ABSTRACT
The survival rates of many cancers have significantly improved due to recent advancements in cancer screening and therapeutics. Although better cancer outcomes are encouraging, additional health challenges have surfaced, the utmost of which is the burden imposed by various cardiovascular and renal toxicities of anticancer therapies. To improve the overall outcome of patients with cancer, it is essential to understand and manage these treatment-related adverse effects. The cardiovascular side effects of antineoplastic therapies are well-known and include left ventricular dysfunction, heart failure, myocardial ischaemia, QT prolongation, arrhythmia and hypertension. Among these, hypertension is the most common complication, prevalent in about 40% of all cancer patients, yet frequently overlooked and undertreated. This review explores the intricate connection between cancer and hypertension and provides distinct approaches to diagnosing, monitoring and managing hypertension in patients with cancer. We also outline the challenges and considerations that are relevant to the care of patients receiving anticancer drugs with prohypertensive potential.
Keywords: blood pressure management, cancer, cardio-oncology, onco-hypertension, onco-nephrology, VEGF signalling pathway inhibitors
Graphical Abstract
Graphical Abstract.
THE RELATIONSHIP BETWEEN CANCER AND HYPERTENSION
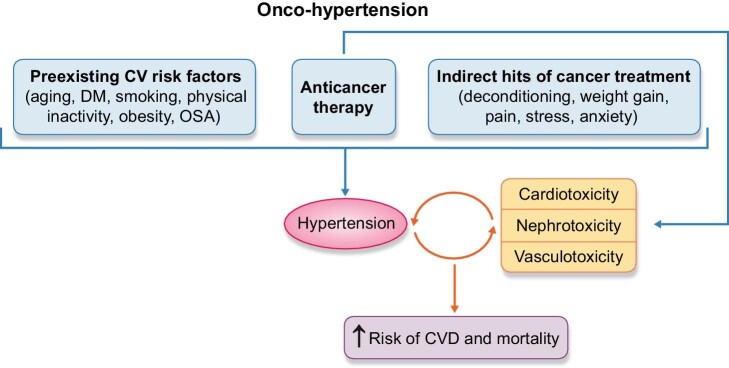
Cancer and hypertension are intricately linked, so much so that their association has inspired its own field of onco-hypertension [1, 2]. They share overlapping pathophysiological mechanisms, including inflammation and oxidative stress, which are associated with common risk factors: diabetes, smoking, obesity, physical inactivity and obstructive sleep apnoea [3]. Additional sequelae of cancer, including deconditioning, pain, anxiety and sleep disorders, may also indirectly promote hypertension. Various anticancer therapies and adjunctive therapies exert prohypertensive effects [4]. Both hypertension and certain anticancer drugs can increase the risk of direct toxicities on the heart, vasculature and kidney, which in turn, exacerbate hypertension in a vicious cycle (Fig. 1).
Figure 1:
Onco-hypertension: the complex interplay between cancer and hypertension. Hypertension in cancer patients can occur due to overlapping risk factors and the direct or indirect effects of cancer therapy. The end-organ toxicity caused by either hypertension or chemotherapy further amplifies the hypertensive response in a vicious cycle.
Furthermore, hypertension may be a risk factor for some cancers. An association between hypertension and the development of renal cell cancer has been demonstrated by several observational studies [5, 6]. The Metabolic Syndrome and Cancer Project, which prospectively followed a cohort of nearly 580 000 participants for 12 years, indicated that elevated blood pressure (BP) was independently associated with a slightly higher risk of cancer incidence in men and cancer-associated mortality in both men and women [7]. However, a clear causal relationship between hypertension and cancer has not been established.
Several observational studies have tried to determine whether there is an association between antihypertensive medications and an increased risk of cancer. Interestingly, three of the drug classes [calcium channel blockers (CCBs), angiotensin-converting enzyme inhibitors (ACE-I)/angiotensin receptor blockers (ARBs) and thiazide diuretics] that were investigated for carcinogenic potential are frequently used for the treatment of hypertension in cancer patients. Notably, there was a large-scale recall of several ARBs in 2018 due to concerns of potentially carcinogenic nitrosamine impurities [8]. However, various studies showed contradictory results and were fraught with confounders [1]. At present, there is no conclusive evidence to suggest a significantly elevated cancer risk, and the long-proven cardiovascular (CV) benefits of these drugs outweigh the minuscule potential risk of cancer.
CAUSES OF HYPERTENSION IN PATIENTS WITH CANCER
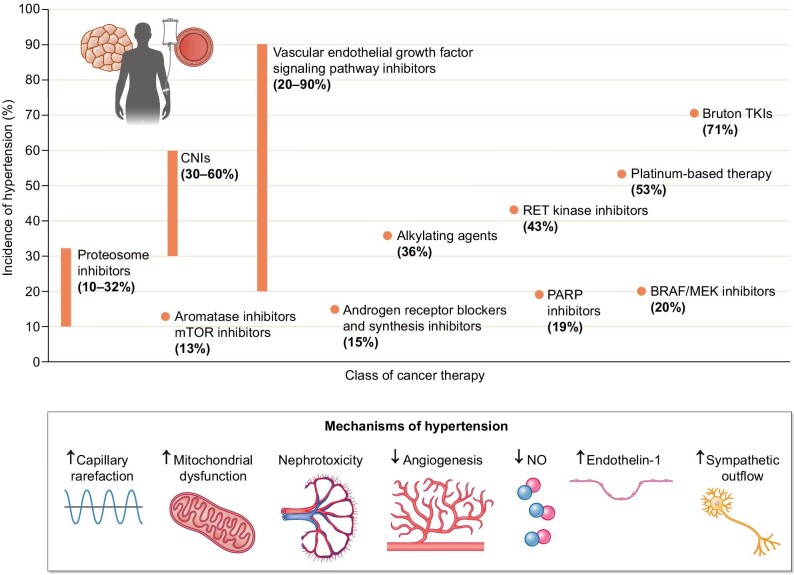
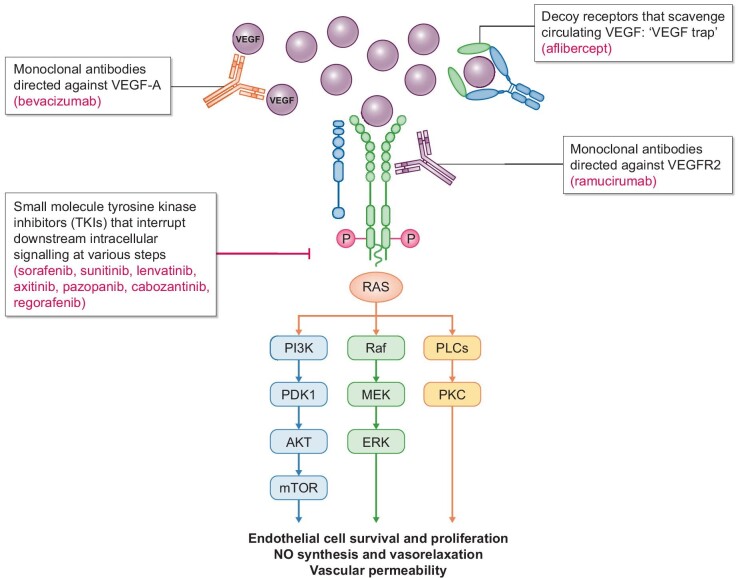
Cancer therapy–related hypertension (CTRH) is the most extensively studied aspect of onco-hypertension. Several classes of cancer therapeutics have been associated with the development of hypertension (Fig. 2), of which vascular endothelial growth factor (VEGF) inhibitors, also known as VEGF signalling pathway inhibitors (VSPIs), are the most common. In fact, an increase in BP from baseline is seen in almost all patients on VSPIs, with new or worsening hypertension in up to 80% of the patients [9, 10]. A meta-analysis by Abdel-Qadir et al. reported a number needed to harm of 6 for the development of hypertension and 17 for severe hypertension [11]. The incidence of life-threatening hypertensive crises with bevacizumab has been estimated to be around 1% [10]. VEGF binding to its receptor (VEGFR) activates downstream intracellular signalling pathways critical for vasodilation and maintenance of vascular integrity via endothelial cell survival, proliferation and permeability. VEGF plays an important role in angiogenesis, the lymphatic system, and the glomerular filtration barrier [12] but also promotes tumour growth and metastasis. VSPIs exert anticancer effects by impairing tumour angiogenesis. Figure 3 illustrates the targets of various VSPIs along the VEGF pathway. The hypertensive effect of VSPIs is believed to be due to a combination of impaired nitric oxide (NO) production and vasodilation, abnormal endothelin-1 and prostacyclin signalling, microvessel rarefaction, increased vascular stiffness, and renal effects that include impaired renovascular homeostasis, decreased natriuresis, increased podocyte permeability (and proteinuria) and eventually glomerular endotheliosis and renal damage [9]. Anti-VEGF ligands such as bevacizumab and aflibercept can cause thrombotic microangiopathy (TMA), while VEGF-tyrosine kinase inhibitors (TKIs) have been associated with minimal change nephropathy and focal segmental glomerulosclerosis [13]. The early hypertensive response likely occurs due to dysfunction of vascular tone and natriuresis rather than glomerular damage, although observational studies suggest a possible association between impaired glomerular filtration rate and increased risk of VSPI-mediated rise in BP [14].
Figure 2:
Incidence and mechanism of hypertension due to various classes of cancer therapy [62].
Figure 3:
Different types of VEGF signalling pathway inhibitors and their actions.
Apart from VSPIs, there are additional classes of cancer therapeutics that promote hypertension, ranging from traditional chemotherapeutic agents (platinum-based therapy, nucleoside analogues and alkylating agents [15, 16]) to targeted therapies. These include proteasome inhibitors for multiple myeloma [17, 18], Bruton’s tyrosine kinase (BTK) inhibitors for chronic lymphocytic lymphoma (CLL) [19], rapidly accelerated fibrosarcoma B-type/mitogen-activated kinase kinase (BRAF/MEK) inhibitors for melanoma and colorectal cancer [20], rearranged during transfection (RET) kinase inhibitors for thyroid and non-small-cell lung cancer [21, 22], poly-ADP ribose polymerase (PARP) inhibitors for ovarian cancer [23, 24], and phosphatidylinositol-3-kinase (PI3K) inhibitors for CLL and breast cancer [25, 26], among others. While the prohypertensive mechanisms of these various medications are still being elucidated, most inhibit common intracellular signalling pathways that result in endothelial dysfunction and NO dysregulation (Table 1). Traditional chemotherapy also causes direct renal toxicity which can eventually contribute to hypertension, although certain agents like cisplatin can conversely lead to orthostatic hypotension and hyponatremia in the acute setting due to renal salt wasting and poor oral intake, especially in head and neck cancer patients [27]. Multivariate logistic regression analysis showed that low BP and the use of renin–angiotensin–aldosterone system (RAAS) inhibitors were associated with a higher incidence of cisplatin nephrotoxicity [28]. Proteasome inhibitors (e.g. carfilzomib, ixazomib or bortezomib) [29] and gemcitabine, a nucleoside analogue, can also cause TMA [30]. Many of these agents are used concurrently and compound the hypertensive effect.
Table 1:
Anticancer drugs and adjunctive therapies commonly associated with hypertension. Listed are example drugs, hypothesized mechanisms of drug-induced hypertension, and suggested first-line treatment of hypertension.
| Anticancer drug class | Example drugs | Putative mechanisms | Suggested first-line treatmenta | Ref |
|---|---|---|---|---|
| VEGF signalling pathway inhibitors | Bevacizumab, sorafenib, sunitinib, lenvatinib, axitinib, pazopanib, regorafinib | ↑ Vasoconstriction (ET-1), ↓ vasodilation (NO), endothelial dysfunction, capillary rarefaction, ↓ lymphangiogenesis, renal injury and TMA | ACE-I/ARB, dihydropyridine CCB | [3, 9, 12, 85–89] |
| Proteosome inhibitors | Bortezomib, carfilzomib, ixazomib | Cellular toxicity, endothelial dysfunction, ↓ NO bioavailability, TMA | ACE-I/ARB | [18, 90, 91] |
| Alkylating agents | Cyclophosphamide, ifosfamide, Busulfan | Oxidative stress, endothelial dysfunction, abnormal vascular remodelling, renal injury | ACE-I/ARB, dihydropyridine CCB | [92, 93] |
| Platinum-based compounds | Cisplatin, carboplatin | ↓ NO bioavailability, endothelial dysfunction, renal injury | ACE-I/ARB, dihydropyridine CCB | [82, 94, 95] |
| Nucleoside analogues | Gemcitabine | Endothelial cell damage, TMA | ACE-I/ARB | [1, 30, 96] |
| BTK inhibitors | Ibrutinib, acalabrutinib | ↓ hsp 70, ↓ NO bioavailability, endothelial dysfunction | ACE-I/ARB, BB (nebivolol or carvedilol) | [19, 97] |
| BRAF/MEK inhibitors | Vemurafenib, dabrafenib, encorafenib | CD47 upregulation, ↓ cGMP, ↓ NO bioavailability | ACE-I/ARB, dihydropyridine CCB | [20, 98, 99] |
| Androgen synthesis inhibitors | Abiraterone, leuprolide | CYP17A inhibition with diversion of steroid precursors to mineralocorticoid production → increased fluid retention | MRA (eplerenone), diuretics | [79, 100] |
| Androgen receptor blockers | Enzalutamide | Endothelial dysfunction, ↓ testosterone-induced vasodilation mediated by L-type calcium channels | Dihydropyridine CCB, ACE-I/ARB, MRA | [59, 101, 102] |
| mTOR inhibitors | Everolimus, sirolimus | ↓ VEGF bioavailability | ACE-I/ARB, dihydropyridine CCB | [103, 104] |
| PI3K inhibitorsb | Copanlisib | ↓ NO production by inhibition of PI3K/AKT/eNOS pathway | ACE-I/ARB, dihydropyridine CCB | [26, 104] |
| RET kinase inhibitors | Selpercatinib, pralsetinib | Inhibition of BRAF/MEK/ERK → CD47 upregulation, ↓ cGMP, ↓ NO bioavailability | ACE-I/ARB, dihydropyridine CCB | [105–107] |
| PARP inhibitorsc | Niraparib | ↓ Dopamine, norepinephrine and serotonin reuptake, inhibition of DYRK1A | BB (nebivolol or carvedilol), ACE-I/ARB | [108–110] |
| Calcineurin inhibitors | Cyclosporine, tacrolimus | ↑ Proximal tubule Na reabsorption, renal dysfunction, ↓ NO bioavailability, ↑ ET-1 and ROS, ↑ RAAS | Dihydropyridine CCB, thiazide diuretics | [41, 111–113] |
| Glucocorticoids | Prednisone, dexamethasone | ↑ Na and water retention, ↑ vasoconstriction, ↑ sensitivity to endogenous vasopressors | MRA, diuretics, ACE-I/ARB, steroid dose reduction or discontinuation | [114] |
| Erythropoiesis-stimulating agents | Erythropoietin, darbopoietin | ↑ Blood viscosity, ↑ vasoconstriction, ↑ sensitivity to endogenous vasopressors | Dihydropyridine CCB, EPO discontinuation | [38] |
In the absence of contraindication.
Idelalisib does not typically cause hypertension.
Olaparib may have antihypertensive and cardioprotective effects.
ET-1, endothelin 1; hsp, heat shock protein; CD47, cluster of differentiation; cGMP, cyclic guanosine monophosphate; CYP17A, cytochrome 17A; DYRK1A, dual-specificity tyrosine phosphorylated and regulated kinase 1A; Na, sodium; EPO, erythropoietin.
Endocrine therapies can also cause hypertension, most notably androgen synthesis inhibitors (leuprolide, abiraterone) and androgen receptor blockers (enzalutamide), used in the treatment of metastatic prostate cancer [31, 32]. There are conflicting data regarding the association of hypertension with aromatase inhibitors. While prior studies suggested an increased risk of arterial hypertension [33] and CV events [34] compared with tamoxifen, a recent meta-analysis did not identify a statistically significant association [35]. Instead, this perceived difference between the two drug classes may be due to the cardioprotective effects of tamoxifen. Similarly, there is no conclusive evidence that immunotherapy causes hypertension. A meta-analysis of 32 randomized controlled trials involving 19 000 patients did not demonstrate an association between the use of immune checkpoint inhibitors and short-term hypertension [36].
Adjunctive medications commonly administered in conjunction with chemotherapy can also raise BP. Glucocorticoids are widely used in anticancer regimens to increase the efficacy of chemotherapy and minimize therapy-related side effects. They contribute to hypertension by promoting sodium and water retention via mineralocorticoid receptor stimulation [15]. Erythropoiesis-stimulating agents (ESAs) are recommended by the American Society of Clinical Oncology/American Society of Hematology for selected patients with chemotherapy-associated anaemia [37]. Recent decades have seen a decline in their use because of increased risk of hypertension, thromboembolic events and mortality [38]. ESA-induced hypertension occurs due to increased blood viscosity, intrinsic vasoconstrictive properties, increased sensitivity to endogenous vasopressors and vascular resistance to NO [15]. ESA discontinuation is advised when antihypertensives are ineffective in managing elevated BP [38]. Frequently used in cancer patients, non-steroidal anti-inflammatory drugs (NSAIDs) can cause or exacerbate hypertension by increased salt and water retention and diminished production of prostaglandins [39]. They should be used judiciously in patients with preexisting CV disease (CVD) or chronic kidney disease (CKD). Calcineurin inhibitors (CNIs) such as cyclosporine and tacrolimus represent another class of drugs used in cancer management, most commonly for immunosuppression following bone marrow transplantation, occasionally for cancer-associated autoimmune haemolytic anaemia and pure red cell aplasia. CNIs can cause hypertension in 30%–60% of patients [40], primarily through their effects on the renal tubules, RAAS and NO–endothelin balance [41].
Radiation therapy has been implicated in hypertension through various pathways. Carotid baroreflex denervation and failure from craniocervical irradiation can lead to labile hypertension or hypertensive crisis. Endothelial cell injury and baroreceptor dysfunction are associated with elevated sympathetic activity, increased reactive oxygen species (ROS) and reduced parasympathetic activity [42], which may also manifest as BP variability, orthostatic intolerance and tachycardia. Radiation-induced renal artery stenosis is rare; however, it should be suspected in patients who develop hypertension following abdominal radiotherapy [43]. Radiation nephropathy can occur in around 20% of patients following kidney irradiation and may present as either acute radiation nephritis (ARN) or chronic radiation nephropathy (CRN) [1]. ARN usually occurs between 6–18 months after radiation exposure. It may be associated with vague symptoms of fatigue, oedema and headaches, or symptoms due to malignant hypertension like encephalopathy and heart failure (HF). Renal biopsy shows loss of endothelial cells with subendothelial expansion and TMA. In contrast, CRN occurs after 18 months of exposure and is often asymptomatic. Hypertension is one of the first signs of CRN, with evidence of interstitial fibrosis and arteriolar sclerosis on histopathology [44]. Hypertension due to radiation nephropathy is usually treated with ACE-I/ARBs.
There are additional nontherapy-related factors that can contribute to increased BP. Cancer patients are at a higher risk for pain, anxiety, depression and sleep problems, all of which can cause transient or chronic BP elevations. They may also have other characteristics which place them at risk for new-onset or exacerbated hypertension, including a high-sodium diet, sedentary lifestyle, tobacco or alcohol use, and weight gain, particularly seen with breast and prostate cancer. Paraneoplastic hypertension can occur due to the release of various vasoactive peptides like endothelin-1, urotensin-II and adrenomedullin (renal cell cancer) [45], renin and angiotensinogen (hepatocellular cancer) [46, 47], corticotropin-releasing hormone (small-cell lung cancer and carcinoid tumours) [48, 49] and catecholamines (phaeochromocytomas and paragangliomas) [50].
DEFINING ONCO-HYPERTENSION
Cancer patients have traditionally been excluded from large-scale hypertension trials. Therefore, there is little evidence to inform the therapeutic thresholds and BP targets in this highly specialized population. Diagnostic and treatment recommendations are based on expert opinion and extrapolation of the general hypertension guidelines to onco-hypertension. There are several limitations to this approach. The pathophysiologic pathways responsible for CTRH may differ fundamentally from those of essential hypertension. For instance, patients receiving VSPIs can develop an abrupt rise in BP within days of starting treatment, resulting in acute end-organ damage at lower BP compared with chronic hypertensives with well-conditioned autoregulatory mechanisms. In one study, 54 normotensive patients treated with sorafenib underwent 24-h ambulatory BP monitoring (ABPM); 93% had a rise in BP by Day 6, and most experienced an increase within the first 24 h of therapy [51]. This phenomenon has been recognized in the Common Terminology Criteria for Adverse Events (CTCAE), and a symptomatic increase in diastolic BP by >20 mmHg is an indication for therapy [52]. The recent International Cardio-Oncology Society (IC-OS) guidelines also define this exaggerated hypertensive response as an increase in systolic BP by >20 mmHg and in mean arterial pressure by >15 mmHg [53].
In addition, inaccurate BP measurements due to cancer-related pain and anxiety often confound hypertension diagnosis. In a small retrospective study that investigated the difference between BP measurements recorded by physicians and nurses in breast cancer patients before initiation of chemotherapy, almost 60% of the patients were noted to have a significant white coat effect [54]. Similarly, masked hypertension is also quite prevalent among cancer patients and can cause underdiagnosis in an office-based setting. This is especially true for hypertension caused by VSPIs and proteasome inhibitors, which can be dose-dependent and transient. Azizi et al. examined home BP monitoring (HBPM) in 14 patients receiving sunitinib; BP increased by Week 1 of treatment initiation and decreased within 1–2 weeks after discontinuing therapy [55]. Another prospective observational study by Mir et al. noted that twice daily HBPM was associated with more than two times higher detection rates of early hypertension compared with in-clinic measurements (55% versus 24%, P <.001) [56]. As a result, most guidelines recommend ABPM or HBPM, especially when initiating or titrating medications like VSPIs, which can potentially cause a rapid rise in BP and hypertensive emergency if undetected.
Another controversy in the management of CTRH involves the use of BP as a biomarker of antineoplastic efficacy. Several studies have demonstrated that patients who develop hypertension with VSPIs have better overall and progression-free survival compared with those who remain normotensive [57, 58]. This observation has established hypertension as a favourable sign of effective VSPI therapy and encouraged the practice of dose titration guided by changes in BP. The package insert for axitinib suggests that the dose may be increased for patients who have tolerated therapy for 2 weeks and remained normotensive without the need for antihypertensive medications [59]. Current data suggest that treating hypertension does not negate the survival benefits conferred by VSPIs [14, 60] and may, in fact, prevent cancer therapy interruption or dose reduction due to the sequelae of severe uncontrolled hypertension.
Lastly, the treatment of CTRH should be guided by the cancer prognosis in an individualized manner. The 2022 European Society of Cardiology (ESC) guidelines on cardio-oncology acknowledge this need and suggest more lenient treatment thresholds (>160/100 mmHg) for asymptomatic patients with metastatic cancer who have an expected survival of less than 1 year [61].
APPROACH TO MANAGEMENT
Diagnosis, evaluation and monitoring
Before starting prohypertensive cancer therapy, patients should be counselled about the risk of developing new or worsening hypertension so that they can participate in BP monitoring and understand the potential need for rapid institution and escalation of antihypertensive therapy. In addition, a thorough assessment of CV risk should be performed at the first visit, as this initial risk stratification drives BP management and targets.
Baseline BP must be obtained in all patients. Not only is this necessary to detect an exaggerated hypertensive response with chemotherapy, but it also helps identify patients with preexisting hypertension who could benefit from early BP management. A recent study that evaluated BP trends after initiation of various VSPIs found that patients receiving treatment for known hypertension had three times lower odds of developing VSPI-induced BP rise compared with baseline normotensive patients [14].
BP should be measured in both arms after being seated for 5 min, without any exercise, smoking or caffeine consumption in the previous 30 min [62]. The patient should be seated comfortably with legs uncrossed and the arm resting at the level of the heart. Elevated BP should be confirmed on at least two occasions before diagnosing hypertension. ABPM is more accurate and carries a stronger association with CV outcomes than office-based monitoring, and it should be performed in all patients when available. Some centres have established remote patient monitoring (RPM) programs to closely monitor the response to cancer therapy [63]. Home BP self-monitoring is an acceptable alternative to ABPM and RPM. In general, BP should be checked at least every week in the clinic when receiving the first cycle of chemotherapy and every 2–3 weeks during the remaining cycles [64]. This should be supplemented by twice-daily BP monitoring at home with a validated device [65].
The 2017 American College of Cardiology/American Heart Association (ACC/AHA) Clinical Practice Guidelines and the 2018 ESC/European Society of Hypertension guidelines are generally used for the diagnosis and grading of hypertension in patients with cancer. Hypertension is diagnosed if the appropriately measured office-based or average home BP is ≥130/80 mmHg [65] or the average BP on ABPM is ≥125/75 mmHg [66]. Subsequent evaluation is like that of noncancer patients. Workup includes ECG, echocardiography and evaluation for secondary causes of hypertension, including hyperaldosteronism, pheochromocytoma, hypercortisolism, CKD, renal artery stenosis and obstructive sleep apnea, as indicated.
Therapeutic goals
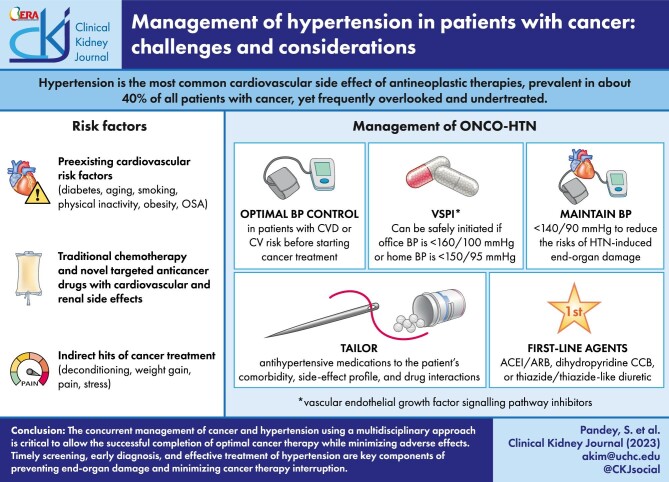
Patients with preexisting CVD [coronary artery disease (CAD); peripheral vascular disease; HF or stroke], proteinuric CKD, diabetes mellitus or atherosclerotic CVD (ASCVD) risk >10% should receive antihypertensive therapies if BP is ≥130/80 mmHg. Patients without known CVD and low ASCVD risk can be treated at a higher BP threshold of ≥140/90 mmHg [62]. BP should be controlled before starting cancer therapy to minimize the risk of cardiotoxicity. The presence of hypertension, particularly poorly controlled hypertension, increases the risk of anthracycline- and trastuzumab-associated cardiomyopathy and HF [67]. A recent study by Kaneko et al. confirmed an association between hypertension and an increased risk of heart failure and other CVD in cancer patients, with an odds ratio of 1.24 for ACC/AHA stage 1 hypertension (130–139/80–89 mmHg) and 1.99 for stage 2 hypertension (>140/90 mmHg) [68]. Effective treatment of hypertension also helps patients tolerate maximum doses of the planned anticancer therapies, yielding better control of the tumour.
Once started on antihypertensive therapy, the goal BP is <130/80 mmHg in most individuals [62]. A goal BP of <140/90 mmHg may be reasonable [61], especially for patients who are unable to either tolerate or achieve stricter BP control due to ongoing chemotherapy. For asymptomatic patients with metastatic cancer with an expected survival of 1–3 years, the target may be relaxed further to 140–159/90–99 mmHg [61]. BP >160/100 mmHg should be treated in all patients, irrespective of the oncologic prognosis, to prevent life-threatening complications, hospitalizations and interruptions to cancer therapy due to uncontrolled hypertension. An acute BP rise (diastolic BP increase >20 mmHg) caused by agents like VSPIs may also benefit from treatment [52].
Prohypertensive anticancer agents should be held if BP rises above 180/110 mmHg and should not be restarted until BP is controlled to <160/100 mmHg. Once BP is better controlled, a multidisciplinary team should assess the competing risks of cancer and CVD and determine whether to rechallenge with dose reduction or switch to an alternative agent [53, 61].
Hypertensive emergency is defined by IC-OS as ‘very high BP elevations associated with acute hypertension-mediated organ damage’ and is an indication for hospital admission for immediate BP-lowering therapy [53]. The occurrence of acute organ damage, including pulmonary oedema, cardiac ischaemia, acute renal failure, papilledema, hypertensive encephalopathy, neurologic deficits or posterior reversible encephalopathy syndrome, may warrant permanent discontinuation of cancer therapy [4, 69].
There are insufficient data to determine a BP threshold predictive of an increased risk of hypertensive emergency with VSPIs. Plummer et al. have reported that VSPI may be safe to start if BP is <160/100 mmHg in the office and <150/95 mmHg at home [69]. However, all efforts must be made to control BP to a level <140/90 mmHg while on VSPI. It is not necessary to delay cancer therapy to achieve this BP goal; instead, BP-lowering and cancer treatment goals can be attained in parallel. Patients with uncontrolled BP despite three antihypertensive medications may benefit from referral to a specialized onco-hypertension clinic.
In addition to BP monitoring, patients should be screened for proteinuria before and during VSPI therapy. Treatment with VSPIs can be continued in most cases of non–nephrotic range proteinuria and can also be managed with ACE-I/ARBs. The implications of more significant proteinuria during VSPI therapy are poorly understood, which is reflected in the lack of consensus and guidance regarding management strategies. A suggested approach includes holding therapy for proteinuria >2 g/day [70, 71]. Proteinuria often disappears upon stopping VSPI therapy; however, persistence beyond 3 months, high-grade proteinuria >3 g/day or microscopic hematuria should prompt a renal biopsy to evaluate for chemotherapy-induced TMA and may require permanent discontinuation of VSPI [70, 72]. A biopsy may also help to identify paraneoplastic glomerular diseases, such as membranous nephropathy, as the cause of proteinuria, which may instead require escalation of chemotherapy.
Choosing antihypertensive medications
There are no randomized controlled trials that prove the superiority of one antihypertensive agent over the rest. As in noncancer patients, antihypertensive medications should be tailored to comorbidities (Table 2). ACE-I/ARBs and dihydropyridine CCBs are the most frequently used agents for the treatment of CTRH because of their vasodilatory effect. While both are believed to have equal BP-lowering effect [14], ACE-I/ARBs are preferred as the first choice because of their beneficial role in concomitant cancer therapy–related cardiac dysfunction [61, 65]. Additionally, some data suggest that the use of ARBs in patients receiving VSPIs has been associated with improved overall and progression-free survival [45, 73]. It has been postulated that ARBs may augment the antitumour efficacy of VSPIs [64, 74]. ACE-I/ARBs may also be beneficial for VSPI-induced proteinuria and vasoreactivity [63]. There are insufficient data pertaining to the use of sodium-glucose cotransporter-2 inhibitors (SGLT2i) in patients with VSPI-induced proteinuria.
Table 2:
Suggested choice of antihypertensive drugs by comorbidity.
| Comorbidity | Suggested antihypertensive medication |
|---|---|
| DM, diabetic nephropathy, proteinuria, CKD | ACE-I/ARBSGLT2i |
| CHF, LV systolic dysfunction | ACE-I/ARB/ARNI |
| SGLT2i | |
| BB | |
| MRA | |
| Loop diuretic | |
| CAD | BB |
| ACE-I/ARB | |
| Nitrates | |
| Arrhythmia | BB |
| Resistant HTN | MRA |
| Nitrates and/or hydralazine | |
| Elderly, isolated systolic HTN | Dihydropyridine CCB |
DM, diabetes mellitus; CHF, congestive heart failure; HTN, hypertension; LV, left ventricular; ARNI, angiotensin receptor/neprilysin inhibitor; BB, beta blocker.
Patients with BP >160/100 mmHg should be treated with a combination therapy of ACE-I/ARBs and dihydropyridine CCBs [61]. Thiazide diuretics may also be used, although caution must be exercised in patients at risk for chemotherapy-induced diarrhoea and electrolyte loss, as they can result in volume depletion and QT prolongation, respectively [62]. For resistant CTRH, spironolactone, nitrates and/or hydralazine are effective agents [61]. Beta-blockers are typically reserved for coexisting indications of CAD, HF and arrhythmias. They may be a good choice for BTK inhibitors which cause both hypertension and atrial fibrillation. They may also be useful for hypertension caused by high sympathetic tone, often associated with pain and stress. Nebivolol is the preferred beta-blocker because it promotes NO bioavailability. Carvedilol may also be a reasonable choice because of its alpha-blocking action. Mineralocorticoid receptor antagonists (MRAs) and other potassium-sparing diuretics are effective agents for hypertension caused by androgen blockers and glucocorticoids, as they counter the effects of sodium-water retention and hypokalemia associated with mineralocorticoid excess. In case of persistent steroid-induced hypertension, loop diuretics or steroid discontinuation may be needed. Table 1 summarizes the preferred antihypertensive medications based on the mechanism of CTRH.
Providers must exercise caution to avoid choosing antihypertensive agents that have a known drug interaction with cancer therapy [63]. For example, non-dihydropyridine CCBs (verapamil, diltiazem) inhibit CYP3A4 enzymes which increase plasma levels of TKIs. In addition, verapamil decreases the excretion of doxorubicin, paclitaxel and irinotecan, and worsens cardiotoxicity. Amlodipine should not be used in patients with VEGF-induced hepatotoxicity. ACE-I have a higher risk of angioedema with mammalian target of rapamycin (mTOR) inhibitors. Loop diuretics have a higher risk of causing ototoxicity and nephrotoxicity with platinum-based therapy. Thiazide diuretics may potentiate cyclophosphamide-induced myelosuppression. Certain TKIs cause QT prolongation, which can be worsened by beta-blocker use, increasing the risk of pause-dependent torsades. Certain non-VEGF TKIs, such as imatinib and gefitinib, increase metoprolol levels and worsen bradycardia. This effect is even more pronounced with crizotinib and ceritinib, which can cause additional bradycardia. Spironolactone should not be used for abiraterone-induced hypertension as it interferes with the antiandrogen effect of abiraterone and can cause an increase in prostate cancer growth. While spironolactone typically carries anti-androgenic properties and is believed to be protective against prostate cancer [75], in an androgen-deprived environment created by androgen synthesis inhibitors, it behaves as a selective androgen receptor modulator and exerts a paradoxical pro-androgenic effect [76–78]. Eplerenone is a safe alternative to spironolactone [79].
Lifestyle modifications
The benefits of lifestyle modifications, such as dietary sodium restriction and exercise, cannot be overemphasized and should be encouraged in all patients [62, 63]. Patients receiving VSPIs are more sensitive to sodium load due to impaired natriuresis, and a sodium-restricted diet has been shown to reduce the risk of VSPI-induced hypertension [80]. Patients should be counselled to avoid substances that can exacerbate hypertension, including caffeine, smoking, alcohol and NSAIDs, especially when receiving cancer therapy.
LONG-TERM FOLLOW-UP
After discontinuation of VSPIs or proteasome inhibitors, BP may decrease in a matter of days [55] and may require a rapid dose reduction of antihypertensive medications. Therefore, these patients should be closely monitored on ABPM or daily HBPM to avoid rebound hypotension and ischaemic events. There is insufficient evidence to identify the risk factors that predispose to this phenomenon.
In contrast, hypertension due to BTK inhibitors can occur several months after treatment initiation [19] and can be persistent [81], especially as the treatment for CLL may continue for several years. Similarly, cisplatin can persist in the bloodstream for up to 20 years after drug exposure, leading to late-onset hypertension due to chronic endothelial dysfunction [82, 83]. Cancer survivors have a higher prevalence of hypertension compared with the general population, accompanied by a higher incidence of CVD if untreated [15, 84]. A meta-analysis revealed that the incidence of HF was 12 times higher in childhood cancer survivors who developed hypertension compared with their normotensive counterparts [84]. Therefore, these patients should be closely followed long after the discontinuation of anticancer therapy. Larger prospective studies are needed to identify the agents that can cause persistent or late-onset hypertension.
CONCLUSION
In summary, the burden of hypertension in patients with cancer is exceedingly high. Many of these patients have underlying CV risk factors; various anticancer therapies can exert prohypertensive effects; and direct cardiac, renal and/or vascular toxicity of cancer treatment can further exacerbate hypertension. All these effects can increase the risk of CV disease and mortality in this vulnerable patient population. The concurrent management of cancer and CV comorbidities, including hypertension, is critical to allow the successful completion of optimal cancer therapy while minimizing adverse effects (Graphical Abstract). The joint efforts of the oncologist, cardio-oncologist, onco-nephrologist, speciality pharmacist and other players are vital to providing optimal care for these patients, especially with new cancer therapeutics being discovered every day. Timely screening, early diagnosis and effective treatment of hypertension are key components of preventing end-organ damage and minimizing cancer therapy dose reduction or interruption. The overarching goal is to prevent short-term and late CV events while achieving the maximum benefits of cancer treatment.
ACKNOWLEDGEMENTS
We thank Dr Mythri Shankar for creation of the Graphical Abstract.
Contributor Information
Shubhi Pandey, Department of Internal Medicine, Calhoun Cardiology Center, University of Connecticut Health, Farmington, CT, USA; University of Connecticut School of Medicine, Farmington, CT, USA.
Amar Kalaria, University of Connecticut School of Medicine, Farmington, CT, USA.
Kenar D Jhaveri, Division of Kidney Diseases and Hypertension, Zucker School of Medicine at Hofstra/Northwell, Great Neck, NY, USA.
Sandra M Herrmann, Division of Nephrology and Hypertension, Mayo Clinic, Rochester, MN, USA.
Agnes S Kim, Department of Internal Medicine, Calhoun Cardiology Center, University of Connecticut Health, Farmington, CT, USA; University of Connecticut School of Medicine, Farmington, CT, USA.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article and in its online supplementary material.
CONFLICT OF INTEREST STATEMENT
A.S.K. is supported by the Letts O'Brien Fund for Breast Cancer Research. K.D.J. is a founder and co-president of the American Society of Onco-Nephrology; and reports consultancy agreements with Secretome, George Clinicals, PMV pharmaceuticals and Calliditas. K.D.J. reports honoraria from the American Society of Nephrology and UpToDate.com; reports serving on the editorial boards of American Journal of Kidney Diseases, Clinical Journal of the American Society of Nephrology, Clinical Kidney Journal, Journal of Onconephrology, Kidney International and Nephrology Dialysis Transplantation; and reports serving as Editor-in-Chief of ASN Kidney News and section editor for onconephrology for Nephrology Dialysis Transplantation. All the authors declared no competing interests. S.M.H. is supported by the National Institute of Health (K08 DK118120).
FUNDING
This study has been funded by the Letts O'Brien Fund for Breast Cancer Research. S.M.H. is supported by the National Institute of Health.
REFERENCES
- 1. Gudsoorkar P, Ruf R, Adnani H et al. Onco-hypertension: an emerging specialty. Adv Chronic Kidney Dis 2021;28:477–89.e1. 10.1053/j.ackd.2021.09.011 [DOI] [PubMed] [Google Scholar]
- 2. Fraeman KH, Nordstrom BL, Luo W et al. Incidence of new-onset hypertension in cancer patients: a retrospective cohort study. Int J Hypertens 2013;2013:379252. 10.1155/2013/379252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DCHv D, Dobbin SJH, Neves KB et al. Hypertension and prohypertensive antineoplastic therapies in cancer patients. Circ Res 2021;128:1040–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Izzedine H, Ederhy S, Goldwasser F et al. Management of hypertension in angiogenesis inhibitor-treated patients. Ann Oncol 2009;20:807–15. 10.1093/annonc/mdn713 [DOI] [PubMed] [Google Scholar]
- 5. Kim CS, Han K-D, Choi HS et al. Association of hypertension and blood pressure with kidney cancer risk: a nationwide population-based cohort study. Hypertension 2020;75:1439–46. 10.1161/HYPERTENSIONAHA.120.14820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sanfilippo KM, Mctigue KM, Fidler CJ et al. Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension 2014;63:934–41. 10.1161/HYPERTENSIONAHA.113.02953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stocks T, Van Hemelrijck M, Manjer J et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension 2012;59:802–10. 10.1161/HYPERTENSIONAHA.111.189258 [DOI] [PubMed] [Google Scholar]
- 8. Gunasekaran PM, Chertow GM, Bhalla V et al. Current status of angiotensin receptor blocker recalls. Hypertension 2019;74:1275–8. 10.1161/HYPERTENSIONAHA.119.13955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Camarda N, Travers R, Yang VK et al. VEGF receptor inhibitor-induced hypertension: emerging mechanisms and clinical implications. Curr Oncol Rep 2022;24:463–74. 10.1007/s11912-022-01224-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Touyz RM, Herrmann SMS, Herrmann J. Vascular toxicities with VEGF inhibitor therapies-focus on hypertension and arterial thrombotic events. J Am Soc Hypertens 2018;12:409–25. 10.1016/j.jash.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abdel-Qadir H, Ethier J-L, Lee DS et al. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: a systematic review and meta-analysis. Cancer Treat Rev 2017;53:120–7. 10.1016/j.ctrv.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 12. Pandey AK, Singhi EK, Arroyo JP et al. Mechanisms of VEGF (vascular endothelial growth factor) inhibitor-associated hypertension and vascular disease. Hypertension 2018;71:e1–8. 10.1161/HYPERTENSIONAHA.117.10271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Izzedine H, Mangier M, Ory V et al. Expression patterns of RelA and c-mip are associated with different glomerular diseases following anti-VEGF therapy. Kidney Int 2014;85:457–70. 10.1038/ki.2013.344 [DOI] [PubMed] [Google Scholar]
- 14. van Dorst DCH, Kabadayi S, Oomen-de Hoop E et al. Treatment and implications of vascular endothelial growth factor inhibitor-induced blood pressure rise: a clinical cohort study. J Am Heart Assoc 2023;12:e028050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen JB, Geara AS, Hogan JJ et al. Hypertension in cancer patients and survivors: epidemiology, diagnosis, and management. JACC CardioOncol 2019;1:238–51. 10.1016/j.jaccao.2019.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knijnenburg SL, Jaspers MW, Van Der Pal HJ et al. Renal dysfunction and elevated blood pressure in long-term childhood cancer survivors. Clin J Am Soc Nephrol 2012;7:1416–27. 10.2215/CJN.09620911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dimopoulos MA, Goldschmidt H, Niesvizky R et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol 2017;18:1327–37. 10.1016/S1470-2045(17)30578-8 [DOI] [PubMed] [Google Scholar]
- 18. Georgiopoulos G, Makris N, Laina A et al. Cardiovascular toxicity of proteasome inhibitors: underlying mechanisms and management strategies. JACC CardioOncol 2023;5:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dickerson T, Wiczer T, Waller A et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood 2019;134:1919–28. 10.1182/blood.2019000840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glen C, Tan YY, Waterston A et al. Mechanistic and clinical overview cardiovascular toxicity of BRAF and MEK inhibitors. JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol 2022;4:1–18. 10.1016/j.jaccao.2022.01.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gainor JF, Curigliano G, Kim D-W et al. et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol 2021;22:959–69. 10.1016/S1470-2045(21)00247-3 [DOI] [PubMed] [Google Scholar]
- 22. Wirth LJ, Sherman E, Robinson B et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med 2020;383:825–35. 10.1056/NEJMoa2005651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mirza MR, Monk BJ, Herrstedt J et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 2016;375:2154–64. 10.1056/NEJMoa1611310 [DOI] [PubMed] [Google Scholar]
- 24. Wu XH, Zhu JQ, Yin RT et al. Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): a randomized, double-blind, placebo-controlled phase III trial☆. Ann Oncol 2021;32:512–21. 10.1016/j.annonc.2020.12.018 [DOI] [PubMed] [Google Scholar]
- 25. Dreyling M, Santoro A, Mollica L et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol 2017;35:3898–905. 10.1200/JCO.2017.75.4648 [DOI] [PubMed] [Google Scholar]
- 26. Greenwell IB, Ip A, Cohen JB. PI3K inhibitors: understanding toxicity mechanisms and management. Oncology (Williston Park) 2017;31:821–8. [PubMed] [Google Scholar]
- 27. Hutchison FN. Renal salt wasting in patients treated with cisplatin. Ann Intern Med 1988;108:21–5. 10.7326/0003-4819-108-1-21 [DOI] [PubMed] [Google Scholar]
- 28. Komaki K, Kusaba T, Tanaka M et al. Lower blood pressure and risk of cisplatin nephrotoxicity: a retrospective cohort study. BMC Cancer 2017;17:144. 10.1186/s12885-017-3135-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hobeika L, Self SE, Velez JCQ. Renal thrombotic microangiopathy and podocytopathy associated with the use of carfilzomib in a patient with multiple myeloma. BMC Nephrol 2014;15:156. 10.1186/1471-2369-15-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Izzedine H, Isnard-Bagnis C, Launay-Vacher V et al. Gemcitabine-induced thrombotic microangiopathy: a systematic review. Nephrol Dial Transplant 2006;21:3038–45. 10.1093/ndt/gfl507 [DOI] [PubMed] [Google Scholar]
- 31. Iacovelli R, Ciccarese C, Bria E et al. The Cardiovascular toxicity of abiraterone and enzalutamide in prostate cancer. Clin Genitourin Cancer 2018;16:e645–53. 10.1016/j.clgc.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 32. Lee HY, Chen H-L, Teoh JY-C et al. Abiraterone and enzalutamide had different adverse effects on the cardiovascular system: a systematic review with pairwise and network meta-analyses. Prostate Cancer Prostatic Dis 2021;24:244–52. 10.1038/s41391-020-00275-3 [DOI] [PubMed] [Google Scholar]
- 33. Van De Velde CJ, Rea D, Seynaeve C et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet 2011;377:321–31. 10.1016/S0140-6736(10)62312-4 [DOI] [PubMed] [Google Scholar]
- 34. Khosrow-Khavar F, Filion KB, Bouganim N et al. Aromatase inhibitors and the risk of cardiovascular outcomes in women with breast cancer: a population-based cohort study. Circulation 2020;141:549–59. 10.1161/CIRCULATIONAHA.119.044750 [DOI] [PubMed] [Google Scholar]
- 35. Boszkiewicz K, Piwowar A, Petryszyn P. Aromatase inhibitors and risk of metabolic and cardiovascular adverse effects in breast cancer patients-a systematic review and meta-analysis. J Clin Med 2022;11:3133. 10.3390/jcm11113133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Minegishi S, Kinguchi S, Horita N et al. Immune checkpoint inhibitors do not increase short-term risk of hypertension in cancer patients: a systematic literature review and meta-analysis. Hypertension 2022;79:2611–21. 10.1161/HYPERTENSIONAHA.122.19865 [DOI] [PubMed] [Google Scholar]
- 37. Bohlius J, Bohlke K, Castelli R et al. Management of cancer-associated anemia with erythropoiesis-stimulating agents: ASCO/ASH clinical practice guideline update. Blood Adv 2019;3:1197–210. 10.1182/bloodadvances.2018030387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schoen MW, Hoque S, Witherspoon BJ et al. End of an era for erythropoiesis-stimulating agents in oncology. Int J Cancer 2020;146:2829–35. 10.1002/ijc.32917 [DOI] [PubMed] [Google Scholar]
- 39. Mohammed T, Singh M, Tiu JG et al. Etiology and management of hypertension in patients with cancer. Cardiooncology 2021;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arslansoyu Camlar S, Soylu A, Kavukcu S. Cyclosporine in pediatric nephrology. Iran J Kidney Dis 2018;12:319–30. [PubMed] [Google Scholar]
- 41. Zhai Y-J, Wu M-M, Linck VA et al. Intracellular cholesterol stimulates ENaC by interacting with phosphatidylinositol‑4,5‑bisphosphate and mediates cyclosporine A-induced hypertension. Biochim Biophys Acta Mol Basis Dis 2019;1865:1915–24. 10.1016/j.bbadis.2018.08.027 [DOI] [PubMed] [Google Scholar]
- 42. Barazzuol L, Coppes RP, Luijk P. Prevention and treatment of radiotherapy-induced side effects. Mol Oncol 2020;14:1538–54. 10.1002/1878-0261.12750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Izzedine H, Cluzel P, Deray G. Renal radiation-induced arterial stenosis. Kidney Int 2007;71:1188. 10.1038/sj.ki.5002137 [DOI] [PubMed] [Google Scholar]
- 44. Klaus R, Niyazi M, Lange-Sperandio B. Radiation-induced kidney toxicity: molecular and cellular pathogenesis. Radiat Oncol 2021;16:43. 10.1186/s13014-021-01764-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ikuerowo SO, Ojewuyi OO, Omisanjo OA et al. Paraneoplastic syndromes and oncological outcomes in renal cancer. Niger J Clin Pract 2019;22:1271–5. [DOI] [PubMed] [Google Scholar]
- 46. Ueno N, Yoshida K, Hirose S et al. Angiotensinogen-producing hepatocellular carcinoma. Hypertension 1984;6:931–3. 10.1161/01.HYP.6.6.931 [DOI] [PubMed] [Google Scholar]
- 47. Kew MC. Arterial hypertension as a paraneoplastic phenomenon in hepatocellular carcinoma. Arch Intern Med 1989;149:2111–3. 10.1001/archinte.1989.00390090135028 [DOI] [PubMed] [Google Scholar]
- 48. Ilias I, Torpy DJ, Pacak K et al. Cushing's syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab 2005;90:4955–62. [DOI] [PubMed] [Google Scholar]
- 49. Delisle L. Ectopic corticotropin syndrome and small-cell carcinoma of the lung: clinical features, outcome, and complications. Arch Intern Med 1993;153:746–52. 10.1001/archinte.1993.00410060054009 [DOI] [PubMed] [Google Scholar]
- 50. Baguet J, Hammer L, Mazzuco T et al. Circumstances of discovery of phaeochromocytoma: a retrospective study of 41 consecutive patients. Eur J Endocrinol 2004;150:681–6. 10.1530/eje.0.1500681 [DOI] [PubMed] [Google Scholar]
- 51. Maitland ML, Kasza KE, Karrison T et al. Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res 2009;15:6250–7. 10.1158/1078-0432.CCR-09-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf [Google Scholar]
- 53. Herrmann J, Lenihan D, Armenian S et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J 2022;43:280–99. 10.1093/eurheartj/ehab674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Costa LJM, Varella PCS, Del Giglio A. White coat effect in breast cancer patients undergoing chemotherapy. Eur J Cancer Care (Engl) 2003;12:372–3. 10.1046/j.1365-2354.2003.00416.x [DOI] [PubMed] [Google Scholar]
- 55. Azizi M, Chedid A, Oudard S. Home blood-pressure monitoring in patients receiving sunitinib. N Engl J Med 2008;358:95–7. 10.1056/NEJMc072330 [DOI] [PubMed] [Google Scholar]
- 56. Mir O, Coriat R, Cabanes L et al. An observational study of bevacizumab-induced hypertension as a clinical biomarker of antitumor activity. Oncologist 2011;16:1325–32. 10.1634/theoncologist.2010-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Scartozzi M, Galizia E, Chiorrini S et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol 2009;20:227–30. 10.1093/annonc/mdn637 [DOI] [PubMed] [Google Scholar]
- 58. Friberg G, Kasza K, Vokes EE et al. Early hypertension (HTN) as a potential pharmacodynamic (PD) marker for survival in pancreatic cancer (PC) patients (pts) treated with bevacizumab (B) and gemcitabine (G). J Clin Oncol 2005;23:3020. 10.1200/jco.2005.23.16_suppl.3020 [DOI] [Google Scholar]
- 59. Zhu X, Wu S. Risks and management of hypertension in cancer patients undergoing targeted therapy: a review. Clin Hypertens 2022;28:14. 10.1186/s40885-022-00197-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rini BI, Cohen DP, Lu DR et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 2011;103:763–73. 10.1093/jnci/djr128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lyon AR, López-Fernández T, Couch LS et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur Heart J 2022;43:4229–361. [DOI] [PubMed] [Google Scholar]
- 62. Cohen JB, Brown NJ, Brown S-A et al. Cancer therapy–related hypertension: a scientific statement from the american heart association. Hypertension 2023;80:e46–57. 10.1161/HYP.0000000000000224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sahni G. Onco-hypertension: changing paradigm of treating hypertension in patients with cancer. J Clin Oncol 2023;41:958–63. 10.1200/JCO.22.01875 [DOI] [PubMed] [Google Scholar]
- 64. Maitland ML, Bakris GL, Black HR et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst 2010;102:596–604. 10.1093/jnci/djq091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rao VU, Reeves DJ, Chugh AR et al. Clinical approach to cardiovascular toxicity of oral antineoplastic agents. J Am Coll Cardiol 2021;77:2693–716. 10.1016/j.jacc.2021.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Whelton PK, Carey RM, Aronow WS et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J Am Coll Cardiol 2018;71:e127–248. 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 67. Von Hoff DD. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 1979;91:710–7. 10.7326/0003-4819-91-5-710 [DOI] [PubMed] [Google Scholar]
- 68. Kaneko H, Yano Y, Lee H et al. Blood pressure classification using the 2017 ACC/AHA guideline and heart failure in patients with cancer. J Clin Oncol 2023;41:980–90. 10.1200/JCO.22.00083 [DOI] [PubMed] [Google Scholar]
- 69. Plummer C, Michael A, Shaikh G et al. Expert recommendations on the management of hypertension in patients with ovarian and cervical cancer receiving bevacizumab in the UK. Br J Cancer 2019;121:109–16. 10.1038/s41416-019-0481-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kandula P, Agarwal R. Proteinuria and hypertension with tyrosine kinase inhibitors. Kidney Int 2011;80:1271–7. 10.1038/ki.2011.288 [DOI] [PubMed] [Google Scholar]
- 71. Brandes AA, Bartolotti M, Tosoni A et al. Practical management of bevacizumab-related toxicities in glioblastoma. Oncologist 2015;20:166–75. 10.1634/theoncologist.2014-0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rashidi A, Wanchoo R, Izzedine H. How I manage hypertension and proteinuria associated with VEGF inhibitor. Clin J Am Soc Nephrol 2023;18:121–3. 10.2215/CJN.05610522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mckay RR, Rodriguez GE, Lin X et al. Angiotensin system inhibitors and survival outcomes in patients with metastatic renal cell carcinoma. Clin Cancer Res 2015;21:2471–9. 10.1158/1078-0432.CCR-14-2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Deshayes F, Nahmias C. Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab 2005;16:293–9. 10.1016/j.tem.2005.07.009 [DOI] [PubMed] [Google Scholar]
- 75. Lainscak M, Pelliccia F, Rosano G et al. Safety profile of mineralocorticoid receptor antagonists: spironolactone and eplerenone. Int J Cardiol 2015;200:25–9. 10.1016/j.ijcard.2015.05.127 [DOI] [PubMed] [Google Scholar]
- 76. Dhondt B, Buelens S, Van Besien J et al. Abiraterone and spironolactone in prostate cancer: a combination to avoid. Acta Clin Belg 2019;74:439–44. 10.1080/17843286.2018.1543827 [DOI] [PubMed] [Google Scholar]
- 77. Walsh PC, Siiteri PK. Suppression of plasma androgens by spironolactone in castrated men with carcinoma of the prostate. J Urol 1975;114:254–6. 10.1016/S0022-5347(17)67001-0 [DOI] [PubMed] [Google Scholar]
- 78. Sundar S, Dickinson PD. Spironolactone, a possible selective androgen receptor modulator, should be used with caution in patients with metastatic carcinoma of the prostate. BMJ Case Rep 2012;2012:bcr1120115238. 10.1136/bcr.11.2011.5238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gill D, Gaston D, Bailey E et al. Efficacy of eplerenone in the management of mineralocorticoid excess in men with metastatic castration-resistant prostate cancer treated with abiraterone without prednisone. Clin Genitourin Cancer 2017;15:e599–602. 10.1016/j.clgc.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Van Doorn L, Visser WJ, Van Dorst DCH et al. Dietary sodium restriction prevents vascular endothelial growth factor inhibitor-induced hypertension. Br J Cancer 2023;128:354–62. 10.1038/s41416-022-02036-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gashonia LM, Carver JR, O'Quinn R et al. Persistence of ibrutinib-associated hypertension in CLL pts treated in a real-world experience. J Clin Oncol 2017;35:7525. 10.1200/JCO.2017.35.15_suppl.7525 [DOI] [Google Scholar]
- 82. Boer H, Proost JH, Nuver J et al. Long-term exposure to circulating platinum is associated with late effects of treatment in testicular cancer survivors. Ann Oncol 2015;26:2305–10. 10.1093/annonc/mdv369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gietema J, Meinardi M, Messerschmidt J et al. Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. Lancet 2000;355:1075–6. 10.1016/S0140-6736(00)02044-4 [DOI] [PubMed] [Google Scholar]
- 84. Chuquin D, Abbate A, Bottinor W. Hypertension in cancer survivors: a review of the literature and suggested approach to diagnosis and treatment. J Cardiovasc Pharmacol 2022;80:522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li W, Croce K, Steensma DP et al. Vascular and metabolic implications of novel targeted cancer therapies: focus on kinase inhibitors. J Am Coll Cardiol 2015;66:1160–78. 10.1016/j.jacc.2015.07.025 [DOI] [PubMed] [Google Scholar]
- 86. Kappers Marië HW, Van Esch JHM, Sluiter W et al. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension 2010;56:675–81. 10.1161/HYPERTENSIONAHA.109.149690 [DOI] [PubMed] [Google Scholar]
- 87. Neves KB, Rios FJ, Van Der Mey L et al. VEGFR (vascular endothelial growth factor receptor) inhibition induces cardiovascular damage via redox-sensitive processes. Hypertension 2018;71:638–47. 10.1161/HYPERTENSIONAHA.117.10490 [DOI] [PubMed] [Google Scholar]
- 88. Mourad J-J, Des Guetz G, Debbabi H et al. Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann Oncol 2008;19:927–34. 10.1093/annonc/mdm550 [DOI] [PubMed] [Google Scholar]
- 89. Lankhorst S, Severs D, Markó L et al. Salt sensitivity of angiogenesis inhibition-induced blood pressure rise: role of interstitial sodium accumulation? Hypertension 2017;69:919–26. 10.1161/HYPERTENSIONAHA.116.08565 [DOI] [PubMed] [Google Scholar]
- 90. Wei Q, Xia Y. Proteasome inhibition down-regulates endothelial nitric-oxide synthase phosphorylation and function. J Biol Chem 2006;281:21652–9. 10.1074/jbc.M602105200 [DOI] [PubMed] [Google Scholar]
- 91. Stangl K, Stangl V. The ubiquitin-proteasome pathway and endothelial (dys)function. Cardiovasc Res 2010;85:281–90. 10.1093/cvr/cvp315 [DOI] [PubMed] [Google Scholar]
- 92. Kooijmans EC, Bökenkamp A, Tjahjadi NS et al. Early and late adverse renal effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst Rev 2019;3:CD008944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sagstuen H, Aass N, Fosså SD et al. Blood pressure and body mass index in long-term survivors of testicular cancer. J Clin Oncol 2005;23:4980–90. 10.1200/JCO.2005.06.882 [DOI] [PubMed] [Google Scholar]
- 94. Nuver J, De Haas EC, Van Zweeden M et al. Vascular damage in testicular cancer patients: a study on endothelial activation by bleomycin and cisplatin in vitro. Oncol Rep 2010;23:247–53. [PubMed] [Google Scholar]
- 95. Yu M, Han J, Cui P et al. Cisplatin up-regulates ICAM-1 expression in endothelial cell via a NF-kappaB dependent pathway. Cancer Sci 2008;99:391–7. 10.1111/j.1349-7006.2008.00696.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Glezerman I, Kris MG, Miller V et al. Gemcitabine nephrotoxicity and hemolytic uremic syndrome: report of 29 cases from a single institution. Clin Nephrol 2009;71:130–9. 10.5414/CNP71130 [DOI] [PubMed] [Google Scholar]
- 97. Lipsky A, Lamanna N. Managing toxicities of Bruton tyrosine kinase inhibitors. Hematology Am Soc Hematol Educ Program 2020;2020:336–45. 10.1182/hematology.2020000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Liu F, Jiang CC, Yan XG et al. BRAF/MEK inhibitors promote CD47 expression that is reversible by ERK inhibition in melanoma. Oncotarget 2017;8:69477–92. 10.18632/oncotarget.17704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Isenberg JS, Ridnour LA, Dimitry J et al. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem 2006;281:26069–80. 10.1074/jbc.M605040200 [DOI] [PubMed] [Google Scholar]
- 100. Attard G, Reid AHM, Yap TA et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol 2008;26:4563–71. 10.1200/JCO.2007.15.9749 [DOI] [PubMed] [Google Scholar]
- 101. Navarro-Dorado J, Orensanz LM, Recio P et al. Mechanisms involved in testosterone-induced vasodilatation in pig prostatic small arteries. Life Sci 2008;83:569–73. 10.1016/j.lfs.2008.08.009 [DOI] [PubMed] [Google Scholar]
- 102. Traish AM, Saad F, Feeley RJ et al. The dark side of testosterone deficiency: III. Cardiovascular disease. J Androl 2009;30:477–94. 10.2164/jandrol.108.007245 [DOI] [PubMed] [Google Scholar]
- 103. Motzer RJ, Hutson TE, Glen H et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 2015;16:1473–82. 10.1016/S1470-2045(15)00290-9 [DOI] [PubMed] [Google Scholar]
- 104. Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci 2011;4:51. 10.3389/fnmol.2011.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Højer Wang L, Wehland M, Wise PM et al. Cabozantinib, vandetanib, pralsetinib and selpercatinib as treatment for progressed medullary thyroid cancer with a main focus on hypertension as adverse effect. Int J Mol Sci 2023;24:2312. 10.3390/ijms24032312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Drilon A, Subbiah V, Gautschi O et al. Selpercatinib in patients with RET fusion-positive non-small-cell lung cancer: updated safety and efficacy from the registrational LIBRETTO-001 phase I/II trial. J Clin Oncol 2023;41:385–94. 10.1200/JCO.22.00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev 2001;12:361–73. 10.1016/S1359-6101(01)00012-0 [DOI] [PubMed] [Google Scholar]
- 108. Chen X, Wen Q, Kou L et al. Incidence and risk of hypertension associated with PARP inhibitors in cancer patients: a systematic review and meta-analysis. BMC Cancer 2023;23:107. 10.1186/s12885-023-10571-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lafargue CJ, Dal Molin GZ, Sood AK et al. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol 2019;20:e15–28. 10.1016/S1470-2045(18)30786-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sandhu D, Antolin AA, Cox AR et al. Identification of different side effects between PARP inhibitors and their polypharmacological multi-target rationale. Br J Clin Pharmacol 2022;88:742–52. 10.1111/bcp.15015 [DOI] [PubMed] [Google Scholar]
- 111. Kaye D, Thompson J, Jennings G et al. Cyclosporine therapy after cardiac transplantation causes hypertension and renal vasoconstriction without sympathetic activation. Circulation 1993;88:1101–9. 10.1161/01.CIR.88.3.1101 [DOI] [PubMed] [Google Scholar]
- 112. Lassila M. Interaction of cyclosporine A and the renin-angiotensin system; new perspectives. Curr Drug Metab 2002;3:61–71. 10.2174/1389200023337964 [DOI] [PubMed] [Google Scholar]
- 113. Hošková L, Málek I, Kopkan L et al. Pathophysiological mechanisms of calcineurin inhibitor-induced nephrotoxicity and arterial hypertension. Physiol Res 2017;66:167–80. 10.33549/physiolres.933332 [DOI] [PubMed] [Google Scholar]
- 114. Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol 2012;27:1059–66. 10.1007/s00467-011-1928-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.