Abstract
Xylanase is widely used in various industries such as food processing, paper, textiles, and leather tanning. In this study, Bacillus cereus L-1 strain was isolated and identified as capable of producing low molecular weight xylanase through 16 s rRNA sequencing. Maximum xylanase yield of 15.51 ± 2.08 U/mL was achieved under optimal fermentation conditions (5% inoculum, 20 g/L xylan, pH 6.0, for 24 h). After purification via ammonium sulfate precipitation and High-S ion exchange chromatography, electrophoretic purity xylanase was obtained with a 28-fold purification and specific activity of 244.97 U/mg. Xylanase had an optimal pH of 6.5 and temperature of 60 °C and displayed thermostability at 30 °C and 40 °C with 48.56% and 45.97% remaining activity after 180 min, respectively. The xylanase retained more than 82.97% of its activity after incubation for 24 h at pH 5.0 and was sensitive to metal ions, especially Mg2+ and Li+. Purified xylanase showed a molecular weight of 23 kDa on SDS-PAGE, and partial peptide sequencing revealed homology to the endo-1,4-beta-xylanase with a molecular weight of 23.3 kDa through LC/MS–MS (liquid chromatography-tandem mass spectrometry). This study suggests that the purified xylanase is easier to purify and enriches low molecular weight xylanases from bacteria source.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-023-01129-5.
Keywords: Bacillus cereus, Enzyme property, Low molecular weight, Production, Purification, Xylanase
Introduction
Hemicellulose is the second most abundant source of biomass after cellulose [1]. Numerous studies have focused on hemicellulose degradation for various applications, and several methods have been developed to increase efficiency and obtain more xylose, including physical, chemical, and biological methods [2]. Biological methods use enzymes, which are highly selective and produce fewer by-products than other methods that require extreme conditions such as high temperature and pressure, or acidic or basic environment [3]. Xylanase, a key enzyme that degrades xylan into xylose, has wide application in the textile processing, energy conversion, feed, detergent, and pulp production industries [4–6].
Xylanases are produced by microorganisms, protozoans, mollusks, and are also found in the rumen of higher animals [7]. Among them, microbial-derived xylanase is mainly divided into bacteria xylanase and fungal xylanase based on their sources [8–12]. Bacteria xylanase has attracted more attention due to its advantages of shorter fermentation period, richer products, and easier fermentation techniques. Bacteria xylanase belongs to the hydrolase group of enzymes, mainly including endoxylanases and exoxylanases that degrade the xylan. Low molecular weight endo-xylanases are preferred because then can effectively hydrolyze xylan in pulp bleaching, diffuse well into biomass structures or fiber pulp, and have less metabolic burden during synthesis [13]. Moreover, low molecular xylanases are stable at a wide range of pH and temperature, making the process more economical [14]. In addition, purification is critical for obtaining the protein, with common methods including ammonium sulfate precipitation, ultrafiltration, ion chromatography, and gel filtration chromatograph. Efficient and low-cost techniques for xylanases recycling and purification are necessary, especially those that reduce production time and costs [15].
In this study, we isolated and identified a strain from cigar leaves, named Bacillus cereus L-1, using molecular identification technique based on the 16S rDNA sequence. We tested and analyzed the morphologies and biochemical characteristics of L-1, which secreted xylanase degrading xylan into monose. We then conducted fermentation parameters of L-1, enzyme properties of xylanase, purification processes, molecular weight of xylanase, and xylanase identification. This study provided a strain capable of secreting low molecular weight xylanase, enriching the low molecular weight xylanases from bacteria.
Materials and methods
Media and regents
Primary screening medium, also named xylan agar plates (XAP), contained 20 g/L xylan (xylan ≥ 85.0%, Yuanye Biochemical Co., Ltd, China), 0.5 g/L K2HPO4, 0.5 g/L KH2PO4, 2.0 g/L NH4Cl, 0.02 g/L CaCl2·2H2O, 0.01 g/L K2SO4, 0.02 g/L NaCl, 3.0 g/L MgSO4·7H2O, and 20.0 g/L agar powder, sterilized at 115 °C for 30 min. Original fermentation medium contained 20.0 g/L xylan, 0.2 g/L MgSO4·7H20, 2.0 g/L NH4Cl, 1.0 g/L K2HPO4, and 1.0 g/L KH2PO4, pH 6.6, sterilized at 115 °C for 30 min. LB (Luria–Bertani) medium 10.0 g/L tryptone, 5.0 g/L yeast extract, and 10.0 g/L NaCl are sterilized at 115 °C for 30 min.
Isolation and identification of strain
The samples were from cigar wrappers (Hainan, China), soil (Qingdao, China), and decaying leaves (Qingdao, China). The samples were treated with normal saline in a 1:10 (g/mL), and then the suspension was coated on XAP media by dilution coating method. The media were placed at 37 °C for 72 h to obtain colonies with transparent circles. The colonies were inoculated on XAP media and placed at same conditions to get single strain.
Primary screening all the strain were primarily screened for the xylanase producing ability by Congo red assay and detecting the culture using DNS regent. The diameter value of the transparent circle divided by the diameter of the colony was named as Hc value, which indicated the ability to produce xylanase. The ability of strains to produce xylanase in original fermentation medium was also tested using the DNS reagent.
The strain was identified according to the 16S ribosomal RNA (rRNA) method and tested the biochemical characteristics using microbial biochemical identification tubes (Zhaoyuan Top Biotech. Co., Ltd.) according to the instruction, including methyl red, voges–proskauer, mannitol, glucose utilization, casein, catalase, nitrate reduction, lactose, phenylalanine, starch, and gelatin, giving some metabolism characteristics for L-1. Morphology characteristics contain shape, color, and margin [16].
The 16S rDNA sequence was amplified by polymerase chain reaction (PCR) using primer 27F(5′-3′ AGAGTTTGATCCTGGCTCAG) and primer 1492R (5′-3′ ACGGCTACCTTGTTACGACTT). Afterwards, the final volume of PCR reaction, including 21.0 µL double distilled H2O (ddH2O), 1.0 µL 27F (10 µM), 1.0 µL 1492R (10 µM), 25.0 µL Premix Taq™ (LA Taq™ Version 2.0, Takara Bio, Japan), and 1.0 µL genomic template of the strain, was amplified. Then, the fragment was sequenced by Tsingke Biological (Tsingke Biotechnology Co., Ltd.). The sequence was blasted in NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and submitted to NCBI database with the accession number MW570877.1. The phylogenetic tree of the strain was constructed by MEGA (version 7.0) by the way of neighbor-joining. The analysis of bootstrap was based on 1000 resamples [17].
The assay of xylanase activity
Xylanase activity was measured by DNS (3,5-dinitrosalicylic acid) method at 540 nm using automatic microplate reader (TECAN SPARK 10 M) [18]. Supernatant without cell (100 µL) was mixed with 0.9 mL 1% xylan solution as the substrate (50 mmol/L citric acid-Na2HPO3, pH 7.0). Subsequently, the mixture was placed in the incubator at 60 °C for 30 min. Next, the volume of 0.5 mL was taken out from the reaction system mixing with 0.5 mL DNS solution, boiling for 4 min, and cooling it to room temperature. Then, 5.5 mL ddH2O was added, mixed, and detected the absorbance at 540 nm. As a comparison, boil the supernatant for 10 min to inactivate the xylanase.
In this experiment, the one unit (1 U) was expressed as the amount of the xylanase that generated 1 µmol reducing sugar which was xylose by breaking down 1% of the xylanase solution per min. The following formula was the calculation method of xylanase activity:
where ΔA was according to the standard curve to calculate after deducting contrast of xylose production (µmol), N was the dilution factor, T was 30 min, and M was a xylose molecular mass of 150.
Definition of the fermentation conditions
To obtain the fermentation condition with Bacillus cereus L-1, it was important to determine its fermentation period. Xylanase activity was measured every 8 h for up to 64 h to obtain the desired period. A single colony of the L-1 strain was incubated in LB (Luria–Bertani) medium at 37 °C for 10 h to obtain the seed liquid with approximately 0.395 (OD600) after being diluted tenfold. This was added as a 5% (v/v) inoculum volume into 300-mL shaking flask containing 100 mL original fermentation medium. The flasks were then placed and cultured at 37 °C and 180 rpm for up to 64 h, and all results were repeated three times. One-way ANOVA (analysis of variance) using Tukey’s test was used to text significant between SAS (Statistical Analysis System) treatments (p < 0.05).
The effect of different inoculation concentration (1%, 3%, 5%, 7%, and 9% v/v) on xylanase production was studied under the same conditions as before. Similarly, different concentrations of xylan (5.0 g/L, 10.0 g/L, 15.0 g/L, 20.0 g/L, and 30.0 g/L) in the fermentation medium at an initial pH of 6.7 were studied under a 5% (v/v) inoculation concentration. The culture was placed at 37 °C and 180 rpm for 24 h. Finally, the effect of fermentation medium pH on the xylanase yield was performed, ranging from 4.0 to 8.0 at one pH unit intervals. The initial culture medium was sterilized at 115 °C for 30 min before adjustment, and the culture was placed at 37 °C and 180 rpm for 24 h.
Purification of xylanase
After optimizing the fermentation parameters (xylan 20.0 g/L, 5% inoculation concentration, pH 7.0, fermentation period of 24 h), approximately 1.70 L of fermentation liquid was obtained. The supernatant containing xylanase was then obtained by centrifuge (Hunan Xiang Yi Laboratory Instrument Development Co., Ltd., China, 2050R) at 4 °C and 6000 g for 15 min.
Partial purification of xylanase was achieved using ammonium sulfate precipitation and ion exchange chromatography. Ammonium sulfate saturation at 20%, 30%, 40%, 50%, 60%, 70%, 80%, and 90% was applied slowly in 20 mL supernatant under 0 °C, respectively, overnight at 4 °C. The precipitate and supernatant were then obtained by centrifuging at 6000 × g for 20 min. Xylanase activity of precipitation and supernatant with different saturation was determined using DNS method, determining the fractionation curve of ammonium sulfate. Base on the results, the saturation of ammonium sulfate was determined to obtain the target protein. The crude enzyme was obtained by dialysis of the precipitate several times in the equilibration buffer.
The crude xylanase was further purified by Mini Macro-Prep High-S ion exchange chromatography (Bio-Rad Laboratories). Tris–HCl equilibration buffer (0.5 mM, pH 7.0) with 0 M and 1 M NaCl was prepared, and linear gradient elution was carried out at a flow rate of 0.5 mL/min. Protein was detected at UV 280 nm, recording absorption curve, and the xylanase activity in the collection was measured using DNS method.
Enzyme properties
Enzyme activity is greatly influenced by temperature, pH, and metal ions, which are significant characteristics of biocatalysts for industrial application. In this study, various xylanase properties, including optimal pH, pH stability, optimal temperature, thermal stability, and the effect of metal ions on xylanase activity, were assessed. Xylanase’s optimal pH was measured using a citrate–phosphate buffer (pH 3.0–8.0, 50 mmol/L, 1% xylan) at 60 °C. To determine pH stability, xylanase was pre-incubated in the same buffers at 4 °C for 12 h and 24 h to assess residual activities. The optimum temperature range of xylanase was measured at the optimum pH condition, ranging from 30 to 70 °C. Thermal stability was evaluated by incubating xylanase at 30 °C, 40 °C, 50 °C, 60 °C, and 70 °C and analyzing samples taken at 20 min intervals. The remaining xylanase activity was determined under standard conditions. The effect of metal ions (K+, Mg2+, Ba2+, Ca2+, Mn2+, Cu2+, Zn2+, Ni2+, Co2+, Ag+, and Li+) on xylanase activity was determined at a concentration of 1.0 mM.
Determination of molecular weight by SDS-PAGE and protein concentration by BCA
Protein concentration was determined using a bovine serum albumin (BCA) standard curve (Beijing Solaibao) according to the instruction [19]. Xylanase molecular weight was identified via sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) using 12% gel and protein marker [20]. The resulting protein was visualized by coomassie brilliant blue (CBB) R-250 staining.
LC–MS/MS detection of xylanase
To analyze the xylanase signal band, it was cut from the gel and subjects to liquid chromatographic-tandem mass spectrometric (LC–MS/MS) analysis by BGI Life Technology [21]. Protein specimens were processed with trypsin for 20 h at 37 °C, and the peptides were captured, desalted, and separated as described by Zhang [22]. A sample NLC system (Thermo Fisher Scientific) was used to transport mobile phase A (0.1% formic acid) and B (0.1% formic acid in 84% acetonitrile) in accordance with the traditional approach [23]. The mass spectrometer operated in MS/MS mode with a scanning range of 380–1800 amu. Top 20 charge ions from each scan were selected for MS/MS analysis [24]. The MS/MS spectrum was searched using MASCOT 2.2 (Matrix Science, London, UK), and the protein was identified by searching the UniProt database (https://www.uniprot.org/).
Statistics analysis
All the statistics and graphics were analyzed by Microsoft 2016 and Origin 2018. The data analysis of variance was supported by ANOVA (p = 0.05).
Results
Screening of strains with higher xylanase activity
On XAP (xylan agar plates) medium, we obtained six strains with transparent circles and determined their enzyme activity in the original fermentation medium as shown in Tab. S1. Notably, L-1 isolated from a cigar wrapper exhibited significantly higher activity than the other strains. This bacterium was named as L-1, and identification was carried out.
Identification and biochemical characteristics of the strain L-1
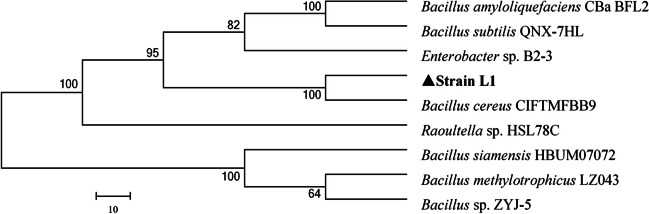
We subsequently identified this bacterium using the 16S rDNA sequence stored in the NCBI database (accession number: MW570877.1). The phylogenetic tree of L-1 revealed 100% similarity to Bacillus cereus CIFTMFBB9 (Fig. 1), leading us to name it Bacillus cereus L-1. Previous studies have reported various xylanase-producing strains, including Bacillus subtilis, Bacillus pumilus, Bacillus halodurans TSEV1, Trichoderma, Streptomyces, and others [8, 9, 11, 12, 25]. Our funding that Bacillus cereus strains also have potential for xylanase production expands the microbial resources available for producing this xylanase [26].
Fig. 1.
Phylogenetic tree of L-1 using MEGA 7.0. Numbers at branching points refer to bootstrap values (1000 resamplings) with 0.10 as the sequence divergence
We characterized L-1 biochemical, and the results are shown in Tab. S2. These tests included methyl red, voges–proskauer, mannitol, glucose utilization, casein, catalase, nitrate reduction, lactose, phenylalanine, starch, and gelatin. Voges–proskauer, glucose utilization, casein, catalase, and nitrate reduction yielded positive results; the remaining tests were negative. We observed that L-1 was a gram-negative bacterium with rod-shaped cells and formed a reticular membrane on the surface of liquid medium. On LB medium, the colony had an opalescent, convex shape with uneven edges and folds on the surface.
Production of xylanase
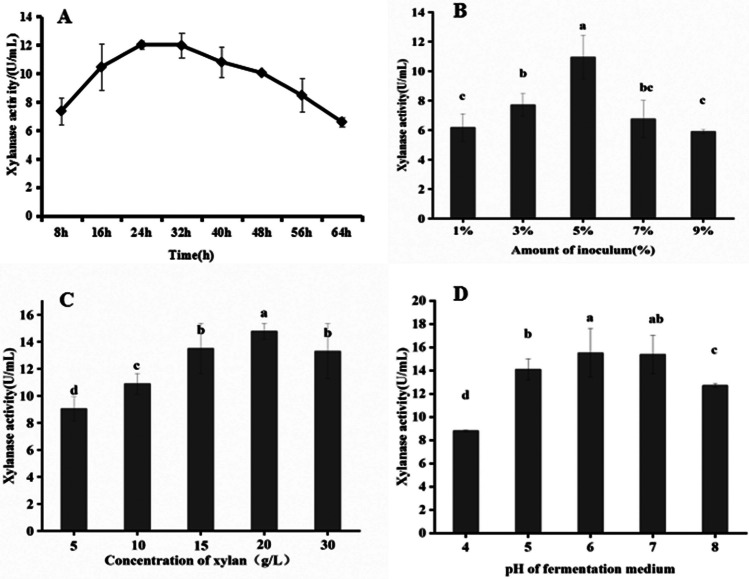
To increase xylanase production, the single-factor-at-one-time method was used in the fermentation process, optimizing the fermentation period, inoculation volume, xylan concentration, and pH of medium (Fig. 2). We found that Bacillus cereus L-1 had the highest xylanase activity (12.02 ± 0.26 U/mL) after 24 h of fermentation (Fig. 2A). The effect of inoculation volume on xylanase activity was investigated, with the highest activity (10.95 ± 1.48 U/mL) obtained at 5% (v/v) inoculum, while higher inoculum does lead to decreased xylanase production due to nutrient competition among bacterial populations (Fig. 2B) [27]. Xylan concentration in the fermentation medium also had a significant effect on xylanase activity, with the highest activity (14.76 ± 0.58 U/mL) observed at a concentration of 20 g/L xylan (Fig. 2C). Furthermore, the initial pH of the medium affected xylanase production, with the highest activity observed at pH 6.0. It was found that at the pH 6.0 was not significantly different from that at pH 7.0 (Fig. 2D).
Fig. 2.
Xylanase activity of L-1 in the optimization fermentation conditions. A Fermentation period of L-1 on xylanase activity. B Effects of inoculum volume of L-1 on xylanase activity. C Effects of xylan concentration in medium on enzyme activity. D Effects of the initial pH of fermentation medium on enzyme activity. Note: Different letters indicate significant differences at 5%
Purification of xylanase
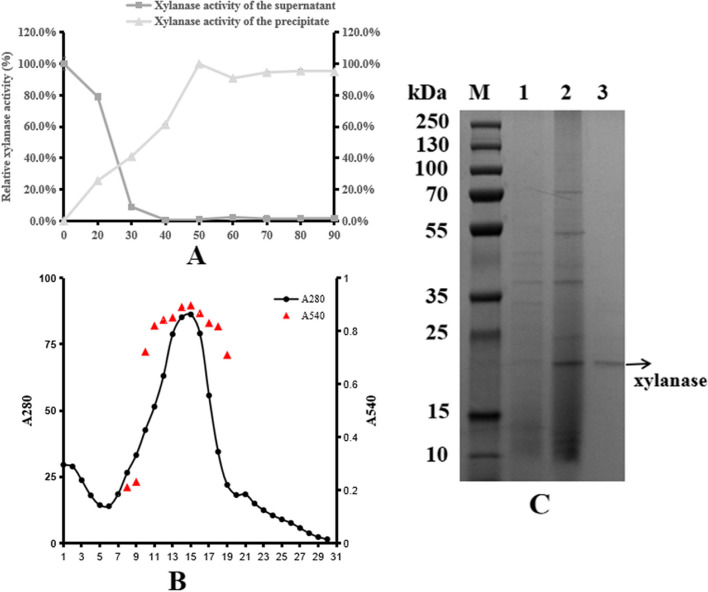
After centrifugation, ammonium sulfate fractional precipitation, and ion exchange chromatography, electrophoresis pure xylanase was obtained. Initially, the supernatant obtained after centrifugation had a xylanase activity of 17.05 U/mL from 1.7 L of culture broth, and the total xylanase activity reached 28,996 U.
After fractionation with ammonium sulfate, the xylanase activity of the precipitate increased significantly, reaching a maximum at 50% saturation, while further saturation did not affect the xylanase activity significantly (Fig. 3A). The crude xylanase was renatured by dialysis, and the resulting activity was 62.8 U/mL, with a total enzyme activity of 2073 U after ammonium sulfate precipitation.
Fig. 3.
The purification of xylanase from L-1. A Ammonium sulfate fractionation curve; B elution curve of Mini Macro-Prep High-S ion exchange chromatography; and C the SDS-PAGE of partial purification xylanase from L-1. (M) makers, (1) fermented liquid, (2) concentrated ammonium sulfate salting out solution, and (3) Mini Macro-Prep High-S ion exchange chromatography with pH 8.5
The crude protein was then purified by Mini Macro-Prep High-S ion exchange chromatography, with a total xylanase activity of 926 U obtained (Fig. 3B). The purified xylanase had an estimated molecular weight of approximately 20–25 kDa as determined by SDS-PAGE (Fig. 3C). The summarized results for each step of purification are presented in Table 1.
Table 1.
The partial purification results of xylanase
| Total activity (U) | Total protein (mg) | Specific activity | Purification fold | Yield (%) | |
|---|---|---|---|---|---|
| Fermentation liquid | 28,996.00 | 3252.00 | 8.92 | 1.00 | |
| Ammonium sulfate | 2073.00 | 148.00 | 14.01 | 1.57 | 7.15 |
| High-S-8.0 | 926.00 | 3.78 | 244.97 | 27.47 | 3.19 |
Xylanase properties
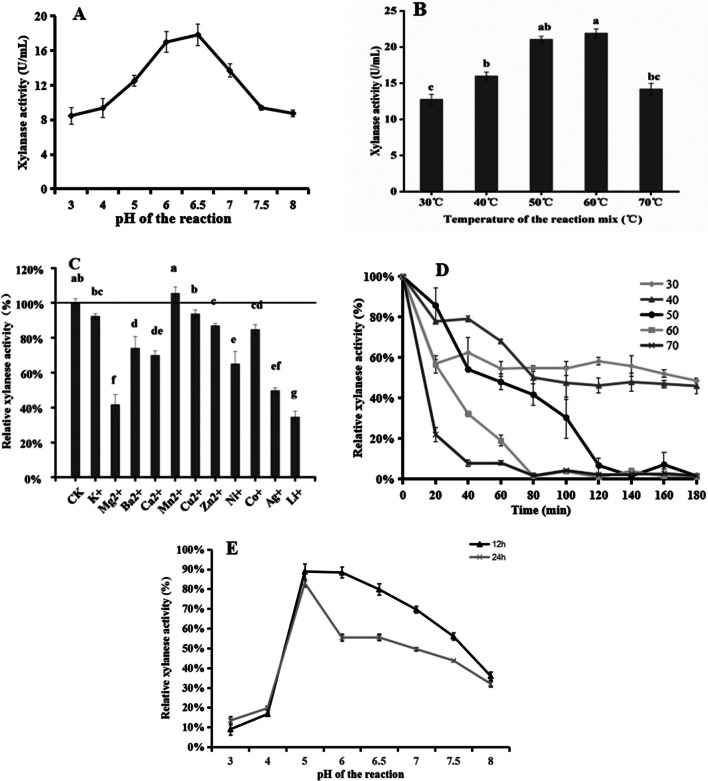
The xylanase’s properties are presented in Fig. 4. It displayed pH activity ranging from 3.0 to 8.0, with maximum activity at pH 6.5 (17.84 ± 1.24 U/mL) (Fig. 4A). Xylanase activity increased with temperature, peaking at 60 °C (Fig. 4B). The xylanase activity at 50 °C was slightly lower than at 60 °C, but no significant difference between 50 and 60 °C (Fig. 4B), suggesting that the xylanase had a better selectivity in reaction temperature. Mn2+ enhanced xylanase activity by 5.42%, while Zn2+, K+, Cu2+, Mg2+, Ba2+, Ca2+, Co2+, Ni2+, Mg2+, Cd2+, and Li+ inhibited activity, especially Mg2+ and Li+ (Fig. 4C). Interestingly, this result of inhibition by most metal ions was quite consistent with the properties of many Streptomyces xylanases [28]. The enzyme was stable at 30 °C and 40 °C for 180 min, remaining 48.56% and 45.97% xylanase activity, respectively (Fig. 4D). At 50 °C, the enzyme lost about 46% of its activity after 40 min (Fig. 4D). The xylanase was stable over a pH range of 5.0 to 8.0, retaining over 82.97% of its activity after 24 h at pH 5.0 (Fig. 4E).
Fig. 4.
Enzyme properties of xylanase. A Effects of pH on xylanase activity. B Effects of temperature on xylanase activity. C Effects of metal ions on xylanase activity. D Temperature stability of xylanase from L-1. E Stability of xylanase acid base. Note: Values are given as the means ± standard deviation (n = 3). Different letters indicate significant differences at 5%
LC–MS/MS analysis of the xylanase
The molecular weight of the endo-1,4-beta-xylanase was 23.3 kDa with the accession number P09850 (https://www.uniprot.org/uniprotkb/P09850/entry), consistent with SDS-PAGE results. MS–MS analysis identified the peptide sequence (22% cover of P09850), and matched proteins with a score ≥ 32 were shown in Tab. S3.
Discussion
The production of xylanase from L-1
In this study, the fermentation conditions and medium components for low molecular weight xylanase secreted by L-1 were optimized using the single-factor-at-one-time method. The optimal fermentation condition resulted in the highest production of xylanase at 15.51 ± 2.08 U/mL. However, compared to previous reports, the xylanase from L-1 did not slow any advantage in production. Further research is needed to increase production yield by analyzing additional elements that influence xylanase yield, such as nitrogen and concentration, the trace elements, and vitamins [29]. Carbon source also plays a crucial role in xylanase production, particularly with agricultural resources like wheat bran and corn cob [29, 30]. Statistical analysis techniques such as response surface methodology and Plackett–Burman design can be utilized to improve yield [18, 31].
Low molecular weight xylanase from bacteria
Low molecular weight xylanases (< 30 kDa) have advantages over high-molecular weight proteins due to their ease in substrate accessibility and decreased metabolic burden during synthesis [32]. Bacteria, in particular, offer significant advantages over fungi for xylanase production due to their rapid growth, easy genetic manipulation, and short fermentation period. To our knowledge, fungi have been found to produce more low molecular weight xylanases than bacteria, with the lowest molecular weight recorded at 12 kDa [33]. The low molecular weights of bacterial xylanases range from 15 to 29.8 kDa [34, 35]. Roy and Rowshanul reported a 32 kDa xylanase from Bacillus cereus that was purified by ammonium sulfate precipitation, DEAE-sepharose, Phenyl-5PW, and hydroxyapatite column chromatography [36]. Xylanase from L-1 showed better selectivity in reaction temperature (50 to 60 °C) and could be easily purified by ammonium sulfate precipitation and ion exchange chromatography. However, obtaining electrophoretic pure low molecular weight xylanases secreted by bacteria required many purification steps in most studies. Currently, only three low molecular weight xylanases produced by bacteria, including those from Bacillus licheniformis, Bacillus Methylotrophicus CSB40, and Bacillus arseniciselenatis DSM 15340, have been purified using a two-step process [35, 37, 38]. This study enriched the pool of low molecular weight xylanases from bacteria, which may help reduce cost in industrial production [39], as fewer purification steps would reduce time and costs.
Conclusion
In this study, a strain identified as Bacillus cereus L-1 that secretes xylanase was isolated. Using a single-factor-at-one-time method, the fermentation conditions of xylanase production from L-1 were optimized to yield 15.51 ± 2.08 U/mL under 5% inoculum, 20 g/L xylan, pH 6.0, for 24 h. The enzyme was purified through ammonium sulfate precipitation and High-S ion exchange chromatography, resulting in a single band of electrophoretic purity. Using LC–MS/MS, the purified enzyme was identified as endo-1,4-beta-xylanase with a molecular weight of 23.3 kDa. The purified xylanase from Bacillus cereus L-1 showed maximum activity at pH 6.5 and 60 °C, and only Mn2+ ions increased enzyme activity by 105.42% at 1 mM. The enzyme was relatively stable at pH 6.5 and had a better selectivity in reaction temperature between 50 and 60 °C. This study enriched the pool of low molecular weight xylanases from bacteria by providing a strain that secretes such an enzyme.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
GZ and ZL contributed equally to this work. GZ and ZL carried out the experiments in this study and drafted the manuscript. HL, LW, and HL conducted the design of the experiment and helped draft and finalized the manuscript. WC, LZ, and HZ revised the manuscript. ZL and GZ assisted with the screening and fermentation experiments. GC and SD participated in the xylanase activity detection and data analysis work. All authors read and approved the final manuscript.
Funding
The work was supported by Key Project of Yunnan Academy of Tobacco Sciences (2021YL04).
Data availability
All data generated or analyzed during this study are included in this published article and its additional files. The datasets generated during the study are available in the NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ge Zhang and Zhihao Li contributed equally to this work.
Contributor Information
Lijun Wu, Email: wallis8@126.com.
Hongtao Li, Email: lihongtao022@126.com.
Haobao Liu, Email: liuhaobao@caas.cn.
References
- 1.Huang Y, Zhang NJ, Zhao Z. Immobilization of mutated xylanase from Neocallimastix patriciarum in E. coli and application for kraft pulp biobleaching. Braz J Biol. 2021;83:e243629. doi: 10.1590/1519-6984.243629. [DOI] [PubMed] [Google Scholar]
- 2.Blanch HW, Simmons BA, Klein-Marcuschamer D. Biomass deconstruction to sugars. Biotechnol J. 2011;9:1086–1102. doi: 10.1002/biot.201000180. [DOI] [PubMed] [Google Scholar]
- 3.Qeshmi FI, Homaei A, Fernandes P, Hemmati R, Dijkstra BW, Khajeh K. Xylanases from marine microorganisms: a brief overview on scope, sources, features and potential applications. Biochim Biophys Acta Proteins. Proteomics. 2020;2:140312. doi: 10.1016/j.bbapap.2019.140312. [DOI] [PubMed] [Google Scholar]
- 4.Polizeli ML, Rizzatti AC, Monti R, Terenzi HF, Jorge JA, Amorim DS. Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol. 2005;5:577–591. doi: 10.1007/s00253-005-1904-7. [DOI] [PubMed] [Google Scholar]
- 5.Collins T, Gerday C, Feller G. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev. 2005;1:3–23. doi: 10.1016/j.femsre.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Nagar S, Mittal A, Kumar D, Kumar L, Gupta VK. Immobilization of xylanase on glutaraldehyde activated aluminum oxide pellets for increasing digestibility of poultry feed. Process Biochem. 2012;9:1402–1410. doi: 10.1016/j.procbio.2012.05.013. [DOI] [Google Scholar]
- 7.Bhardwaj N, Kumar B, Verma P. A detailed overview of xylanases: an emerging biomolecule for current and future prospective. Bioresour Bioprocess. 2019;1:1–36. doi: 10.1186/s40643-019-0276-2. [DOI] [Google Scholar]
- 8.Saleem M, Aslam F, Akhtar MS, Tariq M, Rajoka MI. Characterization of a thermostable and alkaline xylanase from Bacillus sp. and its bleaching impact on wheat straw pulp. World J Microbio Biotechnol. 2012;2:513–522. doi: 10.1007/s11274-011-0842-z. [DOI] [PubMed] [Google Scholar]
- 9.Subramaniyan S. Isolation, purification and characterisation of low molecular weight xylanase from Bacillus pumilus SSP-34. Appl Biochem Biotechnol. 2012;7:1831–1842. doi: 10.1007/s12010-012-9600-4. [DOI] [PubMed] [Google Scholar]
- 10.Taneja K, Gupta S, Kuhad RC. Properties and application of a partially purified alkaline xylanase from an alkalophilic fungus Aspergillus nidulans KK-99. Bioresour Technolgy. 2002;2002:39–42. doi: 10.1016/S0960-8524(02)00064-0. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Hosseindoust A, Laxman Ingale S, Rathi PC, Yoon SY, Choi JW, Kim JS. Thermostable xylanase derived from Trichoderma citrinoviride increases growth performance and non-starch polysaccharide degradation in broiler chickens. Br Poult Sci. 2020;1:57–62. doi: 10.1080/00071668.2019.1673316. [DOI] [PubMed] [Google Scholar]
- 12.Simkhada JR, Yoo H-Y, Choi YH, Kim SW, Yoo JC. An extremely alkaline novel xylanase from a newly isolated Streptomyces strain cultivated in corncob medium. Appl Biochem Biotechnol. 2012;7:2017–2027. doi: 10.1007/s12010-012-9914-2. [DOI] [PubMed] [Google Scholar]
- 13.Joo JC, Pack SP, Kim YH, Yoo YJ. Thermostabilization of Bacillus circulans xylanase: computational optimization of unstable residues based on thermal fluctuation analysis. J Biotechnol. 2011;1:56–65. doi: 10.1016/j.jbiotec.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Boonrung S, Katekaew S, Mongkolthanaruk W, Aimi T, Boonlue S. Purification and characterization of low molecular weight extreme alkaline xylanase from the thermophilic fungus Myceliophthora thermophila BF1-7. Mycoscience. 2016;6:408–416. doi: 10.1016/j.myc.2016.07.003. [DOI] [Google Scholar]
- 15.Kocabaş DS, Yurtdaş E. Crystallization studies of a low molecular weight xylanase from Scytalidium thermophilum. J Biol Environ Sci. 2017;33:129–136. [Google Scholar]
- 16.El-Sayed MH. Thermoalkali-stable pectinase from Bacillus subtilis strain NVFO 19 isolated from agricultural waste dump soil. Curr Res Microbiol Biotechn. 2015;6:805–815. [Google Scholar]
- 17.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;7:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar V, Satyanarayana T. Production of thermo-alkali-stable xylanase by a novel polyextremophilic Bacillus halodurans TSEV1 in cane molasses medium and its applicability in making whole wheat bread. Bioprocess Biosyst Eng. 2014;37:1043–1053. doi: 10.1007/s00449-013-1075-3. [DOI] [PubMed] [Google Scholar]
- 19.Dao TMA, Cuong NT, Nguyen TT, Nguyen NPD, Tuyen DT. Purification, identification, and characterization of a glycoside hydrolase family 11-xylanase with high activity from Aspergillus niger VTCC 017. Mol Biotechnol. 2022;2:187–198. doi: 10.1007/s12033-021-00395-8. [DOI] [PubMed] [Google Scholar]
- 20.Narra M, Dixit G, Divecha J, Kumar K, Madamwar D, Shah AR. Production, purification and characterization of a novel GH 12 family endoglucanase from Aspergillus terreus and its application in enzymatic degradation of delignified rice straw. Int Biodeterior Biodegrad. 2014;88:150–161. doi: 10.1016/j.ibiod.2013.12.016. [DOI] [Google Scholar]
- 21.Gao L, Li Z, Xia C, Qu Y, Liu M, Yang P, Yu L, Song X. Combining manipulation of transcription factors and overexpression of the target genes to enhance lignocellulolytic enzyme production in Penicillium oxalicum. Biotechnol Biofuels. 2017;1:100–117. doi: 10.1186/s13068-017-0783-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang G, Li S, Xu Y, Wang J, Wang F, Xin Y, Shen Z, Zhang H, Ma M, Liu H. Production of alkaline pectinase: a case study investigating the use of tobacco stalk with the newly isolated strain Bacillus tequilensis CAS-MEI-2-33. BMC Biotechnol. 2019;1:1–11. doi: 10.1186/s12896-019-0526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pejchinovski M, Klein J, Ram’ırez-Torres A, Bitsika V, George M, Vlahou A, Mullen W, Mischak H, Jankowski V. Comparison of higher energy collisional dissociation and collision-induced dissociation MS/MS sequencing methods for identification of naturally occurring peptides in human urine. Proteomics Clin Appl. 2015;9:531–542. doi: 10.1002/prca.201400163. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Liu T, Li K, Song X, Yan R, Xu L, Li X. Proteomic analysis of protein interactions between Eimeria maxima sporozoites and chicken jejunal epithelial cells by shotgun LC-MS/MS. Parasite Vector. 2018;1:226–235. doi: 10.1186/s13071-018-2818-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar V, Satyanarayana T. Thermo-alkali-stable xylanase of a novel polyextremophilic Bacillus halodurans TSEV1 and its application in biobleaching. Int Biodegrad. 2012;75:138–145. doi: 10.1016/j.ibiod.2012.09.007. [DOI] [Google Scholar]
- 26.Feng Y, Wang L, Khan A, Zhao R, Wei S, Jing X. Fermented wheat bran by xylanase-producing Bacillus cereus boosts the intestinal microflora of broiler chickens. Poult Sci. 2020;1:263–271. doi: 10.3382/ps/pez482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang J, Ou Y, Zhang D, Zhang G, Pan Y. Optimization of the culture condition of Bacillus mucilaginous using Agaricus bisporus industrial wastewater by Plackett-Burman combined with Box-Behnken response surface method. AMB Expr. 2018;1:1–12. doi: 10.1186/s13568-018-0671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puls J. Chemistry and biochemistry of hemicelluloses relationship between hemicellulose. Macromol Symp. 2011;120:183–196. doi: 10.1002/masy.19971200119. [DOI] [Google Scholar]
- 29.Alokika SB. Production, characteristics, and biotechnological applications of microbial xylanases. Appl Microbiol Biotechnol. 2019;21:8763–8784. doi: 10.1007/s00253-019-10108-6. [DOI] [PubMed] [Google Scholar]
- 30.Rahman MA, Choi YH, Pradeep GC, Choi YS, Choi EJ, Cho SS, Yoo JC. A novel low molecular weight endo-xylanase from Streptomyces sp. CS628 cultivated in wheat bran. Appl Biochem Biotechnol. 2014;6:1469–1480. doi: 10.1007/s12010-014-0916-0. [DOI] [PubMed] [Google Scholar]
- 31.Dhaver P, Pletschke B, Sithole B, Govinden R. Optimization, purification, and characterization of xylanase production by a newly isolated Trichoderma harzianum strain by a two-step statistical experimental design strategy. Scientific reports. 2022;1:17791. doi: 10.1038/s41598-022-22723-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutay Kocabaş D, Güder S, Özben N. Purification strategies and properties of a low-molecular weight xylanase and its application in agricultural waste biomass hydrolysis. J Molecul Cataly B: Enzymat. 2015;115:66–75. doi: 10.1016/j.molcatb.2015.01.012. [DOI] [Google Scholar]
- 33.Soren D, Jana M, Sengupta S, Ghosh AK. Purification and characterization of a low molecular weight endo-xylanase from mushroom Termitomyces clypeatus. Appl Biochem Biotechnol. 2010;2:373–389. doi: 10.1007/s12010-009-8763-0. [DOI] [PubMed] [Google Scholar]
- 34.Prakash B, Vidyasagar M, Jayalakshmi SK, Sreeramulu K. Purification and some properties of low-molecular-weight extreme halophilic xylanase from Chromohalobacter sp. TPSV 101. J Mol Catal B: Enzym. 2012;3:192–198. doi: 10.1016/j.molcatb.2011.10.004. [DOI] [Google Scholar]
- 35.Kamble RD, Jadhav AR. Isolation, purification, and characterization of xylanase produced by a new species of bacillus in solid state fermentation. Int J Microbiol. 2012;2012:683193. doi: 10.1155/2012/683193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy N, Rowshanul HM. Isolation and characterization of xylanase producing strain of Bacillus cereus from soil. Iran J Microbiol. 2009;2:49–53. [Google Scholar]
- 37.Malhotra G, Chapadgaonkar SS. Partial purification and characterization of a thermostable xylanase from Bacillus licheniformis isolated from hot water geyser. J Genet Eng Biotechnol. 2022;1:1–6. doi: 10.1186/s43141-022-00333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panthi S, Choi YS, Choi YH, Kim M, Yoo JC. Biochemical and thermodynamic characterization of a novel, low molecular weight xylanase from Bacillus Methylotrophicus CSB40 isolated from traditional Korean food. Appl Biochem Biotechnol. 2016;1:126–142. doi: 10.1007/s12010-016-1983-1. [DOI] [PubMed] [Google Scholar]
- 39.Martins MD, Guimarães MW, de Lima VA, Gaglioti AL, Da-Silva PR, Kadowaki MK, Knob A. Valorization of passion fruit peel by-product: xylanase production and its potential as bleaching agent for kraft pulp. Biocatal Agric Biotechnol. 2018;16:172–180. doi: 10.1016/j.bcab.2018.07.033. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its additional files. The datasets generated during the study are available in the NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Not applicable.