Abstract
The nucleoskeleton forms a filamentous meshwork under the nuclear envelope and contributes to the regulation of nuclear shape and gene expression. To understand how the Arabidopsis (Arabidopsis thaliana) nucleoskeleton physically connects to the nuclear periphery in plants, we investigated the Arabidopsis nucleoskeleton protein KAKU4 and sought for functional regions responsible for its localization at the nuclear periphery. We identified 3 conserved peptide motifs within the N-terminal region of KAKU4, which are required for intermolecular interactions of KAKU4 with itself, interaction with the nucleoskeleton protein CROWDED NUCLEI (CRWN), localization at the nuclear periphery, and nuclear elongation in differentiated tissues. Unexpectedly, we find these motifs to be present also in NUP82 and NUP136, 2 plant-specific nucleoporins from the nuclear pore basket. We further show that NUP82, NUP136, and KAKU4 have a common evolutionary history predating nonvascular land plants with KAKU4 mainly localizing outside the nuclear pore suggesting its divergence from an ancient nucleoporin into a new nucleoskeleton component. Finally, we demonstrate that both NUP82 and NUP136, through their shared N-terminal motifs, interact with CRWN and KAKU4 proteins revealing the existence of a physical continuum between the nuclear pore and the nucleoskeleton in plants.
The KAKU4 protein, a component of the plant nucleoskeleton, diverged from an ancestral nucleoporin and provides a continuum between the nucleoskeleton and the nuclear pore complex basket via short peptide motifs.
IN A NUTSHELL.
Background: The nuclear envelope is made up of a double membrane that separates the nucleus from the cytoplasm. It isolates and protects the DNA and creates 2 functional compartments with DNA replication and transcription taking place inside the nucleus and translation in the cytoplasmic compartment. The nuclear envelope is associated inside the nucleus with the nucleoskeleton, a nuclear structure composed of lamins and their associated proteins. The nuclear envelope is punctuated by thousands of nuclear pore complexes (NPCs) that not only regulate the trafficking of macromolecules in and out of the nucleus but also play significant roles in transcription regulation.
Question: To function in a coordinated manner, the nuclear envelope, NPCs, and nucleoskeleton must be closely linked by a network of protein–protein interactions. These interactions are poorly documented in plants as the plant-specific proteins within these structures have only recently been described. This motivated us to investigate the characteristics and functions of these interactions.
Findings: One such plant-specific protein is KAKU4, a component of the nucleoskeleton in which, using Arabidopsis as a model species, we identified 3 short peptide motifs of about 30 amino acids each. These motifs allow protein–protein interaction with the CROWDED NUCLEI proteins, the main component of the plant nucleoskeleton, and are required for elongation of the nucleus in differentiating tissues. The motifs were also found in NUP82 and NUP136, 2 plant-specific nucleoporins, suggesting that KAKU4 diverged from an ancestral nucleoporin, which had the ability to interact with the nucleoskeleton and led to the emergence of KAKU4 as a component of the plant nucleoskeleton.
Next steps: In future, it will be important to assess if the NPC–nucleoskeleton physical interactions identified are essential to anchor the nucleoskeleton at the nuclear envelope and to control NPC composition and distribution. Whether they regulate gene expression or chromatin organization in response to stress should also be investigated.
Introduction
The nuclear envelope is an iconic structure of the eukaryotic cell. It separates the nucleus from the cytoplasm, creating 2 functional compartments with DNA replication and transcription taking place inside the nucleus and translation in the cytoplasmic compartment (De Magistris and Antonin 2018). However, the nuclear envelope is made of a double membrane that is more than a simple barrier. The outer nuclear membrane is connected to the cytoskeleton and the inner nuclear membrane to the nucleoskeleton, a nuclear structure constituted of intermediate filament proteins called lamins in animals and their associated proteins (Briand and Collas 2020). The nuclear envelope also contains several hundred nuclear pore complexes (NPCs) that regulate the trafficking of macromolecules in and out of the nucleus (Knockenhauer and Schwartz 2016) and play roles in transcription regulation, e.g. through the interaction with the Spt-Ada-Gcn5 Acetyltransferase transcriptional coactivator complex (Raices and D’Angelo 2017) or by binding to Polycomb and Trithorax complexes (Pascual-Garcia et al. 2014; Jacinto et al. 2015). There is increasing evidence in eukaryotes that the nuclear envelope, NPCs, and the nucleoskeleton are closely connected via protein–protein interactions (PPIs) to function in a coordinated manner (Kittisopikul et al. 2021), but the characteristics and the roles of these interactions remain poorly understood in photosynthetic organisms given the presence of several plant-specific proteins within these structures. Indeed, plant cells, like animal cells, have a cytoskeleton, a nuclear envelope, NPCs, and a nucleoskeleton (Meier et al. 2017). However, one of the challenges addressed in recent years has been the identification of the main components of the nucleoskeleton, which, unlike in animals, is not made up of lamins and lamin-associated proteins (Meier et al. 2017; Tang et al. 2020). Indeed, the plant nucleoskeleton includes specific proteins called CROWDED NUCLEI 1 to 4 (CRWN 1 to 4) (Dittmer et al. 2007; Ciska et al. 2013) that share long coiled-coil regions with the lamins in animal cells, as well the plant-specific CRWN-binding protein KAKU4 (Goto et al. 2014). Despite their different composition, the nucleoskeletons in animals and plants share common functions such as maintenance of nuclear shape (Shumaker et al. 2006; Dittmer et al. 2007; Goto et al. 2014) and interaction with specific chromatin domains called lamin-associated domains (LADs) situated at the nuclear periphery (van Steensel and Belmont 2017; Hu et al. 2019). Plant LADs have been identified by ChIP for chromatin domains enriched in CRWN1 and comprise transcriptionally silent genes and transposable elements belonging to the repressed B chromatin compartment (Hu et al. 2019). Chromatin regions located at the nuclear periphery have also been characterized by restriction enzyme-mediated Chromatin ImmunoPrecipitation (ChIP)-sequencing (RE-ChIP) targeting NUCLEOPORIN (NUP) 136, a component of the nuclear basket, the nucleoplasmic part of the NPC (Bi et al. 2017). Chromatin regions bound by NUP136 overlap considerably with those of CRWN1 raising the possibility of a dual contribution of the NPC and the nucleoskeleton in promoting contact between repressed chromatin and the nuclear envelope. Interestingly, elongated nuclei often observed in plant differentiated tissues become more rounded in crwn (Wang et al. 2013), kaku4 (Goto et al. 2014), and nup136 mutants (Tamura and Hara-Nishimura 2011), indicating that the NPC and the nucleoskeleton might fulfill shared cellular functions. More recently, combining subtractive proteomics and BioID2-based Proximity Labeling (PL), label-free Quantitative Mass Spectrometry (LFQMS), the protein interactome at the Arabidopsis (Arabidopsis thaliana) nuclear periphery was characterized and shown to include up to 200 PLANT NUCLEAR ENVELOPE TRANSMEMBRANE (PNET) proteins (Tang et al. 2020). Among them, PNET2 interacts with CRWN1 and KAKU4, and KAKU4 is associated with the CRWNs; PNET10 and PNET11; NUP82, NUP136, NUP50a, and NUP50c (nucleoporins from the NPC basket); nucleosome assembly proteins; histone H2A and H2B; histone chaperones; and DNA-binding proteins (Tang et al. 2021). This suggests that interactions between CRWNs and KAKU4 with other nuclear periphery or with chromatin-associated proteins may be more frequent than originally thought, which may allow fine-tuning nucleoskeleton–chromatin interactions and gene expression regulation.
Here, we used KAKU4 protein as an entry point to explore in more detail its physical interactions with CRWNs in an attempt to explain how KAKU4 and CRWNs localize to the nuclear periphery. Since KAKU4 does not possess an identifiable transmembrane region that could account for its enrichment at the nuclear periphery, we searched for protein regions responsible for KAKU4 localization. We identified 3 peptide motifs in the N-terminus of KAKU4, which mediate its peripheral localization, are involved in PPI with the CRWN proteins, and are mandatory for elongation of the nuclei in differentiated tissues. We find that KAKU4 shares these motifs with NUP82 and NUP136, 2 nucleoporins situated in the NPC basket, and that these motifs mediate interaction with the CRWN proteins, hence demonstrating the existence of a physical connection between the plant nucleoskeleton and the NPC basket. Genetic interactions further support a functional link between these 2 cell structures as the kaku4 mutation enhances the nup136 phenotype in vegetative and reproductive tissues. Finally, phylogenetic analyses predict that the N-terminal regions of KAKU4, NUP82, and NUP136 diverged from an ancestral plant-specific nucleoporin, which had the ability to interact with the nucleoskeleton and led to the emergence of KAKU4 as a new component of the plant nucleoskeleton.
Results
N-terminal region of KAKU4 is required for PPI and contains 3 conserved peptide motifs
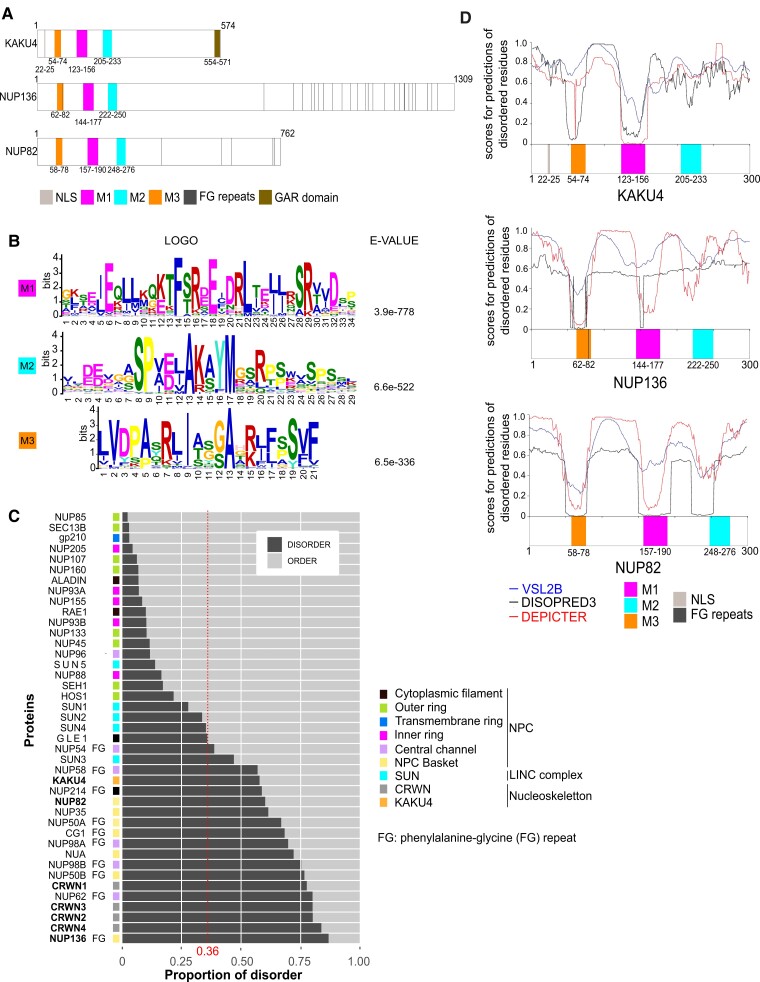
The nucleoskeleton component KAKU4 from Arabidopsis participates in several protein complexes at the nuclear periphery (Goto et al. 2014; Tang et al. 2020, 2021). To define the protein regions of KAKU4 allowing its intermolecular protein interaction and its interaction with CRWN proteins, we performed yeast-2-hybrid (Y2H) assays with 3 constructs expressing the full-length (amino acids 1 to 574), the N-terminus (1 to 310), or the C-terminus (311 to 574) of the KAKU4 protein (see ‘Materials and methods’ section and Supplemental Fig. S1A). We found that the N-terminal region of KAKU4 was sufficient to promote both intermolecular interactions of KAKU4 with itself (Supplemental Fig. S1B) and interaction of KAKU4 with CRWN1, CRWN2, and CRWN3 (Supplemental Fig. S1C). The expression of the N- or C-terminal part of KAKU4 as a GFP fusion in Nicotiana benthamiana leaf cells further revealed that the N-terminal domain of KAKU4 is necessary and sufficient to mediate localization at the nuclear periphery (Supplemental Fig. S1, D and E). We then used the Multiple Em for Motif Elicitation (MEME) suite (Bailey et al. 2015) to predict motifs in the N-terminal region based on their conservation among 16 KAKU4 orthologs from 6 major sister groups of the green lineage (see ‘Materials and methods’ section). This approach revealed several small, evolutionarily conserved regions in the N-terminal domain of KAKU4 (Fig. 1A). The 3 most conserved motifs (M) were selected and numbered according to their E-value ranking (M1, M2, and M3) (Fig. 1, A and B). The PEP-FOLD3 de novo structure modeling engine (Lamiable et al. 2016) was then used to predict secondary structures of the short motifs (Fig. 1C). The 3 motifs are expected to fold into one or several alpha helices that were also predicted by alphaFold and are available in the Uniprot database under KAKU4 accession number Q949W6. PEP-FOLD3 revealed that the M1 motif forms a longer helix region (I127-V154) with a possible break around residues 135-TFA-137; the M2 motif comprises a shorter helix (P213-A223) and the M3 motif 2 smaller helices (I51-V55 and A59-G65). As alpha helices are frequently involved in PPI (Jochim and Arora 2009), we explored whether the short KAKU4 motifs contribute to the observed intermolecular interactions of KAKU4. To investigate this, we designed 2 sets of constructs, one expressing only the M1, M2, and/or M3 motifs and other expressing full-length KAKU4 comprising deletions of these motifs (see ‘Materials and methods’ section and Fig. 1D). We found that in Y2H assays, the M1 motif is required for intermolecular protein interactions of 2 N-terminal regions of KAKU4 and that the M1 motif alone is sufficient to promote interaction with the N-terminal part of KAKU4 (Fig. 1E and Supplemental Fig. S2A). Instead, the M2 motif is necessary for interactions with CRWN1, CRWN2, and CRWN3 proteins, but the presence of the M1 and M3 motifs enhances these interactions, particularly in the case of CRWN2 and CRWN3 (Fig. 1F and Supplemental Fig. S2B). These results identify M1 and M2, located within the N-terminal region of KAKU4 and forming short alpha helices, as key functional elements that drive intermolecular interactions between KAKU4 with itself and with the CRWN proteins.
Figure 1.
Arabidopsis KAKU4 contains conserved motifs required for PPI. A) Domains and motifs organization of KAKU4: N- (1 to 310) and C-terminal region (311 to 574), nuclear localization signal (NLS, 22 to 25), glycine arginine rich domain (GAR domain, 554 to 571), and 3 conserved motifs named M1, M2, and M3. B) Consensus motifs identified by MEME from 16 species representative of the green lineage (see ‘Materials and methods’ section). Position of M1 (123 to 155), M2 (209 to 237), and M3 (50 to 69) in Arabidopsis KAKU4 and logos corresponding to the probability of each possible amino acid at each position in the peptide sequence. E-values are indicated for the 3 motifs. C) PEP-FOLD3 prediction with the peptide sequences used for prediction (top) and the predicted helices (boxes) as well as the superposition of the 5 best models of the helical organization of the motifs (bottom). D) Schematic representation of KAKU4 constructs on the left deletion constructs (deletion as dashed lines) and on the right constructs expressing the motifs alone. E and F) Summary of Y2H experiments testing interaction of M1, M2, and M3 motifs (white cells) or M1 and M2 deletions (gray cells) and the N-terminal region of KAKU4 E) or the CRWN proteins F) (see also Supplemental Fig. S2 for original data) by growth of yeast cell on restrictive medium (SD/-Leu/-Trp/-Ade/-His). Strong interaction (++), interaction (+), and no interaction (−) are indicated. Empty vectors pGBKT7 and pGADT7 are used, respectively, as bait and prey negative controls.
Short peptide motifs are required to localize KAKU4 at the nuclear periphery and for nuclear elongation
We then investigated the contribution of the 3 conserved motifs within the N-terminal region to KAKU4 localization at the nuclear periphery and to the nuclear phenotypes triggered by misexpression of KAKU4. Two types of nuclear phenotypes were recorded in previous studies including: (i) nuclear membrane overgrowth and deformation upon KAKU4 overexpression and (ii) reduced nuclear elongation upon loss of KAKU4 (Goto et al. 2014). We generated a set of KAKU4 GFP-fusion constructs containing either the full-length coding sequence of KAKU4 or the full-length sequence with different motifs deleted as in Fig. 1D. First, these different constructs were expressed transiently in N. benthamiana. In our experimental conditions, full-length KAKU4 caused membrane overgrowth in 80.4% of the analyzed nuclei (Fig. 2A, left). In 57.1% of the observed nuclei, KAKU4 localized exclusively at the nuclear periphery (Fig. 2A, right), whereas in the remaining nuclei, KAKU4 was enriched at the nuclear periphery, but also showed weak nucleoplasmic localization. Expression of any of the 3 motifs M1, M2, or M3 alone or M1 and M2 simultaneously (M1M2) did not localize the GFP fusion constructs at the nuclear periphery (Fig. 2B). In contrast, expression of all 3 motifs together (M3M1M2) was sufficient to restrict KAKU4 to the nuclear periphery revealing a role for these 3 motifs in mediating KAKU4 localization. However, neither combination of these motifs induced membrane overgrowth (Fig. 2B), suggesting that the short motifs alone cannot induce this phenotype. Furthermore, deletion of M1, M2, M3, M1M2, or M3M1M2 motifs abolished the peripheral localization of KAKU4 (Fig. 2C). Instead, membrane overgrowth still occurred when M1, M2, or M1M2 were deleted, and only removal of M3 alone or in combination with M1 and M2 prevents membrane overgrowth (Fig. 2C), suggesting that M3 contributes to this phenotype although it is not sufficient to induce membrane overgrowth on its own (Fig. 2B).
Figure 2.
Function of short motifs in nuclear shape and KAKU4 localization in Arabidopsis. A to C) Transient expression assays in N. benthamiana of different KAKU4 constructs expressed as GFP fusions as described in Fig. 1D. For each panel, representative nuclei for the most prominent nuclear phenotypes are shown. The percentage of nuclei displaying membrane overgrowth (white arrowhead) or localization exclusively at the nuclear periphery (NP) is indicated for each construct. A) When highly expressed, full-length KAKU4 can cause membrane overgrowth in N. benthamiana (left). When KAKU4 is expressed at low to moderate levels, localization is observed exclusively at the NP with no membrane overgrowth (right). B and C) Quantification of NP localization and membrane overgrowth for various combinations of B) motifs alone or C) motif deletions. D) Representative nuclei from Arabidopsis anther filaments stained with DAPI in WT (elongated nuclei) or kaku4-2 mutant (rounded nuclei). E) Quantification of nuclear shape using the aspect ratio (major axis/minor axis) calculated with the Fiji plugin Shape Descriptors of DAPI-stained anther filament nuclei in WT and kaku4-2 mutants. F and G) Complementation assay using Arabidopsis transgenic lines expressing different KAKU4 constructs as GFP fusions in kaku4-2 mutant background (representative nuclei in Supplemental Fig. S3). F) Aspect ratio based on the localization of the GFP signal. A dashed line indicates the aspect ratio value (2.06) for kaku4-2 mutant. G) Quantification of the localization of GFP-tagged KAKU4 constructs. Values are expressed as the log2 ratio of fluorescence intensity at the NP versus the nucleoplasm. Statistical significance is indicated for the kaku4-2 mutant relative to WT using Wilcoxon test (E, P-value = 2.02e−11) or relative to p35S-GFP-K4 in kaku4-2F) and G) using the Kruskal–Wallis test with P-value = 7.97e−05 F) and 8.04e−04 G), followed by pairwise comparisons using the Wilcoxon test with Holm correction for F) and G). The box represents the 25 to 75th percentiles, the median is indicated as a horizontal line across the box, and the dark red diamond and value represent the mean. The whiskers are equal to 1.5× the interquartile range. Outliers are represented by dots. Ns, not significant (P-value >0.05), *P-value <0.05, **P-value <0.01, ****P-value <0.0001. Scale bar = 10 µm. n = number of nuclei.
To confirm these observations in Arabidopsis, we established transgenic lines for a selection of these constructs in kaku4-2 mutant plants. Complementation analysis for nuclear shape and KAKU4 localization was then performed in anther filament cells, which contain characteristic elongated nuclei in wild-type (WT) plants, as previously described (Dittmer et al. 2007; Choi et al. 2019). First, we confirmed by DAPI staining that anther filament nuclei are rounder in the kaku4-2 mutant compared with in the WT (average aspect ratio of 3.65 in WT, 2.06 in kaku4-2, P < 0.0001, Fig. 2, D and E). When observing anther filament cell nuclei expressing the different GFP constructs, we first observed that no membrane overgrowth was induced in our transgenic Arabidopsis plants. We then scored nuclear shape with the help of the KAKU4-GFP signal and found that the reduced nuclear elongation in kaku4-2 mutants was partially complemented upon expression of full-length KAKU4 (mean aspect ratio of 2.69, Fig. 2F). Partial complementation of the altered nuclear shape was also observed when the 3 motifs were expressed together (mean aspect ratio of 3.07). However, more rounded nuclei were observed when M3 or M1M2 were deleted (mean aspect ratios of 1.98 and 1.87, respectively, Fig. 2F), revealing that in Arabidopsis, at least M3 and M1 in combination with M2 (M1M2) participate in the establishment or maintenance of elongated nuclei.
Using the same nuclei data set, we then investigated whether the conserved motifs contribute to the localization of KAKU4 at the nuclear periphery by computing the ratio of fluorescence intensity at the nuclear periphery versus the nucleoplasm, a ratio >1 indicating an enrichment at the nuclear periphery. Expression of full-length KAKU4 or only the 3 motifs (M3M1M2) results in preferential localization of the fusion protein at the nuclear periphery (mean ratio 1.56, Fig. 2G). Deleting M2 or M3 motifs does not delocalize KAKU4 from the periphery, suggesting that these 2 motifs are dispensable for the KAKU4 localization in Arabidopsis. In contrast, deleting M1 or M1M2 (ratios: 0.85 < 1) delocalizes KAKU4 to the nucleoplasm, confirming the important role of M1 in determining the localization of KAKU4 at the nuclear periphery in Arabidopsis.
Finally, in a complementary analysis using the same data set, we simultaneously assessed nuclear localization and morphology for each nucleus to investigate the possible interdependence of these 2 nuclear phenotypes. We noticed that the deletion of M1 or M1M2 renders nuclei more spherical, and about half of these nuclei also show delocalization of KAKU4 to the nucleoplasm, suggesting a possible link between the 2 phenotypes (Supplemental Fig. S3).
Taken together, although we noticed certain differences between the 2 plant experimental systems, our results show that in Arabidopsis, nuclear elongation requires the presence of the 3 motifs, in particular M1 and M3. M1 is further critical for KAKU4 localization at the nuclear periphery in Arabidopsis, while in N. benthamiana, the 3 motifs are needed for its localization at the periphery and M3 contributes to membrane overgrowth.
Short peptide motifs of KAKU4 are conserved in NUP82 and NUP136, 2 plant-specific proteins of the NPC basket
To determine whether M1, M2, and M3 are specific to KAKU4, we used the protein sequences of the 3 motifs from Arabidopsis KAKU4 as queries in BLASTp (Altschul et al. 1990) or in FIMO (Grant et al. 2011) searches. Unexpectedly, we found regions of similarity in NUP82 and NUP136, 2 paralogous proteins described as components of the NPC basket (Tamura et al. 2010, 2017) (Fig. 3A). We then refined our motif predictions by conducting a new MEME analysis for the 3 protein families, this time using a set of 52 orthologs representing the NUP82, NUP136, and KAKU4 protein families (see ‘Materials and methods’ section, Fig. 3B, and Supplemental Table S1). This confirmed that the 3 peptide motifs discovered in KAKU4 are also conserved in size, order, and amino acid sequences within the N-terminal ends of NUP82 and NUP136 nucleoporins.
Figure 3.
The 3 motifs of KAKU4 are conserved in NUP136 and NUP82. A) Schematic representation of Arabidopsis KAKU4, NUP136, and NUP82 proteins including the 3 motifs M1, M2, and M3. B) MEME logos and associated E-values for 3 motifs conserved between KAKU4, NUP82, and NUP136 from 16 species representative of the green lineage (see ‘Materials and methods’ section). C) Proportion of IDRs and ordered regions in NUP, CRWN, KAKU4, and SUN proteins from Arabidopsis. The median value (0.36) of disorder proportion of the Arabidopsis proteome is indicated as a red dashed line. NUPs containing phenylalanine-glycine (FG) repeats and NPC substructures are indicated by FG and with different colors, respectively. D) Profiles of intrinsically disordered residues predicted by VSL2B, DISOPRED3, and DEPICTER within the N-terminal regions (1 to 300) of KAKU4, NUP82, and NUP136 containing M3, M1, and M2 peptide motifs.
As short peptide motifs are often found in intrinsically disordered proteins (IDPs) (Tompa et al. 2014), we investigated whether KAKU4, NUP82, and NUP136 are IDPs. To this aim, we took advantage of DescribePROT, a database providing access to precomputed IDP predictions (Peng et al. 2006) for the Arabidopsis proteome (Zhao et al. 2021). Within a collection of 41 proteins representative of the nuclear periphery proteome, KAKU4, NUP82, and NUP136 were shown to contain a high proportion of disordered regions, respectively with 57%, 60%, and 87% compared with the whole proteome, which has a median value of 36% (Fig. 3C and Supplemental Table S2). We then refined our analysis using VSL2B, the meta predictors of DISOPRED3 (Jones and Cozzetto 2015) and DEPICTER (Barik et al. 2020) to predict intrinsically disordered regions (IDRs) in the N-terminal part of KAKU4, NUP82, and NUP136 (amino acids 1 to 300). From this analysis, we found that several IDRs interspersed with more structured regions close to or overlapping the motifs (Fig. 3D and Supplemental Data Set 1). IDP/IDR searches predict KAKU4, NUP82, and NUP136 as disordered proteins that may not adopt spontaneously stable secondary structures except in the regions containing the conserved motifs that are ordered.
Taken together, KAKU4 shares the M1, M2, and M3 motifs with 2 nucleoporins of the NPC basket. In a context of highly IDP regions surrounding M1, M2, and M3, the motifs may therefore serve as docking sites for PPI.
NUP82 and NUP136 drive PPI between the NPC and the nuclear periphery through their short peptide motifs
Since M1 and M2 of KAKU4 are involved in PPI with components of the nuclear periphery, we tested if these motifs in the 2 NPC basket proteins enable interaction with KAKU4 and CRWN proteins. We first performed Y2H assays testing interactions among KAKU4, NUP82, and NUP136 full-length proteins. NUP136, when used as bait, auto activated the reporter construct, impeding our ability to test its intermolecular interactions, whereas the interactions could be recorded for NUP82 (Fig. 4A and Supplemental Fig. S4A). NUP82 and NUP136 interact physically in Y2H. The interaction was confirmed by bimolecular fluorescence complementation (BiFC) as previously shown (Tamura et al. 2017) as well as the intermolecular interaction of KAKU4 with itself (Fig. 4, A and B and Supplemental Table S3). In Y2H assays on high stringency medium, we did not detect an interaction between either NUP82 or NUP136 and KAKU4 (Fig. 4A and Supplemental Fig. S4A), but we recorded an interaction of the 2 NUPs with KAKU4 in planta using BiFC assays (Fig. 4B and Supplemental Table S3). In N. benthamiana, interactions among NUP82, NUP136, and KAKU4 were all found to take place at the nuclear periphery (Fig. 4B). We then tested the ability of the 2 NUPs to interact with CRWN proteins and demonstrated that, like KAKU4, the 2 NUPs interact directly with CRWN1, CRWN2, and CRWN3 proteins (Fig. 4C). The interaction between NUP82 and CRWN2 was confirmed in planta by BiFC and also shown to take place at the nuclear periphery (Fig. 4B and Supplemental Table S3). Altogether, Y2H and BiFC experiments revealed multiple PPI among KAKU4, NUP82, NUP136, and the CRWNs (Fig. 4D) revealing the existence of a continuum between the nucleoskeleton and the NPC basket.
Figure 4.
NUP82 and NUP136 interact with CRWN proteins via their motifs M1 and M2. A, C, and F) Y2H assays showing growth on test (SD/-Leu/-Trp/-Ade/-His) or permissive (SD/-Leu/-Trp) media. Empty vectors pGBKT7 and pGADT7 are used, respectively, as bait and prey negative controls. Interactions (strong interaction [++], interaction [+], and no interaction [−]) among KAKU4, NUP136, and NUP82 proteins A) (Supplemental Fig. S4 for original data) and CRWN proteins C). B) PPI assay in N. benthamiana using BiFC. Different proteins fused to the N-terminal (nY) or C-terminal (cY) half of yellow fluorescent protein (YFP) are coexpressed with mRFP-fibrillarin2 (Fib2) as a positive marker for transformed cells. D) Schematic representation of the PPI between NUP136, NUP82, KAKU4, and CRWNs proteins recorded by BiFC and Y2H in this study. E) Schematic representation of NUP136 and NUP82 constructs with deletions (dashed line) of motifs M1 (magenta) and M2 (cyan) or motifs M1 and M2 only. F) Y2H assay testing the role of M1 and M2 motifs from NUP136 and NUP82 in the interaction with CRWN proteins (Supplemental Fig. S4 for original data). G) Transient coexpression of mRFP-NLS/GFP-CRWN1 and mRFP-NLS/GFP-KAKU4 in Arabidopsis root protoplasts from WT and nup136-2 nup82-1 mutant. Scale bar = 10 µm.
We then focused on the M1 and M2 motifs, which in KAKU4 are involved in PPI, of NUP82 and NUP136 and generated different constructs expressing either the full-length proteins, deletions for their corresponding M1 and M2 motifs, or only the region including M1M2 (see ‘Materials and methods’ section and Fig. 4E). In Y2H assays, we found that the regions encompassing M1 and M2 of NUP82 or NUP136 are sufficient to interact with CRWN2 and CRWN3 proteins (Fig. 4F and Supplemental Fig. S4B). Deletion of the 2 motifs completely impaired interactions between NUP136 and the CRWNs, while weak interactions between NUP82 and CRWN2 remained suggesting that other regions of NUP82 could participate in interactions with CRWN2 (Fig. 4F and Supplemental Fig. S4B). These results indicate that formation of a physical link between the CRWN components of the nucleoskeleton and the 2 nucleoporins from the NPC basket requires their M1 and M2 motifs. Finally, to investigate whether the localization of CRWN proteins and/or KAKU4 at the nuclear periphery depends on the anchoring of the nucleoskeleton at the NPC via interactions with NUP136 and NUP82, we expressed GFP-KAKU4 and GFP-CRWN1 as a representative member of the CRWN family in WT and nup136 nup82 root protoplasts from Arabidopsis. In this system, even in the absence of the 2 NUPs, localization of CRWN1 and KAKU4 was observed at the nuclear periphery (Fig. 4G).
Taken together, we revealed here a physical link between the nucleoskeleton and the NPC basket that is mediated by the conserved M1 and M2 motifs of NUP136 and NUP82. These PPIs, however, are not the only determinants of KAKU4 and CRWN localization at the nuclear periphery.
KAKU4 and NUP136 genetically interact with each other, and loss of KAKU4 enhances growth and fertility defects of nup136 mutants
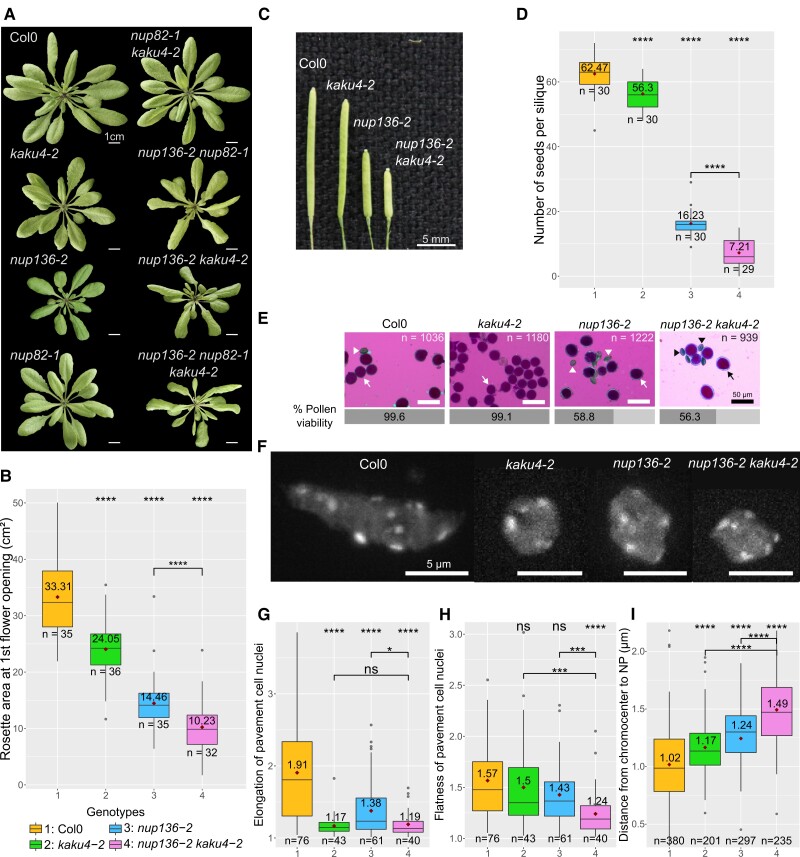
Since we identified that KAKU4, NUP82, and NUP136 proteins show reciprocal binding, we next investigated their genetic interaction, i.e. we determined whether the phenotypes of different higher order mutant combinations involving nup136-2, nup82-1, and kaku4-2 differ from those of the single mutants. Both KAKU4 and NUP136 loss-of-function mutations affect nuclear shape, impact vegetative development, and reduce seed production, whereas these phenotypes were not observed in nup82 mutant plants (Tamura et al. 2010, 2017; Goto et al. 2014, 2020). At the vegetative stage, the nup136-2 nup82-1, nup136-2 kaku4-2, and nup136-2 nup82-1 kaku4-2 mutants have laterally inward curved leaves aggravating weak leaf curling occasionally observed in the nup136 single mutant (Fig. 5A). The nup136 single mutant and all mutants containing nup136 (nup136-2 nup82-1 [Tamura et al. 2017], nup136-2 kaku4-2, and nup136-2 nup82-1 kaku4-2) have reduced rosette size compared with the WT and the nup82 and kaku4 mutants (Fig. 5, A and B, Supplemental Fig. S5A).
Figure 5.
Analysis of genetic interactions between NUP136 and KAKU4. A) Photos of WT and single (kaku4-2, nup136-2, and nup82-1), double (nup136-2 kaku4-2, nup82-1 kaku4-2, and nup136-2 nup82-1), and triple mutant (nup136-2 nup82-1 kaku4-2) combinations at the day of first flower opening. Photo scale = 1 cm. B to D) Quantification of different plant morphology parameters in WT and kaku4-2, nup136-2 single mutants and nup136-2 kaku4-2 double mutants. B) Rosette area at the day of first flower opening (see also Supplemental Fig. S5A). C) Size of mature siliques (Supplemental Fig. S5B for quantification). Scale bar = 5 mm. D) Number of seeds per silique. n = number of siliques. E) Microscopy observation of Alexander's stained pollen and quantification of the percentage of viable pollen. n = number of pollen grain scored. Scale bar = 50 µm. Viable pollen appears in dark pink (arrow) and dead pollen in blue (arrowhead). F) Images of pavement cell nuclei from 14-d-old seedlings stained with DAPI in WT and mutants. Scale bar = 5 µm. G to I) Quantification of nuclear flatness G) and nuclear elongation H) computed by NucleusJ 2.0 software and distance from chromocenter to nuclear periphery I) determined by NODeJ 1.0 software. The box represents the 25 to 75th percentiles, the median is indicated as a horizontal line across the box, and the dark red diamond and value represent the mean. The whiskers are equal to 1.5× the interquartile range. Outliers are represented by dots. n = number of nuclei G to H) or chromocenters I). Statistics analyses were done by comparing to the WT or by comparing the specified genotypes using Wilcoxon tests; ns, not significant (P-value >0.05), *P-value <0.05, ***P-value <0.001, ****P-value <0.0001.
As no phenotypic difference was observed at the rosette stage between the nup136-2 nup82-1 kaku4-2 and the nup136-2 kaku4-2 mutants (Supplemental Fig. S5A), we then decided to focus on the nup136-2 kaku4-2 double mutant and the corresponding single mutants. In reproductive tissues, a significant decrease in silique size was observed for nup136-2 compared with the WT, and siliques were even shorter in the nup136-2 kaku4-2 double mutants (Fig. 5C and Supplemental Fig. S5B). In line with the shorter siliques, seed number was reduced in all mutants compared with in the single mutants, with an additive effect for the double mutant nup136-2 kaku4-2 displaying on average only 7.21 seeds per silique (Fig. 5D). In addition, Alexander staining assays showed that 41% and 44% of pollen grains were nonviable in nup136-2 and nup136-2 kaku4-2, respectively, while pollen in kaku4-2 was fully viable supporting a major effect of the nup136-2 mutation on pollen viability (Fig. 5E). Finally, we investigated the impact of loss of NUP136 and KAKU4 on nuclear shape in pavement cells of the cotyledon epidermis in 14-d-old seedlings. While the nuclear volume was largely unaffected by loss of NUP136, KAKU4, and both proteins (Supplemental Fig. S5C), we found that nuclear elongation was significantly reduced in nup136-2, kaku4-2, and nup136-2 kaku4-2 mutants that show rounder nuclei compared with the WT (Fig. 5, F and G). Additionally, the nup136-2 kaku4-2 mutant displayed an additive effect compared with both single mutants with a decrease in nuclear flatness (Fig. 5H) and an increase in the distance between chromocenters and the nuclear periphery (Fig. 5I), suggesting a contribution from KAKU4 and NUP136 to the positioning of these heterochromatic structures at the nuclear periphery.
Taken together, loss of KAKU4 enhances certain developmental phenotypes of nup136 and aggravates nuclear shape defects present in the NPC mutant.
N-terminal regions of NUP82, NUP136, and KAKU4 originate from an ancestral nucleoporin
As NUP82 and NUP136 have already been described as paralogs (Tamura et al. 2017) and we identified structural similarities in the N-terminal region of KAKU4, NUP82, and NUP136, we explored if these proteins have a common evolutionary origin.
To demonstrate that the 3 protein families derived from a common ancestor, the occurrence of KAKU4, NUP82, and NUP136 orthologs was sought in a set of representative species of the green lineage selected for their fully sequenced genomes (see ‘Materials and methods’ section). We observed that NUP136 is present in Marchantiophyta, which are nonvascular land plants, and conserved in Bryophyta and in all angiosperms. KAKU4 and NUP82 emerged more recently and were first detected in the Amborella trichopoda genome. According to TimeTree (Kumar et al. 2017), this first result supports NUP136 as the most ancestral protein family of the 3 that appeared about 500 to 600 Ma, while NUP82 and KAKU4 emerged 200 to 300 Ma (Fig. 6A). The phylogenetic tree performed using full-length proteins grouped NUP136, NUP82, and KAKU4 in 3 connected clades supporting the divergence of the 3 protein families from a common ancestor (Fig. 6B). In this tree, NUP136 is the most ancestral family, while a closer relationship is observed between NUP82 and KAKU4, supporting the hypothesis that they diverged from an ancestral NUP136 protein through 2 successive gene duplications.
Figure 6.
Phylogenetic relationships among KAKU4, NUP82, and NUP136. A) Presence or absence of NUP136, NUP82, and KAKU4 orthologs in 6 groups of species representative of the green lineage (groups indicated as different colors). The 22 plant species used in this study (left) and numbers of paralogs per species (right) for NUP136, NUP82, and KAKU4 proteins are indicated. B) Maximum likelihood tree constructed with IQ-TREE from a MAFFT alignment of amino acid sequences using orthologs of NUP136, NUP82, and KAKU4 proteins. Clades are distinguished by thin black (NUP136), thick black (NUP82), and thick light gray branches (KAKU4). Evolution rates expressed as substitutions per site are given for each clade at the right of the tree. Tree scale: 1 substitution per site. C) Evolution rates expressed as dN/dS ratio for full length, N-terminal, and C-terminal regions of KAKU4, NUP82, and NUP136. Genetic drift (M0) hypothesis is rejected if P-value <0.5. D) Schematic representation of NUP82, NUP136, and KAKU4 divergence from an ancestral gene that has undergone 2 successive rounds of duplication and subsequent divergence of paralogs. Proteins are represented by a N-terminal region corresponding to the first 300 amino acids (white box with M1, M2, and M3, respectively in magenta, cyan, and orange) and a C-terminal region as a gray box (>300 amino acids). Two hypotheses are proposed to explain KAKU4 divergence (in red): (i) the C-terminal region of KAKU4 derived from the same ancestral gene as NUP82 and NUP136 or (ii) the C-terminal region was acquired through a recombination event from another yet unknown gene. E) Exon structures of KAKU4, NUP82, and NUP136 genes from Arabidopsis (Ath) drawn with the Gene Structure Display Server (GSDS). N- and C-terminal regions of KAKU4, NUP82, and NUP136 are indicated at the bottom of the figure. Exons (rectangles) are drawn to scale, realigned manually to include M1, M2, and M3 motifs and FG repeats; exon scale = 1 kb. Introns are not drawn to scale and are depicted as a black line. NW global alignment for the N-terminal part (exons 1 to 5 or 1 to 6, left) and the C-terminal part (exons 6 to 8 or 7 to 13, right) shown as a similarity matrix among the 3 Arabidopsis proteins with the percentage of identity on the top and NW scores on the bottom of the matrix. Exon 9 of NUP136 containing the FG repeats was analyzed separately (right panel).
Quantification of evolution rates for each clade as the sum of amino acid substitutions per site indicates higher conservation of NUP136 protein sequences (9.34 substitution per site) consistent with the preservation of its original function within the NPC basket, while NUP82 and KAKU4 diverged faster (11.48 and 13.41 substitutions per site, respectively). To determine if the observed divergence is the result of genetic drift or if selection caused nonsynonymous changes, we then used codon substitution modeling (Yang 2007) and analyzed the coding sequences using Codeml NSsites. To avoid underestimation of synonymous mutations observed when long evolutionary periods are investigated, we restricted our analysis to 9 closely related Brassicaceae species (see ‘Materials and methods’ section). This analysis indicated that selection occurs in the 3 gene families when the whole coding sequence was analyzed or when the sequences encoding only the N- (first 300 amino acids) or C-terminal parts (>300 amino acids) of the proteins were analyzed, showing that not only the N-terminal part comprising the 3 short motifs but also the remaining protein sequence is under selection (Fig. 6C). From this phylogenetic analysis, we propose that NUP82, NUP136, and KAKU4 diverged from a common ancestor with a gene structure resembling the actual gene structure of NUP136. After the duplication events, our analyses indicate that both the sequences encoding the N- and the C-terminal regions from all 3 proteins were kept under selective pressure to preserve their newly acquired biological functions.
We summarized these evolutionary scenarios in a model (Fig. 6D). Starting from an ancestral gene encoding a N-terminal region that includes M1, M2, and M3 and a C-terminal region enriched in FG repeats, a first duplication occurred with, for 1 of the 2 paralogs, loss of the FG repeat region to result in an ancestral form of NUP82 and KAKU4. A second duplication of this ancestor of NUP82 and KAKU4 would then have resulted in the current version of the genes (Fig. 6D).
Despite the existence of selection pressure on the C-terminal region of KAKU4, its origin remains uncertain and could either be explained by sequence divergence after 2 gene duplications (first hypothesis as proposed above) or by a new C-terminal sequence gained by recombination from an unrelated sequence (gene X in our second hypothesis) (Fig. 6D, right). In favor of the second hypothesis, we observed that the conservation between the 3 gene families is higher for the N-terminal region of the respective proteins (8.78 to 10.87 substitution per site), while the C-terminal region of KAKU4 diverged faster (21.41 substitution per site) (Supplemental Fig. S6). Second, by looking at NUP82, NUP136, and KAKU4 gene structures in Arabidopsis, we confirmed that the exon structures of the N-terminal region are well conserved between the 3 gene families while they diverged in the C-terminal region especially for KAKU4 raising the possibility that its C-terminal region could have been gained from another unknown gene (Fig. 6, D and E). Using the Needleman–Wunsch (NW) algorithm, one of the most sensitive tools for global alignment of protein sequences (Needleman and Wunsch 1970), we observed identities ranging from 16% to 25% in the N- and C-terminal regions of Arabidopsis KAKU4, NUP82, and NUP136 proteins (Fig. 6E, matrices). However, the NW scores corresponding to the sum of every match (+1), mismatch (−1), and indel (−1) gave positive values when comparing the N-terminal part of all 3 proteins while the scores remained positive for the C-terminal region only for the comparison of NUP82 with NUP136 supporting the second hypothesis. Nevertheless, homology searches using the C-terminal region of KAKU4 from Arabidopsis or A. trichopoda by BLASTp, tBLASTn, or mmseq2 failed to identify another protein sharing sequence similarities with KAKU4. Finally, despite the homologies observed in the N-terminal part of our 3 proteins, KAKU4 shows a very different localization from NUP136, with KAKU4 forming a continuous ring at the nuclear periphery, while NUP136 localizes in spots at the nuclear periphery, again underlining that KAKU4 has acquired a new function associated with the nucleoskeleton (Supplemental Fig. S7, A and B).
In summary, our phylogenetic analyses suggest a scenario of functional diversification of KAKU4, NUP82, and NUP136, in which the current NUP136 protein retained a structure close to that of the ancestral protein and, together with NUP82, establishes a continuum between the NPC basket and the nucleoskeleton. Instead, KAKU4, while retaining the ability to interact with CRWN proteins, diverged in terms of structure and biological function and became an integral component of the nucleoskeleton.
Discussion
Our initial investigation of the KAKU4 protein led us to discover 3 conserved motifs within its N-terminal region that we termed M1, M2, and M3 and which range in length from 20 to 30 amino acids according to the MEME prediction. We were able to assign at least 4 molecular functions to these motifs including PPI (M1 and M2), membrane overgrowth (M3 in N. benthamiana), localization of KAKU4 at the nuclear periphery (M1 in Arabidopsis), and nuclear envelope elongation (M1M2 and M3 in Arabidopsis). M1 and M2 are further involved in different types of interactions, including intermolecular interactions of KAKU4 with itself (M1) and multimeric complex formation with the CRWN proteins (M2) summarized in Fig. 7. During experiments to decipher the biological function of the different motifs, we observed some differences between results obtained during transient expression in N. benthamiana and in stable transgenic Arabidopsis lines. M3M1M2 promoted an almost perfect enrichment of KAKU4 at the nuclear periphery in N. benthamiana, but not in Arabidopsis, and M3 effectively contributed to membrane overgrowth, a phenotype we observed only in N. benthamiana in this study. However even if some of our observations were specific to N. benthamiana, the results from the 2 model systems support our conclusion that the 3 short conserved regions fulfil molecular functions in KAKU4 localization and the modulation of nuclear shape previously observed for full-length KAKU4 in Arabidopsis (Goto et al. 2014) and in maize (McKenna et al. 2021). Finally, from the results revealing the importance of M1 for intermolecular interaction of KAKU4 with itself and localization at the periphery, it is tempting to speculate that KAKU4 intermolecular interactions with itself could promote its aggregation or polymerization to achieve its function within the nucleoskeleton. Unexpectedly, we discovered that these 3 motifs are not only found in KAKU4 homologs but also in the N-terminal part of NUP82 and NUP136, 2 nucleoporins of the NPC basket, and that KAKU4 interacts with NUP82 and NUP136. In addition to the presence of shared motifs and the physical interaction between KAKU4 and the 2 NUPs, we also showed genetic interactions between KAKU4 and NUP136, as we observed additive developmental defects in the double nup136 kaku4 mutant compared with in the respective single mutants. Together, these results indicate that KAKU4 and NUP136 not only share common motifs and interact with CRWN proteins but also are involved in shared biological processes.
Figure 7.
Model for the plant-specific continuum between nucleoskeleton and nuclear pores. During plant evolution, KAKU4 emerged as a new nucleoskeleton component from an ancestral nucleoporin (NUP). KAKU4 contains the short peptide motifs M1, M2 and M3 shared with the nucleoporins NUP82 and NUP136 from the nuclear pore basket. M1 and M2 from KAKU4, NUP82, and NUP136 are required for interaction with CRWN proteins establishing a continuum between the nucleoskeleton and the nuclear pore basket through direct PPI (arrows).
Short peptide motifs have been described in NUPs from other species such as NUP93/Nic96, NUP98/Nup145N, and NUP35/Nup53 (human/yeast, respectively) where they mediate key interactions with NUP188/Nup188, NUP205/Nup192, and NUP155/Nup170, respectively, needed for the assembly of the inner ring complex of the nuclear pore basket (Lin et al. 2016; Beck and Hurt 2017). Interestingly, although the 3 motifs of the plant NUP82, NUP136, and KAKU4 do not share sequence similarities with the motifs in yeast and human NUPs, they are functionally similar in promoting PPI. Extending the research of short protein motifs to all plant NUPs may help unravel the structural basis for protein assembly at the plant NPC.
We also found large IDRs in NUP82, NUP136, and KAKU4 proteins, which are interspersed with structured regions containing the identified peptide motifs in the N-terminal tail. IDRs may function as flexible linkers favoring the exposure of the motifs to facilitate interactions with other proteins while also providing a conformational separation of these protein-docking regions compatible with the formation of a multimeric complex. We hypothesize that for NUP82, NUP136, and KAKU4, conformational transitions between fully disordered and partially structured folding states triggered by motif-mediated PPI may allow alternating between NPC and nucleoskeleton-specific functions. The occurrence of motifs with functional significance within disordered proteins may be more widespread in plants. While it is well documented in other species (van der Lee et al. 2014), few examples are known in plants (Hatos et al. 2020). One recent example is GUANYLATE BINDING PROTEIN-LIKE 3 (GBPL3), a component of the NPC basket that interacts with CRWN1, CRWN4, and KAKU4 (Reimann et al. 2023) and forms a larger complex by phase separation (Huang et al. 2021). This complex includes components of the NPC basket (GBPL3, NUP136, and NUP82) and the nucleoskeleton (CRWNs and KAKU4) and interacts with PWWP-DOMAININTERACTOR OF POLYCOMBS1 (PWO1), a histone reader that recruits POLYCOMB REPRESSIVE COMPLEX 2 (PRC2) and interacts with CRWN1 (Hohenstatt et al. 2018; Mikulski et al. 2019). It is tempting to speculate that this protein network forms domains at the nuclear periphery to promote transcriptional repression by deposition of H3K27 trimethylation by PRC2 (Reimann et al. 2023).
We found that M1 and M2 identified in KAKU4, NUP82, and NUP136 are involved in direct interactions with nucleoskeleton components CRWN and KAKU4 (Fig. 7). These PPIs of CRWNs with KAKU4, NUP136, NUP82, and GBPL3 establish a continuum between the NPC and the nucleoskeleton and may help to anchor the nucleoskeleton at the nuclear envelope. However, when we experimentally tested whether interactions with NUP82 and NUP136 are necessary for CRWNs or KAKU4 localization at the nuclear periphery, we found that KAKU4 and CRWN1 remained located at the nuclear periphery even in the absence of these 2 NPC proteins, which also indicates that the phenotype of nup136 mutants is not caused by KAKU4 or CRWN mislocalization. NUP82 and NUP136 are therefore not the only protein components of the nuclear periphery that contribute to anchor the nucleoskeleton at the nuclear periphery. Besides nuclear pores, the inner nuclear envelope proteins, Sad1-UNC84 homology (SUN) and PNET2, which were shown to interact with CRWN1 (Graumann 2014; Tang et al. 2021), could play such a role. Indeed, using subtractive proteomics between NE-enriched and NE-depleted extracts as well as proximity-labeling proteomics, CRWNs, KAKU4, and PNET2 were found to be in close proximity at the nuclear envelope (Tang et al. 2020, 2021). As PNET2 sits within the inner nuclear membrane through its transmembrane domains, it can be expected that PNET2 contributes to attach the nucleoskeleton at the nuclear periphery. Following this line of thought, KAKU4 may localize to the nuclear periphery not only through its interaction with CRWN proteins but also with complexes involving PNET2 (Tang et al. 2020, 2021).
We identified alternative, but not exclusive, roles for the interactions between nucleoporins and the nucleoskeleton, namely, to control the nuclear basket composition or to achieve a regular distribution of NPCs within the nuclear envelope. Mammalian NUP153, the functional counterpart of NUP136 (Neumann et al. 2007), interacts with lamins A and B, and this interaction is required to recruit and maintain NUP153 at the NPC (Smythe et al. 2000; Al-Haboubi et al. 2011). Furthermore, in mouse embryonic fibroblasts, removal of all lamins leads to clustering of the NPCs, which can be rescued by reexpression of either A- or B-type lamin (Guo and Zheng 2015). Whether such a mechanism exists in plants remains to be investigated.
What might be the functional role of the molecular connection between the nucleoskeleton and the NPC? One hypothesis is that this connection facilitates gene expression regulation in response to external stimuli. Indeed, rapid transcriptional responses involving one or several of the NUP, KAKU4, and CRWN proteins have been reported during hyperosmotic stress (Goswami et al. 2020), in response to copper (Sakamoto et al. 2020) or upon biotic stress (Choi et al. 2019). Consistently, several pieces of evidence suggest that gene expression in plants is influenced by the positioning of genes in respect to the nuclear periphery (Feng et al. 2014; Smith et al. 2015; Nützmann et al. 2020; Sakamoto et al. 2020). As such, the nucleoskeleton may play a central role by recruiting specific, repressive chromatin domains at the nuclear periphery (Hu et al. 2019; Mikulski et al. 2019). NPCs are also known to participate in stress responses (Yang et al. 2017). More specifically, NUP82 and NUP136 control the expression of defense-related genes. This can be achieved either by direct interaction between NUPs and target genes (Pascual-Garcia et al. 2014) or by modulating the nuclear import of specific regulators (Tamura et al. 2017; Tamura 2020; Wang et al. 2023). Thus, the physical link between the NPC basket and the nucleoskeleton may contribute to rapid transitions between an inactive (i.e. near the nucleoskeleton) and an active state (i.e. near the NPCs) in response to stress, as suggested during the formation of gene loops in yeast and animals (Yang et al. 2017). Investigations of the subnuclear localization of NUP82, NUP136, and CRWN together with different chromatin marks may help to determine in the future if specific chromatin domains with different transcription activities can be found at the NPC–nucleoskeleton boundary.
Finally, our phylogenetic analysis traced NUP136 back to Marchantia polymorpha and suggested that NUP82 and KAKU4 emerged from an ancestral duplication. One interesting hypothesis postulates that 2 subsequent polyploidy events termed ξ (∼319 Ma) and ε (∼192 Ma), which according to TimeTree occurred respectively soon before (∼355 Ma) and after angiosperm divergence (∼170 Ma), were responsible for these new duplication events (Kumar et al. 2017). During evolution, KAKU4 diverged into a new component of the nucleoskeleton but still conserved its ability to interact with the NPC. This work highlights the tight relationship among the nuclear envelope, nuclear pores, and the nucleoskeleton in plants. As part of this network, the KAKU4 protein represents an intriguing case of neofunctionalization of an ancestral nucleoporin into a new component of the nucleoskeleton.
Materials and methods
Plant materials
All mutants used in this study are T-DNA insertion alleles in the Arabidopsis (A. thaliana) Columbia-0 (Col-0) genetic background. Mutant combinations were produced by crosses using kaku4-2 (SALK_076754), nup82-1 (SALK_001707), and nup136-2 (SAIL_796_H02) alleles described in previous work (Goto et al. 2014; Tamura et al. 2017). Plants were grown in long-day light conditions (16 h of light/8 h of dark) at 23 °C on soil in Aralab growth chambers. Genotyping of individual T-DNA alleles was performed by standard PCR using allele-specific primers (Supplemental Table S4).
Constructs and cloning
cDNAs of CRWN1 (AT1G67230), CRWN2 (AT1G13220), CRWN3 (AT1G68790), CRWN4 (AT5G65770), KAKU4 (AT4G31430), NUP136 (AT3G10650), NUP82 (AT5G20200), and FIBRILLARIN2 (AT4G25630) were cloned in pDONR vectors using gene-specific primer sequences (Supplemental Table S4). Motifs were amplified from the corresponding cDNA using specific primers (Supplemental Table S4) and cloned in pDONR vectors, while the domain deletions were built from initial cDNA constructs using specific primers or using the site-directed mutagenesis kit QuikChange (Agilent Technologies) and appropriate primers (Supplemental Table S4). All cloning procedures rely on Gateway technology. After initial cloning, full-length coding sequences and other constructs were cloned into appropriate expression vectors for Y2H assays (bait vector pDEST-GBKT7 or prey vector pDEST-GADT7), expression in planta (pGWB606-GFP and pGWB555-RFP), or BiFC (pB4cYGW, pB4nYGw, pB4GWnY, and pB4GWcY). The lists of all the plasmids used in this study can be found in Supplemental Tables S5 and S6.
Gene and protein sequences
Orthologs of KAKU4, NUP136, and NUP82 were collected from 22 plant species that best represent the evolutionary history of the green lineage: Arabidopsis (A.th.), A. lyrata (A.ly.), Brassica rapa (B.ra.), Carica papaya (C.pa.), Glycine max (G.ma.), Theobroma cacao (T.ca.), Vitis vinifera (V.vi.), Populus trichocarpa (P.tr.), Prunus persica (P.pe.), Solanum lycopersicum (S.ly.), Oryza sativa (O.sa.), Zea mays (Z.ma.), Sorghum bicolor (S.bi.), Ananas comosus (A.co.), Musa acuminata (M.ac.), A. trichopoda (A.tr.), Picea abies (P.ab.), Pinus taeda (P.ta.), M. polymorpha (M.po.), Physcomitrella patens (P.pa.), Oestrococcus lucimarinus (O.lu.), and Chlamydomonas reinhardtii (C.re.). Most genomes of these plant species are available at Phytozome 7.0 (Goodstein et al. 2012) and Monocots or Dicots PLAZA 4.5 (Van Bel et al. 2018). The gymnosperm genomes were collected from conGenIE.org (Conifer Genome Integrative Explorer) (Sundell et al. 2015). Gene and protein accession numbers used in this study are listed in Supplemental Table S1.
Protein structure predictions
Protein structures of KAKU4, NUP82, and NUP136 were defined using Uniprot (The UniProt Consortium 2019) and pfam (El-Gebali et al. 2019) databases for domain predictions. Secondary structures were predicted using PEP-FOLD3 (Lamiable et al. 2016). IDRs were predicted using DEPICTER, DISOPRED3, and VSL2B (Peng et al. 2006; Jones and Cozzetto 2015; Barik et al. 2020) (Supplemental Data Set 1). VSL2B is the baseline predictor of VSL2, which uses only the amino acid composition-based features that can be calculated directly from the protein sequence (Peng et al. 2006). DescribePROT database was used to collect precomputed IDR predictions for 27,466 proteins of the Arabidopsis proteome including those predicted by VSL2B (Zhao et al. 2021). VSL2B predictions were extracted for 31 NUPs, 5 nucleoskeleton proteins, and 5 inner nuclear membrane proteins. All schemes of protein structures were drawn to scale using the drawProteins R package (Brennan 2018).
Phylogenetic studies
Phylogenetic trees were constructed using protein sequences collected from 52 species using MAFFT 7.407 (Katoh and Standley 2013) for multiple alignment and IQ-TREE v2.2.0.3 (Minh et al. 2020) with the LG substitution model with 1,000 bootstrap replicates (Supplemental Data Set 2). Trees were refined using the Interactive Tree Of Life (ITOL) software (Letunic and Bork 2011). The amino acid substitution rate was computed as the sum of the branch lengths (i.e. sum of the amino acid substitutions per site) of the phylogenetic trees and by considering separately KAKU4, NUP82, and NUP136 clades. Nonsynonymous (dN) and synonymous (dS) substitution rates were calculated to determine if selection or genetic drift occurred within the KAKU4, NUP82, or NUP136 gene families. To avoid underestimation of dS, the analysis was restricted to 9 close relative species from the Brassicaceae family (Arabidopsis, A. lyrata, Camelina sativa, Capsella rubella, Eutrema salsugineum, B. napus, B. oleraceae, B. rapa, and Raphanus sativus). The Codeml program of the Phylogenetic Analysis by Maximum Likelihood (PAML) package v.4.4c (Yang 2007) was used with the variable NSsites to compare the observed dN/dS ratio for KAKU4, NUP82, and NUP136 phylogenetic tree with the null model M0 where all tree branches have the same dN/dS set to 1 (no selection). The M0 model was rejected if P-value <0.05. Gene structures (exon/intron structure) were defined and drawn to scale with the Gene Structure Display Server (GSDS) 2.0 (Hu et al. 2015). The NW algorithm available at the ncbi BLAST server was used for global alignment of protein sequences to compute protein identities and NW scores.
Domain and motif search in proteins
The orthologs of KAKU4, NUP82, and NUP136 were identified using the Arabidopsis proteins as a query by performing an all-against-all protein sequence similarity search using BLASTP with an E-value cutoff of 10−10. Ortholog occurrence in distant species was refined using sequences from A. trichopoda with the same approach. The MEME 5.1.1 suite was used for de novo motif predictions (Bailey et al. 2015). From a list of orthologous proteins, MEME was parameterized to define 10 motifs, each with a maximum length of 100 amino acids. Protein sequences from each clade (KAKU4, NUP82, and NUP136) were submitted to MEME either individually or in combination of 2 or 3 with the assumption that the 3 protein families contain common conserved regions.
Y2H assay
Yeast cultures were grown at 30 °C on YPD or on selective SD media. Saccharomyces cerevisiae strains AH109 Gold and Y187 (Clontech, MATCHMAKER GAL4 Two-Hybrid System) were transformed according to the classical protocol (Gietz and Woods 2002) separately with the bait vector pDEST-GBKT7 or the prey vector pDEST-GADT7 (Rossignol et al. 2007) and grown on appropriate medium (SD-Trp or SD-Leu plates, respectively). After mating on YPD and selection of diploids on SD-Leu-Trp medium, interactions were recorded on stringent medium SD-Leu-Trp-His-Ade. Empty pDEST-GBKT7 or pDEST-GADT7 vectors were used as negative controls.
Nuclear shape analysis
Three-dimensional images were obtained from whole mount preparations of cotyledons at 13 d after germination as previously described (Poulet et al. 2017; Dubos et al. 2020, 2022). Briefly, pairs of cotyledons were collected and fixed using 1% formaldehyde (v/v) and 10% DMSO (v/v) in 1× PBS with 6.7 mm EGTA (pH 7.5) under vacuum for 5 min and incubated for 25 min at room temperature. Tissues were then washed with methanol and ethanol to obtain transparent tissue preparations. DNA in whole-mount preparations was stained with Hoechst (Hoechst 33258 overnight at 1 μg/mL final concentration, at 4 °C). Microscopic observations were performed by structured illumination microscopy using an optigrid module (Leica Microsystems MAAF DM 16000B) to produce wide-field stacks. All stacks were acquired using an X63 oil N.A. 1.4 objective and a digital CMOS Camera (ORCA-Flash4.0 V2 C11440-22 CU—Hamamatsu) at an optimal resolution such that lateral and axial resolution were respectively XY = 0.103 μm and Z = 0.2 μm. The ImageJ plugin NucleusJ2.0 (Dubos et al. 2020) was used to characterize elongation and flatness ratio for each nucleus as major/medium axis and major/minor axis, respectively. The ImageJ plugin NODeJ 1.0 was used to compute the chromocenter distance in respect to the nuclear periphery (Dubos et al. 2022).
Pollen viability assay
Pollen viability was studied via Alexander staining (ethanol 10% v/v, malachite green 0.01% w/v, distilled water 50% v/v, glycerol 25% v/v, phenol 5.5% v/v, chloral hydrate 5% w/v, fuchsin acid 0.05% w/v, orange G 0.005% w/v, and glacial acetic acid 2% v/v). For this purpose, buds with their white petals starting to appear were dissected to recover the stamens with their mature unopened anthers. The stamens were then placed in Alexander stain, and the anthers were crushed to release the pollen grains. The slides were observed under a Zeiss Imager M2 microscope in white light (bright field with an RGB camera) with X10 and/or X20 objectives, and at least 1,000 pollen grains per genotype from different plants were analyzed.
Transient expression of fusion proteins in N. benthamiana
Vectors pGWB606 (GFP Nter), pGWB555 (mRFP Nter) (Nakagawa et al. 2007) or pB4GWnY (Nter of YFP/nYFP Cter), pB4nYGW (Nter of YFP/nYFP Nter), pB4GWcY (Cter of YFP/cYFP Cter), and pB4cYGW (Cter of YFP/cYFP Nter) (Kamigaki et al. 2016) were used for transient expression studies. Expression vectors were transformed in the Agrobacterium tumefaciens strain GV3101. The p19 suppressor of gene silencing was used to enhance expression (Norkunas et al. 2018). Transient coexpression of the expression vectors and p19 constructs was performed by infiltration Agrobacterium cultures at OD600 of 0.1 and 0.05, respectively, into 5- to 6-wk-old leaves of N. benthamiana as described before (Sparkes et al. 2006).
Stable expression of KAKU4-GFP fusions, nuclear shape, and localization complementation
Full length and deletion derivatives of KAKU4 were expressed under the 35S promoter as stable transformants in the kaku4-2 mutant using similar constructs as in transient expression (pGWB506, GPF Nter). Observation of nuclear shape was performed in filament cells of stamens, which are nonpigmented and contain elongated nuclei in WT plants (Dittmer et al. 2007; Choi et al. 2019). For each construct, 2 independent transgenic lines were selected (at various generations: T2, T3, and T4 transgenic plants) except for plants expressing M3M1M2 or deleted of M3 for which only T1 transgenic plants were available. After collection, filaments were placed in PBS 1× and mounted in PBS 1× and observed using confocal microscopy (Zeiss LSM 800). In WT and kaku4-2 mutant plants, anther filaments were fixed with ethanol:acetic acid solution (3:1, v/v) for 10 min and then washed in PBS 1× for 10 min and mounted in Vectashield with DAPI. The images (0.31 × 0.31 × 1) were analyzed with the Fiji plugin Shape Descriptors to compute 2D shape parameters.
To quantify the localization of GFP-tagged proteins expressed in Arabidopsis transgenic lines, the ratio of fluorescence intensity at the nuclear envelope in respect to the nucleoplasm was computed. For each nucleus, 4 equally sized regions of interest were selected at the nuclear envelope and 4 in the nucleoplasm as previously described (Graumann et al. 2014). The ratio is expressed as log2 of nuclear periphery to nucleoplasm intensity.
Protoplast isolation and transformation
Roots of 7-d-old seedlings were used to perform protoplast isolation and transformation according to the method described by Kolářová (Kolářová et al. 2021). Protoplasts were cotransformed for 25 min at 50 rpm at room temperature in the dark with a plasmid expressing a nuclear localization signal coupled to mRFP and another expressing either the KAKU4 protein or CRWN1 fused to GFP. The next day, the protoplasts were observed under a Zeiss LSM 800 confocal microscope with X40 and X63 oil immersion objectives.
Image acquisition, storage, and analysis
Fluorescence images were obtained using an inverted confocal laser-scanning microscope (LSM800; Carl Zeiss). The 488-nm line of a 40-mWAr/Kr laser and the 544-nm line of a 1-mW He/Ne laser were used to excite GFP/YFP and RFP, respectively. Images were acquired with 40× or 63× oil immersion objectives. All images are stored in an in house OMERO server, and figures were produced using OMERO.figure (Allan et al. 2012). Imaris 9.7 (Bitplane AG) was used for image rendering.
Statistical analysis
Statistical analyses were performed as described in each figure legend. Statistical data are provided in Supplemental Data Set 3.
Supplementary Material
Acknowledgments
C.T. would like to thank Lukasz Kurgan for his advices on the use of DescribeProt and Antoine Molaro for advice on the phylogenetic analysis and critical reading of the manuscript. C.T. and A.V.P. would like to thank Martina Dvořáčková and Martina Nešpor Dadejová for training S.M. in the preparation of A. thaliana protoplasts.
Contributor Information
Sarah Mermet, iGReD, Université Clermont Auvergne, CNRS, INSERM, 63001 Clermont-Ferrand, France.
Maxime Voisin, iGReD, Université Clermont Auvergne, CNRS, INSERM, 63001 Clermont-Ferrand, France.
Joris Mordier, iGReD, Université Clermont Auvergne, CNRS, INSERM, 63001 Clermont-Ferrand, France.
Tristan Dubos, iGReD, Université Clermont Auvergne, CNRS, INSERM, 63001 Clermont-Ferrand, France.
Sylvie Tutois, iGReD, Université Clermont Auvergne, CNRS, INSERM, 63001 Clermont-Ferrand, France.
Pierre Tuffery, Université Paris Cité, CNRS UMR 8251, INSERM ERL U1133, 75013 Paris, France.
Célia Baroux, Department of Plant and Microbial Biology, Zürich-Basel Plant Science Center, University of Zürich, 8008 Zürich, Switzerland.
Kentaro Tamura, Department of Environmental and Life Sciences, University of Shizuoka, Shizuoka 422-8526, Japan.
Aline V Probst, iGReD, Université Clermont Auvergne, CNRS, INSERM, 63001 Clermont-Ferrand, France.
Emmanuel Vanrobays, iGReD, Université Clermont Auvergne, CNRS, INSERM, 63001 Clermont-Ferrand, France.
Christophe Tatout, iGReD, Université Clermont Auvergne, CNRS, INSERM, 63001 Clermont-Ferrand, France.
Author contributions
E.V. and C.T. supervised the study. S.M., E.V., S.T., and M.V. performed experiments. J.M., T.D., and C.T. performed the bio-informatics work on evolution and IDR. P.T. performed the PEPS-FOLD3 analysis. K.T. provided the initial plant lines and constructs used in this study. C.B. performed the IMARIS analysis. C.T., S.M., E.V., and A.V.P. wrote the manuscript.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. The N-terminal region of KAKU4 is necessary for PPI and localization.
Supplemental Figure S2. PPI within the nucleoskeleton probed by yeast 2-hybrid assay.
Supplemental Figure S3. Role of KAKU4 motifs in its localization at the nuclear periphery in Arabidopsis.
Supplemental Figure S4. PPI between the nucleoporins NUP82 and NUP136 and the nucleoskeleton components KAKU4 and CRWNs.
Supplemental Figure S5. Analysis of genetic interactions among KAKU4, NUP82, and NUP136.
Supplemental Figure S6. Phylogenetic relationships among N- and C-terminal region of KAKU4, NUP82, and NUP136 proteins.
Supplemental Figure S7. Localization of KAKU4 and NUP136 when transiently expressed in N. benthamiana.
Supplemental Table S1. Genes and proteins IDs.
Supplemental Table S2. Intrinsically disorder prediction by VSL2 for NUPs, nucleoskeleton, and SUN domain proteins.
Supplemental Table S3. BiFC assay in N. benthamiana leaves.
Supplemental Table S4. Primer sequences used in this study.
Supplemental Table S5. Plasmid backbones used in this study.
Supplemental Table S6. Overview of constructs used in this study.
Supplemental Data Set 1. Intrinsically disorder prediction by VSL2, DEPICTER, and DISOPRED3.
Supplemental Data Set 2. mafft sequence alignments and tree files in Newick formats.
Supplemental Data Set 3. Statistical tests.
Funding
This work was supported by , , Clermont-Auvergne (UCA), 16-IDEX-0001 CAP20-25 CIR 1, Pack Ambition Recherche project Noyau-HD from the Auvergne-Rhône-Alpes, (JP23H04205, 22K06269, and 18K06283) and by the Human Frontier Science Program (RGP0009/2018 to KT) to K.T. and by the Swiss National Science Foundation (SNSF-COST project number IZCOZ0_198171) to CB. All pictures were acquired on the CLIC microscopy facility (CLermont Imagerie Confocale).
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Al-Haboubi T, Shumaker DK, Köser J, Wehnert M, Fahrenkrog B. Distinct association of the nuclear pore protein Nup153 with A- and B-type lamins. Nucleus. 2011:2(5):500–509. 10.4161/nucl.2.5.17913 [DOI] [PubMed] [Google Scholar]
- Allan C, Burel J-M, Moore J, Blackburn C, Linkert M, Loynton S, MacDonald D, Moore WJ, Neves C, Patterson A, et al. OMERO: flexible, model-driven data management for experimental biology. Nat Methods. 2012:9(3):245–253. 10.1038/nmeth.1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990:215(3):403–410. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- Bailey TL, Johnson J, Grant CE, Noble WS. The MEME suite. Nucleic Acids Res. 2015:43(W1):W39–W49. 10.1093/nar/gkv416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik A, Katuwawala A, Hanson J, Paliwal K, Zhou Y, Kurgan L. DEPICTER: intrinsic disorder and disorder function prediction server. J Mol Biol. 2020:432(11):3379–3387. 10.1016/j.jmb.2019.12.030 [DOI] [PubMed] [Google Scholar]
- Beck M, Hurt E. The nuclear pore complex: understanding its function through structural insight. Nat Rev Mol Cell Biol. 2017:18(2):73–89. 10.1038/nrm.2016.147 [DOI] [PubMed] [Google Scholar]
- Bi X, Cheng Y-J, Hu B, Ma X, Wu R, Wang J-W, Liu C. Nonrandom domain organization of the Arabidopsis genome at the nuclear periphery. Genome Res. 2017:27(7):1162–1173. 10.1101/gr.215186.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. Drawproteins: a Bioconductor/R package for reproducible and programmatic generation of protein schematics. F1000Res. 2018:7:1105. 10.12688/f1000research.14541.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand N, Collas P. Lamina-associated domains: peripheral matters and internal affairs. Genome Biol. 2020:21(1):85. 10.1186/s13059-020-02003-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Strickler SR, Richards EJ. Loss of CRWN nuclear proteins induces cell death and salicylic acid defense signaling. Plant Physiol. 2019:179(4):1315–1329. 10.1104/pp.18.01020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciska M, Masuda K, Moreno Díaz de la Espina S. Lamin-like analogues in plants: the characterization of NMCP1 in Allium cepa. J Exp Bot. 2013:64(6):1553–1564. 10.1093/jxb/ert020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Magistris P, Antonin W. The dynamic nature of the nuclear envelope. Currnt Biol. 2018:28(8):R487–R497. 10.1016/j.cub.2018.01.073 [DOI] [PubMed] [Google Scholar]
- Dittmer TA, Stacey NJ, Sugimoto-Shirasu K, Richards EJ. LITTLE NUCLEI genes affecting nuclear morphology in Arabidopsis thaliana. Plant Cell. 2007:19(9):2793–2803. 10.1105/tpc.107.053231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos T, Poulet A, Gonthier-Gueret C, Mougeot G, Vanrobays E, Li Y, Tutois S, Pery E, Chausse F, Probst AV, et al. Automated 3D bio-imaging analysis of nuclear organization by NucleusJ 2.0. Nucleus. 2020:11(1):315–329. 10.1080/19491034.2020.1845012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos T, Poulet A, Thomson G, Péry E, Chausse F, Tatout C, Desset S, van Wolfswinkel JC, Jacob Y. NODej: an ImageJ plugin for 3D segmentation of nuclear objects. BMC Bioinformatics. 2022:23(1):216. 10.1186/s12859-022-04743-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, Potter SC, Qureshi M, Richardson LJ, Salazar GA, Smart A, et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019:47(D1):D427–D432. 10.1093/nar/gky995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C-M, Qiu Y, Van Buskirk EK, Yang EJ, Chen M. Light-regulated gene repositioning in Arabidopsis. Nat Commun. 2014:5(1):3027. 10.1038/ncomms4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002:350:87–96. 10.1016/S0076-6879(02)50957-5 [DOI] [PubMed] [Google Scholar]
- Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012:40(D1):D1178–D1186. 10.1093/nar/gkr944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami R, Asnacios A, Milani P, Graindorge S, Houlné G, Mutterer J, Hamant O, Chabouté M-E. Mechanical shielding in plant nuclei. Curr Biol. 2020:30(11):2013–2025.e3. 10.1016/j.cub.2020.03.059 [DOI] [PubMed] [Google Scholar]
- Goto C, Tamura K, Fukao Y, Shimada T, Hara-Nishimura I. The novel nuclear envelope protein KAKU4 modulates nuclear morphology in Arabidopsis. Plant Cell. 2014:26(5):2143–2155. 10.1105/tpc.113.122168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto C, Tamura K, Nishimaki S, Maruyama D, Hara-Nishimura I. The nuclear envelope protein KAKU4 determines the migration order of the vegetative nucleus and sperm cells in pollen tubes. J Exp Bot. 2020:71(20):6273–6281. 10.1093/jxb/eraa367 [DOI] [PubMed] [Google Scholar]
- Grant CE, Bailey TL, Noble WS. FIMO: scanning for occurrences of a given motif. Bioinformatics. 2011:27(7):1017–1018. 10.1093/bioinformatics/btr064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graumann K. Evidence for LINC1-SUN associations at the plant nuclear periphery. PLoS One. 2014:9(3):e93406. 10.1371/journal.pone.0093406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graumann K, Vanrobays E, Tutois S, Probst AV, Evans DE, Tatout C. Characterization of two distinct subfamilies of SUN-domain proteins in Arabidopsis and their interactions with the novel KASH-domain protein AtTIK. J Exp Bot. 2014:65(22):6499–6512. 10.1093/jxb/eru368 [DOI] [PubMed] [Google Scholar]
- Guo Y, Zheng Y. Lamins position the nuclear pores and centrosomes by modulating dynein. Mol Biol Cell. 2015:26(19):3379–3389. 10.1091/mbc.E15-07-0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatos A, Hajdu-Soltész B, Monzon AM, Palopoli N, Álvarez L, Aykac-Fas B, Bassot C, Benítez GI, Bevilacqua M, Chasapi A, et al. Disprot: intrinsic protein disorder annotation in 2020. Nucleic Acids Res. 2020:48(D1):D269–D276. 10.1093/nar/gkz975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenstatt ML, Mikulski P, Komarynets O, Klose C, Kycia I, Jeltsch A, Farrona S, Schubert D. PWWP-DOMAIN INTERACTOR OF POLYCOMBS1 interacts with polycomb-group proteins and histones and regulates Arabidopsis flowering and development. Plant Cell 2018:30:117–133. https://doi-org/10.1105/tpc.17.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Jin J, Guo A-Y, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015:31(8):1296–1297. 10.1093/bioinformatics/btu817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Wang N, Bi X, Karaaslan ES, Weber A-L, Zhu W, Berendzen KW, Liu C. Plant lamin-like proteins mediate chromatin tethering at the nuclear periphery. Genome Biol. 2019:20(1):87. 10.1186/s13059-019-1694-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Zhu S, Kumar P, MacMicking JD. A phase-separated nuclear GBPL circuit controls immunity in plants. Nature. 2021:594(7863):424–429. 10.1038/s41586-021-03572-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto FV, Benner C, Hetzer MW. The nucleoporin Nup153 regulates embryonic stem cell pluripotency through gene silencing. Genes Dev. 2015:29(12):1224–1238. 10.1101/gad.260919.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochim AL, Arora PS. Assessment of helical interfaces in protein-protein interactions. Mol Biosyst. 2009:5(9):924–926. 10.1039/b903202a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Cozzetto D. DISOPRED3: precise disordered region predictions with annotated protein-binding activity. Bioinformatics. 2015:31(6):857–863. 10.1093/bioinformatics/btu744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamigaki A, Nito K, Hikino K, Goto-Yamada S, Nishimura M, Nakagawa T, Mano S. Gateway vectors for simultaneous detection of multiple protein-protein interactions in plant cells using bimolecular fluorescence complementation. PLoS One. 2016:11(8):e0160717. 10.1371/journal.pone.0160717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013:30(4):772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittisopikul M, Shimi T, Tatli M, Tran JR, Zheng Y, Medalia O, Jaqaman K, Adam SA, Goldman RD. Computational analyses reveal spatial relationships between nuclear pore complexes and specific lamins. J Cell Biol. 2021:220(4):e202007082. 10.1083/jcb.202007082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knockenhauer KE, Schwartz TU. The nuclear pore complex as a flexible and dynamic gate. Cell. 2016:164(6):1162–1171. 10.1016/j.cell.2016.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolářová K, Nešpor Dadejová M, Loja T, Lochmanová G, Sýkorová E, Dvořáčková M. Disruption of NAP1 genes in Arabidopsis thaliana suppresses the fas1 mutant phenotype, enhances genome stability and changes chromatin compaction. The Plant J. 2021:106(1):56–73. 10.1111/tpj.15145 [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Suleski M, Hedges SB. Timetree: a resource for timelines, timetrees, and divergence times. Mol Biol Evol. 2017:34(7):1812–1819. 10.1093/molbev/msx116 [DOI] [PubMed] [Google Scholar]
- Lamiable A, Thévenet P, Rey J, Vavrusa M, Derreumaux P, Tufféry P. PEP-FOLD3: faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res. 2016:44(W1):W449–W454. 10.1093/nar/gkw329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Bork P. Interactive tree of life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res. 2011:39(Web Server issue):W475–W478. 10.1093/nar/gkr201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DH, Stuwe T, Schilbach S, Rundlet EJ, Perriches T, Mobbs G, Fan Y, Thierbach K, Huber FM, Collins LN, et al. Architecture of the symmetric core of the nuclear pore. Science. 2016:352(6283):aaf1015. 10.1126/science.aaf1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JF, Gumber HK, Turpin ZM, Jalovec AM, Kartick AC, Graumann K, Bass HW. Maize (Zea mays L.) nucleoskeletal proteins regulate nuclear envelope remodeling and function in stomatal Complex development and pollen viability. Front Plant Sci. 2021:12:645218. 10.3389/fpls.2021.645218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier I, Richards EJ, Evans DE. Cell biology of the plant nucleus. Annu Rev Plant Biol. 2017:68(1):139–172. 10.1146/annurev-arplant-042916-041115 [DOI] [PubMed] [Google Scholar]
- Mikulski P, Hohenstatt ML, Farrona S, Smaczniak C, Stahl Y, Kalyanikrishna, Kaufmann K, Angenent G, Schubert D. The chromatin-associated protein PWO1 interacts with plant nuclear lamin-like components to regulate nuclear size. Plant Cell. 2019:31(5):1141–1154. 10.1105/tpc.18.00663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 2020:37(5):1530–1534. 10.1093/molbev/msaa015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Suzuki T, Murata S, Nakamura S, Hino T, Maeo K, Tabata R, Kawai T, Tanaka K, Niwa Y, et al. Improved gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci Biotechnol Biochem. 2007:71(8):2095–2100. 10.1271/bbb.70216 [DOI] [PubMed] [Google Scholar]
- Needleman SB, Wunsch CD. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970:48(3):443–453. 10.1016/0022-2836(70)90057-4 [DOI] [PubMed] [Google Scholar]
- Neumann N, Jeffares DC, Poole AM. Outsourcing the nucleus: nuclear pore complex genes are no longer encoded in nucleomorph genomes. Evol Bioinform Online. 2007:2:22–34. 10.1177/117693430600200023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkunas K, Harding R, Dale J, Dugdale B. Improving agroinfiltration-based transient gene expression in Nicotiana benthamiana. Plant Methods. 2018:14(1):71. 10.1186/s13007-018-0343-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nützmann H-W, Doerr D, Ramírez-Colmenero A, Sotelo-Fonseca JE, Wegel E, Di Stefano M, Wingett SW, Fraser P, Hurst L, Fernandez-Valverde SL, et al. Active and repressed biosynthetic gene clusters have spatially distinct chromosome states. Proc Natl Acad Sci U S A. 2020:117(24):13800–13809. 10.1073/pnas.1920474117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Garcia P, Jeong J, Capelson M. Nucleoporin Nup98 associates with Trx/MLL and NSL histone-modifying complexes and regulates Hox gene expression. Cell Rep. 2014:9(2):433–442. 10.1016/j.celrep.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Peng K, Radivojac P, Vucetic S, Dunker AK, Obradovic Z. Length-dependent prediction of protein intrinsic disorder. BMC Bioinformatics. 2006:7(1):208. 10.1186/1471-2105-7-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet A, Duc C, Voisin M, Desset S, Tutois S, Vanrobays E, Benoit M, Evans DE, Probst AV, Tatout C. The LINC complex contributes to heterochromatin organisation and transcriptional gene silencing in plants. J Cell Sci. 2017:130(3):590–601. 10.1242/jcs.194712 [DOI] [PubMed] [Google Scholar]
- Raices M, D’Angelo MA. Nuclear pore complexes and regulation of gene expression. Curr Opin Cell Biol. 2017:46:26–32. 10.1016/j.ceb.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann TM, Müdsam C, Schachtler C, Ince S, Sticht H, Herrmann C, Stürzl M, Kost B. The large GTPase AtGBPL3 links nuclear envelope formation and morphogenesis to transcriptional repression. Nat Plants. 2023:9(5):766–784. 10.1038/s41477-023-01400-5 [DOI] [PubMed] [Google Scholar]
- Rossignol P, Collier S, Bush M, Shaw P, Doonan JH. Arabidopsis POT1A interacts with TERT-V(I8), an N-terminal splicing variant of telomerase. J Cell Sci. 2007:120(20):3678–3687. 10.1242/jcs.004119 [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Sato M, Sato Y, Harada A, Suzuki T, Goto C, Tamura K, Toyooka K, Kimura H, Ohkawa Y, et al. Subnuclear gene positioning through lamina association affects copper tolerance. Nat Commun. 2020:11(1):5914. 10.1038/s41467-020-19621-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker DK, Dechat T, Kohlmaier A, Adam SA, Bozovsky MR, Erdos MR, Eriksson M, Goldman AE, Khuon S, Collins FS, et al. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc Natl Acad Sci U S A. 2006:103(23):8703–8708. 10.1073/pnas.0602569103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Galinha C, Desset S, Tolmie F, Evans D, Tatout C, Graumann K. Marker gene tethering by nucleoporins affects gene expression in plants. Nucleus. 2015:6(6):471–478. 10.1080/19491034.2015.1126028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe C, Jenkins HE, Hutchison CJ. Incorporation of the nuclear pore basket protein nup153 into nuclear pore structures is dependent upon lamina assembly: evidence from cell-free extracts of Xenopus eggs. EMBO J. 2000:19(15):3918–3931. 10.1093/emboj/19.15.3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes IA, Runions J, Kearns A, Hawes C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat Protoc. 2006:1(4):2019–2025. 10.1038/nprot.2006.286 [DOI] [PubMed] [Google Scholar]
- Sundell D, Mannapperuma C, Netotea S, Delhomme N, Lin Y-C, Sjödin A, Van de Peer Y, Jansson S, Hvidsten TR, Street NR. The plant genome integrative explorer resource: plantGenIE.org. New Phytol. 2015:208(4):1149–1156. 10.1111/nph.13557 [DOI] [PubMed] [Google Scholar]
- Tamura K. Nuclear pore complex-mediated gene expression in Arabidopsis thaliana. J Plant Res. 2020:133(4):449–455. 10.1007/s10265-020-01177-0 [DOI] [PubMed] [Google Scholar]
- Tamura K, Fukao Y, Hatsugai N, Katagiri F, Hara-Nishimura I. Nup82 functions redundantly with Nup136 in a salicylic acid-dependent defense response of Arabidopsis thaliana. Nucleus. 2017:8(3):301–311. 10.1080/19491034.2017.1279774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Fukao Y, Iwamoto M, Haraguchi T, Hara-Nishimura I. Identification and characterization of nuclear pore complex components in Arabidopsis thaliana. Plant Cell. 2010:22(12):4084–4097. 10.1105/tpc.110.079947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Hara-Nishimura I. Involvement of the nuclear pore complex in morphology of the plant nucleus. Nucleus. 2011:2(3):168–172. 10.4161/nucl.2.3.16175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Dong Q, Wang T, Gong L, Gu Y. PNET2 Is a component of the plant nuclear lamina and is required for proper genome organization and activity. Dev Cell. 2021:57(1):19–31.e6. 10.1016/j.devcel.2021.11.002 [DOI] [PubMed] [Google Scholar]
- Tang Y, Huang A, Gu Y. Global profiling of plant nuclear membrane proteome in Arabidopsis. Nat Plants. 2020:6(7):838–847. 10.1038/s41477-020-0700-9 [DOI] [PubMed] [Google Scholar]
- Tompa P, Davey NE, Gibson TJ, Babu MM. A million peptide motifs for the molecular biologist. Mol Cell. 2014:55(2):161–169. 10.1016/j.molcel.2014.05.032 [DOI] [PubMed] [Google Scholar]
- The UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019:47:D506–D515. https://doi-org/10.1093/nar/gky1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bel M, Diels T, Vancaester E, Kreft L, Botzki A, Van de Peer Y, Coppens F, Vandepoele K. PLAZA 4.0: an integrative resource for functional, evolutionary and comparative plant genomics. Nucleic Acids Res. 2018:46(D1):D1190–D1196. 10.1093/nar/gkx1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lee R, et al. Classification of intrinsically disordered regions and proteins. Chem Rev. 114:6589–6631. https://doi-org/10.1021/cr400525m [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel B, Belmont AS. Lamina-associated domains: links with chromosome architecture, heterochromatin, and gene repression. Cell. 2017:169(5):780–791. 10.1016/j.cell.2017.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Dittmer TA, Richards EJ. Arabidopsis CROWDED NUCLEI (CRWN) proteins are required for nuclear size control and heterochromatin organization. BMC Plant Biol. 2013:13(1):200. 10.1186/1471-2229-13-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Pei G, Wang Y, Wu D, Liu X, Li G, He J, Zhang X, Shan X, Li P, et al. Phase separation of the nuclear pore complex facilitates selective nuclear transport to regulate plant defense against pathogen and pest invasion. Mol Plant. 2023:16(6):1016–1030. 10.1016/j.molp.2023.04.008 [DOI] [PubMed] [Google Scholar]
- Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007:24(8):1586–1591. 10.1093/molbev/msm088 [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang W, Chu Z, Zhu J-K, Zhang H. Roles of nuclear pores and nucleo-cytoplasmic trafficking in plant stress responses. Front Plant Sci. 2017:8:574. 10.3389/fpls.2017.00574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Katuwawala A, Oldfield CJ, Dunker AK, Faraggi E, Gsponer J, Kloczkowski A, Malhis N, Mirdita M, Obradovic Z, et al. DescribePROT: database of amino acid-level protein structure and function predictions. Nucleic Acids Res. 2021:49(D1):D298–D308. 10.1093/nar/gkaa931 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.