scholar]
- 3. Jadoul M, Albert JM, Akiba T et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2006;70:1358–66. 10.1038/sj.ki.5001754 [DOI] [PubMed] [Google Scholar]
- 4. Fukuma S, Kurita N, Fukagawa M et al. Impact of Cinacalcet introduction on MBD management: the MBD-5D study in Japan. Kidney Int Suppl 2013;3:436–41. 10.1038/kisup.2013.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akizawa T, Ikejiri K, Kondo Y et al. Evocalcet: a new oral calcimimetic for dialysis patients with secondary hyperparathyroidism. Ther Apher Dial 2020;24:248–57. 10.1111/1744-9987.13434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Block GA, Bushinsky DA, Cheng S et al. Effect of Etelcalcetide vs Cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA 2017;317:156–64. 10.1001/jama.2016.19468 [DOI] [PubMed] [Google Scholar]
- 7. Palmer SC, Nistor I, Craig JC et al. Cinacalcet in patients with chronic kidney disease: a cumulative meta-analysis of randomized controlled trials. PLoS Med 2013;10:e1001436. 10.1371/journal.pmed.1001436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palmer SC, Mavridis D, Johnson DW et al. Comparative effectiveness of calcimimetic agents for secondary hyperparathyroidism in adults: a systematic review and network meta-analysis. Am J Kidney Dis 2020;76:321–30. 10.1053/j.ajkd.2020.02.439 [DOI] [PubMed] [Google Scholar]
- 9. Hoy SM. Upacicalcet: first approval. Drugs 2021;81:1593–6. 10.1007/s40265-021-01578-y [DOI] [PubMed] [Google Scholar]
- 10. Kazama JJ, Koiwa F, Yokoyama K et al. First-in-patient phase I/II study of Upacicalcet in Japanese patients with secondary hyperparathyroidism undergoing hemodialysis: pharmacokinetic and pharmacodynamic properties. Clin Pharmacokinet 2022;61:1271–84. 10.1007/s40262-022-01139-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koiwa F, Yazawa R, Fukagawa M et al. First-in-human phase I study of the novel injectable calcimimetic agent Upacicalcet in healthy adult Japanese participants. Drugs R D 2022;22:131–40. 10.1007/s40268-022-00385-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sato H, Murakami S, Horii Y et al. Upacicalcet is a novel secondary hyperparathyroidism drug that targets the amino acid binding site of calcium-sensing receptor. Mol Pharmacol 2022;102:183–95. 10.1124/molpharm.122.000522 [DOI] [PubMed] [Google Scholar]
- 13. Fukagawa M, Yokoyama K, Koiwa F et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial 2013;17:247–88. 10.1111/1744-9987.12058 [DOI] [PubMed] [Google Scholar]
- 14. Payne RB, Little AJ, Williams RB et al. Interpretation of serum calcium in patients with abnormal serum proteins. Br Med J 1973;4:643–6. 10.1136/bmj.4.5893.643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fukagawa M, Yokoyama K, Shigematsu T et al. A phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of etelcalcetide (ONO-5163/AMG 416), a novel intravenous calcimimetic, for secondary hyperparathyroidism in Japanese haemodialysis patients. Nephrol Dial Transplant 2017;32:1723–30. 10.1093/ndt/gfw408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shigematsu T, Fukagawa M, Yokoyama K et al. Long-term effects of etelcalcetide as intravenous calcimimetic therapy in hemodialysis patients with secondary hyperparathyroidism. Clin Exp Nephrol 2018;22:426–36. 10.1007/s10157-017-1442-5 [DOI] [PubMed] [Google Scholar]
- 17. Yokoyama K, Shimazaki R, Fukagawa M et al. , Evocalcet Study Group . Long-term efficacy and safety of Evocalcet in Japanese patients with secondary hyperparathyroidism receiving hemodialysis. Sci Rep 2019;9:6410. 10.1038/s41598-019-42017-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fukagawa M, Yumita S, Akizawa T et al. Cinacalcet (KRN1493) effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patients. Nephrol Dial Transplant 2008;23:328–35. 10.1093/ndt/gfm534 [DOI] [PubMed] [Google Scholar]
- 19. Yokoyama K, Fukagawa M, Shigematsu T et al. A 12-week dose-escalating study of etelcalcetide (ONO-5163/AMG 416), a novel intravenous calcimimetic, for secondary hyperparathyroidism in Japanese hemodialysis patients. Clin Nephrol 2017;88:68–78. 10.5414/CN108974 [DOI] [PubMed] [Google Scholar]
- 20. Yokoyama K, Fukagawa M, Shigematsu T et al. A single- and multiple-dose, multicenter study of Etelcalcetide in Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int Rep 2017;2:634–44. 10.1016/j.ekir.2017.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Subramanian R, Zhu X, Hock MB et al. Pharmacokinetics, biotransformation, and excretion of [14C]Etelcalcetide (AMG 416) following a single microtracer intravenous dose in patients with chronic kidney disease on hemodialysis. Clin Pharmacokinet 2017;56:179–92. 10.1007/s40262-016-0433-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group . KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2017;7:1–59. 10.1016/j.kisu.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Floege J, Tsirtsonis K, Iles J et al. Incidence, predictors and therapeutic consequences of hypocalcemia in patients treated with Cinacalcet in the EVOLVE trial. Kidney Int 2018;93:1475–82. 10.1016/j.kint.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 24. Itano Y, Kato S, Tsuboi M et al. A prospective, randomized clinical trial of Etelcalcetide in patients receiving hemodialysis with secondary hyperparathyroidism (the DUET trial). Kidney Int Rep 2020;5:2168–77. 10.1016/j.ekir.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bellasi A, Cozzolino M, Malberti F et al. New scenarios in secondary hyperparathyroidism: etelcalcetide. Position paper of working group on CKD-MBD of the Italian Society of Nephrology. J Nephrol 2020;33:211–21. 10.1007/s40620-019-00677-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tabacco G, Bilezikian JP. Osteoanabolic and dual action drugs. Br J Clin Pharmacol 2019;85:1084–94. 10.1111/bcp.13766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shigematsu T, Akizawa T, Uchida E et al. Long-term Cinacalcet HCl treatment improved bone metabolism in japanese hemodialysis patients with secondary hyperparathyroidism. Am J Nephrol 2009;29:230–6. 10.1159/000156717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shigematsu T, Fukagawa M, Yokoyama K et al. Effects of the intravenous calcimimetic etelcalcetide on bone turnover and serum fibroblast growth factor 23: post hoc analysis of an open-label study. Clin Ther 2018;40:2099–111. 10.1016/j.clinthera.2018.10.016 [DOI] [PubMed] [Google Scholar]
- 29. Díaz-Tocados JM, Rodríguez-Ortiz ME, Almadén Y et al. Calcimimetics maintain bone turnover in uremic rats despite the concomitant decrease in parathyroid hormone concentration. Kidney Int 2019;95:1064–78. 10.1016/j.kint.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 30. National Kidney Foundation . K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42:S1–S201. 10.1016/S0272-6386(03)00905-3 [DOI] [PubMed] [Google Scholar]
- 31. Block GA, Bushinsky DA, Cunningham J et al. Effect of Etelcalcetide vs Placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA 2017;317:146–55. 10.1001/jama.2016.19456 [DOI] [PubMed] [Google Scholar]
- 32. Louie KS, Erhard C, Wheeler DC et al. Cinacalcet-induced hypocalcemia in a cohort of European haemodialysis patients: predictors, therapeutic approaches and outcomes. J Nephrol 2020;33:803–16. 10.1007/s40620-019-00686-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset from this study is not available in any open data repository.