ABSTRACT
Background
Data on belimumab efficacy in patients with lupus nephritis (LN) according to diagnosis duration or induction therapy are limited. Post hoc analyses of the phase 3, randomized, double-blind BLISS-LN study (GSK BEL114054; NCT01639339) were performed to assess belimumab efficacy on kidney-related outcomes in newly diagnosed and relapsed LN subgroups and according to the use of glucocorticoid (GC) pulses at induction.
Methods
BLISS-LN randomized 448 patients with active LN to monthly intravenous belimumab 10 mg/kg or placebo plus standard therapy. Post hoc analyses assessed primary efficacy renal response (PERR) and complete renal response (CRR) at week 104, time to kidney-related event or death and time to first LN flare from week 24 in newly diagnosed and relapsed patients and patients with/without GC pulses at induction.
Results
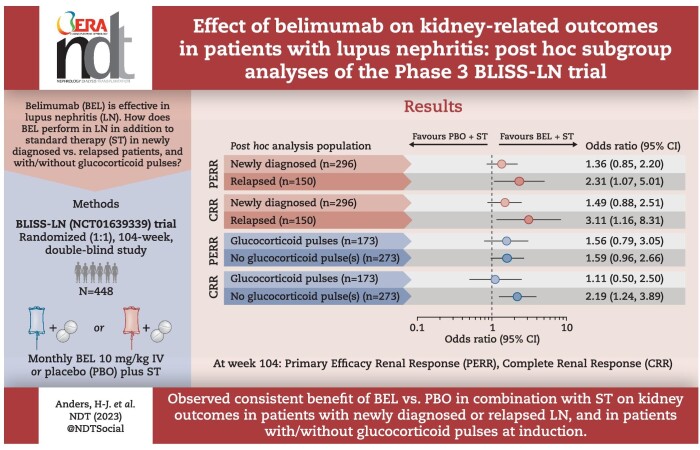
A greater proportion of patients achieved a PERR with belimumab versus placebo in the newly diagnosed {69/148 [46.6%] versus 55/148 [37.2%]; odds ratio [OR] 1.36 [95% confidence interval (CI) 0.85–2.20]} and relapsed [27/75 (36.0%) versus 17/75 (22.7%); OR 2.31 (95% CI 1.07–5.01)] subgroups. Similarly for CRR [newly diagnosed: 50/148 (33.8%) versus 36/148 (24.3%); OR 1.49 (95% CI 0.88–2.51) and relapsed: 17/75 (22.7%) versus 8/75 (10.7%); OR 3.11 (95% CI 1.16–8.31)]. The probability of kidney-related event or death, or LN flare was lower with belimumab versus placebo in both subgroups. Belimumab was associated with improved kidney outcomes versus placebo with or without GC pulses at induction.
Conclusion
Data suggest consistent benefits of belimumab on kidney outcomes for newly diagnosed and relapsed patients, and irrespective of GC pulses at induction.
Keywords: B cells, belimumab, glucocorticoids, lupus nephritis, proteinuria
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
Lupus nephritis (LN) is the most common serious manifestation of systemic lupus erythematosus (SLE), affecting ≈40% of patients with SLE.
The phase 3 BLISS-LN study (GSK study BEL114054; NCT01639339) demonstrated that addition of intravenous belimumab to standard therapy improved kidney outcomes in patients with active LN.
This study adds:
Belimumab plus standard therapy improved kidney outcomes compared with placebo plus standard therapy, regardless of whether patients had newly diagnosed or relapsed LN.
Compared with placebo plus standard therapy, belimumab plus standard therapy improved kidney outcomes irrespective of glucocorticoid (GC) pulse administration during induction.
Potential impact:
These data support the use of belimumab in clinical practice for all patients with LN, whether newly diagnosed or relapsed.
The benefit of belimumab was evident with or without the concomitant use of GC pulses during the induction treatment regimen.
INTRODUCTION
Lupus nephritis (LN) is a serious manifestation of systemic lupus erythematosus (SLE) and a major cause of morbidity and mortality [1, 2]. LN occurs in ≈40% of patients with SLE [1]; however, the prevalence and incidence can vary depending on the population and whether LN is assessed via clinical criteria alone or using histopathological findings [2].
To preserve kidney function, LN requires rapid and sustained suppression of SLE disease activity [2]. A modest proportion of patients with LN achieve a complete renal response after 12 months of treatment [3, 4], while studies have shown 13–37% of patients experienced renal flare within 3–5 years of standard therapy [5, 6]. Novel treatments are a medical necessity, as the risk of patients with LN progressing to end-stage kidney disease (ESKD) is notable; ≈44% of patients with class IV LN progress to ESKD within 15 years [7].
Belimumab is a recombinant human immunoglobulin G1λ monoclonal antibody that binds to the soluble B lymphocyte stimulator protein [8]. Based on clinical trials that demonstrated favourable safety and efficacy, belimumab is approved in numerous countries, including the USA, for the treatment of patients ≥5 years of age with active autoantibody-positive SLE [9–13] and for adults with active LN who are receiving standard therapy [14]. Belimumab has also recently been approved in the USA for the treatment of paediatric patients with active LN receiving standard therapy [15].
The phase 3 BLISS-LN study (GSK study BEL114054; NCT01639339), which enrolled patients with both de novo and relapsed LN, demonstrated that addition of intravenous (IV) belimumab to standard therapy improved kidney outcomes in patients with active LN [14]. A post hoc analysis of the BLISS-LN study further established the benefits of belimumab in patients with LN, with patients receiving belimumab plus standard therapy having a reduced risk over time of an LN flare and better preservation of kidney function compared with patients receiving placebo plus standard therapy [16].
Relapse has been shown to be a predictor of progression to ESKD in children with LN [17]. Thus, newly diagnosed and relapsed patients might have discordant responses to belimumab due to differing proteinuria levels, stage of chronic kidney disease, immunosenescence, T-cell exhaustion or otherwise altered immune pathways. Although data are limited regarding clinical differences between patients with newly diagnosed versus relapsed LN, a recent study showed that patients with newly diagnosed SLE were more likely to have new-onset hypertension than patients with relapsed SLE [18].
Guidelines recommend minimization of glucocorticoids (GCs) owing to the possible detrimental effects associated with high corticosteroid use, including organ damage accrual [19–22]. The BLISS-LN study included optional administration of high-dose GC pulses as part of standard therapy during induction therapy for LN [14].
The aims of these post hoc subgroup analyses of the BLISS-LN trial were to compare the efficacy of belimumab with placebo on kidney outcomes in newly diagnosed and relapsed patients with LN who received standard therapy. In addition, the effect of belimumab versus placebo, as an add-on to standard therapy, on kidney outcomes was assessed in patients with or without GC pulses received during induction therapy.
MATERIALS AND METHODS
Study design
BLISS-LN was a phase 3, randomized, double-blind, placebo-controlled, 104-week study (GSK study BEL114054; NCT01639339) that evaluated the efficacy and safety of belimumab 10 mg/kg IV plus standard therapy in adult patients with active LN. Full details of the study methods have been published previously [14]. The trial was performed in accordance with the principles of the Declaration of Helsinki, all the trial sites received approval from ethics committees or institutional review boards and written informed consent was obtained from all patients.
Patients
The eligibility criteria for the BLISS-LN study are summarized in Supplementary Fig. 1. Patients excluded from the study included those who had previously failed both cyclophosphamide (CYC) and mycophenolate mofetil (MMF) induction therapies based on the investigator's opinion and those with an estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2 at screening.
Treatments
Patients were randomized 1:1 to receive belimumab 10 mg/kg IV or placebo plus standard therapy (details appear in Supplementary Fig. 1). Of note, standard therapy included induction therapy with either CYC or MMF plus high-dose oral GCs, with optional administration of high-dose GC pulses (up to three IV pulses of methylprednisolone 500–1000 mg each) during induction. All patients received oral prednisone (0.5–1.0 mg/kg/day, total daily dose ≤60 mg/day). By week 24, GCs were required to be at a prednisone equivalent dose of ≤10 mg/day or the patient was considered a treatment failure. Study treatment was administered on day 1 (baseline), day 15, day 29 and every 28 days thereafter to week 100.
Endpoints and assessments
The primary endpoint of the BLISS-LN study was primary efficacy renal response (PERR) at week 104, defined as a urinary protein:creatinine ratio (UPCR) ≤0.7 g/g, eGFR ≤20% below the pre-flare value or ≥60 ml/min/1.73 m2 (per the simplified Modification of Diet in Renal Disease formula) and no receipt of prohibited (rescue) therapy resulting in treatment failure [14]. Additionally, confirmation of a PERR across two consecutive visits was required for the endpoint to be considered met.
Major secondary endpoints included complete renal response (CRR) at week 104 [which is a more stringent, composite endpoint defined as UPCR <0.5 g/g, eGFR ≤10% below the pre-flare value or ≥90 ml/min/1.73 m2 and no receipt of prohibited (rescue) therapy resulting in treatment failure, including (similar to PERR) the reduction of GCs to ≤10 mg/day at 24 weeks and requiring confirmation across two consecutive visits] and the time to kidney-related event or death through week 104 (defined as the first event occurring after day 1 among any of the following: ESKD, doubling of serum creatinine, increase in proteinuria and/or impaired excretory kidney function, kidney disease-related treatment failure or death). The stringency of the PERR and CRR endpoints are discussed in the BLISS-LN primary manuscript [14].
The post hoc endpoint of time to first LN flare from week 24 was defined as the first event occurring after week 24 among any of the following: impaired kidney function [reproducible eGFR decrease >20% from week 24 accompanied by proteinuria (UPCR >1 g/g) and/or cellular casts (red blood cells, white blood cells in the absence of infection or both)], increased proteinuria (UPCR >1 g/g if week 24 UPCR was <0.2 g/g, UPCR >2 g/g if week 24 UPCR was 0.2–1 g/g or more than twice the week 24 value if the week 24 UPCR was >1 g/g) or kidney disease-related treatment failure. Full definitions of the study endpoints have been published previously [14, 16].
These endpoints were assessed post hoc in subgroups of patients with newly diagnosed versus relapsed LN and patients with or without IV GC pulses during induction therapy. In addition, the mean combined (IV and oral) daily prednisone equivalent dose during the first 6 months was assessed in patients with and without IV GC pulses during induction therapy. While all patients were required to have active LN at baseline, per the inclusion criteria, newly diagnosed LN was defined as the patients’ first episode of LN (first kidney biopsy–established diagnosis of LN) and relapsed LN was defined as the second or any subsequent episode of active LN confirmed by rebiopsy.
Statistical analyses
Post hoc analyses were performed in the modified intention-to-treat (mITT) population, which included all randomized patients who received at least one dose of belimumab or placebo. Site compliance issues resulted in two patients being excluded from the mITT population.
Comparisons included belimumab versus placebo in newly diagnosed and relapsed LN subgroups and belimumab versus placebo in the subgroup of patients who received GC pulses during induction therapy and those who did not. No formal comparisons between subgroups were performed; the study was not powered to investigate subgroups and therefore any analyses by subgroup should be regarded as descriptive.
PERR and CRR were analysed using logistic regression models. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were determined within subgroups for the comparison between belimumab and placebo, with covariates for treatment group, induction regimen (CYC versus MMF), race (Black African ancestry versus other), baseline UPCR and baseline eGFR. Discontinuation of belimumab or placebo, withdrawal from the study or treatment failure were considered as no response.
Time to a kidney-related event or death was analysed using a Cox proportional hazards regression model for the comparison between belimumab and placebo, adjusting for induction regimen (CYC versus MMF), race (Black African ancestry versus other), baseline UPCR and baseline eGFR. Data were censored following discontinuation of belimumab or placebo, withdrawal from the study or having a treatment failure unrelated to a kidney event.
Time to first LN flare from week 24 was analysed using a Cox proportional hazards regression model for the comparison between belimumab and placebo, adjusting for induction regimen (CYC versus MMF), race (Black African ancestry versus other), week 24 UPCR and week 24 eGFR. Data were censored following discontinuation of treatment, withdrawal from the study or having a treatment failure unrelated to kidney disease.
RESULTS
Baseline demographics and clinical characteristics
Overall, 296 patients had newly diagnosed LN and 150 had relapsed LN. With the obvious exception of LN and SLE disease duration, baseline demographics and clinical characteristics were generally similar between the newly diagnosed and relapsed subgroups.
Baseline demographics and clinical characteristics of patients with GC pulses (n = 173) and with no GC pulses (n = 273) at induction were generally similar; however, more patients with GC pulses received CYC as standard therapy [belimumab, n = 27/76 (35.5%); placebo, n = 36/97 (37.1%)] compared with patients with no GC pulses [belimumab, n = 32/147 (21.8%); placebo, n = 23/126 (18.3%)]. There were also country-specific differences; a greater proportion of patients who received GC pulses were from Europe [belimumab, n = 24/76 (31.6%); placebo, n = 29/97 (29.9%)] than patients without GC pulses [belimumab, n = 17/147 (11.6%); placebo, n = 16/126 (12.7%)]. The reverse applied in the USA and Canada, where there was a greater proportion of patients from these countries among those without GC pulses [belimumab, n = 30/147 (20.4%); placebo, n = 28/126 (22.2%)] than those with GC pulses [belimumab, n = 8/76 (10.5%); placebo, n = 10/97 (10.3%)]. Patients with GC pulses had a lower mean eGFR {belimumab, 98.1 [standard deviation (SD) 36.1]; placebo, 90.7 [SD 41.5]} than patients without GC pulses [belimumab, 101.0 (SD 38.6); placebo, 108.9 (SD 42.1)].
Baseline patient demographics and clinical characteristics for all four patient subgroups (newly diagnosed, relapsed, GC pulses, no GC pulses) are shown in Table 1.
Table 1:
Baseline demographic and clinical characteristics (mITT population; post hoc analyses).
| Newly diagnosed | Relapsed | GC pulses | No GC pulses | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Placebo (n = 148) |
Belimumab 10 mg/kg IV (n = 148) |
Placebo (n = 75) |
Belimumab 10 mg/kg IV (n = 75) |
Placebo (n = 97) |
Belimumab 10 mg/kg IV (n = 76) |
Placebo (n = 126) |
Belimumab 10 mg/kg IV (n = 147) |
| Region, n (%) | ||||||||
| Asia | 72 (48.6) | 75 (50.7) | 33 (44.0) | 31 (41.3) | 46 (47.4) | 33 (43.4) | 59 (46.8) | 73 (49.7) |
| Europe | 24 (16.2) | 23 (15.5) | 21 (28.0) | 18 (24.0) | 29 (29.9) | 24 (31.6) | 16 (12.7) | 17 (11.6) |
| USA/Canada | 26 (17.6) | 23 (15.5) | 12 (16.0) | 15 (20.0) | 10 (10.3) | 8 (10.5) | 28 (22.2) | 30 (20.4) |
| Americas excluding USA/Canada | 26 (17.6) | 27 (18.2) | 9 (12.0) | 11 (14.7) | 12 (12.4) | 11 (14.5) | 23 (18.3) | 27 (18.4) |
| Female, n (%) | 129 (87.2) | 133 (89.9) | 67 (89.3) | 64 (85.3) | 88 (90.7) | 66 (86.8) | 108 (85.7) | 131 (89.1) |
| Age (years), mean (SD) | 33.0 (10.7) | 33.9 (11.1) | 33.3 (10.7) | 33.5 (10.0) | 33.1 (9.5) | 34.5 (11.7) | 33.1 (11.5) | 33.4 (10.2) |
| Standard therapy, n (%) | ||||||||
| MMF | 111 (75.0) | 116 (78.4) | 53 (70.7) | 48 (64.0) | 61 (62.9) | 49 (64.5) | 103 (81.7) | 115 (78.2) |
| CYC | 37 (25.0) | 32 (21.6) | 22 (29.3) | 27 (36.0) | 36 (37.1) | 27 (35.5) | 23 (18.3) | 32 (21.8) |
| SLE duration (years)a, median (IQR) | 0.9 (0.2–4.3) |
1.1 (0.1–5.6) |
7.8 (4.9–11.2) |
7.3 (4.2–11.1) |
2.9 (0.2–7.3) |
4.1 (0.3–8.0) |
3.4 (0.2–8.2) |
3.2 (0.3–8.5) |
| LN duration (years)a, median (IQR) | 0.1 (0.1–0.2) |
0.1 (0.1–0.2) |
5.5 (3.3–8.7) |
5.1 (2.8–7.9) |
0.2 (0.1–2.7) |
0.3 (0.1–4.1) |
0.2 (0.1–3.4) |
0.2 (0.1–2.7) |
| LN class, n (%) | ||||||||
| Class III or IV | 88 (59.5) | 86 (58.1) | 44 (58.7) | 40 (53.3) | 59 (60.8) | 42 (55.3) | 73 (57.9) | 84 (57.1) |
| Class III + V or class IV + V | 34 (23.0) | 40 (27.0) | 21 (28.0) | 21 (28.0) | 29 (29.9) | 24 (31.6) | 26 (20.6) | 37 (25.2) |
| Class V | 26 (17.6) | 22 (14.9) | 10 (13.3) | 14 (18.7) | 9 (9.3) | 10 (13.2) | 27 (21.4) | 26 (17.7) |
| UPCR (g/g), mean (SD) | 3.5 (3.8) | 2.9 (2.4) | 3.5 (3.0) | 3.9 (3.3) | 4.0 (4.6) | 3.2 (2.9) | 3.2 (2.5) | 3.2 (2.7) |
| UPCR category (≥3 g/g), n (%) | 61 (41.2) | 55 (37.2) | 31 (41.3) | 36 (48.0) | 41 (42.3) | 30 (39.5) | 51 (40.5) | 61 (41.5) |
| SLEDAI-2K score, mean (SD) | 12.4 (5.1) | 12.7 (5.4) | 11.8 (4.2)b | 12.0 (5.0) | 12.9 (4.6)c | 13.0 (5.7) | 11.6 (4.9) | 12.2 (5.0) |
| eGFR (ml/min/1.73 m2), mean (SD) | 100.4 (42.7) | 101.6 (36.5) | 102.1 (43.0) | 96.9 (40.0) | 90.7 (41.5) | 98.1 (36.1) | 108.9 (42.1) | 101.0 (38.6) |
| eGFR category (≥60 ml/min/1.73 m2), n (%) | 116 (78.4) | 131 (88.5) | 66 (88.0) | 59 (78.7) | 70 (72.2) | 65 (85.5) | 112 (88.9) | 125 (85.0) |
| eGFR category (≥90 ml/min/1.73 m2), n (%) | 87 (58.8) | 89 (60.1) | 46 (61.3) | 42 (56.0) | 48 (49.5) | 45 (59.2) | 85 (67.5) | 86 (58.5) |
| Average daily prednisone equivalent dose (mg/day), mean (SD)d | 80.2 (156.3) | 67.8 (98.1) | 57.3 (65.9) | 63.9 (103.2) | – | – | – | – |
Duration defined as (Treatment start date–Diagnosis date + 1)/365.25.
n = 74.
n = 96.
GCs were converted to prednisone equivalents. Baseline values take into account only the last 7 days prior to randomization; baseline values are not available for GC pulse subgroups as pulses or highest dose of GC (which could be administered between 60 days prior to baseline or at day 1) would not be captured.
SLEDAI-2K: SLE Disease Activity Index 2000.
Belimumab demonstrated efficacy in both newly diagnosed and relapsed patients
To determine whether patients with newly diagnosed LN respond differently to treatment with belimumab than patients with relapsed LN, PERR and CRR at week 104 as well as the time to kidney-related event or death and the time to first LN flare from week 24 were evaluated in the newly diagnosed and relapsed patient subgroups.
More patients achieved PERR or CRR at week 104 with belimumab than with placebo in both subgroups
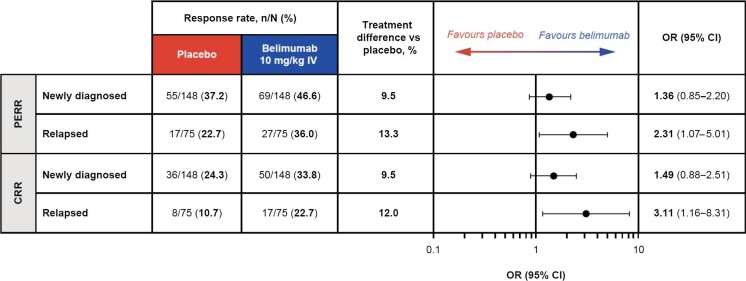
The proportions of patients who achieved PERR were greater with belimumab versus placebo in both the newly diagnosed [69/148 (46.6%) versus 55/148 (37.2%); OR 1.36 (95% CI 0.85–2.20)] and relapsed [27/75 (36.0%) versus 17/75 (22.7%); OR 2.31 (95% CI 1.07–5.01)] subgroups (Fig. 1). Similarly, a greater proportion of patients achieved CRR with belimumab compared with placebo in both the newly diagnosed [50/148 (33.8%) versus 36/148 (24.3%); OR 1.49 (95% CI 0.88–2.51)] and relapsed [17/75 (22.7%) versus 8/75 (10.7%); OR 3.11 (95% CI 1.16–8.31)] subgroups (Fig. 1). For both PERR and CRR, the treatment difference versus placebo tended to be greater in relapsed compared with newly diagnosed patients; however, no formal comparisons between subgroups were performed.
Figure 1:
PERR and CRR at week 104 in patients with relapsed and newly diagnosed LN (mITT population; post hoc analyses). OR and 95% CI values are from a logistic regression model run within the subgroup level for the comparison between belimumab and placebo with covariates treatment group, induction regimen (CYC versus MMF), race (Black African ancestry versus other), baseline UPCR and baseline eGFR.
The risk of experiencing a kidney-related event or death was lower with belimumab than with placebo in both subgroups
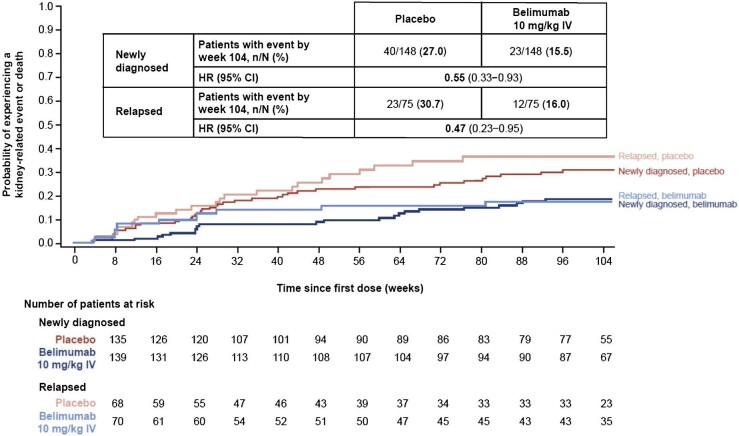
The risk of experiencing a kidney-related event or death through week 104 was reduced by 45% among patients who received belimumab compared with those who received placebo in the newly diagnosed subgroup [hazard ratio (HR) 0.55 (95% CI 0.33–0.93)] and by 53% in the relapsed subgroup [HR 0.47 (95% CI 0.23–0.95)] (Fig. 2).
Figure 2:
Time to kidney-related event or death through week 104 in patients with newly diagnosed and relapsed LN (mITT population; post hoc analyses). HR and 95% CI values are from Cox proportional hazards model for the comparison between belimumab and placebo adjusting for induction regimen (CYC versus MMF), race (Black African ancestry versus other), baseline UPCR and baseline eGFR. Investigational product discontinuations, treatment failures not related to kidney disease and withdrawals were censored on the date of the event. Patients who completed the study were censored at week 104.
The risk of experiencing an LN flare from week 24 was lower with belimumab than with placebo in both subgroups
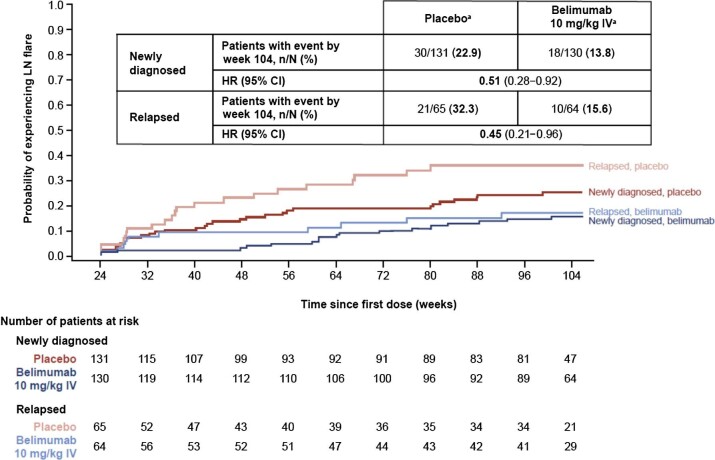
The risk of experiencing an LN flare from week 24 through week 104 was reduced by 49% among patients who received belimumab compared with those on placebo in the newly diagnosed subgroup [HR 0.51 (95% CI 0.28–0.92)] and by 55% in the relapsed subgroup [HR 0.45 (95% CI 0.21–0.96)] (Fig. 3).
Figure 3:
Time to first LN flare from week 24 in patients with newly diagnosed and relapsed LN (mITT population among patients on treatment at week 24; post hoc analyses). aWeek 24 values are used as ‘baseline’ in the definition of LN flare. HR and 95% CI values are from Cox proportional hazards model for the comparison between belimumab and placebo adjusting for induction regimen (CYC versus MMF), race (Black African ancestry versus other), week 24 UPCR and week 24 eGFR. Investigational product discontinuations, treatment failures not related to kidney disease and withdrawals were censored on the date of the event. Patients who completed the study were censored at week 104. Renal-related treatment failures were considered flares.
These data demonstrate that treatment with belimumab was associated with greater proportions of patients achieving PERR or CRR, a reduced risk of a kidney-related event or death and a reduced risk of experiencing an LN flare through week 104 compared with placebo, regardless of whether patients had newly diagnosed or relapsed LN.
Belimumab demonstrated efficacy in patients with and without GC pulses during induction therapy
To determine whether patients with and without GC pulses during induction therapy responded differently to belimumab, we evaluated PERR and CRR at week 104 as well as the time to kidney-related event or death and the time to first LN flare from week 24 in the GC pulses and no GC pulses subgroups. The mean combined (IV and oral) daily prednisone equivalent dose in the first 6 months is presented in Supplementary Table 1.
More patients achieved PERR or CRR at week 104 with belimumab than with placebo regardless of GC pulse use during induction
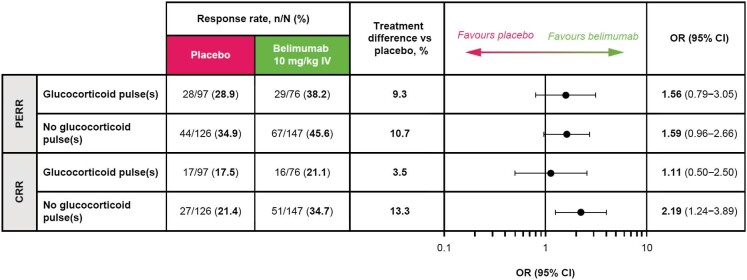
Greater proportions of patients achieved PERR with belimumab versus placebo in both the GC pulses [29/76 (38.2%) versus 28/97 (28.9%); OR 1.56 (95% CI 0.79–3.05)] and no GC pulses [67/147 (45.6%) versus 44/126 (34.9%); OR 1.59 (95% CI 0.96–2.66)] subgroups (Fig. 4). Similarly, the proportions of patients who achieved CRR were greater with belimumab versus placebo in both the GC pulses [16/76 (21.1%) versus 17/97 (17.5%); OR 1.11 (95% CI 0.50–2.50)] and no GC pulses [51/147 (34.7%) versus 27/126 (21.4%); OR 2.19 (95% CI 1.24–3.89)] subgroups (Fig. 4). More patients achieved PERR or CRR in the subgroup with no GC pulses compared with the subgroup that received GC pulses, irrespective of the treatment allocation (Fig. 4).
Figure 4:
PERR and CRR at week 104 in patients with and without GC pulses at induction (mITT population; post hoc analyses). OR and 95% CI values are from a logistic regression model run within the subgroup level for the comparison between belimumab and placebo with covariates treatment group, induction regimen (CYC versus MMF), race (Black African ancestry versus other), baseline UPCR and baseline eGFR.
The risk of experiencing a kidney-related event or death was lower with belimumab than with placebo regardless of GC pulse during induction
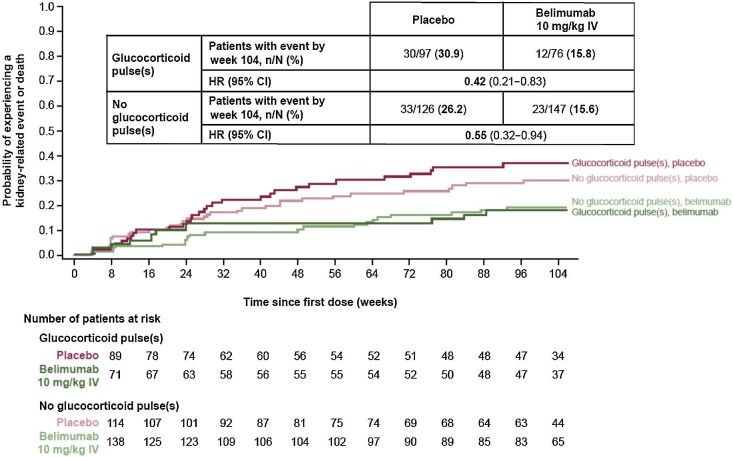
The risk of experiencing a kidney-related event or death through week 104 was lower with belimumab versus placebo in both the subgroup of patients with GC pulses [HR 0.42 (95% CI 0.21–0.83)] and the subgroup with no GC pulses [HR 0.55 (95% CI 0.32–0.94)] administered during induction (Fig. 5).
Figure 5:
Time to kidney-related event or death through week 104 in patients with and without GC pulses at induction (mITT population; post hoc analyses). HR and 95% CI values are from Cox proportional hazards model for the comparison between belimumab and placebo adjusting for induction regimen (CYC versus MMF), race (Black African ancestry versus other), baseline UPCR and baseline eGFR. Investigational product discontinuations, treatment failures not related to kidney disease and withdrawals were censored on the date of the event. Patients who completed the study were censored at week 104.
The risk of experiencing an LN flare from week 24 was lower with belimumab than with placebo regardless of GC pulse during induction
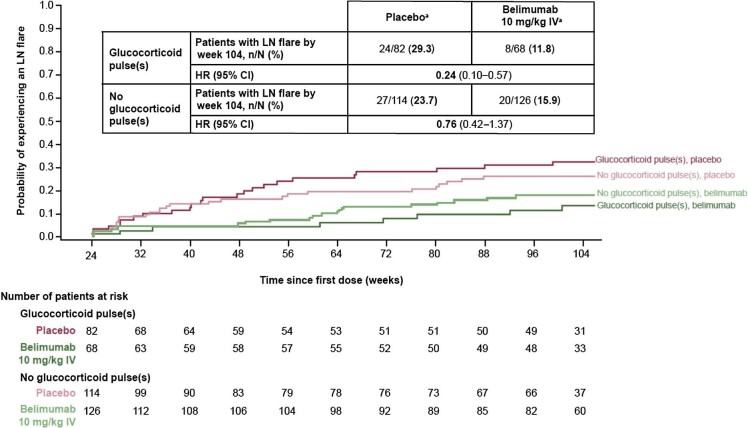
The risk of experiencing an LN flare from week 24 was reduced by 76% among patients who received belimumab compared with those who received placebo in the subgroup of patients with GC pulses [HR 0.24 (95% CI 0.10–0.57)] and by 24% in the subgroup of patients with no GC pulses [HR 0.76 (95% CI 0.42–1.37)] administered during induction (Fig. 6).
Figure 6:
Time to first LN flare from week 24 in patients with and without GC pulses at induction (mITT population; post hoc analyses). aWeek 24 values are used as ‘baseline’ in the definition of LN flare. HR and 95% CI values are from Cox proportional hazards model for the comparison between belimumab and placebo adjusting for induction regimen (CYC versus MMF), race (Black African ancestry versus other), week 24 UPCR and week 24 eGFR. Investigational product discontinuations, treatment failures not related to kidney disease and withdrawals were censored on the date of the event. Patients who completed the study were censored at week 104. Renal-related treatment failures were considered flares.
These data demonstrate that, compared with placebo, belimumab treatment was associated with greater proportions of patients achieving PERR or CRR, a reduced risk of a kidney-related event or death and a reduced risk of experiencing an LN flare through week 104, regardless of GC pulse administration during induction therapy.
DISCUSSION
These post hoc analyses of data from the BLISS-LN study investigated whether the addition of belimumab to standard therapy improved kidney outcomes compared with standard therapy alone in patients with newly diagnosed LN as well as in those with relapsed LN. In addition, we investigated whether belimumab plus standard therapy improved kidney outcomes irrespective of administration of GC pulses during induction.
These analyses are among the first to assess clinical outcomes of patients based on whether patients were newly diagnosed or experiencing a relapse of LN. For both newly diagnosed and relapsed patients with LN, greater proportions of patients treated with belimumab compared with placebo achieved PERR or CRR at week 104, in line with previous findings from the overall BLISS-LN patient cohort [14]. Similar findings were also observed for the time to kidney-related event or death and the time to first LN flare endpoints. The observed improvement in patients with relapsed LN is particularly notable given this population is usually more refractory to treatment than patients with newly diagnosed LN. Furthermore, patients with relapsed disease are generally considered to have more advanced chronic kidney lesions, which hinders attaining any of the study endpoints. Of note, this study did not show baseline demographic differences in sex or age between newly diagnosed and relapsed LN, which have been shown previously [18]. In addition, the average daily prednisone dose at baseline with placebo in patients with newly diagnosed LN was high (80 mg/day), although the reason for this has remained undetermined.
The use of methylprednisolone pulses and medium-dose prednisone is associated with a lower toxicity than treatment with high doses of oral steroids [23, 24]. In this study, a greater proportion of patients achieved PERR or CRR when administered belimumab, and the risk of experiencing a kidney-related event or death or LN flare was reduced with belimumab versus placebo in patients with or without GC pulses at induction. Interestingly, in both treatment groups, greater proportions of patients who did not receive GC pulses at induction achieved PERR or CRR compared with those who received GC pulses at induction, and the observed treatment differences favoured belimumab. As the average combined daily (oral and/or IV) GC dose over the first 6 months of the study was similar between belimumab and placebo in patients with and without GC pulses, the observed findings do not appear to be driven by differences in GC exposure. These findings could also be a result of patients who received GC pulses having more active and/or severe kidney disease and therefore possessing a lower potential to achieve renal responses. If so, this should be considered in future study designs, which should account for histologic activity and chronicity in estimating response rates. There were some country-specific differences in the receipt of GC pulses, likely due to regional differences in clinical practice. In addition, although the study was not designed to assess this, more patients who received CYC as initial standard therapy received GC pulses, whereas more patients who received MMF as initial standard therapy did not receive pulses; this could reflect the aforementioned assumption regarding the severity of LN or simply a preference of clinicians to administer pulse GCs with CYC regimens. Still, the prospect of not requiring GC pulses at induction is tantalising and recapitulates the results of a small prospective cohort study that showed the addition of methylprednisolone to rituximab as induction therapy did not affect complete remission rates (although many patients had existing high steroid exposure) [25]. Our data suggest that regardless of whether pulse GCs were administered, benefit was derived with belimumab treatment.
Limitations of our analyses should be considered when interpreting these results. First, the BLISS-LN study was not designed to detect treatment differences between the patient subgroups assessed here. These analyses were post hoc in nature and not powered for statistical analyses between subgroups. Overlapping 95% CIs were observed and should be considered when interpreting these results. Furthermore, sample sizes were not comparable between all subgroups, although patient numbers were similar across those receiving placebo and belimumab for newly diagnosed and relapsed patients.
In conclusion, these data show consistent benefit of belimumab versus placebo, in addition to standard therapy, on kidney outcomes in both newly diagnosed and relapsed patients and irrespective of whether patients received GC pulses at induction.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the participating patients and their families, clinicians and study investigators. Medical writing support was provided by Nicholas Thomas and Laura Fullerton-Batten of Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by GSK.
Contributor Information
Hans-Joachim Anders, Department of Medicine IV, Hospital of Ludwig Maximilians University Munich, Munich, Germany.
Richard Furie, Division of Rheumatology, Northwell Health, Great Neck, NY, USA.
Ana Malvar, Nephrology Research Unit, Organización Médica de Investigación, Buenos Aires, Argentina.
Ming-Hui Zhao, Renal Division, Peking University First Hospital, Beijing, China.
Keiju Hiromura, Department of Nephrology and Rheumatology, Gunma University Graduate School of Medicine, Maebashi, Japan.
Julia Weinmann-Menke, Department of Medicine I, Division of Nephrology, University Medical Center Mainz, Mainz, Germany.
Yulia Green, Clinical Development, GSK, Brentford, Middlesex, UK.
Angela Jones-Leone, Specialty Care, Global Medical Affairs, GSK, Collegeville, PA, USA.
Daniela Negrini, Immunology Biostatistics, GSK, Stevenage, UK.
Roger A Levy, Specialty Care, Global Medical Affairs, GSK, Collegeville, PA, USA.
Liz Lightstone, Department of Immunology and Inflammation, Imperial College London, London, UK.
Yoshiya Tanaka, The First Department of Internal Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan.
Brad H Rovin, Division of Nephrology, The Ohio State University Wexner Medical Center, Columbus, OH, USA.
FUNDING
This study (GSK Study BEL114054) was funded by GSK.
AUTHORS’ CONTRIBUTIONS
All authors analysed and interpreted the data, contributed intellectually towards the preparation of the manuscript, approved the final submitted version and agreed to be listed as authors. H.-J.A., R.F., A.M., M.-H.Z., Y.T. and B.H.R. were involved in the acquisition of data. Y.G., A.J.-L. and R.A.L. contributed to the conception and design of the analysis.
DATA AVAILABILITY STATEMENT
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
CONFLICT OF INTEREST STATEMENT
H.-J.A. has received consultancy fees or honoraria from AstraZeneca, Bayer, Boehringer, GSK, Idorsia, Janssen, Kezar, Sanofi, Otsuka, Novartis and PreviPharma; is a member of the Scientific Advisory Board of the European Renal Association and an associate editor at Journal of the American Society of Nephrology and NDT. R.F. has received advisory board fees and travel support from GSK. L.L. has received consulting fees from Alexion, AstraZeneca, Biogen, Bristol-Myers Squibb, GSK, Kezar, Novartis and Pfizer; honoraria from Alexion, AstraZeneca, Bristol-Myers Squibb, GSK and Novartis; serves as Chair, DMC of a trial in LN for Novartis and Deputy Chair for the Western Europe Regional Board, ISN; Member ExCom ISN, Trustee Kidney Research UK. A.M. has received consultancy fees from GSK, Roche and Biogen. M.-H.Z. has received consultancy fees from AstraZeneca, GSK, Kira, Novartis and Roche. K.H. has received consultancy fees from GSK; grant/research support from Bayer, Chugai, GSK and Kyowa Kirin and honoraria from Astellas, AstraZeneca, Chugai, GSK and Sanofi. J.W.-M. participated in advisory boards and has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Chiesi, GSK, Novartis and Otsuka. Y.T. has received speaker fees and/or honoraria from AbbVie, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, GSK, Mitsubishi-Tanabe and Pfizer and has received research grants from AbbVie, Asahi-Kasei, Boehringer Ingelheim, Chugai, Daiichi-Sankyo, Eisai and Takeda. B.H.R. has received grants or contracts from Biogen, National Institute on Aging, National Institute of Arthritis and Musculoskeletal and Skin Diseases and National Institute of Diabetes and Digestive and Kidney Diseases; consulting fees from Aurinia, Alexion, AstraZeneca, Biocryst, Biogen, Bristol-Myers Squibb, Calliditas, Chemocentryx, Corrona, EMD Serono, Exagen, Galapagos, Genentech-Roche, GSK, Janssen, Kezar, Lilly, Morphosys, Novartis, Omeros, Otsuka, Pfizer, Travere and the University of Minnesota; honoraria for Medicine, Nephrology, Rheumatology Grand Rounds Lectures at various academic centres; support for attending meetings from the American Society of Nephrology and leadership/fiduciary roles for the Lupus Foundation of American and Kidney Disease: Improving Global Outcomes Glomerulonephritis Guideline. A.J.-L., Y.G. and R.A.L. are employees of GSK and hold stock and shares in the company. D.N. was a contract worker at GSK at the time of the study.
REFERENCES
- 1. Hanly JG, O'Keeffe AG, Su L et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford) 2016;55:252–62. 10.1093/rheumatology/kev311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anders HJ, Saxena R, Zhao MH et al. Lupus nephritis. Nat Rev Dis Primers 2020;6:7. 10.1038/s41572-019-0141-9 [DOI] [PubMed] [Google Scholar]
- 3. Parikh SV, Rovin BH. Current and emerging therapies for lupus nephritis. J Am Soc Nephrol 2016;27:2929–39. 10.1681/ASN.2016040415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rovin BH, Furie R, Latinis K et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 2012;64:1215–26. 10.1002/art.34359 [DOI] [PubMed] [Google Scholar]
- 5. El Hachmi M, Jadoul M, Lefebvre C et al. Relapses of lupus nephritis: incidence, risk factors, serology and impact on outcome. Lupus 2003;12:692–6. 10.1191/0961203303lu444oa [DOI] [PubMed] [Google Scholar]
- 6. Dooley MA, Jayne D, Ginzler EM et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med 2011;365:1886–95. 10.1056/NEJMoa1014460 [DOI] [PubMed] [Google Scholar]
- 7. Tektonidou MG, Dasgupta A, Ward MM. Risk of end-stage renal disease in patients with lupus nephritis, 1971–2015: a systematic review and Bayesian meta-analysis. Arthritis Rheumatol 2016;68:1432–41. 10.1002/art.39594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baker KP, Edwards BM, Main SH et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum 2003;48:3253–65. 10.1002/art.11299 [DOI] [PubMed] [Google Scholar]
- 9. Wallace DJ, Stohl W, Furie RA et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum 2009;61:1168–78. 10.1002/art.24699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Furie R, Petri M, Zamani O et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63:3918–30. 10.1002/art.30613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Navarra SV, Guzmán RM, Gallacher AE et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:721–31. 10.1016/S0140-6736(10)61354-2 [DOI] [PubMed] [Google Scholar]
- 12. Ginzler E, Guedes Barbosa LS, D'Cruz D et al. Phase III/IV, randomized, fifty-two-week study of the efficacy and safety of belimumab in patients of black African ancestry with systemic lupus erythematosus. Arthritis Rheumatol 2021;74:112–23. 10.1002/art.41900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brunner HI, Abud-Mendoza C, Viola DO et al. Safety and efficacy of intravenous belimumab in children with systemic lupus erythematosus: results from a randomised, placebo-controlled trial. Ann Rheum Dis 2020;79:1340–8. 10.1136/annrheumdis-2020-217101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Furie R, Rovin BH, Houssiau F et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med 2020;383:1117–28. 10.1056/NEJMoa2001180 [DOI] [PubMed] [Google Scholar]
- 15. GlaxoSmithKline . Benlysta prescribing information. https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Benlysta/pdf/BENLYSTA-PI-MG-IFU.PDF (01 August 2023, date last accessed). [Google Scholar]
- 16. Rovin BH, Furie R, Teng YO et al. A secondary analysis of the Belimumab International Study in Lupus Nephritis trial examined effects of belimumab on kidney outcomes and preservation of kidney function in patients with lupus nephritis. Kidney Int 2022;101:403–13. 10.1016/j.kint.2021.08.027 [DOI] [PubMed] [Google Scholar]
- 17. Gibson KL, Gipson DS, Massengill SA et al. Predictors of relapse and end stage kidney disease in proliferative lupus nephritis: focus on children, adolescents, and young adults. Clin J Am Soc Nephrol 2009;4:1962–7. 10.2215/CJN.00490109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tonsawan P, Sawanyawisuth K. Clinical comparisons between previously diagnosed SLE and newly diagnosed SLE by kidney biopsy. Auto Immun Highlights 2020;11:18. 10.1186/s13317-020-00140-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bruce IN, O'Keeffe AG, Farewell V et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis 2015;74:1706–13. 10.1136/annrheumdis-2013-205171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen HL, Shen LJ, Hsu PN et al. Cumulative burden of glucocorticoid-related adverse events in patients with systemic lupus erythematosus: findings from a 12-year longitudinal study. J Rheumatol 2018;45:83–9. 10.3899/jrheum.160214 [DOI] [PubMed] [Google Scholar]
- 21. Fanouriakis A, Kostopoulou M, Alunno A et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78:736–45. 10.1136/annrheumdis-2019-215089 [DOI] [PubMed] [Google Scholar]
- 22. Kidney Disease: Improving Global Outcomes Glomerular Diseases Work Group . KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int 2021;100(4 Suppl):S1–276. 10.1016/j.kint.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 23. Scheinberg M. The history of pulse therapy in lupus nephritis (1976–2016). Lupus Sci Med 2016;3:e000149. 10.1136/lupus-2016-000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruiz-Irastorza G, Danza A, Perales I et al. Prednisone in lupus nephritis: how much is enough? Autoimmun Rev 2014;13:206–14. 10.1016/j.autrev.2013.10.013 [DOI] [PubMed] [Google Scholar]
- 25. Pepper R, Griffith M, Kirwan C et al. Rituximab is an effective treatment for lupus nephritis and allows a reduction in maintenance steroids. Nephrol Dial Transplant 2009;24:3717–23. 10.1093/ndt/gfp336 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.