Abstract
Antimicrobial resistance is a major global public health problem, with fluoroquinolone-resistant strains of Escherichia coli posing a significant threat. This study examines the genetic characterization of ESBL-producing E. coli isolates in Mexican hospitals, which are resistant to both cephalosporins and fluoroquinolones. A total of 23 ESBL-producing E. coli isolates were found to be positive for the qepA gene, which confers resistance to fluoroquinolones. These isolates exhibited drug resistance phenotypes and belonged to specific sequence types and phylogenetic groups. The genetic context of the qepA gene was identified in a novel genetic context flanked by IS26 sequences. Mating experiments showed the co-transfer of qepA1 and chrA determinants alongside blaCTX-M-15 genes, emphasizing the potential for these genetic structures to spread among Enterobacterales. The emergence of multidrug-resistant Gram-negative bacteria carrying these resistance genes is a significant clinical concern for public healthcare systems.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-023-01115-x.
Keywords: Efflux pump qepA, ESBL, Escherichia coli, Fluoroquinolone
Introduction
Antimicrobial resistance (AMR) is one of the main global public health problems worldwide. In low-income countries, including Latin America, the indiscriminate use of antimicrobials has augmented the rates of AMR [1]. In healthcare systems, the introduction and subsequent use of fluoroquinolones as antimicrobial agents have been widely adopted [2]. The emergence and increase of fluoroquinolone-resistant strains and their subsequent spread have been reported in several countries [2]. Escherichia coli is the most common cause of urinary tract infections (UTI) and is found in around 70–90% of cases [3, 4]. The first treatment in patients with UTI is frequently empirical, and one of the most widely used antimicrobials is fluoroquinolone [5, 6]. The increase in these multidrug-resistant bacteria in community and hospital settings is becoming an important issue for healthcare systems [7].
The main mechanism for quinolone resistance in E. coli is chromosomal mutations in the DNA gyrase gyrA gene, and less frequently, mutations in the gyrB gene, as well as in both genes encoding topoisomerase IV parC and parE. However, the description of horizontal transfer of quinolone resistance determinants by plasmids initiated concern about the quinolone resistance mechanism [8]. These mechanisms reduce bacterial susceptibility to quinolones and are described as plasmid-mediated quinolone resistance genes (PMQRs). The three main mechanisms, the qnr protein that protects the binding site in type II DNA topoisomerases, the enzymatic modification of the drug by the aac(6')-Ib-cr, and the efflux pumps oqxAB and qepA, are considered the main PMQRs [9]. The PMQR genes have been found in bacterial isolates worldwide, and they reduce bacterial susceptibility to fluoroquinolones, usually not to the level of clinical non-susceptibility, but they facilitate bacterial survival and subsequently generate mutants with higher-level fluoroquinolone resistance and promote treatment failure [10]. Currently, several reports describe ESBL-producing E. coli isolates resistant to both cephalosporins and fluoroquinolones spreading in the community and hospitals [7]. Clinical isolates containing multidrug and fluoroquinolone resistance mechanisms generate therapeutic failure in both cephalosporins and quinolones and/or fluoroquinolones. QepA is an efflux pump that decreases susceptibility to hydrophilic fluoroquinolones, especially ciprofloxacin and norfloxacin [11]. The qepA gene has been described on large conjugative IncF group plasmids with the encoding aminoglycoside ribosomal methylase rmtB and blaTEM flanked by IS26 and ISCR3C insertion sequences as part of compound transposons [12, 13]. In this work, we performed a genetic characterization of ESBL-producing E. coli clinical isolates harboring emerging plasmids with new genetic context bearing qepA, chrA, and blaCTX-M genes that could become a serious clinical concern.
Materials and methods
Clinical isolates included in the study
A total of 628 ESBL-producing E. coli isolates were collected from patients during the period 2005–2020 from six hospitals in Mexico: ISTE-S, ISSSTESON, Hermosillo, Sonora; International Reference Laboratory (CAPERMOR), Ciudad de México; Instituto Nacional de Cancerología (INCan), Ciudad de México; Sanatorio Durango (SD-DF), Ciudad de México; Hospital de Pediatría, Centro Médico Nacional Siglo XXI, Ciudad de México; and Hospital Regional de Alta Especialidad, Oaxaca. All the bacterial species identification and susceptibility pattern were initially detected by the Dade MicroScan and VITEK 2 compact system (BioMérieux, Durham, USA). The ESBL-producing phenotype was determined using the double-disc synergism method according to guidelines of the Clinical and Laboratory Standards Institute (CLSI) (M100-S21) [14].
PCR amplification and DNA sequences
The qepA, aac(6´)-Ib-cr and chrA genes were screened by single PCR with specific primers for each gene. In the qepA-positive isolates, the mutations in gyrA and parC chromosomal genes were determined by PCR using specific primers and confirmed by nucleotide sequencing [14]. The 5′ region in the class 1 integrons was determined with the oligonucleotide Intl1 (CGTTCCATACAGAAGCTGG) and qepA-R (CTGCAGGTACTGCGTCATG). The relationship of the qepA with the insertion sequence ISCR3C was identified with the oligonucleotide qepA-F (CGTGTTGCTGGAGTTCTTC) and ISCR3C-F (CCACTGCGGTGGCACCGT). In addition, TEM, SHV, CTX-M, and TLA-lactamases genes were screened by PCR, as well as PMQR qnrA, qnrB, qnrC, qnrD, and qnrS genes using specific oligonucleotides described previously [15, 16].
The PCR products were purified by the commercial Kit from Roche (Roche, the USA) and sequenced by the BigDye Terminator v3.1 Cycle Sequencing Kit in the automated system ABIPrisma 3100 (Applied Biosystem, the USA). The Translate Tool (http://ca.expasy.org/tools/dna.html) was used for each nucleotide sequence to obtain the amino acid sequences and was compared by BLASTp in the GenBank database (http://www.ncbi.nlm.nih.gov/).
Susceptibility determination
The minimal inhibitory concentration (MIC) to cefotaxime, ceftazidime, imipenem, meropenem, nalidixic acid, ciprofloxacin, levofloxacin, gentamicin, polymyxin B, and tigecycline were determined by micro-dilution in broth according to CLSI [14].
Phylogenetic grouping
Phylogenetic grouping of the E. coli isolates was performed using the triplex PCR for chuA and yjaA genes and DNA fragment tspE4C2, which allows the classification of four different groups, as previously described by Clermont et al. [17].
Genetic characterization
Random amplified polymorphic DNA (RAPD) analysis was performed to identify the genetic diversity of qepA-positive E. coli isolates. The RAPD was performed using decameric primers P1254 and PCR conditions described by Betancor et al. [18]. The correlation by similarity was computed based on the band positions using the Dice coefficient, and the dendrogram was generated using the UPGMA clustering method, both within the GelCompar II program. The MultiLocus Sequencing Typing (MLST) was performed in all qepA-positive isolates using the MLST tools (https://enterobase.warwick.ac.uk) [19].
Plasmid analysis and mating experiments
Mating assays for the horizontal transfer of quinolone resistance were performed using the E. coli J53-2 (met-, pro-, Rifr) as the recipient strain, in solid-phase mating as described by Miller [20]. Transconjugants were selected on Luria-Bertani (LB) agar supplemented with rifampin (100 μg/ml) and nalidixic acid (8 mg/L), ampicillin (100 mg/L), or cefotaxime (1 mg/L). All transconjugants were verified by their auxotrophic requirements (Pro and Met), and plasmids were analyzed according to the method described by Kieser [21].
Plasmid typing
Incompatibility groups of transconjugants were detected by PCR replicon typing. Specific primers were used including the following: HI1, HI2, I1, X, L/M, N, FIA, FIB, W, P, FIC, Y, FIIA, A/C, T, K, B/O, and F, previously described by Carattoli et al. [22].
Plasmid sequence
Plasmid DNA was obtained from transconjugants pEC8020 and sequenced on Platform 454 (Roche). The assembly was obtained using the PHRED-PHRAP-CONSED and Newbler programs. The prediction of open reading frames (ORFs) was done with the Glimmer3 and RAST programs and compared with the GenBank nr database. In silico plasmid analysis was performed using the Center for Genomic Epidemiology tools (https://cge.cbs.dtu.dk/) to identify antimicrobial resistance genes (ResFinder) and plasmid replicons (PlasmidFinder) [23].
Results
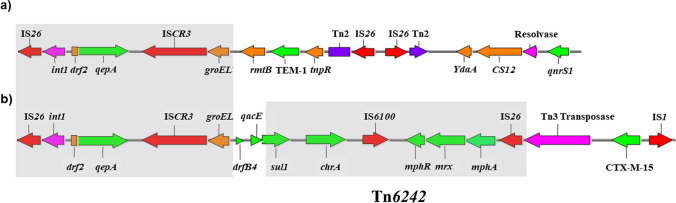
A total of 23/628 (3.6%) ESBL-producing E. coli isolates were positive for the qepA gene (Table 1). All ESBL-producing E. coli isolates were positive for the chromate resistance chrA. The blaCTX-M-15 was identified in 91% (21/23) of isolates, while blaSHV-12 and blaTEM-55 genes were identified in one isolate each (Table 1). The blaTLA, and PMQR qnrA, qnrB, qnrC, qnrD, qnrS, and aac(6′)-Ib-cr genes were not identified. Substitutions in GyrA (Ser83→Leu) and (Asp87→Asn) and ParC (Ser80→Ile) were identified in all qepA-positive isolates (Table 1). The isolates showed a drug resistance phenotype profile to cefotaxime (> 256 μg/mL), nalidixic acid (> 256 μg/mL), ciprofloxacin (> 64 μg/mL), and levofloxacin (8 μg/mL). Of the isolates, 21/23 were resistant to ceftazidime (16 μg/mL), and all were susceptible to imipenem, meropenem, polymyxim B, gentamicin, and tigecycline (Table 1). The susceptibility results for ciprofloxacin and levofloxacin were consistent with the identification of substitutions in gyrA and parC genes. To identify genetic diversity and relationship, among ESBL-producing E. coli isolates, we performed RAPD, MLST, and phylogenetic group analyses. The results of all three analyses were consistent. Isolates 7530, 7505, 7514, and 03212 were clustered in clone A by RAPD and belonged to phylogroup B1 and ST205. Isolates 03210, 8020, and 8019 were clustered in clone B, belonged to phylogroup D, and ST405. The remaining isolates were clustered in clone C and belonged to phylogroup A. However, the sequence types observed in this group were as follows: strain 16211 belonged to ST46; strains 16201, 16206, and 16257 belonged to ST167; strains 16243, 16710, 16715, 16721, and 17188 belonged to ST361; strains 7537, 7544, 09220, and 10246 belonged to ST617 (Supplementary Figure 1). To characterize the plasmids harboring the qepA gene, we performed plasmid profile, type of ESBL-producing, and incompatibility group studies. We observed that the isolates contained plasmids of 60, 81, 85, 94, 100, and 127 kb. We selected five isolates (7530, 8019, 8020, and 09220) with blaCTX-M and one isolate (03210) with blaSHV genes for mating experiments. The mating experiments were successful, and all transconjugants co-transferred the qepA1 gene and chrA gene determinants along with the blaCTX-M-15 in the four mating experiments. The mating experiments with the blaSHV-12 gene were negative in terms of ESBL gene transfer but positive in terms of co-transferred qepA1 and chrA determinants. PCR-based replicon typing showed the presence of FIA, FIB, and FII replicons among all qepA-positive transconjugants in a plasmid of 127 kb obtained in this study (Table 1). All the transconjugants presented resistance to quinolones and were positive for the determination of class 1 integrons in the 5′ region and the qepA gene with the insertion sequence ISCR3C (Fig. 1). The complete nucleotide sequence of plasmid pEC8020 obtained from ESBL-producing E. coli (8020) isolate was 127,611 bp. In silico analysis by ResFinder showed the antimicrobial resistance genes qepA1, mphA, dfrB4, sul1, and blaCTX-M-15 conferring resistance to the antibiotics fluoroquinolones, macrolides, trimethoprim, sulphonamides, and cephalosporins, respectively. The PlasmidFinder analysis identified the replicons IncFIA, IncFIB (pB171), and IncFII with 99.38%, 99.74%, and 96.18% identity, respectively. The genetic context of the qepA gene was determined and corresponded to a complete transfer region with an IS26, followed by a truncated class 1 integrase and truncated dfr2, located upstream of the qepA gene. Downstream from the qepA gene, we identified an ISCR3, followed by a truncated intI1-groEL, the dfrB4, qacED1-sul1 genes, the chromate ion transporter chrA, the transposase insertion sequence IS6100, three macrolide-resistant genes (mphR, the erythromycin resistance repressor; mrx, a transmembrane transport protein of MFS family; and mphA, the macrolide-2′-phosphotransferase), and IS26. Based on this genetic structure, we identified a Tn3 family transposase, blaCTX-M-15, and two IS1 family transposases (Fig. 1b). The genetic context between the class 1 integron in the 5′ region and the distal insertion sequence IS6100 was identified and confirmed in all the qepA-positive E. coli isolates by PCR mapping and sequencing.
Table 1.
Molecular characteristics and antimicrobial resistance of qepA producers Escherichia coli clinical isolates
| Isolate | Year of isolation | Mexican Region | Phylo group | MLST | Plasmid profile (kb) | Inc groupa | GyrA | ParC | ESBL | MIC (μg/ml) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ser83 | Asp87 | Ser80 | CTX | CAZ | NAL | CIP | LEV | GEN | ||||||||
| 7530 | 2006 | Edo Mex | B1 | 205 | 127 | FIA, FIB, FII | Leu | Asn | Ile | CTXM-15 | > 256 | 16 | > 256 | > 64 | 32 | 4 |
| 7505 | 2007 | MC | B1 | 205 | 127 | ND | Leu | Asn | Ile | CTXM-15 | > 256 | 32 | > 256 | > 64 | 32 | 2 |
| 7514 | 2007 | MC | B1 | 205 | 127 | ND | Leu | Asn | Ile | CTXM-15 | > 256 | 64 | > 256 | > 64 | 32 | 1 |
| 03212 | 2008 | Sonora | B1 | 205 | 127 | ND | Leu | Asn | Ile | CTXM-15 | > 256 | 16 | > 256 | > 64 | 32 | 0.5 |
| 03210 | 2008 | Sonora | D | 405 | 127 | FIA, FIB, FII | Leu | Asn | Ile | SHV-12 | > 256 | 2 | > 256 | > 64 | 64 | 64 |
| 8020 | 2009 | MC | D | 405 | 127 | FIA, FIB, FII | Leu | Asn | Ile | CTXM-15 | > 256 | 64 | > 256 | > 64 | 32 | 2 |
| 8019 | 2009 | MC | D | 405 | 100, 127 | FIA, FIB, FII | Leu | Asn | Ile | CTXM-15 | > 256 | 64 | > 256 | > 64 | 32 | 1 |
| 7537 | 2006 | Edo Mex | A | 617 | 127 | FIA, FIB, FII | Leu | Asn | Ile | TEM-55 | > 256 | 1 | > 256 | > 64 | 64 | 64 |
| 7544 | 2007 | Edo Mex | A | 617 | 127 | ND | Leu | Asn | Ile | CTXM-15 | > 256 | 64 | > 256 | > 64 | 64 | 0.5 |
| 09220 | 2008 | MC | A | 617 | 100, 127 | FIA, FIB, FII | Leu | Asn | Ile | CTXM-15 | > 256 | 128 | > 256 | > 64 | 64 | 0.5 |
| 10246 | 2008 | MC | A | 617 | 60, 100, 127 | ND | Leu | Asn | Ile | CTXM-15 | > 256 | 64 | > 256 | > 64 | 64 | 2 |
| 16201 | 2018 | Oaxaca | A | 167 | 94, 85, 60 | FII, FIA, FIB | Leu | Asn | Ile | CTXM-15 | > 256 | 16 | > 256 | > 64 | 32 | 4 |
| 16206 | 2018 | Oaxaca | A | 167 | 94 | FII, FIA, FIB | Leu | Asn | Ile | CTXM-15 | > 256 | 32 | > 256 | > 64 | 32 | 2 |
| 16211 | 2018 | Oaxaca | A | 46 | 94 | FII, FIA, FIB | Leu | Asn | Ile | CTXM-15 | > 256 | 64 | > 256 | > 64 | 32 | 1 |
| 16230 | 2018 | Oaxaca | A | ND | 94, 85 | FII, FIA, FIB | Leu | Asn | Ile | CTXM-15 | > 256 | 16 | > 256 | > 64 | 32 | 4 |
| 16243 | 2018 | Oaxaca | A | 361 | 85, 81 | FII, Frep, IncY,FIA, FIB | Leu | Asn | Ile | CTXM-15 | > 256 | 64 | > 256 | > 64 | 32 | 2 |
| 16257 | 2018 | Oaxaca | A | 167 | 94 | FII,FIA, FIB | Leu | Asn | Ile | CTXM-15 | > 256 | 64 | > 256 | > 64 | 32 | 1 |
| 16707 | 2019 | MC | A | ND | 85 | FII, Frep, FIA, FIB | Leu | Asn | Ile | CTXM-15 | > 256 | 64 | > 256 | > 64 | 32 | 2 |
| 16710 | 2019 | MC | A | 361 | 85, 81 | FII, Frep, IncY, FIA, FIB | Leu | Asn | Ile | CTXM-15 | > 256 | 64 | > 256 | > 64 | 32 | 1 |
| 16715 | 2019 | MC | A | 361 | 85 | FII, Frep, IncY, FIA, FIB | Leu | Asn | Ile | CTXM-15 | > 256 | 64 | > 256 | > 64 | 32 | 2 |
| 16721 | 2019 | MC | A | 361 | 85, 81 | FII, Frep, IncY, FIB | Leu | Asn | Ile | CTXM-15 | > 256 | 64 | > 256 | > 64 | 32 | 1 |
| 16722 | 2019 | MC | A | ND | 85 | FII, Frep, IncY, FIA, FIB | Leu | Asn | Ile | CTXM-15 | > 256 | 64 | > 256 | > 64 | 32 | 2 |
| 17188 | 2020 | Oaxaca | A | 361 | 94, 81 | FII, Frep, IncY, FIA, FIB | Leu | Asn | Ile | CTXM-15 | > 256 | 64 | > 256 | > 64 | 32 | 2 |
aMC Mexico City. Edo Mex Estado de Mexico, RAPD random amplified polymorphic DNA, MLST multilocus sequence typing, ESBL extended-spectrum B-lactamase, MIC minimum inhibitory concentration, ND undetermined, Ser serine, Leu leucine, Asp asparagine, Asn aspartic acid, Ile isoleucine, CTX cefotaxime, CAZ ceftazidime, NAL nalidixic acid; CIP, ciprofloxacin, LEV levofloxacin, GEN gentamicin
All the isolates were susceptible to imipenem, meropenem, polymyxim B, gentamicin, and tigecycline b transconjugants incompatibility groups. ND, undeterminated. Substitutions in GyrA (Ser83→Leu) and (Asp87→Asn) and ParC (Ser80→Ile) were identified in all qepA-positive isolates
Fig. 1.
Comparison of the genetic context of the qepA gene in a plasmid pHPA (28) and b plasmid pT2080 from ESBL-producing E. coli. Open reading frames of resistance genes and genetic mobile elements are indicated in green and red, respectively. Shaded areas show the genetic context shared between the two plasmid structures
Discussion
The prevalence of qepA reported in recent literature fluctuates between 8.3 and 10% and is still low in some cases [24–26]. In Egypt, qepA was identified in 10% of a collection of 39 MDR isolates [24]. In the Pediatric Hospital in Doha, the qepA gene was identified in 10% of 19 E. coli isolates from a neonatal intensive care unit [25]. A similar percentage of 8.3% qepA-positive cases was reported in a collection of 144 ESBL-producing E. coli isolates from Tabriz University in Iran [26]. In addition, the susceptibility results to fluoroquinolone antibiotics were consistent with the identification of substitutions in gyrA and parC found in all isolates [27]. As suggested by Jacoby et al. [28], the horizontal transfer of PMQR determinants accelerates the selection of higher levels of quinolone resistance, which facilitates bacterial survival and the subsequent generation of mutants in GyrA and ParC with higher-level quinolone resistance that produces therapeutic failure [10].
The twenty-three isolates formed three specific groups; the first corresponds to clone A by RAPD and belongs to phylogroup B1 and ST205. The members of this clone were isolated from at least two geographically separated regions (northwest and central Mexico) in three different years. A similar characteristic was observed in the second group, clone B, which belongs to phylogroup D and ST405. The third group, clone C, belongs to phylogroup A and sequence types ST46, ST167, ST361, and ST617. The genetic relationship between the members of each clone suggests low-frequency identification; however, the spread of resistance of ESBL-producing E. coli isolates to fluoroquinolones in Mexican hospitals has remained constant [29, 30].
Plasmid sequence analysis of pEC8020 showed a genetic environment for the qepA gene flanked by IS26 sequences not yet described. The 5′ region corresponds to the arrangement observed in plasmid pHPA (Fig. 1a), with an IS26, Int1, truncated dfr2 located upstream of qepA, and flanked downstream by ISCR3 and truncated intI1-groEL [31]. Instead of truncated rmtB, blaTEM-1, and tnpR genes flanked by IS26 downstream of ISCR3 and intI1-groEL described in plasmid pHPA, we identified drfB4, qacED1-sul1, chrA, IS6100, mphR, mrx, mphA, and insertion sequences IS26 (Fig. 1b). The arrangement of these genes was previously described as part of the transposon structure Tn6242 flanked by two IS26 sequences.
Remarkably, the Tn6242 inserted in the chromosome was identified after whole genome sequencing of one E. coli ST405 and belongs to phylogroup D obtained from a urine sample [28]. Curiously, the plasmid pEC8020 was obtained from an ESBL-producing E. coli ST405 and belongs to phylogroup D isolated from blood culture. We hypothesize a possible recombination between an E. coli ST405 with a chromosomal or plasmidic Tn6242 and IncFII type plasmid harboring the conserved qepA gene flanked by IS26. The mechanism of how this novel structure acquires fragments of plasmid like pHPA and the recombination with Tn6242 will require deeper study in the future.
The novel genetic context previously described flanked by the IS26 was identified in the twenty-three isolates by PCR and sequencing analysis. However, the Tn3, blaCTX-M-15, and IS1 were absent in the isolates with blaSHV and blaTEM genes.
The recruitment of multiple resistance mechanisms bordered by diverse insertion sequences was evident in this structure (IS26, Int1, dfr2, qepA, ISCR3, intI1-groEL, drfB4, qacED1-sul1, chrA, IS6100, mphR, mrx, mphA, IS26, Tn3, blaCTX-M-15, and IS1) (Fig. 1b).
In the case of chrA, the heterologous expression in a plasmid from Shewanella sp. conferred increased chromate resistance in E. coli and Pseudomonas aeruginosa [32]. Caballero et al. suggested that the use of metal derivatives as antiseptics in hospitals is an important factor in the selection of bacteria that acquire genes to confer and spread metal resistance among bacteria in hospitals [33].
The previously identified qepA and rmtB genes flanked by the transposable element IS26 in transferable plasmids belonging to incompatibility group IncFII, such as pHPA, suggested the efficient dissemination of these genetic structures [11, 34]. In this work, the description of a novel genetic structure of the qepA gene in plasmid pEC8020, which belongs to the IncFII type, may be an efficient means for the dissemination of resistance genes and the constant spread among Enterobacterales.
Our research provides a characterization of the novel genetic context harboring the PMQR qepA gene. However, to fully understand the successful widespread mechanism of plasmids carrying this genetic structure, further investigation will be necessary in the future. Our observations about the genetic relatedness among different E. coli strains, as revealed by phylogroups, RAPD, and ST analysis, suggest that these correlations are mainly associated with the clonal lineage of the E. coli strains themselves, rather than being attributed to the presence of specific plasmids carrying the qepA as an independent mechanism of widespread dissemination.
The presence of PMQR determinants, such as qepA, in bacteria confers reduced susceptibility to quinolones or fluoroquinolones. Even though these determinants may not exceed the breakpoints for quinolone resistance on their own, they may play an accessory role in contributing to higher resistance when combined with other chromosomally encoded mechanisms. The presence of qepA gene would increase fluoroquinolone resistance and improves bacterial fitness. The PMQR determinants are transferrable by plasmid, making their spread faster than chromosomal mutations, contributing to the interspecies dissemination of resistance globally. Overall, understanding the mechanisms and spread of PMQR is essential for effectively combating antimicrobial resistance. The emergence of multidrug-resistant Gram-negative bacteria that harbor and spread plasmids with qepA, chrA, and blaCTX-M-15 genes could become a serious clinical concern for all public healthcare systems.
Conclusion
This study has demonstrated the presence of ESBL-producing E. coli isolates carrying the qepA gene, which confers resistance to fluoroquinolones, in Mexican hospitals. The isolates exhibited drug resistance phenotypes and belong to the main Clonal Complex ST10, ST46, and ST205. The genetic context of the qepA gene was identified in a novel arrangement flanked by IS26 sequences, highlighting the importance of horizontal gene transfer in the dissemination of resistance genes. Moreover, the co-transfer of qepA1 and chrA determinants alongside blaCTX-M-15 genes in mating experiments emphasizes the potential of these genetic structures to spread among Enterobacterales. The emergence of such multidrug-resistant Gram-negative bacteria poses a significant clinical concern for public healthcare systems. Further studies are required to understand the recombination mechanisms and the role of insertion sequences in the dissemination of resistance genes. Increased surveillance and the development of novel therapeutic strategies are crucial to combat the growing threat of multidrug-resistant bacterial infections.
Supplementary information
(PNG 32 kb)
Acknowledgements
Bacterial Resistance Consortium: Carpermor, Laboratorio de Referencia Internacional, Cd de México. Felipe J. Uribe-Salas; ISTE-S, ISSSTESON, Hermosillo, Sonora. M.C. Moisés Navarro; Instituto Nacional de Cancerología (INCan), Cd de México. Dra. Patricia Cornejo-Juárez; Sanatorio Durango (SD-DF), Cd de México. Dr. Octavio Novoa-Farías; Hospital de Pediatría, Centro Médico Nacional Siglo XXI; Ciudad de México. Dr. Fortino Solórzano; Hospital Regional de Alta Especialidad, Oaxaca, Q. Sofía Cruz
Author contribution
Conceptualization: HBC, JSS, UGR; methodology: HBC, JDB, LL, FRF, ASP; writing—original draft preparation: HBC, JDB; writing—review and editing: HBC, JDB, UGR; funding acquisition: JSS; supervision: HBC.
Funding
This work was supported by grant 256988 from SEP-CONACyT (Secretaría de Educación Pública, Consejo Nacional de Ciencia y Tecnología).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Responsible Editor: Beatriz Ernestina Cabilio Guth
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Josefina Duran-Bedolla Ulises and Garza-Ramos contributed equally to this work.
Contributor Information
Humberto Barrios-Camacho, Email: humberto.barrios@insp.mx.
Bacterial Resistance Consortium:
Felipe J. Uribe-Salas, Moisés Navarro, Patricia Cornejo-Juárez, Octavio Novoa-Farías, Fortino Solórzano, and Sofía Cruz
References
- 1.Pokharel S, Raut S, Adhikari B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob Health. 2019;4(6):e002104. doi: 10.1136/bmjgh-2019-002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vieira DC, Lima WG, de Paiva MC. Plasmid-mediated quinolone resistance (PMQR) among Enterobacteriales in Latin America: a systematic review. Mol Biol Rep. 2020;47(2):1471–1483. doi: 10.1007/s11033-019-05220-9. [DOI] [PubMed] [Google Scholar]
- 3.Larramendy S, Deglaire V, Dusollier P, Fournier JP, Caillon J, Beaudeau F, Moret L. Risk factors of extended-spectrum beta-lactamases-producing Escherichia coli community acquired urinary tract infections: a systematic review. Infect Drug Resist. 2020;3(13):3945–3955. doi: 10.2147/IDR.S269033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tandogdu Z, Wagenlehner FM. Global epidemiology of urinary tract infections. Curr Opin Infect Dis. 2016;29(1):73–79. doi: 10.1097/QCO.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 5.Daneman N, Chateau D, Dahl M, Zhang J, Fisher A, Sketris IS, Quail J, Marra F, Ernst P, Bugden S. Canadian Network for Observational Drug Effect Studies (CNODES) Investigators. Fluoroquinolone use for uncomplicated urinary tract infections in women: a retrospective cohort study. Clin Microbiol Infect. 2020;26(5):613–618. doi: 10.1016/j.cmi.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Bader MS, Hawboldt J, Brooks A. Management of complicated urinary tract infections in the era of antimicrobial resistance. Postgrad Med. 2010;122(6):7–15. doi: 10.3810/pgm.2010.11.2217. [DOI] [PubMed] [Google Scholar]
- 7.Kawamura K, Nagano N, Suzuki M, Wachino JI, Kimura K, Arakawa Y. ESBL-producing Escherichia coli and its rapid rise among healthy people. Food Saf (Tokyo) 2017;5(4):122–150. doi: 10.14252/foodsafetyfscj.2017011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rincón G, Radice M, Giovanakis M, Di Conza JA, Gutkind G. First report of plasmid-mediated fluoroquinolone efflux pump QepA in Escherichia coli clinical isolate ST68 in South America. Diagn Microbiol Infect Dis. 2014;79(1):70–72. doi: 10.1016/j.diagmicrobio.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Hooper DC, Jacoby GA. Mechanisms of drug resistance: quinolone resistance. Ann N Y Acad Sci. 2015;1354(1):12–31. doi: 10.1111/nyas.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poirel L, Cattoir V, Nordmann P. Is plasmid-mediated quinolone resistance a clinically significant problem? Clin Microbiol Infect. 2008;14(4):295–297. doi: 10.1111/j.1469-0691.2007.01930.x. [DOI] [PubMed] [Google Scholar]
- 11.Yamane K, Wachino J, Suzuki S, Kimura K, Shibata N, Kato H, Shibayama K, Konda T, Arakawa Y. New plasmid-mediated fluoroquinolone efflux pump, QepA, found in an Escherichia coli clinical isolate. Antimicrob Agents Chemother. 2007;51(9):3354–3360. doi: 10.1128/AAC.00339-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Périchon B, Bogaerts P, Lambert T, Frangeul L, Courvalin P, Galimand M. Sequence of conjugative plasmid pIP1206 mediating resistance to aminoglycosides by 16S rRNA methylation and to hydrophilic fluoroquinolones by efflux. Antimicrob Agents Chemother. 2008;52(7):2581–2592. doi: 10.1128/AAC.01540-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cattoir V, Poirel L, Nordmann P. Plasmid-mediated quinolone resistance pump QepA2 in an Escherichia coli isolate from France. Antimicrob Agents Chemother. 2008;52(10):3801–3804. doi: 10.1128/AAC.00638-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. Clinical and Laboratory Standards Institute; Wayne, PA: 2017. Performance standard for antimicrobial susceptibility testing. CLSI supplement M100.
- 15.Silva-Sanchez J, Barrios H, Reyna-Flores F, Bello-Diaz M, Sanchez-Perez A, Rojas T, Bacterial Resistance Consortium. Garza-Ramos U. Prevalence and characterization of plasmid-mediated quinolone resistance genes in extended-spectrum β-lactamase-producing Enterobacteriaceae isolates in Mexico. Microb Drug Resist. 2011;17(4):497–505. doi: 10.1089/mdr.2011.0086. [DOI] [PubMed] [Google Scholar]
- 16.Cattoir V, Poirel L, Rotimi V, Soussy CJ, Nordmann P. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J Antimicrob Chemother. 2007;60(2):394–397. doi: 10.1093/jac/dkm204. [DOI] [PubMed] [Google Scholar]
- 17.Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000;66:4555–4558. doi: 10.1128/AEM.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Betancor L, Schelotto F, Martinez A, Pereira M, Algorta G, Rodríguez MA, Vignoli R, Chabalgoity JA. Random amplified polymorphic DNA and phenotyping analysis of Salmonella enterica serovar enteritidis isolates collected from humans and poultry in Uruguay from 1995 to 2002. J Clin Microbiol. 2004;42(3):1155–1162. doi: 10.1128/JCM.42.3.1155-1162.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol. 2006;60(5):1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller JH. Experiments in molecular genetics. (1972) Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory. xvi, 466 p. p
- 21.Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- 22.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods. 2005;63(3):219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, Møller Aarestrup F, Hasman H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58(7):3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elshamy AA, Aboshanab KM, Yassien MA, Hassouna NA. Prevalence of plasmid-mediated resistance genes among multidrug-resistant uropathogens in Egypt. Afr Health Sci. 2020;20(1):190–198. doi: 10.4314/ahs.v20i1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Lopez A, Sundararaju S, Al-Mana H, Tsui KM, Hasan MR, Suleiman M, Janahi M, Al Maslamani E, Tang P. Molecular characterization of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae among the pediatric population in Qatar. Front Microbiol. 2020;11:581711. doi: 10.3389/fmicb.2020.581711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azargun R, Sadeghi MR, Soroush Barhaghi MH, Samadi Kafil H, Yeganeh F, Ahangar Oskouee M, Ghotaslou R. The prevalence of plasmid-mediated quinolone resistance and ESBL-production in Enterobacteriaceae isolated from urinary tract infections. Infect Drug Resist. 2018;23(11):1007–1014. doi: 10.2147/IDR.S160720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu Y, Zhang W, Wang H, Zhao S, Chen Y, Meng F, Zhang Y, Xu H, Chen X, Zhang F. Specific patterns of gyrA mutations determine the resistance difference to ciprofloxacin and levofloxacin in Klebsiella pneumoniae and Escherichia coli. BMC Infect Dis. 2013;7(13):8. doi: 10.1186/1471-2334-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy Chowdhury P, McKinnon J, Liu M, Djordjevic SP. Multidrug resistant uropathogenic Escherichia coli ST405 with a novel, composite IS26 transposon in a unique chromosomal location. Front Microbiol. 2019;8(9):3212. doi: 10.3389/fmicb.2018.03212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reyna-Flores F, Barrios H, Garza-Ramos U, Sánchez-Pérez A, Rojas-Moreno T, Uribe-Salas FJ, Fagundo-Sierra R, Silva-Sanchez J. Molecular epidemiology of Escherichia coli O25b-ST131 isolates causing community-acquired UTIs in Mexico. Diagn Microbiol Infect Dis. 2013;76(3):396–398. doi: 10.1016/j.diagmicrobio.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 30.Silva-Sánchez J, Cruz-Trujillo E, Barrios H, Reyna-Flores F, Sánchez-Pérez A, Bacterial Resistance Consortium. Garza-Ramos U. Characterization of plasmid-mediated quinolone resistance (PMQR) genes in extended-spectrum β-lactamase-producing Enterobacteriaceae pediatric clinical isolates in Mexico. PLoS One. 2013;8(10):e77968. doi: 10.1371/journal.pone.0077968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim ES, Jeong JY, Choi SH, Lee SO, Kim SH, Kim MN, Woo JH, Kim YS. Plasmid-mediated fluoroquinolone efflux pump gene, qepA, in Escherichia coli clinical isolates in Korea. Diagn Microbiol Infect Dis. 2009;65(3):335–338. doi: 10.1016/j.diagmicrobio.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Aguilar-Barajas E, Paluscio E, Cervantes C, Rensing C. Expression of chromate resistance genes from Shewanella sp. strain ANA-3 in Escherichia coli. FEMS Microbiol Lett. 2008;285(1):97–100. doi: 10.1111/j.1574-6968.2008.01220.x. [DOI] [PubMed] [Google Scholar]
- 33.Caballero-Flores GG, Acosta-Navarrete YM, Ramírez-Díaz MI, Silva-Sánchez J, Cervantes C. Chromate-resistance genes in plasmids from antibiotic-resistant nosocomial enterobacterial isolates. FEMS Microbiol Lett. 2012;327(2):148–154. doi: 10.1111/j.1574-6968.2011.02473.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, He L, Li Y, Zeng Z, Deng Y, Liu Y, Liu JH. Complete sequence of a F2:A-:B- plasmid pHN3A11 carrying rmtB and qepA, and its dissemination in China. Vet Microbiol. 2014;174(1-2):267–271. doi: 10.1016/j.vetmic.2014.08.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PNG 32 kb)