Abstract
R-(+)-Perillic acid, a promising anticancer and immunomodulatory agent, is the major product from the biotransformation of R-(+)-limonene-rich orange essential oil by the yeast Yarrowia lipolytica. Due to the abundance and low cost of orange essential oil, which is a byproduct of the citrus industry, we attempted to improve the biotransformation process by optimizing yeast cell mass production. Then, the whole process was transposed and adapted to a 2-L instrumented bioreactor. Cell mass production was optimized in shaker flasks using a statistical experimental design. The optimized medium (g·L−1: 22.9 glucose, 7.7 peptone, 4.1 yeast extract and 1.0 malt extract) resulted in a 13.0 g·L−1 final cell concentration and 0.18 g cell·L−1·h−1 productivity. A further increase to 18.0 g·L−1 was achieved in a 2-L bioreactor upon fed-batch culture. High-purity limonene bioconversion was performed in the same bioreactor utilizing top aeration to diminish terpene volatilization; as a result, 839.6 mg·L−1 perillic acid accumulated after 48 h. Under the same conditions, industrial orange essential oil afforded 806.4 mg·L−1 perillic acid. The yeast growth medium optimization resulted in a twofold increase in biomass accumulation and a reduction in growth medium nitrogen sources, which lowered the catalytic biomass production cost. Compared with conventional bottom aeration, the bioreactor top aeration strategy resulted in higher bioconversion rates. The conditions developed for high-purity limonene bioconversion were successfully applied to low-cost orange essential oil, showing the robustness of Y. lipolytica yeast.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-023-01108-w.
Keywords: Orange essential oil, Limonene, Biotransformation, Yarrowia lipolytica, Perillic acid, Top-aerated bioreactor
Introduction
The perillic series of monoterpenes is derived from the limonene molecule through the oxidation of its exocyclic methyl group. Limonene [1] and the p-menthane derivatives perillyl alcohol [2], peryllaldehyde [3], and perillic acid [4, 5] have shown anticancer potential. The latter, which is the major metabolite found in human plasma after the administration of limonene or perillyl alcohol [6], also showed immunomodulatory activity in vitro [7] and in vivo [8].
Perillic derivatives can be obtained via bio-oxidation processes performed by microorganisms [9, 10]. These bioprocesses usually generate a single or a major product under mild reaction conditions and avoid hazardous reagents. In particular, the microbial oxidation of the exocyclic methyl group of limonene has been achieved by bacteria of the genera Pseudomonas, Bacillus and Mycobacterium [11–13], fungi of the genus Aspergillus [11], and yeasts of the genera Yarrowia and Arxula [14]. Limonene is converted to perillic acid by Y. lipolytica through oxidation‒reduction reactions that most likely involve P450 monooxygenases [11, 15] present in the yeast smooth endoplasmic reticulum [15, 16]. Heme-containing oxygenases require cofactor regeneration and therefore the use of viable cells.
In a previous study by our laboratory, the pharmaceutically suitable yeast Yarrowia lipolytica American Type Culture Collection (ATCC) 18942 was utilized for the selective oxidation of limonene [17]. A total of 855 mg·L−1 of perillic acid was exclusively produced with 10 g (dry basis) of yeast cells·L−1 in shaker flasks at 25 °C, with 6 stepwise additions of 0.5% (v/v) limonene over 48 h. Subsequently, the bioconversion reaction parameters were optimized by experimental design to generate a final perillic acid yield of 24%. Utilizing orange essential oil instead of pure limonene enhanced the accumulation and yield of perillic acid accumulation to 0.872 g·L−1 and 29.7%, respectively [18]. The Y. lipolytica ATCC 18942 cell mass was produced upon yeast cultivation in standard yeast malt broth (YMB) medium (glucose 10 g·L−1, yeast extract 3 g·L−1, malt extract 3 g·L−1 and peptone 5 g·L−1) at 28 °C for 48 h.
Aiming to advance the feasibility and cost of the overall bioconversion process, we first investigated the improvement of Y. lipolytica ATCC 18942 cell mass production, which was carried out in shaker flasks, using a statistical experimental design. The selected conditions were transposed to an instrumented 2-L bioreactor to operate the cell growth and limonene bioconversion processes. The bioconversion step was carried out using limonene (97% purity) as the substrate, which was added stepwise to minimize its toxicity to the yeast cells. Crude and much cheaper orange essential oil was also tested as a substrate using the same stepwise addition. In both cases, the bioreactor was top aerated to prevent limonene from evaporating.
Materials and methods
Microorganism and reagents
Yarrowia lipolytica ATCC 18942 was provided by the Microbial Culture Collection of the National Institute for Quality Control in Health, Oswaldo Cruz Foundation, Rio de Janeiro, RJ, Brazil (INCQS) 40,149). R-(+)-Limonene (97%) and (S)-(-)-perillic acid (95%) were purchased from Sigma‒Aldrich (St. Louis, MO, USA). Orange essential oil originated from Tropfruit Nordeste SA, from Estância, SE, Brazil. Antifoam A was purchased from Sigma‒Aldrich. Peptone, D-(+)-glucose, yeast extract and malt extract were obtained from Himedia Laboratories (Kelton, PA, USA). All other reagents used were of analytical grade.
Standard shaker-flask cultivation of Y. lipolytica and limonene bioconversion
For the standard shaker-flask experiments, yeast cells were cultivated (150 mL/1000 Erlenmeyer flask) in yeast malt broth (YMB) medium formulated to a defined chemical composition (glucose 10 g·L−1, yeast extract 3 g·L−1, malt extract 3 g·L−1 and peptone 5 g·L−1) at 28 °C and 200 rpm for 48 h, separated by centrifugation (3000 × g, 15 min at 8 °C) and resuspended at pH 6.9 (50 mM phosphate buffer) at 20 g yeast cells·L−1 (dry basis). For the bioconversion reaction that was carried out at 25 °C and 200 rpm for 48 h, 97% R-(+)-limonene was added to the cell suspension to a final concentration of 0.16% (v/v) at reaction times of 0 and 24 h.
The yeast bioconversion ability was assessed using cells collected within 12, 24, 42 and 48 h of cultivation, corresponding to different growth phases; these cells were used for 24 h bioconversion reactions, as described above, with a single feed of limonene at the onset of the reaction.
Optimization of Y. lipolytica growth medium
A 24–1 fractional factorial design was carried out to screen the significant components of the culture medium for Y. lipolytica ATCC 18942 cultivation. Glucose, peptone, yeast extract and malt extract concentrations were tested at the levels shown in Table 1. The cell mass concentration was the response variable.
Table 1.
Real and coded values of variables by 24–1 fractional factorial design
| Component (g·L−1) | −1 | 0 | 1 |
|---|---|---|---|
| Yeast extract | 1.0 | 3.0 | 5.0 |
| Malt extract | 1.0 | 3.0 | 5.0 |
| Peptone | 3.0 | 5.0 | 7.0 |
| Glucose | 5.0 | 10.0 | 15.0 |
For the culture medium optimization, a central composite rotatable design (CCRD) was performed by taking the initial glucose, peptone, and yeast extract concentrations as variables at the levels shown in Table 2. The malt extract concentration was fixed at 1.0 g·L−1. Design settings and calculations were performed in Statistica 10 software (StatSoft, Tulsa, OK, USA). The cell mass concentration was considered for the statistical analysis, and the prediction model was calculated as follows:
where Y is the predicted response, β0 is the result (response) at the central point, βj and βjj measure the main effects and interactions of the codified variables, xi and xj are the independent variables or codified factors, and ε is the random error. Triplicate experiments under the optimum conditions were carried out to validate the predicted model.
Table 2.
Culture medium optimization by central composite design
| Variable (g·L−1)* | −1.68 | −1 | 0 | 1 | 1.68 |
|---|---|---|---|---|---|
| Glucose | 15.0 | 17.0 | 20.0 | 22.9 | 25.0 |
| Peptone | 3.0 | 4.2 | 6.0 | 7.7 | 9.0 |
| Yeast extract | 1.0 | 1.8 | 3.0 | 4.1 | 5.0 |
*The initial concentration of malt extract was fixed at 1 g·L−1 at 28 °C
The optimized culture media were evaluated for cell biomass yield and productivity in comparison with YMB culture. The cells grown in the different culture media were evaluated for their ability to convert limonene into perillic acid in shaker flasks using the standardized conditions described above.
Y. lipolytica fed-batch cultivation in the 2-L bioreactor
The experiments were conducted in a Biostat B fermentor (Sartorius AG, Gõttingen, Germany) with a working volume of 1.5 L; this fermentor was equipped with a conventional aeration system in which air was bubbled from the bottom of the reactor. After the Y. lipolytica ATCC 18942 cells were activated in a shaker-flask cultivation (YMB medium at 28 °C and 200 rpm for 24 h), the culture was transferred to the bioreactor containing 1.4 L of optimized medium with the temperature set at 28 °C and pH at 6.0, which was controlled by the automatic addition of 1 M NaOH. The dissolved oxygen was maintained at 30% by varying the stirring speed between 250 and 700 rpm and the air flow rate at 0.5 vvm. For fed-batch cultivation, optimized medium (50 mL of tenfold concentrated medium) was added to the bioreactor 24 h and 42 h after the onset of yeast culturing. Antifoam A was added when necessary. The fermentation time course was monitored by measuring the yeast cell mass accumulation and the glucose concentration along the process. The cell mass concentration was determined by optical density at 600 nm (OD600) using a Shimadzu-UV 1601 spectrophotometer (Shimadzu, Kyoto, Japan), in which the OD600 unit corresponded to a dry cell concentration of 0.444 g·L−1. The glucose concentration was measured using a YSI 2700 Select Biochemistry Analyser (YSI, Yellow Springs, OH, USA) equipped with a membrane YSI-2365 (YSI, Yellow Springs, OH, USA).
Limonene bioconversion by cells grown in the 2-L bioreactor
Within 33 h of bioreactor cultivation, when the maximum Y. lipolytica ATCC 18942 cell concentration was reached, the whole bioreactor content (approximately 1700 mL) was withdrawn, and a portion of 1600 mL was centrifuged (3000 × g, 15 min at 8 °C) for cell separation and resuspension at 20 g·L−1 (dry basis) in 50 mM phosphate buffer (pH 6.9) for the bioconversion experiment in the bioreactor. The cell suspension was transferred back to the bioreactor, and 97% R-(+)-limonene was added to a final concentration of 0.16% (v/v) at 0 and 24 h of reaction time. The temperature was maintained at 25 °C, and the air flow rate was 0.2 vvm. The dissolved oxygen concentration was controlled at 5% by varying the stirring speed between 250 and 300 rpm. To clarify the biotransformation capacity of the bioreactor-grown cells, 100 mL of the bioreactor content was separated into 50 mL samples. In the first set, in triplicate experiments, the pH was adjusted to 6.9 by adding 1 M NaOH, and limonene at 0.16% (v/v) was directly added at 0 and 24 h. The other 50 mL sample was used as a triplicate control (cell separation and buffer resuspension before the bioconversion reaction).
Bioconversion of limonene and orange essential oil in a surface-aerated 2-L bioreactor
Within 33 h of fed-batch cell cultivation (Item 2.4), the temperature and pH were adjusted to 25 °C and 6.9, respectively, and 97% R-( +)-limonene was directly added at 0 and every 24 h up to 96 h, without cell separation, to a final concentration of 0.16% (v/v). The bioreactor bottom aeration was replaced by surface aeration [19]. Different air flow rates and dissolved oxygen concentrations were tested with a stirring speed of 300–600 rpm. The optimized conditions were applied to assay perillic acid production by using industrial orange essential oil.
High-performance liquid chromatography (HPLC) of limonene and perillic acid
The limonene content in the crude orange essential oil and perillic acid from the bioconversion supernatants were determined in Ultimate 3000 HPLC equipment (Thermo Fisher Scientific, Dreieich, Germany) equipped with a precolumn LiChroCART RP-18e (4.0 mm I.D. × 4.0 mm) and a reversed-phase column Hibar RT Purospher Star RP18 (3 mm I.D. × 125 mm) (Merck, Darmstadt, GE). Detection was performed with a DAD-3000 photodiode array detector at 207 nm. Commercial R-( +)-limonene or (S)-(-)-perillic acid were used as standards. Calibration curves were made for both standards using six different concentrations, ranging from 25 mg·L−1 to 250 mg·L−1. Samples were cleared using a 0.2-µm polytetrafluoroethylene (PTFE) filter and diluted in acetonitrile whenever necessary using the following conditions: oven temperature of 30 °C, postcolumn cooler temperature of 35 °C, automatic sampler temperature of 15 °C, and run time of 25 min. Acetonitrile and deionized water (90:10), basified with NH4OH at pH 9.0, were used as the mobile phase at a flow rate of 1.0 mL·min−1.
Statistical analysis
The experimental data are expressed in terms of arithmetic average of three replicate experiments and the standard deviation or the average of duplicate experiments, as specified in the text. When applicable, the results were evaluated using the one-way analysis of variance (ANOVA) followed by Tukey’s test. Significance levels were set at 0.05.
Results and discussion
Limonene bioconversion by Y. lipolytica from different growth phases using YMB shaker-flask cultivations
Cells grown for 12, 20, 24, 42 and 48 h (mid-exponential, end of exponential, start of stationary and stationary growth phases, respectively) were tested for limonene bioconversion under standard conditions. Figure 1 shows that yeast cells grown for periods from 20 h onwards resulted in perillic acid accumulation in the range of 368.2 to 411.5 mg·L−1 with no significant differences according to one-way analysis of variance (ANOVA) followed by Tukey’s test (p > 0.05). These values are close to that reported by Tappin et al. [18] of 368 mg·L−1 using 48 h grown cells in a 24 h bioconversion reaction.
Fig. 1.
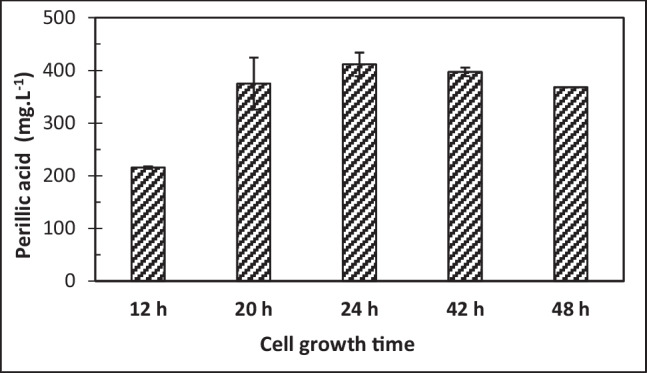
Perillic acid accumulation by Y. lipolytica ATCC 18942 cells collected at different growth phases in YMB medium. The results represent the average of three experiments and the standard deviation
Optimization of Y. lipolytica growth medium
Cell mass production was optimized in shaker flasks using statistical experimental design as detailed in the Online Resource. The optimized medium (g·L−1: 22.9 glucose, 7.7 peptone, 4.1 yeast extract and 1.0 malt extract) resulted in a 13.0 g·L−1 final cell concentration and 0.18 g cell·L−1·h−1 productivity. Optimizing the culture medium exhibited a positive effect on Y. lipolytica ATCC 18942 biomass accumulation, which roughly doubled in relation to the standard YMB medium; in addition, the necessity of expensive N-rich sources was reduced regarding equivalent cell mass production. Thus, savings of 24% peptone, 32% yeast extract and 84% malt extract were observed for the optimized medium, thereby lowering the overall cost for biomass production.
Perillic acid production upon limonene bioconversion using yeast cells grown in YMB (411,3 mg·L−1 within 24 h and 799,4 mg·L−1 within 48 h) and optimized medium (382,9 mg·L−1 within 24 h and 882.9 mg·L−1 within 48 h) did not present significant differences according to one-way ANOVA followed by Tukey’s test (p > 0.05). These results, which correspond with those previously obtained in our laboratories [18], show that the cheaper optimized medium can be used to obtain yeast cell mass without impairing the subsequent limonene bioconversion step.
Y. lipolytica cell mass production in the 2-L bioreactor
The optimized medium was used for fed-batch Y. lipolytica ATCC 18942 cultivation in a 2-L bioreactor equipped with a conventional bottom aeration system. As shown in Fig. 2, exponential cell growth was observed during the first 10 h of culture, with a specific growth rate (μ) of 0.20 h−1, corresponding to a doubling time of 3.47 h. The mean rate of glucose consumption was 0.68 g·L−1·h−1. A 50-mL pulse of tenfold concentrated medium was fed to the culture 24 h after culture onset, when the residual glucose concentration approached 10% of the initial value. As a result, the cell concentration reached 18.0 g·L−1 within 33 h of culture, which is close to the value used in the standard bioconversion procedure (20 g·L−1). The cell concentration increased 1 g·L−1 in the next 6 h and remained in this plateau even after another 50-mL pulse of tenfold concentrated medium was fed, indicating that a maximum cell accumulation was reached for the conditions employed.
Fig. 2.
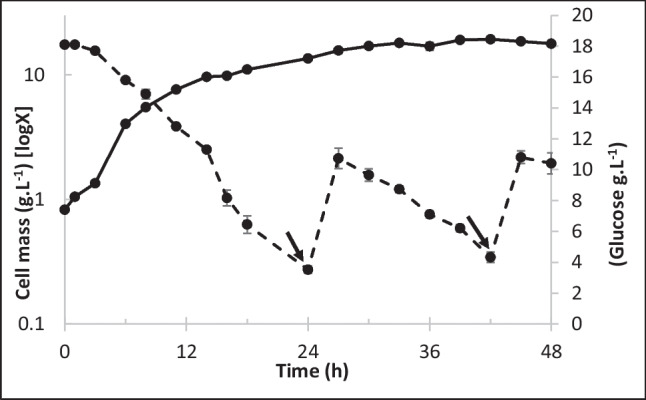
Kinetics of cell mass accumulation (solid line) and glucose consumption (dashed line) during Yarrowia lipolytica ATCC 18942 fed-batch cultivation in the 2-L bioreactor using the optimized medium. The arrows indicate the pulse addition of 50 ml of tenfold concentrated medium to the culture. The bioreactor was run at 28 °C, 1 vvm aeration, 30% pO2, and stirring speed between 250–700 rpm. The results represent the average of three experiments and the standard deviation
Workman et al. [20] studied Y. lipolytica batch culture in an instrumented bottom aerated bioreactor using glucose as a carbon source and reported a growth rate of 0.24 h−1, which was somewhat higher than that obtained in the present work. In another study, Cordova et al. [21] cultivated an engineered strain of Y. lipolytica on glucose in a fed-batch culture aiming to produce fatty alcohols. In this work, a final cell mass concentration of 20 g L−1 was found, which was similar to our results. Most studies on Y. lipolytica bioreactor cultivation have used glycerol as a carbon source to produce lipids or lipases. As such, the working conditions and the reported results are less straightforward compared to those of the present work.
Limonene bioconversion by cells grown in the 2-L bioreactor
Y. lipolytica ATCC 18942 cells cultivated for 33 h in the 2-L bioreactor were handled as described above. Limonene bioconversion in the bioreactor (perillic acid accumulation of 142.5 mg·L−1 within 24 h and 170.9 mg·L−1 within 48 h) was much lower than that observed in the shaker flask when the same bioreactor-grown cells were applied (485 mg·L−1 within 24 h and 725 mg·L−1 within 48 h). The bioconversion results obtained with the shaker flask indicate that the yeast cells grown in the bioreactor oxidize limonene with the same efficiency as those grown in the rotary shaker (382,9 mg·L−1 within 24 h and 882.9 mg·L−1 within 48 h); therefore, exposure to antifoam and the possible changes in cell morphology did not hinder the process. The lower bioconversion yields in the bioreactor could be attributed to the decrease in limonene concentration caused by the effect of the bottom aeration system on this highly volatile substrate.
Moreover, the results obtained in the shaker flasks showed that cell separation prior to bioconversion was unnecessary, as equivalent perillic acid accumulation (530 mg·L−1 within 24 h and 700 mg·L−1 within 48 h) was observed; thus, the presence of the cell culture supernatant, which contains cell metabolites and antifoam, did not inhibit the bioconversion step. Furthermore, performing the cell growth and biotransformation steps sequentially without intermediate cell separation could benefit the overall productivity and costs.
Use of surface-aerated bioreactor
To minimize limonene loss by volatilization in the bioreactor, a top aeration system was used. Aligned with the shaker-flask outcomes, the substrate was added without a previous step to separate the yeast cell mass. In these experiments, after the Y. lipolytica ATCC 18942 growth step (using the conventional bottom aeration approach) was concluded, the pH was adjusted to 6.9, the temperature was adjusted to 25 °C and the stirring speed was adjusted to 300–600 rpm. Then, the air injection system was shifted to top aeration, and the bioconversion step was started by limonene feeding at 0.16% (v/v) at 24 h intervals.
Three different aeration conditions were tested by varying the dissolved oxygen concentration (pO2) and the aeration rate. Table 3 shows that utilizing 1.0% pO2 and a 0.5 vvm aeration rate resulted in a perillic acid accumulation of 840.0 mg·L−1, which was approximately fivefold higher than that of the bottom aeration and equivalent to that obtained for the bioconversion performed in shaker flasks in the same reaction period. The observation that the highest perillic acid concentration was achieved with the highest values of pO2 and aeration rate suggests the importance of oxygenation for selective limonene oxidation; this was expected because the P450 enzyme system, which is putatively involved in the oxidation of limonene by yeasts [11], depends on molecular oxygen.
Table 3.
Influence of operational parameters on the bioconversion of limonene by Yarrowia lipolytica ATCC 18942 in a bioreactor after 48 h of bioconversion
| Aeration mode | Dissolved oxygen (pO2) (%) | Aeration rate (vvm) | Perillic acid (mg·L−1) |
|---|---|---|---|
| Bottom | 0.5 | 0.2 | 170.9 |
| Surface | 0.5 | 0.2 | 449.7 |
| Surface | 1.0 | 0.2 | 618.1 |
| Surface | 1.0 | 0.5 | 840.0 |
Figure 3 shows the kinetics of perillic acid accumulation in the bioreactor using surface aeration under the best tested conditions, which produced 596.6 mg·L−1 and 839.9 mg·L−1 after 24 h and 48 h, respectively. These values are 13% and 20% higher than the values obtained in shaker flasks (530 mg·L−1 and 700 mg·L−1) within the corresponding periods. After a further 48 h period, the product concentration reached 1223.6 mg·L−1. The molar yields (calculated with reference to the added amount of limonene) for each reaction time were 37.5%, 26.4% and 19.1%, respectively. The sharp drop in the bioconversion yield over time, also seen in the kinetic profile (Fig. 3), suggests that cell viability has decrease and/or the product exhibits an inhibitory effect as it accumulates in the medium. At 96 h, the cell viability was 76.8%, showing that Y. lipolytica ATCC 18942 could withstand the harmful reaction conditions without further addition of nutrients.
Fig. 3.
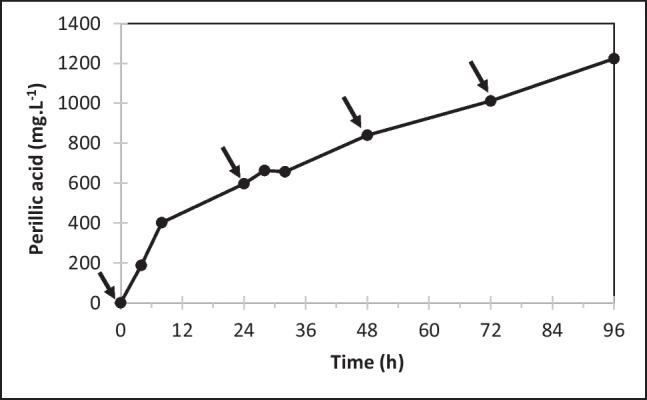
Perillic acid accumulation from high-purity limonene in surface aerated bioreactor. Operational conditions: 20 g·L−1 cell mass, pH 6.9, 1% oxygen saturation, pO2 1%, aeration rate 0.5 vvm. Arrows indicate limonene pulses at 0.16% (v/v) every 24 h. The numerical results are the average of duplicate experiments
Regarding the effect of perillic acid on the microorganism, Mirata et al. [13] reported an inhibitory effect on the growth of Pseudomonas putida DSM 12264 and its oxidizing capacity towards limonene at concentrations above 2.5 g·L−1 and 0.3 g·L−1, respectively. These authors circumvented this issue by using a fluidized bed anion exchange resin column coupled to the bioreactor to remove and recover the product in situ. The results obtained by Mirata et al. [13] suggest that removing perillic acid during the bioconversion of limonene by Y. lipolytica in a bioreactor with surface aeration could further increase the yield and productivity found in the present work.
Perillic acid production from orange essential oil
The same optimized conditions were used for the conversion of orange essential oil containing 89.1% limonene, as measured by HPLC. The results presented in Fig. 4 show that the concentration of perillic acid increased from 675 mg·L−1 to 806.4 mg·L−1 and 1061.7 mg·L−1 after 24, 48 and 96 h, respectively, which correspond to molar yields of 46.2%, 27.6% and 18.1%. These yields were similar to the yields found for the pure R-(+)-limonene bioconversion except that a higher value (23%) was obtained for the crude oil at 24 h. The profile of the curve is also very similar to the profile observed for the high purity substrate (Fig. 3).
Fig. 4.
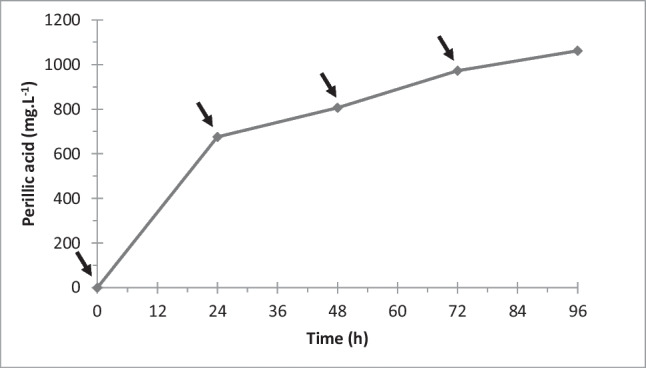
Perillic acid production from orange essential oil in surface-aerated 2-L bioreactor. Operational conditions: 20 g·L−1 cell mass, pH 6.9, 1% oxygen saturation, pO2 of 1%, and aeration rate of 0.5 vvm. The arrows indicate limonene pulses at 0.16% (v/v) every 24 h. The numerical results are the average of duplicate experiments
Crude orange oil presents a wide variety of components, such as oxidized monoterpenes and additional sesquiterpenes [22–24]. Although these compounds are present in low amounts, they may putatively contribute to an increasing availability of the limonene molecule to the yeast cells. However, some of these compounds, such as aliphatic aldehydes [25], could inhibit the microbial bioconversion process. Anyhow, the results of the present work suggest that Y. lipolytica ATCC 18942 was not significantly affected by the presence of such substances.
To the best of our knowledge, studies on the use of orange essential oil to produce perillic derivatives have not been published thus far. However, Marostica Junior and Pastore [26] evaluated the production of R-(+)-α-terpineol through the biotransformation of this agro-industrial by-product using filamentous fungi, such as Aspergillus sp, Penicillium sp and Fusarium oxysporum. The latter produced 450 mg·L−1 (+)-α-terpineol when 1 mL·L−1 of substrate was added every 24 h for 72 h of processing.
Aiming at (+)-nootkatone production, Palmerín-Carreño et al. [27] assayed the bioconversion of (+)-valencene (a minor orange oil constituent) by Y. lipolytica using a three-phase partitioning bioreactor and experimental conditions relatively similar to those of the present work. The highest product yield was 773 mg·L−1 after 96 h of conversion.
The present study contributes to research on industrial waste usage, in which low-cost industrial raw materials are of particular interest for the fine chemicals industry [28]. Orange essential oil is widely available due to orange production worldwide, which is 76.2 million tons per year [29]. The orange industry generates residues corresponding to 50% of the processed fruit (approximately 28 million tons/year), and orange oil represents approximately 5% (dry basis) [30]. In this scenario, orange essential oil, which is highly available, could be coupled to an environmentally friendly process based on a safe yeast to produce perillic acid; as such, this method could be exploited to establish an economical synthesis of pharmaceutically valuable molecules.
Conclusions
This study showed that optimizing the growth medium for Y. lipolytica ATCC 18942 resulted in a twofold increase in biomass accumulation and improved cell mass productivity; in addition, the necessity of more expensive nitrogen sources was significantly reduced. These aspects collectively represent an overall reduction in the cost of catalytic biomass production. Moreover, the present work reports the successful bioconversion of limonene by Y. lipolytica ATCC 18942 using a top-aerated instrumented bioreactor; this process decreased limonene loss via evaporation, which was beneficial for higher bioconversion rates. The conditions developed for the bioconversion of high-purity limonene and the surface aeration strategy were also successfully applied to orange essential oil as a substrate, showing the robustness of Y. lipolytica ATCC 18942 yeast for the use of this agro-industrial by-product.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to Daniel Santos Pereira and Maria Alice Santos Cerullo for the HPLC analysis and to Lucas Tupi Caldas Pereira for the valuable assistance in the bioreactor experiments.
Author contribution
F.M. Knopp contributed to the design of the studies, performed the experiments, analysed the experimental data, and contributed to writing the manuscript; R.R.O. Barros contributed to the design of the optimization studies and the critical revision of the manuscript; B.S. Drummond contributed to the performance of the experiments; A.C. Siani acquired funding for this work and contributed to the critical revision of the manuscript; M.A. Ferrara contributed to the supervision, conception and design of the studies and writing of the manuscript; E.P.S. Bon contributed to the supervision, provided lab space, acquired funding for this work and contributed to the critical revision of the manuscript.
All authors read and approved the final manuscript.
Funding
This work was supported by the National Council for Scientific and Technological Development (CNPq) and the Pharmaceutical Technology Institute of the Oswaldo Cruz Foundation under grant PROEP/FAR/CNPq 407841/2017–2; Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (FAPERJ) under grants E-26/110.856/2013 and E-26/101.210/2013; CNPq under grant 307766/2014/4; and PIBIC/CNPq/FIOCRUZ 2014–2016.
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Conflict of interest
The authors report no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sun J. D-Limonene: safety and clinical applications. Altern Med Rev. 2007;12:259–264. [PubMed] [Google Scholar]
- 2.Ariazi EA, Satomi Y, Ellis MJ, Haag JD, Shi W, Sattler CA, Gould MN. Activation of the transforming growth factor beta signaling pathway and induction of cytostasis and apoptosis in mammary carcinomas treated with the anticancer agent perillyl alcohol. Cancer Res. 1999;59:1917–1928. [PubMed] [Google Scholar]
- 3.Tsai KD, Liu YH, Chen TW, Yang SM, Wong HY, Cherng J, Chou KS, Cherng JM. Cuminaldehyde from Cinnamomum verum induces cell death through targeting topoisomerase 1 and 2 in human colorectal adenocarcinoma COLO 205 cells. Nutrients. 2016;8:318. doi: 10.3390/nu8060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardcastle I, Rowlands M, Barber AM, Grimshaw R, Mohan M, Nutley B, Jarman M. Inhibition of protein prenylation by metabolites of limonene. Biochem Pharmacol. 1999;57:801–809. doi: 10.1016/s0006-2952(98)00349-9. [DOI] [PubMed] [Google Scholar]
- 5.Mukhtar YM, Adu-Frimpong M, Xu X, Yu J (2018) Biochemical significance of limonene and its metabolites: future prospects for designing and developing highly potent anticancer drugs. Biosci Rep 38. 10.1042/BSR20181253 [DOI] [PMC free article] [PubMed]
- 6.Yeruva L, Pierre KJ, Elegbede A, Wang RC, Carper SW. Perillyl alcohol and perillic acid induced cell cycle arrest and apoptosis in non-small cell lung cancer cells. Cancer Lett. 2007;257:216–226. doi: 10.1016/j.canlet.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Lappas CM, Lappas NT. D-Limonene modulates T lymphocyte activity and viability. Cell Immunol. 2012;279:30–41. doi: 10.1016/j.cellimm.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Del Toro-Arreola S, Flores-Torales E, Torres-Lozano C, Del Toro-Arreola A, Tostado-Pelayo K, Ramirez-Dueñas MG, Daneri-Navarro A. Effect of D-limonene on immune response in BALB/c mice with lymphoma. Int Immunopharmacol. 2005;5:829–838. doi: 10.1016/j.intimp.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Marmulla R, Harder J. Microbial monoterpene transformations-a review. Front Microbiol. 2014;5:346. doi: 10.3389/fmicb.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grabarczyk M, Mączka W, Żołnierczyk AK, Wińska K. Transformations of monoterpenes with the p-menthane skeleton in the enzymatic system of bacteria, fungi and insects. Molecules. 2020;25:4840. doi: 10.3390/molecules25204840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duetz WA, Bouwmeester H, Van Beilen JB, Witholt B. Biotransformation of limonene by bacteria, fungi, yeasts, and plants. Appl Microbiol Biotechnol. 2003;61:269–277. doi: 10.1007/s00253-003-1221-y. [DOI] [PubMed] [Google Scholar]
- 12.Van Beilen JB, Holtackers R, Lüscher D, Bauer U, Witholt B, Duetz WA. Biocatalytic production of perillyl alcohol from limonene by using a novel Mycobacterium sp. cytochrome P450 alkane hydroxylase expressed in Pseudomonas putida. Appl Environ Microbiol. 2005;71:1737–1744. doi: 10.1128/AEM.71.4.1737-1744.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirata MA, Heerd D, Schrader J. Integrated bioprocess for the oxidation of limonene to perillic acid with Pseudomonas putida DSM 12264. Process Biochem. 2009;44:764–771. doi: 10.1016/j.procbio.2009.03.013. [DOI] [Google Scholar]
- 14.Van Rensburg E, Molekeli N, Van Der Walt JP, Botes PJ, Van Dyk MS. Biotransformation of (+) limonene and (−) piperitone by yeasts and yeast-like fungi. Biotechnol Lett. 1997;19:779–782. doi: 10.1023/A:1018344411069. [DOI] [Google Scholar]
- 15.Szczesna-Skorupa E, Chen C, Liu H, Kemper B. Gene expression changes associated with the endoplasmic reticulum stress response induced by microsomal cytochrome P450 overproduction. J Biol Chem. 2004;279:13953–13961. doi: 10.1074/jbc.M312170200. [DOI] [PubMed] [Google Scholar]
- 16.Linder T. Taxonomic distribution of cytochrome P450 monooxygenases (CYPs) among the budding yeasts (sub-phylum Saccharomycotina) Microorganisms. 2019;7:247. doi: 10.3390/microorganisms7080247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrara MA, Almeida DS, Siani AC, Lucchetti L, Lacerda PSB, Freitas A, Tappin MR, Bon EPS. Bioconversion of R-(+)-limonene to perillic acid by the yeast Yarrowia lipolytica. Braz J Microbiol. 2013;44:1075–1080. doi: 10.1590/S1517-83822014005000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tappin MRR, Knopp FM, Cardoso IC, Santos RT, Drummond BS, Siani AC, Bon EPS, Ferrara MA. Synthesis of the prospective anticancer molecule perillic acid from orange essential oil by the yeast Yarrowia lipolytica. Green Sustain Chem. 2017;7:172–184. doi: 10.4236/gsc.2017.72013. [DOI] [Google Scholar]
- 19.Pescheck M, Mirata MA, Brauer B, Krings U, Berger RG, Schrader J. Improved monoterpene biotransformation with Penicillium sp. by use of a closed gas loop bioreactor. J Ind Microbiol Biotechnol. 2009;36:827–836. doi: 10.1007/s10295-009-0558-3. [DOI] [PubMed] [Google Scholar]
- 20.Workman M, Holt P, Thykaer J. Comparing cellular performance of Yarrowia lipolytica during growth on glucose and glycerol in submerged cultivations. AMB Expr. 2013;3:58. doi: 10.1186/2191-0855-3-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cordova LT, Butler J, Alper HS. Direct production of fatty alcohols from glucose using engineered strains of Yarrowia lipolytica. Metab Eng Commun. 2020;10:e00105. doi: 10.1016/j.mec.2019.e00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galvão JG, Silva VF, Ferreira SG, França FRM, Santos DA, Freitas LS, Alves PB, Araujo AAS, Cavalcanti SCH, Nunes RS. β-cyclodextrin inclusion complexes containing Citrus sinensis (L.) Osbeck essential oil: An alternative to control Aedes aegypti larvae. Thermochim Acta. 2015;608:14–19. doi: 10.1016/j.tca.2015.04.001. [DOI] [Google Scholar]
- 23.Negro V, Mancini G, Ruggeri B, Fino D. Citrus waste as feedstock for bio-based products recovery: review on limonene case study and energy valorization. Biores Technol. 2016;214:806–815. doi: 10.1016/j.biortech.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Kringel DH, Antunes MD, Klein B, Crizel LR, Wagner R, Oliveira RP, Dias ARG, Zavareze ER. Production, characterization, and stability of orange or eucalyptus essential oil/β-cyclodextrin inclusion complex. J Food Sci. 2017;82:2598–2605. doi: 10.1111/1750-3841.13923. [DOI] [PubMed] [Google Scholar]
- 25.Kunjapur AM, Prather KLJ. Microbial engineering for aldehyde synthesis. Appl Environ Microbiol. 2015;81:1892–1901. doi: 10.1128/AEM.03319-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marostica MR, Jr, Pastore GM. Biotransformation of limonene: a review of the main metabolic pathways. Quim Nova. 2007;30:382–387. doi: 10.1590/S0100-40422007000200027. [DOI] [Google Scholar]
- 27.Palmerín-Carreño DM, Rutiaga-Quiñones OM, Verde-Calvo JR, Prado-Barragán A, Huerta-Ochoa S. Whole cell bioconversion of (+)-valencene to (+)-nootkatone in 100% organic phase using Yarrowia lipolytica 2.2ab. Int J Chem React Eng. 2016;29:36–45. doi: 10.1515/ijcre-2016-0013. [DOI] [Google Scholar]
- 28.Kruhne U, Heintz S, Ringborg R, Rosinha IP, Tufvesson P, Gernaey KV, Woodley JM. Biocatalytic process development using microfluidic miniaturized systems. Green Process Synth. 2014;3:23–31. doi: 10.1515/gps-2013-0089. [DOI] [Google Scholar]
- 29.FAO (2021) Citrus Fruit Statistical Compendium 2020. Rome. Food and Agriculture Organization of the United Nations. https://www.fao.org/3/cb6492en/cb6492en.pdf. Accessed 22 March 2022
- 30.Ferreira-Leitão V, Gottschalk LMF, Ferrara MA, Nepomuceno AL, Molinari HBC, Bon EPS. Biomass residues in Brazil: availability and potential uses. Waste Biomass Valorization. 2010;1:65–76. doi: 10.1007/s12649-010-9008-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.


