ABSTRACT
Throughout the history of nephrology, little attention has been paid to the sex and gender differences in kidney disease. This lack of awareness prevents optimal diagnosis and management of kidney disease. In today's world of precision medicine, it is imperative to appreciate the differential factors regarding gender and kidney disease.
This editorial summarizes the up-to-date literature regarding sex and gender differences in kidney disease and considers areas where knowledge is incomplete and where further research is needed. We address sex-specific effects on chronic kidney disease epidemiology; risks of dialysis underdosing and medication overdosing in women; unexplained loss of female sex advantage in life expectancy during dialysis, and impact of sex on diagnosis and management of genetic kidney disease. We also aim to highlight the impact of gender on kidney health and raise awareness of disparities that may be faced by women, and transgender and gender-diverse persons when a male-model approach is used by healthcare systems. By understanding the link between sex and kidney disease, kidney specialists can improve the care and outcomes of their patients. In addition, research on this topic can inform the development of targeted prevention and intervention strategies that address the specific needs and risk factors of different populations.
Keywords: dialysis, disparity, gender, pharmacokinetics, women
INTRODUCTION
Although women and men share nearly identical genetic information, their phenotypes are distinct. Women may react differently to treatment, may manifest disease symptoms in a different way and may have profoundly different needs from a social or cultural perspective. In 1991, McMurray et al. claimed gender disparities in clinical decision-making and pointed to the fact that medical treatments for women are based on the male model, regardless of biological differences between sexes [1].
In the modern era, biological (sex) and sociocultural (gender) conditioning are readily separated although both may fundamentally modulate healthcare needs and outcomes. The term sex is the one most established in terms of pre-clinical and clinical research. Sex influences choice, efficacy and outcome of therapy in many clinical areas (Table 1). This is a consequence of sex-specific effects of genetic polymorphisms, different function of ion channels in heart or kidney, variation in body composition, and sex hormones’ influence on pharmacokinetics and pharmacodynamics [2].
Table 1:
Examples of known therapeutical areas influenced by sex.
| Therapeutic area | Examples of differences | References |
|---|---|---|
| Cardiovascular disease | Influence of sex hormones on regulation β-adrenergic receptors in cardiovascular system | [49, 50, 51] |
| Increased hypotensive action of β-blockers in women | ||
| Higher incidence of cough after ACEi in women | ||
| Anticoagulation and antithrombotic treatment | The bioavailability of acetylsalicylic acid is greater but platelet inhibition is lower in women | [52, 53] |
| Antiarrhythmic | Influence of sex hormones on the length of QT | [54, 55] |
| Increased risk of Torsade de Pointes in women | ||
| Higher risk of tachycardia in women | ||
| Pain and anesthesia | Women tend to experience more severely chronic pain | [56, 57] |
| Depressive disorders | Sex hormones influence the pharmacokinetic of antidepressants | [58] |
| More adverse events after TCA in women | ||
| Oncologic diseases | Checkpoint inhibitors are less beneficial in women | [59] |
| COPD | Better response to anticholinergic bronchodilators due to greater expression of M2 over M3 muscarinic receptors in women | [60] |
| Anti-inflammatory therapy | Higher risk of serious infection during biological treatment in men | [61] |
| Anti-viral therapy | Humoral immune response after vaccination higher in women | [62, 63] |
| More adverse effects and toxicity of anti-viral drugs in women | ||
| Thyroid disorders | Thyroxine requirements are higher in men | [64] |
COPD: chronic obstructive pulmonary disease; TCA: tricyclic antidepressant.
Understanding those processes and utilizing the knowledge of sex differences warrants enhanced therapeutic effectiveness and minimizes drug side effects (Table 2). Based on this knowledge one may expect that women need higher initial doses of calcineurin inhibitors per kilogram of body weight, have lower probability of developing severe infection after biological therapy and experience greater suppressive effects of cortisol. Nevertheless, the relevance and consequences of these hypotheses are largely unknown.
Table 2:
Mechanisms underlying sex differences in pharmacokinetics and pharmacodynamics of drugs—adapted from [2].
| Pharmacokinetics | Absorption | Gastric enzymes |
| Gut motility | ||
| Microbiota | ||
| Transporting proteins | ||
| Distribution | Body composition | |
| Cardiac output | ||
| Organ blood flow | ||
| Liver metabolism | CYP3A4, CYP2D6, CYP2C19, CYP1A2 | |
| Elimination | Kidney clearance | |
| Liver function | ||
| Pulmonary expiration | ||
| Plasma proteins concentration | ||
| Pharmacodynamics | Sex-specific conditions | Contraception |
| Pregnancy | ||
| Menopause |
CYP3A4, CYP2D6, CYP2C19, CYP1A2: cytochrome P 450 isoenzymes named according to their coding chromosomes (activity in women CYP3A4 ↓, CYP2D6↑, CYP2C19↑, CYP1A2↑).
AWARENESS OF SEX DIFFERENCES IN NEPHROLOGY
Sex differences have not been extensively explored in nephrology. In 2018, World Kidney Day coincided with International Women's Day and led to increased awareness of women's health by publishing a document entitled ‘Women and Kidney Diseases: Questions Unanswered and Answers Unquestioned’ [3]. That initiative paved the road towards incorporating sex disparities in nephrology into the research agenda at both basic and clinical levels. Nevertheless, kidney disease is misidentified as not being influenced by sex or gender by 44.1% of 1323 European internists who were surveyed by the Internal Medicine Assessment of Gender differences in Europe (IMAGINE) working group [4].
In February 2023 the first KDIGO Controversies Conference on Women and Kidney Health took place in Athens. A multidisciplinary team involving nephrologists, obstetricians, specialists in reproductive health and patients, amongst others, came together with the primary aim of identifying gender and sex issues in kidney care. There was a strong focus on improving the reproductive care of women with established chronic kidney disease (CKD) and women who develop hypertensive disorders of pregnancy or pregnancy-related acute kidney injury (AKI). Ultimately, this KDIGO meeting sought to describe current best practice, to identify areas of uncertainty and controversial issues, and to outline essential areas of research required to improve the management of women with CKD [5].
How do men and women differ? Sex associated differences of the kidney
Sex differences in kidney structure and function are known across species. Kidney mass, including volume of cortex, is greater in males in humans and rodents [6], while the size of medulla and length of thick ascending limbs prevails in female rats [7]. Interestingly, the latter dimorphism does not translate into better concentration capacity, which is greater in males [7]. This could be explained by the higher (up to 80%) expression of aquaporin 1 in male kidneys. Sex differences have also been described in other transporters. For example, expression of sodium-glucose cotransporter 2 is higher in kidneys of female rats [6].
Haemodynamics
Kidney haemodynamics may also differ across sexes, with higher glomerular vascular resistance reported in female rodents which presumably explains greater urinary protein excretion in males [8, 9]. Other functional differences include the role of nitric oxide (NO), which is higher in pre-menopausal women than in men, related to increased expression of endothelial NO synthase activity [10]. NO contributes to kidney hemodynamics, regulating the medullary blood flow, pressure natriuresis, tubulo-glomerular feedback and sympathetic system activity [11, 12].
Sex hormones
Furthermore, sex steroid hormones are believed to play a critical role in aggravation or inhibition of kidney damage [13]. In experimental models of polycystic kidney disease (PKD) or ischaemic–reperfusion kidney injury, oestrogens delayed processes of apoptosis and fibrosis [14]. In humans, the course of immunoglobulin A nephropathy, membranous nephropathy or PKD is more aggressive in men [14]. In general, testosterone is believed to increase oxidative stress and activate renin–angiotensin system (RAS) while oestrogens exhibit renoprotective effects [13, 14]. Counterintuitively, the use of oestrogen–progesterone oral contraception seems to be a risk factor for CKD progression, pointing to the fact that there are many gaps in our understanding of those processes and their clinical importance is largely unknown.
Chronic kidney disease
The recent analysis the SCREAM (Stockholm Creatinine Measurement from outpatient care project) cohort (n = 227 847; 45% men) revealed sex differences in detection, recognition, monitoring and treatment of CKD [15]. They discriminated against women, persisted over time and were difficult to explain [15]. To some extent, this knowledge is not new. The paradox of women experiencing a higher prevalence of CKD, yet being less likely to be treated with dialysis or kidney transplantation has been found repeatedly and in diverse geographical locations [16, 17]. For many years, researchers were puzzled by this finding and hypothesized that formulas estimating glomerular filtration rate (eGFR) may overestimate CKD in women, or that kidney disease may progress more slowly in women [18]. Recently, the latter theory has been confirmed in a study that measured GFR decline with the use of iohexol clearance (Renal Iohexol Clearance Survey, Norway) and found it to be 25% steeper in men [19]. As the rate of kidney function decline does not fully explain the observed sex differences in epidemiology, cultural and social factors have been highlighted as possible contributors. This suggests that the differences may in part be explained by men having better education, higher income, better access to healthcare facilities and improved health literacy. However, this seems improbable given that the observed differences are reproducible across all geographical regions and are stable over decades of profound social change [20]. Interestingly, CKD is repeatedly reported among the 10 leading causes of death for women but not for men [21]. Thus, it is conceivable that biological sex does indeed contribute to the pathogenesis of kidney disease.
THE EXTENT OF THE PROBLEM
In the era of precision and personalized medicine, the obvious biological difference between sexes should not be ignored. The known differences regarding sex and kidney disease are summarized in Table 3.
Table 3:
Established facts about sex and gender differences in kidney diseases.
| Characteristic in women | Supporting evidence |
|---|---|
| Higher prevalence of CKD | Carrero et al., Nat Rev Nephrol 2018 [17] |
| Melsom et al., J Am Soc Nephrol 2022 [19] | |
| Bikbov et al., Nephron 2018 [16] | |
| Autoimmune diseases targeting kidneys are more prevalent | Piccoli et al., Kidney Int Rep 2018 [3] |
| Lower probability of testing for albuminuria | Bello et al., JAMA Netw Open 2019 [22] |
| Less common initiation of ACEi/ARB | Qiao et al., Hypertension 2020 [23] |
| Pregnancy associated complications including CKD | Zhang et al., Clin J Am Soc Nephrol 2015 [65] |
| GFR decline is slower | Melsom et al., J Am Soc Nephrol 2022 [19] |
| Lower eGFR at referral | John et al., Am J Kidney Dis 2004 [66] |
| Lower number of women starting KRT | Carrero et al., Nat Rev Nephrol 2018 [17] |
| Higher mortality on dialysis | De La Mata et al., BMJ 2021 [29] |
| Chen et al., Perit Dial Int 2021 [67] | |
| Carrero et al., Clin J Am Soc Nephrol 2011 [31] | |
| Lower probability to have an arteriovenous fistula as a vascular access | Shah et al., Am J Nephrol 2018 [68] |
| Markell et al., Hemodial Int 2018 [35] | |
| Higher risk of peritonitis on PD | Kotsanas et al., Nephrology (Carlton) 2007 [69] |
| Higher risk of encapsulating peritoneal sclerosis | Guest et al., Perit Dial Int 2009 [70] |
| Higher ESA demands on dialysis | Ryta et al., Int Urol Nephrol 2017 [33] |
| Higher incidence of depression and restless leg syndrome | Guglielmi, Adv Chronic Kidney Dis 2013 [71] |
| Gitto et al., Int J Health Policy Manag 2015 [72] | |
| Merlino et al., Neurol Sci 2012 [73] | |
| Lower dialysis adequacy | Lowrie et al., Kidney Int 2004 [74] |
| Depner et al., Kidney Int 2004 [75] | |
| Lower probability to receive kidney transplant | Wolfe et al., Am J Kidney Dis 2000 [76] |
| Higher probability to become a living kidney donor | Gill et al., J Am Soc Nephrol 2018 [77] |
PD: peritoneal dialysis.
Standard of care
Stratification by sex revealed that CKD care was more likely to conform to recommendations for men than for women in testing, monitoring of kidney function and use of recommended medications [22]. RAS inhibitors (RASi) are less frequently prescribed to eligible women [odds ratio (OR) 0.89, 95% confidence interval (CI) 0.83–0.94] [22, 23]. The rationale for the underutilization of angiotensin converting enzyme inhibitors/angiotensin-receptor blocker (ACEi/ARB) could in part be ascribed to less frequent urinary/creatinine albumin ratio testing (OR 0.93, 95% CI 0.91–0.96) [22], but could also be explained by the fact that these medications are contraindicated in women of childbearing potential without reliable contraception [24, 25]. Alternatively, some women may not get prescribed these medicines at all due to indecisiveness as regards future pregnancy, or may experience a higher burden of side effects. Additionally, even higher disproportion exists in non-prescribing statins between sexes (OR 0.90, 95% CI 0.87–0.93 for eligible men vs women) [23].
The use of combined oestrogen–progesterone oral contraceptive pills can be associated with a significant increase in blood pressure and albuminuria as well as kidney function decline [26–28], and for that reason it seems that women of childbearing age may be trapped in a vicious circle. For many of them, non-initiation of RASi is the comfortable or ‘easiest’ option. This ambiguity applies also to initiation hesitancy and premature cessation of other crucial medications, for example mycophenolate mofetil and tolvaptan. For the latter, a demand for reliable contraception can confuse certain patients who may have previously been told to avoid oestrogens due to the risk of triggering lupus flare or progression of liver cystic disease.
Mortality
Although the life expectancy of women in the general population exceeds that of men, this does not hold true in kidney replacement therapy (KRT) patients. Data from the Australian and New Zealand Dialysis and Transplant Registry (ANZDATA) show that standardized mortality rate compared with the general population is higher in women on dialysis than in men [29]. Women have 11% (95% CI 11.2–11.5) higher excess mortality at any given time, and on average had four more years of life lost than their male counterparts [29]. The excess mortality in women were amplified in those who were younger [29]. The detailed analysis of the same dataset revealed a higher risk of all-cause mortality was driven by higher mortality from infections and dialysis withdrawals [30].
The loss of the survival advantage of women on dialysis was observed also in comparison of mortality rate in the European incident dialysis patients from European Renal Association Registry with the European general population (Eurostat) [31]. Again, the difference was attributed to increased non-cardiovascular mortality in women [31].
Finally, the mortality risk was higher in women versus men among kidney graft recipients of all ages in three large transplant databases [32].
Disparity in kidney replacement therapy
There is a risk of overestimating dialysis adequacy in women. Men have greater total body water volume (V) than women with the same body surface area. This occurs because woman have proportionally greater total body fat mass, thus for a given weight, the male V is larger than the female V. However, there is no provision for adjusting Kt/V targets accordingly.
Furthermore, the same haemoglobin targets are used in both sexes, risking overexposure of erythropoietin-stimulating agent (ESA) in women [33]. Indeed, the prescription of ESA per kg body weight has been reported to be significantly higher in women than men.
Women with CKD have a 2-fold increase in bone fractures, which does not translate into sex-specific vigilance in guidelines on CKD–metabolic bone disease or osteoporosis [34].
Any of these examples may be enough to explain why mortality rates on dialysis are higher in women than men. Unfortunately, there are many other explanations, including less frequent use of arterio-venous fistulas (AVF) (OR 0.69, 95% CI 0.67–0.71) [35], higher incidence of depression and overall very poor quality of life (Table 3). Women are arbitrarily disqualified from AVF procedures due to misconception that vessel diameter is smaller, overlooking that outcomes are equally successful for both sexes [36].
Possibly due to cultural and social conditioning, women choose conservative treatment of CKD much more frequently than their male counterparts and are less likely to receive a kidney transplant. On the other hand, women are more likely to donate a kidney, even although the outcomes of transplantation of sex-mismatched kidneys seems less optimal [37, 38]. The mortality risk seems to be higher in women receiving a kidney transplant from men [32].
Inherited kidney diseases
Some ambivalence exists when considering inherited kidney diseases. For many years, women with Alport syndrome or Fabry disease were carelessly referred to as ‘carriers’. It has only recently been acknowledged that a significant percentage of these ‘carriers’ may eventually need KRT and deserve careful medical surveillance [39, 40]. Additionally, women with X-linked kidney diseases are also underdiagnosed. The algorithms used to diagnose rare conditions based on clinical traits rely on male symptoms which are more severe and of earlier onset. Therefore these tools perform very poorly for making a diagnosis in women with X-linked diseases [41].
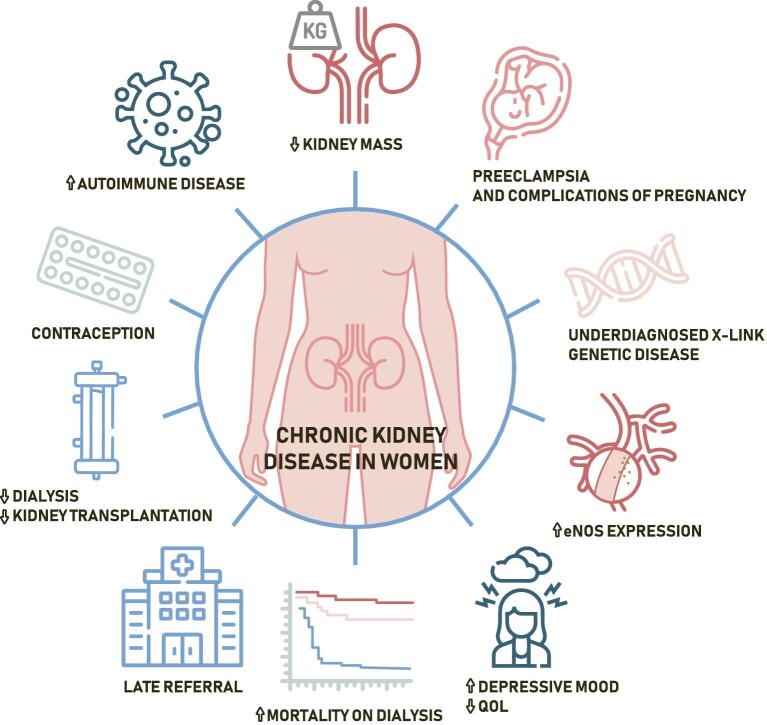
Figure 1:
Chronic kidney disease in women.
Complications of pregnancy
Pregnancy is the most prevalent cause of AKI in women of childbearing age and remains the leading cause of maternal morbidity and mortality [42]. There is a wide variety of pregnancy-specific causes of AKI that comprises, among others:
hyperemesis gravidarum
preeclampsia, HELLP (haemolysis, elevated liver enzymes and low platelets syndrome), TTP (thrombotic thrombocytopenic purpura) and hemolytic uremic syndrome
septic abortion
placenta abruption
Pregnancy-related AKI is associated with adverse fetal outcomes, increased mortality and prolonged hospital stay, but also with higher risk of cardiovascular events [43]. AKI preceding pregnancy increases the risk of preeclampsia and pre-term birth [42], while pregnancy-associated AKI and hypertensive disorders of pregnancy are risk factors for CKD [44, 45]. The latter finding should warrant CKD screening in women with pregnancy complications in medical history.
Gender matters
Focusing on biological aspects of sex divergence should not overshadow the gender construct, which plays a role in maintaining social, economic and cultural barriers. This is reflected in suboptimal health of transgender and gender diverse individuals [46]. In a recent analysis of University of Alabama database, the prevalence of CKD and AKI in a transgender cohort was as high as 36% and 32% [47]. This could be attributed to side effects of treatment with gender-affirming hormonal therapy but likely also to stress, discriminatory policies and unmet healthcare needs, particularly secondary to the lack of awareness amongst medical professionals.
There are a few specific aspects that nephrologists should be aware of:
the prevalence of AKI and CKD in transgender population is higher than in their cisgender counterparts [47]
eGFR measures may be subject to bias due to changed muscle mass after initiation of hormone therapy which could be critical in qualification for kidney donation
testosterone administration may have deleterious kidney effects
there is an increased thromboembolic risk of oestrogen therapy
spironolactone is often used as adjunctive anti-androgenic therapy and carries a risk of several side effects [48]
WHICH QUESTIONS ARE UNANSWERED AND WHAT SOLUTIONS COULD BE PROPOSED?
Mechanisms and medical consequences of differences between men and women receive increased attention among nephrologists. There are several potentially important sex-associated risk factors for CKD that are not modifiable (kidney mass, sex hormones and NO production) but there are modifiable risk factors too, including complications of pregnancy and poor health-related quality of life (HRQOL).
Regardless, crucial questions remain unanswered:
Are there any sex differences in responses to kidney therapeutic measures?
Should we make dose adjustments according to sex?
Should we provide gender oriented psychological support?
How can we use collected knowledge in guidelines?
What can we do to avoid discrimination and prevent disparities?
Despite the complexity of the issue, we should be much more proactive in identifying solutions for the benefit of patients:
nephrologists could gain from including some of the presented points into training curricula
unraveling the role of physiological differences between sexes should receive higher priority in basic science studies
sex-based equity in planned clinical trials may be achieved, by promoting recruitment of women, avoiding women-specific exclusion criteria and addressing barriers that affect women
editorial boards of scientific journals may require sex-stratified analysis before considering accepting articles for publication
every effort should be made to analyse existing evidence for differences in outcomes between men and women
task force of could be set-up for generating best practice guidelines
CONCLUSIONS
Sex differences in nephrology are vastly underexplored. Our main focus was to highlight this issue. As researchers and clinicians, we need to be aware of the potential bias and should be able to offer our patients the best healthcare to meet their individual needs. That must include personalization of therapies by taking account of sex and gender. Promoting the representation of women in clinical trials, increasing awareness of sex and gender disparities, improving pregnancy care, and performing sex-stratified analyses of existing and future studies might be effective tools in attaining this goal. It is plausible that exploring hypotheses and seeking answers could be time consuming and difficult to achieve in this complex field blurred by many confounders. This does not mean that we should not start.
Contributor Information
Magdalena Jankowska, Department of Nephrology, Transplantology and Internal Medicine, Faculty of Medicine, Medical University of Gdansk, Gdańsk, Poland.
María José Soler, Department of Nephrology, Vall d'Hebron Hospital Universitari, Vall d'Hebron Barcelona Hospital Campus, Nephrology and Transplantation Group, Vall d'Hebron Institut de Recerca (VHIR), Barcelona, Spain.
Kate I Stevens, The Renal and Transplant Unit, Queen Elizabeth University Hospital, Glasgow, UK.
Roser Torra, Inherited Kidney Diseases, Nephrology Department, Fundació Puigvert, Institut d'Investigacions Biomèdiques Sant Pau (IIB-Sant Pau), Universitat Autónoma de Barcelona, Barcelona, Spain.
DATA AVAILABILITY STATEMENT
No new data were generated or analysed in support of this research.
CONFLICT OF INTEREST STATEMENT
M.J.S. is Editor Emeritus of CKJ and reports honorarium for conferences, consulting fees and advisory boards from AstraZeneca, NovoNordsik, Esteve, Vifor, Bayer, Mundipharma, Ingelheim Lilly, Jansen, ICU Medical, Fresenius, Travere therapeutics and Boehringer. M.J. reports a grant (2018/30/M/NZ5/00 480) from the National Science Centre, Poland and honorarium for lectures from Chiesi, Fresenius Kabi and Swixx. R.T.’s research is funded by Fundació la Marató de TV3 202036-30, Instituto de Salud Carlos III PI 22/0031, RICORS40, Instituto de Salud Carlos III, Funded by EU-Next Generation, Mechanism of recuperation and resilience (MRR). K.I.S. is member of the CKJ editorial board.
REFERENCES
- 1. Gender disparities in clinical decision making. Council on Ethical and Judicial Affairs, American Medical Association. JAMA 1991;266:559–62. [PubMed] [Google Scholar]
- 2. Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet 2009;48:143–57. 10.2165/00003088-200948030-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piccoli GB, Alrukhaimi M, Liu ZH et al. Women and kidney diseases: questions unanswered and answers unquestioned. Kidney Int Rep 2018;3:225–35. 10.1016/j.ekir.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biskup E, Marra AM, Ambrosino I et al. Awareness of sex and gender dimensions among physicians: the European federation of internal medicine assessment of gender differences in Europe (EFIM-IMAGINE) survey. Intern Emerg Med 2022;17:1395–404. 10.1007/s11739-022-02951-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. KDIGO Controversies Conference on Women and Kidney Health. https://kdigo.org/conferences/controversies-conference-on-womens-kidney-health-issues/ (13 February 2023, date last accessed). [Google Scholar]
- 6. Bairey Merz CN, Dember LM, Ingelfinger JR et al. Sex and the kidneys: current understanding and research opportunities. Nat Rev Nephrol 2019;15:776–83. 10.1038/s41581-019-0208-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oudar O, Elger M, Bankir L et al. Differences in rat kidney morphology between males, females and testosterone-treated females. Ren Physiol Biochem 1991;14:92–102. [DOI] [PubMed] [Google Scholar]
- 8. Remuzzi A, Puntorieri S, Mazzoleni A et al. Sex related differences in glomerular ultrafiltration and proteinuria in Munich-Wistar rats. Kidney Int 1988;34:481–6. 10.1038/ki.1988.206 [DOI] [PubMed] [Google Scholar]
- 9. Halbesma N, Brantsma AH, Bakker SJ et al. Gender differences in predictors of the decline of renal function in the general population. Kidney Int 2008;74:505–12. 10.1038/ki.2008.200 [DOI] [PubMed] [Google Scholar]
- 10. Neugarten J, Ding Q, Friedman A et al. Sex hormones and renal nitric oxide synthases. J Am Soc Nephrol 1997;8:1240–6. 10.1681/ASN.V881240 [DOI] [PubMed] [Google Scholar]
- 11. Kone BC. Nitric oxide synthesis in the kidney: isoforms, biosynthesis, and functions in health. Semin Nephrol 2004;24:299–315. 10.1016/j.semnephrol.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 12. Baylis C. Sexual dimorphism of the aging kidney: role of nitric oxide deficiency. Physiology (Bethesda) 2008;23:142–50. [DOI] [PubMed] [Google Scholar]
- 13. Mauvais-Jarvis F, Bairey MN, Barnes PJ et al. Sex and gender: modifiers of health, disease, and medicine. Lancet North Am Ed 2020;396:565–82. 10.1016/S0140-6736(20)31561-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valdivielso JM, Jacobs-Cachá C, Soler MJ. Sex hormones and their influence on chronic kidney disease. Curr Opin Nephrol Hypertens 2019;28:1–9. 10.1097/MNH.0000000000000463 [DOI] [PubMed] [Google Scholar]
- 15. Swartling O, Yang Y, Clase CM et al. Sex differences in the recognition, monitoring, and management of CKD in health care: an observational cohort study. J Am Soc Nephrol 2022;33:1903–14. 10.1681/ASN.2022030373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bikbov B, Perico N, Remuzzi G et al. Disparities in chronic kidney disease prevalence among males and females in 195 countries: analysis of the Global Burden of Disease 2016 Study. Nephron 2018;139:313–8. 10.1159/000489897 [DOI] [PubMed] [Google Scholar]
- 17. Carrero JJ, Hecking M, Chesnaye NC et al. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 2018;14:151–64. 10.1038/nrneph.2017.181 [DOI] [PubMed] [Google Scholar]
- 18. Tomlinson LA, Clase CM. Sex and the incidence and prevalence of kidney disease. Clin J Am Soc Nephrol 2019;14:1557–9. 10.2215/CJN.11030919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Melsom T, Norvik JV, Enoksen IT et al. Sex differences in age-related loss of kidney function. J Am Soc Nephrol 2022;33:1891–902. 10.1681/ASN.2022030323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Antlanger M, Noordzij M, van de Luijtgaarden M et al. Sex differences in kidney replacement therapy initiation and maintenance. Clin J Am Soc Nephrol 2019;14:1616–25. 10.2215/CJN.04400419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heron M. Deaths: leading causes for 2018. Natl Vital Stat Rep 2021;70:1–115. [PubMed] [Google Scholar]
- 22. Bello AK, Ronksley PE, Tangri N et al. Quality of chronic kidney disease management in Canadian primary care. JAMA Netw Open 2019;2:e1910704. 10.1001/jamanetworkopen.2019.10704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qiao Y, Shin JI, Chen TK et al. Association of albuminuria levels with the prescription of renin-angiotensin system blockade. Hypertension 2020;76:1762–8. 10.1161/HYPERTENSIONAHA.120.15956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williams B, Mancia G, Spiering W et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). G Ital Cardiol (Rome) 2018;19(11 Suppl 1):3S–73S. [DOI] [PubMed] [Google Scholar]
- 25. Hypertension in pregnancy: diagnosis and management. London: National Institute for Health and Care Excellence (NICE). 2019. [PubMed] [Google Scholar]
- 26. Cífková R, Strilchuk L. Sex differences in hypertension. Do we need a sex-specific guideline? Front Cardiovasc Med 2022;9:960336. 10.3389/fcvm.2022.960336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Monster TB, Janssen WM, de Jong PE et al. Oral contraceptive use and hormone replacement therapy are associated with microalbuminuria. Arch Intern Med 2001;161:2000–5. 10.1001/archinte.161.16.2000 [DOI] [PubMed] [Google Scholar]
- 28. Attini R, Cabiddu G, Montersino B et al. Contraception in chronic kidney disease: a best practice position statement by the Kidney and Pregnancy Group of the Italian Society of Nephrology. J Nephrol 2020;33:1343–59. 10.1007/s40620-020-00717-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De La Mata NL, Rosales B, MacLeod G et al. Sex differences in mortality among binational cohort of people with chronic kidney disease: population based data linkage study. BMJ 2021;375:e068247. 10.1136/BMJ-2021-068247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lim WH, Chen JHC, Minas K et al. Sex disparity in cause-specific and all-cause mortality among incident dialysis patients. Am J Kidney Dis 2023;81:156–67.e151. 10.1053/j.ajkd.2022.07.007 [DOI] [PubMed] [Google Scholar]
- 31. Carrero JJ, de Jager DJ, Verduijn M et al. Cardiovascular and noncardiovascular mortality among men and women starting dialysis. Clin J Am Soc Nephrol 2011;6:1722–30. 10.2215/CJN.11331210 [DOI] [PubMed] [Google Scholar]
- 32. Vinson AJ, Zhang X, Dahhou M et al. A multinational cohort study uncovered sex differences in excess mortality after kidney transplant. Kidney Int 2023;103:1131–43. 10.1016/j.kint.2023.01.022 [DOI] [PubMed] [Google Scholar]
- 33. Ryta A, Chmielewski M, Debska-Slizien A et al. Impact of gender and dialysis adequacy on anaemia in peritoneal dialysis. Int Urol Nephrol 2017;49:903–8. 10.1007/s11255-016-1499-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brar A, Markell M. Impact of gender and gender disparities in patients with kidney disease. Curr Opin Nephrol Hypertens 2019;28:178–82. 10.1097/MNH.0000000000000482 [DOI] [PubMed] [Google Scholar]
- 35. Markell M, Brar A, Stefanov DG et al. Gender disparity in fistula use at initiation of hemodialysis varies markedly across ESRD networks-analysis of USRDS data. Hemodial Int 2018;22:168–75. 10.1111/hdi.12579 [DOI] [PubMed] [Google Scholar]
- 36. Caplin N, Sedlacek M, Teodorescu V et al. Venous access: women are equal. Am J Kidney Dis 2003;41:429–32. 10.1053/ajkd.2003.50052 [DOI] [PubMed] [Google Scholar]
- 37. Miller AJ, Kiberd BA, Alwayn IP et al. Donor-recipient weight and sex mismatch and the risk of graft loss in renal transplantation. Clin J Am Soc Nephrol 2017;12:669–76. 10.2215/CJN.07660716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mudalige NL, Brown C, Marks SD. The impact of donor and recipient sex on kidney allograft survival in pediatric transplant recipients. Pediatr Nephrol 2022;37:209–16. 10.1007/s00467-021-05071-2 [DOI] [PubMed] [Google Scholar]
- 39. Participants KC. Genetics in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int 2022;101:1126–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sánchez R, Ripoll-Vera T, López-Mendoza M et al. The Spanish Fabry women study: a retrospective observational study describing the phenotype of females with GLA variants. Orphanet J Rare Dis 2023;18:8. 10.1186/s13023-022-02599-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Köhler S, Carmody L, Vasilevsky N et al. . et al. Expansion of the Human Phenotype Ontology (HPO) knowledge base and resources. Nucleic Acids Res 2019;47:D1018–27. 10.1093/nar/gky1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shah S, Verma P. Pregnancy-related acute kidney injury: do we know what to do? Nephron 2023;147:35–8. 10.1159/000525492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shah S, Meganathan K, Christianson AL et al. Pregnancy-related acute kidney injury in the United States: clinical outcomes and health care utilization. Am J Nephrol 2020;51:216–26. 10.1159/000505894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barrett PM, McCarthy FP, Evans M et al. Hypertensive disorders of pregnancy and the risk of chronic kidney disease: a Swedish registry-based cohort study. PLoS Med 2020;17:e1003255. 10.1371/journal.pmed.1003255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khashan AS, Evans M, Kublickas M et al. Preeclampsia and risk of end stage kidney disease: a Swedish nationwide cohort study. PLoS Med 2019;16:e1002875. 10.1371/journal.pmed.1002875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Winter S, Diamond M, Green J et al. Transgender people: health at the margins of society. Lancet North Am Ed 2016;388:390–400. 10.1016/S0140-6736(16)00683-8 [DOI] [PubMed] [Google Scholar]
- 47. Eckenrode HE, Gutierrez OM, Osis G et al. Kidney disease prevalence in transgender individuals. Clin J Am Soc Nephrol 2022;17:280–2. 10.2215/CJN.04660421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eckenrode HE, Flynn JT, Mohottige D. Advancing kidney health justice through gender-affirming care. Nat Rev Nephrol 2022;18:343–4. 10.1038/s41581-022-00575-y [DOI] [PubMed] [Google Scholar]
- 49. Thürmann PA, Haack S, Werner U et al. Tolerability of beta-blockers metabolized via cytochrome P450 2D6 is sex-dependent. Clin Pharmacol Ther 2006;80:551–3. 10.1016/j.clpt.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 50. Santema BT, Ouwerkerk W, Tromp J et al. Identifying optimal doses of heart failure medications in men compared with women: a prospective, observational, cohort study. Lancet North Am Ed 2019;394:1254–63. 10.1016/S0140-6736(19)31792-1 [DOI] [PubMed] [Google Scholar]
- 51. Fernandez-Rando M, Herrera MD, Almeida-González CV et al. Days needed for the disappearance of a cough due to the use of an angiotensin-converting enzyme inhibitor and identification of predisposing factors associated with its appearance in a clinical cohort of hypertensive patients. J Clin Pharmacol 2021;61:591–7. 10.1002/jcph.1786 [DOI] [PubMed] [Google Scholar]
- 52. Miners JO, Grgurinovich N, Whitehead AG et al. Influence of gender and oral contraceptive steroids on the metabolism of salicylic acid and acetylsalicylic acid. Br J Clin Pharmacol 1986;22:135–42. 10.1111/j.1365-2125.1986.tb05240.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Friede KA, Infeld MM, Tan RS et al. Influence of sex on platelet reactivity in response to aspirin. J Am Heart Assoc 2020;9:e014726. 10.1161/JAHA.119.014726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rautaharju PM, Zhou SH, Wong S et al. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol 1992;8:690–5. [PubMed] [Google Scholar]
- 55. Makkar RR, Fromm BS, Steinman RT et al. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA 1993;270:2590–7. 10.1001/jama.1993.03510210076031 [DOI] [PubMed] [Google Scholar]
- 56. Racine M, Tousignant-Laflamme Y, Kloda LA et al. A systematic literature review of 10 years of research on sex/gender and experimental pain perception—Part 1: are there really differences between women and men? Pain 2012;153:602–18. 10.1016/j.pain.2011.11.025 [DOI] [PubMed] [Google Scholar]
- 57. Packiasabapathy S, Sadhasivam S. Gender, genetics, and analgesia: understanding the differences in response to pain relief. J Pain Res 2018;1:2729–39. 10.2147/JPR.S94650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Damoiseaux VA, Proost JH, Jiawan VC et al. Sex differences in the pharmacokinetics of antidepressants: influence of female sex hormones and oral contraceptives. Clin Pharmacokinet 2014;53:509–19. 10.1007/s40262-014-0145-2 [DOI] [PubMed] [Google Scholar]
- 59. Conforti F, Pala L, Bagnardi V et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol 2018;19:737–46. 10.1016/S1470-2045(18)30261-4 [DOI] [PubMed] [Google Scholar]
- 60. Li X, Obeidat M, Zhou G et al. Responsiveness to ipratropium bromide in male and female patients with mild to moderate chronic obstructive pulmonary disease. EBioMedicine 2017;19:139–45. 10.1016/j.ebiom.2017.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Simon TA, Askling J, Lacaille D et al. Infections requiring hospitalization in the abatacept clinical development program: an epidemiological assessment. Arthritis Res Ther 2010;12:R67. 10.1186/ar2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morgan R, Klein SL. The intersection of sex and gender in the treatment of influenza. Curr Opin Virol 2019;35:35–41. 10.1016/j.coviro.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shapiro JR, Privor-Dumm L, Rosser EN et al. The intersection of gender and race in older adults' decision to receive COVID-19 vaccines. Vaccine 2023;41:211–8. 10.1016/j.vaccine.2022.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mistry D, Atkin S, Atkinson H et al. Predicting thyroxine requirements following total thyroidectomy. Clin Endocrinol (Oxf) 2011;74:384–7. 10.1111/j.1365-2265.2010.03940.x [DOI] [PubMed] [Google Scholar]
- 65. Zhang JJ, Ma XX, Hao L et al. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol 2015;10:1964–78. 10.2215/CJN.09250914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. John R, Webb M, Young A et al. Unreferred chronic kidney disease: a longitudinal study. Am J Kidney Dis 2004;43:825–35. 10.1053/j.ajkd.2003.12.046 [DOI] [PubMed] [Google Scholar]
- 67. Chen JH, Johnson DW, Wong G et al. Associations between diabetes and sex with peritoneal dialysis technique and patient survival: results from the Australia and New Zealand Dialysis and Transplant Registry cohort study. Perit Dial Int 2021;41:57–68. 10.1177/0896860820918708 [DOI] [PubMed] [Google Scholar]
- 68. Shah S, Leonard AC, Meganathan K et al. Gender and racial disparities in initial hemodialysis access and outcomes in incident end-stage renal disease patients. Am J Nephrol 2018;48:4–14. 10.1159/000490624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kotsanas D, Polkinghorne KR, Korman TM et al. Risk factors for peritoneal dialysis-related peritonitis: can we reduce the incidence and improve patient selection? Nephrology 2007;12:239–45. 10.1111/j.1440-1797.2006.00756.x [DOI] [PubMed] [Google Scholar]
- 70. Guest S. Hypothesis: gender and encapsulating peritoneal sclerosis. Perit Dial Int 2009;29:489–91. 10.1177/089686080902900502 [DOI] [PubMed] [Google Scholar]
- 71. Guglielmi KE. Women and ESRD: modalities, survival, unique considerations. Adv Chronic Kidney Dis 2013;20:411–8. 10.1053/j.ackd.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 72. Gitto L, Noh YH, Andrés AR. An instrumental variable probit (IVP) analysis on depressed mood in Korea: the impact of gender differences and other socio-economic factors. Int J Health Policy Manag 2015;4:523–30. 10.15171/ijhpm.2015.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Merlino G, Lorenzut S, Romano G et al. Restless legs syndrome in dialysis patients: a comparison between hemodialysis and continuous ambulatory peritoneal dialysis. Neurol Sci 2012;33:1311–8. 10.1007/s10072-012-0953-9 [DOI] [PubMed] [Google Scholar]
- 74. Lowrie EG, Ofsthun N, Li Z. Dialysis dose and gender: a different hypothesis. Kidney Int 2004;66:1291; author reply 1292. 10.1111/j.1523-1755.2004.884_8.x [DOI] [PubMed] [Google Scholar]
- 75. Depner T, Daugirdas J, Greene T et al. Dialysis dose and the effect of gender and body size on outcome in the HEMO Study. Kidney Int 2004;65:1386–94. 10.1111/j.1523-1755.2004.00519.x [DOI] [PubMed] [Google Scholar]
- 76. Wolfe RA, Ashby VB, Milford EL et al. Differences in access to cadaveric renal transplantation in the United States. Am J Kidney Dis 2000;36:1025–33. 10.1053/ajkd.2000.19106 [DOI] [PubMed] [Google Scholar]
- 77. Gill J, Joffres Y, Rose C et al. The change in living kidney donation in women and men in the United States (2005-2015): a population-based analysis. J Am Soc Nephrol 2018;29:1301–8. 10.1681/ASN.2017111160 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.