Abstract
Background
Maternal inflammation can result from immune dysregulation and metabolic perturbations during pregnancy. Whether conditions associated with inflammation during pregnancy increase the likelihood of autism spectrum disorder (ASD) or other neurodevelopmental disorders (DDs) is not well understood.
Methods
We conducted a case-control study among children born in California from 2011 to 2016 to investigate maternal immune-mediated and cardiometabolic conditions during pregnancy and risk of ASD (n = 311) and DDs (n = 1291) compared with children from the general population (n = 967). Data on maternal conditions and covariates were retrieved from electronic health records. Maternal genetic data were used to assess a causal relationship.
Results
Using multivariable logistic regression, we found that mothers with asthma were more likely to deliver infants later diagnosed with ASD (odds ratio [OR] = 1.62, 95% CI: 1.15–2.29) or DDs (OR = 1.30, 95% CI: 1.02–1.64). Maternal obesity was also associated with child ASD (OR = 1.51, 95% CI: 1.07–2.13). Mothers with both asthma and extreme obesity had the greatest odds of delivering an infant later diagnosed with ASD (OR = 16.9, 95% CI: 5.13–55.71). These increased ASD odds were observed among female children only. Polygenic risk scores for obesity, asthma, and their combination showed no association with ASD risk. Mendelian randomization did not support a causal relationship between maternal conditions and ASD.
Conclusions
Inflammatory conditions during pregnancy are associated with risk for neurodevelopmental disorders in children. These risks do not seem to be due to shared genetic risk; rather, inflammatory conditions may share nongenetic risk factors with neurodevelopmental disorders. Children whose mothers have both asthma and obesity during pregnancy may benefit from earlier screening and intervention.
Keywords: Asthma, ASD, Cardiometabolic, Immune dysregulation, Obesity, Prenatal
While the nongenetic causes of neurodevelopmental disorders are largely unknown, there is increasing evidence that lifestyle factors, environmental exposures, and maternal conditions during pregnancy that induce inflammation are associated with increased risk (1). Epidemiological research has found associations between autism spectrum disorder (ASD) and other neurodevelopmental disorders (DDs) and maternal immune-related conditions around the time of pregnancy including infection (2), asthma, allergy, and autoimmune disease (1,3,4). Evidence is accumulating regarding associations between ASD and DDs and maternal obesity (1,5) and specific cardiometabolic disorders preceding or developing during pregnancy, such as hypertension, type 2 diabetes, gestational diabetes, and preeclampsia (6, 7, 8, 9, 10, 11, 12, 13, 14, 15). The diversity of conditions associated with altered neurodevelopment suggests that maternal inflammation during pregnancy, which is often triggered by these conditions, may provide a common link to altered neurodevelopment in children.
Under normal homeostatic conditions, the maternal immune system maintains a pathogen-free and noninflammatory environment for the developing fetus (16,17). However, maternal immune activation during pregnancy may adversely affect programming of the fetal immune, metabolic, and neurological systems, with long-lasting effects into adulthood (18, 19, 20). Fluctuations in levels of maternal inflammatory molecules, including cytokines, chemokines, and antibodies, have been shown to have adverse developmental consequences for the fetus (21, 22, 23).
Most prior epidemiological studies investigating maternal immune activation have focused on single maternal conditions and their associations with specific neurodevelopmental disorders. Many have been limited by small sample sizes, lack of rigorous definitions of exposure or outcomes, retrospective study design, and inability to control for important covariates. Determination of whether shared genetic risk, shared environmental risk, or the physiology of the conditions explain any association remains unanswered.
In the current study, we explored the risk of ASD and DDs in the context of common maternal conditions associated with inflammation and maternal genetics. We hypothesized that maternal inflammation during pregnancy stemming from immune or metabolic dysregulation adversely affects child neurodevelopment and that individual and combinations of maternal conditions are differentially associated with neurodevelopmental outcomes. We integrated maternal genetic data to parse whether genetic or nongenetic risk factors are shared between maternal conditions and neurodevelopmental disorders.
Methods and Materials
The study population for the IMPaCT (Immune and Metabolic Markers during Pregnancy and Child Development) Study was selected from children born at Kaiser Permanente Northern California (KPNC) from January 2011 to January 2016, who survived to age 2 years and whose mothers received health care during the 2 years prior to delivery. KPNC is a large integrated health care system serving >4.5 million members and with a sociodemographic profile similar to the local and statewide California population, although the extremes of the income distribution are underrepresented (24). All mothers had previously consented to participate in the Research Program on Genes, Environment, and Health (RPGEH) pregnancy cohort (25), including donation of a blood sample during the first and second trimesters of pregnancy and permission to access their own and their child’s KPNC electronic health records (EHRs) for future studies. In December 2019, we retrieved information on demographic and clinical characteristics of mothers and their children prospectively recorded in their KPNC EHRs. At the time of data extraction, children were 3 to 8 years old. Study procedures were approved by the KPNC Institutional Review Board.
Child Outcomes
Three groups of children were included: children with ASD (n = 311), children with DDs (n = 1291), and children from the general population (GP) (n = 967) (Table S1). ASD diagnoses were based on the DSM criteria that were in effect at the time of diagnosis (DSM-IV or DSM-5) (26). Children with ASD had an ASD diagnosis recorded in their EHR on at least one occasion; 80% were diagnosed at a KPNC ASD evaluation center by a multidisciplinary team using a standardized protocol, including the Autism Diagnostic Observation Schedule (27). The remaining 20% were diagnosed by developmental behavioral pediatricians, child psychiatrists, pediatric neurologists, or general pediatricians.
Children included in the DD group had at least one diagnosis of intellectual disability, cerebral palsy, language delay, motor disorder, global delay, or learning disorder recorded in their KPNC EHR and no diagnoses of ASD. Attention-deficit/hyperactivity disorder (ADHD) was not included given the young age at the time neurodevelopmental diagnoses were ascertained. GP control participants were randomly sampled in proportion to the birth-year distribution of cases from the children in the study birth cohort who had no diagnoses of ASD or DDs recorded in their KPNC EHR.
Maternal Conditions During Pregnancy
We examined 10 maternal immune-mediated and cardiometabolic conditions: prenatal infections, asthma, allergy, autoimmune disease, gestational diabetes (GDM), preeclampsia, gestational hypertension, and preexisting chronic hypertension, diabetes, and obesity (Table S1). Clinician diagnoses were identified from the maternal inpatient and outpatient EHRs. Pregnancy was defined as the time between the last menstrual period and the date of delivery. Obesity class was determined by prepregnancy body mass index (BMI) recorded in the year prior to the last menstrual period and closest to the start of pregnancy (class I, BMI = 30.0–34.9; class II, BMI = 35.0–39.9; class III, BMI ≥ 40) (28). For participants with missing BMI (8.4%), BMI was imputed using the fully conditional specification method (29).
Covariates
Data on maternal sociodemographic and child characteristics shown to be significantly associated with risk of neurodevelopmental disorders or maternal immune-mediated and cardiometabolic conditions in previous studies were extracted from KPNC EHRs and birth certificate files (Table 1). Data on maternal psychiatric conditions (yes or no) and maternal anemia (yes or no) during pregnancy were extracted from the EHRs.
Table 1.
Characteristics of the IMPaCT Case-Control Study Sample, Kaiser Permanente Northern California
| Characteristic | All, N = 2569 | ASD, n = 311 | DD, n = 1291 | GP, n = 967 |
|---|---|---|---|---|
| Maternal Age at Birth, Years | 31.30 (5.21) | 31.67 (5.34) | 31.49 (5.32) | 30.92 (5.00) |
| Maternal Race/Ethnicity | ||||
| Asian | 534 (20.79%) | 64 (20.58%) | 266 (20.60%) | 204 (21.10%) |
| Black | 148 (5.76%) | 20 (6.43%) | 80 (6.20%) | 48 (4.96%) |
| Hispanic | 619 (24.09%) | 69 (22.19%) | 323 (25.02%) | 227 (23.47%) |
| Other | 107 (4.17%) | 11 (3.54%) | 52 (4.03%) | 44 (4.55%) |
| White | 1145 (44.57%) | 144 (46.30%) | 561 (43.45%) | 440 (45.50%) |
| Unknown | 16 (0.62%) | 3 (0.96%) | 9 (0.70%) | 4 (0.41%) |
| Maternal Education | ||||
| Less than high school | 66 (2.57%) | 5 (1.61%) | 38 (2.94%) | 23 (2.38%) |
| High school | 309 (12.03%) | 37 (11.90%) | 163 (12.63%) | 109 (11.27%) |
| College | 1527 (59.44%) | 192 (61.74%) | 757 (58.64%) | 578 (59.77%) |
| Postgraduate | 422 (16.43%) | 46 (14.79%) | 212 (16.42%) | 164 (16.96%) |
| Unknown | 245 (9.54%) | 31 (9.97%) | 121 (9.37%) | 93 (9.62%) |
| Parity | ||||
| 0 | 1164 (45.31%) | 167 (53.70%) | 561 (43.45%) | 436 (45.09%) |
| 1 | 880 (34.25%) | 87 (27.97%) | 451 (34.93%) | 342 (35.37%) |
| 2 | 373 (14.52%) | 38 (12.22%) | 195 (15.10%) | 140 (14.48%) |
| 3 | 105 (4.09%) | 11 (3.54%) | 60 (4.65%) | 34 (3.52%) |
| 4+ | 38 (1.48%) | 6 (1.93%) | 17 (1.32%) | 15 (1.55%) |
| Unknown | 9 (0.35%) | 2 (0.64%) | 7 (0.54%) | 0 (0%) |
| Plurality | ||||
| Singleton | 2468 (96.07%) | 294 (94.53%) | 1223 (94.73%) | 951 (98.34%) |
| Multiplea | 101 (3.93%) | 17 (5.47%) | 68 (5.27%) | 16 (1.65%) |
| Gestational Age | ||||
| <35 weeks (very preterm) | 61 (2.37%) | 8 (2.57%) | 46 (3.56%) | 7 (0.72%) |
| 35–37 weeks (preterm) | 214 (8.33%) | 32 (10.29%) | 130 (10.07%) | 52 (5.38%) |
| ≥38 weeks (term) | 2294 (89.30%) | 271 (87.14%) | 1115 (86.37%) | 908 (93.90%) |
| Child Sex | ||||
| Female | 1031 (40.13%) | 69 (22.19%) | 474 (36.72%) | 488 (50.47%) |
| Male | 1538 (59.87%) | 244 (77.81%) | 817 (63.28%) | 479 (49.53%) |
| Child Year of Birth | ||||
| 2011 | 330 (12.85%) | 32 (10.29%) | 178 (13.79%) | 120 (12.41%) |
| 2012 | 396 (15.41%) | 45 (14.47%) | 215 (16.65%) | 136 (14.06%) |
| 2013 | 663 (25.81%) | 79 (25.40%) | 326 (25.25%) | 258 (26.68%) |
| 2014 | 689 (26.82%) | 92 (29.58%) | 326 (25.25%) | 271 (28.02%) |
| 2015 | 483 (18.80%) | 63 (20.26%) | 238 (18.44%) | 182 (18.82%) |
| 2016 | 8 (0.31%) | 0 (0.00%) | 8 (0.62%) | 0 (0%) |
Values are presented as mean (SD) or n (%).
ASD, autism spectrum disorder; DD, other neurodevelopmental disorders; GP, general population control; IMPaCT, Immune and Metabolic Markers during Pregnancy and Child Development.
Only one child per multiple pregnancy was included in the analytic dataset.
Statistical Analyses
We fit crude and adjusted logistic regression models to estimate associations between maternal pregnancy conditions and child neurodevelopmental outcomes. The final adjustment set (child sex, birth year, maternal age, maternal race, and maternal education) included variables not considered intermediaries in the pathway under investigation. We first examined the association of individual maternal conditions with each child outcome in separate logistic regression models. We further examined maternal obesity by modeling the association by obesity class and by continuous BMI using restricted cubic splines and a linearity test. For individual maternal conditions that showed a statistically significant association with either ASD or DD, we examined joint effects by assessing both multiplicative interaction by including two-way interaction terms and additive interactions by computing the RERI (relative excess risk due to interaction) (30). To assess whether estimated associations varied by child’s sex, we conducted all analyses stratified by child’s sex and ran analyses with two-way interaction terms between child sex and the maternal condition.
Genetic Analyses
Genetic analyses were pursued for conditions significant in the primary analysis. See the Supplemental Methods for sample collection and genotyping methodology. Genome-wide association study (GWAS) summary statistics for asthma (31) and BMI (32) were used to generate polygenic risk scores (PRSs) for individuals in the IMPaCT dataset using PRS-CSx (33) with 1000 Genomes linkage disequilibrium reference panels. Meta-analysis of the asthma and BMI GWAS was performed with METAL (34). All linear associations were done using lm or glm from the R stats package, and pseudo-R2 values were calculated with the pscl package (35). Genetic principal components and ancestries were computed with PLINK (36) and used as covariates in analyses in addition to child’s sex, mother’s age, and year of birth.
Mendelian randomization (MR) was performed using the TwoSampleMR package (37) comparing the asthma and BMI meta-analysis and summary statistics from a large ASD GWAS (38). After pruning for linkage disequilibrium using the LDlinkR package (39), single nucleotide polymorphisms were selected using 3 approaches that are described in the Results. The power of MR analyses was calculated with mRnd (40).
Analysis of genetic correlation between meta-analysis and GWAS summary statistics was performed with linkage disequilibrium score regression (41,42).
Results
The study population was diverse in maternal race/ethnicity (approximately 55% non-White) (Table 1). Most mothers (76%) had at least some college education. Maternal race/ethnicity and education level did not differ across study groups; however, the percentage of first-born children (53.7%) and the male:female ratio (3.5:1) were highest in the ASD group. Children with ASD and DDs were more likely to have been born preterm (12.9% and 13.6%, respectively) and to be a twin (5.5% and 5.3%, respectively) than GP control participants.
Maternal Conditions Associated With ASD and DDs
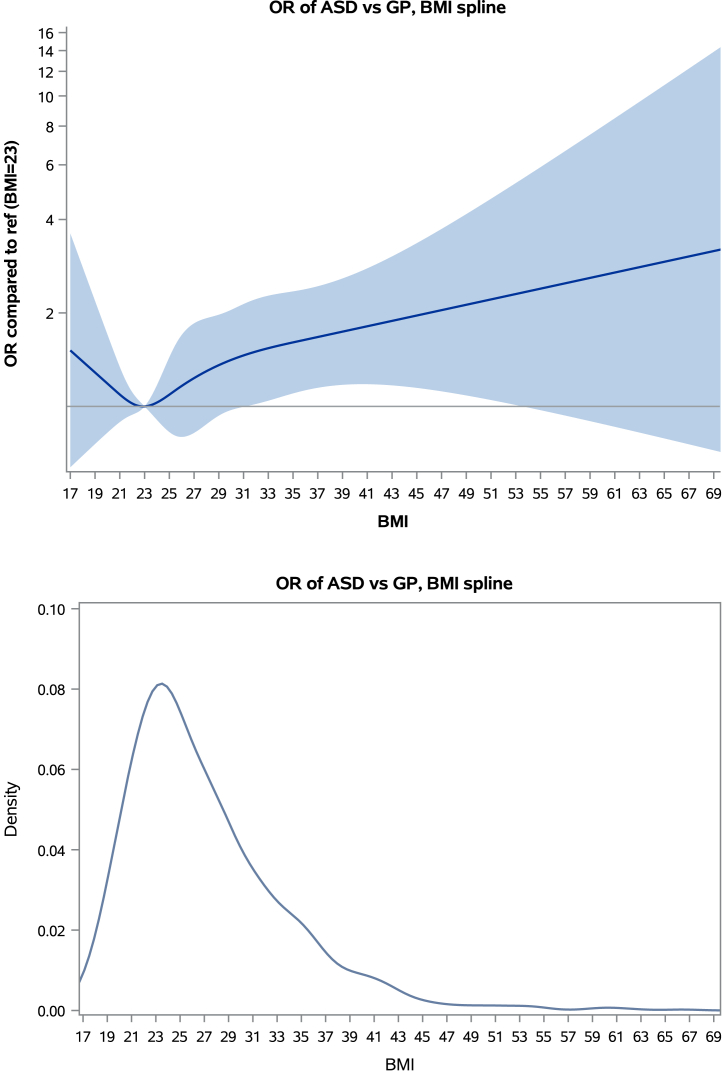
Compared with the GP control group, maternal asthma was associated with increased odds of child ASD (adjusted odds ratio [adj-OR] = 1.62, 95% CI: 1.15–2.29) (Table 2). Results were similar when conducted separately by asthma treatment status (Table S3). Maternal obesity was also associated with increased odds of child ASD (adj-OR = 1.51, 95% CI: 1.07–2.13) (Table 2). We observed a trend of increasing ASD odds with increasing level of maternal obesity, with the highest ASD odds associated with obesity class III (adj-OR = 2.27, 95% CI: 1.21–4.24). When maternal obesity was modeled as continuous BMI, the association showed an increasing linear trend (Figure 1); (nonlinearity p value = .53). In race/ethnicity-stratified analyses, asthma and obesity were associated only in the White subset, although interactions were not statistically significant (Table S2).
Table 2.
Associations of Individual Maternal Immune-Mediated and Cardiometabolic Conditions With Child Neurodevelopmental Outcomes
| Maternal Conditions | ASD, n = 311 |
DD, n = 1291 |
GP, n = 967 |
ASD vs. GP |
DD vs. GP |
||
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | Crude OR (95% CI) | Adjusteda OR (95% CI) | Crude OR (95% CI) | Adjusteda OR (95% CI) | |
| Immune-Mediated | |||||||
| Allergy | 55 (17.7%) | 204 (15.8%) | 134 (13.9%) | 1.34 (0.95–1.88) | 1.23 (0.86–1.77) | 1.17 (0.92–1.48) | 1.16 (0.91–1.48) |
| Asthma | 64 (20.6%) | 223 (17.3%) | 136 (14.1%) | 1.58 (1.14–2.20)b | 1.62 (1.15–2.29)b | 1.28 (1.01–1.61)b | 1.30 (1.02–1.64)b |
| Autoimmune | 27 (8.7%) | 140 (10.8%) | 96 (9.9%) | 0.86 (0.55–1.35) | 0.82 (0.52–1.31) | 1.10 (0.84–1.45) | 1.09 (0.82–1.44) |
| Infection | 163 (52.4%) | 635 (49.2%) | 464 (48.0%) | 1.19 (0.92–1.54) | 1.18 (0.90–1.54) | 1.05 (0.89–1.24) | 1.06 (0.89–1.26) |
| Cardiometabolic | |||||||
| GDM | 39 (12.5%) | 202 (15.7%) | 108 (11.2%) | 1.14 (0.77–1.69) | 1.01 (0.67–1.52) | 1.48 (1.15–1.89)b | 1.37 (1.06–1.77)b |
| Diabetes | 3 (0.96%) | 20 (1.55%) | 13 (1.34%) | 0.71 (0.20–2.52) | 0.60 (0.16–2.22) | 1.15 (0.57–2.33) | 1.05 (0.51–2.15) |
| Obesity | 97 (31.2%) | 356 (27.6%) | 233 (24.1%) | 1.51 (1.11–2.07)b | 1.51 (1.07–2.13)b | 1.20 (0.98–1.48) | 1.19 (0.95–1.49) |
| Obesity class I | 50 (16.1%) | 186 (14.4%) | 137 (14.2%) | 1.33 (0.91–1.94) | 1.31 (0.87–1.99) | 1.07 (0.83–1.38) | 1.09 (0.83–1.42) |
| Obesity class II | 26 (8.4%) | 102 (7.9%) | 58 (6.0%) | 1.63 (0.98–2.70) | 1.43 (0.81–2.52) | 1.38 (0.98–1.96) | 1.35 (0.94–1.94) |
| Obesity class III | 21 (6.8%) | 68 (5.3%) | 38 (3.9%) | 2.01 (1.14–3.55)b | 2.27 (1.21–4.24)b | 1.41 (0.93–2.14) | 1.36 (0.87–2.11) |
| Preeclampsia | 14 (4.5%) | 57 (4.4%) | 36 (3.7%) | 1.22 (0.65–2.29) | 0.99 (0.51–1.90) | 1.19 (0.78–1.83) | 1.15 (0.75–1.77) |
| Hypertension | 40 (12.9%) | 141 (10.9%) | 93 (9.6%) | 1.39 (0.93–2.05) | 1.32 (0.87–2.00) | 1.15 (0.87–1.52) | 1.12 (0.85–1.49) |
| Chronic | 13 (4.2%) | 42 (3.3%) | 27 (2.8%) | 1.52 (0.77–2.98) | 1.20 (0.59–2.44) | 1.17 (0.72–1.91) | 1.00 (0.61–1.66) |
| Gestational | 28 (9.0%) | 113 (8.8%) | 75 (7.8%) | 1.18 (0.75–1.85) | 1.16 (0.72–1.86) | 1.14 (0.84–1.55) | 1.14 (0.84–1.56) |
ASD, autism spectrum disorder; DD, other neurodevelopmental disorders; GDM, gestational diabetes; GP, general population control; OR, odds ratio.
Adjusted models include child sex, birth year, maternal age, maternal race, and maternal education. The reference group for each condition is the absence of the condition.
Statistically significant association.
Figure 1.
Spline model for continuous body mass index (BMI). When modeled using restricted cubic splines, the association of BMI with autism spectrum disorder (ASD) risk showed an increasing linear trend (p value for nonlinearity = .53). Although the plot shows a slight U-shape with a potential increase in ASD risk among women who were underweight, we note that the confidence bands below a BMI of 21 are extremely wide, showing no statistically significant difference as confirmed by the test for nonlinearity. GP, general population control; OR, odds ratio.
Maternal asthma was also associated with increased odds of child DDs compared with the GP control group (adj-OR = 1.30, 95% CI: 1.02–1.64) (Table 2) and in sensitivity analyses accounting for asthma treatment (Table S3). GDM was also associated with increased odds of child DD (adj-OR = 1.37, 95% CI: 1.06–1.77).
Joint Associations Between Maternal Conditions and Child ASD and DDs
Compared with women with neither condition, the odds of having a child with ASD were higher among women with both asthma and obesity (adj-OR = 2.72, 95% CI: 1.57–4.71, interaction p value = .47). Women with asthma and obesity class III had more than a 16-fold increased odds of having a child with ASD (adj-OR = 16.9, 95% CI: 5.13–55.71, interaction p value = .005) (Table 3). The combination of asthma and obesity was also associated with higher odds of child DD (adj-OR = 1.64, 95% CI: 1.11–2.43, interaction p value = .72). The relative excess risk due to interaction showed no statistically significant additive interactions (Table 3). Additional adjustment for maternal diagnosis of psychiatric conditions or anemia during pregnancy did not alter the results (data not shown).
Table 3.
Joint Effects of Maternal Asthma During Pregnancy and Prepregnancy Obesity on Child Neurodevelopmental Outcomes
| Obesity Class | No Asthma |
Asthma |
Interaction p Value | RERI (95% CI) | ||
|---|---|---|---|---|---|---|
| nASDor DD/nGPa | Adjusted OR (95% CI)b | nASDor DD/nGPa | Adjusted OR (95% CI)b | |||
| ASD vs. GP | ||||||
| Not obese | 179/642 | 1.0 (ref) | 35/92 | 1.37 (0.88–2.15) | – | – |
| Obese all classes | 68/189 | 1.29 (0.91–1.84) | 29/44 | 2.72 (1.57–4.71)c | .47 | 0.79 (−0.76 to 2.33) |
| Obese class I | 39/113 | 1.25 (0.81–1.93) | 11/24 | 1.85 (0.84–4.11) | .80 | −0.11 (−1.70 to 1.48) |
| Obese class II | 20/43 | 1.48 (0.81–2.72) | 6/15 | 1.34 (0.47–3.76) | .48 | −0.62 (−2.50 to 1.27) |
| Obese class III | 9/33 | 1.06 (0.47–2.37) | 12/5 | 16.90 (5.13–55.71)c | .005 | 11.47 (−3.50 to 26.43) |
| DD vs. GP | ||||||
| Not obese | 803/642 | 1.0 (ref) | 132/92 | 1.20 (0.89–1.60) | – | – |
| Obese all classes | 265/189 | 1.14 (0.91–1.42) | 91/44 | 1.64 (1.11–2.43)c | .72 | 0.20 (−0.61 to 1.01) |
| Obese class I | 144/113 | 1.06 (0.80–1.40) | 42/24 | 1.42 (0.84–2.42) | .97 | 0.03 (−0.88 to 0.95) |
| Obese class II | 69/43 | 1.25 (0.83–1.89) | 33/15 | 1.72 (0.91–3.23) | .91 | 0.00 (−1.27 to 1.27) |
| Obese class III | 52/33 | 1.22 (0.77–1.95) | 16/5 | 2.62 (0.92–7.43) | .46 | 1.01 (−1.76 to 3.79) |
ASD, autism spectrum disorder; DD, other neurodevelopmental disorders; GP, general population control; OR, odds ratio; ref, reference category; RERI, relative excess risk due to interaction.
n represents counts for ASD, DDs, or GPs.
Adjusted models include child sex, birth year, and maternal age, race, and education. Obesity and asthma were both included in the models simultaneously.
Statistically significant association.
Neither the combination of asthma and GDM nor the combination of obesity and GDM further elevated the odds of ASD or DDs above those observed for each condition individually, and there were no statistically significant multiplicative or additive interactions (Table S4).
Sex Differences
The odds of ASD associated with maternal asthma and hypertension were much higher among female offspring than male offspring, representing statistically significant differences by child sex (interaction p values of .02 and .001, respectively) (Table 4). Higher odds of ASD among female offspring were also observed for maternal obesity, although sex differences were not statistically significant. Maternal allergy was associated with increased odds of DDs among female offspring only (interaction p value = .02).
Table 4.
Sex Differences in the Associations of Individual Maternal Conditions With Child Neurodevelopmental Outcomes
| Conditions | ASD vs. GP |
DD vs. GP |
||||
|---|---|---|---|---|---|---|
| Adjusted ORa in Male Children | Adjusted ORa in Female Children | p Valueb | Adjusted ORa in Male Children | Adjusted ORa in Female Children | p Valueb | |
| Allergy | 1.05 (0.69–1.61) | 1.87 (0.98–3.59) | .15 | 0.90 (0.66–1.23) | 1.61 (1.12–2.31)c | .02c |
| Asthma | 1.23 (0.81–1.87) | 2.93 (1.63–5.25)c | .02c | 1.04 (0.76–1.43) | 1.68 (1.18–2.38)c | .05c |
| Autoimmune | 0.67 (0.39–1.17) | 1.38 (0.62–3.07) | .15 | 1.01 (0.70–1.45) | 1.21 (0.79–1.86) | .52 |
| Fever | 1.27 (0.84–1.90) | 1.05 (0.55–2.01) | .63 | 1.04 (0.76–1.42) | 0.72 (0.51–1.02) | .12 |
| Infection | 1.16 (0.85–1.59) | 1.21 (0.73–2.01) | .89 | 0.93 (0.74–1.17) | 1.26 (0.97–1.62) | .08 |
| Metabolic | 0.78 (0.35–1.76) | 1.17 (0.39–3.53) | .56 | 1.45 (0.85–2.47) | 1.09 (0.61–1.95) | .48 |
| GDM | 1.02 (0.64–1.62) | 0.99 (0.42–2.29) | .95 | 1.37 (0.99–1.91) | 1.37 (0.91–2.04) | .99 |
| Diabetes | 0.81 (0.20–3.33) | –d | .98 | 1.51 (0.58–3.92) | 0.56 (0.16–1.93) | .21 |
| Obesity | 1.34 (0.89–2.02) | 1.96 (1.08–3.55)c | .29 | 1.04 (0.78–1.40) | 1.40 (1.01–1.93)c | .18 |
| Obesity class I | 1.14 (0.69–1.88) | 1.76 (0.87–3.54) | .32 | 0.85 (0.59–1.22) | 1.41 (0.97–2.06) | .05 |
| Obesity class II | 1.47 (0.78–2.77) | 1.29 (0.41–4.10) | .84 | 1.17 (0.73–1.87) | 1.64 (0.94–2.85) | .35 |
| Obesity class III | 1.77 (0.79–3.94) | 3.22 (1.28–8.11)c | .33 | 1.62 (0.88–3.00) | 1.10 (0.58–2.10) | .38 |
| Preeclampsia | 0.98 (0.48–2.04) | 1.00 (0.22–4.56) | .98 | 0.85 (0.49–1.46) | 1.83 (0.91–3.66) | .09 |
| Hypertension | 0.82 (0.49–1.39) | 3.26 (1.70–6.24)c | .001c | 0.93 (0.64–1.34) | 1.44 (0.94–2.22) | .12 |
| Chronic hypertension | 0.58 (0.23–1.42) | 6.23 (2.00–19.4)c | .001c | 0.70 (0.38–1.26) | 2.15 (0.86–5.35) | .04c |
| Gestational hypertension | 0.84 (0.47–1.51) | 2.21 (1.03–4.71)c | .05c | 0.97 (0.65–1.47) | 1.40 (0.88–2.22) | .25 |
ASD, autism spectrum disorder; DD, other neurodevelopmental disorders; GDM, gestational diabetes; GP, general population control group; OR, odds ratio.
Adjusted models include child sex, birth year, maternal age, maternal race, and maternal education, and the interaction term for child sex by maternal condition.
p Value for two-way interaction term between maternal condition and child sex.
Statistically significant association.
Model did not converge due to data sparsity within cells.
In combination with asthma, obesity was associated with a 5-fold increased odds of ASD (adj-OR = 5.6, 95% CI: 2.5–12.8; interaction p value = .03), and obesity class III was associated with a 10-fold increase in ASD risk (adj-OR = 10.8, 95% CI 2.7–44.1) (Table 5), both among female offspring only. Asthma and GDM did not jointly increase the odds of ASD or DDs in either male or female offspring (Table 5). However, GDM and obesity were jointly associated with higher odds of DDs in female offspring only (Table 5). There were no statistically significant additive interactions (Table 5).
Table 5.
Joint Effects of Maternal Prepregnancy Obesity, Asthma During Pregnancy, and Gestational Diabetes on Child Neurodevelopmental Outcomes Stratified by Child Sex
| Maternal Condition | No asthma |
Asthma |
p-int | RERI (95% CI) | ||
|---|---|---|---|---|---|---|
| Female |
Male |
Female |
Male |
|||
| OR (95% CI)a | OR (95% CI)a | OR (95% CI)a | OR (95% CI)a | |||
| ASD vs. GP | ||||||
| Not obese | 1.0 (ref) | 1.0 (ref) | 2.54 (1.15–5.61)b | 1.08 (0.64–1.84) | .08 | −1.12 (−4.57 to 2.32) |
| Obese all classes | 1.70 (0.89–3.27) | 1.17 (0.78–1.76) | 5.60 (2.45–12.82)b | 1.75 (0.89–3.45) | .03 | −0.83 (−7.93 to 6.27) |
| Obese class I | 1.74 (0.80–3.76) | 1.10 (0.66–1.83) | 4.53 (1.46–14.05)b | 1.04 (0.37–2.89) | .06 | −3.35 (−10.72 to 4.01) |
| Obese class II | 0.96 (0.21–4.36) | 1.63 (0.83–3.19) | 4.38 (0.82–23.26) | 0.86 (0.25–2.91) | .12 | −4.09 (−13.26 to 5.07) |
| Obese class III | 2.44 (0.76–7.88) | 0.65 (0.23–1.85) | 10.81 (2.66–44.05)b | –c | – | –c |
| DD vs. GP | ||||||
| Not obese | 1.0 (ref) | 1.0 (ref) | 1.68 (1.08–2.60)b | 0.91 (0.62–1.34) | .04 | −0.85 (−1.89 to 0.18) |
| Obese all classes | 1.28 (0.92–1.77) | 1.03 (0.76–1.39) | 1.98 (1.14–3.43)b | 1.36 (0.80–2.33) | .33 | −0.25 (−2.03 to 1.54) |
| Obese class I | 1.26 (0.85–1.87) | 0.91 (0.62–1.32) | 2.28 (1.11–4.70)b | 0.79 (0.37–1.68) | .05 | −1.71 (−3.77 to 0.34) |
| Obese class II | 1.49 (0.81–2.76) | 1.09 (0.64–1.87) | 2.05 (0.73–5.76) | 1.54 (0.70–3.39) | .66 | −0.04 (−3.18 to 3.26) |
| Obese class III | 1.10 (0.54–2.25) | 1.32 (0.71–2.45) | 1.23 (0.35–4.38) | –c | – | –c |
| ASD vs. GP | ||||||
| No GDM | 1.0 (ref) | 1.0 (ref) | 3.00 (1.58–5.70) | 1.38 (0.88–2.16) | .04 | −0.33 (−3.42 to 2.77) |
| GDM | 1.01 (0.36–2.80) | 1.18 (0.70–1.96) | 1.89 (0.36–9.88) | 0.59 (0.19–1.88) | .26 | −2.55 (−7.01 to 1.90) |
| DD vs. GP | ||||||
| No GDM | 1.0 (ref) | 1.0 (ref) | 1.67 (1.15–2.45)b | 1.08 (0.76–1.53) | .09 | −0.53 (−1.48 to 0.42) |
| GDM | 1.46 (0.92–2.30) | 1.48 (1.02–2.15)b | 1.94 (0.81–4.64) | 1.05 (0.52–2.12) | .38 | −0.61 (−2.70 to 1.48) |
| No GDM | GDM | |||||
|---|---|---|---|---|---|---|
| Female |
Male |
Female |
Male |
|||
| OR (95% CI)a | OR (95% CI)a | OR (95% CI)a | OR (95% CI)a | p-int | RERI (95% CI) | |
| ASD vs. GP | ||||||
| Not obese | 1.0 (ref) | 1.0 (ref) | –d | 0.82 (0.43–1.56) | – | – |
| Obese all classes | –d | 1.10 (0.72–1.66) | –d | 1.39 (0.70–2.75) | – | – |
| Obese class I | – d | 0.89 (0.52–1.54) | –d | 1.43 (0.59–3.48) | – | – |
| Obese class II | –d | 1.10 (0.55–2.19) | –d | 1.80 (0.55–5.84) | – | – |
| Obese class III | –d | 2.04 (0.87–4.80) | –d | 0.49 (0.05–4.57) | – | – |
| DD vs. GP | ||||||
| Not obese | 1.0 (ref) | 1.0 (ref) | 1.63 (1.04–2.54)b | 0.90 (0.61–1.32) | .53 | −0.12 (−1.35 to 1.10) |
| Obese all classes | 1.33 (0.95–1.86) | 1.03 (0.76–1.40) | 2.02 (1.16–3.53)b | 1.30 (0.76–2.24) | .72 | 0.69 (−0.93 to 2.32) |
| Obese class I | 1.31 (0.87–1.96) | 0.91 (0.62–1.33) | 2.44 (1.17–5.11)b | 0.73 (0.34–1.59) | .30 | 0.87 (−0.96 to 2.70) |
| Obese class II | 1.57 (0.84–2.93) | 1.05 (0.61–1.82) | 1.94 (0.69–5.47) | 1.49 (0.67–3.32) | .26 | −1.86 (−7.82 to 4.10) |
| Obese class III | 1.15 (0.56–2.36) | 1.30 (0.69–2.45) | 1.28 (0.36–4.59) | –d | – | – |
ASD, autism spectrum disorder; DD, other neurodevelopmental disorders; GDM, gestational diabetes; GP, general population control; OR, odds ratio; RERI, relative excess risk due to interaction.
Adjusted models include child sex, birth year, and maternal age, race, and education. For each combination of maternal conditions, both conditions (i.e., obesity and asthma, GDM and asthma, GDM and obesity) were included in the model simultaneously.
Statistically significant association.
No male GP control participants were exposed to both asthma and obese class III.
Model did not converge due to data sparsity within cells.
Polygenic Risk Scoring
We first performed a set of PRS analyses to determine whether the observed associations could be explained by shared genetic risk, i.e., the same alleles happen to predispose mothers to both asthma/BMI and to having offspring with ASD. Because evidence for asthma and obesity associations were found only in White individuals (Table S2), all PRS and subsequent analyses were performed on the subset with European ancestry (n = 571) as determined by genetic principal component clustering. We used external summary statistics from asthma and BMI GWAS (32) to predict maternal diagnosis using the IMPaCT genetic data. The PRS for asthma explained 2.36% (p = 1.06 × 10−6) of the variance in asthma in the IMPaCT mothers. The PRS for BMI explained 6.07% (p = 8.02 × 10−13) of the variance in BMI. The PRS for the meta-analyzed combination of asthma and BMI explained 4.92% (p = 8.54 × 10−7) of the variance of a combined asthma-obesity (BMI > 30) phenotype. We tested the association of each PRS generated with the child ASD outcome to assess shared genetic risk, and none were significant (p > .05), although we may have had low power. We observed a moderate correlation (rG = 0.108, p = .0041) between the meta-analyzed combination of asthma and BMI and the ASD proband GWAS. This correlation would place it in the ∼30th percentile of 235 phenotypes tested with the same ASD dataset, making it an unlikely explanation for the strong association with maternal conditions (50% shared genetics).
Mendelian Randomization
To test whether the physiology of maternal asthma, BMI, or their combination could be causal for the ASD outcome, we performed MR analyses. This rationale relies on the assumption that any cause of asthma/BMI (including genetic causes) would proportionally increase risk for ASD outcomes, and thus, we can use genetic risk factors for asthma/BMI as instruments. Power for an MR study relating the obesity-asthma phenotype meta-analysis to the ASD GWAS (38) was determined to be 0.97 for the European-ancestry data based on the proportion of variance explained by the PRS. Because the ASD GWAS measures proband rather than maternal genetic risk, the estimated variance explained was reduced by half after adjusting for the sharing of 50% of genetics between children and mothers. In this conservative case, the power was found to be 0.78. Because it is not advisable to perform two-sample MR with the full PRS (inclusion of invalid instruments can bias results), both MR-Egger and inverse variance-weighted MR were performed on 3 sets of overlapping single nucleotide polymorphisms between the autism GWAS summary statistics and the asthma and BMI GWAS: 1) all genome-wide significant (capturing maximum variance explained), 2) the top 20 single nucleotide polymorphisms (balancing variance explained and strong instruments), and 3) genome-wide significant filtered for F-statistic > 10 (a criterion for a strong MR instrument) (maximizing instrument strength). MR-Egger p values were .656, .0947, and .742, respectively. Inverse variance-weighted MR p values were .292, .972, and .508, respectively. Therefore, the relationship between the obesity-asthma phenotype and child ASD was not supported as being causal in nature.
Discussion
This study investigated common immune-mediated and cardiometabolic conditions during pregnancy and their associations with distinct neurodevelopmental outcomes within a large, well-characterized integrated health care delivery system. Asthma and obesity were independently associated with a higher likelihood of child ASD. Furthermore, women with both asthma and obesity were substantially more likely to deliver infants who were later diagnosed with ASD or DDs. The odds of having a child with ASD, but not DDs, increased among women with extreme obesity and asthma. Furthermore, results differed by child sex, with the combination of maternal asthma and obesity increasing the odds of ASD among female offspring only.
When investigating causal relationships underlying these associations, PRSs for asthma, obesity, and their combination in mothers were not found to be associated with ASD in children, and the genetic correlation was modest. Similarly, an MR analysis of the combination of asthma and obesity and the relationship with ASD showed no significant relationship. The results of MR do not support the hypothesis that the association with ASD is due to the physiology of the condition(s) because in that scenario genetic risk factors would be expected to proportionately increase ASD risk; similarly, risk did not seem to be equivalently elevated across race/ethnic groups. These results do not invalidate the detected associations; rather, they suggest that associations may not be driven by shared genetic risk or by the conditions themselves, but instead by other shared risk factors. One plausible factor is air pollution, which has been shown to be associated with asthma (43), obesity (44), and ASD and other neurodevelopmental disorders (45,46). This study was not able to address air pollution directly; however, future studies with data on maternal inflammatory conditions during pregnancy, environmental exposures such as air pollution, and child neurodevelopmental outcomes are warranted.
We may not have had sufficient power to rule out shared genetics or causality in several plausible scenarios. First, the strongest PRS associations expected under those hypotheses would be observed directly in the ASD probands, whose genetic data are not included in IMPaCT. Second, there may be a small subset of overlapping pathways, and given the small amount of variance predicted by PRS, we could be underpowered to detect partial correlation. In our MR analysis, the strongest associations expected would be with maternal genetics, but we had only proband ASD GWAS available. The power calculations were performed based on the prediction of the full PRS for BMI-asthma meta-analysis; however, we do not know whether meta-analysis best captures the true model of the BMI and asthma relationship to ASD risk, and the MR instruments may not have the full r2 of the PRS upon which our power analysis was based. In addition, there are biological subtypes of both asthma and obesity that this analysis does not recognize. Further refining relevant subtypes and corresponding GWAS data could improve our power in the future.
Asthma has been rising in prevalence among reproductive-aged individuals and can be exacerbated during pregnancy (47), increasing risk of perinatal complications (48). Our finding of a relationship between maternal asthma and child ASD and DDs is consistent with epidemiological literature linking asthma during pregnancy to child neurodevelopmental conditions, including ASD, intellectual disability, and ADHD (49, 50, 51, 52, 53, 54, 55). This evidence is corroborated by a rodent asthma model showing that induction of allergic asthma during early and late gestation increased anxiety-like and repetitive behaviors in offspring (56, 57, 58). While some studies have shown no association between maternal asthma and ASD (59,60), 2 large studies, conducted in Sweden and the United States, have strengthened the evidence in support of a link between the conditions (49,51). Furthermore, these studies are consistent with our findings of no confounding by shared familial factors or use of asthma medications. However, in a separate U.S.-based study relying on retrospective report of medication use, mothers who used asthma medication during pregnancy had slightly elevated odds of having a child with ASD compared with mothers who had asthma but did not use treatment (51). Studies incorporating information on asthma severity and medication may help clarify these data.
Obesity affects 29% of reproductive-aged women (61) and is linked to greater cardiometabolic risk and inflammation. Our findings of higher odds of ASD among children of mothers with obesity are consistent with systematic reviews and meta-analyses finding strong support for the idea that prepregnancy obesity is a risk factor for child ASD, ADHD, and cognitive delays (62, 63, 64). Our analysis also replicated the linear relationship between higher BMI and odds of ASD observed across populations (65,66). However, other studies suggest that the strength of association between maternal obesity and ASD diminishes after adjusting for paternal BMI or in sibling study designs (67,68).
To our knowledge, this is the first study to examine the joint association of asthma and obesity and their underlying genetics with ASD and DD risk. The interrelationship and co-occurrence of obesity and asthma have been recognized for some time (69). Several prospective studies have demonstrated that high BMI increases the risk of incident asthma and exacerbation of symptoms, especially among women (69, 70, 71). Studies also suggest a genetic underpinning of obesity with adult-onset nonatopic asthma (72). Furthermore, a unique immune and metabolic profile seems to distinguish obesity-related asthma from other asthma endophenotypes (73), which may have implications for neurodevelopmental outcomes.
We found that GDM was associated with significantly higher odds of DDs but not ASD. The literature suggests that GDM is associated with a range of developmental delays and psychiatric conditions in offspring (10,11,74, 75, 76, 77, 78). In contrast, our null ASD finding conflicts with a meta-analysis of 18 studies supporting an increased risk of ASD associated with GDM (79). However, one previous large U.S.-based case-control study found no association between GDM during pregnancy and ASD (80). Given the high heterogeneity across these studies (79), future work should consider how the timing, severity, and pharmacologic clinical management of GDM and variation in phenotypes among racial and ethnic groups may affect these relationships.
Multiple studies have observed an interaction between maternal obesity and GDM on risk of neurodevelopmental conditions (7,10,64,81). However, our analysis did not replicate this joint association or find evidence of an interaction between GDM and asthma with respect to ASD or DDs. Larger future studies could consider active management of asthma and GDM during pregnancy and the severity of these conditions. Furthermore, pregestational diabetes mellitus was not associated with either child outcome in our study, but previous studies have found evidence that maternal overweight/obesity and pregestational diabetes may jointly increase the risk of child ASD (81,82). We did not adjust for multiple comparisons because the primary hypotheses were 1) prespecified and 2) preceded by prior relevant data in earlier studies following the rationale outlined by Rothman and Savitz (83, 84, 85). The 10 maternal conditions selected for this study were all based on evidence from prior studies, and we conducted 20 primary tests of association, 10 for ASD and 10 for DDs.
Animal studies suggest that maternal inflammation has a more deleterious impact on the neurodevelopment of male offspring (86, 87, 88), although some evidence suggests unique responses in female offspring (89,90). To date, few epidemiological studies have explored these sex differences in humans, where the maternal inflammation from chronic conditions may be very different from the short-term inflammation induced in animal models (4). Our findings indicate that certain maternal conditions, including asthma, obesity, and preexisting and gestational hypertension, may increase the odds of ASD and DDs in girls but not boys. However, it is worth noting that other studies have reported conflicting findings, with stronger correlations of maternal obesity and ASD in boys or no sex differences (91,92).
Emerging evidence suggests that fetal sex, possibly through genetic and hormonal influences on the placenta and immune signaling, may not only modulate fetal susceptibility to maternal inflammation but also shape the maternal inflammatory response (93, 94, 95, 96, 97, 98). Maternal asthma demonstrates an example of this complex interplay. In a cohort of children with ASD, maternal asthma was more common among boys than girls; however, maternal asthma was more strongly associated with behavioral and emotional problems in girls than in boys (4). Furthermore, studies have documented greater asthma exacerbations and inflammation among pregnant women with asthma carrying a female compared with a male fetus (96,97). These relationships require greater scrutiny.
Strengths and Limitations
This study has several key strengths. First, we used a large, well-characterized pregnancy cohort within the membership of an integrated health delivery system that is generally representative of pregnancies across the insured population of California. Using comprehensive longitudinal clinical information on mothers and children, we were able to look at child outcomes with respect to multiple maternal medical conditions prospectively documented by clinicians during pregnancy while controlling for key confounders. Diagnoses of ASD were ascertained by rigorous clinical assessment, thereby reducing possible misclassification. The observed ASD prevalence of approximately 2% among KPNC members <10 years old approximates recent figures from multisource surveillance systems (99), and the demographic profile of KPNC’s patients with autism is similar to that of other populations (e.g., 80% male). Our genetic approaches of using PRS and MR further take advantage of large external resources relevant to ASD, asthma, and obesity.
Our findings should also be interpreted in light of several limitations. First, we lacked data on potential confounders and moderators, such as breastfeeding, maternal diet, physical activity, multivitamin use, smoking, and air pollution. We did not have information on asthma severity, although adjustment for use of asthma medications did not appreciably alter results. We did not have a sufficient sample size to rigorously examine developmental phenotype differences, and the age of study children precluded robust examination of ADHD. Because associations with asthma and obesity were primarily observed in individuals of European ancestry and external genetic resources are primarily available to match European ancestry, genetic analyses were limited to our European ancestry subset. Furthermore, despite sex differences in associations, due to power limitations and availability of high-quality sex-stratified summary statistics, genetic analyses were not performed in a sex-stratified manner.
Conclusions
In summary, we found evidence that common maternal inflammatory-related conditions during pregnancy indicate risk for neurodevelopmental disorders in children. Children of women with both asthma and obesity may be especially vulnerable to adverse outcomes. Future studies should consider asthma endophenotypes, specifically the unique inflammatory state of obesity-related asthma. Studies should also continue to explore how fetal responses to maternal inflammation may differ by biological sex. Future analyses in the IMPaCT study will integrate maternal and child genetic profiles with environmental exposures, maternal inflammatory conditions, and pregnancy and newborn immune biomarkers, which may shed light on key biological pathways and pregnancy time points and inform early detection and prevention strategies.
Acknowledgments and Disclosures
This work was supported by the National Institute of Child Health and Human Development (Grant No. R01HD095128 [to LAC, principal investigator]).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institutes of Health.
Data used in this study were provided by the Kaiser Permanente Research Bank from the Kaiser Permanente Research Bank collection, which includes the Kaiser Permanente Research Program on Genes, Environment, and Health, funded by the Robert Wood Johnson Foundation, the Wayne and Gladys Valley Foundation, The Ellison Medical Foundation, and the Kaiser Permanente Community Benefits Program. Access to data used in this study may be obtained by application to the Kaiser Permanente Research Bank at kp.org/researchbank/researchers.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2023.09.008.
Supplementary Material
References
- 1.Han V.X., Patel S., Jones H.F., Nielsen T.C., Mohammad S.S., Hofer M.J., et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: A systematic review. Transl Psychiatry. 2021;11:71. doi: 10.1038/s41398-021-01198-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tioleco N., Silberman A.E., Stratigos K., Banerjee-Basu S., Spann M.N., Whitaker A.H., Turner J.B. Prenatal maternal infection and risk for autism in offspring: A meta-analysis. Autism Res. 2021;14:1296–1316. doi: 10.1002/aur.2499. [DOI] [PubMed] [Google Scholar]
- 3.Chen S.W., Zhong X.S., Jiang L.N., Zheng X.Y., Xiong Y.Q., Ma S.J., et al. Maternal autoimmune diseases and the risk of autism spectrum disorders in offspring: A systematic review and meta-analysis. Behav Brain Res. 2016;296:61–69. doi: 10.1016/j.bbr.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 4.Patel S., Dale R.C., Rose D., Heath B., Nordahl C.W., Rogers S., et al. Maternal immune conditions are increased in males with autism spectrum disorders and are associated with behavioural and emotional but not cognitive co-morbidity. Transl Psychiatry. 2020;10:286. doi: 10.1038/s41398-020-00976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edlow A.G. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat Diagn. 2017;37:95–110. doi: 10.1002/pd.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jo H., Schieve L.A., Sharma A.J., Hinkle S.N., Li R., Lind J.N. Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics. 2015;135:e1198–e1209. doi: 10.1542/peds.2014-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connolly N., Anixt J., Manning P., Ping-I Lin D., Marsolo K.A., Bowers K. Maternal metabolic risk factors for autism spectrum disorder-An analysis of electronic medical records and linked birth data. Autism Res. 2016;9:829–837. doi: 10.1002/aur.1586. [DOI] [PubMed] [Google Scholar]
- 8.Li M., Fallin M.D., Riley A., Landa R., Walker S.O., et al. The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics. 2016;137 doi: 10.1542/peds.2015-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nahum Sacks K., Friger M., Shoham-Vardi I., Abokaf H., Spiegel E., Sergienko R., et al. Prenatal exposure to gestational diabetes mellitus as an independent risk factor for long-term neuropsychiatric morbidity of the offspring. Am J Obstet Gynecol. 2016;215:380.e1–380.e7. doi: 10.1016/j.ajog.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Dionne G., Boivin M., Séguin J.R., Pérusse D., Tremblay R.E. Gestational diabetes hinders language development in offspring. Pediatrics. 2008;122:e1073–e1079. doi: 10.1542/peds.2007-3028. [DOI] [PubMed] [Google Scholar]
- 11.Krakowiak P., Walker C.K., Bremer A.A., Baker A.S., Ozonoff S., Hansen R.L., Hertz-Picciotto I. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129:e1121–e1128. doi: 10.1542/peds.2011-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyall K., Pauls D.L., Spiegelman D., Ascherio A., Santangelo S.L. Pregnancy complications and obstetric suboptimality in association with autism spectrum disorders in children of the Nurses’ Health Study II. Autism Res. 2012;5:21–30. doi: 10.1002/aur.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mann J.R., Pan C., Rao G.A., McDermott S., Hardin J.W. Children born to diabetic mothers may be more likely to have intellectual disability. Matern Child Health J. 2013;17:928–932. doi: 10.1007/s10995-012-1072-1. [DOI] [PubMed] [Google Scholar]
- 14.Xiang A.H., Wang X., Martinez M.P., Walthall J.C., Curry E.S., Page K., et al. Association of maternal diabetes with autism in offspring. JAMA. 2015;313:1425–1434. doi: 10.1001/jama.2015.2707. [DOI] [PubMed] [Google Scholar]
- 15.Xu G., Jing J., Bowers K., Liu B., Bao W. Maternal diabetes and the risk of autism spectrum disorders in the offspring: A systematic review and meta-analysis. J Autism Dev Disord. 2014;44:766–775. doi: 10.1007/s10803-013-1928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaouat G. The Th1/Th2 paradigm: Still important in pregnancy? Semin Immunopathol. 2007;29:95–113. doi: 10.1007/s00281-007-0069-0. [DOI] [PubMed] [Google Scholar]
- 17.Wegmann T.G., Lin H., Guilbert L., Mosmann T.R. Bidirectional cytokine interactions in the maternal-fetal relationship: Is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 18.Rose D.R., Careaga M., Van de Water J., McAllister K., Bauman M.D., Ashwood P. Long-term altered immune responses following fetal priming in a non-human primate model of maternal immune activation. Brain Behav Immun. 2017;63:60–70. doi: 10.1016/j.bbi.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solek C.M., Farooqi N., Verly M., Lim T.K., Ruthazer E.S. Maternal immune activation in neurodevelopmental disorders. Dev Dyn. 2018;247:588–619. doi: 10.1002/dvdy.24612. [DOI] [PubMed] [Google Scholar]
- 21.Irwin J.L., McSorley E.M., Yeates A.J., Mulhern M.S., Strain J.J., Watson G.E., et al. Maternal immune markers during pregnancy and child neurodevelopmental outcomes at age 20 months in the Seychelles Child Development Study. J Neuroimmunol. 2019;335 doi: 10.1016/j.jneuroim.2019.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones K.L., Croen L.A., Yoshida C.K., Heuer L., Hansen R., Zerbo O., et al. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol Psychiatry. 2017;22:273–279. doi: 10.1038/mp.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghassabian A., Albert P.S., Hornig M., Yeung E., Cherkerzian S., Goldstein R.B., et al. Gestational cytokine concentrations and neurocognitive development at 7 years. Transl Psychiatry. 2018;8:64. doi: 10.1038/s41398-018-0112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon N. Kaiser Permanente Division of Research; Oakland, CA: 2015. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics From the 2011–12 California Health Interview Survey. [Google Scholar]
- 25.Hedderson M.M., Ferrara A., Avalos L.A., Van den Eeden S.K., Gunderson E.P., Li D.K., et al. The Kaiser Permanente Northern California research program on genes, environment, and health (RPGEH) pregnancy cohort: Study design, methodology and baseline characteristics. BMC Pregnancy Childbirth. 2016;16:381. doi: 10.1186/s12884-016-1150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Psychiatric Association, DSM-5 Task Force . 5th ed. American Psychiatric Publishing; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [Google Scholar]
- 27.Lord C., Risi S., Lambrecht L., Cook E.H., Jr., Leventhal B.L., DiLavore P.C., et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 28.NHLBI Classification of Overweight and Obesity by BMI, Waist Circumference, and Associated Disease Risks. US Department of Health & Human Services. https://www.nhlbi.nih.gov/files/docs/guidelines/prctgd_c.pdf Available at:
- 29.Van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 30.Knol M.J., VanderWeele T.J. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41:514–520. doi: 10.1093/ije/dyr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuo K., Zhou W., Wang Y., Kanai M., Namba S., Gupta R., et al. Multi-ancestry meta-analysis of asthma identifies novel associations and highlights the value of increased power and diversity. Cell Genomics. 2022;2 doi: 10.1016/j.xgen.2022.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locke A.E., Kahali B., Berndt S.I., Justice A.E., Pers T.H., Day F.R., et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruan Y., Lin Y.F., Feng Y.A., Chen C.Y., Lam M., Guo Z., et al. Improving polygenic prediction in ancestrally diverse populations. Nat Genet. 2022;54:573–580. doi: 10.1038/s41588-022-01054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willer C.J., Li Y., Abecasis G.R. METAL: Fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeileis A., Kleiber C., Jackman S. Regression models for count data in R. J Stat Softw. 2008;27:1–25. [Google Scholar]
- 36.Chang C.C., Chow C.C., Tellier L.C., Vattikuti S., Purcell S.M., Lee J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemani G., Zheng J., Elsworth B., Wade K.H., Haberland V., Baird D., et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018;7 doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grove J., Ripke S., Als T.D., Mattheisen M., Walters R.K., Won H., et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51:431–444. doi: 10.1038/s41588-019-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myers T.A., Chanock S.J., Machiela M.J. LDlinkR: An R package for rapidly calculating linkage disequilibrium statistics in diverse populations. Front Genet. 2020;11:157. doi: 10.3389/fgene.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brion M.J., Shakhbazov K., Visscher P.M. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42:1497–1501. doi: 10.1093/ije/dyt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Bulik-Sullivan B.K., Loh P.R., Finucane H.K., Ripke S., Yang J., et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bulik-Sullivan B., Finucane H.K., Anttila V., Gusev A., Day F.R., Loh P.R., et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47:1236–1241. doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guarnieri M., Balmes J.R. Outdoor air pollution and asthma. Lancet. 2014;383:1581–1592. doi: 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McConnell R., Shen E., Gilliland F.D., Jerrett M., Wolch J., Chang C.C., et al. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: The Southern California Children’s Health Study. Environ Health Perspect. 2015;123:360–366. doi: 10.1289/ehp.1307031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dutheil F., Comptour A., Morlon R., Mermillod M., Pereira B., Baker J.S., et al. Autism spectrum disorder and air pollution: A systematic review and meta-analysis. Environ Pollut. 2021;278 doi: 10.1016/j.envpol.2021.116856. [DOI] [PubMed] [Google Scholar]
- 46.McGuinn L.A., Wiggins L.D., Volk H.E., Di Q., Moody E.J., Kasten E., et al. Pre- and postnatal fine particulate matter exposure and childhood cognitive and adaptive function. Int J Environ Res Public Health. 2022;19:3748. doi: 10.3390/ijerph19073748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen J.M., Bateman B.T., Huybrechts K.F., Mogun H., Yland J., Schatz M., et al. Poorly controlled asthma during pregnancy remains common in the United States. J Allergy Clin Immunol Pract. 2019;7:2672–2680.e10. doi: 10.1016/j.jaip.2019.05.043. [DOI] [PubMed] [Google Scholar]
- 48.Bohács A., Cseh A., Stenczer B., Müller V., Gálffy G., Molvarec A., et al. Effector and regulatory lymphocytes in asthmatic pregnant women. Am J Reprod Immunol. 2010;64:393–401. doi: 10.1111/j.1600-0897.2010.00878.x. [DOI] [PubMed] [Google Scholar]
- 49.Gong T., Lundholm C., Rejnö G., Bölte S., Larsson H., D’Onofrio B.M., et al. Parental asthma and risk of autism spectrum disorder in offspring: A population and family-based case-control study. Clin Exp Allergy. 2019;49:883–891. doi: 10.1111/cea.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Croen L.A., Grether J.K., Yoshida C.K., Odouli R., Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: A case-control study. Arch Pediatr Adolesc Med. 2005;159:151–157. doi: 10.1001/archpedi.159.2.151. [DOI] [PubMed] [Google Scholar]
- 51.Croen L.A., Qian Y., Ashwood P., Daniels J.L., Fallin D., Schendel D., et al. Family history of immune conditions and autism spectrum and developmental disorders: Findings from the study to explore early development. Autism Res. 2019;12:123–135. doi: 10.1002/aur.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lyall K., Ashwood P., Van de Water J., Hertz-Picciotto I. Maternal immune-mediated conditions, autism spectrum disorders, and developmental delay. J Autism Dev Disord. 2014;44:1546–1555. doi: 10.1007/s10803-013-2017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theoharides T.C., Tsilioni I., Patel A.B., Doyle R. Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl Psychiatry. 2016;6:e844. doi: 10.1038/tp.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.May-Benson T.A., Koomar J.A., Teasdale A. Incidence of pre-, peri-, and post-natal birth and developmental problems of children with sensory processing disorder and children with autism spectrum disorder. Front Integr Neurosci. 2009;3:31. doi: 10.3389/neuro.07.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langridge A.T., Glasson E.J., Nassar N., Jacoby P., Pennell C., Hagan R., et al. Maternal conditions and perinatal characteristics associated with autism spectrum disorder and intellectual disability. PLoS One. 2013;8 doi: 10.1371/journal.pone.0050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Church J.S., Tamayo J.M., Ashwood P., Schwartzer J.J. Repeated allergic asthma in early versus late pregnancy differentially impacts offspring brain and behavior development. Brain Behav Immun. 2021;93:66–79. doi: 10.1016/j.bbi.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwartzer J.J., Careaga M., Chang C., Onore C.E., Ashwood P. Allergic fetal priming leads to developmental, behavioral and neurobiological changes in mice. Transl Psychiatry. 2015;5:e543. doi: 10.1038/tp.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartzer J.J., Careaga M., Coburn M.A., Rose D.R., Hughes H.K., Ashwood P. Behavioral impact of maternal allergic-asthma in two genetically distinct mouse strains. Brain Behav Immun. 2017;63:99–107. doi: 10.1016/j.bbi.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larsson M., Weiss B., Janson S., Sundell J., Bornehag C.G. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6–8 years of age. Neurotoxicology. 2009;30:822–831. doi: 10.1016/j.neuro.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mouridsen S.E., Rich B., Isager T., Nedergaard N.J. Autoimmune diseases in parents of children with infantile autism: A case-control study. Dev Med Child Neurol. 2007;49:429–432. doi: 10.1111/j.1469-8749.2007.00429.x. [DOI] [PubMed] [Google Scholar]
- 61.Driscoll A.K., Gregory E.C.W. Increases in prepregnancy obesity: United States, 2016–2019. NCHS Data Brief. 2020;392:1–8. [PubMed] [Google Scholar]
- 62.Tong L., Kalish B.T. The impact of maternal obesity on childhood neurodevelopment. J Perinatol. 2021;41:928–939. doi: 10.1038/s41372-020-00871-0. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez C.E., Barry C., Sabhlok A., Russell K., Majors A., Kollins S.H., Fuemmeler B.F. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obes Rev. 2018;19:464–484. doi: 10.1111/obr.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kong L., Chen X., Gissler M., Lavebratt C. Relationship of prenatal maternal obesity and diabetes to offspring neurodevelopmental and psychiatric disorders: A narrative review. Int J Obes (Lond) 2020;44:1981–2000. doi: 10.1038/s41366-020-0609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y., Tang S., Xu S., Weng S., Liu Z. Maternal body mass index and risk of autism spectrum disorders in offspring: A meta-analysis. Sci Rep. 2016;6 doi: 10.1038/srep34248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andersen C.H., Thomsen P.H., Nohr E.A., Lemcke S. Maternal body mass index before pregnancy as a risk factor for ADHD and autism in children. Eur Child Adolesc Psychiatry. 2018;27:139–148. doi: 10.1007/s00787-017-1027-6. [DOI] [PubMed] [Google Scholar]
- 67.Surén P., Gunnes N., Roth C., Bresnahan M., Hornig M., Hirtz D., et al. Parental obesity and risk of autism spectrum disorder. Pediatrics. 2014;133:e1128–e1138. doi: 10.1542/peds.2013-3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gardner R.M., Lee B.K., Magnusson C., Rai D., Frisell T., Karlsson H., et al. Maternal body mass index during early pregnancy, gestational weight gain, and risk of autism spectrum disorders: Results from a Swedish total population and discordant sibling study. Int J Epidemiol. 2015;44:870–883. doi: 10.1093/ije/dyv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang L., Wang K., Gao X., Paul T.K., Cai J., Wang Y. Sex difference in the association between obesity and asthma in U.S. adults: Findings from a national study. Respir Med. 2015;109:955–962. doi: 10.1016/j.rmed.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 70.Klepaker G., Svendsen M.V., Hertel J.K., Holla Ø.L., Henneberger P.K., Kongerud J., Fell A.K.M. Influence of obesity on work ability, respiratory symptoms, and lung function in adults with asthma. Respiration. 2019;98:473–481. doi: 10.1159/000502154. [DOI] [PubMed] [Google Scholar]
- 71.Hendler I., Schatz M., Momirova V., Wise R., Landon M., Mabie W., et al. Association of obesity with pulmonary and nonpulmonary complications of pregnancy in asthmatic women. Obstet Gynecol. 2006;108:77–82. doi: 10.1097/01.AOG.0000223180.53113.0f. [DOI] [PubMed] [Google Scholar]
- 72.Zhu Z., Guo Y., Shi H., Liu C.L., Panganiban R.A., Chung W., et al. Shared genetic and experimental links between obesity-related traits and asthma subtypes in UK Biobank. J Allergy Clin Immunol. 2020;145:537–549. doi: 10.1016/j.jaci.2019.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y., Zheng J., Zhang H.P., Zhang X., Wang L., Wood L., Wang G. Obesity-associated metabolic signatures correlate to clinical and inflammatory profiles of asthma: A pilot study. Allergy Asthma Immunol Res. 2018;10:628–647. doi: 10.4168/aair.2018.10.6.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DeBoer T., Wewerka S., Bauer P.J., Georgieff M.K., Nelson C.A. Explicit memory performance in infants of diabetic mothers at 1 year of age. Dev Med Child Neurol. 2005;47:525–531. doi: 10.1017/s0012162205001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Girchenko P., Tuovinen S., Lahti-Pulkkinen M., Lahti J., Savolainen K., Heinonen K., et al. Maternal early pregnancy obesity and related pregnancy and pre-pregnancy disorders: Associations with child developmental milestones in the prospective PREDO Study. Int J Obes (Lond) 2018;42:995–1007. doi: 10.1038/s41366-018-0061-x. [DOI] [PubMed] [Google Scholar]
- 76.Deregnier R.A., Nelson C.A., Thomas K.M., Wewerka S., Georgieff M.K. Neurophysiologic evaluation of auditory recognition memory in healthy newborn infants and infants of diabetic mothers. J Pediatr. 2000;137:777–784. doi: 10.1067/mpd.2000.109149. [DOI] [PubMed] [Google Scholar]
- 77.Xiang A.H., Wang X., Martinez M.P., Getahun D., Page K.A., Buchanan T.A., Feldman K. Maternal gestational diabetes mellitus, type 1 diabetes, and type 2 diabetes during pregnancy and risk of ADHD in offspring. Diabetes Care. 2018;41:2502–2508. doi: 10.2337/dc18-0733. [DOI] [PubMed] [Google Scholar]
- 78.Nogueira Avelar E Silva R., Yu Y., Liew Z., Vested A., Sørensen H.T., Li J. Associations of maternal diabetes during pregnancy with psychiatric disorders in offspring during the first 4 decades of life in a population-based Danish birth cohort. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.28005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rowland J., Wilson C.A. The association between gestational diabetes and ASD and ADHD: A systematic review and meta-analysis. Sci Rep. 2021;11:5136. doi: 10.1038/s41598-021-84573-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cordero C., Windham G.C., Schieve L.A., Fallin M.D., Croen L.A., Siega-Riz A.M., et al. Maternal diabetes and hypertensive disorders in association with autism spectrum disorder. Autism Res. 2019;12:967–975. doi: 10.1002/aur.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kong L., Norstedt G., Schalling M., Gissler M., Lavebratt C. The risk of offspring psychiatric disorders in the setting of maternal obesity and diabetes. Pediatrics. 2018;142 doi: 10.1542/peds.2018-0776. [DOI] [PubMed] [Google Scholar]
- 82.Li M., Fallin M.D., Riley A., Landa R., Walker S.O., Silverstein M., et al. The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics. 2016;137 doi: 10.1542/peds.2015-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Savitz D.A. Oxford University Press; Oxford, UK: 2003. Interpreting Epidemiologic Evidence: Strategy for Study Design and Analysis. [Google Scholar]
- 84.Rothman K.J., Greenland S., Lash T.L. Lippincott Williams & Wilkins; Philadelphia: 2008. Modern Epidemiology. [Google Scholar]
- 85.Rothman K.J. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 86.Xuan I.C., Hampson D.R. Gender-dependent effects of maternal immune activation on the behavior of mouse offspring. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schwartzer J.J., Careaga M., Onore C.E., Rushakoff J.A., Berman R.F., Ashwood P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl Psychiatry. 2013;3 doi: 10.1038/tp.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haida O., Al Sagheer T., Balbous A., Francheteau M., Matas E., Soria F., et al. Sex-dependent behavioral deficits and neuropathology in a maternal immune activation model of autism. Transl Psychiatry. 2019;9:124. doi: 10.1038/s41398-019-0457-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vogel Ciernia A., Careaga M., LaSalle J.M., Ashwood P. Microglia from offspring of dams with allergic asthma exhibit epigenomic alterations in genes dysregulated in autism. Glia. 2018;66:505–521. doi: 10.1002/glia.23261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Breach M.R., Dye C.N., Joshi A., Platko S., Gilfarb R.A., Krug A.R., et al. Maternal allergic inflammation in rats impacts the offspring perinatal neuroimmune milieu and the development of social play, locomotor behavior, and cognitive flexibility. Brain Behav Immun. 2021;95:269–286. doi: 10.1016/j.bbi.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carter S.A., Lin J.C., Chow T., Yu X., Rahman M.M., Martinez M.P., et al. Maternal obesity, diabetes, preeclampsia, and asthma during pregnancy and likelihood of autism spectrum disorder with gastrointestinal disturbances in offspring. Autism. 2023;27:916–926. doi: 10.1177/13623613221118430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Panjwani A.A., Ji Y., Fahey J.W., Palmer A., Wang G., Hong X., et al. Maternal obesity/diabetes, plasma branched-chain amino acids, and autism spectrum disorder risk in urban low-income children: Evidence of sex difference. Autism Res. 2019;12:1562–1573. doi: 10.1002/aur.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Global Pregnancy Collaboration. Schalekamp-Timmermans S., Arends L.R., Alsaker E., Chappell L., Hansson S., et al. Fetal sex-specific differences in gestational age at delivery in pre-eclampsia: A meta-analysis. Int J Epidemiol. 2017;46:632–642. doi: 10.1093/ije/dyw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clifton V.L. Review: Sex and the human placenta: Mediating differential strategies of fetal growth and survival. Placenta. 2010;31(suppl):S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 95.Bangma J.T., Hartwell H., Santos H.P., Jr., O’Shea T.M., Fry R.C. Placental programming, perinatal inflammation, and neurodevelopment impairment among those born extremely preterm. Pediatr Res. 2021;89:326–335. doi: 10.1038/s41390-020-01236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bakhireva L.N., Schatz M., Jones K.L., Tucker C.M., Slymen D.J., Klonoff-Cohen H.S., et al. Fetal sex and maternal asthma control in pregnancy. J Asthma. 2008;45:403–407. doi: 10.1080/02770900801971826. [DOI] [PubMed] [Google Scholar]
- 97.Scott N.M., Hodyl N.A., Murphy V.E., Osei-Kumah A., Wyper H., Hodgson D.M., et al. Placental cytokine expression covaries with maternal asthma severity and fetal sex. J Immunol. 2009;182:1411–1420. doi: 10.4049/jimmunol.182.3.1411. [DOI] [PubMed] [Google Scholar]
- 98.Baines K.J., West R.C. Sex differences in innate and adaptive immunity impact fetal, placental, and maternal health. Biol Reprod. 2023;109:256–270. doi: 10.1093/biolre/ioad072. [DOI] [PubMed] [Google Scholar]
- 99.Maenner M.J., Shaw K.A., Baio J., EdS1, Washington A., Patrick M., et al. Prevalence of autism spectrum disorder among children aged 8 years - Autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveill Summ. 2020;69:1–12. doi: 10.15585/mmwr.ss6904a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.