ABSTRACT
There is growing evidence that chronic kidney disease (CKD) is an independent risk factor for cognitive impairment, especially due to vascular damage, blood–brain barrier disruption and uremic toxins. Given the presence of multiple comorbidities, the medication regimen of CKD patients often becomes very complex. Several medications such as psychotropic agents, drugs with anticholinergic properties, GABAergic drugs, opioids, corticosteroids, antibiotics and others have been linked to negative effects on cognition. These drugs are frequently included in the treatment regimen of CKD patients. The first review of this series described how CKD could represent a risk factor for adverse drug reactions affecting the central nervous system. This second review will describe some of the most common medications associated with cognitive impairment (in the general population and in CKD) and describe their effects.
Keywords: adverse drug reaction, chronic kidney disease, cognitive impairment, drug prescription, medications
Graphical Abstract
Graphical Abstract.
INTRODUCTION
There is growing evidence showing that chronic kidney disease (CKD) is an independent risk factor for cognitive impairment, especially due to vascular damage, blood–brain barrier (BBB) disruption and uremic toxins [1]. A better understanding of the risk factors associated with cognitive impairment could lead to new avenues to prevent further cognitive impairment in CKD patients. In particular, adverse drug reactions (ADRs) affecting the central nervous system (CNS) could represent another modifiable risk factor as these may be prevented by re-evaluation of a patient's drug prescription.
Among ADRs affecting the CNS, cognitive adverse effects are frequent and could present as sedation, decreased performance skills, hallucinations or delirium [2]. Any small molecule that can cross the BBB has the potential to affect the CNS and therefore alter cognitive functioning. Agents that modulate the brain's neurotransmitter systems (acetylcholine, serotonin, dopamine and glutamate) could either enhance or impair several cognitive functions. In addition to brain modulators, blockers of neurotransmitter receptors can also result in cognitive impairment and include compounds such as cholinergic, dopaminergic and histaminergic antagonists. Finally, sedative properties of certain drug classes (such as antihistaminic agents and hypnotics) have been associated with cognitive impairment. Since patients with CKD tend to have multiple comorbidities and treatments, their medication regimen often becomes very complex and they may be highly exposed to these drugs [3].
In the first review of this series, we discuss how CKD represents a risk factor for ADRs on the CNS via BBB disruption and alteration of drug pharmacokinetics. In this narrative review, we will describe some of the most common medications linked with negative effects on cognition (in the general population and in CKD) and describe their effects (Fig. 1).
Figure 1:
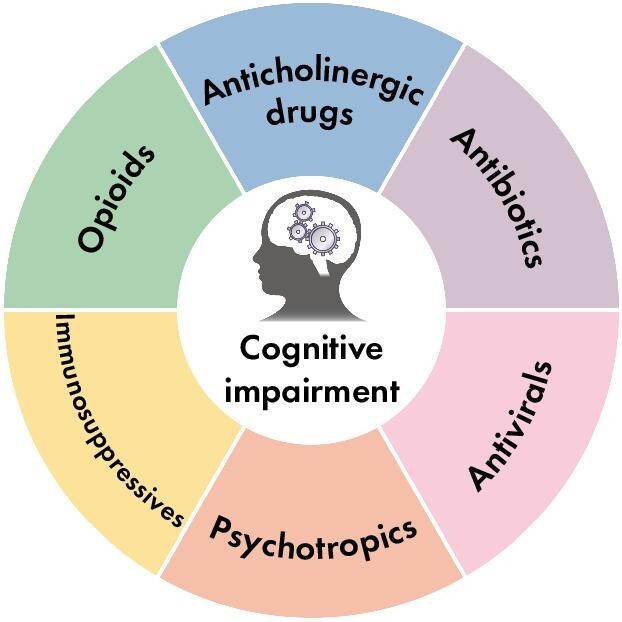
The most common medications with negative impacts on cognition in the general population.
DRUGS WITH ANTICHOLINERGIC EFFECTS
Pathophysiology of anticholinergic drugs in the brain
There are two types of receptors in the cholinergic system: muscarinic and nicotinic, named after their exogenous ligands. Acetylcholine stimulates both types of receptors, exerting both muscarinic and nicotinic effects. Medications with an anticholinergic effect refer to drugs that bind muscarinic receptors but not nicotinic receptors. Most of them are nonselective for receptor binding and are not tissue selective.
Muscarinic receptors (mAChRs) belong to a group of G-protein-coupled membrane metabotropic receptors [4]. Five types of mAChRs have been identified based on amino acid sequences: M1, M2, M3, M4 and M5 [5, 6]. All subtypes of M1–M5 muscarinic receptors are distributed in the brain. The M1 subtype is found in the cortex, hippocampus, striatum and thalamus, where it occurs postsynaptically, while the M2 subtype is found primarily in the brainstem and thalamus, but also in the cortex, hippocampus and striatum, at synaptic terminals. The other receptors are expressed at much lower levels. M3 receptors are found in the cortex and hippocampus, M4 in many brain regions, including the cortex and hippocampus, but most abundantly in the striatum, while M5 is found in the substantia nigra [4]. The M1 receptors (Gq) in the brain are the most involved in the anticholinergic response that causes delirium, cognitive impairment, dizziness, sedation and confusion.
Many commonly used drugs have primary or secondary anticholinergic effects. Anticholinergic drugs are the principal treatments of some clinical conditions, such as urinary incontinence. However, anticholinergic adverse effects frequently occur with medications prescribed with other intended mechanisms of action, including antihistamines, antidepressants and antipsychotics. These drugs are commonly prescribed to older people and CKD patients and unfortunately these drugs are not routinely recognized as having anticholinergic activity.
Evaluation of anticholinergic drugs burden
The cumulative effect of taking one or more drugs with anticholinergic properties is referred as anticholinergic burden. There are different techniques to indirectly assess the anticholinergic activity of drugs. The evaluation of serum anticholinergic activity (SAA) is the laboratory technique [7] recognized as the gold standard. However, this evaluation is not routinely available, in part due to the method using a radioreceptor assay. As a consequence, different drug scales for anticholinergic drugs have been developed [8–20] (Table 1) enabling the calculation of the “anticholinergic burden.” Recently, 13 different scales have been documented and accepted by the international academic community; however, only three of them are commonly used: the Anticholinergic Drug Scale (ADS), the Anticholinergic Risk Scale (ARS) and the Anticholinergic Burden Scale (ACB) (Table 1). One of the most commonly used scales in research studies to evaluate central adverse events is the Anticholinergic Cognitive Burden scale (ACB) [8] where drugs are classified based on an expert-based list according to SAA or in vitro affinity to muscarinic receptors. Drugs with possible anticholinergic effects are given a score of 1. Drugs are classified as score 2 when some cognitive effects can be found and as score 3 when these cognitive effects are very significant. Table 2 reports drugs with anticholinergic effects based on the ACB scale. Anticholinergic drugs are used for different medical conditions such as urinary dysfunction, peptic ulcer disease, Parkinson's disease, and neurologic and psychiatric conditions. Drugs with anticholinergic properties are widely prescribed especially in the elderly [21].
Table 1:
Different anticholinergic scales.
| Score name | Princeps publication | Country | Princeps study population | Methods to assess anticholinergic activity | Princeps study main results | Type of anticholinergic adverse effects: central and peripheral | Observations |
|---|---|---|---|---|---|---|---|
| Anticholinergic Cognitive Burden Scale (ACB) | Boustani et al. 2008 [8] | USA | Older adults attending primary care clinics in Indianapolis (n = 3013) | Expert-based list | High level of anticholinergic burden. More than 23% of older adults were receiving at least one medication with anticholinergic activity | Central | One of the most used scale in elderly population |
| Anticholinergic Risk Scale (ARS) | Rudolph et al. 2008 [9] | USA | Older men adults (≥65 years) attending geriatric at the Veterans Affairs Boston Healthcare System or primary care ambulatory clinics (n = 117) | Expert-based list | Higher ARS scores are associated with increased risk of anticholinergic adverse effects | Peripheral and central | One of the most used scale in elderly population |
| Anticholinergic Drug Scale (ADS) | Carnahan et al. 2002 [10] | USA | Older adults from rural long term care facilities (n = 98) | List created using association with the SAA | Significant correlation between SAA and anticholinergic drug scale | Peripheral | One of the most used scale in elderly population |
| - Focused on peripheral ADR | |||||||
| - ADS did not use the clinical input of expert clinicians and did not focus on the cognitive effects of anticholinergics | |||||||
| Clinician-rated Anticholinergic Score (CrAS) | Han et al. 2001 [11] | Canada | Older inpatients (≥65 years) with diagnosed incident or prevalent delirium (n = 278) | Expert-based list | Exposure to anticholinergic medications is independently and specifically associated with a subsequent increase in delirium symptom severity | Central | Limited number of publications using this scale |
| Anticholinergic Impregnation Scale (AIS) | Briet et al. 2017 [12] | France | Psychiatric patients (n = 7278) | Expert-based list | A high anticholinergic burden (AIS score >5) was associated with a higher rate of prescription of two anticholinergic adverse drug reaction correctors: laxatives and drugs against xerostomia | Peripheral | Limited number of publications using this scaleSpecific to psychiatric population and focused on peripheral ADR |
| Anticholinergic Burden Classification (ABC) | Ancelin et al. 2006 [13] | France | Older adults participants (aged >60 years) through 63 randomly selected general practitioners in the Montpellier region of southern France (n = 372) | Expert-based list | Association between high anticholinergic burden and mild cognitive impairment | Central | Limited number of publications using this scale |
| Anticholinergic Activity Scale (AAS) | Ehrt et al. 2010 [14] | Norway | A community-based cohort of patients with Parkinson's disease (n = 235) | Expert-based list | Association between anticholinergic drug use and cognitive decline in Parkinson's disease | Central | Limited number of publications using this scaleSpecific to patients with Parkinson's disease |
| Anticholinergic Effect on Cognition (AEC) | Bishara et al. 2017 [15] | UK | Not applicable. List developed for older patients | Expert-based list | Not applicable | Central | Limited number of publications using this scale |
| Muscarinic Acetylcholine Receptor Antagonist Exposure (MARANTE) | Klamer et al. 2017 [16] | Belgium | Not applicable. List developed for older patients | Expert-based list | Not applicable | Central | Limited number of publications using this scale |
| Anticholinergic Load scale (ACL) | Sittironnarit et al. 2011 [17] | Australia | Older adults participants (aged >60 years): a cohort of 211 Alzheimer's disease patients, 133 mild cognitive impairment patients and 768 healthy controls | Expert-based list | Association between ACL and psychomotor speed and executive function in healthy older adults | Central | Limited number of publications using this scale |
| Anticholinergic Burden (ACB) | Kiesel et al. 2018 [18] | Germany | Not applicableList developed for older patients | Expert-based listBy different scores comparison and adapted to German prescription | Not applicable | Peripheral and central | Limited number of publications using this scaleSpecific to Germany |
| Brazilian Anticholinergic Activity scale (BAA) | Nery et al. 2019 [19] | Brazil | Not applicable. List developed for older patients | Expert-based listBy different scores comparison and adapted to Brazilian prescription | Not applicable | Peripheral and central | Limited number of publications using this scaleSpecific to Brazil |
| Anticholinergic Burden Scale (KABS) | Jun et al. 2019 [20] | South Korea | Not applicable. List developed for older patients | Expert-based listBy different scores comparison and adapted to South Korean prescription | Not applicable | Peripheral and central | Limited number of publications using this scaleSpecific to South KoreaDrug dosages use in this evaluation |
Table 2:
Anticholinergic cognitive burden scoring drugs based on ACB scale [8].
| Pharmacologic groups | ATC code | International no-proprietary name | ACB score |
|---|---|---|---|
| Parasympatholytics or sympathomimetics | A04 | Scopolamine | 3 |
| Drugs for gastrointestinal disorders | A02 | Cimetidine | 1 |
| Ranitidine | 1 | ||
| A03 | Alverine | 1 | |
| Atropine | 3 | ||
| Dimenhydrinate | 3 | ||
| Propantheline | 3 | ||
| A07 | Hyoscyamine | 3 | |
| loperamide | 1 | ||
| Drugs related to cardiovascular system | B01 | Coumadine | 1 |
| C01 | Dipyridamole | 1 | |
| Digoxin | 1 | ||
| Disopyramide | 1 | ||
| Isosorbide | 1 | ||
| C02 | Hydralazine | 1 | |
| C03 | Furosemide | 1 | |
| Quinidine | 1 | ||
| Chlortalidone | 1 | ||
| C07 | Triamterene | 1 | |
| Atenolol | 1 | ||
| C08 | Metoprolol | 1 | |
| C09 | Nifedipine | 1 | |
| Captopril | 1 | ||
| Urinary antispasmodics | G04 | Dariferacin | 3 |
| Flavoxate | 3 | ||
| Oxybutynine | 3 | ||
| Tolterodine | 3 | ||
| Corticoids | H02 | Hydrocortisone | 1 |
| Prednisone | 1 | ||
| Analgesic | N02 | Codeine | 1 |
| Fentanyl | 1 | ||
| Morphine | 1 | ||
| Pethidine | 2 | ||
| Meperidine | 2 | ||
| Antiepileptic | N03 | Carbamazepine | 2 |
| Oxcarbazepine | 2 | ||
| Antiparkinsonian | N04 | Amantadine | 2 |
| Benztropine | 3 | ||
| Orphenadrine | 3 | ||
| Procyclidine | 3 | ||
| Trihexyphenidyl | 3 | ||
| Psycholeptic | N05A antipsychotic | Clozapine | 3 |
| Chlorpromazine | 3 | ||
| Haloperidol | 1 | ||
| Levomepromazine | 2 | ||
| Loxapine | 2 | ||
| Molindone | 2 | ||
| Olanzapine | 3 | ||
| Perphenazine | 3 | ||
| Pimozide | 2 | ||
| Promazine | 3 | ||
| Quetiapine | 3 | ||
| Risperidone | 1 | ||
| Thiorizadine | 3 | ||
| Trifluoperazine | 3 | ||
| N05B anxiolytic | Alprazolam | 1 | |
| Clorazepate | 1 | ||
| Diazepam | 1 | ||
| Hydroxyzine | 3 | ||
| Antidepressant | N06A | Amitriptylline | 3 |
| Amoxapine | 3 | ||
| Buproprion | 1 | ||
| Clomipramine | 3 | ||
| Desipramine | 3 | ||
| Doxepin | 3 | ||
| Fluvoxamine | 1 | ||
| Imipramine | 3 | ||
| Nortriptyline | 3 | ||
| Paroxetine | 2 | ||
| Trazodone | 1 | ||
| Trimipramine | 3 | ||
| Antiasthmatic drugs | R03 | Theophylline | 1 |
| H1 antihistaminic | R06A | Alimemazine | 1 |
| Brompheniramine | 3 | ||
| Carbinoxamine | 3 | ||
| Chlorpheninamine | 3 | ||
| Clemastine | 3 | ||
| Cyproheptadine | 2 | ||
| Diphenhydramine | 3 | ||
| Meclozine | 3 | ||
| Promethazine | 3 | ||
| Pyrilamine | 3 | ||
| Others | M03 | Cyclobenzaprine | 2 |
| M04 | Colchicine | 1 |
A Drugs in bold are drugs with ACB score at 3.
TC: anatomical therapeutic chemical; ACB: Anticholinergic Cognitive Burden.
Anticholinergic ADRs of drugs with anticholinergic activity can be peripheral, with typical symptoms including dry mouth, constipation, urinary retention, bowel obstruction, dilated pupils, blurred vision, increased heart rate and decreased sweating, or central side effects such as impairment in cognitive function.
Association between anticholinergic medication use and cognitive impairment
The biological basis for the cognitive effects of anticholinergic drugs is partially known. Given the importance of the cholinergic system in cognition, researchers speculate that direct impairment of the cholinergic neurons may underlie these effects. In addition, Risacher et al. suggested that the use of anticholinergic medication was associated with increased brain atrophy and dysfunction, and clinical decline [22]. Indeed, the authors observed that cognitively normal older adults taking medications with medium or high anticholinergic activities (evaluated by the ACB scale) showed poorer cognition, reduced cerebral glucose metabolism, increased brain atrophy and increased clinical decline in comparison with those not taking these medications. The kidney function was, however, not provided in this analysis.
Most studies evaluating the association between anticholinergic drug burden and outcomes have been performed in elderly patients. They report a high prevalence of use of anticholinergic drugs and an association with the risk of hospitalization, cognitive impairment and higher mortality [21, 23, 24]. In a recent study on 19 114 participants form Australia and the USA aged 70 years and older, the anticholinergic burden calculated by using the ACB predicted worse cognitive function over time, particularly for executive function and episodic memory [25].
Frequency of use of anticholinergics in CKD patients
As discussed above, anticholinergic drugs are mostly used in the elderly, and a high anticholinergic burden is associated with more adverse effects, including cognitive impairment, confusion, forgetfulness, delirium and falls. In a medication review study of 155 consecutive hospitalized patients, hypoalbuminemia and kidney dysfunction were shown to be independently associated with an ACB score. Patients with a higher anticholinergic burden score had a lower estimated glomerular filtration rate (eGFR) and were also prescribed higher doses of anticholinergic medications than those with a lower anticholinergic burden score [26]. In a study conducted with 5196 advanced CKD patients over 75 years of age, polypharmacy was measured, and the average number of drugs prescribed per day was determined as 9. Among common potentially inappropriate medications with renal contraindications, dose adjustments or precautions for the study population, the frequency of anticholinergic drugs prescribed was as follows: hydroxyzine (4.9%), mirtazapine (1.6%), amitriptyline (0.7%) dexchlorpheniramine (0.5%), alimemazine (0.4%) and ropinirole (0.2%). Consequently, it was recommended that physicians should limit the number of these drugs as much as possible [27].
OPIOID AGENTS
Chronic pain is a consequence of CKD and its complications, and a comprehensive treatment strategy is required in these patients. Dose control of drugs used in pain management in CKD and end-stage kidney disease (ESKD) patients not only increases the effects of drugs, but also reduces adverse effects including mild cognitive impairment. Many studies have shown that pain on its own has negative effects on cognition, but there are also studies that show some regression/decline of cognitive functions after the use of opioids as pain relievers [28]. In a systematic review, anticholinergics, antihistamines, GABAergic drugs, tricyclic antidepressants and opioids were associated with mild cognitive impairment with amnesic and non-amnesic effects in adults without an underlying CNS disease, and this systematic review summarizes the role of high doses of oral opioids cause impairment in concentration and memory storage [29].
Table 3 summarizes the possible CNS-related adverse effects of some opioids used in pain management in CKD and the pharmacokinetic changes of these drugs in CKD patients. In particular, morphine and codeine most be avoided in patients with CKD with an eGFR ≤60 mL/min/1.73 m2 due to neurotoxic adverse effects. If opioids are to be used in CKD patients, a low dose should be started and titrated slowly, as CNS-related adverse effects may appear more prominently at the start of opioid therapy or with dose increases [30].
Table 3:
Possible CNS-related ADRs and pharmacokinetics changes of opioids in CKD or ESKD [110].
| Drug | ADR in CKD or ESKD | Pharmacokinetics data in CKD |
|---|---|---|
| Buprenorphine | Safer profile [31] | No dose adjustment required [31] |
| Codeine | Prolonged CNS depression | Not recommended in CKD [31] |
| Dihydrocodeine | CNS depression | N/A |
| Fentanyl | Prolonged sedation | Its clearance may be altered |
| Hydrocodone | Limited information available [111] | Metabolized to hydromorphone; caution in CKD [112] |
| Hydromorphone or active metabolite H3G | Myoclonus, mental status changes; neuro-excitatory side effects [30] | Active metabolite may accumulate in CKD [112, 113], close monitoring is required [31] |
| Meperidine | CNS depression, psychosis, hyperactivity, seizures, myoclonus, mental status changes [111] | Active metabolite excretion depends on GFR. Dose adjustment is recommended. It should be avoided in ESKD |
| Methadone | PK is unaffected but poorly removed by hemodialysis | |
| Morphine or M3G (active metabolite) | CNS depression, morphine encephalopathy, neuroexcitatory effects such as allodynia, myoclonus, and seizures, cognitive impairment [30] | Disposition or sensitivity is altered. Not recommended in CKD [31] |
| Oxycodone | Lower risk of hallucinations and mental confusion [31] | Active metabolite excretion depends on GFR. Dose adjustment is recommended. Close monitoring is required [31] |
| Oxymorphone | Bioavailability is increased in kidney dysfunction. Caution in CKD | |
| Pethidine | Risk of CNS depression or convulsions | Dose recommendation: in ESKD avoid if possible, if not, decrease dose in 50% in 8-h intervals |
| Propoxyphene | Active metabolite excretion depends on GFR. Dose adjustment is recommended. It should be avoided in ESKD | |
| Tapentadol | Low risk of withdrawal | Low risk of drug–drug interaction |
| Tramadol (centrally acting) | CNS depression | 2-fold prolonged t1/2 in reduced GFR; recommendations: decrease the dose, increase the intervals between doses, and careful monitoring |
M3G, morphine-3-glucuronide; H3G, hydromorphone-3-glucuronide; N/A, not available PK, pharmacokinetics; t1/2, half life.
Clinical diagnosis of morphine encephalopathy in CKD patients under morphine includes the presence of cognitive troubles, coma, bilateral myosis and bradycardia. A recent ingestion of sustained-release morphine sulfate is highly suggestive of the diagnosis. The rapid aggravation of neurologic signs in a short time makes the diagnosis of uremic encephalopathy less probable. The lack of other signs of CNS disorders (e.g. hemiplegia, and/or agitation, infection), cerebral computed tomography scans and cerebrospinal fluid evaluations helps to exclude the diagnosis of intracranial bleeding or ischemic stroke, or infection. The best strategy to confirm the diagnosis of morphine encephalopathy is a positive naloxone test (i.e. rapid involvement of consciousness after naloxone injection). The evaluation of blood morphine level is not available as an emergency test, since its often done by high-performance liquid chromatography or gas chromatography–mass spectrometry, which are time-consuming techniques.
In the liver, morphine is metabolized into two primary metabolites named 3 and 6 glucuronide (M3G and M6G), which are eliminated almost exclusively by the kidneys. In the CKD condition, M6G may accumulate and cause severe opioid ADRs with insidious onset and long persistence because M6G accumulation may cause neurotoxic symptoms [31]. Since codeine is a prodrug and forms the same morphine metabolites, it is also not recommended in CKD patients. Other opioids, even if some are renally excreted and could induce CNS ADRs, could be use in CKD patients after dose adjustments (Table 3).
The severity of ADRs after morphine administration is also due to enhanced opioid receptor sensitivity in CKD patients. Not all patients with kidney dysfunction suffer from ADRs after morphine administration because of the accumulation of the active metabolite M6G. The single nucleotide polymorphism A118G of the mu-opioid-receptor gene (OPRM1), associated with decreased potency of M6G, could be among protective factors against M6G-related opioid toxicity [32, 33]. Many CKD patients are prescribed a high number of drugs, leading to potential drug–drug interactions, increasing the effects of opioids and CNS adverse effects. This risk is increased when they are co-administered with cytochrome P450 (CYP450) enzyme inducers or inhibitors. Concomitant use of gabapentinoids such as gabapentin and pregabalin, used for neuropathic pain in CKD patients, with opioids may increase CNS depressive effects [31].
Frequency of use of opioid and gabapentinoids in CKD patients
In a large population-based retrospective cohort study that included adults with CKD defined by eGFR <60 mL/min/1.73 m2, opioid use was common, with 24.3% of patients being prescribed repeated or long-term opioids [34]. The Centers for Disease Control guideline recommends that opioid use should be reduced in patients with CKD because these patients have a higher risk of mental problems, falls and fractures [35]. Opioids are generally less used in CKD and dialysis patients than in the general population because of their adverse effect profile and possible changes in pharmacokinetics with reduced clearance [31]. Opioid use in pain management in CKD patients should be utilized with caution on an individual basis to avoid CNS adverse effects including cognitive impairment.
Gabapentinoids are frequently prescribed in CKD patients for chronic neuropathic pain, but because they are excreted by the kidneys, dose adjustment may be necessary due to the risk of toxicity. In a retrospective study evaluating frequency of ADRs in 200 patients with CKD and ESKD, it was shown that appropriate dosing of these drugs is particularly important to minimize the risk of adverse events in patients of older age, with a history of seizures or concomitant antipsychotic use. Dose reduction would be appropriate and physicians should be aware that high-dose gabapentinoids should not be used in CKD patients [36]. A population-based study examined the risk of adverse effects after 30 days of administration of high- and low-dose gabapentin and pregabalin in 74 084 CKD patients with a eGFR <60 mL/min/1.73 m2 was examined and demonstrated a slight increase in hospitalization with encephalopathy, falls or fractures, with or without respiratory depression with high-dose administration [37]. Silva-Almodóvar et al. investigated potentially inappropriately prescribed medications in patients with CKD. They examined the prescriptions of 3624 CKD patients and found that 14% of all medications were potentially inappropriately prescribed medications, and that among the most prominent of these drugs were levetiracetam, pregabalin and gabapentin [38]. Therefore, if gabapentinoid is to be used in CKD patients, it would be appropriate to consider both patient factors and dose and adverse effect profiles, and consider the risk–benefit ratio.
PSYCHOTROPIC AGENTS
Psychotropic drugs such as anxiolytics, antidepressants and antipsychotics are frequently associated with cognitive impairment through different mechanisms; they may have direct effects on cognition due to their own therapeutic properties or because of their anticholinergic properties. The question of the direct impact of psychotropic drugs and cognition is still controversial.
Antidepressants and Anxiolytics
There are many studies showing that cognition is also impaired in depression. These patients may also use anxiolytics, sleeping pills, in addition to antidepressants. Due to this multi-drug use, it becomes difficult to delineate the effects of antidepressants on cognition in patients treated for depression [39]. In advanced CKD patients, especially older adults, in addition to cognitive impairment, psychiatric disorders such as anxiety and depression may also frequently be seen. The prevalence of depression in this patient group has shown to be between 14% and 30%, whereas it varies between 22.8% and 39.3% in dialysis patients [40]. The risk of hospitalization and death increases in those with CKD and depression comorbidities [41, 42]. In general, some antidepressants require dose adjustment and some CNS adverse events require close monitoring, as decreased clearance due to decreased kidney function affects the pharmacokinetics of drugs [43]. Fluoxetine due to its long half-life, tricyclic antidepressants due to the risk of prolonging the QT interval and causing arrhythmia, monoamine oxidase inhibitors (MAOIs) due to drug interactions, serotonin and norepinephrine reuptake inhibitors due to the risk of formation of toxic metabolites in predialysis CKD patients, and paroxetine due to the need for dose adjustment are not preferred for the treatment of depression in CKD patients. Citalopram is also not recommended in CKD patients with a GFR ≤20 mL/min/1.73 m2 due to its active metabolite. Sertraline has been used in studies that have considered it safe in CKD and hemodialysis patients because it is metabolized in the liver and its active metabolite becomes inactive before it is excreted renally [42, 44]. However, antidepressants seem to be of limited efficacy in the CKD population: according to the CAST (Chronic Kidney Disease Antidepressant Sertralin Trial) double-blind placebo-controlled clinical trial, sertraline had no significant effect over placebo for reducing depressive symptoms. However, the authors suggested that sertraline may be used as an effective agent, since there is currently no effective treatment alternative for CKD and ESKD patients with depression [43]. In the ASCEND study, where sertraline was compared with cognitive behavior therapy, some patients experienced CNS side effects such as dizziness, headache and insomnia [45].
Benzodiazepines are used to treat anxiety and insomnia, which may also be seen in CKD and hemodialysis patients. Several studies in non-CKD patients have shown a link between long-term benzodiazepine use and dementia and cognitive impairment [46, 47], but results from a community-based retrospective cohort study from Spain have questioned this. Short half-life drugs, however, increased the risk of dementia at the highest doses, especially in female patients, showing a dose–response relationship [48]. In rats, diazepam has recently been shown to impair the structural plasticity of dendritic spines, causing cognitive impairment [49]. This newer study contradicts a previous animal study, where diazepam did not appear to irreversibly affect functions such as working memory, visual recognition memory, spatial reference learning and memory, and visuospatial memory [50].
Antipsychotics
Lithium is a mood stabilizer and a first-choice medication in bipolar disorder treatment and an additional therapy for resistant depression and unipolar depression. The dose and serum lithium levels are important to follow and monitor due to its adverse effects including mainly kidney, thyroid and cognitive disturbances. On the other hand, in recent publications it has been shown to have immunomodulatory, neuroprotective and neurotrophic properties [51, 52]. However, there is also a perception and studies showing that it has negative effects on cognition in the long term [53]. Therefore, the dose and duration of lithium treatment are clinically important [51].
Overall, since psychiatric problems such as anxiety, insomnia and depression are frequent in CKD and hemodialysis patients, psychotropic drugs are often used and could have negative effects on cognition, as the pharmacokinetics of drugs may change with impaired kidney function.
Frequency of use of psychotropic agents in CKD patients
In a large study based on data from 242 349 patients in UK general practice, the rate of antidepressant prescription in CKD patients was found to be 1.5 times higher than in the general population [54]. The choice of antidepressants was similar in patients with and without CKD [54, 55]. In a cross-sectional study examining the frequency of psychotropic drug use in 195 hemodialysis patients, the frequency of use of benzodiazepines, hypnotic drugs, antidepressants, antipsychotics and mood stabilizers, was found to be 42.6%, 20.0%, 5.6%, 1.0% and 3.1%, respectively [56]. However, it was observed that only 24% of the patients were diagnosed with a major depressive disorder and 6.4% of them were treated in a psychiatry clinic and used medication [56]. Therefore, it may be concluded that the presence of depression in hemodialysis patients may not be recognized well enough, and perhaps major depressive disorder treatment is not well-managed [57] .
ANTIBACTERIALS
Antibiotics (AB) represent one of the most frequently used drug classes globally [58]. In addition to well-known side effects on various organ systems such as the gastrointestinal, cardiovascular or musculoskeletal system, these substances have been associated with adverse effects on both the peripheral and the central nervous system [59]. Until now, AB-induced neurotoxic ADRs have been observed with the majority of AB classes including betalactams (penicillins, cephalosporins, carbapenems), fluoroquinolones, sulfonamides, macrolides, metronidazole, aminoglycosides, oxazolidinones, polymyxins and antimycobacterials [60–70]. However, a detailed review of neurotoxic effects according to AB classes is beyond the scope of the present work.
Mechanisms of AB-induced central neurotoxicity include inhibition of gamma-aminobutyric acid (GABA)-ergic neurotransmission (e.g. betalactams, fluoroquinolones), interference with N-methyl-d-aspartate (NMDA)-ergic neurotransmission (fluoroquinolones, aminoglycosides), inhibition of MAO (linezolid) as well as drug interactions, e.g. with anti-convulsive drugs (carbapenems) or ciclosporin (imipenem) [71–77]. However, exact neurotoxic mechanisms have not been elucidated for all AB classes. While specific molecule characteristics, i.e. lipophilia, may favor the occurrence of central neurotoxicity (e.g. polymyxins, sulfonamides), AB classes without BBB penetration might still lead to neurotoxicity in the context of high-dose administration or in the context of CKD-related BBB dysfunction (penicillins).
Clinical manifestations of CNS neurotoxicity may range from mild to life-threatening presentations including confusion, acute delirium and acute psychosis, as well as seizures, myoclonus and focal symptoms such as aphasia [59]. A particular clinical picture linked to metronidazole use involves signs of encephalopathy and prominent cerebellar dysfunction [66, 78]. However, AB-induced neurotoxicity may be difficult to recognize, particularly in CKD patients and in cases of atypical presentations (hypoactive delirium, non-convulsive status epilepticus), and may mimic a wide spectrum of neurological or psychiatric illnesses in this high-risk population with high comorbid burden.
The onset of symptoms is rapid (within days) for most of the described central neurotoxic effects induced by AB while symptom onset might be late, within weeks of exposure (metronidazole) [79]. Similarly, whereas most symptoms are rapidly reversible after stopping the offending agent, long-lasting or even durable effects have been described (e.g. ertapenem, metronidazole) [66, 80]. The role of extracorporeal therapies in the treatment of AB-induced neurotoxicity is currently not clearly established.
CKD patients are particularly prone to infectious problems and hence, AB are commonly used in this population, both in the ambulatory and in the hospital setting [81]. Due to the renal route of elimination of the large majority of AB drugs in addition to intrinsic CKD-related factors discussed in our partner review, CKD patients are at high risk for AB-induced ADR [82]. However, neurotoxic effects have been reported in CKD patients even despite adequate dosing [83, 84]. On the other hand, drug elimination in the context of kidney replacement therapies have to be considered for treatment adaptation. In addition to vancomycin and aminoglycosides, therapeutic drug monitoring is nowadays increasingly being used for several additional AB substances, particularly in critically ill patients, in patients undergoing kidney replacement therapy or with impaired kidney function [85–87].
In conclusion, a high index of suspicion for neurotoxic drug effects in CKD patients treated with AB should be maintained. Therapeutic drug monitoring should be considered in CKD patients where available to reduce the risk of toxicity while achieving pharmacokinetics/pharmacodynamics optimization and potential reduction in the development of antimicrobial resistance.
ANTIVIRAL DRUGS
Antivirals are frequently prescribed in CKD patients for both prophylactic and therapeutic purposes, particularly in transplanted patients and those immunocompromised by treatments for the kidney disease.
The most frequently prescribed are antivirals against viruses of the herpes group: acyclovir and valacyclovir for Herpes Simplex Virus and Varicella Zoster Virus infections, and ganciclovir and valganciclovir for Cytomegalovirus infections. These drugs cross the BBB. Acyclovir and valacyclovir are particularly known for their potential but rare neurological toxicity. A systematic review of published cases of neurotoxicity associated with these two drugs found that neurotoxicity was related mostly to intravenous acyclovir (74%) rather than oral valacyclovir (29%). The main symptoms are confusion, lethargy, tremor and hallucinations. Kidney dysfunction was the main factor associated with neurotoxicity as it was documented in 83% of cases. In this series the administered dose was higher than the recommendation for kidney function adjustment in 60% [88], highlighting the importance of dose adaptation of CKD patients to prevent neurotoxicity. This may be related to an increased half-life of acyclovir, and in particular to the serum accumulation of the main metabolite of acyclovir 9-carboxymethoxymethylguanine (CMMG) in CKD patients, which is related to neuropsychiatric symptoms of acyclovir toxicity [89]. An increased level of CMMG is a valuable diagnostic marker of this toxicity and can guide physicians to drug withdrawal or prescription of dialysis in acute cases as both acyclovir and CMMG are readily dialyzed and more than 50% can be removed by a single dialysis [90]. Ganciclovir neurotoxicity is much less described, but a few case reports indicate acute neurotoxicity, especially in patients with impaired kidney function [91]. Ganciclovir dose also needs to be adapted to kidney function.
In patients with HIV, tenofovir disoproxil fumarate, a nucleoside reverse transcriptase inhibitor, is commonly prescribed and is the antiretroviral associated with the most important risk of proximal tubular dysfunction and CKD [92] but does not seem to be associated with neurotoxicity per se [93]. Ritonavir, a protease inhibitor frequently used as a booster for other antivirals, seems to be associated with less neurotoxicity but could enhance the neurotoxicity of other antivirals [94].
Also, nirmatrelvir/ritonavir (Paxlovid®), indicated for the treatment of early COVID-19 in patients at high risk of severe forms (notably the immunocompromised), is associated with many drug–drug interactions. Particularly, it increases the blood concentrations of potentially other neurotoxic drugs like tacrolimus, antipsychotics and cytotoxic chemotherapies (vinblastine) [95].
However, very few data are available on the exact frequency of prescription of antivirals in CKD patients, and specific studies are needed in the CKD population.
IMMUNOSUPPRESSIVE DRUGS
Immunosuppressive drugs are commonly used after organ transplantation, and kidney transplantation is one of the most common solid organ transplants in the world, followed by liver, lung and heart. According to the European Renal Association (ERA), a total of 24 013 kidney transplantations were performed in Europe in 2019 [96].
Immunosuppressive drugs greatly improve survival after kidney transplantation, but can cause numerous and frequent neurological complications [97]. Immunosuppressive agents may affect the central and peripheral nervous systems and thereby cause both direct and indirect neurotoxic effects [98]. Calcineurin inhibitors (CNIs), e.g. tacrolimus (TAC), cyclosporine (CsA) and corticosteroids, are the immunosuppressants most commonly associated with neurological complications [99]. It is known that TAC and CsA do not readily cross the BBB, but in CKD this barrier can be disrupted, thereby CNIs can exert a toxic effect on the nervous system [100]. TAC and CsA inhibit calcineurin activation, which is highly expressed in the CNS, and leads to the blockage of interleukin-2 production [101]. The neurotoxic effect of CNIs is mediated by several mechanisms, including changes in neurotransmission, disruption of the BBB and vascular endothelium, or alteration of mitochondrial function [102]. Usually, the severity of symptoms in most patients is mild or moderate, such as tremor, headache, dizziness, paraesthesia and peripheral neuropathy. Some patients may experience serious neurological side effects associated with CNIs, including posterior reversible encephalopathy syndrome, seizures, psychosis, blindness, confusion or coma [101]. Much less is known about the effects of immunosuppressive drugs on cognitive function. Martínez-Sanchis and colleagues compared three main immunosuppressants (CsA, TAC and sirolimus) and their impact on cognition after kidney transplantation [103]. They showed that patients treated with TAC and sirolimus had impaired performance on multidomain cognitive tasks. In contrast, such cognitive side effects were not reported in subjects receiving CsA, whose cognitive test scores were similar to those not receiving immunosuppression. In another publication, researchers investigated cognitive function, brain structure and metabolism in patients treated with standard-dose tacrolimus therapy 10 years after kidney transplantation and compared with kidney recipients 1 and 5 years after surgery, tacrolimus patients after liver transplantation and healthy controls [104]. In all patients after kidney and liver transplantation, cognitive decline was observed, mainly in visuospatial and constructional domains. However, the results indicated that many years after kidney transplantation, kidney recipients may experience cognitive decline, although tacrolimus therapy alone is not sufficient to lead to cognitive impairment and changes in brain energy metabolism.
Apart from CNIs, corticosteroids are the second group of immunosuppressive drugs that most often cause neurological complications [98]. Corticosteroids inhibit many cytokines, e.g. interleukin-1, interleukin-2, interleukin-6, tumour necrosis factor–α and interferon-gamma [105]. Glucocorticoids cross the BBB and bind to corticosteroid receptors in the CNS [106]. Common neurological side effects associated with corticosteroid use are myopathies, visual blurring, tremor and psychiatric disorders, such as mood changes, insomnia, anxiety, mania, psychosis, decreased concentration or cognitive impairment [107]. Corticosteroids are known to negatively affect cognition [108]. Elevated cortisol levels are associated with impairments in several cognitive domains such as selective attention processing and visual components [108]. Moreover, chronic corticosteroid therapy is associated with decreased hippocampal volume in brain imaging, which plays a major role in learning and memory and impaired declarative memory performance [109].
Finally, other immunosuppressants, i.e. mammalian target of rapamycin inhibitors (mTOR) (sirolimus, everolimus), monoclonal antibodies (rituximab, alemtuzumab) and purine synthesis inhibitors (mycophenolate, azathioprine), have relatively low neurotoxicity or neurological complications are rare and mild [101]. The influence of immunosuppressive drugs on cognition has not been fully investigated and it is likely that other confounding factors should be considered, including age, diverse etiology of cognitive disorders or multimorbidity of kidney recipients.
CONCLUSION
Neurocognitive disorders are common among CKD patients. Identifying risk factors for cognitive impairment can help to assess the ability of adherence to CKD risk reduction strategy. As discussed above, re-evaluation of drug prescription could represent an interesting tool to prevent or improve cognitive disorder in CKD patients. Early diagnosis of drug-induced confusion, and withdrawal of the offending agent or agents is essential and might finally result in a substantial decrease in socio-economic burden and patient mortality. As discussed elsewhere, as kidney function declines pharmacokinetics of drugs change and CKD is associated with BBB disruption that could increase the risk of ADRs.
Because cognition is not routinely recorded in electronic healthcare records, there is very limited pharmacoepidemiologic evidence for CNS ADRs in CKD patients (except for immunosuppression treatment). Nevertheless, we have highlighted that CKD patients are frequently treated with drugs that are commonly recognized as at risk of leading to cognitive impairment in the general population such as opioids, psychotropics, AB, antivirals and drugs with anticholinergic properties, suggesting that more research on drug dosing consideration in CKD is needed.
ACKNOWLEDGEMENTS
The authors would like to thank Prof. Giovambattista Capasso, acting chair of Cognitive Decline in Nephro-Neurology: European Cooperative Target (CONNECT) Action and members of COST Action for their support.
APPENDIX
The CONNECT collaborators are: Giovambattista Capasso, Alexandre Andrade, Maie Bachmann, Inga Bumblyte, Adrian Constantin Covic, Pilar Delgado, Nicole En dlich, Andreas Engvig, Denis Fouque, Casper Franssen, Sebastian Frische, Liliana Garneata, Loreto Gesualdo, Konstantinos Giannakou, Dimitrios Goumenos, Ayşe Tuğba Kartal, Sophie Liabeuf, Laila-Yasmin Mani, Hans-Peter Marti, Christopher Mayer, Rikke Nielsen, Vesna Pešić, Merita Rroji (Molla), Giorgos Sakkas, Goce Spasovski, Kate Stevens, Evgueniy Vazelov, Davide Viggiano, Lefteris Zacharia, Ana Carina Ferreira, Jolanta Malyszko, Ewout Hoorn, Andreja Figurek, Robert Unwin, Carsten Wagner, Christoph Wanner, Annette Bruchfeld, Marion Pépin, Andrzej Wiecek, Dorothea Nitsch, Ivo Fridolin, Gaye Hafez, Maria José Soler Romeo, Michelangela Barbieri, Bojan Batinić, Laura Carrasco, Sol Carriazo, Ron Gansevoort, Gianvito Martino, Francesco Mattace Raso, Ionut Nistor, Alberto Ortiz, Giuseppe Paolisso, Daiva Rastenytė, Gabriel Stefan, Gioacchino Tedeschi, Ziad Massy, Boris Bikbov, Karl Hans Endlich, Olivier Godefroy, Anastassia Kossioni, Justina Kurganaite, Norberto Perico, Giuseppe Remuzzi, Tomasz Grodzicki, Francesco Trepiccione, Carmine Zoccali, Mustafa Arıcı, Peter Blankestijn, Kai-Uwe Eckardt, Danilo Fliser, Eugenio Gutiérrez Jiménez, Maximilian Konig, Ivan Rychlik, Michela Deleidi, George Reusz, Michele Farisco, Norberto Perico, Pedro Imenez Silva, Mickaël Bobot, Aleksandra Golenia, Alessandra Perna, Alma Idrizi, Brian Hansen and Mariadelina Simeoni.
Notes
See Appendix for CONNECT Action collaborators.
Contributor Information
Gaye Hafez, Department of Pharmacology, Faculty of Pharmacy, Altinbas University, Istanbul, Turkey.
Jolanta Malyszko, Department of Nephrology, Dialysis and Internal Medicine, Medical University of Warsaw, Warsaw, Poland.
Aleksandra Golenia, Department of Neurology, Medical University of Warsaw, Warsaw, Poland.
Aleksandra Klimkowicz-Mrowiec, Department of Internal Medicine and Gerontology, Jagiellonian University Medical College, Cracow, Poland.
Ana Carina Ferreira, Nephrology Department, Centro Hospitalar e Universitário de Lisboa Central, Lisbon, Portugal; Universidade Nova de Lisboa-Faculdade de Ciências Médicas-Nephology, Lisbon, Portugal.
Mustafa Arıcı, Department of Internal Medicine, Division of Nephrology, Faculty of Medicine, Hacettepe University, Ankara, Turkey.
Annette Bruchfeld, Department of Health, Medicine and Caring Sciences, Linköping University, Linköping, Sweden; Department of Renal Medicine, Karolinska University Hospital and CLINTEC Karolinska Institutet, Stockholm, Sweden.
Dorothea Nitsch, Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, UK.
Ziad A Massy, Paris-Saclay University, UVSQ, Inserm, Clinical Epidemiology Team, Centre de Recherche en Epidémiologie et Santé des Populations (CESP), Villejuif, France; Department of Nephrology, Ambroise Paré University Medical Center, APHP, Paris, France.
Marion Pépin, Department of Nephrology, Ambroise Paré University Medical Center, APHP, Paris, France; Department of Geriatrics, Ambroise Paré University Medical Center, APHP, Boulogne-Billancourt, France.
Giovambattista Capasso, Department of Translational Medical Sciences, University of Campania Luigi Vanvitelli, Naples, Italy; Biogem Research Institute, Ariano Irpino, Italy.
Laila-Yasmin Mani, Department of Nephrology and Hypertension, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Sophie Liabeuf, Pharmacoepidemiology Unit, Department of Clinical Pharmacology, Amiens University Medical Center, Amiens, France; MP3CV Laboratory, EA7517, Jules Verne University of Picardie, Amiens, France.
CONNECT Action (Cognitive Decline in Nephro-Neurology European Cooperative Target):
Giovambattista Capasso, Alexandre Andrade, Maie Bachmann, Inga Bumblyte, Adrian Constantin Covic, Pilar Delgado, Nicole Endlich, Andreas Engvig, Denis Fouque, Casper Franssen, Sebastian Frische, Liliana Garneata, Loreto Gesualdo, Konstantinos Giannakou, Dimitrios Goumenos, Ayşe Tuğba Kartal, Sophie Liabeuf, Laila-Yasmin Mani, Hans-Peter Marti, Christopher Mayer, Rikke Nielsen, Vesna Pešić, Merita Rroji (Molla), Giorgos Sakkas, Goce Spasovski, Kate Stevens, Evgueniy Vazelov, Davide Viggiano, Lefteris Zacharia, Ana Carina Ferreira, Jolanta Malyszko, Ewout Hoorn, Andreja Figurek, Robert Unwin, Carsten Wagner, Christoph Wanner, Annette Bruchfeld, Marion Pepin, Andrzej Wiecek, Dorothea Nitsch, Ivo Fridolin, Gaye Hafez, Maria José Soler Romeo, Michelangela Barbieri, Bojan Batinić, Laura Carrasco, Sol Carriazo, Ron Gansevoort, Gianvito Martino, Francesco Mattace Raso, Ionut Nistor, Alberto Ortiz, Giuseppe Paolisso, Daiva Rastenytė, Gabriel Stefan, Gioacchino Tedeschi, Ziad Massy, Boris Bikbov, Karl Hans Endlich, Olivier Godefroy, Anastassia Kossioni, Justina Kurganaite, Norberto Perico, Giuseppe Remuzzi, Tomasz Grodzicki, Francesco Trepiccione, Carmine Zoccali, Mustafa Arici, Peter Blankestijn, Kai-Uwe Eckardt, Danilo Fliser, Eugenio Gutiérrez Jiménez, Maximilian Konig, Ivan Rychlik, Michela Deleidi, George Reusz, Michele Farisco, Norberto Perico, Pedro Imenez Silva, Mickaël Bobot, Aleksandra Golenia, Alessandra Perna, Alma Idrizi, Brian Hansen, and Mariadelina Simeoni
FUNDING
This article is published as financially supported by the Horizon EU COST Action CA19127-Cognitive Decline in Nephro-Neurology: European Cooperative Target (CONNECT).
AUTHORS’ CONTRIBUTIONS
S.L. and G.H. were responsible for the research idea and supervision of review writing. L.-Y.M., J.M., A.G., A.K.-M., A.C.F., M.A., Z.A.M. and M.P. contributed to writing parts of the manuscript. A.B., D.N. and G.C. critically revised the manuscript. All authors reviewed and approved the manuscript for publication.
DATA AVAILABILITY STATEMENT
Not applicable.
CONFLICT OF INTEREST STATEMENT
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
REFERENCES
- 1. Berger I, Wu S, Masson Pet al. Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Med 2016;14:206. 10.1186/s12916-016-0745-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strassels SA. Cognitive effects of opioids. Curr Pain Headache Rep 2008;12:32–6. 10.1007/s11916-008-0007-4 [DOI] [PubMed] [Google Scholar]
- 3. Liabeuf S, Laville M.. Drug prescription in patients with chronic kidney disease: a true challenge. Nephrol Dial Transplant 2021;36:385–6. 10.1093/ndt/gfaa164 [DOI] [PubMed] [Google Scholar]
- 4. Langmead CJ, Watson J, Reavill C.. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther 2008;117:232–43. 10.1016/j.pharmthera.2007.09.009 [DOI] [PubMed] [Google Scholar]
- 5. Santiago LJ, Abrol R.. Understanding G protein selectivity of muscarinic acetylcholine receptors using computational methods. Int J Mol Sci 2019;20:5290. 10.3390/ijms20215290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kruse AC, Kobilka BK, Gautam Det al. Muscarinic acetylcholine receptors: novel opportunities for drug development. Nat Rev Drug Discov 2014;13:549–60. 10.1038/nrd4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tune L, Coyle JT.. Serum levels of anticholinergic drugs in treatment of acute extrapyramidal side effects. Arch Gen Psychiatry 1980;37:293–7. 10.1001/archpsyc.1980.01780160063007 [DOI] [PubMed] [Google Scholar]
- 8. Boustani M, Campbell N., Munger Set al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health 2008;4:311–20. https://doi:10.2217/1745509X.4.3.311 [Google Scholar]
- 9. Rudolph JL, Salow MJ, Angelini MCet al. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 2008;168:508–13. 10.1001/archinternmed.2007.106 [DOI] [PubMed] [Google Scholar]
- 10. Carnahan RM, Lund BC, Perry PJet al. The relationship of an anticholinergic rating scale with serum anticholinergic activity in elderly nursing home residents. Psychopharmacol Bull 2002;36:14–9. [PubMed] [Google Scholar]
- 11. Han L, McCusker J, Cole Met al. Use of medications with anticholinergic effect predicts clinical severity of delirium symptoms in older medical inpatients. Arch Intern Med 2001;161:1099–105. 10.1001/archinte.161.8.1099 [DOI] [PubMed] [Google Scholar]
- 12. Briet J, Javelot H, Heitzmann Eet al. The anticholinergic impregnation scale: towards the elaboration of a scale adapted to prescriptions in French psychiatric settings. Therapies 2017;72:427–37. 10.1016/j.therap.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 13. Ancelin ML, Artero S, Portet Fet al. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ 2006;332:455–9. 10.1136/bmj.38740.439664.DE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ehrt U, Broich K, Larsen JPet al. Use of drugs with anticholinergic effect and impact on cognition in Parkinson's disease: a cohort study. J Neurol Neurosurg Psychiatry 2010;81:160–5. 10.1136/jnnp.2009.186239 [DOI] [PubMed] [Google Scholar]
- 15. Bishara D, Harwood D, Sauer Jet al. Anticholinergic effect on cognition (AEC) of drugs commonly used in older people. Int J Geriatr Psychiatry 2017;32:650–6. 10.1002/gps.4507 [DOI] [PubMed] [Google Scholar]
- 16. Klamer TT, Wauters M, Azermai Met al. A novel scale linking potency and dosage to estimate anticholinergic exposure in older adults: the muscarinic acetylcholinergic receptor ANTagonist exposure scale. Basic Clin Pharmacol Toxicol 2017;120:582–90. 10.1111/bcpt.12699 [DOI] [PubMed] [Google Scholar]
- 17. Sittironnarit G, Ames D, Bush AIet al. Effects of anticholinergic drugs on cognitive function in older Australians: results from the AIBL study. Dement Geriatr Cogn Disord 2011;31:173–8. 10.1159/000325171 [DOI] [PubMed] [Google Scholar]
- 18. Kiesel EK, Hopf YM, Drey M.. An anticholinergic burden score for German prescribers: score development. BMC Geriatr 2018;18:239. 10.1186/s12877-018-0929-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nery RT, Reis AMM.. Development of a Brazilian anticholinergic activity drug scale. Einstein (Sao Paulo) 2019;17:eAO4435. 10.31744/einstein_journal/2019AO4435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jun K, Hwang S, Ah Y-Met al. Development of an anticholinergic burden scale specific for Korean older adults. Geriatr Gerontol Int 2019;19:628–34. 10.1111/ggi.13680 [DOI] [PubMed] [Google Scholar]
- 21. López-Álvarez J, Sevilla-Llewellyn-Jones J, Agüera-Ortiz L.. Anticholinergic drugs in geriatric psychopharmacology. Front Neurosci 2019;13:1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Risacher SL, McDonald BC, Tallman EFet al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016;73:721–32. 10.1001/jamaneurol.2016.0580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Welk B, McClure JA.. The impact of anticholinergic use for overactive bladder on cognitive changes in adults with normal cognition, mild cognitive impairment, or dementia. Eur Urol Open Sci 2022;46:22–9. 10.1016/j.euros.2022.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Naja M, Zmudka J, Hannat Set al. In geriatric patients, delirium symptoms are related to the anticholinergic burden. Geriatr Gerontol Int 2016;16:424–31. 10.1111/ggi.12485 [DOI] [PubMed] [Google Scholar]
- 25. Broder JC, Ryan J, Shah RCet al. Anticholinergic medication burden and cognitive function in participants of the ASPREE study. Pharmacotherapy 2022;42:134–44. 10.1002/phar.2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parasca A, Doogue MP, Woodman RJet al. Hypoalbuminaemia and impaired renal function are associated with increased anticholinergic drug prescribing. Int J Clin Pract 2009;63:1110–4. 10.1111/j.1742-1241.2009.02067.x [DOI] [PubMed] [Google Scholar]
- 27. Roux-Marson C, Baranski JB, Fafin Cet al. Medication burden and inappropriate prescription risk among elderly with advanced chronic kidney disease. BMC Geriatr 2020;20:87. 10.1186/s12877-020-1485-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marco CA, Mann D, Rasp Jet al. Effects of opioid medications on cognitive skills among Emergency Department patients. Am J Emerg Med 2018;36:1009–13. 10.1016/j.ajem.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 29. Tannenbaum C, Paquette A, Hilmer Set al. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging 2012;29:639–58. [DOI] [PubMed] [Google Scholar]
- 30. Davison SN. Clinical pharmacology considerations in pain management in patients with advanced kidney failure. Clin J Am Soc Nephrol 2019;14:917–31. 10.2215/CJN.05180418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coluzzi F, Caputi FF, Billeci Det al. Safe use of opioids in chronic kidney disease and hemodialysis patients: tips and tricks for non-pain specialists. Ther Clin Risk Manag 2020;16:821–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lötsch J, Zimmermann M, Darimont Jet al. Does the A118G polymorphism at the mu-opioid receptor gene protect against morphine-6-glucuronide toxicity? Anesthesiology 2002;97:814–9. [DOI] [PubMed] [Google Scholar]
- 33. Andersen G, Christrup LL, Sjøgren P.. Morphine metabolism—pharmacokinetics and pharmacodynamics. Ugeskr Laeger 1997;159:3383–6. [PubMed] [Google Scholar]
- 34. Molnar AO, Bota SE, Naylor Ket al. Opioid prescribing practices in chronic kidney disease: a population-based cohort study. Nephrol Dial Transplant 2022;37:2408–17. 10.1093/ndt/gfab343 [DOI] [PubMed] [Google Scholar]
- 35. Leja N, Genord CK, Berriman SMet al. Tramadol and opioid prescription rates in chronic kidney disease patients before and after the 2016. Centers for Disease Control and Prevention Opioid Guideline. Hosp Pharm 2022;57:101–6. 10.1177/0018578720985427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wells DA, Washington K, Cave Bet al. Gabapentinoid dosing and adverse events in patients with chronic kidney disease. Clin Nephrol 2022;98:147–54. 10.5414/CN110815 [DOI] [PubMed] [Google Scholar]
- 37. Muanda FT, Weir MA, Ahmadi Fet al. Higher-dose gabapentinoids and the risk of adverse events in older adults with CKD: a population-based cohort study. Am J Kidney Dis 2022;80:98–107.e1. 10.1053/j.ajkd.2021.11.007 [DOI] [PubMed] [Google Scholar]
- 38. Silva-Almodóvar A, Hackim E, Wolk Het al. Potentially inappropriately prescribed medications among Medicare medication therapy management eligible patients with chronic kidney disease: an observational analysis. J Gen Intern Med 2021;36:2346–52. 10.1007/s11606-020-06537-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ziad A, Berr C, Ruiz Fet al. Anticholinergic activity of psychotropic drugs and cognitive impairment among participants aged 45 and over: the CONSTANCES Study. Drug Saf 2021;44:565–79. 10.1007/s40264-021-01043-5 [DOI] [PubMed] [Google Scholar]
- 40. de Menezes RRPPB, Sampaio TL, Martins AMCet al. Prescription drug overdose, depression, and other mental disorders in the context of kidney disease. Contrib Nephrol 2021;199:155–61. 10.1159/000517700 [DOI] [PubMed] [Google Scholar]
- 41. Nagler EV, Webster AC, Vanholder Ret al. Antidepressants for depression in stage 3-5 chronic kidney disease: a systematic review of pharmacokinetics, efficacy and safety with recommendations by European Renal Best Practice (ERBP). Nephrol Dial Transplant 2012;27:3736–45. 10.1093/ndt/gfs295 [DOI] [PubMed] [Google Scholar]
- 42. Jain N, Trivedi MH, Rush AJet al. Rationale and design of the Chronic Kidney Disease Antidepressant Sertraline Trial (CAST). Contemp Clin Trials 2013;34:136–44. 10.1016/j.cct.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gregg LP, Hedayati SS.. Pharmacologic and psychological interventions for depression treatment in patients with kidney disease. Curr Opin Nephrol Hypertens 2020;29:457–64. 10.1097/MNH.0000000000000629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kubanek A, Paul P, Przybylak Met al. Use of sertraline in hemodialysis patients. Medicina (Kaunas) 2021;57:949. 10.3390/medicina57090949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hedayati SS, Daniel DM, Cohen Set al. Rationale and design of A trial of Sertraline vs. Cognitive Behavioral therapy for End-stage Renal Disease Patients with Depression (ASCEND). Contemp Clin Trials 2016;47:1–11. 10.1016/j.cct.2015.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bierman EJM, Comijs HC, Gundy CMet al. The effect of chronic benzodiazepine use on cognitive functioning in older persons: good, bad or indifferent? Int J Geriat Psychiatry 2007;22:1194–200. 10.1002/gps.1811 [DOI] [PubMed] [Google Scholar]
- 47. Ferreira P, Ferreira AR, Barreto Bet al. Is there a link between the use of benzodiazepines and related drugs and dementia? A systematic review of reviews. Eur Geriatr Med 2022;13:19–32. 10.1007/s41999-021-00553-w [DOI] [PubMed] [Google Scholar]
- 48. Torres-Bondia F, Dakterzada F, Galván Let al. Benzodiazepine and Z-drug use and the risk of developing dementia. Int J Neuropsychopharmacol 2022;25:261–8. 10.1093/ijnp/pyab073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shi Y, Cui M, Ochs Ket al. Long-term diazepam treatment enhances microglial spine engulfment and impairs cognitive performance via the mitochondrial 18 kDa translocator protein (TSPO). Nat Neurosci 2022;25:317–29. 10.1038/s41593-022-01013-9 [DOI] [PubMed] [Google Scholar]
- 50. Carton L, Niot C, Kyheng Met al. Lack of direct involvement of a diazepam long-term treatment in the occurrence of irreversible cognitive impairment: a pre-clinical approach. Transl Psychiatry 2021;11:612. 10.1038/s41398-021-01718-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rybakowski JK. Challenging the negative perception of lithium and optimizing its long-term administration. Front Mol Neurosci 2018;11:349. 10.3389/fnmol.2018.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Quiroz JA, Machado-Vieira R, Zarate CAet al. Novel insights into lithium's mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology 2010;62:50–60. 10.1159/000314310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ferensztajn-Rochowiak E, Rybakowski JK.. Long-term lithium therapy: side effects and interactions. Pharmaceuticals (Basel) 2023;16:74. 10.3390/ph16010074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Iwagami M, Tomlinson LA, Mansfield KEet al. Prevalence, incidence, indication, and choice of antidepressants in patients with and without chronic kidney disease: a matched cohort study in UK Clinical Practice Research Datalink. Pharmacoepidemiol Drug Saf 2017;26:792–801. 10.1002/pds.4212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Oosten MJM, Koning D, Logtenberg SJJet al. Chronic prescription of antidepressant medication in patients with chronic kidney disease with and without kidney replacement therapy compared with matched controls in the Dutch general population. Clin Kidney J 2022;15:778–85. 10.1093/ckj/sfab242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yeh C-Y, Chen C-K, Hsu H-Jet al. Prescription of psychotropic drugs in patients with chronic renal failure on hemodialysis. Ren Fail 2014;36:1545–9. 10.3109/0886022X.2014.949762 [DOI] [PubMed] [Google Scholar]
- 57. Hedayati SS, Yalamanchili V, Finkelstein FO.. A practical approach to the treatment of depression in patients with chronic kidney disease and end-stage renal disease. Kidney Int 2012;81:247–55. 10.1038/ki.2011.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Van Boeckel TP, Gandra S, Ashok Aet al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014;14:742–50. 10.1016/S1473-3099(14)70780-7 [DOI] [PubMed] [Google Scholar]
- 59. Bhattacharyya S, Darby R, Berkowitz AL.. Antibiotic-induced neurotoxicity. Curr Infect Dis Rep 2014;16:448. 10.1007/s11908-014-0448-3 [DOI] [PubMed] [Google Scholar]
- 60. Walker AE, Johnson HC, Kollros JJ.. Penicillin convulsions; the convulsive effects of penicillin applied to the cerebral cortex of monkey and man. Surg Gynecol Obstet 1945;81:692–701. [PubMed] [Google Scholar]
- 61. Mani L-Y, Kissling S, Viceic Det al. Intermittent hemodialysis treatment in cefepime-induced neurotoxicity: case report, pharmacokinetic modeling, and review of the literature. Hemodial Int 2015;19:333–43. 10.1111/hdi.12198 [DOI] [PubMed] [Google Scholar]
- 62. Norrby SR. Neurotoxicity of carbapenem antibacterials. Drug Saf 1996;15:87–90. 10.2165/00002018-199615020-00001 [DOI] [PubMed] [Google Scholar]
- 63. Tomé AM, Filipe A.. Quinolones: review of psychiatric and neurological adverse reactions. Drug Saf 2011;34:465–88. 10.2165/11587280-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 64. Little S. Nervous and mental effects of the sulfonamides. JAMA 1942;119:467–74. 10.1001/jama.1942.02830230001001 [DOI] [Google Scholar]
- 65. Bandettini di Poggio M, Anfosso S, Audenino Det al. Clarithromycin-induced neurotoxicity in adults. J Clin Neurosci 2011;18:313–8. 10.1016/j.jocn.2010.08.014 [DOI] [PubMed] [Google Scholar]
- 66. Sørensen CG, Karlsson WK, Amin FMet al. Metronidazole-induced encephalopathy: a systematic review. J Neurol 2020;267:1–13. [DOI] [PubMed] [Google Scholar]
- 67. Bischoff A, Meier C, Roth F.. Gentamicin neurotoxicity (polyneuropathy—encephalopathy). Schweiz Med Wochenschr 1977;107:3–8. [PubMed] [Google Scholar]
- 68. Ferry T, Ponceau B, Simon Met al. Possibly linezolid-induced peripheral and central neurotoxicity: report of four cases. Infection 2005;33:151–4. 10.1007/s15010-005-4057-9 [DOI] [PubMed] [Google Scholar]
- 69. Falagas ME, Kasiakou SK.. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care 2006;10:R27. 10.1186/cc3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kass JS, Shandera WX.. Nervous system effects of antituberculosis therapy. CNS Drugs 2010;24:655–67. 10.2165/11534340-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 71. Chow KM, Hui AC, Szeto CC.. Neurotoxicity induced by beta-lactam antibiotics: from bench to bedside. Eur J Clin Microbiol Infect Dis 2005;24:649–53. 10.1007/s10096-005-0021-y [DOI] [PubMed] [Google Scholar]
- 72. De Sarro A, De Sarro G.. Adverse reactions to fluoroquinolones. An overview on mechanistic aspects. Curr Med Chem 2001;8:371–84. 10.2174/0929867013373435 [DOI] [PubMed] [Google Scholar]
- 73. Schmuck G, Schürmann A, Schlüter G.. Determination of the excitatory potencies of fluoroquinolones in the central nervous system by an in vitro model. Antimicrob Agents Chemother 1998;42:1831–6. 10.1128/AAC.42.7.1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Segal JA, Harris BD, Kustova Yet al. Aminoglycoside neurotoxicity involves NMDA receptor activation. Brain Res 1999;815:270–7. 10.1016/S0006-8993(98)01123-8 [DOI] [PubMed] [Google Scholar]
- 75. Moellering RC. Linezolid: the first oxazolidinone antimicrobial. Ann Intern Med 2003;138:135–42. 10.7326/0003-4819-138-2-200301210-00015 [DOI] [PubMed] [Google Scholar]
- 76. Al-Quteimat O, Laila A.. Valproate interaction with carbapenems: review and recommendations. Hosp Pharm 2020;55:181–7. 10.1177/0018578719831974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bösmüller C, Steurer W, Königsrainer Aet al. Increased risk of central nervous system toxicity in patients treated with ciclosporin and imipenem/cilastatin. Nephron 1991;58:362–4. [DOI] [PubMed] [Google Scholar]
- 78. Patel L, Batchala P, Almardawi Ret al. Acute metronidazole-induced neurotoxicity: an update on MRI findings. Clin Radiol 2020;75:202–8. 10.1016/j.crad.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 79. Bhattacharyya S, Darby RR, Raibagkar Pet al. Antibiotic-associated encephalopathy. Neurology 2016;86:963–71. 10.1212/WNL.0000000000002455 [DOI] [PubMed] [Google Scholar]
- 80. Wen M-J, Sung C-C, Chau Tet al. Acute prolonged neurotoxicity associated with recommended doses of ertapenem in 2 patients with advanced renal failure. Clin Nephrol 2013;80:474–8. 10.5414/CN107247 [DOI] [PubMed] [Google Scholar]
- 81. Naqvi SB, Collins AJ.. Infectious complications in chronic kidney disease. Adv Chronic Kidney Dis 2006;13:199–204. 10.1053/j.ackd.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 82. St Peter WL, Redic-Kill KA, Halstenson CE.. Clinical pharmacokinetics of antibiotics in patients with impaired renal function. Clin Pharmacokinet 1992;22:169–210. 10.2165/00003088-199222030-00002 [DOI] [PubMed] [Google Scholar]
- 83. Lemaire-Hurtel A, Gras-Champel V, Hary Let al. Recommended dosage adaptation based on renal function is not always sufficient to avoid betalactam antibiotics side effects. Nephrol Ther 2009;5:144–8. 10.1016/j.nephro.2008.10.004 [DOI] [PubMed] [Google Scholar]
- 84. Huang W-T, Hsu Y-J, Chu P-Let al. Neurotoxicity associated with standard doses of piperacillin in an elderly patient with renal failure. Infection 2009;37:374–6. 10.1007/s15010-009-8373-3 [DOI] [PubMed] [Google Scholar]
- 85. Abdul-Aziz MH, Alffenaar J-WC, Bassetti Met al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med 2020;46:1127–53. 10.1007/s00134-020-06050-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Suttels V, André P, Thoma Yet al. Therapeutic drug monitoring of cefepime in a non-critically ill population: retrospective assessment and potential role for model-based dosing. JAC Antimicrob Resist 2022;4:dlac043. 10.1093/jacamr/dlac043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Matusik E, Boidin C, Friggeri Aet al. Therapeutic drug monitoring of antibiotic drugs in patients receiving continuous renal replacement therapy or intermittent hemodialysis: a critical review. Ther Drug Monit 2022;44:86–102. 10.1097/FTD.0000000000000941 [DOI] [PubMed] [Google Scholar]
- 88. Brandariz-Nuñez D, Correas-Sanahuja M, Maya-Gallego Set al. Neurotoxicity associated with acyclovir and valacyclovir: a systematic review of cases. J Clin Pharm Ther 2021;46:918–26. 10.1111/jcpt.13464 [DOI] [PubMed] [Google Scholar]
- 89. Helldén A, Odar-Cederlöf I, Diener Pet al. High serum concentrations of the acyclovir main metabolite 9-carboxymethoxymethylguanine in renal failure patients with acyclovir-related neuropsychiatric side effects: an observational study. Nephrol Dial Transplant 2003;18:1135–41. 10.1093/ndt/gfg119 [DOI] [PubMed] [Google Scholar]
- 90. von Euler M, Axelsson G, Helldén A.. Differential diagnosis of central nervous system involvement in a patient treated with acyclovir. Ther Drug Monit 2013;35:417–9. 10.1097/FTD.0b013e31828faa35 [DOI] [PubMed] [Google Scholar]
- 91. Ernst ME, Franey RJ.. Acyclovir- and ganciclovir-induced neurotoxicity. Ann Pharmacother 1998;32:111–3. 10.1345/aph.17135 [DOI] [PubMed] [Google Scholar]
- 92. Scherzer R, Shlipak MG.. Risk factors: individual assessment of CKD risk in HIV-positive patients. Nat Rev Nephrol 2015;11:392–3. 10.1038/nrneph.2015.75 [DOI] [PubMed] [Google Scholar]
- 93. Robertson K, Liner J, Meeker RB.. Antiretroviral neurotoxicity. J Neurovirol 2012;18:388–99. 10.1007/s13365-012-0120-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Danner SA, Carr A, Leonard JMet al. A short-term study of the safety, pharmacokinetics, and efficacy of ritonavir, an inhibitor of HIV-1 protease. European-Australian Collaborative Ritonavir Study Group. N Engl J Med 1995;333:1528–34. 10.1056/NEJM199512073332303 [DOI] [PubMed] [Google Scholar]
- 95. National Institutes of Health . COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19). Treatment Guidelines. Available from: https://www.covid19treatmentguidelines.nih.gov/ (3 October 2023, last date accessed) [PubMed] [Google Scholar]
- 96. Boenink R, Astley ME, Huijben JAet al. The ERA Registry Annual Report 2019: summary and age comparisons. Clin Kidney J 2022;15:452–72. 10.1093/ckj/sfab273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ponticelli C, Campise MR.. Neurological complications in kidney transplant recipients. J Nephrol 2005;18:521–8. [PubMed] [Google Scholar]
- 98. Piotrowski PC, Lutkowska A, Tsibulski Aet al. Neurologic complications in kidney transplant recipients. Folia Neuropathol 2017;2:86–109. 10.5114/fn.2017.68577 [DOI] [PubMed] [Google Scholar]
- 99. Jurgensen A, Qannus AA, Gupta A.. Cognitive function in kidney transplantation. Curr Transpl Rep 2020;7:145–53. 10.1007/s40472-020-00284-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Stolp HB, Dziegielewska KM, Ek CJet al. Long-term changes in blood-brain barrier permeability and white matter following prolonged systemic inflammation in early development in the rat. Eur J Neurosci 2005;22:2805–16. 10.1111/j.1460-9568.2005.04483.x [DOI] [PubMed] [Google Scholar]
- 101. Faravelli I, Velardo D, Podestà MAet al. Immunosuppression-related neurological disorders in kidney transplantation. J Nephrol 2021;34:539–55. 10.1007/s40620-020-00956-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Meena P, Bhargava V, Rana Det al. An approach to neurological disorders in a kidney transplant recipient. Kidney360 2020;1:837–44. 10.34067/KID.0002052020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Martínez-Sanchis S, Bernal MC., Montagud JVet al. Effects of immunosuppressive drugs on the cognitive functioning of renal transplant recipients: a pilot study. J Clin Exp Neuropsychol 2011;33:1016–24. [DOI] [PubMed] [Google Scholar]
- 104. Pflugrad H, Nösel P, Ding Xet al. Brain function and metabolism in patients with long-term tacrolimus therapy after kidney transplantation in comparison to patients after liver transplantation. PLoS One 2020;15:e0229759. 10.1371/journal.pone.0229759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Shoskes A, Wilson R.. Neurologic complications of kidney transplantation. Transl Androl Urol 2019;8:164–72. 10.21037/tau.2018.08.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Uhr M, Holsboer F, Müller MB.. Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b P-glycoproteins. J Neuroendocrinol 2002;14:753–9. 10.1046/j.1365-2826.2002.00836.x [DOI] [PubMed] [Google Scholar]
- 107. Dietrich J, Rao K, Pastorino Set al. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol 2011;4:233–42. 10.1586/ecp.11.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Forget H, Lacroix A, Somma Met al. Cognitive decline in patients with Cushing's syndrome. J Int Neuropsychol Soc 2000;6:20–9. 10.1017/S1355617700611037 [DOI] [PubMed] [Google Scholar]
- 109. Brown ES, Woolston J, D, Frol A. et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biol Psychiatry 2004;55:538–45. 10.1016/j.biopsych.2003.09.010 [DOI] [PubMed] [Google Scholar]
- 110. Kurella M, Bennett WM, Chertow GM.. Analgesia in patients with ESRD: a review of available evidence. Am J Kidney Dis 2003;42:217–28. 10.1016/S0272-6386(03)00645-0 [DOI] [PubMed] [Google Scholar]
- 111. Koncicki HM, Unruh M, Schell JO.. Pain management in CKD: a guide for nephrology providers. Am J Kidney Dis 2017;69:451–60. 10.1053/j.ajkd.2016.08.039 [DOI] [PubMed] [Google Scholar]
- 112. Nagar VR, Birthi P, Salles Set al. Opioid use in chronic pain patients with chronic kidney disease: a systematic review. Pain Med 2017;18:1416–49. 10.1093/pm/pnw238 [DOI] [PubMed] [Google Scholar]
- 113. Smith MT. Neuroexcitatory effects of morphine and hydromorphone: evidence implicating the 3-glucuronide metabolites. Clin Exp Pharmacol Physiol 2000;27:524–8. 10.1046/j.1440-1681.2000.03290.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.