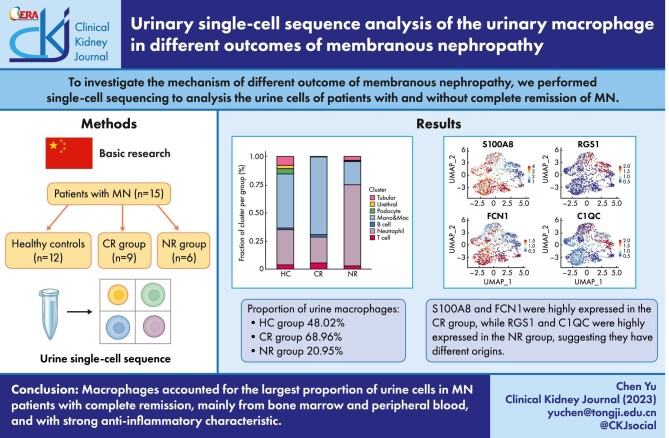
ABSTRACT
Background
Great progress has been made in the diagnosis and treatment of membranous nephropathy (MN). However, a significant number of patients do not respond to immunosuppressive therapy and eventually progress to end-stage kidney disease. To investigate the mechanism of different outcome of MN, we performed single-cell sequencing to analyze the urine cells of patients with and without complete remission of MN.
Methods
Urine single-cell RNA sequencing was performed on 12 healthy controls (HC) and 15 patients with MN. The patients were divided into a complete remission group (CR, n = 9) and a no remission group (NR, n = 6).
Results
(i) Macrophages were the largest group in urine cells, comprising 48.02%, 68.96% and 20.95% in the HC, CR and NR groups, respectively. (ii) Urinary macrophages expressing FIColin-1 and S100 calcium-binding protein A8 were mainly found in the HC and CR groups, indicating that they were derived from bone marrow and peripheral blood, while the urinary macrophages expressing the regulator of G-protein signaling 1 (RGS1) and HLA-DPA1, mainly found in the NR group, were derived from renal resident macrophages. (iii) In healthy adults, urine macrophages expressed the metallothionein family, indicating that they can regulate anti-inflammatory and proinflammatory functions bidirectionally. In the CR group, the urine macrophages showed strong proinflammatory properties. In the NR group, the urinary macrophages mainly associated with the level of proteinuria and the impaired renal function.
Conclusions
Our study firstly delineated the differences in urinary cell maps between healthy individuals and MN patients with CR or NR outcomes. Not only the origin but also the function of urine macrophages were different in the HC, CR and NR groups.
Keywords: macrophages, membranous nephropathy, proteinuria, urine, urine single-cell sequencing
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Membranous nephropathy (MN) is one of the most common pathological types of nephrotic syndrome. Over the years, breakthroughs have been made in the diagnosis and treatment of MN. At present, it is generally accepted that risk assessment should be carried out when the diagnosis has been made. For the treatment of intermediate-, high- and very high–risk patients, in addition to basic supportive treatment with angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), glucocorticoid combined immunosuppressant is the main treatment method [1]. Many patients respond well to treatment; however, there is still a subset of patients who respond poorly to treatment, the reasons for which we need to explore.
The pathogenesis of MN has not been fully elucidated. It is generally believed that B cells are activated to produce autoantibodies, which bind to certain components of podocytes and then deposit under epithelial cells to activate the attack complex of complement, thereby damaging podocytes and causing kidney damage [2]. Many antigens have been identified, such as phospholipase A2 antigen (PLA2R), thrombospondin type 1 domain-containing 7A (THSD7A), exostosin 1/2 (EXT1/2), neural epidermal growth-like 1 protein (NELL1), semaphorin 3B (SEMA3B), protocadherin 7 (PCDH7) and neural cell adhesion molecule 1 (NCAM1) [3–9]. PLA2R antibodies account for 70%–80% of primary MN, and the frequency of anti-PLA2R testing has increased from 40% in 2011 to 93.3% in 2014 [10]. The different antigen-associated MNs represent distinct clinical and pathologic characteristics, suggesting that each antigen-associated MN is a specific disease entity [11].
However, the role of other immune cells, such as macrophages, remains unclear. B cells and T cells account for only a small portion of the inflammatory cells in the renal interstitial infiltrate in MN, while most are macrophages [12]. It has also been reported that an increase in the number of glomerular macrophage migration inhibitory factor–positive cells is associated with ineffective treatment in MN patients [13].
We used single-cell sequencing technology to identify urinary cells in MN patients and compared the differences between a group with complete remission (CR) and one with no remission (NR), using healthy adults as controls (HC). We not only performed urinary single-cell mapping of patients with MN for the first time but also further analyzed the correlations between macrophage subpopulations and the different outcomes of treatment in MN patients. We expect our findings to be helpful for the clinical treatment of this patient population.
MATERIALS AND METHODS
Study design
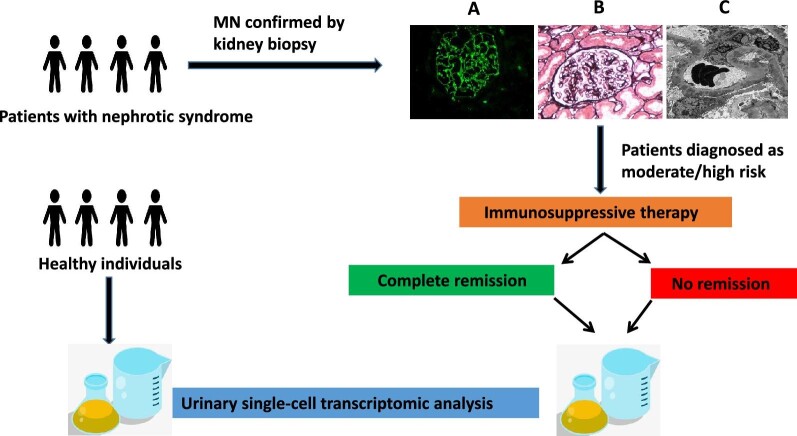
Fifteen MN patients with intermediate-risk and high-risk were enrolled in this study. After 6 months of supportive care patients could be divided into three categories: low risk [<4 g/day, stable golerular filtration rate (GFR)], moderate risk (4–8 g/day with stable GFR) or high risk (>8 g/day, <50% decrease from baseline or >30% decline in GFR since baseline) [2, 14]. All patients underwent basic therapy (ACEI/ARB) and glucocorticoid combined immunosuppressant therapy. They were divided into the CR group (n = 9) and NR group (n = 6) based on the therapeutic effect.
Criteria for CR
After more than 6 months of immunosuppressive treatment, the following criteria were met: (i) 24-h urinary protein <0.3 g, which was confirmed at least twice at an interval of >1 week; (ii) serum albumin level >3.5 g/dL; and (iii) normal serum creatinine level.
Criteria for NR
After more than 6 months of immunosuppressive treatment, the decrease in the level of 24-h urinary protein was <50% from the baseline, and the clinical manifestations were still nephrotic-range proteinuria.
Exclusion criteria
(i) Patients with secondary kidney diseases, including systemic lupus erythematosus, sicca syndrome, hepatitis and malignant tumors; (ii) patients with severe diseases of other organs and those who were considered unsuitable to participate in this study by the investigators such as patients with poor adherence.
Healthy adults (n = 12) were enrolled as the control group. All subjects signed the informed consent form.
Urine sample collection and processing
For single-cell RNA sequencing (scRNA-seq) analysis, fresh morning urine (300–900 mL) was collected and separated, and single-cell suspensions were prepared for urinary scRNA-seq (Fig. 1). Urine samples were pooled together to test according to grouping. Samples were transferred into a 50 mL conical tube and centrifuged immediately at a speed of 390 g. The cell pellets were washed twice with cold wash buffer including F12 medium, 5% FBS (Hyclone, Australia), 1%P/S (Life, 15070-063) and 1% l-glutamine (Life, 25030-081), and resuspended in cold phosphate-buffered saline (PBS). Then the cells were passed through a 40-μm Nylon mesh (Falcon, USA) to remove aggregates and casts in the urine. Cell viability was assessed by the exclusion of trypan blue dye. To remove dead cells and cell debris, urinary cells were subjected to fluorescent dye staining by Calcein AM (Solarbio, C8950, 1 mg) and DAPI for 30 min at 37°C. Then the cells were washed twice with cold PBS and resuspended in PBS with 2% FBS (Hyclone, Australia) for flow cytometry sorting of Calcein AM–stained living cells. Sorted cells were immediately subjected to scRNA-seq.
Figure 1:
Study design and histopathological findings on the renal biopsy. (A) Granular deposition of IgG along capillary wall (immunofluorescence assay, 400×). (B) Diffuse thickening of the glomerular basement membrane and spike formation (PASM, 400×). (C) Diffuse and irregular thickening of glomerular basement membrane and electron-dense deposits in the subepithelial areas (electron microscopy, 5000×).
Immunohistochemistry
Samples of kidney tissue were obtained from biopsies of the patients with MN. The samples of control cases were from patients with minimal change disease. Kidney samples were fixed in 4% paraformaldehyde. Immunohistochemistry was performed in 3 μm paraffin sections using a water-bath heating for antigen retrieval. The primary antibodies used in this study included: CD3 [GT200202, 1:200, Gene Tech (Shanghai) Company Ltd], CD20 [GM075502, 1:1, Gene Tech (Shanghai) Company Ltd], CD68 [GM081402, 1:200, Gene Tech (Shanghai) Company Ltd] and S100A8/A9 complex (ab22506, 1:1000, Abcam). The positive cells for CD3, CD20, CD68 and S100A8/A9 staining were counted under a 400-fold microscope in 10 random areas of kidney tissues.
ScRNA-seq and bioinformatic analysis
Single cells were captured and barcoded in 10× Chromium Controller (10× Genomics). Subsequently, RNA from the barcoded cells was reverse-transcribed and sequencing libraries were prepared using Chromium Single Cell 3′ v3 Reagent Kit (10× Genomics) according to the manufacturer's instructions. Sequencing libraries were loaded on an Illumina NovaSeq with 2 × 150 paired-end kits at Novogene, China. Raw sequencing reads were processed using the Cell Ranger v.3.1.0 pipeline from 10× Genomics. In brief, reads were demultiplexed, aligned to the human GRCh38 genome and Unique Molecular Identifiers (UMI) counts were quantified per gene per cell to generate a gene-barcode matrix. Data were aggregated and normalized to the same sequencing depth, resulting in a combined gene–barcode matrix of all samples.
The following criteria were then applied to HC, CR and NR samples: gene number between 200 and 5000 and mitochondrial gene percentage <0.3. Genes were filtered out that were detected in fewer than three cells. Finally, a filtered gene–barcode matrix of HC, CR and NR sample was integrated with Seurat v.3 to remove batch effects across different sample. In parameter settings, the first 30 dimensions of canonical correlation analysis and principal component analysis (PCA) were used.
The filtered gene–barcode matrix was first normalized using “LogNormalize” method in Seurat v.3 with default parameters. The top 2000 variable genes were then identified using the “vst” method in the Seurat FindVariableFeatures function. A linear transformation (ScaleData) was applied as a pre-processing step and PCA was performed using the top 2000 variable genes. We then selected the top 30 significant principal components for two-dimensional uniform manifold approximation and projection. We used FindCluster in Seurat to identify cell clusters. To identify the marker genes, differential expression analysis was performed by the function FindAllMarkers in Seurat with the likelihood-ratio test. Differentially expressed genes that were expressed at least in 25% cells within the cluster and with a fold-change >0.25 (log scale) were considered to be marker genes.
Gene Ontology (GO) enrichment analysis and Gene Set Enrichment Analysis of differentially expressed genes (DEGs) were implemented by the ClusterProfiler R package. GO terms with corrected P-value <.05 were considered significantly enriched by DEGs.
The Macspectrum (https://macspectrum.uconn.edu) model was used to assess the macrophage polarization index (MPI) of macrophages in each sample [15]. The projection of each cell on this axis was indexed as MPI: higher MPI suggesting more “M1-like” (more inflammatory) states and lower MPI suggesting more “M2-like” (less inflammatory) states.
RESULTS
Clinicopathological characteristics of MN in the CR and NR groups
In the CR group (n = 9) there were two females and seven males, with an average age of 62.8 years (52–74 years) and disease duration of 2–5 years; there were five patients with hypertension, one patient with both hypertension and diabetes, and one patient with gout. Two patients had no comorbidities. The average estimated GFR (eGFR) [Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula] during renal biopsy was 74.7 mL/min/1.73 m2 (58.2–102.8 mL/min/1.73 m2), the average 24-h urinary protein was 7.46 g (4.34–11.2 g), the average serum albumin was 20.7 g/L (14.1–32.1 g/L), eight patients were positive for the PLA2R antibody in their kidney tissues, and one patient was negative (secondary factors were excluded). The serum PLA2R antibody level was >20 RU/mL (24.37–427.98 RU/mL) in six patients, <20 RU/mL (0.87 RU/mL) in one patient and was not detected in two patients at the time of diagnosis. The proportion of glomerulosclerosis under light microscopy was 0%–10%, and segmental sclerosis was observed in one patient, in whom there was no crescent. The proportion of renal tubular atrophy and interstitial fibrosis was 5%–15%.
In the NR group (n = 6) there were one female and five males, with an average age of 47 years (23–73 years) and disease duration of 2–7 years; there was one patient with hypertension, one patient with diabetes, one patient with gout, and four patients without comorbidities. The mean eGFR (CKD-EPI formula) during renal biopsy was 90.6 mL/min/1.73 m2 (69–132.9 mL/min/1.73 m2), the mean 24-h urinary protein was 8.69 g (5.74–13.53 g) and the mean serum albumin was 23.4. g/L (16–27.4 g/L). All six patients were positive for the PLA2R antibody in the kidney tissues, two patients had serum PLA2R antibodies >20 RU/mL (91.24, 521.09 RU/mL), three patients had serum PLA2R antibody levels <20 RU/mL, and one patient had undetectable serum PLA2R antibodies. The proportion of glomerulosclerosis under light microscopy was 3%–17%, there was no segmental sclerosis or crescent formation, and the proportion of renal tubular atrophy and interstitial fibrosis was 5%–40%.
Patients in both groups were treated with maximum dose of renin–angiotensin–aldosterone system blockers for 3–6 months. After patients were evaluated as moderate or high risk, glucocorticoids combined with calcineurin inhibitors/glucocorticoids combined with cyclophosphamide/rituximab were added. The duration of remission after treatment in the CR group was 3–45 months. The average immunosuppressive treatment time of patients in the NR group was 11–77 months. Among the six NR patients, four patients underwent two immunosuppressive regimens, one patient underwent three regimens and one patient underwent one immunosuppressive regimen.
The clinical data and pathological characteristics of the 15 patients are shown in Tables 1 and 2.
Table 1:
The clinical and pathological characteristics of CR patients.
| CR patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics/number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Age at diagnosis, years | 60 | 58 | 56 | 74 | 52 | 65 | 59 | 74 | 67 |
| Gender | M | M | F | F | M | M | M | M | M |
| Course of disease, years | 5 | 4 | 4 | 4 | 3 | 4 | 2 | 2 | 2 |
| BMI at diagnosis, kg/m2 | 24.6 | 26.3 | 24.8 | 27.5 | 25.1 | 22.2 | 26 | 24.5 | 23.7 |
| BP at diagnosis, mmHg | 160/106 | 150/80 | 118/90 | 133/77 | 120/70 | 165/90 | 156/82 | 150/81 | 140/82 |
| eGFR at diagnosis, mL/min/1.73 m2 (CKD-EPI) | 89.2 | 61.3 | 101.3 | 74.8 | 102.8 | 58.7 | 63.7 | 58.2 | 61.9 |
| Creatinine at diagnosis, µmol/L | 82 | 113 | 54 | 69 | 75 | 112 | 109 | 108 | 107 |
| 24 h UTP at diagnosis, g/day | 11.2 | 7.54 | 4.99 | 7.48 | 3.68 | 9.85 | 8.84 | 9.2 | 4.34 |
| Albumin at diagnosis, g/L | 14.1 | 32.1 | 20.5 | 25.3 | 23.2 | 16 | 17.2 | 15 | 22.9 |
| Serum PLA2R at diagnosis, RU/mL | ND | 24.37 | 62.09 | 0.87 | ND | 49.29 | 427.98 | 53.43 | 34.07 |
| Complication | Hypertension | Hypertension/diabetes | No | Hypertension | No | Hypertension | Hypertension | Hypertension | Gout |
| Immunosuppressor | CTX | Tacrolimus/CTX | CTX/rituximab | Tacrolimus | CTX | CTX/Tacrolimus | Tacrolimus | Rituximab | CTX |
| MN class | I | I–II | I | II | I | I | I | II | II |
| IF IgG | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ |
| IF IgA | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| IF C3 | 3+ | 2+ | 1+ | 2+ | Neg | 2+ | 2+ | 2+ | 3+ |
| IF C1q | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Tissue PLA2R | Neg | Pos | Pos | Pos | Pos | Pos | Pos | Pos | Pos |
| Global sclerosis (%) | 10 | 9 | 0 | 6 | 4 | 0 | 6 | 0 | 0 |
| Segmental sclerosis | 0/10 | 0/32 | 0/3 | 0/20 | 0/28 | 1/6 | 0/17 | 0/21 | 0/16 |
| Crescent | 0/10 | 0/32 | 0/3 | 0/20 | 0/28 | 0/6 | 0/17 | 0/21 | 0/16 |
| Interstitial fibrosis (%) | 5 | 5 | 0 | 10 | 0 | 15 | 10 | 10 | 5 |
| Mesangial matrix increase | Neg | Mild | Neg | Neg | Mild | Neg | Neg | Mild | neg |
| Interstitial lymphocytes | Neg | Neg | Mild | Mild | Neg | Mild | Mild | Mild | Mild |
| Arterial sclerosis | Mild | Mild | Mild | Mild | Mild | Neg | Mild | Mild | Neg |
M: male; F: female; BMI: body mass index BP: blood pressure; UTP: urine total protein; CTX: cyclophosphamide; Neg: negative; Pos: positive.
Table 2:
The clinical and pathological characteristics of NR patients.
| NR patients | ||||||
|---|---|---|---|---|---|---|
| Characteristics/number | 1 | 2 | 3 | 4 | 5 | 6 |
| Age at diagnosis, years | 73 | 35 | 51 | 44 | 56 | 23 |
| Gender | M | M | M | M | F | M |
| Course of disease, years | 2 | 2 | 4 | 2 | 2 | 7 |
| BMI at diagnosis, kg/m2 | 23.9 | 30.86 | 24.4 | 25 | 25.3 | 31.8 |
| BP at diagnosis, mmHg | 120/80 | 135/78 | 126/76 | 130/86 | 108/64 | 115/74 |
| eGFR at diagnosis, mL/min/1.73 m2 (CKD-EPI) | 69 | 89.1 | 75.6 | 102.8 | 74.4 | 132.9 |
| Creatinine at diagnosis, µmol/L | 94 | 95 | 99 | 80 | 77 | 62 |
| 24 h UTP at diagnosis, g/day | 5.74 | 12.8 | 10.3 | 4.98 | 4.77 | 13.53 |
| Albumin at diagnosis, g/L | 27.4 | 20.6 | 27.3 | 16 | 24 | 25 |
| Serum PLA2R at diagnosis, RU/mL | 521.09 | 17.61 | ND | 91.24 | <2 | <2 |
| Complication | Hypetension/diabetes/stroke | Gout | No | No | No | No |
| Immunosuppressor | Tacrolimus/CTX | Rituximab/tacrolimus | CTX/cyclosporin | Tacrolimus | Rituximab/tacrolimus | Rituximab/tacrolimus/CTX |
| MN class | II | III | I | I | I | II |
| IF IgG | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ |
| IF IgA | Neg | Neg | Neg | Neg | Neg | Neg |
| IF C3 | 2+ | 3+ | 2+ | 1+ | 1+ | 1+ |
| IF C1q | Neg | Neg | Neg | Neg | Neg | Neg |
| Tissue PLA2R | Pos | Pos | Pos | Pos | Pos | Pos |
| Global sclerosis (%) | 17 | 5 | 6 | 3 | 17 | 0 |
| Segmental sclerosis | 0/29 | 0/20 | 0/18 | 0/31 | 0/30 | 0/7 |
| Crescent | 0/29 | 0/20 | 0/18 | 0/31 | 0/30 | 0/7 |
| Interstitial fibrosis (%) | 10 | 40 | 5 | 5 | 10 | 40 |
| Mesangial matrix increase | Neg | Mild | Neg | Mild | Neg | Neg |
| Interstitial lymphocytes | Mild | Mod | Neg | Neg | Mild | Neg |
| Arterial sclerosis | Mild | Mild | Mild | Mild | Mild | Neg |
M: male; F: female; BMI: body mass index BP: blood pressure; UTP: urine total protein; CTX: cyclophosphamide; Neg: negative; Pos: positive.
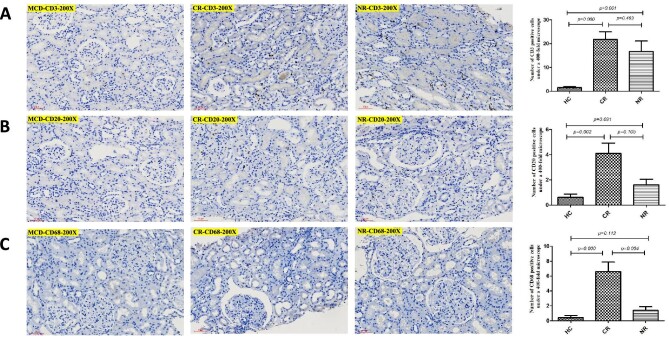
Infiltration of renal interstitial inflammatory cells
The renal interstitium of the 15 patients with MN had varying degrees of inflammatory cell infiltration. The renal biopsy tissues were subjected to CD protein serial immunohistochemical staining. The results showed few inflammatory cells in the renal interstitium of the control group (patient with minimal change disease) (Fig. 2A). There were a small number of inflammatory cells in the CR and NR groups, mainly CD68-positive macrophages, CD3-positive T cells and CD20-positive B cells (Fig. 2B and C). Compared with the control group, the positive cells for CD3, CD20 and CD68 were significantly higher in the CR group (P < .05), while in the NR group, only the number of CD3-positive cells was significantly higher than that in the control group (P < .05). The number of CD68-positive cells in the CR group was the highest in the three groups (Fig. 2C).
Figure 2:
Immunohistochemical staining shows types of inflammatory cells in renal interstitial. (A) CD3; (B) CD20; (C) CD68.
Urinary single-cell mapping of patients with MN
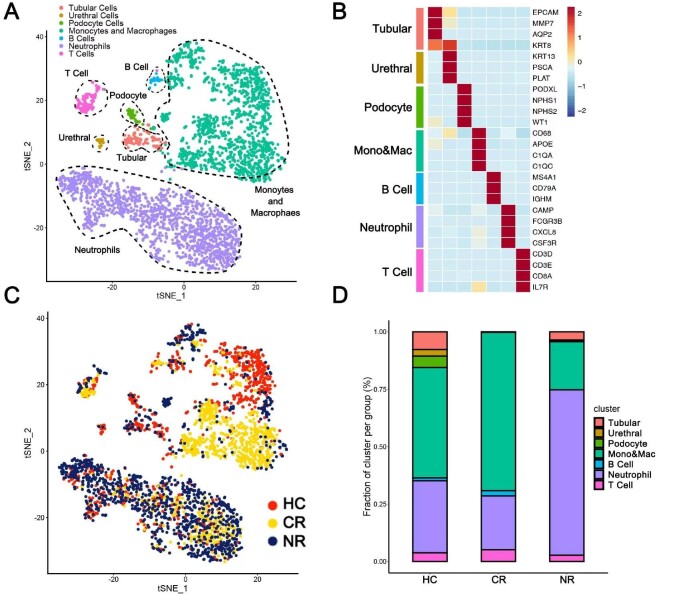
A total of 4089 cells were captured from 27 urine specimens of 15 patients with MN and 12 healthy adults, including 1104 cells in the HC group (n = 12), 1154 cells in the CR group (n = 9) and 1831 cells in the NR group (n = 6). The number of cells used for analysis after quality control filtration was 3299, including 1010 cells in the HC, 1005 cells in the CR group and 1284 cells in the NR group. Comparatively, the NR group had more urinary cells. After clustering, all cells were grouped into seven subpopulations. In descending order of abundance, they were neutrophils (44.77%), macrophages (43.86%), T cells (3.79%), renal tubular epithelial cells (3.76%), podocytes (1.70%), B cells (1.09%) and urethral epithelial cells (1.03%) (Fig. 3A). The cell markers used to cluster the seven cell subpopulations are shown in a heatmap (Fig. 3B). The seven cell subpopulations showed specific enrichment in different patient groups (Fig. 3C). The HC group's urinary single cells were mainly macrophages (48.02%), followed by neutrophils (31.39%) and renal tubular epithelial cells (7.72%). The CR group had a similar ranking, and its subpopulations in descending order were macrophages (68.96%), neutrophils (23.38%), T cells (5.17%) and B cells (2.29%). The distribution of urinary single-cell subpopulations of patients in the NR group was different from that of the HC and the CR groups. Its neutrophil subpopulations was the most abundant (72.04%), followed by macrophages (20.95%), renal tubular epithelial cells (3.58%) and T cells (2.73%) (Fig. 3D).
Figure 3:
Single-cell sequencing showed urinary cell types and their distribution in the HC, CR and NR groups. (A) Number and types of total urinary single cells in all groups. (B) The cell markers used to cluster the seven cell subpopulations are shown in a heatmap. (C) tSNE diagram showing the clustering distribution characteristics of all cells in the HC, CR and NR groups. (D) The histogram shows the proportion of different cell types in the HC, CR and NR groups.
Analysis the composition and source of urinary macrophages in patients in the HC, CR and NR groups
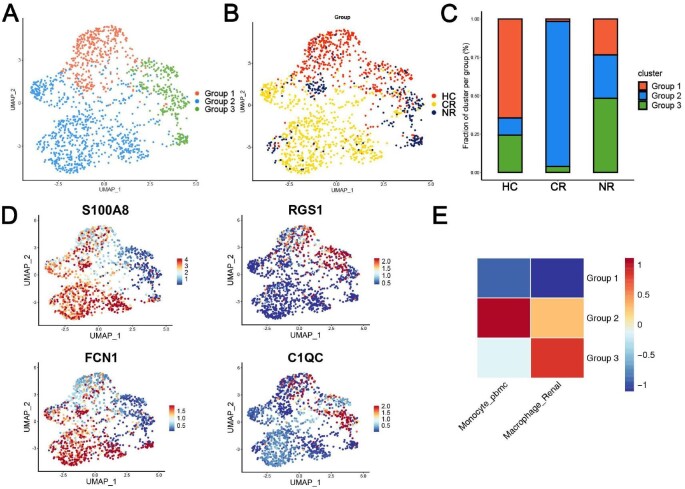
We further performed subclustering analysis on the macrophages population and obtained three subpopulations, Groups 1, 2 and 3 (Fig. 4A). The Group 1 subpopulation was mainly enriched in the HC group, the Group 2 subpopulation was mainly enriched in the CR group and the Group 3 subpopulation was the most abundant in the NR group (Fig. 4B and C). Figure 4D shows the representative markers of the urine macrophages: S100A8 and FCN1 were highly expressed in Groups 1 and 2, suggesting peripheral blood and bone marrow–derived macrophages. RGS1 and C1QC were highly expressed in Group 3, suggesting that they were renal resident macrophages. Heatmap depicted the gene expression correlation between three groups of macrophages and monocytes in peripheral blood mononuclear cells/renal resident macrophages (Fig. 4E).
Figure 4:
The composition and source of urinary macrophages in patients in the HC, CR and NR groups. (A) Subclustering analysis of the macrophage population obtained three subpopulations (Groups 1, 2 and 3). (B) Different distribution of the macrophages subpopulations in the HC, CR and NR groups. (C) The histogram shows the proportion of the macrophages subpopulations in the HC, CR and NR groups. (D) FeaturePlot shows representative markers of the source of the urine macrophages: S100A8 and FCN1 represent peripheral blood and bone marrow–derived macrophages. RGS1 and C1QC represent the sources of renal resident macrophages. (E) The heatmap shows the source of Groups 1, 2 and 3.
The functions and characteristics of urinary macrophages in patients in the HC, CR and NR groups
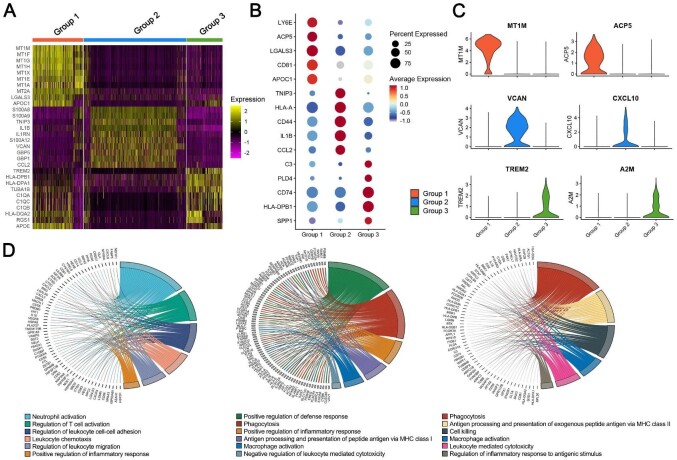
Heat mapping showed the top ranked genes highly expressed in three groups of macrophages (Fig. 5A). Group 1 highly expressed metallothionein 1 (MT1M, MT1F, MT1G, MT1H, MT1X, MT1E and MT1A), metallothionein 2 (MT2A), galectin‐3 (LGALS3) and apolipoprotein C1 (APOC1). Group 2 highly expressed S100 calcium binding protein A8/A9 (S100A8/A9), TNFAIP3-interacting protein 3 (TNIP3), interleukin (IL)-1β, IL-1 receptor antagonist (IL1RA), S100A12, Versican (VCAN), guanylate-binding protein 5 (GBP5), GBP1 and chemokine ligand 2 (CCL2). Group 3 highly expressed triggering receptor expressed on myeloid cells-2 (TREM2), human leukocyte antigens (HLA-DPB1, HLA-DPA1\HLA-DQA2), α-tubulin 1b subtype (TUBA1B), C1QA, C1QC, C1QB, regulator of G-protein signaling 1 (RGS1) and APOE. Dotplot showed the expression of other representative marker genes. The Group 1 subpopulation highly expressed lymphocyte antigen 6 family member E (LY6E), acid phosphatase 5 (ACP5), LGALS3, CD81 and APOC1. LY6E can regulate T-lymphocytes proliferation, differentiation and activation. LGALS3 plays a role in numerous cellular functions including apoptosis, innate immunity, cell adhesion and T-cell regulation. Group 2 subpopulation highly expressed TNIP3, HLA-A, CD44, IL-1β and CCL2. HLA-A play a central role in the immune system by presenting peptides derived from the endoplasmic reticulum lumen so that they can be recognized by cytotoxic T cells. CD44 has many cellular functions including the activation, recirculation and homing of T-lymphocytes, hematopoiesis, inflammation and response to bacterial infection. Group 3 subpopulation highly expressed C3, phospholipase D family member 4 (PLD4), CD74, HLA-DPB1 and secreted phosphoprotein 1 (SPP1) (Fig. 5B). Complement component C3 plays a central role in the activation of complement system. CD74 regulates antigen presentation for immune response and serves as cell surface receptor for the cytokine macrophage migration inhibitory factor (MIF). Violin plots showed Group 1 subpopulation highly expressed MT1M and ACP5. Group 2 subpopulation highly expressed VCAN and CXCL10. The Group 3 subpopulation highly expressed the profibrotic genes TREM2 and the immunoregulatory factor alpha-2-macroglobulin (A2M) (Fig. 5C). GO enrichment shows the biological processes involved in three groups of macrophages. The function of Group 1 is mainly cellular response to cadmium ion, detoxification and response to toxic substance. The function of Group 2 is mainly neutrophil-mediated immunity, neutrophil degranulation and neutrophil activation involved in immune response, while the function of Group 3 is mainly signal recognition particle-dependent cotranslational protein targeting to membrane, cotranslational protein targeting to membrane and protein targeting to endoplasmic reticulum (Fig. 5D).
Figure 5:
The functions and characteristics of urinary macrophages in patients in HC, CR and NR groups. (A) Heat map of the top ranked genes highly expressed in three groups of macrophages. Color scheme is based on z-score distribution from –2 (purple) to 2 (yellow). (B and C) Bubble chart and violin plots of the expression of other representative marker genes related to the function across the three groups. (D) GO enrichment shows the biological processes involved in three groups of macrophages. The left side of the circle represents the DEGs of three groups, while the right side represents different biological processes.
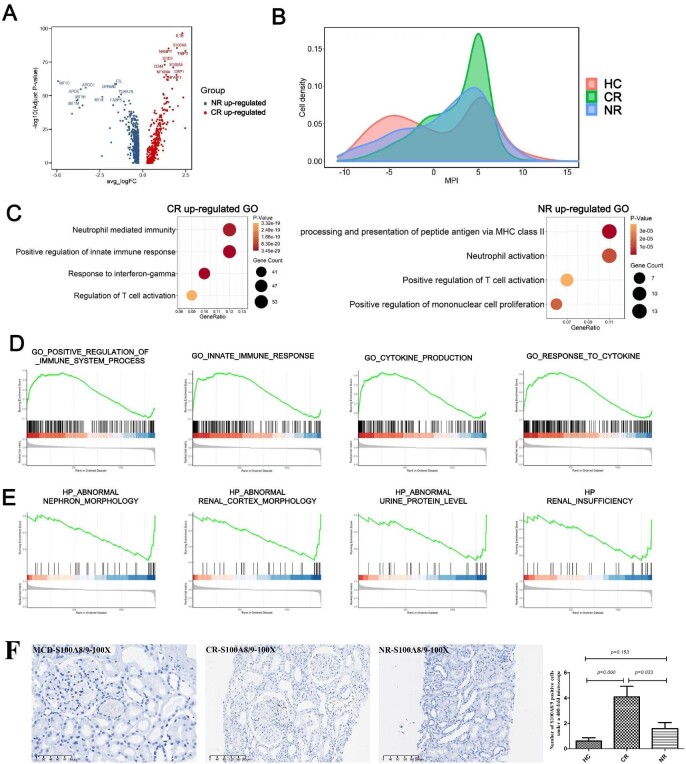
Polarity analysis of urinary macrophages in the CR group and the NR group
We analyzed DEGs in macrophages in the CR and NR groups (Fig. 6A). IL-1β, S100A8, nicotinamide phosphoribosyltransferase (NAMPT), TNIP3, SOD2, CD44, S100A9, NFKB1A, GBP1 and TNFAIP3 were highly expressed in the CR group. MT1G, APOE, APOC1, MT1H, MT1M, MT1F, glycoprotein nonmetastatic melanoma protein B (GPNMB), ferritin light chain (FTL), tubulin alpha 1b (TUBA1B) and human fatty acid-binding protein 5 (FABP5 ) were highly expressed in the NR group. For further verification, immunohistochemical staining of S100A8/9 was performed on the kidney tissues of patients with MN. The results showed the number of S100A8/9-positive cells in CR group was significantly higher than that in the HC and NR groups (P < .05) (Fig. 6F).
Figure 6:
Polarity analysis of urinary macrophages in the CR and NR groups. (A) DEGs analysis on macrophages in the CR and NR groups. (B) Macrophages Macspectrum analysis model was used to analyze the HC, CR and NR groups. (C) GO analysis in the CR and NR groups. (D and E) Gene-set enrichment analysis in the CR and NR groups. (F) Immunohistochemical staining of S100A8/9 in the HC, CR and NR groups.
Next, the “Macspectrum” macrophage analysis model was used to analyze the HC, CR and NR groups (Fig. 6B). In the Macspectrum, a higher macrophage polarization score reflects a more proinflammatory phenotype. The results showed that the macrophages in the urine of healthy people were more balanced in terms of anti-inflammatory and proinflammatory properties. Both the CR group and the NR group showed a more proinflammatory than anti-inflammatory nature, especially the CR group, which showed a stronger proinflammatory nature. GO analysis (Fig. 6C) showed that the upregulated genes in the CR group were enriched in the regulation of neutrophil-mediated immune response, the positive regulation of the innate immune response, the response to γ-interferon and the regulation of T cell activation. The upregulated genes in the NR group were enriched in the processing and presentation of MHC-like peptide antigens, neutrophil activation, positive regulation of T cell activation and positive regulation of monocyte proliferation.
Gene set enrichment analysis (Fig. 6D and E) suggested that the upregulated genes in the CR group were associated with positive regulation of the immune system, secretion of cytokines, and responses to innate immunity and cytokines, while the upregulated genes in the NR group were associated with urinary protein levels and impaired renal function.
DISCUSSION
Our study first used single-cell sequencing technology to compare the differences of urine cells between MN patients with CR and NR outcomes. We found that macrophage was the one of the largest groups in urine cells. Not only the origin but also the function of the urine macrophages were different in the CR group and the NR group, compared with the HC group.
Single-cell sequencing is a powerful emerging research tool that can perform transcriptomic analysis of thousands of cells, leading to a new understanding of kidney diseases. Previous studies have mostly used kidney tissue or blood to prepare single-cell detection samples [16]. Kidney tissue relies on the invasive operation of renal biopsy, which provides a very small sample, and is not conducive to repeated detection. Although specimen collection is simple and feasible for peripheral blood single-cell sequencing, the cells in the blood have a wide range of sources, and its specificity in reflecting kidney injury is low. Urine has abundant diagnostic information, which can specifically reflect the condition of kidney injury, and it is convenient to obtain repeatedly. Our study showed that urinary single-cell sequencing is effective and feasible. In recent years, the application of single-cell sequencing methods to the study of diabetic nephropathy [17], lupus nephritis [18], immunoglobulin A (IgA) nephritis [19] and transplanted kidney [20] has been reported. However, the application of urine single-cell sequencing in kidney disease is rarely reported.
Compared with the previous paper on diabetic nephropathy [17], we collected a single morning urine sample, rather than a 24-h urine collection, which resulted in a smaller number of single cells. So in our study, urine samples from the CR, NR and HC groups were pooled together to test.
As can be seen from our results, the total cell number was significantly increased in the ineffective group, in which neutrophils dominated. However, the proportion of macrophages was highest in the CR group. Therefore, it is necessary to carefully compare the differences of macrophages among the three groups.
Macrophages are derived from CD34-positive hematopoietic stem cells. Promonocytes enter the blood and differentiate into mature monocytes in the peripheral blood. Under the stimulation of inflammatory signals, they enter damaged tissues and undergo a series of changes to differentiate into macrophages [21]. We found that macrophages in the CR group account for 68.96%, and were significantly higher than those in the control group (48.02%) and NR group (20.95%), suggesting that macrophages have special significance in the pathogenesis and the outcome of MN.
We performed cluster analysis of these macrophages and found that the urinary macrophages of the three groups had different sources. The macrophages in the HC and CR groups mainly expressed the markers FCN1 and S100A8, suggesting that they were derived from the bone marrow and peripheral blood [22]. In the CR group, the urine macrophages expressed C1QC, RGS1, APOE and HLA-DPA1, and had a strong antigen-presenting ability, which was more in line with the characteristics of resident macrophages in the kidney [22].
In the HC group, the urine macrophages mainly expressed the metallothionein family, LGALS3 and APOC1. Metallothioneins are a group of metal-binding proteins ubiquitously present in organisms. They can be synthesized and degraded in the kidneys and have the function of scavenging free radicals, protecting cells from oxidative stress–induced cytotoxicity, and preventing senescence [23]. That is, they function in organismal self-protection. Polarity analysis of macrophages showed that the distribution of anti-inflammatory and proinflammatory properties of macrophages in the healthy group was relatively balanced.
The urinary macrophages in CR patients mainly expressed the S100A8/S100A9, TNIP3, IL-1β, IL1RN, and S100A12 genes. These genes are mainly associated with inflammation. S100A8/S100A9, a Toll-like receptor-4 ligand, is seen in neutrophils and monocytes and elevated in inflammatory states. It was reported that it reflected activities of ANCA-associated nephritis [24], and lupus nephritis and its response to rituximab therapy [25]. TNIP3 regulates autophagy in CD4-positive T cells [26]. Both IL-1β and IL1RN are important mediators of the inflammatory process. Gene-set enrichment analysis showed that urinary macrophages of the CR group positively regulate the immune system, secrete cytokines, and respond to innate immunity signals and cytokines. These findings show the strongly proinflammatory nature of urinary macrophages in CR patients. The latest research reports that pro-inflammatory macrophages can improve disease. Macrophages with a pro-inflammatory phenotype in obesity can reduce the metabolic inflammation of adipose tissue, alleviate insulin resistance and improve glucose homeostasis [27]. Pro-inflammatory macrophages can enhance the activity of effector T cells and cooperate with other immune checkpoint inhibitors to limit tumor spread [28]. This may be the reason why these patients are able to achieve CR.
The top 10 genes expressed on the surface of the urine macrophages in NR group were TREM-2, HLA-DPB1, HLA-DPA1, TUBA1B, C1QA, C1QC, C1QB, HLA-DQA2, RGS1 and APOE genes. These genes are mainly divided into three categories. The first is associated with fibrosis and chronic inflammation: TREM-2, HLA-DPB1, HLA-DPA1 and HLA-DQA2. The ratio of TREM-1/TREM-2 in urine is a biological marker of renal interstitial fibrosis in patients with chronic kidney disease [29]. HLA atypia is recognized as an important risk factor for most immune-mediated kidney diseases [30]. Together with environmental factors, they lead to loss of tolerance and autoimmune-mediated renal inflammation. The second is associated with the complement system: C1QA, C1QC and C1QB. Complement C1q participates in inflammatory responses, forms immune complexes and attacks kidney cells. The third, genome-wide association studies have shown that the RGS1 and RASGRP1 genes are heterozygous in the pathogenesis of IgA nephritis [31]. APOE gene mutations are associated with the pathogenesis of lipoprotein nephropathy, and APOE2 homozygous mutations are associated with glomerulosclerosis, significant infiltration of foam cells transformed by macrophages, and nonlaminar lipoprotein emboli [32]. There are also case reports of apoE Toyonaka (Ser197Cys) combined with pure apoE2/2, in which nonimmune MN-like features were observed in the glomeruli [33]. From these analyses, we can see that the urinary macrophages of the patients in the NR group having genetic susceptibility to renal injury factors, which was associated with the level of proteinuria and impaired renal function of the patients.
There is a lot that is new in our study. It confirmed the feasibility and effectiveness of urinary single-cell sequencing technology, obtained a urinary single-cell sequencing mapping of patients with MN, and performed an in-depth analysis of the gene expression differences of macrophages in patients with different treatment outcomes. As for its limitations, first, as a preliminary study, it did not include single-cell sequencing of large samples. Second, we did not perform single-cell sequencing analysis of urine from the time of the initial diagnosis of MN, which will be performed in our future studies. Third, the number of women (22.2% in the CR group and 16.7% in the NR group) in our study was significantly lower than in previous studies, which contributed to the low number of urine single cells in our study.
In conclusion, we established a stable and feasible single-cell sequencing technology for urine specimens and drew the first urinary single-cell map of patients with MN. Urinary macrophages from bone marrow had a strong proinflammatory function in CR patients, which can positively regulate the immune system, secrete cytokines, and respond to innate immunity and cytokines. While in NR patients, the urinary macrophages were proportionally less and from renal resident cells, and had strong upregulated genes associated with the patient's urinary protein level and impaired renal function.
Our study provides a new reference for the pathogenesis and treatment outcome of MN. The proportion, origin and function of urine macrophages were different in the three groups. In the CR group, the macrophage was the highest proportion, mainly from bone marrow/peripheral blood, and with a strong anti-inflammatory characteristic. These results may help us to understand the different responses of different MN patients to immunotherapy.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Natural Science Foundation of China (81900622, 82170696 and 81800631), the Shanghai Sailing Program (Grants 19YF1444200) and the Shanghai Shenkang Hospital Develop Center Project (Grants SHDC12022104).
Contributor Information
Xi Liu, Department of Nephrology, Tongji Hospital, School of Medicine, Tongji University, Shanghai, China.
Yu Zhao, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, China.
Yangyang Niu, Department of Nephrology, Tongji Hospital, School of Medicine, Tongji University, Shanghai, China.
Qionghong Xie, Division of Nephrology, Huashan Hospital, and Nephrology Research Institute, Fudan University, Shanghai, China.
Hao Nie, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, China.
Yun Jin, Department of Nephrology, Tongji Hospital, School of Medicine, Tongji University, Shanghai, China.
Yingying Zhang, Department of Nephrology, Tongji Hospital, School of Medicine, Tongji University, Shanghai, China.
Yuqiu Lu, Department of Nephrology, Tongji Hospital, School of Medicine, Tongji University, Shanghai, China.
Saiya Zhu, Department of Nephrology, Tongji Hospital, School of Medicine, Tongji University, Shanghai, China.
Wei Zuo, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, China.
Chen Yu, Department of Nephrology, Tongji Hospital, School of Medicine, Tongji University, Shanghai, China.
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
CONFLICT OF INTEREST STATEMENT
This paper has not been presented elsewhere expect in abstract format at ASN Kidney Week 2022. The authors declare that there are no other potential conflicts of interest with respect to the research, authorship or publication of this manuscript.
REFERENCES
- 1. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group . KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int 2021;100:S1–276. [DOI] [PubMed] [Google Scholar]
- 2. Couser WG. Primary membranous nephropathy . Clin J Am Soc Nephrol 2017;12:983–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beck LH Jr, Bonegio RG, Lambeau G et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009;361:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tomas NM, Beck LH Jr, Meyer-Schwesinger C et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 2014;371:2277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sethi S, Madden BJ, Debiec H et al. Exostosin 1/exostosin 2-associated membranous nephropathy. J Am Soc Nephrol 2019;30:1123–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sethi S, Debiec H, Madden B. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int 2020;97:163–74. [DOI] [PubMed] [Google Scholar]
- 7. Sethi S, Debiec H, Madden B et al. Semaphorin 3B-associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int 2020;98:1253–64. [DOI] [PubMed] [Google Scholar]
- 8. Sethi S, Madden B, Debiec H et al. Protocadherin 7-associated membranous nephropathy. J Am Soc Nephrol 2021;32:1249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sethi S. New ‘antigens’ in membranous nephropathy. J Am Soc Nephrol 2021;32:268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sethi S. Membranous nephropathy: a single disease or a pattern of injury resulting from different diseases. Clin Kidney J 2021;14:2166–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamilton P, Wilson F, Chinnadurai R et al. The investigative burden of membranous nephropathy in the UK. Clin Kidney J 2020;13:27–34. 10.1093/ckj/sfz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alexopoulos E, Leontsini M, Papadimitriou M. Relationship between interstitial infiltrates and steroid responsiveness of proteinuria in membranous nephropathy. Nephrol Dial Transplant 1994;9:623–9. 10.1093/ndt/9.6.623. [DOI] [PubMed] [Google Scholar]
- 13. L'Imperio V, Smith A, Ajello E. MALDI-MSI pilot study highlights glomerular deposits of macrophage migration inhibitory factor as a possible indicator of response to therapy in membranous nephropathy. Proteomics Clin Appl 2019;13:1800019. 10.1002/prca.201800019. [DOI] [PubMed] [Google Scholar]
- 14. Cattran DC. Idiopathic membranous glomerulonephritis. Kidney Int 2001;59:1983–94. 10.1046/j.1523-1755.2001.0590051983.x. [DOI] [PubMed] [Google Scholar]
- 15. Li C, Menoret A, Farragher C et al. Single cell transcriptomics based-MacSpectrum reveals novel macrophage activation signatures in diseases. JCI Insight 2019;4:e126453. 10.1172/jci.insight.126453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deleersnijder D, Callemeyn J, Arijs I et al. Current methodological challenges of single-cell and singlenucleus rna-sequencing in glomerular diseases. J Am Soc Nephrol 2021;32:1838–52. 10.1681/ASN.2021020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abedini A, Zhu YO, Chatterjee S. Urinary single-cell profiling captures the cellular diversity of the kidney. J Am Soc Nephrol 2021;32:614–27. 10.1681/ASN.2020050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arazi A, Rao DA, Berthier CC. The immune cell landscape in kidneys of patients with lupus nephritis. Nat Immunol 2019;20:902–14. 10.1038/s41590-019-0398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng Y, Lu P, Deng Y et al. Single-cell transcriptomics reveal immune mechanisms of the onset and progression of IgA nephropathy. Cell Rep 2020;33:108525. 10.1016/j.celrep.2020.108525. [DOI] [PubMed] [Google Scholar]
- 20. Chen Z, Zhang T, Mao K et al. A single-cell survey of the human glomerulonephritis. J Cell Mol Med 2021;25:4684–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Epelman S, Lavine KJ, Randolph GJ et al. Origin and functions of tissue macrophages. Immunity 2014;41:21–35. 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zimmerman KA, Bentley MR, Lever JM et al. Single-cell RNA sequencing identifies candidate renal resident macrophage gene expression signatures across species. J Am Soc Nephrol 2019;30:767–81. 10.1681/ASN.2018090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nordberg GF, Jin T, Wu X et al. Prevalence of kidney dysfunction in humans-relationship to cadmium dose, metallothionein, immunological and metabolic factors. Biochimie 2009;91:1282–5. 10.1016/j.biochi.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 24. Pepper RJ, Hamour S, Chavele K-M. Leukocyte and serum S100A8/S100A9 expression reflects disease activity in ANCA-associated vasculitis and glomerulonephritis. Kidney Int 2013;83:1150–8. 10.1038/ki.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davies JC, Midgley A, Carlsson E. Urine and serum S100A8/A9 and S100A12 associate with active lupus nephritis and may predict response to rituximab treatment. RMD Open 2020;6:e001257. 10.1136/rmdopen-2020-001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsuzawa Y, Oshima S, Takahara M et al. TNFAIP3 promotes survival of CD4 T cells by restricting MTOR and promoting autophagy. Autophagy 2015;11:1052–62. 10.1080/15548627.2015.1055439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anderson-Baucum E, Piñeros AR, Kulkarni A et al. Deoxyhypusine synthase promotes a pro-inflammatory macrophage phenotype. Cell Metab 2021;33:1883–93.e7. 10.1016/j.cmet.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qiu Y, Chen T, Hu R et al. Next frontier in tumor immunotherapy: macrophage-mediated immune evasion. Biomark Res 2021;9:72. 10.1186/s40364-021-00327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cao Y, Wang Y, Peng N. The ratio of urinary TREM-1/TREM-2 mRNA expression in chronic kidney disease and renal fibrosis. Ann Med 2021;53:1011–9. 10.1080/07853890.2021.1912384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robson KJ, Ooi JD, Holdsworth SR. HLA and kidney disease: from associations to mechanisms. Nat Rev Nephrol 2018;14:636–55. 10.1038/s41581-018-0057-8. [DOI] [PubMed] [Google Scholar]
- 31. Zhou X-J, Nath SK, Qi Y-Y. Novel identified associations of RGS1 and RASGRP1 variants in IgA nephropathy. Sci Rep 2016;6:35781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saito T, Matsunaga A, Fukunaga M. Apolipoprotein E-related glomerular disorders. Kidney Int 2020;97:279–88. 10.1016/j.kint.2019.10.031. [DOI] [PubMed] [Google Scholar]
- 33. Kato T, Ushiogi Y, Yokoyama H et al. A case of apolipoprotein E Toyonaka and homozygous apolipoprotein E2/2 showing non-immune membranous nephropathy-like glomerular lesions with foamy changes. CEN Case Rep 2019;8:106–11. 10.1007/s13730-019-00380-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.