Abstract
To attend to the growing world demand for mushrooms, it is interesting to increase the system’s productivity, improve quality and reduce production costs. This study aimed to optimize the production and quality of fruiting bodies of the edible and medicinal mushroom Lentinula edodes (shiitake), in agroresidues substrate using appropriate strain and spawn formulation. The evaluation was conducted using two strains under seven different spawn formulations (Control [C]: Sorghum grain + 2.5% CaCO3; (2) C + 2.5% sawdust; (T3) C + 5% sawdust; (T4) C + 2.5% peat; (T5) C + 5% peat; (T6) C + 1.25% sawdust + 1.25% peat; (T7) C + 2.5% sawdust + 2.5% peat) that were inoculated into the blocks at a proportion of 2% (w/w). The substrate was formulated with 63% rice straw, 20% sawdust, 15% wheat bran, and 2% CaCO3 and sterilized. The incubation period was 87 days. Two flushes were obtained. Adding small aliquots of peat and sawdust to the inoculum gave significantly higher morphological results than the control in all variables analyzed. The days required for the first harvest ranged from 87 to 94 days. The average weight of basidiomes ranged from 6.38 to 28.75 g. The productivity data show superior results for the treatments in which the spawn was supplemented with sawdust and peat. Enhanced bioconversion with supplemented spawn shows promises for yield and composition improvement, crucial for commercial viability. It can be concluded that shiitake production using agroresidues such as straw can be increased using a suitable strain/spawn for optimal production.
Keywords: Agroresidues, Axenic rice straw, Edible mushrooms, Strain selection, Supplemented spawn
Introduction
Lentinula edodes (Berkeley) Pegler, known as shiitake, is now the world’s leading cultivated edible mushroom with about 22% of the world’s supply [1]. Protein-rich mushrooms can be produced from many lignocellulosic substrates, including coffee pulp, sugarcane bagasse, straw from various cereals, vineyard pruning residues, and sorghum stubble can also be used, among other substrates [2–6]; however, the substrate most traditionally used is sawdust in a proportion approximately of 50%, to generate synthetic logs or substrates for bag cultivation [7].
About two hundred billion tons of organic matter are generated annually through photosynthesis, and a portion is considered agroindustrial waste [8]. This poses a serious threat to the environment [9] as this material is probably burned or accumulated until it naturally rots. To mitigate this impact, the utilization of agricultural waste can offer a solution for producing high-quality food products, such as L. edodes. [2, 4, 10–13]. These substrates are considered unconventional but have become increasingly important in the cultivation of L. edodes. Furthermore, agro-waste reuse is an eco-friendly way to value biomass that is unsuitable for human and animal consumption [14], in addition to being more energy efficient than reusing wood-wastes [15].
Due to chemical and structural differences in the cultivation substrates, as well as in thermal treatments, the selection of good spawn is critically important to ensure high production of mushrooms in the shortest time possible [12]. To optimize the production of L. edodes in straw, the strain selection for commercial cultivation and breeding is an important step, which is because different strains may differ in mycelial growth rate, spawn-run, cultivation temperature, and nutritional requirements [16–18]. As a production obstacle, this type of substrate is easily contaminated by competing fungi, however, it has been shown that the use of supplemental spawn reduces substrate contamination. The preparation of a supplemented spawn and pre-adaptation of the mycelium allow reducing the contaminants during the fungi colonization in the substrate, since the ability of the fungus to grow on a lignocellulosic substrate is related to the vigor of the spawn mycelium [4].
Mushrooms are nutraceutical foods. Polysaccharides isolated from mushrooms can activate immune responses, stimulating natural killer cells, T cells, B cells, and macrophage-dependent immune system responses [19]. Because of these features, fungal glucans are pharmacologically classified as biological response modifiers (BRM). Glucans, with chitin, represent the greatest quantity of polysaccharides in fungal cell walls [20]. Several studies have focused their attention on the medicinal properties of these polysaccharides. Lentinan is one of the most important metabolites isolated from L. edodes, a β-glucan of high molecular weight with immunomodulatory activity [21].
Additionally, the strain and formulation of the substrate can also define the composition of the fruiting bodies, and consequently the amount of Lentinan [22]. In this study, we hypothesized that well-formulated spawns can increase L. edodes production and provide greater bioconversion efficiency in an unconventional substrate. Therefore, the objective was to evaluate the production and biochemical composition of two strains of L. edodes under different spawn formulations.
Materials and methods
Strains
Two strains of Lentinula edodes were evaluated in this study: LED 19/11 and LED 20/06. The strains were deposited and maintained at Center for Mushroom Studies (CECOG) of Faculty of Agricultural and Technological Sciences (FCAT/ UNESP—Dracena, Brazil). The isolates were maintained in potato-dextrose-agar (BDA) medium (KASVI, Brazil) at 25ºC.
Spawn
Different types of spawn were prepared: Control (C): 97.5% grain sorghum (Sorghum bicolor [L.] Moench/Brazilian origin) + 2.5% CaCO3; T2: C + 2.5% sawdust; T3: C + 5% sawdust; T4: C + 2. 5% black peat (Brazilian origin); T5: C + 5% black peat; T6: C + 1.25% sawdust + 1.25% black peat; T7: C + 2.5% sawdust + 2.5% black peat, percentages are based on dry matter. The control was prepared by cooking the grains until there was no mechanical resistance of the pericarp and then they were drained and mixed with the other ingredients. Each mixture (250 g fresh wt) was placed in plastic bags and sterilized for 4 h at 121 °C. After cooling, the substrate was inoculated with 2% tertiary matrix previously prepared. These units were incubated at room temperature (24–28 ºC) for three weeks. The tertiary matrix had been prepared using sorghum grains (300 g fresh wt) cooked until there was no mechanical resistance of the pericarp, drained, and added 2.5% CaCO3. The material was sterilized for 4 h at 121 °C in glass vials and after cooling, discs of mycelium, previously prepared in PDA medium, were inoculated in each vial.
Substrate preparation
The substrate used consisted of a mixture of rice straw (Oryza sativa), cultivated under dry conditions at the UNESP/FCAT Dracena campus. The straw was chopped (0.5 cm granulometry) and added to 20% sawdust + 15% wheat bran + 2% CaCO3 (dry weight). After homogenization of the substrates, water was added until it reached 65% humidity. The substrate was collected prior to inoculation and subjected to analysis. The analysis protocols employed for these assessments were in accordance with the methodologies outlined by MAPA [23] for chemical analysis (C, N and minerals) and AENOR [24] for physical–chemical properties (pH e EC). High density polypropylene bags with filter were filled with 2 kg of substrate each. Afterwards, they were sealed and autoclaved at 121 °C for 4 h. After cooling to room temperature, the substrates were inoculated with 2% (w/w) spawn per bag and incubated at room temperature (24–26 ºC) in the absence of light for the colonization and browning process. After 87 days of incubation, the bags were opened, and the plastic was removed, leaving a browned substrate, hereafter referred to as ''block'' (Table 1).
Table 1.
Chemical analysis of the used rice straw-based substrate (before autoclaving)
| Unit | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | Ratio C:N | (g/kg) | (mg/kg) | H2O | µS.cm | ||||||||||
| C | N | N | P | Ca | Mg | S | B | Cu | K | Fe | Mn | Zn | pH1 | EC2 | |
| 44 | 1.04 | 42.5 | 10 | 2.6 | 6.3 | 3.3 | 1.7 | 8 | 28 | 11.7 | 357 | 357 | 377 | 6.1 | 2668 |
1Potential hydrogen, 2 Electrical conductivity
Pining and cultivation
Detachments were performed, a practice that consists in carefully detaching the substrate from the wall of the bags, without opening them. This was done from the first week after inoculation, every 15 days. To induce fruiting after opening the blocks, they were washed with water to eliminate exudates and kept in a climate chamber at 18 ± 1 °C and 85–90% RU during the entire cycle. After harvesting the first flush, the blocks were submerged in water for approximately 8 h to induce the fruiting on the second flush, according to the methodology of Kobayashi et al. [25]. Each flush had a duration of 20 days. During the production period, the blocks received full daylight and remained in the dark only during the night.
Mushroom production
Mushrooms were harvested at maturity with the gills exposed and the margin of the pileus slightly turned downward. Untrimmed mushrooms were counted per unit and weighed daily. The yield for each discharge and total was converted according to Dias et al. [26] to biological efficiency (BE%) (fresh weight of mushrooms divided by dry weight of substrate multiplied by 100); production rate (PR%) (BE% divided by days to obtain the first flush, including incubation time); the number of mushrooms (represented by the count of mushrooms picked per 2 kg substrate), and the average weight per mushroom (calculated by the fresh weight of mushrooms per block divided by the number of mushrooms). Production period (PP), earliness (productivity in the first half of the total harvest time divided by productivity in the total harvest time × 100) and the size of the harvested mushrooms according to the diameter of the pileus were also considered: group 1 (G1) < 5 cm, group 2 (G2) 5—9.9 cm and group 3 (G3) 10—15 cm.
Obtaining lentinan polysaccharide
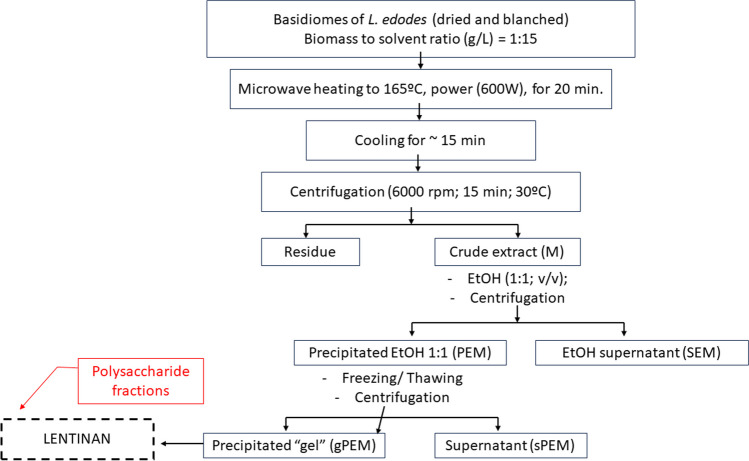
The polysaccharide extracts of the fruiting body samples (Spawn formulation x strain) were obtained by microwave-assisted extraction (MAE). The experiments were performed in Teflon tubes (55 mL capacity) containing the basidiomes, freeze-dried and sieved in 30 mesh particle size, and 15 mL of MilliQ water, in a biomass/solvent ratio of 1:15 (g/mL). Once the mixtures were prepared, each flask was properly sealed and placed in the rotor of the equipment for irradiation, which was performed in two phases. In the first phase, called heating, the tubes were subjected to a power set in the planning for 5 min for the solution + sample to reach 165ºC. In the second phase, called the extraction phase, 165 ºC set was kept constant for 20 min using the 600W of power.
After irradiation, the tubes were immediately cooled (~ 15 min), and the materials were centrifuged (6000 rpm, 15 min, 20 ºC) to separate the residues. The polysaccharide fractions were recovered by precipitation of the aqueous extracts with ethanol (1:1, v/v), centrifugation under the same conditions as above, and lyophilized (Fig. 1). All experiments were performed in triplicate, randomly.
Fig. 1.
Arrangement to obtain lentinan from L. edodes by microwave-assisted extraction
After that, Nuclear Magnetic Resonance (NMR) spectra from the different fractions were obtained using a 400 MHz Bruker model Advance III spectrometer with a 5 mm inverse probe, and the analyses were performed at 70 °C. The samples (30 mg) were dissolved in Me2SO-d6 and were centrifuged (10,000 rpm, 22 °C, 2 min) to remove insoluble material, therefore only the soluble fractions were analyzed. Chemical shifts are expressed in ppm (δ) relative to Me2SO-d6 at 70ºC (13C) and 50ºC (HSQC-DEPT). The results showed two polysaccharides of different sizes, whose structures were revealed using one- and two-dimensional NMR techniques. The two polysaccharides were identified as xylan and lentinan.
Central composite rotatable design
The experiment followed a two x seven factorial design (2 shiitake strains per 7 spawn compositions) with seven replicates for each treatment. Each replication corresponded to a polypropylene bag filled with a 2 kg substrate. Analysis of variance (ANOVA) was performed for all values and comparison of means according to Tukey and Student's t-test (p < 0.05) using the program System for Analysis of Variance—SISVAR 5.6 [27] for the analyzed variables.
Results
Mushroom production
After the incubation period, substrates showed dark colored patches that eventually spread to cover the entire surface. This is consistent with the appearance cited by Przybylowicz and Donoghue [28] and Gaitán-Hernandez et al. [4].
No difference in substrate colonization time was observed between the spawn formulations evaluated. The effects of spawn composition and the interaction between strain and spawn composition, significantly (p < 0.05) influenced the yield for 1st flush, 2nd flush and total yields (ANOVA table not shown). Biological Efficiency (BE) was significantly affected by spawn composition (p < 0.0000) and by the interaction between strain and spawn composition (p < 0.035). The average BE% for axenic rice straw ranged from 2.5% (C—LED 19/11) to 55.8% (T7—LED 19/11) showing an average per formula ranging from 8.53% (C) to 45.59% (T6) with a statistical difference between the seven spawn types, but no statistical difference in the average between the strains (p < 0.05). Each strain manifested its highest BE% when the substrate was inoculated with supplemented spawn, except for T3—LED 20/06 (Table 2). The composition of T4, T5, and T6 spawns was higher on average for both strains evaluated. Strain LED 19/11 showed a higher BE% when associated with spawn compositions T5 and T7 and strain LED 20/06 when associated with T4. Thus, the biological efficiency response is not independent of strain or substrate but requires the correct combination of the two for optimal production.
Table 2.
The biological efficiency [(mushroom weight/substrate wet weight) × 100] from two strains of L. edodes cultivated on seven types of spawn
| Treatments | Strain | Means | |
|---|---|---|---|
| LED 19/11 | LED 20/06 | ||
| C | 2.5 c ± 1.38 | 16.08 b ± 7.83 | 8.53 c |
| T2 | 17.17 bc ± 11.82 | 19.71 ab ± 6.87 | 18.03 bc |
| T3 | 28.77 abc ± 18.21 | 5.5 b ± 1.05 | 17.13 c |
| T4 | 36.91 abc ± 13.05 | 53.19 a ± 8.77 | 45.05 a |
| T5 | 54.33 A a ± 17.46 | 33.07 B ab ± 17.25 | 43.7 a |
| T6 | 50.31 ab ± 26.81 | 39.93 ab ± 26.98 | 45.59 a |
| T7 | 55.8 A a ± 13.31 | 33.02 B ab ± 30.39 | 42.51 ab |
| Means | 35.59 | 30.32 | |
Values are mean and ± are standard deviations. Means followed by lower-case letters indicate statistical difference according to Tukey (p < 0.05) and capital letters indicate significant difference according to the "t" test (p < 0.05)
The Production Rate (PR) was significantly affected by spawn composition (p < 0,000) and by the combination of strain and spawn composition (p = 0,019). The average PR% ranged between 0,03 (C – LED 19/11) and 0,63 (T7 – LED 19/11), showing an average per formula that ranged from 0,09 (C) to 0,52 (T4) (Table 3). Differences were statistically significant between the spawn compositions, but with no statistical difference in average between strains (p < 0.05). The PR% values followed a similar trend to the BE, where the highest PR% value was evident in the case of the substrate inoculated with supplemented spawn. The means were statistically significantly higher under spawn formulations T4, T5, and T6.
Table 3.
Production rate (%) * of two L. edodes strains on axenic rice straw inoculated with seven types of spawn
| Treatments | Strain | Means | |
|---|---|---|---|
| LED 19/11 | LED 20/06 | ||
| C | 0.03 c ± 0.02 | 0.18 b ± 0.09 | 0.09 c |
| T2 | 0.19 bc ± 0.14 | 0.23 ab ± 0.08 | 0.21 bc |
| T3 | 0.32 A abc ± 0.21 | 0.06 B b ± 0.01 | 0.19 bc |
| T4 | 0.43 ab ± 0.15 | 0.61 a ± 0.1 | 0.52 a |
| T5 | 0.6 A a ± 0.22 | 0.37 B ab ± 0.19 | 0.49 a |
| T6 | 0.58 ab ± 0.31 | 0.43 ab ± 0.29 | 0.51 a |
| T7 | 0.63 A a ± 0.17 | 0.35 B ab ± 0.32 | 0.47 ab |
| Means | 0.4 | 0.34 | |
Values are mean and ± are standard deviations. Means followed by lower-case letters indicate statistical difference according to Tukey (p < 0.05) and capital letters indicate significant difference according to the "t" test (p < 0.05)
* Data transformation was used: Square root of Y + 0.5 - SQRT (Y + 0.5)
The number of mushrooms was significantly (p < 0.002) affected by spawn composition (ANOVA table not shown). Average numbers of mushrooms for each spawn composition regardless of strain were significantly different one from the other; and the mushroom numbers means for each strain across all spawn compositions did not differ significantly from each other (Table 4). Treatment T4 showed a higher mean result across spawn compositions for both strains tested.
Table 4.
The number of mushrooms harvested* of L. edodes strains on axenic rice straw inoculated with seven types of spawn
| Treatments | Strain | Means | |
|---|---|---|---|
| LED 19/11 | LED 20/06 | ||
| C | 2.4 c ± 1.02 | 7.5 ab ± 2.29 | 4.67 c |
| T2 | 6.14 bc ± 3.09 | 8.25 ab ± 1.48 | 6.91 bc |
| T3 | 16.4 A ab ± 10.38 | 3.2 B b ± 0.75 | 9.8 abc |
| T4 | 18.33 a ± 4.53 | 14.33 a ± 3.5 | 16.33 a |
| T5 | 12.28 ab ± 4.71 | 9.71 ab ± 4.27 | 11 abc |
| T6 | 14.67 ab ± 8.44 | 10.2 ab ± 5.63 | 12.64 ab |
| T7 | 14.00 ab ± 2.53 | 12.85 ab ± 12.16 | 13.33 ab |
| Means | 11.97 | 9.84 | |
Values are mean and ± are standard deviations. Means followed by lower-case letters indicate statistical difference according to Tukey (p < 0.05) and capital letters indicate significant difference according to the "t" test (p < 0.05)
* Data transformation was used: Square root of Y + 0.5 - SQRT (Y + 0.5 )
Individual mushroom weight was significantly affected by spawn composition (p < 0.0000) and between the interaction of strain and spawn composition (p < 0.001). A T5 spawn composition provided the best average result among the compositions evaluated and showed the highest average mushroom weight for the LED 19/11 strain even though the strain LED 20/06 has presented the highest average weight associated with T4 (Table 5). The response of mushroom numbers to the interaction of strain and spawn composition was significant and therefore not independent of each other, requiring the correct combination of the two for optimal production.
Table 5.
Average weight of mushrooms harvested from L. edodes strains on rice straw-based axenic substrate inoculated with seven types of spawn
| Treatments | Strain | Means | |
|---|---|---|---|
| LED 19/11 | LED 20/06 | ||
| C | 6.38 d ± 4.32 | 12.05 ab ± 3.81 | 8.9 d |
| T2 | 13.93 bcd ± 6.13 | 14 ab ± 2.63 | 13.96 bcd |
| T3 | 10.17 cd ± 1.85 | 10.62 b ± 1.82 | 10.39 cd |
| T4 | 12.41 B bcd ± 3.44 | 23.46 A a ± 5.78 | 17.93 abc |
| T5 | 28.75 A a ± 7.26 | 19.71 B ab ± 8.3 | 24.23 a |
| T6 | 19.54 abc ± 7.29 | 23.16 ab ± 9.47 | 21.18 ab |
| T7 | 24.2 A ab ± 4.92 | 13.54 B ab ± 7.53 | 17.98 abc |
| Means | 16.93 | 17.02 | |
Values are mean and ± are standard deviations. Means followed by lower-case letters indicate statistical difference according to Tukey (p < 0.05) and capital letters indicate significant difference according to the "t" test (p < 0.05)
Table 6 shows the effect of the inoculated substrate for each spawn formula employed during the total mushroom production period (PP) for each strain evaluated. The PP differed significantly among the different spawn compositions (p < 0.002). The PP ranged from 10 to 26 days and days to the first harvest ranged from 87 to 94 days depending on strain and spawn formulation, but with no statistical difference between the results. Mushroom weight was significantly affected by spawn treatments (p < 0.0000) and the relationship between strain and spawn treatment (p < 0.03). Harvested mushroom weight was better at T4 and T6 for LED strain 20/06 and at T5 and T7 for LED strain 19/11 (p < 0.05). Overall, the supplemented spawn formulations provided higher average fresh weight compared to the control (C), except T3 for strain LED 20/06. The number of harvests obtained for the strains from the inoculated substrate using each formula was two. Most total yield was harvested in the first flush for both strains under the spawn compositions tested (Table 6).
Table 6.
The production of fresh L. edodes mushrooms on rice straw-based axenic substrate inoculated with seven types of spawn
| Strain | Treatments | PP 1, 5 | Days to 1st harvest 2, 5 | Average weight (g) 3, 5 | Production by flush (%) 4, 5 | |
|---|---|---|---|---|---|---|
| First | Second | |||||
| LED 20/06 | C | 10 ± 3 | 89 ± 3 | 96.5 ab ± 46.95 | 100 | 0 |
| T2 | 10 ± 3 | 87 ± 0 | 118.25 ab ± 41.24 | 100 | 0 | |
| T3 | 11 ± 1 | 92 ± 4 | 33 B b ± 6.29 | 100 | 0 | |
| T4 | 16 ± 10 | 87 ± 0 | 319.17 a ± 52.6 | 89.75 | 10.25 | |
| T5 | 20 ± 9 | 89 ± 3 | 198.43 B ab ± 103.51 | 94.94 | 5.06 | |
| T6 | 24 ± 12 | 94 A ± 7 | 239.6 a ± 161.88 | 89.09 | 12.91 | |
| T7 | 18 ± 12 | 94 ± 3 | 198.14 B ab ± 182.34 | 89.38 | 10.62 | |
| LED 19/11 | C | 11 ± 2 | 94 ± 3 | 15 c ± 8.29 | 100 | 0 |
| T2 | 12 ± 4 | 89 ± 4 | 103 bc ± 70.92 | 100 | 0 | |
| T3 | 11 ± 2 | 89 ± 4 | 172.6 A ab ± 109.24 | 100 | 0 | |
| T4 | 15 ± 8 | 87 ± 3 | 221.5 ab ± 78.3 | 99.57 | 0.43 | |
| T5 | 20 ± 10 | 92 ± 6 | 326 A a ± 104.74 | 90.73 | 9.27 | |
| T6 | 26 ± 8 | 88 B ± 3 | 301.83 ab ± 160.86 | 92.96 | 7.04 | |
| T7 | 20 ± 12 | 90 ± 5 | 334.8 A a ± 79.89 | 92.98 | 7.02 | |
1 Production Period (time from primordia formation to last harvest)
2 days to the first harvest after inoculation
3 Average fresh weight of mushrooms harvested from seven replicates
4 Distribution of total weight mushrooms obtained in each harvest, estimated in percentage
5 Values are mean and ± are standard deviations. Means followed by lower-case letters indicate statistical difference according to Tukey (p < 0.05) and capital letters indicate significant difference according to the "t" test (p < 0.05)
Mushroom yields per size group were significantly affected by spawn composition (p < 0.0000). Mushrooms produced by the substrate inoculated with the control spawn (C), T2 and T3 were mainly from the G1 group, except for T3—LED 19/11 that produced 60% mushrooms from the G2 group. The two evaluated strains produced on average between treatments, more mushrooms from the G2 group. Only strain LED 20/06, when inoculated with spawn T5 and T7, produced mushrooms of the G3 group. The supplemented spawn formulations T4, T5, T6, and T7 produced more large mushrooms in both strains tested than the other spawn formulations tested (Table 7).
Table 7.
The size group distribution of mushrooms of two strains of L. edodes under seven spawn formulations on rice straw-based axenic substrate
| Strain | Treatments | Production by each size group (%)1 | ||
|---|---|---|---|---|
| G1 | G2 | G3 | ||
| LED 19/11 | T1 | 83,33 | 16,67 | 0 |
| T2 | 50 | 50 | 0 | |
| T3 | 40 | 60 | 0 | |
| T4 | 40 | 60 | 0 | |
| T5 | 40 | 60 | 0 | |
| T6 | 15 | 85 | 0 | |
| T7 | 35 | 65 | 0 | |
| LED 20/06 | T1 | 72,22 | 27,78 | 0 |
| T2 | 66,67 | 33,33 | 0 | |
| T3 | 73,33 | 26,67 | 0 | |
| T4 | 15,79 | 84,21 | 0 | |
| T5 | 10,53 | 78,94 | 10,53 | |
| T6 | 31,58 | 68,42 | 0 | |
| T7 | 47,37 | 47,37 | 5,26 | |
1 Pileus size groups according to diameter: G1 < 5 cm, G2 5—9,9 cm e G3 10–15 cm
Lentinan polysaccharide
It was seen that the use of spawn supplemented with different doses of peat and sawdust (T3, T4, T5, T6, and T7), resulted in higher average results (mg lentinan/gram dried mushroom) of lentinan, when compared to the control treatment for both strains tested. Furthermore, the strains were found to result in different amounts of this polysaccharide, as strain LED 19/11 was shown to be superior to strain LED 20/06 regardless of the spawning formulation used (p < 0.05) (Table 8).
Table 8.
Content of β-glucan (1 → 3) and (1 → 6), lentinan (mg lentinan/gram dried mushroom), obtained from L. edodes strains LED 19/11 and LED 20/06
| Treatments | Strain | Means | |
|---|---|---|---|
| LED 19/11 | LED 20/06 | ||
| C | 140.77 ± 2.5 A b | 90.7 ± 1.2 B b | 115.73 b |
| T2 | 142.97 ± 2.7 A b | 90.97 ± 2.3 B b | 116.97 b |
| T3 | 170.6 ± 3.5 A a | 105.6 ± 6.4 B a | 138.1 a |
| T4 | 176.33 ± 8.1 A a | 103.03 ± 4.3 B a | 139.68 a |
| T5 | 176.1 ± 6.9 A a | 103.57 ± 4 B a | 139.83 a |
| T6 | 173.6 ± 5.6 A a | 104.63 ± 2 B a | 139.11 a |
| T7 | 170.77 ± 2 A a | 101.1 ± 1.2 B a | 135.93 a |
| Means | 164.45 A | 99.74 B | |
| C.V. | 4.1 | ||
Values are mean and ± are standard deviations. Means followed by lower-case letters indicate statistical difference according to Tukey (p < 0.05) and capital letters indicate significant difference according to the "t" test (p < 0.05)
Discussion
This study has demonstrated that mushroom yield, numbers, and individual mushroom weight depend on strain and can be increased using supplemented spawn. In the same vein, millet spawns were used as a supplement to increase the productivity of shiitake in substrates with wood chips [29], and spawn supplementation was also employed to optimize Pleurotus cultivation on coffee pulp, wheat straw, and sawdust, with good results [30–32].
The size or weight of individual mushrooms is an important characteristic for the market and harvest efficiency. Typically, larger mushrooms are desirable over smaller ones and are more rapidly harvested [12]. The two strains behaved differently when submitted to the different spawn formulations. The T4 spawn formulation provided the LED 20/06 strain with not only more larger mushrooms (predominantly G2 and G3), but also higher BE, PR, number of mushrooms harvested and individual weight (p < 0.05). Regarding the LED 19/11 strain, the T5 spawn formulation provided not only more G2 mushrooms but also higher BE, PR, and individual mushroom weight, but did not produce a higher number of mushrooms (p < 0.05).
The studies conducted by Shifeng et al. [13] and Gao et al. [6] offer valuable insights into optimizing shiitake cultivation using alternative substrates. Shifeng et al. [13] examined the effects of substituting varying proportions of rice straw (10%, 20%, 30%, and 40%) for sawdust in a conventional cultivation substrate. While they successfully obtained shiitake fruiting bodies, they also noticed a decrease in BE% values as the substitution of rice straw increased.
On the other hand, Gao et al. [6] demonstrated the feasibility of replacing oak sawdust with finely chopped rice straw, up to 80%, achieving similar or even better results than the control. The BE% values in their study ranged from 36.09% to 49.66%. However, despite the successful outcomes of these cultivation experiments, the authors of the presented study achieved a lower BE% compared to the current study, which employed a substrate formulation containing 63% rice straw. In our study, the highest BE% values were 53.19 and 55.8%, and the mean BE% values of spawning formulations T4, T5, and T6 were significantly (p < 0.05) higher than the control group.
The BE% values reported in this study were lower than those obtained by Royse & Sanchez [33] (80.4—98.9%), but to those obtained by Shifeng et al. [13] (36.4—56.6%), Gaitán-Hernández & Mata [10] (24.8—55.6%) and Gao et al. [6] (36.09—49.66%). The BE% values reported in previous studies vary according to the strain, substrate and treatments used [34–41]. However, very few studies have been conducted to improve spawning and prove its effect on shiitake growth and production [4, 29, 42].
Sawdust and peat were added to the spawns. Peat is a fossil and organomineral material, found in flooded areas such as river floodplains [43]. From a physical–chemical perspective, it is a porous and highly polar material that contains cellulose, hemicellulose, lignin, phenolic compounds, ash, and humic substances with high adsorption capacity for transition metals and polar organic molecules [44]. According to Mata et al. [45] the addition of peat to the growing substrate improved both the defense against Trichoderma attack and BE%, even in the absence of contamination, which the authors indicate occurs due to the composition of peat, which can act as a source of slow-release C and a protective component. Moreover, because L. edodes is a lignolytic fungus [22] it can degrade different forms of phenolic compounds that could have fungistatic effects on Trichoderma spp. Peat characteristics, such as significant ion exchange capacity and adsorptive properties, may have enhanced the adsorption of calcium or another cation on the substrate [46]. Besides this, the adsorption of metabolites produced by Trichoderma spp. that release toxic phenolic compounds could explain the effect of peat addition on the competition between L. edodes and Trichoderma spp [45]. L. edodes is a primary decomposer, i.e., it depends directly on lignin to develop, which makes it difficult to grow on straws and to reduce this effect, sawdust was added to the spawn formulation to evaluate the behavior of the strains to the higher supply of lignin [7]. Because of this study, it was obtained that the spawn formulations that provided superior results (T4, T5, and T6) (p < 0.05) are those in which 2.5% and 5% peat were added, and the mixture of the components in the proportion of 1.25% sawdust and 1.25% peat.
Calcite (CaCO3) was also used as a supplement to the spawn formulations. This ingredient is commonly employed to neutralize acidity, especially during the later stages of substrate decomposition [28]. Fungi typically acidify the environment through various mechanisms, and excessive acidity can become unfavorable for the metabolism of L. edodes. The use of CaCO3, in turn, aids in neutralizing these acids [47]. The Ca2+ ion is essential for the growth, metabolism, and differentiation of fungi [48]. Additionally, it functions as a secondary messenger for intracellular signal transduction involved in various cellular processes [49]. Beyer [44] observed that the adsorption of calcium or another cation in the substrate was linked to increased later yields of Agaricus bisporus. In this regard, the addition of calcite to the spawn formulations may have also influenced the improved production outcomes in this study. This study shows that considerable productivity, BE% and PR% can be obtained by adding 63% rice straw to the substrate and adopting adequate strain and spawn formulation. The reason for this remains unknown but may be related to the supplementation with nutrients added to each spawn type (peat, sawdust and CaCO3). However, the strain’s capacity to invade this substrate is related to the shiitake’s strain adaptation to the system [4]. The two strains tested in this study are suitable for use in the methodologies proposed here, but a specific spawn formulation should be adopted.
Changes in fungal metabolism and in the composition of cell wall polysaccharides occur under stress or adverse conditions [50]. In response to stress signals, such as the presence of phenols in the culture substrate, fungi have mechanisms that preserve cell wall integrity [51, 52]. The main fungal responses to cell wall stress are related to the synthesis of cell wall components, as well as the activation and regulation of these components [47, 50]. The alteration of the cell wall structure could increase glucan synthesis, as reported in the case of L. edodes grown on olive mill wastewaters [22, 48].
Reverberi et al. [22] observed a delay in the initial growth of L. edodes, that the author attributes to certain mechanisms developed by the fungus to overcome the unfavorable environmental conditions created by the presence of toxic compounds. This mechanism is evidenced by the increased concentration of certain enzymes (laccase, superoxidedismutase and catalase), suggesting that the oxidizing and degrading activities of the enzymes were preparing more favorable conditions for the growth of the organism [49].
The alteration of the cell wall structure can increase β-glucan synthesis, induced by the composition of the culture substrate and environmental conditions in which the fungi grown, as reported in L. edodes by Reverberi et al. [22]. It is known that β-glucan synthases have specificity for the substrate, optimal pH (5.8– 7.8), temperature (20–37 °C) and activation or inactivation by different divalent metal ions (Mg, Ca, Fe) [20]. Just as phenols are stress-inducing molecules and alter the cell wall by stimulating the biosynthesis of its components [47, 52], in this work, the addition of small aliquots of peat and/or sawdust to the spawn formulation, resulted in higher amounts of lentinan. Reverberi et al. [22] observed that increased β-glucan synthesis activity did not appear to correlate with increased fungal growth, in this study, the increase in the amounts of the lentinan polysaccharide was shown to be higher when the strains were subjected to spawn supplemented with different doses of peat and sawdust. Furthermore, the results showed that both strains of L. edodes tested produce different amounts of the polysaccharide according to the spawn formulation. The increased β-glucan synthase and β-glucans production could be considered a fungal defense mechanism for overcoming stress conditions [22]. According to this author, fungal growth on a potentially pollutant substrate could have a double effect: the degradation of agro-wastes and the production of pharmacologically active compounds.
Conclusion
The selection of a good spawn adapted the production methodology conditions is critically important to ensure a high production of bodies in the shortest time possible. The bioconversion process of axenic rice straw using supplemented spawn with optimum aliquots of black peat and sawdust, offers the possibility to increase yield and chemical composition of fruiting bodies, important features for commercialization.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, Finance Code 001, and by São Paulo Research Foundation (FAPESP) Process number #2020/13230-8.
Data Availability
All data generated or analyzed during this study are included in this published article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Royse DJ, Baars J, Tan Q (2017) Current overview of mushroom production in the world. In: Zied DC, Pardo-Gimenez A. Edible and medicinal mushrooms: technology and applications p 5–13. 10.1002/9781119149446.ch2
- 2.Gaitán-Hernández R, Esqueda M, Gutiérrez A, Sánchez A, Beltrán-García M, Mata G. Bioconversion of agrowastes by Lentinula edodes: The high potential of viticulture residues. Appl Microbiol Biot. 2006;71:432–439. doi: 10.1007/s00253-005-0241-1. [DOI] [PubMed] [Google Scholar]
- 3.Neelam, Upadhyay V, Kushwaha KPS (2014) Effect of alkaligens faecalis supplementation to different casing mixtures on its physicochemical properties and yield stimulation of Agaricus bisporus. The Bioscan 9(2):659–661. https://epubs.icar.org.in/index.php/MR/article/view/57365. Accessed 30 Aug 2023
- 4.Gaitán-Hernández R, Cortés GMN. Improvement of yield of the edible and medicinal mushroom Lentinula edodes on wheat straw by use of supplemented spawn. Braz J Micro [online] 2014;45(2):467–474. doi: 10.1590/S1517-83822014000200013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaitán-Hernández R, Zavaleta MAB, Aquino-Bolaños EN. Productivity, physicochemical changes, and antioxidant activity of shiitake culinary-medicinal mushroom Lentinus edodes (Agaricomycetes) cultivated on lignocellulosic residues. Inter J Med Mush. 2017;19(11):1041–1052. doi: 10.1615/IntJMedMushrooms.2017024521. [DOI] [PubMed] [Google Scholar]
- 6.Gao S, Huang Z, Feng X, Bian Y, Huang W, Liu Y (2020) Bioconversion of rice straw agroresidues by Lentinula edodes and evaluation of non-volatile taste compounds in mushrooms. 10:1814. 10.1038/s41598-020-58778-x [DOI] [PMC free article] [PubMed]
- 7.Gong WB, Xu R, Xiao Y, Zhou Y, Bian YB. Phenotypic evaluation and analysis of important agronomic traits in the hybrid and natural populations of Lentinula edodes. Sci Hortic. 2014;179:271–276. doi: 10.1016/j.scienta.2014.09.044. [DOI] [Google Scholar]
- 8.Zhang YHP. Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. J Ind Microbiol Biotechnol. 2008;35:367–375. doi: 10.1007/s10295-007-0293-6. [DOI] [PubMed] [Google Scholar]
- 9.Koopmans A, Koppejan J (1997) Agricultural and forest residues – Generation, utilization, and availability. Regional Consultation on Modern Applications of Biomass Energy, 6, 10, Kuala Lumpur, Malaysia
- 10.Gaitán-Hernández R, Mata G. Cultivation of edible mushroom Lentinula edodes (shiitake) in pasteurized wheat straw alternative use of geothermal energy in Mexico. Eng Life Sci. 2004;4:363–367. doi: 10.1590/S1517-83822014000200013. [DOI] [Google Scholar]
- 11.Özçelik E, Pekşen A. Hazelnut husk as a substrate for the cultivation of shiitake mushroom (Lentinula edodes) Bioresour Technol. 2007;98:2652–2658. doi: 10.1016/j.biortech.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Zied DC, Maciel WP, Marques SC, Santos DMS, Rinker DL, Dias ES. Selection of strains for shiitake production in axenic substrate. World J Microbiol Biotechnol. 2016;32:168. doi: 10.1007/s11274-016-2115-3. [DOI] [PubMed] [Google Scholar]
- 13.Shifeng Y, Yang X, Tianji H, Jian Z, Junpeng L, Yuhua G, Yinbing B (2017) Effects of incorporating rice straw in Lentinula edodes cultivation substrates on mycelial growth and selected fruit body parameters. Acta Edulis Fungi 24:30–34. https://www.cabdirect.org/cabdirect/abstract/20173346112. Accessed 30 Aug 2023
- 14.Rashad FM, El Kattan MH, Fathy HM, Abd El-Fattah DA, El Tohamy M, Farahat AA. Recycling of agro-wastes for Ganoderma lucidum mushroom production and Ganoderma post mushroom substrate as soil amendment. Wast Manag. 2019;88:147–159. doi: 10.1016/j.wasman.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 15.Kosre A, Mahish KD, Chandrawanshi NP (2021) Current perspective of sustainable utilization of agro-waste and biotransformation of energy in mushroom. In: Energy: crises, challenges and solutions. 10.1002/9781119741503.ch15
- 16.Gbolagade JS, Fasid IO, Ajayi EJ, Sobowale AA. Effect of physico-chemical factors and semisynthetic media on vegetative of Lentinus subnudus (Berk.), and edible mushroom from Nigeria. Food Chem. 2006;99:742–747. doi: 10.1016/j.foodchem.2005.08.052. [DOI] [Google Scholar]
- 17.Llarena-Hernández CR, Largeteau ML, Ferrer N, Regnault-Roger C, Savoie J. Optimization of the cultivation conditions for mushroom production with European wild strains of Agaricus subrufescens and Brazilian cultivars. J Sci Food Agric. 2013 doi: 10.1002/jsfa.6200. [DOI] [PubMed] [Google Scholar]
- 18.Pardo-Giménez A, Pardo JE, Dias ES, Rinker DL, Caitano CEC, Zied DC. Optimization of cultivation techniques improves the agronomic behavior of Agaricus subrufescens. Sci Rep. 2020;10:8154. doi: 10.1038/s41598-020-65081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borchers AT, Stern JS, Hackman RM, Keen CL, Gershwin EM. Mushrooms, tumors, and immunity. Proc Soc Exp Biol Med. 1999;221:281–293. doi: 10.1046/j.1525-1373.1999.d01-86.x. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Herrera J, Sentandreu R. Fungal cell wall synthesis and assembly. In: McGinnis MR, Borgers M, editors. Current topics in medical mycology, vol three. Berlin Heidelberg New York: Springer; 1991. pp. 169–217. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Li S, Wang X, Zhang L, Cheung P. Advances in lentinan: isolation, structure, chain conformation and bioactivities. Food Hyd. 2011;25:196–206. doi: 10.1016/j.foodhyd.2010.02.001. [DOI] [Google Scholar]
- 22.Reverberi M, Di Mario F, Tomati U. β-Glucan synthase induction in mushrooms grown on olive mill wastewaters. Appl Microbial Cell Physiol. 2004;66:217–225. doi: 10.1007/s00253-004-1662-y. [DOI] [PubMed] [Google Scholar]
- 23.MAPA, Ministério da Agricultura, Pecuária e Abastecimento (2014) Manual de métodos analíticos oficiais para fertilizantes minerais, orgânicos, organominerais e corretivos. Brasília:MAPA/SDA/CGAL. https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/fertilizantes/legislacao/manual-de-metodos_2017_isbn-978-85-7991-109-5.pdf. Accessed 30 Aug 2023
- 24.AENOR, Asociación Española de Normalización y Certificación (2001) Mejoradores de suelos y sustratos de cultivo. Determinación del pH, ed. (AENOR) - Norma Española UNE-EN 13037, Madrid. https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0049121. Accessed 30 Aug 2023
- 25.Kobayashi T, Oguro M, Akiba M, Taki H, Kitajima H, Ishihara H. Mushroom yield of cultivated shiitake (Lentinula edodes) and fungal communities in logs. J For Res. 2020;25(4):269–275. doi: 10.1080/13416979.2020.1759886. [DOI] [Google Scholar]
- 26.Dias ES, Zied DC, Rinker DL. Physiologic response of Agaricus subrufescens using different casing materials and practices applied in the cultivation of Agaricus bisporus. Fungal Biol. 2013;117:569–575. doi: 10.1016/j.funbio.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira DF. Sisvar: A computer analysis system to fixed effects split plot type designs. R Bras Biom. 2019;37(4):529–535. doi: 10.28951/rbb.v37i4.450. [DOI] [Google Scholar]
- 28.Przybylowicz P, Donoghue J. Shiitake growers handbook. The art and science of mushroom cultivation. Dubuque: Kendall/Hunt; 1990. [Google Scholar]
- 29.Royse DJ (1996) Yield stimulation of shiitake by millet supplementation of wood chip substrate. In: Royse DJ (Ed.), Proceedings of the Second International Conference on Mushroom Biology and Mushroom Products. 9–12 June, University Park, PA, 277–283. 10.1590/S1517-83822003000100014
- 30.Sainos E, Díaz-Godínez G, Loera O, Montiel-González AM, Sánchez C. Growth of Pleurotus ostreatus on wheat straw and wheat-grain-based media: Biochemical aspects and preparation of mushroom inoculum. Appl Microbiol Biotechnol. 2006;72:812–815. doi: 10.1007/s00253-006-0363-0. [DOI] [PubMed] [Google Scholar]
- 31.Mata G, Ortega SC, Pérez MR (2011) Inóculo suplementado: Evaluación de un método para optimizar la producción de inóculo para el cultivo de Pleurotus en pulpa de café. Rev Mex Mic 34:53–61. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0187-31802011000200008&lng=es. Accessed 30 Aug 2023
- 32.Narh DL, Obodai M, Baka D, Dzomeku M (2011) The efficacy of sorghum and millet grains in spawn production of Pleurotus ostreatus (Jacq. Ex. Fr.) Kummer. Int Food Res J 18:1143–1148. http://www.ifrj.upm.edu.my/18%20(03)%202011/(41)IFRJ-2010-289.pdf
- 33.Royse DJ, Sanchez JE. Ground wheat straw as a substitute for portions of oak wood chips used in shiitake (Lentinula edodes) substrate formulae. Bioresource Technol. 2007;98:2137–2141. doi: 10.1016/j.biortech.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Mata G, Salmones D, Pérez-Merlo R. (2016) Hydrolytic enzyme activities in shiitake mushroom (Lentinula edodes) strains cultivated on coffee pulp. Rev Arg Microb 48(3):191–195. 10.1016/j.ram.2016.05.008. Accessed 30 Aug 2023 [DOI] [PubMed]
- 35.Salmones D, Mata G, Ramos LM, Waliszeski KN (1999) Cultivation of shiitake mushroom, Lentinula edodes, in several lignocellulosic materials originating from the subtropics. Agronomie 19:13–19. https://hal.science/hal-00885909. Accessed 30 Aug 2023
- 36.Leifa F, Pandey A, Soccol CR. Solid state cultivation-an-efficient method to use toxic agroindustrial residues. J Basic Microb. 2000;40:187–197. doi: 10.1002/1521-4028(200007)40:33.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 37.Pire DG, Wright JE, Albertó E. Cultivation on shiitake using sawdust from widely available local woods in Argentine. Micol Apl Int. 2001;13:87–91. [Google Scholar]
- 38.Philippoussis AN, Diamantopoulou PA, Zervakis GL. Correlation of the properties of several lignocellulosic substrates to the crop performance of the shiitake mushroom Lentinula edodes. World J Microb Biot. 2003;19:551–557. doi: 10.1023/A:1025100731410. [DOI] [Google Scholar]
- 39.Philippoussis AN, Diamantopoulou PA, Israilides C. Productivity of agricultural residues used for the cultivation of the medicinal fungus Lentinula edodes. Int Biodeter Biodegr. 2007;59:216–219. doi: 10.1016/j.ibiod.2006.10.007. [DOI] [Google Scholar]
- 40.Rossi IH, Monteiro AC, Machado JO, Andrioli JL, Barbosa JC. Shiitake Lentinula edodes production on a sterilized bagasse substrate enriched with rice bran and sugarcane molasses. Braz J Microbiol. 2003;34:66–71. doi: 10.1590/S1517-83822003000100014. [DOI] [Google Scholar]
- 41.Mata G, Gaitán-Hernández R, Pérez-Merlo R, Ortega C. Improvement of shiitake spawn for culturing on pasteurized wheat straw. In: Sánchez JE, Huerta G, Montiel E, editors. Mushroom biology and mushroom products. México: UAEM; 2002. pp. 303–309. [Google Scholar]
- 42.Franchi J, Sígolo J, Motta J. Diagnóstico das turfas no Brasil: histórico da utilização, classificação, geologia e dados econômicos. Rev Bras Geoc. 2006;36:179–190. doi: 10.25249/0375-7536.200636S1179190. [DOI] [Google Scholar]
- 43.Mata G, Savoie JM, Delpech P, Olivier JM (1998) Reduction in the incidence of Trichoderma spp. using substrate supplementation with peat and an alternative spawn during cultivation of Lentinula edodes on pasteurized wheat straw. Agronomie 18:515–520. https://hal.archives-ouvertes.fr/hal-00885900. Accessed 30 Aug 2023
- 44.Beyer MB. The effect of chelating agents on the later break yields of Agaricus bisporus. Can J Bot. 1997;75:402–407. doi: 10.1139/b97-043. [DOI] [Google Scholar]
- 45.Smits GJ, van der Ende H, Klis FM. Differential regulation of cell wall biogenesis during growth and development in yeast. Microbiology. 2001;147:781–794. doi: 10.1099/00221287-147-4-781. [DOI] [PubMed] [Google Scholar]
- 46.Elisashvili V, Kachlishvili E, Tsiklauri N, Khardziani T, Bakradze M (2002) Physiological regulation of edible and medicinal higher Basidiomycetes lignocellulolytic enzyme activity. Int J Med Mushroom 4:159–166. https://www.researchgate.net/publication/233866467_Physiological_Regulation_of_Edible_and_MedicinalHigher_Basidiomycetes_LignocellulolyticEnzyme_Activity. Accessed 30 Aug 2023
- 47.Li J, Duan Y, Hu Z, Yang F, Wu X, Zhang R (2022) Physiological mechanisms by which gypsum increases the growth and yield of L. edodes . App Microb Biot 106(7):2677–2688. https://pubmed.ncbi.nlm.nih.gov/35338385/. Accessed 30 Aug 2023 [DOI] [PubMed]
- 48.Dzurendova S, Zimmermann B, Kohler A, Reitzel K, Nielsen UG, Dupuy-Galet BX, Leivers S, Horn SJ, Shapaval V (2021) Calcium affects polyphosphate and lipid accumulation in mucoromycota fungi. J Fung 7(4). https://www.mdpi.com/2309-608X/7/4/300. Accessed 30 Aug2023 [DOI] [PMC free article] [PubMed]
- 49.Roy A, Kumar A, Baruah D, Tamuli R. Calcium signaling is involved in diverse cellular processes in fungi. Mycology. 2021;12(1):10–24. doi: 10.1080/21501203.2020.1785962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bickle M, Delley PA, Schmidt A, Hall MN. Cell wall integrity modulates RHO1 activity via exchange factor ROM2. EMBO J. 1998;17:2235–2245. doi: 10.1093/emboj/17.8.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zjalic S, Fabbri AA, Ricelli A, Reverberi M, Galli E, Fanelli C. Effect of olive oil mill waste waters on the edible and medicinal mushroom Lentinus edodes (Berk.) Sing. Growth and lignin degrading enzymes. Int J Med Mushroom. 2002;4:85–94. doi: 10.1615/IntJMedMushr.v4.i2.20. [DOI] [Google Scholar]
- 52.Soleas GJ, Diamandis EP, Goldberg DM. Resveratrol: a molecule whose time has come? And gone? Clin Biochem. 1997;30:91–113. doi: 10.1016/s0009-9120(96)00155-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.