Abstract
Background
NIS793 is a human IgG2 monoclonal antibody that binds to transforming growth factor beta (TGF-β). This first-in-human study investigated NIS793 plus spartalizumab treatment in patients with advanced solid tumors.
Methods
Patients received NIS793 (0.3–1 mg/kg every 3 weeks (Q3W)) monotherapy; following evaluation of two dose levels, dose escalation continued with NIS793 plus spartalizumab (NIS793 0.3–30 mg/kg Q3W and spartalizumab 300 mg Q3W or NIS793 20–30 mg/kg every 2 weeks [Q2W] and spartalizumab 400 mg every 4 weeks (Q4W)). In dose expansion, patients with non-small cell lung cancer (NSCLC) resistant to prior anti-programmed death ligand 1 or patients with microsatellite stable colorectal cancer (MSS-CRC) were treated at the recommended dose for expansion (RDE).
Results
Sixty patients were treated in dose escalation, 11 with NIS793 monotherapy and 49 with NIS793 plus spartalizumab, and 60 patients were treated in dose expansion (MSS-CRC: n=40; NSCLC: n=20). No dose-limiting toxicities were observed. The RDE was established as NIS793 30 mg/kg (2100 mg) and spartalizumab 300 mg Q3W. Overall 54 (49.5%) patients experienced ≥1 treatment-related adverse event, most commonly rash (n=16; 13.3%), pruritus (n=10; 8.3%), and fatigue (n=9; 7.5%). Three partial responses were reported: one in renal cell carcinoma (NIS793 30 mg/kg Q2W plus spartalizumab 400 mg Q4W), and two in the MSS-CRC expansion cohort. Biomarker data showed evidence of target engagement through increased TGF-β/NIS793 complexes and depleted active TGF-β in peripheral blood. Gene expression analyses in tumor biopsies demonstrated decreased TGF-β target genes and signatures and increased immune signatures.
Conclusions
In patients with advanced solid tumors, proof of mechanism of NIS793 is supported by evidence of target engagement and TGF-β pathway inhibition.
Trial registration number
Keywords: Drug Therapy, Combination; Immunotherapy; Programmed Cell Death 1 Receptor; Therapies, Investigational; Non-Small Cell Lung Cancer
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Transforming growth factor beta (TGF-β) plays a critical role in the regulation of the tumor microenvironment (TME), being implicated in fibroblast activation, immune exclusion, and immune suppression. Given the immunomodulatory properties of TGF-β, the combination of TGF-β-blocking agents with checkpoint inhibitors has the potential to rescue immunological activity in the TME, leading to better immunological control of tumor.
NIS793 is a human, anti-TGF-β, IgG2 monoclonal antibody that binds to TGF-β1 and TGF-β2 with high affinity, and TGF-β3 with a lower affinity.
WHAT THIS STUDY ADDS
This trial is the first-in-human study of NIS793 in combination with spartalizumab (anti-programmed cell death protein 1 (anti-PD-1)) in patients with advanced solid tumors. In this study, we report the first biomarker data in paired tumor biopsies for TGF-β inhibition and provide insight into the safety and clinical implications of TGF-β blockade.
Results were consistent with preclinical data that support proof of mechanism for NIS793 in target engagement and TGF-β pathway inhibition and indicated that NIS793 was well tolerated in combination with spartalizumab, and no dose-limiting toxicities were observed during dose escalation.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These data support the safety and tractability of TGF-β-inhibition in combination with PD-1 blockade in the clinic. Preliminary biomarker data also support the immunomodulatory properties of NIS793, highlighting its use as a potential combinatorial agent in the context of immunotherapies. Further studies are warranted.
Introduction
Transforming growth factor beta (TGF-β) is a homeostatic cytokine, controlling fundamental aspects of cellular behavior such as proliferation, migration, adhesion, and differentiation.1 Three isoforms of TGF-β ligands are found in mammals (TGF-β1-3). TGF-β is secreted as an inactive molecule within the latent TGF-β complex. Tissue-specific signaling events are required to dislodge the ligand from the complex, releasing the active form. On activation, TGF-β ligands bind to their heteromeric serine/threonine kinase receptors to promote phosphorylation of the intracellular mediators Smad2 and Smad3 and propagate downstream signaling events.2 3
TGF-β was originally identified as a tumor suppressor, due to its strong cytostatic and apoptotic potential. Somatic mutations that disrupt the TGF-β pathway are often found in human cancers and have been shown to relieve cancer cells from the antiproliferative effects of TGF-β while promoting the pro-invasive and metastatic functions of TGF-β. In addition to its tumor cell-centric role, TGF-β has a critical role in the regulation of the tumor microenvironment, favoring angiogenesis, fibrosis, and immune evasion. In this context, the direct action of TGF-β on immune cells has been shown to antagonize cytotoxic lymphocytes and promote the recruitment of inhibitory immune cells that favor tumor growth and progression.4–7 Emerging evidence also points to TGF-β as a pivotal activator of cancer-associated fibroblasts (CAFs), inciting the progressive accumulation of collagen and extracellular matrix. This phenomenon, referred to as desmoplastic reaction, has profound effects on the structural and metabolic properties of tumors, and has been shown to contribute to immune exclusion and immune suppression even in the context of therapeutic intervention.3 8 Therefore, combining TGF-β inhibitors with checkpoint blockade therapies offers the opportunity to reduce the tissue barriers that limit immunotherapy efficacy, favoring an environment that is permissive of immune cell activation.3
NIS793 is a fully human, anti-TGF-β, immunoglobulin (Ig)G2 monoclonal antibody that binds TGF-β1 and TGF-β2 with high affinity, and TGF-β3 with lower affinity. NIS793 binds to the active forms of the TGF-β ligands without interfering with the secreted molecules in their latent forms. Notably, preclinical data generated with NIS793 demonstrated that the antibody in tumor-bearing mice is sufficient to disrupt CAF differentiation and the formation of a thick collagen network, while promoting T-cell infiltration and activity with sustained efficacy, in combination with programmed cell death protein-1 (PD-1) immunotherapy.9
Herein, we present the first-in-human (FIH), dose-escalation/dose-expansion study of NIS793 in combination with spartalizumab (anti-PD-1) in adult patients with advanced solid tumors. Our data demonstrate that NIS793 in combination with spartalizumab is well tolerated in patients. Furthermore, the extensive biomarker analyses demonstrate favorable pharmacokinetics (PK), target engagement, and microenvironmental changes supportive of proof of mechanism for NIS793. As the roles of TGF-β in cancer progression and response to therapeutic intervention continue to emerge, this study provides insight into the tractability, safety, and implications of TGF-β inhibition in patients, demonstrating proof of mechanism for NIS793.
Methods
Preclinical characterization of NIS793
Binding of NIS793 to recombinant TGF-β isoforms was assessed in vitro using surface plasmon resonance. The inhibitory activity of NIS793 was assessed using a SMAD reporter cell-based assay (CAGA-Luc assay), with another assay to monitor TGF-β-mediated interleukin 11 release from A549 cells. Further details are described in the online supplemental methods.
jitc-2023-007353supp001.pdf (470.6KB, pdf)
Study design
This is an FIH, open-label, multicenter, phase I/Ib study of NIS793 in combination with spartalizumab. The 0.3 mg/kg starting dose of NIS793 corresponded to 1/30 of the no-observed adverse effect level in animal models and at least 1/10 of the dose causing minimal pharmacological effects in the most sensitive species (rat). Once the safety of the first two dose levels of single-agent NIS793 was evaluated, dose escalation continued in the combination arm only. NIS793 was mainly explored in combination with spartalizumab on a treatment cycle of every 3 weeks (Q3W) in dose escalation. However, based on emerging data, alternative dosing regimens, such as NIS793 every 2 weeks (Q2W), were evaluated in combination with spartalizumab. In such cases, the recommended phase 2 dose of spartalizumab (400 mg every 4 weeks (Q4W)) was used to align with NIS793 dosing. Per protocol, patients treated with single-agent NIS793 were switched to NIS793 plus spartalizumab at the combination dose level that met the Escalation with Overdose Control (EWOC) principle based on dose-limiting toxicity (DLT) data, either on disease progression or after two cycles, whichever occurred earlier, unless unacceptable toxicity occurred. If a patient had no Grade ≥2 treatment-related adverse event (TRAE) and no clinical evidence of disease progression within the first two cycles or had clinical evidence of disease progression within the second cycle, but had no TRAE ≥Grade 2, the switch to a combination of NIS793 plus spartalizumab at the next cycle was considered. Safety data from dose escalation were used to establish the recommended dose for expansion (RDE) of the combination prior to the dose expansion part.
In dose expansion, patients with either non-small cell lung cancer (NSCLC), resistant to prior anti-PD-1/programmed death-ligand 1 (PD-L1) therapy or microsatellite-stable colorectal cancer (MSS-CRC) were recruited.
This study is registered in ClinicalTrials.gov. This trial was conducted in Austria, Canada, Germany, Hong Kong, Italy, Japan, Switzerland, Taiwan and the USA. The data cut-off date (last patient last visit (LPLV)) was on June 18, 2021.
Study objectives
The primary objectives of this study were to characterize the safety and tolerability of NIS793 with or without spartalizumab and to identify recommended doses for future studies of NIS793 in combination with spartalizumab. Secondary objectives were to characterize the preliminary antitumor activity and PK, and assess immunogenicity, of NIS793 with or without spartalizumab, as well as to evaluate modulation of tumor-infiltrating lymphocytes (by NIS793 with or without spartalizumab).
Patient eligibility
Eligible patients were aged ≥18 years with an Eastern Cooperative Oncology Group performance status (ECOG PS) of ≤2. Patients with advanced/metastatic solid tumors who had progressed despite standard therapy or were intolerant to standard therapy, or for whom no standard therapy existed, were eligible. In dose expansion, two groups were enrolled: patients with NSCLC with known ALK/EGFR mutations, resistant to anti-PD-1/PD-L1 therapy (defined as documented progressive disease (PD) occurring while on/or within 6 months after anti-PD-1 and/or anti-PD-L1 agent (single or combination) received as the last therapy prior to enrollment) and patients with MSS-CRC (not mismatch repair deficient, by local assay including PCR and/or immunohistochemistry (IHC)). Within dose escalation, patients could have measurable/non-measurable disease as per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST V.1.1); in dose expansion, patients had to have at least one measurable lesion. To be included, patients were required to be willing to provide tumor biopsies at baseline and during treatment. Key exclusion criteria were: patients with presence of symptomatic central nervous system (CNS) metastases or CNS metastases requiring local therapy (patients with treated brain metastases must have been neurologically stable and off steroids for at least 4 weeks before entering the study); impaired cardiac function or clinically significant cardiac disease or a history of severe hypersensitivity reactions to study treatment ingredients or monoclonal antibodies; history of drug-induced pneumonitis or current pneumonitis; current HIV infection; and active hepatitis B or hepatitis C infection. Other exclusion criteria included active, known, or suspected autoimmune disease; receipt of immunosuppressive medication (such as steroid therapy; ≥10 mg/day of prednisone or equivalent), prior radiotherapy, or major surgery within 2 weeks of the first day of study treatment (C1D1); and prior cytotoxic therapy or antineoplastic surgery within 3 weeks of C1D1.
Dosing regimens
For dose escalation, in the single-agent arm, NIS793 was administered at 0.3 or 1 mg/kg Q3W; in the combination arm, dosing was as follows: NIS793 0.3–30 mg/kg Q3W plus spartalizumab 100–300 mg Q3W, or NIS793 20–30 mg/kg Q2W plus spartalizumab 400 mg Q4W (online supplemental table 1). The RDE was declared based on all available clinical data (safety, PK, and pharmacodynamic) from the NIS793 plus spartalizumab combination arm. Patients who started on single-agent NIS793 were allowed to switch to combination therapy applying the EWOC principle. In dose expansion, patients were treated at the RDE of the combination. Treatment was administered until unacceptable toxicity, PD as per immune-related response criteria (irRC), death, loss to follow-up, or due to patient/physician decision. Treatment was discontinued if adverse events (AEs)/any other protocol deviation occurred that resulted in a significant risk to a patient’s safety, or due to pregnancy or death.
Safety assessments
Regular safety assessments were performed, based on physical examinations, vital signs, ECOG PS, laboratory parameters, and cardiac assessments. AEs, defined by the National Cancer Institute Common Terminology Criteria for Adverse Events V.4.03, were assessed at every visit.
Response assessments
Efficacy was evaluated by local investigator’s assessment per RECIST V.1.1 and irRC. Tumor assessments were performed every two cycles (ie, every 6–8 weeks) from Cycle 3 Day 1 up to Cycle 9 Day 1, then every three cycles afterwards (ie, every 9–12 weeks). Disease progression follow-up was conducted every 8 weeks for up to 40 weeks, then every 12 weeks until progression of disease per irRC, withdrawal of consent, or loss to follow-up.
Pharmacokinetic assessments
Blood samples for PK analyses were collected pre-dose and post-dose (PK profiles) in Cycles 1 and 3. The following PK parameters were determined for all treatment arms: maximum plasma concentration (Cmax); time to peak drug concentration; area under the curve from the time of dosing to the last measurable concentration (AUClast); area under the plasma concentration-time curve extrapolated to infinity; area under the plasma concentration-time curve from time zero to the end of the dosing interval; half-life; and the accumulation of NIS793 and spartalizumab.
Pharmacodynamic analyses
An archival tumor sample or a newly obtained, pretreatment tumor biopsy was collected at screening. On-treatment biopsies were collected between Days 2 and 4 of Cycle 3, and blood and plasma samples were obtained pre-dose and post-dose of Cycles 1 and 3. Active TGF-β was detected in the serum pre-dose and post-dose of NIS793 to assess target engagement using the Simoa platform from Quanterix (run at Myriad RBM), with NIS793 binding to the ligand known to block its detection in this assay. Total TGF-β (bound to NIS793) was measured using a validated ELISA. RNA sequencing and assessment of TGF-β pathway and immune activation gene signatures were performed.
RNA sequencing was performed on RNA extracted from formalin-fixed, paraffin-embedded tumor biopsies from patients, as previously described.10 Gene expression data were normalized using the trimmed mean of M-value normalization, as implemented in the edgeR R/Bioconductor package V.3.20.9. Gene expression profiling of biopsy tumors at screening was used to identify the consensus molecular subtype (CMS) of patients with colorectal cancer (CRC).10 IHC was performed on biopsies, looking at cluster of differentiation 8 (CD8) and PD-L1, to assess baseline levels and changes following treatment. Published and in-house gene sets were used (online supplemental table 2) to investigate pathway expression levels at baseline across patients (Z-scaled counts per million) and for paired tumor biopsies, comparing post-treatment biopsies versus screening (log2 fold change in expression as compared with baseline) for treatment-specific modulation. All calculations and plots were generated in R using the ComplexHeatmap package. Activity of TGF-β in the biopsies was assessed by looking at gene expression signatures. The EMT,11 TGF-β (Genentech),12 and IFNγ10 gene signatures are described. The IFNγ hallmark gene signature is described in the Gene Set Enrichment Analysis.13
In vitro treatment of tumor samples with bulk RNA sequencing and derivation of Novartis TGF-ß gene signature
Dissociated, viable tumor tissues from six patients with clear cell renal cell carcinomas were cultured overnight with TGF-β-blocking antibodies, including NIS793; PD-1-blocking antibody (spartalizumab); or TGF-β ligand. Bulk RNA was isolated and sequenced. Genes modulated by PD-1 blockade were excluded from further analysis. Public databases were used to assess expression of NIS793-responsive genes in tumor and normal tissues. The eight genes in the Novartis TGF-β gene signature are: FOXS1, SOX4, PMEPA1, HEYL, FAP, ALOX5AP, COL1A1, and TBC1D2B (online supplemental table 2). Further details are provided in the online supplemental methods.
Statistical methods
Assuming a true AE incidence rate ≥5% and a probability of detecting an AE greater than 0.99, this study planned to enroll approximately 160 patients. In the dose-escalation part, cohorts of 1–6 or 3–6 patients were enrolled to receive NIS793 or NIS793 plus spartalizumab, respectively. For the dose-expansion part, 20–40 patients were planned in each indication to allow for a robust assessment of the overall response rate. The full analysis set, and safety set included all patients who received ≥1 dose of NIS793 or ≥1 full/partial dose of NIS793 plus spartalizumab and patients were analyzed according to the treatment most frequently taken in cycle 1 for NIS793 and cycles 1 and 2 for NIS793 plus spartalizumab. Dose-escalation was guided by a Bayesian logistic regression model (BLRM), applying the EWOC principle. The DLT relationship for NIS793 single agent was described by a two-parameter BLRM, whereas the relationship for NIS793 plus spartalizumab with a dosing regimen of Q3W was described by a five-parameter model. When the dosing regimen changed to Q2W, a Bayesian hierarchical logistic regression model was used to estimate the dose–DLT relationship, to incorporate information from both dosing regimens. Further specifications of the model priors and how dose-escalation decisions were determined are given in the CNIS793×2101 Clinical Study Report. Additional information on AEs, efficacy and PK parameters are detailed in the additional online supplemental information section.
Results
NIS793 is a human monoclonal antibody that binds to TGF-β1 and TGF-β2 with high affinity, and to TGF-β3 with lower affinity (online supplemental figure 1A). NIS793 has shown significant activity in different in vitro cell-based assays (online supplemental figure 1B). In murine models of cancer, NIS793 remodeled the tumor microenvironment and enhanced the efficacy of PD-1 blockade, supporting the rationale for its combination with spartalizumab that was tested in this FIH trial.9
Patient characteristics
Between April 25, 2017, and June 18, 2021 (LPLV), 120 patients had been treated across both dose escalation and dose expansion at 12 sites, in 9 countries (online supplemental figure 2). Sixty patients were treated in escalation (11 with single-agent NIS793 and 49 with NIS793 plus spartalizumab) and 60 patients were treated in expansion (40 in the MSS-CRC group and 20 in the NSCLC group).
Patient demographics and baseline characteristics are shown in table 1 and were similar across patients treated with single-agent NIS793 and in combination with spartalizumab during dose escalation/expansion. Across all study parts, the median age was 62 years (range, 32–84) and most patients (98.3%) had an ECOG PS of 0 or 1. All patients had received prior antineoplastic therapy (table 1), with a median of three prior medications (range, 1–11). The most common primary cancer types were CRC (44.2%), NSCLC (18.3%), and pancreatic cancer (8.3%) while the most frequent sites of metastasis for all patients were the liver (51.7%) and lung (55.8%; table 1).
Table 1.
Baseline patient demographics, characteristics, prior therapies, and disease history
| All dose escalation patients | All dose expansion patients | All patients | |
| n=60 | n=60 | n=120 | |
| Median age, years (range) | 61.0 (32–84) | 62.0 (33–80) | 62.0 (32–84) |
| Sex (male), n (%) | 38 (63.3) | 37 (61.7) | 75 (62.5) |
| Race, n (%) | |||
| Caucasian | 33 (55.0) | 50 (83.3) | 83 (69.2) |
| Asian | 25 (41.7) | 10 (16.7) | 35 (29.2) |
| Black | 1 (1.7) | 0 | 1 (0.8) |
| Unknown | 1 (1.7) | 0 | 1 (0.8) |
| ECOG PS, n (%) | |||
| 0 | 21 (35.0) | 27 (45.0) | 48 (40.0) |
| 1 | 37 (61.7) | 33 (55.0) | 70 (58.3) |
| 2 | 2 (3.3) | 0 | 2 (1.7) |
| Prior therapy, n (%) | |||
| Any therapy | 60 (100) | 60 (100) | 120 (100.0) |
| Surgery | 51 (85.0) | 50 (83.3) | 101 (84.2) |
| Radiotherapy | 28 (46.7) | 31 (51.7) | 59 (49.2) |
| Immunotherapy | 26 (43.3) | 24 (40.0) | 50 (41.7) |
| Time from last therapy to start of study, months, median (range) | 3.7 (2.4–17.0) | 4.1 (0.8–24.1) | 4.9 (1.7–62.5) |
| Type of cancer, n (%) | |||
| Colorectal cancer | 13 (21.7) | 40 (66.7) | 53 (44.2) |
| Non-small cell lung cancer | 2 (3.3) | 20 (33.3) | 22 (18.3) |
| Adrenocortical carcinoma | 2 (3.3) | 0 | 2 (1.7) |
| Cholangiocarcinoma | 4 (6.7) | 0 | 4 (3.3) |
| Cutaneous melanoma | 2 (3.3) | 0 | 2 (1.7) |
| Endometrial cancer | 1 (1.7) | 0 | 1 (0.8) |
| Esophageal cancer | 4 (6.7) | 0 | 4 (3.3) |
| Gall bladder cancer | 1 (1.7) | 0 | 1 (0.8) |
| Gastrointestinal stromal | 1 (1.7) | 0 | 1 (0.8) |
| Head and neck cancer | 1 (1.7) | 0 | 1 (0.8) |
| Hepatocellular carcinoma | 3 (5.0) | 0 | 3 (2.5) |
| Melanoma | 3 (5.0) | 0 | 3 (2.5) |
| Pancreatic cancer | 10 (16.7) | 0 | 10 (8.3) |
| Prostate cancer | 1 (1.7) | 0 | 1 (0.8) |
| Site of metastasis, n (%) | |||
| Adrenal | 6 (10.0) | 5 (8.3) | 11 (9.2) |
| Bone | 12 (20.0) | 17 (28.3) | 29 (24.2) |
| Brain | 2 (3.3) | 6 (10.0) | 8 (6.7) |
| Liver | 32 (53.3) | 30 (50.0) | 62 (51.7) |
| Lung | 35 (58.3) | 32 (53.3) | 67 (55.8) |
| Other lymph node | 9 (15.0) | 8 (13.3) | 17 (14.2) |
| Peritoneum | 13 (21.7) | 7 (11.7) | 20 (16.7) |
| Mediastinum lymph nodes | 6 (10.0) | 6 (10.0) | 12 (10.0) |
| Paratracheal lymph nodes | 4 (6.7) | 5 (8.3) | 9 (7.5) |
| Pleural | 4 (6.7) | 5 (8.3) | 9 (7.5) |
Please note for sites of metastasis, only the 10 most common are reported in the above table.
ECOG PS, Eastern Cooperative Oncology Group performance status.
Patient disposition
Most patients (102 patients; 85.0%) discontinued study treatment due to PD; 7 (5.8%) discontinued due to AEs, 6 (5.0%) due to patient/guardian decision, and 5 (4.2%) due to death due to study indication. AEs that led to study drug discontinuation included Grade 2 unrelated tumor hemorrhage, Grade 3 related anemia, Grade 4 related hyperglycemia, Grade 1 related keratoacanthoma, Grade 2 related colitis, Grade 3 related diarrhea, Grade 1 related rash, and Grade 1 related pruritus.
Eight of the 11 (72.7%) patients treated with single-agent NIS793 in the dose escalation group discontinued treatment due to PD and three (27.3%) due to patient/guardian decision. Of the 11 patients treated with single-agent NIS793 in the dose escalation group, eight patients switched to NIS793 plus spartalizumab following disease progression or two cycles (whichever was earlier).
Safety and tolerability
Overall, 119/120 (99.2%) treated patients experienced an AE of any grade and 69 (57.5%) patients experienced an AE Grade ≥3, regardless of relationship to study treatment, as detailed in online supplemental table 3.
Of patients treated with single-agent NIS793 (n=11), 6 (54.5%) reported a TRAE (three in the 0.3 mg/kg cohort and three in the 1 mg/kg cohort) of which 1 (9.1%) was Grade ≥3 (decreased appetite; 1 mg/kg cohort).
Across patients treated with NIS793 plus spartalizumab in dose escalation, 21 (42.9%) patients reported a TRAE, with 3 (6.1%) being Grade ≥3 in severity (drug eruption, hepatic enzyme increase, and rash). TRAE profiles were comparable between dosing cohorts, with the majority being skin toxicity or gastrointestinal events. When comparing the NIS793 Q3W and Q2W regimens, there did not appear to be a higher frequency or grade of AEs; therefore, because the Q3W regimen allowed for more convenient combination with Q3W dosing of spartalizumab, the Q3W regimen was taken forward to dose expansion (table 2).
Table 2.
Treatment-related adverse events across dosing levels and regimens (any grade events in ≥5% of patients, all Grade ≥3 events)
| Preferred term, n (%) | Dose escalation | Dose expansion | All patients N=120 |
|||||||||||||||||||||||||
| All NIS793 single agent (0.3–1 mg/kg), n=11 |
NIS793 0.3 mg/kg Q3W+sparta 100 mg Q3W, n=5 |
NIS793 0.3 mg/kg Q3W+sparta 300 mg Q3W, n=5 |
NIS793 1 mg/kg Q3W+sparta 300 mg Q3W, n=5 |
NIS793 3 mg/kg Q3W+sparta 300 mg Q3W, n=5 |
NIS793 10 mg/kg Q3W+sparta 300 mg Q3W, n=5 |
RDE NIS793 30 mg/kg Q3W+sparta 300 mg Q3W, n=7 |
NIS793 20 mg/kg Q2W+sparta 400 mg Q4W, n=6 |
NIS793 30 mg/kg Q2W+sparta 400 mg Q4W, n=11 |
All combination dose escalation, n=49 |
MSS-CRC, NIS793 2100 mg+Sparta 300 mg Q3W, n=40 |
NSCLC, NIS793 2100 mg+Sparta 300 mg Q3W, n=20 |
All expansion, n=60 |
||||||||||||||||
| Any | G≥3 | Any | G≥3 | Any | G≥3 | Any | G≥3 | Any | G≥3 | Any | G≥3 | Any | G≥3 | Any | G≥3 | Any | G≥3 | Any | G≥3 | Any | G≥3 | Any | G≥3 | Any | G≥3 | Any | G≥3 | |
| Patients with≥1 TRAE | 6 (54.5) |
1 (9.1) |
2 (40.0) |
0 | 2 (40.0) |
1 (20.0) |
1 (20.0) |
0 | 3 (60.0) |
0 | 3 (60.0) |
0 | 4 (57.1) |
0 | 2 (33.3) |
1 (16.7) |
4 (36.4) |
1 (9.1) |
21 (42.9) |
3 (6.1) |
19 (47.5) |
4 (10.0) |
14 (70.0) |
6 (30.0) |
33 (55.0) |
10 (16.7) |
60 (50.0) |
14 (11.7) |
| Rash | 1 (9.1) |
0 | 1 (20.0) |
0 | 1 (20.0) |
0 | 0 | 0 | 1 (20.0) |
0 | 0 | 0 | 0 | 0 | 1 (16.7) |
0 | 1 (9.1) |
1 (9.1) |
5 (10.2) |
1 (2.0) |
4 (10.0) |
0 | 6 (30.0) |
3 (15.0) |
10 (16.7) | 3 (5.0) |
16 (13.3) |
4 (3.3) |
| Pruritus | 1 (9.1) | 0 | 0 | 0 | 1 (20.0) | 0 | 0 | 0 | 0 | 0 | 1 (20.0) | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (9.1) |
0 | 4 (8.2) |
0 | 2 (5.0) |
0 | 3 (15.0) |
1 (5.0) |
5 (8.3) | 1 (1.7) |
10 (8.3) |
1 (0.8) |
| Fatigue | 1 (9.1) | 0 | 0 | 0 | 1 (20.0) | 0 | 0 | 0 | 1 (20.0) | 0 | 1 (20.0) | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (9.1) |
0 | 5 (10.2) | 0 | 3 (7.5) |
0 | 0 | 0 | 3 (5.0) | 0 | 9 (7.5) | 0 |
| Nausea | 1 (9.1) | 0 | 1 (20.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (28.6) | 0 | 1 (16.7) | 0 | 0 | 0 | 4 (8.2) |
0 | 3 (7.5) |
0 | 0 | 0 | 3 (5.0) | 0 | 8 (6.7) | 0 |
| Decreased appetite | 2 (18.2) | 1 (9.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (14.3) | 0 | 1 (16.7) | 0 | 1 (9.1) |
0 | 3 (6.1) |
0 | 1 (2.5) |
0 | 0 | 0 | 1 (1.7) | 0 | 6 (5.0) | 1 (0.8) |
| Fever | 0 | 0 | 0 | 0 | 1 (20.0) | 0 | 0 | 0 | 1 (20.0) | 0 | 0 | 0 | 1 (14.3) | 0 | 0 | 0 | 2 (18.2) |
0 | 5 (10.2) | 0 | 1 (2.5) |
0 | 0 | 0 | 1 (1.7) | 0 | 6 (5.0) | 0 |
| Maculopapular rash | 2 (18.2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (20.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.0) |
0 | 2 (5.0) |
0 | 1 (5.0) |
0 | 3 (5.0) | 0 | 6 (5.0) | 0 |
| Lipase increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (5.0) |
1 (2.5) |
1 (5.0) |
0 | 3 (5.0) |
1 (1.7) |
3 (2.5) |
1 (0.8) |
| Adrenal insufficiency | 0 | 0 | 0 | 0 | 1 (20.0) |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.0) |
0 | 0 | 0 | 1 (5.0) |
1 (5.0) |
1 (1.7) |
1 (1.7) |
2 (1.7) |
1 (0.8) |
| Amylase increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (5.0) |
1 (2.5) |
0 | 0 | 2 (3.3) |
1 (1.7) |
2 (1.7) |
1 (0.8) |
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.5) |
0 | 1 (5.0) |
1 (5.0) |
2 (3.3) |
1 (1.7) |
2 (1.7) |
1 (0.8) |
| Hematuria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.5) |
0 | 1 (5.0) |
1 (5.0) |
2 (3.3) |
1 (1.7) |
2 (1.7) |
1 (0.8) |
| Hyponatremia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (5.0) |
2 (5.0) |
0 | 0 | 2 (3.3) |
2 (3.3) |
2 (1.7) |
2 (1.7) |
| Drug eruption | 0 | 0 | 0 | 0 | 1 (20.0) |
1 (20.0) |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.0) |
1 (2.0) |
0 | 0 | 0 | 0 | 0 | 0 | 1 (0.8) |
1 (0.8) |
| Hepatic enzyme increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (16.7) |
1 (16.7) |
0 | 0 | 1 (2.0) |
1 (2.0) |
0 | 0 | 0 | 0 | 0 | 0 | 1 (0.8) |
1 (0.8) |
| Hyperglycemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.5) |
1 (2.5) |
0 | 0 | 1 (1.7) |
1 (1.7) |
1 (0.8) | 1 (0.8) |
G, Grade; MSS-CRC, microsatellite stable colorectal cancer; NSCLC, non-small cell lung cancer; Q2W, every 2 weeks; Q3W, every 3 weeks; Q4W, every 4 weeks; RDE, recommended dose for expansion; sparta, spartalizumab; TRAE, treatment-related adverse event.
Within the dose expansion cohorts, 19 (47.5%) patients with MSS-CRC and 14 (70.0%) patients with NSCLC, treated at the RDE, reported a TRAE of any grade; Grade≥3 TRAE were reported in 4 (10.0%) and 6 (30.0%) patients, respectively (table 2).
TRAEs in all patients treated with NIS793 plus spartalizumab across both dose escalation and dose expansion are shown in online supplemental figure 3. Combining spartalizumab with NIS793 did not appear to increase the frequency or severity of the AEs reported as compared with spartalizumab monotherapy.14 There was one Grade 4 TRAE in dose expansion (hyperglycemia in a patient with MSS-CRC): this case was further assessed, and it was concluded that there were no confounding factors affecting it.
Serious AEs (SAEs), regardless of causality, were observed with NIS793 plus spartalizumab in 22 (44.9%) patients in dose escalation and in 27 (45.0%) patients in dose expansion. In the dose-escalation combination cohorts, SAEs suspected to be related to study treatment were reported in three patients who experienced rash (n=1; NIS793 30 mg/kg Q2W plus spartalizumab 400 mg Q4W); drug eruption (n=1; NIS793 0.3 mg/kg Q3W plus spartalizumab 300 mg Q3W); and increased hepatic enzyme (n=1; NIS793 20 mg/kg Q2W plus spartalizumab 400 mg Q4W). In dose expansion, six patients experienced an SAE suspected to be treatment related, which included rash (n=3), colitis, diarrhea, hematuria, and hyperglycemia (n=1 each). One patient reported both colitis and diarrhea.
No DLTs were reported, supporting that NIS793 was well tolerated at the doses explored in this FIH study. Dose escalation was guided by an adaptive BLRM following the EWOC principle, to ensure that the RDE did not exceed the maximum tolerated dose (MTD). To identify the MTD/RDE, at least six patients had to be treated at this dose or combination for the corresponding regimen, and a minimum of 18 patients had to be treated with the combination of NIS793 plus spartalizumab; however, determination of the RDE could be made with fewer patients prior to the identification of the MTD. Due to the absence of observed DLTs, the MTD was not reached. The RDE for NIS793 plus spartalizumab was declared as NIS793 30 mg/kg Q3W in combination with spartalizumab 300 mg Q3W, which translates to NIS793 2100 mg Q3W and spartalizumab 300 mg Q3W as fixed flat doses.
Preliminary efficacy
The best overall response (BOR) across all evaluable patients (N=119) was three patients with partial response (PR) (2.5%), 29 (24.2%) patients with stable disease (SD) (included one patient with unconfirmed PR in dose escalation), 67 (55.8%) patients with PD, and 21 (17.5%) patients with unknown responses (figure 1, online supplemental table 4). Of the patients with unknown responses (n=21), 4 started new antineoplastic therapy before the first post-baseline assessment, 14 had no valid post-baseline assessment (mainly due to early progression), 2 patients had SD too early, and 1 had PD too late for evaluation.
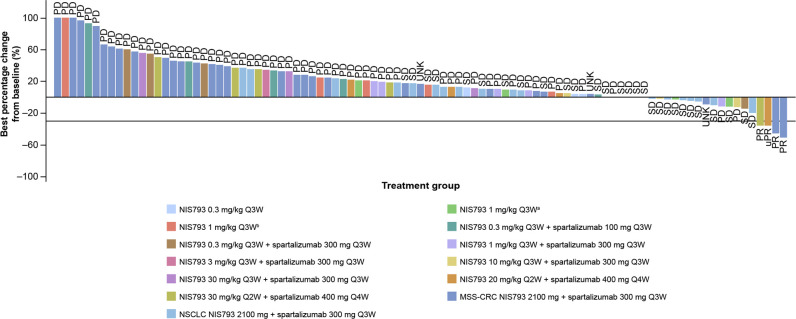
Figure 1.
Waterfall plot for best overall response for dose expansion (n=119) (investigator assessed). A change in formulation (NIS793 originally provided as aliquid for infusion and subsequently as blyophilisate for infusion), which occurred at the first dose level of the study, and proven to be safe, with no difference in the formulation. During dose-escalation, one patient with non-measurable disease as per RECIST V.1.1 was recruited (per protocol). MSS-CRC, microsatellite stable colorectal cancer; NSCLC, non-small cell lung cancer; PD, progressive disease; PR, partial response; Q3W, every 3 weeks; Q4W, every 4 weeks; RECIST V.1.1, Response Evaluation Criteria in Solid Tumors version 1.1; SD, stable disease; UNK, unknown; uPR; unconfirmed partial response.
BORs of patients in dose escalation (n=60) were: PR (n=1; 1.7%); SD (n=14; 23.3%); the disease control rate (DCR) was 25.0% (95% CI, 14.7 to 37.9). Across all patients in dose escalation of NIS793 plus spartalizumab (n=49), BORs were: PR (n=1; 2.0%); SD (n=8; 16.3%). The DCR was 18.4% (95% CI, 8.8 to 32.0). Within dose escalation, the duration of response (DOR) for the patient with a confirmed PR was 113 days. In the dose expansion, BORs for patients with MSS-CRC (n=40) were: PR (n=2; 5.0%); SD (n=3; 7.5%). The DCR was 12.5% (95% CI, 4.2 to 26.8). For the two patients with confirmed PRs, DORs were 111 days and 195 days. For patients with NSCLC (n=20) in dose expansion, BORs were: SD (n=12; 60.0%); there were no cases of PR. The DCR was 60.0% (95% CI, 36.1 to 80.9).
Regardless of whether patients received single-agent (dose escalation) or combination treatment (dose escalation and dose expansion), the median progression free survival (PFS) was 1.41 months. For dose escalation, the NIS793 plus spartalizumab combination arm median PFS was 1.41 months (95% CI, 1.25 to 1.81). For dose expansion, median PFS in the MSS-CRC and NSCLC groups were 1.38 months (95% CI, 1.22 to 1.41) and 2.40 months (95% CI, 1.28 to 4.63), respectively. Kaplan-Meier plots by disease group are presented in online supplemental figure 4.
Pharmacokinetics
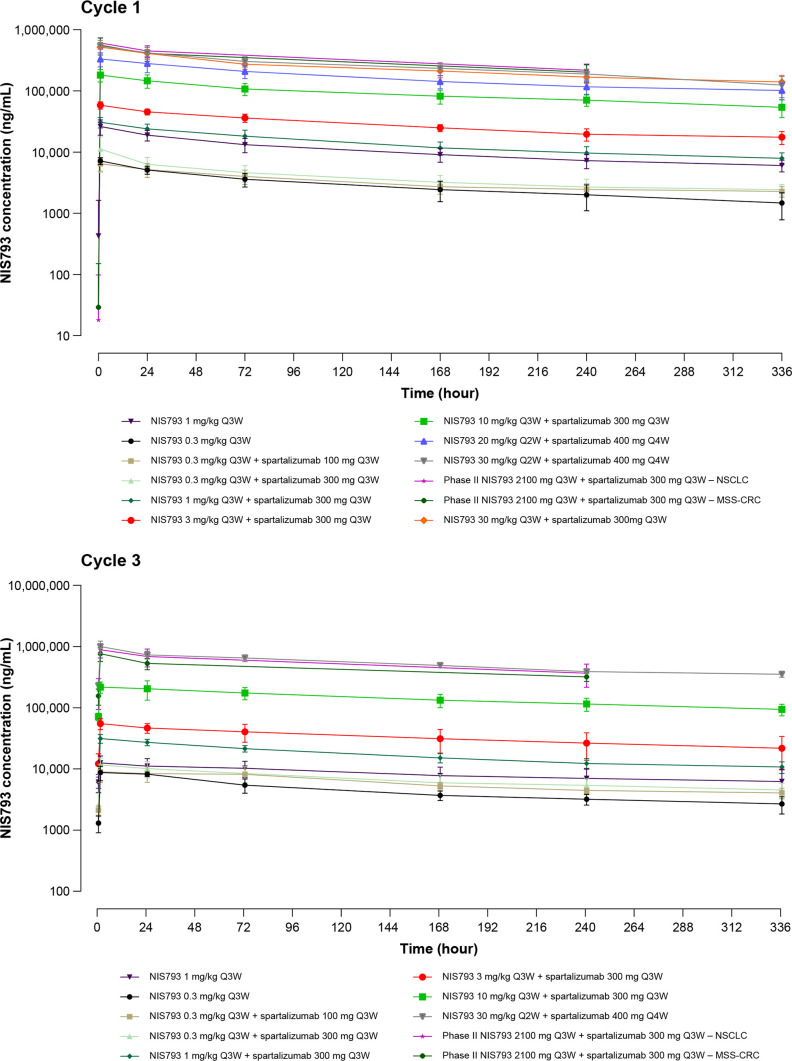
Exposure to NIS793 (AUClast and Cmax) was approximately dose proportional across doses administered in single-agent and combination arms (figure 2). Exposure was comparable when comparing Cycles 1 and 3, indicating no substantial accumulation. This was confirmed with the accumulation ratio ranging from 0.48 to 1.78. The Cmax and AUClast of NIS793 in combination with spartalizumab at 100, 300, or 400 mg doses were comparable to those of single-agent NIS793, indicating a lack of drug–drug interaction with spartalizumab. Based on PK modeling, the predicted exposure and trough concentration at steady state between weight-based and fixed dosing regimens were comparable across different body weight categories. This analysis supports the use of flat dosing on a milligram basis irrespective of patient body weight, as weight-based dosing did not decrease inter-individual variability. Model-based simulations indicated that an RDE for NIS793 of 2100 mg would match exposure observed at 30 mg/kg (data not shown). Furthermore, within the single-agent NIS793 and NIS793 plus spartalizumab cohorts, elimination was comparable, indicating the absence of target-mediated drug disposition.
Figure 2.
Geometric mean and arithmetic mean (SD) of concentration-time profiles of NIS793 by treatment and cycle. MSS-CRC, microsatellite-stable colorectal cancer; NSCLC, non-small cell lung cancer; Q2W, every 2 weeks; Q3W, every 3 weeks; Q4W, every 4 weeks.
In addition, when given in combination with NIS793, exposure to spartalizumab was dose proportional and comparable between cycles (data not shown). These data were consistent with data for single-agent spartalizumab,14 15 indicating a lack of drug–drug interaction of NIS793 on the PK of spartalizumab.
Biomarker analyses
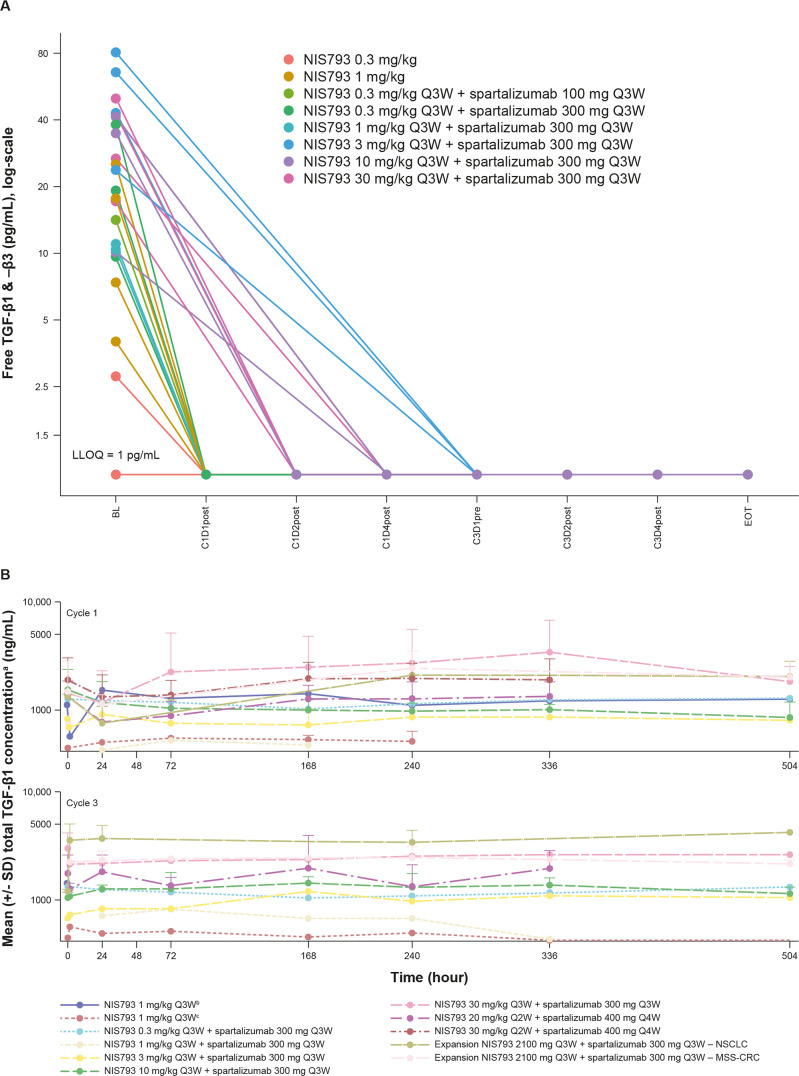
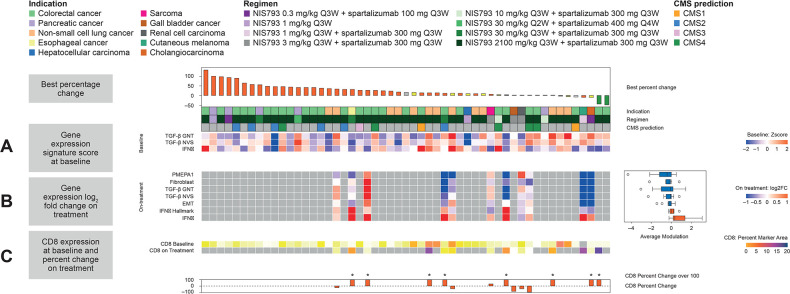
As free TGF-β (unbound to latent complex) is the bioactive molecule in TGF-β signaling, an assay was developed to measure active TGF-β1 in peripheral tissues. Active TGF-β1 was found to be depleted from serum at all doses≥0.3 mg/kg (Q3W; figure 3A). Across Cycles 1 and 3, increases in total TGF-β1 (free, active ligand bound to NIS793) were observed in serum at doses ≥20 mg/kg (Q2W and Q3W; figure 3B), consistent with the fact that binding of NIS793 to the ligand may increase its stability and reduce clearance from circulation. TGF-β pathway activity was assessed using several gene signatures that encompass a disparate set of TGF-β target genes. A decrease was observed in the expression of TGF-β response gene signatures in 6/11 paired samples, alongside activation of interferon gamma transcriptional program, suggesting induction of an active immune response (figure 4). At baseline, COL1A1, SOX4, PMEPA1 and TBC1D2B had the highest level of expression in the Novartis TGF-β gene signature. The greatest change with NIS793 treatment was seen in PMEPA1. Looking at the other gene signatures, genes with the greatest post-treatment depletion were COL1A1, FAP and HEYL (by decreasing order). Moreover, recent publications identified TGF-β activity as leading to immune suppression and T-cell exclusion from human bladder cancer and in animal models.3 8 An increase in CD8+T cells by IHC was apparent in the majority of paired biopsy samples (figure 4C). Although the sample size for on-treatment biopsies was small, the combined tumor and peripheral marker data support the proof of mechanism that NIS793 may increase immune activation, with increased levels of pharmacodynamic markers such as CD8 observed across most tissue samples. Exploratory analysis of CD8 staining in biopsies did not reveal any correlation between response and CD8 staining.3 In patients with MSS-CRC, exploratory analysis of RNA sequencing data using the CMS descriptions16 showed high TGF-β pathway activity in the CMS4 subtype, but no correlation with clinical response to NIS793.
Figure 3.
Serum TGF-β concentration over time on study treatment. (A) Active TGF-β1 and 3 ligands were detected in all but one patient prior to initial dose of NIS793. Post-NIS793 dosing, all serum samples were below limit of quantification, demonstrating that free, active TGF-β was greatly reduced (B) Total TGF-β concentration over time on study treatment. aTGF-β assay measures isoform 1. A change in formulation (NIS793 originally provided as bliquid for infusion and subsequently as clyophilisate for infusion), which occurred at the first dose level of the study, and proven to be safe, with no difference in the formulation. The timing for collection was slightly different when referring to C3, depending on whether the dosing regimen was NIS793 Q2W or Q3W. BL, baseline; C, Cycle; D, Day; EOT, end of treatment; LLOQ, lower limit of quantification; MSS-CRC; microsatellite-stable colorectal cancer; NSCLC, non-small cell lunger cancer; Q2W, every 2 weeks; Q3W, every 3 weeks; Q4W, every 4 weeks; TGF-β, transforming growth factor beta.
Figure 4.
NIS793 proof of mechanism in the tumor. (A) Levels of TGF-β pathway and immune activation at baseline were assessed using two gene expression signatures for TGF-β (one from Genentech and one from Novartis) and an IFN-γ gene signature, respectively, derived from bulk RNA sequencing, (B) on-treatment modulation of gene signatures versus baseline for paired tumor biopsies from 11 patients is shown using five readouts for TGF-β activity (PMEPA1 gene expression and 4 gene signatures capturing fibroblast, TGF-β and EMT activity), as well as two gene signatures for IFN-γ activity, derived from bulk RNA sequencing. The average log2 fold change of those gene signatures across all paired samples in the box plot on the right, and (C) CD8 detection derived from immunohistochemistry per cent marker area at baseline and on treatment is shown in the heat map with percent change shown as a bar graph. CD8, cluster of differentiation 8; CMS, consensus molecular subtypes; EMT, epithelial-to-mesenchymal transition; FC, fold change; GNT, Genentech; IFNγ, interferon gamma; NVS, Novartis; PMEPA1, prostate transmembrane protein, androgen induced 1; Q2W, every 2 weeks; Q3W, every 3 weeks, Q4W, every 4 weeks; TGF-β, transforming growth factor beta.
Discussion
This FIH, open-label, multicenter study consisted of dose escalation of single-agent NIS793, and NIS793 in combination with spartalizumab, followed by dose expansion of NIS793 in combination with spartalizumab in patients with NSCLC resistant to prior anti-PD-1/anti-PD-L1 therapy, or patients with MSS-CRC, a disease with known resistance to PD-1 blockade. Results from dose escalation showed that NIS793 is well tolerated in combination with spartalizumab in patients with advanced solid tumors. The safety profile was acceptable and no DLTs were reported in dose escalation. No difference was observed in the safety profile between the two regimens, Q2W and Q3W, across dose levels. Therefore, due to convenience, the Q3W regimen was moved forward into the dose expansion part. NIS793 dosage was found to have no effect on the safety profile, and overall, the nature, frequency, and grade of AEs showed no differences to those reported in patients receiving spartalizumab monotherapy.14 15
Within this heavily pretreated patient population already exposed to anti-PD-L1 therapy, PRs were achieved in three patients (2.5%): one patient in the NIS793 plus spartalizumab dose-escalation and two patients in the MSS-CRC dose-expansion arm. One unconfirmed PR in the dose-escalation part was included in the SD count. SD was achieved for 29 patients (24.2%), 8 patients in the NIS793 plus spartalizumab escalation, 12 in NSCLC expansion and three in the MSS-CRC expansion part. Encouraging DORs were observed in patients who reported PR; DOR was 113 days (one patient in NIS793 plus spartalizumab dose escalation), and 111 days and 195 days (two patients in the MSS-CRC group). Similar responses were observed in patients receiving spartalizumab monotherapy.14 In comparison, bintrafusp alfa, a bifunctional fusion protein composed of the extracellular domain of TGF-β receptor II fused to a human IgG1 anti-PD-L1 monoclonal antibody (a “TGF-β trap”), has reported no responses (overall response rate; 0%) in microsatellite instability-high tumors in patients who previously progressed on checkpoint inhibitor therapy.17 The design and mechanism of action of NIS793 is considerably different, which may provide explanation into the different response rate versus bintrafusp alfa. NIS793 antagonizes TGF-β1 and TGF-β2 with high affinity, and TGF-β3 with lower affinity, whereas bintrafusp alfa neutralizes all isoforms of TGF-β. Bintrafusp alfa blocks PD-L1, which also differs from this trial, where we blocked PD-1 directly. SAR439459, a human anti-TGF-β monoclonal antibody that has been reported to neutralize TGF-β, also showed limited activity in a phase I trial in advanced solid tumors.18 In terms of safety, similar AEs were reported to those frequently seen with bintrafusp alfa.19 20 Of note, skin-related AEs such as reversible cutaneous keratoacanthomas/squamous-cell carcinomas and hyperkeratosis, were most commonly reported with the pan–TGF-β neutralizing antibody fresolimumab but were not frequently reported in this study.
The PK of NIS793 demonstrated dose proportionality and was typical of a monoclonal antibody. As expected, no PK drug–drug interaction between NIS793 and spartalizumab was seen. Importantly, systemic target engagement and modulation of TGF-β activity in tumors was observed, supporting proof of mechanism. There was also a decrease in free, active TGF-β in circulation, with accumulation of TGF-β/NIS793 complex. Gene signature analyses in paired biopsies from 11 patients suggest potential modulation of TGF-β-driven biological activity. There was a trend toward a decrease in TGF-β-responsive gene expression as well as increased immune activation genes and increased CD8 in the tumors. Furthermore, COL1A1 and FAP are markers of stroma remodeling for which the observed changes are consistent with the mechanism of action of NIS793 that was described in the preclinical manuscript.9 In post-treatment biopsies, the downregulation of TGF-β target genes, including PMEPA1, which is detected in many human tumors and the expression of which is driven by TGF-β signaling,21 was reported. We also noted that in patients with greater clinical responses, we saw a higher level of TGF-β pathway activity in their baseline tumor biopsies, suggesting that the target was active in those lesions. To our knowledge, these are the first reported biomarker data in paired tumor biopsies for TGF-b blockade.
When examining whether patients with MSS-CRC and the CMS4 subtype were more responsive to NIS793, we did not observe any increased activity in this specific subtype, consistent with results reported in clinical trial NCT03436563.22
This FIH study provides preliminary indication of proof of mechanism and supports continuous exploration of NIS793 in combination with other treatments and in other therapeutic settings. Although limitations in data interpretation exist, because of the relatively small number of patients and samples, the data herein reported suggest that NIS793 may offer the potential for effective combination with agents that have different mechanisms of action, allowing treatment combinations to be tailored to best suit specific indications and individual patients.9
Acknowledgments
The authors would like to thank all those involved in the trial including patients, and their families, physicians, nurses, research coordinators and all those who assisted at each site. The authors thank Fabienne Baffert, Vasiliki Katsanou, Kae Ishihara, Nicolas Carlo, Christian Meiss, Florian Villegas, Mirek Dostalek, Ashley Widtfeldt, Tyler Laszewski, Sushma Chalasani and Vishal Gandhi for their contributions to the study. The authors also thank Richard Ducray and Brigitte Schiessl for providing technical input. Editorial support was provided by Lauren O’Brien, of Articulate Science Ltd. and Helen Findlow, PhD, of Novartis Pharmaceuticals UK Ltd which was funded by Novartis Pharmaceuticals Corporation in accordance with the Good Publication Practice (2022) guidelines.
Footnotes
Contributors: Conceptualization of the study was done by ASa, M-EG, VC, MP, and CF. M-EG, DL, VC, MP, CF, and TD designed the methodology. Validation of the data was done by MJ, VC, HE, CF, and TD. Statistical analysis was done by P-EJ, DL, LB, VC, and CF. Data collection was done by TMB, IG-L, MJ, ASp, TY, M-EG, VC, MP, CF, and TD. Provision of resources was done by TMB, C-CL, IG-L, RG, ASp, TY, M-EG, MLH-K, AP, VC, and TD. Data curation was done by C-CL, IG-L, M-EG, and TD. Data visualization was done by DL, LB, VC, MP, CF, and TD. Supervision was done by VC, MP, CF, and TD. Project administration was done by MP, HE, CF, and TD. Writing, review, and/or revision of the manuscript was done by TMB, ASa, C-CL, IG-L, MJ, ASp, TY, M-EG, MLH-K, AP, P-EJ, DL, VC, MP, HE, CF, and TD. MP is responsible for the overall content as guarantor.
Funding: This study was funded by Novartis Pharmaceuticals Corporation.
Competing interests: TMB has received support for the present manuscript from Novartis (to institution); consulting fees from AstraZeneca, Bayer, Blueprint, Lilly, and Pfizer; and has received honoraria from Bayer, Lilly and Pfizer. ASa has received consulting fees from Incyte and Sanofi; has received honoraria from AbbVie, Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Celgene, Eisai, Gilead, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Sandoz, Servier, and Takeda; and has participated in advisory boards for Bayer, Bristol Myers Squibb, Eisai, Gilead, Merck Sharp & Dohme, Pfizer, and Servier. C-CL has received consulting fees from Bayer, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Merck KGaA, Novartis, and PharmaEngine; has received honoraria from Boehringer Ingelheim, Novartis, and Roche; has received attendance/travel support from BeiGene, Daiichi Sankyo, and Eli Lilly; and has participated in an advisory board for Novartis. IG-L has received consulting fees from Jazz, Kanaph, OncXer and SOTIO; has received research support funding from Bayer, BridgeBio Pharma, Bristol Myers Squibb, GlaxoSmithKline, Incyte, Jacobio, Lilly, MedImmune, Novartis, Pfizer, Repare Therapeutics, Sumitomo Dainippon Pharma Oncology (to institution), and Tolero Pharmaceuticals. MJ has received research support from Basilea Pharmaceutica, Bristol Myers Squibb, Immunophotonics, Innomedica, Merck Sharp & Dohme, and Novartis (to institution); has received attendance/travel support from Bristol Myers Squibb and Roche; and has participated on an advisory board for Sanofi. RG has received consulting fees from AbbVie, AstraZeneca, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Gilead, Janssen, Merck, Merck Sharp & Dohme, Novartis, Roche, Sanofi, and Takeda; has received honoraria from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Gilead, Merck, Merck Sharp & Dohme, Novartis, Roche, Sandoz, Sanofi, and Takeda; has received attendance/travel support from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Gilead, Janssen, Merck Sharp & Dohme, Novartis, and Roche; has participated in advisory boards for AbbVie, AstraZeneca, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Gilead, Janssen, Merck, Merck Sharp & Dohme, Novartis, Roche, Sanofi, and Takeda. ASp received research support from Alkermes, Amgen, Array Biopharma/Pfizer, AstraZeneca/Medimmune, Bayer, Bristol Myers Squibb, GlaxoSmithKline, Janssen Oncology/Johnson & Johnson, Merck, Novartis, Northern Biologics, NuBiyota, Oncorus, Surface Oncology, Symphogen, Regeneron, Roche, and Treadwell; has participated in advisory boards for Bristol Myers Squibb, Janssen, Medison & Immunocore Merck, and Oncorus. TY has received support for the present manuscript from Novartis (institution); has received consulting fees from AstraZeneca, Bristol Myers Squibb, Eisai, Ipsen, and Merck Sharp & Dohme; has received support for attending meetings and/or travel from Roche and Bayer; holds stock with Moderna; has received receipt of medical writing from Ipsen and Taiho; has received payments (institution) for clinical trial investigatorship from AstraZeneca, Bristol Myers Squibb, Eli Lilly, Exelixis, Merck Sharp & Dohme, Roche, and Taiho. M-EG has received honoraria from AstraZeneca, Bristol Myers Squibb, Janssen, and Novartis; has participated in advisory boards for Bristol Myers Squibb and Janssen-Cilag. MLH-K has received support for attending meetings and/or travel from Jazz pharmaceuticals; has participated in advisory boards for Grifols. P-EJ and CF are former employees of Novartis. DL, MP, LB, and VC are employees of Novartis and hold stock with Novartis. HE is an employee of Novartis and holds Novartis shares. TD has received research support funding from AbbVie, Bristol Myers Squibb, Boehringer Ingelheim, Chugai Pharma, Daiichi Sankyo, Eisai, IQVIA, Janssen, Lilly Merck, Merck Sharp & Dohme, Novartis, Pfizer, SHIONOGI, Sumitomo Dainippon Pharma Oncology (to institution), and Taiho; consulting fees from A2 Health Care, AbbVie, Bayer, Chugai Pharma, KAKEN Pharma, KYOWA KIRIN, Noil Immune, Otsuka Pharma, PRA Health Science Rakuten Medical, SHIONOGI, Sumitomo Dainippon, Taiho, and Takeda; has received honoraria from AstraZeneca, Bristol Myers Squibbs, Daiichi Sankyo, Ono Pharma, and Rakuten Medical; has participated in advisory boards for AbbVie, Amgen, Astellas Pharma, Bayer Boehringer Ingelheim, Daiichi Sankyo, Janssen Pharma, Merck Sharp & Dohme, and Novartis. AP has no conflicts of interests to declare.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Novartis is committed to sharing, with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in a trial, in line with applicable laws and regulations. This trial data availability is according to the criteria and process described at www.clinicalstudydatarequest.com. The sequencing data described in this publication will be made available for any qualified request. To submit a request, please contact: novartis.datasharing@novartis.com.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Research Ethics Committee, National Taiwan University Hospital, Taipei, City Taiwan, 100, Taiwan (ref no: NTUH-REC No – 201701008MSC) Ethikkommission f.d.Bundesland Salzburg, Salzburg, Salzburg, 5020, Austria (ref no: EK-Nr:415-E/2254) Institut fuer Pharmakologie und Toxikologie, Versbacher Str. 9, Julius-Maximilian-Universitaet Wuerzburg Ethik-Kommission bei der Medizinischen Fakultaet, Wuerzburg, Germany (ref no: 284/17) Helmholtzstr. 20 (Oberer Eselsberg), Universitaet Ulm Ethik-Kommission, Ulm, Germany (ref no: 449/17) Advarra IRB, 6100 MerriweatherAdvarra (formerly IntegReview) Columbia, Maryland (MD), 21044, USA (ref no: CNIS793X2101) University of Utah IRB, Salt Lake City, UT, 84065, USA (ref no: IRB_00100308) IRCCS Ist. Clinico Humanitas, Comitato Etico indipendente IRCCS Ist. Clinico Humanitas, Rozzano, MI, 20089, Italy (ref no: 173/18) Ospedale San Raffaele, Comitato Etico IRCCS Ospedale San Raffaele di Milano, Milano, MI, 20132, Italy (ref no: 102/2018) Review Panel C, UNIVERSITY HEALTH NETWORK Research Ethics Board, Toronto, Ontario, M5G 1Z5, Canada (ref no: CAPCR-ID:17-6048.0) Institutional Review Board of National Cancer Center Hospital, Chuo-ku, Tokyo, 104-0045, Japan (ref no: KO673) Institutional Review Board of the University of Hong Kong/ Hospital Authority Hong Kong West Cluster (HKU/HA HKW IRB), Hong Kong, Hong Kong (ref no: UW 16-528) Ethikkommission Ostschweiz (EKOS) St. Gallen, 9000, Switzerland (ref no: 2019-01157). Participants gave informed consent to participate in the study before taking part.
References
- 1.Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer 2003;3:807–21. 10.1038/nrc1208 [DOI] [PubMed] [Google Scholar]
- 2.Derynck R, Turley SJ, Akhurst RJ. TGFβ biology in cancer progression and Immunotherapy. Nat Rev Clin Oncol 2021;18:9–34. 10.1038/s41571-020-0403-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tauriello DVF, Palomo-Ponce S, Stork D, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018;554:538–43. 10.1038/nature25492 [DOI] [PubMed] [Google Scholar]
- 4.Wojtowicz-Praga S. Reversal of tumor-induced immunosuppression by TGF-beta inhibitors. Invest New Drugs 2003;21:21–32. 10.1023/a:1022951824806 [DOI] [PubMed] [Google Scholar]
- 5.Teicher BA. Transforming growth factor-beta and the immune response to malignant disease. Clin Cancer Res 2007;13:6247–51. 10.1158/1078-0432.CCR-07-1654 [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor Microenvironment and progression. Trends Immunol 2010;31:220–7. 10.1016/j.it.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciardiello D, Elez E, Tabernero J, et al. Clinical development of therapies targeting TGFβ: Current knowledge and future perspectives. Ann Oncol 2020;31:1336–49. 10.1016/j.annonc.2020.07.009 [DOI] [PubMed] [Google Scholar]
- 8.Cremasco V, Chang J. Intratumoral fibrosis: emerging concepts and therapeutic opportunities. In: Brenneman J, Iyer MR, eds. Anti-fibrotic Drug Discovery. Cambridge: Royal Society of Chemistry, 2020: 259–306. [Google Scholar]
- 9.Grauel AL, Nguyen B, Ruddy D, et al. TGFβ-blockade Uncovers Stromal plasticity in tumors by revealing the existence of a subset of interferon-licensed fibroblasts. Nat Commun 2020;11:6315. 10.1038/s41467-020-19920-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dummer R, Lebbé C, Atkinson V, et al. Combined PD-1, BRAF and MEK inhibition in advanced BRAF-mutant Melanoma: safety run-in and biomarker cohorts of COMBI-I. Nat Med 2020;26:1557–63. 10.1038/s41591-020-1082-2 [DOI] [PubMed] [Google Scholar]
- 11.Chae YK, Chang S, Ko T, et al. Epithelial-Mesenchymal transition (EMT) signature is inversely associated with T-cell infiltration in non-small cell lung cancer (NSCLC). Sci Rep 2018;8:2918. 10.1038/s41598-018-21061-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominguez CX, Müller S, Keerthivasan S, et al. Single-cell RNA sequencing reveals Stromal evolution into Lrrc15+ Myofibroblasts as a determinant of patient response to cancer Immunotherapy. Cancer Discov 2020;10:232–53. 10.1158/2159-8290.CD-19-0644 [DOI] [PubMed] [Google Scholar]
- 13.Gene set enrichment analysis. human gene set: HALLMARK_INTERFERON_GAMMA_RESPONSE, Available: https://www.gsea-msigdb.org/gsea/msigdb/cards/HALLMARK_INTERFERON_GAMMA_RESPONSE.html [Accessed 6 2023].
- 14.Naing A, Gainor JF, Gelderblom H, et al. A first-in-human phase 1 dose escalation study of Spartalizumab (Pdr001), an anti-PD-1 antibody, in patients with advanced solid tumors. J Immunother Cancer 2020;8:e000530. 10.1136/jitc-2020-000530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Even C, Wang H-M, Li S-H, et al. Phase II, randomized study of Spartalizumab (Pdr001), an anti-PD-1 antibody, versus chemotherapy in patients with recurrent/metastatic Nasopharyngeal cancer. Clin Cancer Res 2021;27:6413–23. 10.1158/1078-0432.CCR-21-0822 [DOI] [PubMed] [Google Scholar]
- 16.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015;21:1350–6. 10.1038/nm.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris VK, Lam M, Wang X, et al. Phase II trial of bintrafusp alfa in patients with metastatic MSI-H cancers following progression on immunotherapy. JCO 2021;39:79. [Google Scholar]
- 18.Robbrecht D, Doger B, Grob J-J, et al. Safety and efficacy results from the expansion phase of the first-in-human study evaluating TGFβ inhibitor SAR439459 alone and combined with cemiplimab in adults with advanced solid tumors. JCO 2022;40:2524. [Google Scholar]
- 19.Yoo C, Oh D-Y, Choi HJ, et al. Phase I study of Bintrafusp Alfa, a Bifunctional fusion protein targeting TGF-Β and PD-L1, in patients with pretreated biliary tract cancer. J Immunother Cancer 2020;8:e000564. 10.1136/jitc-2020-000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doi T, Fujiwara Y, Koyama T, et al. Phase I study of the Bifunctional fusion protein Bintrafusp Alfa in Asian patients with advanced solid tumors, including a hepatocellular carcinoma safety-assessment cohort. Oncologist 2020;25:e1292–302. 10.1634/theoncologist.2020-0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fournier PGJ, Juárez P, Jiang G, et al. The TGF-Β signaling regulator Pmepa1 suppresses prostate cancer metastases to bone. Cancer Cell 2015;27:809–21. 10.1016/j.ccell.2015.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehrvarz Sarshekeh A, Lam M, Zorrilla IR, et al. Consensus molecular subtype (CMS) as a novel integral biomarker in colorectal cancer: a phase II trial of Bintrafusp Alfa in Cms4 metastatic CRC. JCO 2020;38(15_suppl):4084. 10.1200/JCO.2020.38.15_suppl.4084 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-007353supp001.pdf (470.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Novartis is committed to sharing, with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in a trial, in line with applicable laws and regulations. This trial data availability is according to the criteria and process described at www.clinicalstudydatarequest.com. The sequencing data described in this publication will be made available for any qualified request. To submit a request, please contact: novartis.datasharing@novartis.com.