Abstract
This study describes the discovery of a variety of quinoline2-one derivatives with significant antibacterial action vs a spectrum of multidrug-resistant Gram-positive bacterial strains, especially methicillin-resistant Staphylococcus aureus (MRSA). Compounds 6c, 6l, and 6o exhibited significant antibacterial activity versus the Gram-positive bacterial pathogens evaluated. In comparison to the reference daptomycin, compound 6c demonstrated the most effective activity among the assessed derivatives, with MIC concentrations of 0.75 μg/mL versus MRSA and VRE and 2.50 μg/mL against MRSE. We also reported on these compounds’ biofilm and dihydrofolate reductase inhibitory activities. Compound 6c showed the greatest antibiofilm action in a dose-dependent way and a substantial decrease of biofilm development in the MRSA ACL51 strain at concentrations of 0.5, 0.25, and 0.12 MIC, with reductions of 79%, 55%, and 38%, consecutively, whereas the corresponding values for vancomycin were 20%, 12%, and 9%. These findings imply that these quinoline compounds could be used to develop a new category of antibiotic representatives to prevent Gram-positive drug-resistant bacterial strains.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-023-01132-w.
Keywords: Quinoline, Antibacterial, Resistance, Biofilm, MRSA, MRSE, VRE
Introduction
There is an elevation in the danger of infectious diseases owing to the antibiotic resistance’s continued growth, which in the future will constitute a main threat to world health [1, 2]. Unless proper precautions are followed, antimicrobial-resistant diseases are projected to cause the deaths of millions in the next decades [3, 4]. New antibiotic categories with innovative mechanisms of action may avoid resistance [5–7], so they must be found instantly. Hence, understanding medication resistance processes is one of the most prominent trends in infectious illness research [8, 9].
Antibiotic-resistant Gram-positive bacteria involving vancomycin-resistant Enterococci faecalis (VRE), methicillin-resistant Staphylococcus aureus (MRSA), and methicillin-resistant Staphylococcus epidermidis (MRSE) cause severe public health issues [10]. MRSA is among a number of challenging-to-cure infections that cause elevated mortality and morbidity in the USA [11–13]. MRSE is also recognized as a human opportunistic pathogen, and it can induce a range of fatal diseases [14]. As one of the most common enterococcal species, VRE has been identified as a common reason of endocarditis and the second-greatest contributory factor of wound and nosocomial urinary tract infections in the USA because of its capacity to develop resistance to the overwhelming bulk of antibiotics presently in use [15].
These Gram-positive bacterial infections have caused enormous costs and mortality all over the world [16–18]. The scientific community has made efforts to combat infections caused by these antibiotic-resistant pathogens [19–24]. Innovative antibiotics to treat highly lethal Gram-positive bacterial infections, on the other hand, are desperately needed.
In medicinal chemistry, nitrogen-comprising heterocycles are recognized as one of the most vital components. Quinolines and their physiologically potent isostere have received the most interest among heterocyclic rings [25–27]. Many research groups have continuously evaluated the biological applications and chemistry of the quinoline-2-one scaffold over the last decade [28, 29]. These compounds are used as wide-spectrum antibiotics versus Gram-positive and Gram-negative bacteria, and also numerous fungal strains [30, 31]. Because of their broad antibacterial spectrum and favorable pharmacokinetics, quinolone analogs are widely utilized in clinical practice around the world [32]. While the quest for novel hybrid, next-generation quinolones continue [33], bacterial resistance to these medications is increasing owing to their misuse. These bacterial strains have now become a global concern [34, 35].
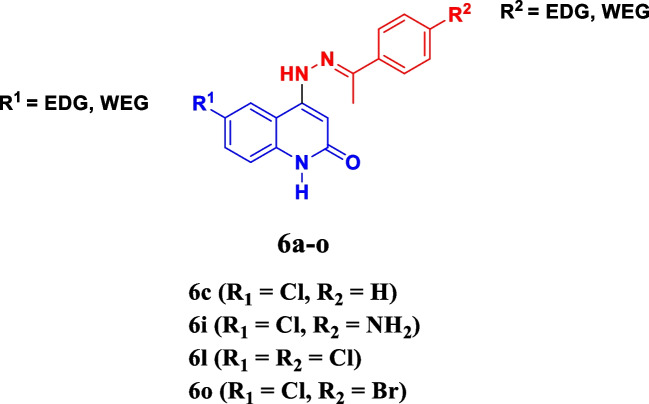
Recently [36], we focused on the design, synthesis, and antimicrobial activity of a brand-new group of quinoline-2-one Schiff-base hybrids 6a–o (Fig. 1). All compounds were evaluated for antimicrobial action versus the Gram-positive bacteria Staphylococcus aureus and Bacillus subtilis, Gram-negative bacteria Escherichia coli and Klebsiella pneumoniae, and the two fungus Aspergillus niger and Candida albicans. Compounds 6c, 6i, 6l, and 6o (Table 1) were noted to be the most efficient derivatives versus S. aureus, with MIC concentrations varying from 0.018 to 0.061 μg/mL, compared to ciprofloxacin’s MIC of 0.018 μg/mL. In addition, the inhibition effectiveness of the most active antibacterial compounds 6i, 6c, 6l, and 6o versus E. coli topoisomerase IV and DNA gyrase as prospective antibacterial targets was examined.
Fig. 1.
Structures of compounds 6c, 6i, 6l and 6o
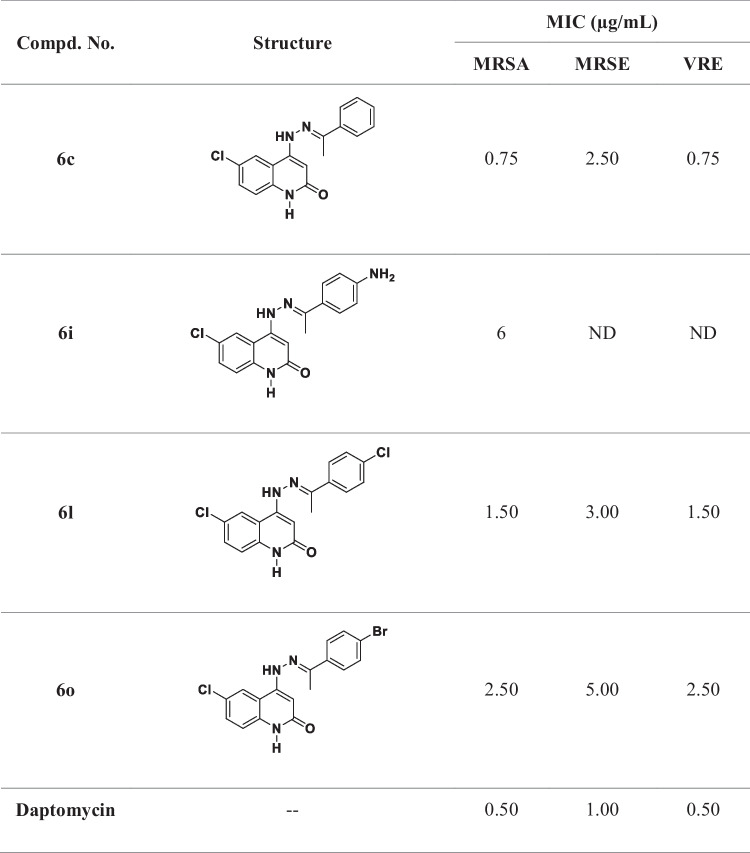
Table 1.
MIC values of compounds 6c, 6i, 6l, and 6o against MRSA, MRSE, and VRE
ND not determined
Compounds 6l and 6c were noted to be the potent derivatives, with inhibitory activities of E. coli DNA gyrase (IC50 = 327 ± 30 nM and 280 ± 30 nM, consecutively) in comparison to the reference novobiocin (IC50 = 170 ± 20 nM). In contrast to the standard novobiocin (IC50 = 11 μM), 6I and 6c demonstrated encouraging inhibitory activity versus E. coli topoisomerase IV, with IC50 values of 22.90 ± 2.50 μM and 17.50 ± 1.50 μM, consecutively [36].
Motivated by the promising antibacterial activity of compounds 6c, 6i, 6l, and 6o, and in continuation of our efforts to identify a novel antibacterial agent capable of overcoming antimicrobial resistance (AMR) [37–42], the most potent antibacterial derivatives, 6c, 6i, 6l, and 6o, were chosen from the newly synthesized series (6a–o) and evaluated versus three clinically appropriate, multidrug-resistant Gram-positive bacterial pathogens, MRSE, VRE, and MRSA, with daptomycin as the control drug. These derivatives’ biofilm and dihydrofolate reductase (DHFR) inhibitory activities were also investigated.
Experimental
Chemistry
General details
All materials were obtained commercially and used without additional purification. TLC was used to monitor the reactions (Kieselgel 60 F254 precoated plates, E. Merck, Germany), and the spots were spotted by exposing them to a UV light at 254 nm. Melting points were calculated uncorrected using an electrothermal melting point instrument (Stuart Scientific Co.). NMR spectra were measured on a Bruker AV-400 spectrometer (Bruker BioSpin Corp., Billerica, MA, USA) (400 MHz for 1H, 101 MHz for 13C) at NMR unit, Mansoura University, Egypt. The 1H and 13C chemical shifts are given relative to internal standard TMS = 0; Vario EL III German CHN Elemental analyzer model was used for elemental analysis.
Synthesis of target compounds 6c, 6i, 6l, and 6o
A suspension of compounds 5a–c (1.0 mmol) in ethanol (20 mL) was treated with the appropriate acetophenone derivative (1.0 mmol) and 3 drops of glacial acetic acid. For 10–12 h, the reaction mixture was refluxed. After cooling, the precipitate was filtered out, washed with diethyl ether, and crystallized from ethanol to yield compounds 6c, 6i, 6l, and 6o.
The supplementary file contains all spectral data related to compounds 6c, 6i, 6l, and 6o.
Biology
Antibacterial activity
The most potent antibacterial derivatives, 6c, 6i, 6l, and 6o, were chosen from the newly synthesized series and evaluated versus 3 clinically appropriate, multidrug-resistant Gram-positive pathogenic bacteria, MRSA, VRE, and MRSE, utilizing daptomycin as the control drug [19]. MRSA (ATCC 33591), MRSE (RP62A), and VRE (ATCC 700802) were employed. The MICs were determined as the lowest concentration that completely inhibits the bacteria growth. Compounds were dissolved in DMSO to make stock drug solution, and the final concentration of DMSO in drug-treated group was less than 0.5%. The experiments were repeated at least three times with duplicates each time.
All bacterial strains were obtained from the American Type Culture Collection (ATCC).
Biofilm inhibitory assay
To evaluate the ability of compound derivatives to inhibit or reduce the biofilm formation of bacteria, MTP method was used against S. aureus clinical isolate (identified potent biofilm producing MRSA strain); the biofilm assay was performed as the previous study with some modifications [43].
DHFR inhibitory activity
The DHFR inhibitory activity of derivatives 6c, 6l, and 6o was tested using the DHFR supercoiling test, and findings were presented as IC50 values in (μM) versus trimethoprim as a control [44]. Appendix A (Supplementary File) contains additional information.
Cell viability assay
The MCF-10A (human mammary gland epithelial) cell line was used in a cell viability assay to examine the impact of compounds 6c, 6i, 6l, and 6o on normal cell lines [45, 46]. See Appendix A.
Results and discussion
Chemistry
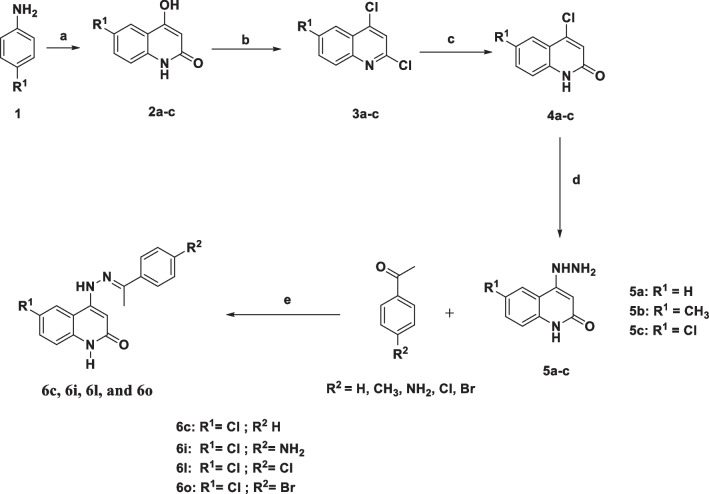
The target compounds 6c, 6i, 6l, and 6o were prepared using previously described procedures [36], Scheme 1. To validate the structures of the synthesized compounds, physical constants as well as spectral data (1H NMR and 13C NMR) were compared to previously reported data.
Scheme 1.
Synthetic pathways for compounds 6c, 6i, 6l, and 6o
Biology
Antibacterial activity
The most potent antibacterial derivatives, 6c, 6i, 6l, and 6o, were chosen from the newly synthesized series and evaluated versus 3 clinically appropriate, multidrug-resistant Gram-positive pathogenic bacteria, MRSA, VRE, and MRSE, utilizing daptomycin as the control drug [19]. Table 1 shows the minimum inhibitory concentration (MIC) value for each compound.
Compounds 6c, 6l, and 6o, with the exception of compound 6i, demonstrated potent antibacterial activity versus the three Gram-positive bacterial pathogens tested. Compound 6c (R1 = Cl, R2 = H, Table 1) revealed the most potent activity among the tested derivatives, with MIC concentrations of 0.75 μg/mL versus MRSA and VRE and 2.50 μg/mL versus MRSE, in comparison to the standard daptomycin, which demonstrated MIC values of 0.50 μg/mL against MRSA and VRE and 1.0 μg/mL against MRSE.
The addition of a chlorine atom to R2 yields compound 6l (R1 = Cl, R2 = Cl). When compared to 6c, the activity of 6l against MRSA and VRE decreased twofold, with a MIC concentration of 1.50 μg/mL, but not significantly against MRSE (MIC = 3.0 μg/mL). Compound 6o (R1 = Cl, R2 = Br) ranked third in activity with a MIC concentration of 2.50 μg/mL against both MRSA and VRE, being 5-fold less potent than the reference daptomycin versus these two pathogens. Moreover, compound 6o demonstrated moderate activity versus MRSE, with a MIC concentration of 5.0 μg/mL.
Finally, when R2 was retained as the amino group, 6i (R1 = Cl, R2 = NH2), only weak activities were detected versus MRSA, with a MIC of 6.0 μg/mL, being 12-fold less potent than the reference daptomycin. These findings demonstrated the importance of the substitution pattern on the phenyl ring’s para position of the benzylidene moiety with increasing activity in the order (R2) = H > Cl > Br > NH2.
S. aureus biofilm inhibition activity
Biofilm was the primary cause of infection in over 80% of cases, comprising microbial cells surface related with an extracellular polymer matrix. They are extremely difficult to treat due to their significant MDR [43].
In a biofilm assay, the most active derivatives 6c, 6l, and 6o were tested, and results were cited in Table 2. When contrasted to vancomycin as a positive control, derivative 6c showed the greatest antibiofilm activity in a dose-dependent way and a substantial decrease of biofilm construction in MRSA ACL51 strain with 79, 55, and 38% at levels of 0.50, 0.25, and 0.12 MIC, consecutively, whereas the corresponding values for vancomycin were 20, 12, and 9%. Compound 6l inhibited biofilms by 48, 35, and 20% at MIC concentrations of 0.5, 0.25, and 0.12, respectively. Finally, at concentrations of 0.5, 0.25, and 0.12 MIC, derivative 6o exhibited weak biofilm inhibition percentages of 18, 10, and 7%, respectively.
Table 2.
Biofilm inhibition percentage of 6c, 6l, 6o and vancomycin against the biofilm formation of S. aureus ACL51 (MRSA) strain
| Compd. no. | Inhibition of biofilm mass (%) | ||
|---|---|---|---|
| 1/2 MIC | 1/4 MIC | 1/8 MIC | |
| 6c | 79 | 55 | 38 |
| 6l | 48 | 35 | 20 |
| 6o | 18 | 10 | 7 |
| Vancomycin | 20 | 12 | 9 |
DHFR inhibitory activity
To validate the antimicrobial mechanism, the DHFR inhibitory activity of derivatives 6c, 6l, and 6o was tested. Table 3 show the DHFR supercoiling test findings as IC50 values in (μM) versus trimethoprim as a control [44].
Table 3.
Inhibitory activity of 6c, 6l, and 6o against DHFR enzyme
| Compd. no. | IC50 (μM) |
|---|---|
| 6c | 3.25 ± 0.15 |
| 6l | 6.30 ± 0.20 |
| 6o | 7.40 ± 0.25 |
| Trimethoprim | 8.40 ± 0.15 |
The findings demonstrated that the evaluated compounds 6c, 6l, and 6o inhibited the DHFR enzyme with IC50 concentrations varying from 3.25 ± 0.15 to 7.40 ± 0.25 μM, which were more potent than trimethoprim (8.40 ± 0.15 μM). Compound 6c was noted to be the most effective DHFR inhibitory derivative, with an IC50 concentration of 3.25 ± 0.15 μM, making it 2.5-fold more potent than trimethoprim. Compound 6l ranked second in terms of DHFR inhibitory activity, with an IC50 concentration of 6.30 ± 0.20 μM, while derivative 6o revealed to be the least potent inhibitor of DHFR with an IC50 concentration of 7.40 ± 0.25 μM.
Cell viability assay
The MCF-10A (human mammary gland epithelial) cell line was used in a cell viability assay to examine the impact of compounds 6c, 6i, 6l, and 6o on normal cell lines [45, 46]. In this investigation, the studied compounds are used at a concentration of 50 μM for 4 days before cell viability is assessed. Table 4 demonstrates that compounds 6c, 6i, 6l, and 6o have a cell viability of more than 89% and no cytotoxic effects.
Table 4.
Cell viability % of compounds 6c, 6i, 6l, and 6o
| Compd. no. | Cell viability (%) |
|---|---|
| 6c | 91 |
| 6i | 89 |
| 6l | 92 |
| 6o | 90 |
Conclusion
Four compounds were selected from a series of quinoline-2-one derivatives 6a–o and tested for antibacterial activity against antibiotic-resistant Gram-positive bacteria including methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant Staphylococcus epidermidis (MRSE), and vancomycin-resistant Enterococci faecalis (VRE). The antibacterial activity of compounds 6c, 6l, and 6o against Gram-positive bacterial pathogens tested was promising. Compound 6c demonstrated the most potent activity among the tested derivatives when compared to the reference daptomycin. More in-depth research into this class of compounds are required to determine the mechanistic pathways underlying antibacterial action.
Supplementary information
Acknowledgements
Professor Dr. Bahaa G. M. Youssif, and Dr. Elbastawesy M.A., Pharmaceutical Organic Chemistry Department, Faculty of Pharmacy, Assiut University, Assiut, Egypt, are gratefully acknowledged for providing compounds 6c, 6i, 6l, and 6o for biological assays.
Declarations
Conflict of interest
The author declares no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lomazzi M, Moore M, Johnson A, Balasegaram M, Borisch B. Antimicrobial resistance–moving forward? BMC pub health. 2019;19:1–6. doi: 10.1186/s12889-019-7173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chokshi A, Sifri Z, Cennimo D, Horng H. Global contributors to antibiotic resistance. J Global Infect Dis. 2019;11(1):36–42. doi: 10.4103/jgid.jgid_110_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Kraker ME, Stewardson AJ, Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016;13(11):e1002184. doi: 10.1371/journal.pmed.1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gwynn MN, Portnoy A, Rittenhouse SF, Payne DJ. Challenges of antibacterial discovery revisited. Ann N Y Acad Sci. 2010;1213(1):5–19. doi: 10.1111/j.1749-6632.2010.05828.x. [DOI] [PubMed] [Google Scholar]
- 6.Abushaheen MA, Fatani AJ, Alosaimi M, Mansy W, George M, Acharya S et al (2020) Antimicrobial resistance, mechanisms and its clinical significance Disease-a-Month. 66(6):100971 [DOI] [PubMed]
- 7.Rello J, Bunsow E, Perez A. What if there were no new antibiotics? A look at alternatives. Expert Rev Clin Pharmacol. 2016;9(12):1547–1555. doi: 10.1080/17512433.2016.1241141. [DOI] [PubMed] [Google Scholar]
- 8.Askari S, B, Krajinovic M. Dihydrofolate reductase gene variations in susceptibility to disease and treatment outcomes. Curr Genomics. 2010;11(8):578–583. doi: 10.2174/138920210793360925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eshetie S, Gizachew M, Dagnew M, Kumera G, Woldie H, Ambaw F, et al. Multidrug resistant tuberculosis in Ethiopian settings and its association with previous history of anti-tuberculosis treatment: a systematic review and meta-analysis. BMC Infect Dis. 2017;17:1–12. doi: 10.1186/s12879-017-2323-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watkins RR, David MZ, Salata RA. Current concepts on the virulence mechanisms of meticillin-resistant Staphylococcus aureus. J Med Microbiol. 2012;61(Pt 9):1179. doi: 10.1099/jmm.0.043513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krute CN. Investigation of post-translational modifications in Staphylococcus aureus. University of South Florida; 2015. [Google Scholar]
- 12.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;13(12):1840. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris SJ, Cormican M, Cummins E. Antimicrobial residues and antimicrobial-resistant bacteria: impact on the microbial environment and risk to human health—a review. Hum Ecol Risk Assess Int J. 2012;18(4):767–809. doi: 10.1080/10807039.2012.688702. [DOI] [Google Scholar]
- 14.Gill SR, Fouts DE, Archer GL, Mongodin EF, DeBoy RT, Ravel J, et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol. 2005;187(7):2426–2438. doi: 10.1128/JB.187.7.2426-2438.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cetinkaya Y, Falk P, Mayhall CG. Vancomycin-resistant enterococci. Clin Microbiol Rev. 2000;13(4):686–707. doi: 10.1128/CMR.13.4.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, et al. Health care–associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 17.Feng T, Lu H, Ye X, Nie C, Zhang J, Yu L, et al. Selective inactivation of Gram-positive bacteria in vitro and in vivo through metabolic labelling. Sci China Mater. 2022;65(1):237–245. doi: 10.1007/s40843-021-1735-0. [DOI] [Google Scholar]
- 18.Kara Ali R, Surme S, Balkan II, Salihoglu A, Sahin Ozdemir M, Ozdemir Y, et al. An eleven-year cohort of bloodstream infections in 552 febrile neutropenic patients: resistance profiles of Gram-negative bacteria as a predictor of mortality. Ann Hematol. 2020;99:1925–1932. doi: 10.1007/s00277-020-04144-w. [DOI] [PubMed] [Google Scholar]
- 19.Teng P, Huo D, Nimmagadda A, Wu J, She F, Su M, et al. Small antimicrobial agents based on acylated reduced amide scaffold. J Med Chem. 2016;59(17):7877–7887. doi: 10.1021/acs.jmedchem.6b00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stokes JM, MacNair CR, Ilyas B, French S, Côté J-P, Bouwman C, et al. Pentamidine sensitizes Gram-negative pathogens to antibiotics and overcomes acquired colistin resistance. Nat Microbiol. 2017;2(5):1–8. doi: 10.1038/nmicrobiol.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Connell KM, Hodgkinson JT, Sore HF, Welch M, Salmond GP, Spring DR. Combating multidrug-resistant bacteria: current strategies for the discovery of novel antibacterials. Angewandte Chemie Int Ed. 2013;52(41):10706–10733. doi: 10.1002/anie.201209979. [DOI] [PubMed] [Google Scholar]
- 22.Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, et al. A new antibiotic kills pathogens without detectable resistance. Nature. 2015;517(7535):455–459. doi: 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin S, Koh J-J, Aung TT, Lim F, Li J, Zou H, et al. Symmetrically substituted xanthone amphiphiles combat gram-positive bacterial resistance with enhanced membrane selectivity. J Med Chem. 2017;60(4):1362–1378. doi: 10.1021/acs.jmedchem.6b01403. [DOI] [PubMed] [Google Scholar]
- 24.LaMarche MJ, Leeds JA, Brewer J, Dean K, Ding J, Dzink-Fox J, et al. Antibacterial and solubility optimization of thiomuracin A. J Med Chem. 2016;59(14):6920–6928. doi: 10.1021/acs.jmedchem.6b00726. [DOI] [PubMed] [Google Scholar]
- 25.Aly AA, Ramadan M, Abuo-Rahma GE-DA, Elshaier YAMM, Elbastawesy MAI, Brown AB, et al. Chapter three-quinolones as prospective drugs: their syntheses and biological applications. Adv Heterocy Chem. 135: Academic Press; (2021).147-196.
- 26.Dib M, Ouchetto H, Ouchetto K, Hafid A, Khouili M. Recent Developments of quinoline derivatives and their potential biological activities. Curr Org Synth. 2021;18(3):248–269. doi: 10.2174/1570179417666201216162055. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava V, Singh PK, Tivari S, Singh PP. Visible light photocatalysis in the synthesis of pharmaceutically relevant heterocyclic scaffolds. Organic Chemistry Front 2022;9(5):1485-1507.
- 28.Ajani OO, Iyaye KT, Ademosun OT. Recent advances in chemistry and therapeutic potential of functionalized quinoline motifs - a review. RSC Adv. 2022;12(29):18594–18614. doi: 10.1039/D2RA02896D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matada BS, Pattanashettar R, Yernale NG. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg Med Chem. 2021;32:115973. doi: 10.1016/j.bmc.2020.115973. [DOI] [PubMed] [Google Scholar]
- 30.Al-Jumaily E, Latif A, Al-Bayati R. Effect of a new quinoline-2-one derivatives (compound 3) on purified DNA gyrase from clinical isolate Pseudomonas aeruginosa PA31. IOSR J Pharmacy. 2016;12:12–18. [Google Scholar]
- 31.Proisl K, Kafka S, Kosmrlj J. Chemistry and applications of 4-hydroxyquinolin-2-one and quinoline-2, 4-dionebased compounds. Curr Org Chem. 2017;21(19):1949–1975. doi: 10.2174/1385272821666170711155631. [DOI] [Google Scholar]
- 32.Estradé O, Vozmediano V, Carral N, Isla A, González M, Poole R, et al. Key factors in effective patient-tailored dosing of fluoroquinolones in urological infections: interindividual pharmacokinetic and pharmacodynamic variability. Antibiotics. 2022;11(5):641–652. doi: 10.3390/antibiotics11050641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Millanao AR, Mora AY, Villagra NA, Bucarey SA, Hidalgo AA. Biological effects of quinolones: a family of broad-spectrum antimicrobial agents. Molecules. 2021;26(23):7153–7163. doi: 10.3390/molecules26237153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fair RJ, Tor Y (2014) Antibiotics and bacterial resistance in the 21st century. Perspect med chem 6; PMC. S14459 [DOI] [PMC free article] [PubMed]
- 35.Hooper DC, Jacoby GA. Mechanisms of drug resistance: quinolone resistance. Ann N Y Acad Sci. 2015;1354(1):12–31. doi: 10.1111/nyas.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elbastawesy MA, Mohamed FA, Zaki I, Alahmdi MI, Alzahrani SS, Alzahrani HA et al (2023) Design, synthesis and antimicrobial activity of novel quinoline-2-one hybrids as promising DNA gyrase and topoisomerase IV inhibitors. J Mol Struct 1278:134902
- 37.Youssif BGM. Synthesis and biological evaluation of some new coumarin derivatives as antimicrobial agents. Bullet Pharmaceut Sci Assiut. 2013;36(2):105–116. doi: 10.21608/bfsa.2013.63201. [DOI] [Google Scholar]
- 38.Youssif B, Abdel-Moty SG, Sayed IM. Synthesis and biological evaluation of some novel 1, 2, 3-triazol-N-arylidene Acetohydrazide incorporating benzimidazole ring moiety as potential antimicrobial agents. J Curr Chem Pharm Sci. 2014;4:54–64. [Google Scholar]
- 39.Hofny HA, Mohamed MF, Gomaa HA, Abdel-Aziz SA, Youssif BG, El-Koussi NA, et al. Design, synthesis, and antibacterial evaluation of new quinoline-1, 3, 4-oxadiazole and quinoline-1, 2, 4-triazole hybrids as potential inhibitors of DNA gyrase and topoisomerase IV. Bioorg Chem. 2021;112:104920. doi: 10.1016/j.bioorg.2021.104920. [DOI] [PubMed] [Google Scholar]
- 40.Frejat FOA, Cao Y, Zhai H, Abdel-Aziz SA, Gomaa HA, Youssif BG, et al. Novel 1, 2, 4-oxadiazole/pyrrolidine hybrids as DNA gyrase and topoisomerase IV inhibitors with potential antibacterial activity. Arab J Chem. 2022;15(1):103538. doi: 10.1016/j.arabjc.2021.103538. [DOI] [Google Scholar]
- 41.Frejat FOA, Zhai H, Cao Y, Wang L, Mostafa YA, Gomaa HA, et al. Novel indazole derivatives as potent apoptotic antiproliferative agents by multi-targeted mechanism: synthesis and biological evaluation. Bioorg Chem. 2022;126:105922. doi: 10.1016/j.bioorg.2022.105922. [DOI] [PubMed] [Google Scholar]
- 42.Al-Wahaibi LH, Amer AA, Marzouk AA, Gomaa HA, Youssif BG, Abdelhamid AA. Design, synthesis, and antibacterial screening of some novel heteroaryl-based ciprofloxacin derivatives as DNA gyrase and topoisomerase IV inhibitors. Pharmaceut. 2021;14(5):399. doi: 10.3390/ph14050399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdelhameed RM, Abu-Elghait M, El-Shahat M. Hybrid three MOFs composites (ZIF-67@ ZIF-8@ MIL-125-NH2): enhancement the biological and visible-light photocatalytic activity. J Environ Chem Eng. 2020;8(5):104107. doi: 10.1016/j.jece.2020.104107. [DOI] [Google Scholar]
- 44.Rashid N, Thapliyal C, Chattopadhyay PC (2016) Dihydrofolate reductase as a versatile drug target in healthcare. J Proteins Proteom 7(4)
- 45.Gomaa HA, Shaker ME, Alzarea SI, Hendawy O, Mohamed FA, Gouda AM, et al. Optimization and SAR investigation of novel 2, 3-dihydropyrazino [1, 2-a] indole-1, 4-dione derivatives as EGFR and BRAFV600E dual inhibitors with potent antiproliferative and antioxidant activities. Bioorg Chem. 2022;120:105616. doi: 10.1016/j.bioorg.2022.105616. [DOI] [PubMed] [Google Scholar]
- 46.Gomaa HA, El-Sherief HA, Hussein S, Gouda AM, Salem OI, Alharbi KS, et al. Novel 1, 2, 4-triazole derivatives as apoptotic inducers targeting p53: synthesis and antiproliferative activity. Bioorg Chem. 2020;105:104369. doi: 10.1016/j.bioorg.2020.104369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.