Abstract
Objective:
To evaluate the association between fluid balance (FB) and health-related quality of life (HRQL) among children at one month following community-acquired septic shock.
Design:
Non-prespecified secondary analysis of the Life After Pediatric Sepsis Evaluation. FB was defined as 100 × [(cumulative PICU Fluid Input – Cumulative PICU Fluid Output)]/PICU Admission Weight]. Three subgroups were identified: low FB (<5%), medium FB (5%–15%) and high FB (>15%) based on cumulative FB on days 0–3 of ICU stay. HRQL was measured at ICU admission and one month after using Pediatric Quality of Life Inventory 4.0 Generic Core or Infant Scales or the Stein-Jessop Functional Status Scale. The primary outcome was a composite of mortality or >25% decline in HRQL one month after admission compared to baseline.
Setting:
Twelve academic PICUs in the United States.
Patients:
Critically ill children between 1 month and 18 years, with community-acquired septic shock who survived to at least day 4.
Interventions:
None
Measurements and Main Results:
293 patients were included of whom 66 (23%) had low FB, 127 (43%) medium FB, and 100 (34%) high FB. There was no difference in PRISM-III scores (median 11 [6,17]), age (median 5[1,12]), or gender (47% female) between FB groups. After adjusting for potential confounders and compared to medium FB, higher odds of mortality or >25% HRQL decline were seen in both the low FB (OR 2.79 [1.20, 6.57]) and the high FB (OR 2.16 [1.06,4.47]), p 0.027. Compared to medium FB, low FB (OR 4.3 [1.62, 11.84} and high FB (OR3.29 [1.42, 8.00]) had higher odds of >25% HRQL decline.
Conclusions:
Over half of children who survived septic shock had low or high FB, which was associated with significant decline in HRQL scores. Prospective studies are needed to determine if optimization of FB can improve HRQL outcomes.
Keywords: Sepsis, Fluid Balance, Health Related Quality of Life, critical care outcomes
Introduction:
Severe sepsis affects over 75,000 children in the U.S. annually and is a significant contributor to mortality and morbidity in children1,2. With improving mortality3 there is an increasing proportion of children who survive sepsis, and functional outcomes and health-related quality of life (HRQL) have become a greater focus for clinicians and researchers. The Life After Pediatric Sepsis Evaluation (LAPSE) trial demonstrated the association between illness severity and organ dysfunction markers with HRQL declines in survivors4, yet did not include fluid balance as a marker of illness severity. Children with sepsis often receive aggressive fluid resuscitation and are at risk of high fluid balance5.
Prior studies have shown an association between extent and timing of fluid balance and associated adverse outcomes including increased risk of in-hospital mortality, longer duration of mechanical ventilation, and development of acute kidney injury5–9. However, the association between fluid balance and short term HRQL has not been evaluated. This secondary analysis of the LAPSE study aimed to evaluate the association between fluid balance and outcomes, including mortality and worse HRQL at 1 month follow up. We hypothesized a priori that among children with septic shock, increased severity of positive fluid balance over the first 4 days of PICU admission would be associated with mortality and HRQL decline.
Research In Context
Fluid balance is an important modifier of in-hospital outcomes in children
Understanding the association between fluid balance and health-related quality of life is important because fluid balance is a modifiable factor that could be optimized to improve these outcomes.
Materials and Methods
The LAPSE trial was a prospective observational study that evaluated the short and long-term morbidity and mortality in children after community acquired septic shock. Upon admission, patients were screened, enrolled, and had data collection as previously published4,10,11. The LAPSE protocol was approved by either central or local institutional review boards (eText1). Informed consent was obtained by the parent or guardian of each patient and assent at time of PICU discharge was also obtained if the patient was developmentally able.
Children aged 1 month to 18 years old admitted with community acquired septic shock at 12 academic PICUs in the U.S between 2013–2017 were included in the LAPSE trial. Patients were diagnosed with septic shock if they met the following inclusion criteria: documented or suspected infection within 48 hours of hospital admission, at least 2 systemic inflammatory response syndrome criteria (1 involving an abnormally high or low patient’s temperature or white blood cell count), need for fluid resuscitation and vasoactive-inotropic support within 48 hours of PICU admission and 72 hours of hospital admission.
For this analysis, patients without complete assessment of fluid balance during the first 4 days of the PICU were excluded; these included those who withdrew consent or if they experienced any of the following by day 4 of PICU admission: discharge from PICU, receipt of renal replacement therapy, or death. We collected baseline clinical data including demographics, proxy-reported pre-illness measurements of functional status measured by the Functional Status Scale Score (FSS), and HRQL measured by Pediatric Quality of Life Inventory 4.0 Generic Core Scales (PedsQL) or Peds QL Infant Scales or the Stein-Jessop Functional Status Scale (FSII-R)12,13 based on pre-sepsis baseline status. Parents were able to select the FSII-R if they felt the items in that scale were more appropriate for their child with severe developmental delay or disability. Patients were classified based on the Pediatric Medical Complexity Algorithm which characterizes children as having a “complex chronic disease”, “non-complex chronic disease” and “no chronic disease”14,15. These categories were collapsed into “complex chronic disease” and “non-complex chronic disease or no chronic disease” for analysis. Organ dysfunction and illness severity estimates including PELOD-2 (Pediatric logistic organ dysfunction score)16 and PRISM-III (Pediatric Risk of Mortality Score)17 scores were collected by chart review. Diagnosis of early severe AKI was made if a patient had serum creatinine elevation consistent with KDIGO (Kidney Disease: Improving Global Outcomes) stage 2 or 3 on day 0 or 1 of PICU admission (termed day 0–1 stage 2–3 AKI)18. If no baseline serum creatinine was available, an estimated normal glomerular filtration rate of 120ml/min/1.73m2 was used to back calculate an estimated creatinine, in line with previously validated methods10,19–21.
Fluid Balance Definitions
Fluid balance (FB) was calculated daily as: FB= [(Net PICU Fluid Input − Net PICU Fluid Output)/PICU Admission Weight] × 10022–25. FB was calculated daily for the first 4 days of PICU admission (Days 0 – 3) and included only fluid administered within the PICU. Fluid balance for any given day was defined as total fluid input-total fluid output from time of PICU admission through the day of interest. Initial analysis showed a non-linear relationship between FB and our outcome measures (eFigure 2), and thus we could not evaluate FB as a continuous variable. Therefore, FB was divided into three categories based on inflection points on this graph: cumulative FB <5% (low FB group), cumulative FB 5%–15% (medium FB group), and cumulative FB >15% (high FB group). We performed a secondary analysis of the high FB group that evaluated on which day peak cumulative FB occurred and divided these patients into three groups: Day 0–1, Day 2, Day 3.
HRQL Outcomes
Baseline HRQL measurements were obtained by parent-proxy assessments at study entry as previously published and reflected the patient status during the month prior to severe sepsis hospitalization 4,10,11. Both PedsQL and FSII-R were collected serially during the year after PICU admission. For this analysis, we a priori chose HRQL data from 1 month after PICU admission as the primary outcome because we hypothesized that this timepoint would demonstrate the maximum effect of early FB perturbations. This timepoint had the most available outcome data due to study design and loss to follow-up after the 1 month point. Additionally, we believed later time-points (3 months, 6 months) to be more affected by other factors such as hospital readmissions and other comorbidities. The persistence or recovery from 1 month HRQL decline was evaluated at 3 months as a secondary outcome measure. A decline of 25% in HRQL was chosen a priori based on previous studies4 and because a 25% decrease in HRQL represents a severe deviation from pre-illness baseline.
Statistical Analysis
The cohort was characterized by the categories low, medium, and high degree of fluid balance. Patient factors were summarized using median and interquartile range for continuous variables and counts and percentages for categorical variables. The association between continuous variables and the outcomes were analyzed using Kruskal-Wallis tests. The association between categorical variables and the outcomes were analyzed using Fisher’s exact test (Monte Carlo approximation). Cumulative FB and day of peak high FB were evaluated as potential predictors in each of the regression models.
The primary outcome was defined a priori as a composite outcome of 28-day mortality or >25% decline in HRQL at 1 month post-enrollment relative to pre-illness baseline. Since the mortality rate was equivalent across all FB groups, post hoc analyses including a secondary outcome of >25% decline in HRQL at 1 month was performed. Additional secondary a priori outcomes included in-hospital outcomes such as 28-day ventilator free days and PICU and hospital lengths of stay. Logistic regression was used to evaluate potential associations between FB and binary outcomes such as mortality or >25% decline in HRQL after adjusting for potentially confounding variables which were identified a priori: age (<1 year, 1 to 11 years, ≥12 years), PRISM-III score, PMCA category, baseline HRQL, and early severe AKI. While prior reports evaluated AKI at any point within 28 days, we chose to focus on early day 0–1 AKI to evaluate the impact of early AKI on HRQL changes. Reported statistics include odds ratios and corresponding 95% confidence intervals (CI). Linear regression was used to evaluate associations between FB and continuous outcomes (ventilator-free days, hospital length of stay). Reported statistics for linear regression included adjusted estimated effect size and 95% CI. Summaries and analyses were performed using SAS (SAS Institute; Cary, NC).
RESULTS:
There were 389 patients in the original LAPSE cohort: 14 were excluded because they withdrew consent, 52 died or were discharged prior to day 4, and 30 were excluded for receiving early renal replacement therapy (eFIgure 1). Table 1 depicts basic characteristics and number of patients in each of the fluid balance groups. We included 293 patients (78% of the original cohort): 66 (23%) were in the low FB group, 127 (43%) in the medium FB group, and 100 (34%) in the high FB group (Table 1). The high FB group had a lower median weight (14kg [9, 33], p <0.003). There was no difference in Day 0 PELOD score, PRISM-III scores, or baseline Functional Status Scale scores amongst the three FB groups. Within the high FB group, 14 had peak FB on day 0–1 (14%), 39 had peak FB on day 2 (39%), and 47 (47%) developed peak FB on day 3 of PICU admission (eTable1).
Table 1.
Demographics and baseline characteristics
| Cumulative fluid balance (Days 0–3) |
||||
|---|---|---|---|---|
| Baseline Characteristics | Overall (N = 293) |
Low FB <5% (N = 66) |
Medium FB 5%-15% (N = 127) |
High FB >15% (N = 100) |
|
| ||||
| Age (years) | 5 [1, 12] | 7 [3, 13] | 6 [2, 12] | 3 [1, 11] |
| Female | 137 (47%) | 25 (38%) | 63 (50%) | 49 (49%) |
| PRISM score | 11 [6, 17] | 11 [6, 15] | 11 [6, 18] | 12 [6, 18] |
| Day 0 PELOD score | 9 [7, 11] | 9 [6, 11] | 9 [7, 10] | 9 [7, 12] |
| Weight at PICU admission (kg) | 20 [10, 40] | 24 [14, 48] | 24 [10, 42] | 14 [9, 33] |
| Height at PICU admission (cm) | 108 [76, 141] | 116 [81, 141] | 114 [77, 144] | 95 [70, 132] |
| PMCA1 category | ||||
| None/Non-complex | 155 (53%) | 37 (56%) | 59 (46%) | 59 (59%) |
| Complex | 137 (47%) | 29 (44%) | 68 (54%) | 40 (40%) |
| Functional Status Scale | ||||
| Good (6 – 7) | 153 (52%) | 31 (47%) | 65 (51%) | 57 (57%) |
| Mildly abnormal (8 – 9) | 28 (10%) | 7 (11%) | 12 (9%) | 9 (9%) |
| Moderately abnormal (10 – 15) | 71 (24%) | 12 (18%) | 33 (26%) | 26 (26%) |
| Severely abnormal (16 – 21) | 34 (12%) | 13 (20%) | 15 (12%) | 6 (6%) |
| Very severely abnormal (≥ 22) | 7 (2%) | 3 (5%) | 2 (2%) | 2 (2%) |
In univariate analyses 17/37 (46%) patients with low FB and 30/72 (42%) patients with high FB either died or had >25% decline in HRQL at 1 month (Table 2). This decline persisted at 3 month measurements in 47% of the overall cohort, 35% in low FB group, 63% in medium FB group, and 43% in high FB group (Figure 1). There was no difference in one month mortality or survival to hospital discharge and so the association between FB and our primary outcome appeared to be driven by HRQL decline. In analysis of 1-month survivors, the medium FB group had the lowest proportion of patients 11/76 (14%) who experienced a >25% decline in HRQL compared to patients in either the high FB group (36%) or the low FB group (41%), p 0.001. Patients in the high FB group also had the longest PICU lengths of stay (12 days [7, 18]) and hospital length of stay (21 [15, 32]) while the low FB group had the shortest durations of PICU stay (8 days [5,12]) and hospital lengths of stay (13 [ 8, 23]). Sub-analysis showed no difference in any of these outcomes based on the day of peak high FB development (eTable2).
Table 2:
Univariate association of outcomes based on cumulative fluid balance
| Cumulative fluid balance (Days 0–3) |
|||||
|---|---|---|---|---|---|
| Outcomes | Overall (N = 293) |
Low FB <5% (N = 66) |
Medium FB 5%-15% (N = 127) |
High FB >15% (N = 100) |
P-value |
|
| |||||
| Mortality or >25% decline in HRQL at 1 month | 66/193 (34%) | 17/37 (46%) | 19/84 (23%) | 30/72 (42%) | 0.0102 |
| >25% decline in HRQL at 1 month | 49/176 (28%) | 14/34 (41%) | 11/76 (14%) | 24/66 (36%) | 0.001 2 |
| Mortality at 1 month | 17 (6%) | 3 (5%) | 8 (6%) | 6 (6%) | |
| Acute kidney injury | 143 (49%) | 29 (44%) | 61 (48%) | 53 (53%) | 0.5262 |
| Cardiopulmonary resuscitation | 22 (8%) | 3 (5%) | 9 (7%) | 10 (10%) | 0.4352 |
| Survival to hospital discharge | 275 (94%) | 62 (94%) | 119 (94%) | 94 (94%) | 1.0002 |
| 1-MONTH SURVIVORS | |||||
| Ventilator-free days | 19 [14, 23] | 22 [16, 23] | 19 [15, 22] | 18 [12, 22] | 0.0581 |
| Vasoactive-inotropic free days | 25 [23, 26] | 25 [23, 27] | 25 [23, 26] | 25 [23, 26] | 0.5951 |
| PICU length of stay | 10 [7, 16] | 8 [5, 12] | 10 [7, 16] | 12 [7, 18] | 0.0121 |
| Hospital length of stay | 17 [11, 28] | 13 [8, 23] | 16 [11, 27] | 21 [15, 32] | <.0011 |
| >25% decline in HRQL at 1 month | 49/176 (28%) | 14/34 (41%) | 11/76 (14%) | 24/66 (36%) | 0.0012 |
| >25% decline in PedsQL at 1 month | 41/105 (39%) | 10/21 (48%) | 8/39 (21%) | 23/45 (51%) | 0.0102 |
| >25% decline in FSII-R at 1 month | 8/71 (11%) | 4/13 (31%) | 3/37 (8%) | 1/21 (5%) | 0.0532 |
Continuous summaries are Median [Q1, Q3].
P-values and percentages are calculated for patients with non-missing measures. Kruskal-Wallis test.
Fisher’s exact test (Monte Carlo approximation).
AKI: Acute kidney injury (KDIGO Stage 2 or Stage 3) at any point within 28 days
Figure 1:
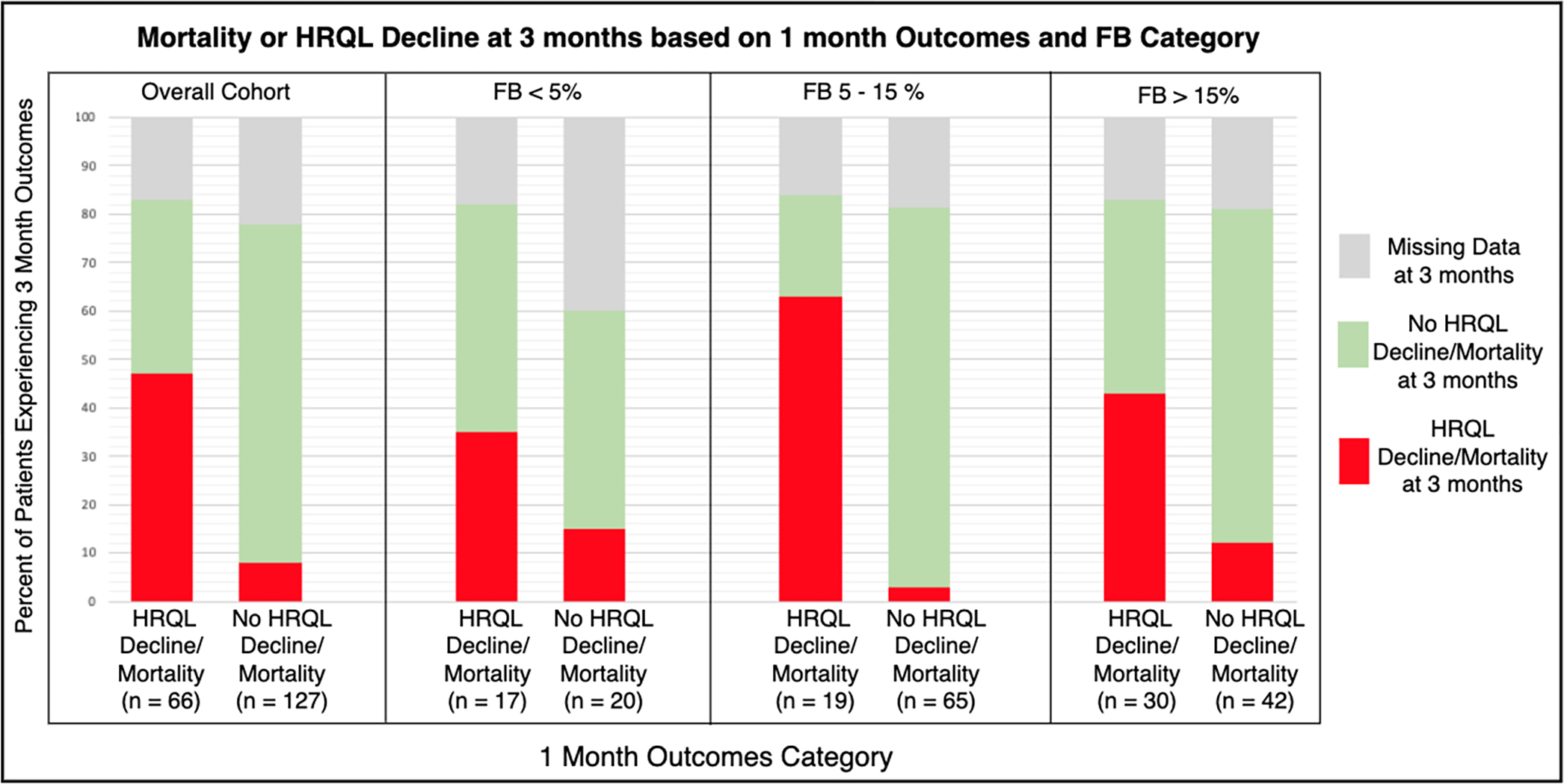
Mortality or HRQL Decline at 3 months based on FB category and 1 month outcomes.
In multivariable analyses adjusting for age, PRISM-III, PMCA, pre-illness baseline HRQL, and day 0–1 stage 2–3 AKI, patients in the high FB group (OR 2.16 [1.06, 4.47]) and the low FB group (OR 2.79 [1.20, 6.57]) had higher odds of mortality or >25% decline in HRQL relative to the medium FB group (Table 3). Multivariable association of cumulative FB with decline in HRQL >25% from baseline showed similar results: high FB group had OR 3.29 [1.42,8.00] and low FB group had OR 4.31 [1.62, 11.84] for HRQL decline >25%. For every 1 point increase in baseline HRQL, the odds of mortality or >25% decline HRQL increased by 2 % (OR 1.02 [1.00,1.04], p 0.037) and the odds of >25% decline HRQL increased by 3% (OR 1.03 [1.01, 1.06], p 0.009) among survivors.
Table 3:
Multivariable association of cumulative fluid balance and mortality or >25% decline in HRQL
| Mortality or >25% decline in HRQL at 1 month |
>25% decline in HRQL at 1 month |
|||
|---|---|---|---|---|
| Patient Characteristic | Odds ratio (95% CI) |
P-value | Odds ratio (95% CI) |
P-value |
|
| ||||
| Cumulative fluid balance (Days 0–3) | 0.027 | 0.003 | ||
| Low FB (<5%) | 2.79 (1.20, 6.57) | 4.31 (1.62, 11.84) | ||
| Medium FB (5%-15%) | Reference | Reference | ||
| High FB (>15%) | 2.16 (1.06, 4.47) | 3.29 (1.42, 8.00) | ||
| Age | 0.715 | 0.286 | ||
| Infant (<1 year) | 0.78 (0.31, 1.88) | 0.48 (0.14, 1.42) | ||
| Child (1–12 years) | Reference | Reference | ||
| Adolescent (12+ years) | 1.17 (0.57, 2.40) | 1.23 (0.55, 2.76) | ||
| PRISM score | 1.01 (0.97, 1.05) | 0.653 | 1.00 (0.95, 1.05) | 0.961 |
| PMCA1 category | 0.329 | 0.323 | ||
| None/Non-complex | 1.39 (0.71, 2.73) | 1.48 (0.68, 3.26) | ||
| Complex | Reference | Reference | ||
| Baseline HRQL | 1.02 (1.00, 1.04) | 0.037 | 1.03 (1.01, 1.06) | 0.009 |
| Early Creatinine AKI (Stage 2+ on Day 0 or Day 1) | 0.570 | 0.987 | ||
| No | Reference | Reference | ||
| Yes | 1.35 (0.47, 3.74) | 0.99 (0.26, 3.38) | ||
Results are based on a multivariable model(s), adjusting for each of the predictors in this table.
Further multivariable analysis was performed on the patients in the highest FB group (Table 4). Patients without complex chronic conditions had higher odds of mortality or >25% decline in HRQL compared to patients with chronic complex PMCA (OR 3.40 [1.03, 12.43]; p 0.044). Multivariable analysis with >25% decline HRQL as the outcome showed similar findings (OR 4.40 [1.18, 19.17]; p 0.027). For every 1 point increase in baseline HRQL, the odds of mortality or >25% decline HRQL increased by 4% (Effect size 1.04 [95% CI 1.00, 1.08]; p 0.035).
Table 4.
Multivariable association of timing of cumulative fluid balance with mortality or decline in HRQL from baseline (among subjects with max cumulative fluid balance > 15 percent in Days 0–3)
| Mortality or >25% decline in HRQL at 1 month |
>25% decline in HRQL at 1 month |
|||
|---|---|---|---|---|
| Patient Characteristic | Odds ratio (95% CI) |
P-value | Odds ratio (95% CI) |
P-value |
|
| ||||
| Day of cumulative fluid balance (Days 0–3) | 0.401 | 0.613 | ||
| Day 0–1 | 0.53 (0.09, 2.52) | 0.73 (0.12, 3.77) | ||
| Day 2 | Reference | Reference | ||
| Day 3 | 1.54 (0.44, 5.75) | 1.62 (0.40, 6.98) | ||
| Age | 0.240 | 0.285 | ||
| Infant (<1 year) | 0.40 (0.08, 1.72) | 0.32 (0.05, 1.67) | ||
| Child (1–12 years) | Reference | Reference | ||
| Adolescent (12+ years) | 1.76 (0.48, 6.81) | 1.42 (0.34, 6.17) | ||
| PRISM score | 0.99 (0.93, 1.06) | 0.846 | 0.97 (0.90, 1.04) | 0.401 |
| PMCA1 category | 0.044 | 0.027 | ||
| None/Non-complex | 3.40 (1.03, 12.43) | 4.40 (1.18, 19.17) | ||
| Complex | Reference | Reference | ||
| Baseline HRQL | 1.04 (1.00, 1.08) | 0.035 | 1.03 (1.00, 1.08) | 0.082 |
| Early Creatinine AKI (Stage 2+ on Day 0 or Day 1) | 0.890 | 0.846 | ||
| No | Reference | Reference | ||
| Yes | 1.12 (0.22, 5.97) | 1.19 (0.19, 7.34) | ||
Results are based on a multivariable model(s), adjusting for each of the predictors in this table.
After adjusting for age, PRISM-III, PMCA, baseline HRQL, and day 0–1 stage 2–3 AKI, the high FB group had an estimated average increase of 7 additional days of hospitalization compared to the medium FB group (Effect size 7.09 [95% CI 1.57, 12.62]) (eTable3). Higher PRISM-III scores and age <1 were independently associated with longer hospital length of stays and fewer ventilator free days.
Among patients in the high FB group, multivariable analysis was performed to determine the association with day of peak FB and outcomes (Table 4). After adjusting for age, PRISM-III, PCMA category, baseline HRQL, and day 0–1 stage 2–3 AKI, there was no difference in mortality or >25% decline in HRQL, ventilator free days, or hospital length of stay amongst the early, middle, or late FB groups.
Discussion:
In this prospective cohort study, a range of fluid balance was observed and both higher and lower fluid balance were associated with worse outcomes relative to positive FB of 5–15% in children with community-acquired septic shock. Among children with septic shock, one in four patients with high FB and one in five patients with low FB developed a >25% decline in HRQL at 1 month. High and low FB was associated with longer PICU and hospital lengths of stay and poor post-discharge outcomes including HRQL decline. Nearly 90% of the patients with high FB developed maximum FB on day 2 or 3 of PICU admission.
AT THE BEDSIDE:
Both high and low fluid balance in children with septic shock is common and associated with HRQL deterioration.
Prospective studies are needed to determine if adverse HRQL outcomes are modifiable as fluid balance is optimized.
This study adds to the literature evaluating HRQL changes after pediatric septic shock. Prior studies have shown that functional outcomes, as measured by Pediatric Overall Performance Category (POPC) were significantly worse at 28 days in nearly 1/3 of surviving children compared to baseline26. Survivors also had significant alterations in neuropsychological performance and educational difficulties27 and both parents and children were more likely to suffer from post-traumatic stress disorder28–31. Given these studies, and ours which shows the decline in HRQL after pediatric septic shock, identifying critical illness factors that can improve these outcomes is imperative. Prior work has shown the association between HRQL decline and AKI within 28 days10, vasotropic-inotropic score, duration of mechanical ventilation, use of renal replacement therapy or extracorporeal life support, in-hospital neurologic events, and in-hospital cardiopulmonary arrest4. While our study did not report the association between AKI and HRQL decline, it may reflect the fact that we removed the most severe cases of early AKI by excluding patients who died or required early CRRT. Our study adds FB, which is a highly modifiable risk factor, to this list. Identifying patients with non-optimal FB may influence fluid management decisions. For example, patients with low FB may benefit from additional fluid resuscitation, while patients with high FB may require earlier fluid de-resuscitation via fluid restriction, diuretic therapy, or renal replacement therapy.
In addition to this study being the first to evaluate the association between fluid balance and post-discharge HRQL, we also demonstrated the association between high fluid balance and worse in-hospital outcomes in a specific pediatric sepsis population. A prior meta-analysis evaluated the association between higher cumulative FB and adverse in-hospital outcomes, including mortality and longer PICU length of stay. This meta-analysis only included 2 retrospective studies that evaluated children with sepsis and neither showed an association with high FB and length of stay32,33. These findings differ from our findings as they used different cut-offs to define high FB (≥5%33 and ≥10%32) and had a higher mortality rate (13%32 and 30%33) than our study which focused on survivorship. We therefore capture a higher rate and number of survivors in our study and demonstrate that a higher, more extreme FB cutoff was associated with adverse in-hospital outcomes (prolonged length of stay). These findings are also consistent with the ARDSNet Clinical Trial which showed increased ventilator free days and fewer ICU days in patients who received a more conservative fluid management strategy34.
There are pathophysiologic explanations for the mechanism by which excessively high FB could lead to deterioration in HRQL. Sepsis alters vascular endothelial permeability and having high FB exacerbates fluid extravasation35, which can lead to pulmonary edema (fewer ventilator free days), acute kidney injury, and alterations in cerebral perfusion pressure affecting neurologic function. Subcutaneous tissue edema, previously characterized in patients with chronically high fluid balance36, may also be seen in patients with sepsis due to capillary leak leading to distorted tissue architecture, obstruction of capillary blood perfusion, and altered tissue oxygenation37. Tissue edema is likely exacerbated in settings of high FB and may impair mobility and ability to participate in early rehabilitation, potentially contributing to decline in physical function sub-score measured in HRQL instruments. This association between high FB and HRQL decline may also be mediated by the association between high FB and longer LOS. We also identified the association between low FB and increased odds of mortality or HRQL deterioration, which may be due to inadequate fluid resuscitation, potentially contributing to clinical and/or sub-clinical ischemia to numerous organs and ultimately HRQL deterioration. The results of this study suggest that fluid can be harmful when administered in quantities either too high or too low relative to a patient’s requirements and objective assessments are needed to aid clinicians in fluid titration38–40.
High FB occurred on day 2 or 3 in almost 90% of patients in the high FB group. While we did not see an association with late FB and outcomes, it highlights the different fluid resuscitation and de-resuscitation patterns. Importantly, these patients had no difference in PRISM-III scores or PELOD scores, suggesting similar baseline illness severity. However, ongoing fluids in excess of the patient’s individual requirements contributes to “fluid creep”, which accounts for upwards of 30% of daily fluid intake in adult ICU patients41. Unrecognized fluids in the form of electrolyte replacements, antibiotics, and “keep venous access open” fluids all contribute to fluid creep41 and are especially important contributors to positive FB during the de-escalation phase of fluid management38. The importance of the de-escalation phase was also seen in the adult ARDSnet trial: patients in conservative fluid management group achieved a net negative fluid balance on ICU day 2 through a combination of fewer fluid intake and higher diuretic doses. Fluid creep may also explain the association between high FB and mortality in patients identified at lower risk based on PERSEVERE biomarker testing in critically ill children with sepsis42.
There are several limitations of this study. First, because this was a secondary analysis, we are missing relevant fluid balance information prior to PICU admission and may have underestimated or overestimated the severity of FB in some patients, which is a known limitation in most of the literature evaluating FB. This may have resulted in patients being misclassified into the FB groups and biased the results. Additionally, while previous studies have shown that decline in HRQL improves but persists by 1 year after hospitalization, we did not evaluate longer term changes in HRQL. Finally, despite our attempts to control for potential confounding variables, there may have been confounding variables we did not measure or include in our analyses.
35% in low FB group, 63% in medium FB group, and 43% in high FB group
Despite these limitations, our study has several strengths that add additional knowledge to the field of FB. Our findings are generalizable as we evaluated a moderately large prospective cohort that included critically ill children from 12 tertiary PICUs throughout the U.S. Second, we had robust data describing enrollment assessments of HRQL at baseline and assessed for numerous potential confounders. Additionally, our comparison groups had similar estimated illness severity and mortality risk scores, which removed much of the confounding that has been seen in other observational studies of FB.
In conclusion, over one third of children in this cohort with community acquired septic shock developed high FB. High FB was an independent risk factor for composite outcome of death or decline in HRQL scores, decline in HRQL score, and longer hospital length of stay. Low FB was also shown to be an independent risk factor for death or decline in HRQL scores. This study adds to the literature detailing the shorter-term adverse outcomes associated with non-optimized FB. Future trials evaluating an optimal FB strategy are needed. In addition to mortality and in-hospital morbidities, post-discharge outcomes like HRQL scores must be measured to evaluate how to optimize HRQL as FB is augmented.
Supplementary Material
Acknowledgements:
The Life After Pediatric Sepsis Evaluation (LAPSE) Investigators thank all subjects and families for participating in the LAPSE investigation. Following is a summary of LAPSE Performance Sites, Principal Investigators (PIs), Coinvestigators (CIs), Research Coordinators (RCs), and Allied Research Personnel (AP). Children’s Hospital of Michigan, Detroit, MI: Kathleen L. Meert, PI; Sabrina Heidemann, CI; Ann Pawluszka, RC; Melanie Lulic, RC. Children’s Hospital of Philadelphia, Philadelphia, PA: Robert A. Berg, PI; Athena Zuppa, CI; Carolann Twelves, RC; Mary Ann DiLiberto, RC. Children’s National Medical Center, Washington, DC: Murray Pollack, PI; David Wessel, PI; John Berger, CI; Elyse Tomanio, RC; Diane Hession, RC; Ashley Wolfe, RC. Children’s Hospital of Colorado, Denver, CO: Peter Mourani, PI; Todd Carpenter, CI; Diane Ladell, RC; Yamila Sierra, RC; Alle Rutebemberwa, RC. Nationwide Children’s Hospital, Columbus, OH: Mark Hall, PI; Andy Yates, CI; Lisa Steele, RC; Maggie Flowers, RC; Josey Hensley, RC. Mattel Children’s Hospital, University of California Los Angeles, Los Angeles, CA: Anil Sapru, PI; Rick Harrison, CI, Neda Ashtari, RC; Anna Ratiu, RC. Children’s Hospital of Pittsburgh, University of Pittsburgh Medical Center, Pittsburgh, PA: Joe Carcillo, PI; Michael Bell, CI; Leighann Koch, RC; Alan Abraham, RC. Benioff Children’s Hospital, University of California, San Francisco, San Francisco, CA: Patrick McQuillen, PI; Anne McKenzie, RC; Yensy Zetino, RC. Children’s Hospital of Los Angeles, Los Angeles, CA: Christopher Newth, PI; Jeni Kwok, RC; Amy Yamakawa, RC. CS Mott Children’s Hospital, University of Michigan, Ann Arbor, MI: Michael Quasney, PI; Thomas Shanley, CI; CJ Jayachandran, RC. Cincinnati Children’s Hospital, Cincinnati, OH: Ranjit Chima PI; Hector Wong, CI; Kelli Krallman, RC; Erin Stoneman, RC; Laura Benken, RC; Toni Yunger, RC. Seattle Children’s Hospital, Seattle Children’s Research Institute (LAPSE Follow-up Center), University of Washington, Seattle, WA: Jerry J Zimmerman, PI; Catherine Chen, RC; Erin Sullivan, RC; Courtney Merritt, RC; Deana Rich, RC; Julie McGalliard, AP; Wren Haaland, AP; Kathryn Whitlock, AP; Derek Salud, AP. University of Utah (LAPSE Data Coordinating Center), Salt Lake City, UT: J Michael Dean, PI; Richard Holubkov, CI; Whit Coleman, RC; Samuel Sorenson, RC; Ron Reeder, AP; Russell Banks, AP; Angie Webster, AP; Jeri Burr, AP; Stephanie Bisping, AP; Teresa Liu, AP; Emily Stock, AP; Kristi Flick, AP. Texas A&M University, College Station, TX: James Varni, AP.
Footnotes
Article Tweet: In children who survive septic shock, fluid balance is associated with deterioration of health-related quality of life one month after ICU admission.
Copyright Form Disclosure: Drs. Stenson, Banks, Reeder, Zimmerman, Meert, and Mourani received support for article research from the National Institutes of Health (NIH). Drs. Banks, Zimmerman, and Mourani’s institutions received funding from the National Institute of Child Health and Human Development (NICHD). Dr. Banks disclosed government work. Drs. Reeder and Meert’s institutions received funding from the NIH. Dr. Maddux’s institution received funding from the NICHD (K23HD096018), the Francis Family Foundation, and the Centers for Disease Control and Prevention. Dr. Zimmerman’s institution received funding from Immunexpress; he received funding from Elsevier. Dr. Mourani’s institution received funding from the National Heart, Lung, and Blood Institute.
References:
- 1.Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis*. Pediatr Crit Care Med. 2013;14(7):686–693. [DOI] [PubMed] [Google Scholar]
- 2.Wong HR, Salisbury S, Xiao Q, et al. The pediatric sepsis biomarker risk model. Crit Care. 2012;16(5):R174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhodes A, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock. Crit Care Med. 2016;45:1–67. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman JJ, Banks R, Berg RA, et al. Critical Illness Factors Associated With Long-Term Mortality and Health-Related Quality of Life Morbidity Following Community-Acquired Pediatric Septic Shock. Crit Care Med. 2020;48(3):319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alobaidi R, Morgan C, Basu RK, et al. Association Between Fluid Balance and Outcomes in Critically Ill Children: A Systematic Review and Meta-analysis. JAMA pediatrics. 2018;172(3):257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alobaidi R, Morgan C, Basu RK, et al. Associations Between Fluid Balance and Outcomes in Critically Ill Children: A Protocol for a Systematic Review and Meta-analysis. Can J Kidney Health Dis. 2017;4:2054358117692560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lima L, Menon S, Goldstein SL, Basu RK. Timing of Fluid Overload and Association With Patient Outcome. Pediatr Crit Care Med. 2021;22(1):114–124. [DOI] [PubMed] [Google Scholar]
- 8.Gist KM, Selewski DT, Brinton J, Menon S, Goldstein SL, Basu RK. Assessment of the Independent and Synergistic Effects of Fluid Overload and Acute Kidney Injury on Outcomes of Critically Ill Children. Pediatr Crit Care Med. 2020;21(2):170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorga SM, Sahay RD, Askenazi DJ, et al. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy: a multicenter retrospective cohort study. Pediatr Nephrol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starr MC, Banks R, Reeder RW, et al. Severe Acute Kidney Injury Is Associated With Increased Risk of Death and New Morbidity After Pediatric Septic Shock. Pediatr Crit Care Med. 2020;21(9):e686–e695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmerman JJ, Banks R, Berg RA, et al. Trajectory of Mortality and Health-Related Quality of Life Morbidity Following Community-Acquired Pediatric Septic Shock. Crit Care Med. 2020;48(3):329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein RE, Jessop DJ. Functional status II(R). A measure of child health status. Med Care. 1990;28(11):1041–1055. [DOI] [PubMed] [Google Scholar]
- 13.Aspesberro F, Fesinmeyer MD, Zhou C, Zimmerman JJ, Mangione-Smith R. Construct Validity and Responsiveness of the Pediatric Quality of Life Inventory 4.0 Generic Core Scales and Infant Scales in the PICU. Pediatr Crit Care Med. 2016;17(6):e272–279. [DOI] [PubMed] [Google Scholar]
- 14.Simon TD, Cawthon ML, Popalisky J, Mangione-Smith R. Development and Validation of the Pediatric Medical Complexity Algorithm (PMCA) Version 2.0. Hosp Pediatr. 2017;7(7):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon TD, Cawthon ML, Stanford S, et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6):e1647–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F . PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med. 2013;41(7):1761–1773. [DOI] [PubMed] [Google Scholar]
- 17.Pollack MM, Holubkov R, Funai T, et al. The Pediatric Risk of Mortality Score: Update 2015. Pediatr Crit Care Med. 2016;17(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.KDIGO Guidelines on AKI. Kidney International Supplements. 2012;2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015;10(4):554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutherland SM, Kwiatkowski DM. Acute Kidney Injury in Children. Adv Chronic Kidney Dis. 2017;24(6):380–387. [DOI] [PubMed] [Google Scholar]
- 21.Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3(4):948–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55(2):316–325. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein SL, Currier H, Graf C, Cosio CC, Brewer ED, Sachdeva R. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107(6):1309–1312. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67(2):653–658. [DOI] [PubMed] [Google Scholar]
- 25.Sinitsky L, Walls D, Nadel S, Inwald DP. Fluid overload at 48 hours is associated with respiratory morbidity but not mortality in a general PICU: retrospective cohort study. Pediatr Crit Care Med. 2015;16(3):205–209. [DOI] [PubMed] [Google Scholar]
- 26.Farris RW, Weiss NS, Zimmerman JJ. Functional outcomes in pediatric severe sepsis: further analysis of the researching severe sepsis and organ dysfunction in children: a global perspective trial. Pediatr Crit Care Med. 2013;14(9):835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Als LC, Nadel S, Cooper M, Pierce CM, Sahakian BJ, Garralda ME. Neuropsychologic function three to six months following admission to the PICU with meningoencephalitis, sepsis, and other disorders: a prospective study of school-aged children. Crit Care Med. 2013;41(4):1094–1103. [DOI] [PubMed] [Google Scholar]
- 28.Rees G, Gledhill J, Garralda ME, Nadel S. Psychiatric outcome following paediatric intensive care unit (PICU) admission: a cohort study. Intensive Care Med. 2004;30(8):1607–1614. [DOI] [PubMed] [Google Scholar]
- 29.Klassen A, Raina P, Reineking S, Dix D, Pritchard S, O’Donnell M. Developing a literature base to understand the caregiving experience of parents of children with cancer: a systematic review of factors related to parental health and well-being. Support Care Cancer. 2007;15(7):807–818. [DOI] [PubMed] [Google Scholar]
- 30.Pochard F, Azoulay E, Chevret S, et al. Symptoms of anxiety and depression in family members of intensive care unit patients: ethical hypothesis regarding decision-making capacity. Crit Care Med. 2001;29(10):1893–1897. [DOI] [PubMed] [Google Scholar]
- 31.Syngal P, Giuliano JS Jr. Health-Related Quality of Life after Pediatric Severe Sepsis. Healthcare (Basel). 2018;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhaskar P, Dhar AV, Thompson M, Quigley R, Modem V. Early fluid accumulation in children with shock and ICU mortality: a matched case-control study. Intensive Care Med. 2015;41(8):1445–1453. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Li X, Bai Z, et al. Association of Fluid Accumulation with Clinical Outcomes in Critically Ill Children with Severe Sepsis. PLoS One. 2016;11(7):e0160093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Xu F, Li S, et al. Influence of fluid balance on the prognosis of patients with sepsis. BMC Anesthesiol. 2021;21(1):269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meler JD, Solomon MA, Steele JR, Yancy CW, Jr., Parkey RW, Fleckenstein JL. The MR appearance of volume overload in the lower extremities. J Comput Assist Tomogr. 1997;21(6):969–973. [DOI] [PubMed] [Google Scholar]
- 37.Claure-Del Granado R, Mehta RL. Fluid overload in the ICU: evaluation and management. BMC Nephrol. 2016;17(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoste EA, Maitland K, Brudney CS, et al. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113(5):740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483–2495. [DOI] [PubMed] [Google Scholar]
- 40.Perez Nieto OR, Wong A, Lopez Fermin J, et al. Aiming for zero fluid accumulation: First, do no harm. Anaesthesiol Intensive Ther. 2021;53(2):162–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Regenmortel N, Verbrugghe W, Roelant E, Van den Wyngaert T, Jorens PG. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: a retrospective study in a tertiary mixed ICU population. Intensive Care Med. 2018;44(4):409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abulebda K, Cvijanovich NZ, Thomas NJ, et al. Post-ICU admission fluid balance and pediatric septic shock outcomes: a risk-stratified analysis. Crit Care Med. 2014;42(2):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


