Abstract
Scedosporium apiospermum is a widespread, emerging, and multidrug-resistant filamentous fungus that can cause localized and disseminated infections. The initial step in the infection process involves the adhesion of the fungus to host cells and/or extracellular matrix components. However, the mechanisms of adhesion involving surface molecules in S. apiospermum are not well understood. Previous studies have suggested that the binding of fungal receptors to fibronectin enhances its ability to attach to and infect host cells. The present study investigated the effects of fibronectin on adhesion events of S. apiospermum. The results revealed that conidial cells were able to bind to both immobilized and soluble human fibronectin in a typically dose-dependent manner. Moreover, fibronectin binding was virtually abolished in trypsin-treated conidia, suggesting the proteinaceous nature of the binding site. Western blotting assay, using fibronectin and anti-fibronectin antibody, evidenced 7 polypeptides with molecular masses ranging from 55 to 17 kDa in both conidial and mycelial extracts. Fibronectin-binding molecules were localized by immunofluorescence and immunocytochemistry microscopies at the cell wall and in intracellular compartments of S. apiospermum cells. Furthermore, a possible function for the fibronectin-like molecules of S. apiospermum in the interaction with host lung cells was assessed. Conidia pre-treated with soluble fibronectin showed a significant reduction in adhesion to either epithelial or fibroblast lung cells in a classically dose-dependent manner. Similarly, the pre-treatment of the lung cells with anti-fibronectin antibodies considerably diminished the adhesion. Collectively, the results demonstrated the presence of fibronectin-binding molecules in S. apiospermum cells and their role in adhesive events.
Keywords: Scedosporium, Fibronectin, Adhesion, Lung cells, Infection, Virulence
Introduction
Scedosporium apiospermum is a filamentous saprophytic fungus that is frequently isolated from soil, polluted water, agricultural, and industrial areas [1–3]. In the last few decades, this fungus has emerged as an important human pathogen, causing infections in both immunocompromised and immunocompetent hosts [1–3]. This fungus can colonize different host anatomical sites such as the lungs, joints, bones, and central nervous system [1]. In immunocompetent individuals, mycetoma, a localized disease, is the most common clinical manifestation and often occurs due to the traumatic inoculation of S. apiospermum conidia and/or hyphal fragments [1]. In individuals with underlying diseases (e.g., neutropenia, sarcoidosis and cystic fibrosis), dissemination may occur, usually beginning with conidia inhalation [1]. Whether in localized or disseminated disease, the success of infection relies on the ability of S. apiospermum conidia to adhere to cells and tissues. After adhesion, conidia germinate into hyphae, causing cell/tissue invasion with severe damage to the host that culminates in host cell death [2, 4–6]. Later on, a biofilm-like structure is formed, composed by a highly resistant mycelial mass that destroys the host tissue architecture [1, 7, 8].
The capacity of pathogenic microorganisms to adhere to host cells and avoid clearance by the host immune system is the initial and most decisive step leading to infections. Adherence to host structures implies that the fungus is able to recognize ligands at the surface of host cells, tissues, and extracellular matrix components (EMC) [9, 10]. The EMC is the proteinaceous part of tissues that is involved in cellular migration, signaling, composing a physical barrier to prevent microorganism’s invasion [10]. The ECM can constitute the basement membrane, an anchoring platform for epithelia, and the interstitial spaces in tissues. Laminin and collagen are found mainly in the subendothelial basement membrane, while fibronectin, elastin, fibrillin, among others, form the connective tissues [10]. The glycoprotein fibronectin presents a molecular mass that can range from 230 to 270 kDa, but can also be a dimer of about 440 kDa [11, 12]. Fibronectin isoforms are found in plasma (soluble) and in ECM, where this glycoprotein anchors the cells [11]. Fibronectin is a ubiquitous glycoprotein that plays an important role in many physiological and pathological processes. There are clear indications that the binding of microorganisms’ receptors to fibronectin promotes attachment to and infection of host cells.
The fibronectin present in the ECM mediates the adhesion of several pathogenic fungi to host tissues, such as Aspergillus fumigatus, Candida albicans, non-albicans Candida species, Sporothrix schenckii, Cryptococcus neoformans, among others [11, 13–15]. Fungi interact with the ECM through surface molecules, like sialic acid-containing glycoconjugates in Penicillium marneffei [16–18]. To invade tissues, fungi secrete proteases capable of cleaving ECM components and spreading through the interstitial space [10]. Previously, our group reported that S. apiospermum secretes metalloproteases capable of degrading several ECM components, including fibronectin [19]. However, little is known about the ability of S. apiospermum cells to bind to fibronectin. In the present study, the capability of S. apiospermum to bind to soluble and immobilized fibronectin was explored. Moreover, the role of surface fibronectin-binding molecules in the interaction with mammalian lung cells was evaluated. Collectively, the results presented herein provide information about the mechanisms of interaction between Scedosporium and mammalian cells. Finally, the authors dedicate this work to the memory of Professor Luiz Rodolpho Raja Gabaglia Travassos, one of the most renowned Brazilian scientists. Professor Travassos traversed a long path in different areas of biological sciences, including mycology. Undoubtedly, his legacy remains alive, and his scientific advice has become present in the lives of many scientists worldwide. Our group was impacted by the studies conducted by Professor Travassos on the surface of opportunistic fungi. The discovery of molecules related to fungi-host adhesion processes was well recognized by our group, which motivated us to develop numerous studies in this field, including the present work.
Materials and methods
Fungi and growth condition
Scedosporium apiospermum (RKI07_0416) was provided by Dr. Bodo Wanke (Evandro Chagas Hospital, Oswaldo Cruz Institute, FIOCRUZ, Rio de Janeiro, Brazil). The fungus was cultured in Sabouraud-dextrose broth (2% glucose, 1% peptone, and 0.5% yeast extract) at 25 °C for 7 days with constant shaking (200 rpm). Then, mycelial cells were filtered with filter paper and washed twice with sterile phosphate-buffered saline (PBS; 150 mM NaCl, 20 mM phosphate buffer, pH 7.2) [19]. Conidial cells were grown on Petri plates containing Sabouraud-dextrose medium at 25 °C. After 7 days of culture, conidial cells were obtained by washing the plate surface with PBS and filtered through gauze to remove hyphal fragments and debris. The conidial cells were counted using a Neubauer chamber.
Adherence to immobilized fibronectin
For adherence assays, 96-well polystyrene plates were initially coated with fibronectin at concentrations ranging from 100 to 400 µg/mL [9, 16, 17]. In parallel, negative control wells were incubated only with BSA (400 µg/mL). The proteins were dissolved in PBS and incubated overnight at 4 °C and then for 1 h at 37 °C. The plates were then blocked with 1% (w/v) bovine serum albumin (BSA) in PBS for 1 h at 37 °C, washed, and a solution containing 1 × 106 conidia was added to each well and incubated for 1 h at 37 °C. Non-adherent conidial cells were removed by washing with PBS containing 0.05% (v/v) Tween-20. The reaction was developed using 3,3′,5,5′-tetramethylbenzidine (BD Biosciences, San Diego, CA) and the color intensity was determined at 490 nm using an automated plate reader. Results were expressed as absorbance at 490 nm (A490).
Interaction with soluble fibronectin
Conidial cells used for these experiments were initially fixed with 4% paraformaldehyde in PBS (pH 7.2) for 30 min, followed by extensive washing in the same buffer. Fixed cells maintained their morphological integrity, as verified by microscopical observation. Subsequently, fungal cells were incubated for 1 h with a solution containing different concentrations of fibronectin (ranging from 100 to 400 µg/mL) [9, 16, 17], and then incubated for an additional hour with a 1:100 dilution of rabbit anti-human fibronectin antibody (Sigma-Aldrich, USA). Finally, conidial cells were incubated for 1 h with a 1:400 dilution of fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit immunoglobulin G (IgG) (Sigma). The conidia were washed three times in PBS and examined in an EPICS ELITE flow cytometer (Coulter Electronics, Hialeah, FL) equipped with 15-mW argon laser emitting at 488 nm. Conidia treated only with the secondary antibody were used as control. Conidial cells were analyzed first to determine their autofluorescence and relative size. The mapped population (10,000 events) was analyzed for log green fluorescence by using a single-parameter histogram. The results were expressed as mean of fluorescence intensity (MFI). In some experiments, the conidial suspensions were incubated with 25 µg/mL of trypsin for 30 min at 37 °C. Proteolytic treatment was stopped by three washes with PBS containing 3% BSA. The trypsin-treated cells were washed with PBS and assayed for fibronectin binding by flow cytometry as described above, using 400 µg/mL of fibronectin for the initial incubation. In parallel, conidia and mycelia of S. apiospermum were incubated with 400 µg/mL, followed by incubation with anti-fibronectin antibody. The samples were then observed using a Zeiss epifluorescence microscope (Axioplan).
Evidence of fibronectin-binding proteins
To prepare the fungal samples, conidia (1 × 108 cells) and mycelia (5 g) were suspended in 1 mL of PBS supplemented with 0.1% Triton X-100. An equivalent volume of glass beads (0.3 mm in diameter) was added to each suspension, and fungal cells were disrupted in a cell homogenizer (Braun Biotech International) by alternating 2-min shaking periods and 2-min cooling intervals (total of 20 cycles). The success of cell disruption was verified using a light microscope (Zeiss, Germany). After removing the glass beads and centrifuging the samples, the protein concentration in the supernatants (whole fungal extracts) was determined using BSA as the protein standard [20]. Next, the protein extracts were separated by 12% SDS-PAGE and the polypeptides electrophoretically transferred to a nitrocellulose membrane at 4 °C at 100 V/300 mA for 2 h. The membrane was blocked with 5% (w/v) low-fat dried milk in PBS containing 0.5% Tween 20 (PBS/Tween) for 1 h at room temperature. Subsequently, the membrane was washed three times (each for 10 min) with the blocking solution and incubated separately with fibronectin at 10 µg/mL. After another three washes with the blocking solution (each for 10 min), the membrane was incubated with primary antibody rabbit anti-human fibronectin at 1:1500 for 1 h. Finally, the membrane was washed again and incubated with the secondary antibody peroxidase-conjugated goat anti-rabbit IgG. Immunoblots were exposed to X-ray film after reaction with ECL reagents for chemiluminescence [21].
Immunolocalization of fibronectin-binding sites
Conidia were fixed for 1 h at 25 °C in 100 mM cacodylate buffer (pH 7.4) containing 0.2% glutaraldehyde, 1% picric acid, 4% paraformaldehyde, and 10 mM calcium chloride. The conidia were then washed in PBS with 3% BSA (PBS-BSA), dehydrated in graded series of ethanol at 4 °C, and embedded in Unicryl resin at – 20 °C. Polymerization was carried out at – 20 °C under UV radiation for 96 h. Ultrathin sections were collected on nickel grids, quenched in 80 mM ammonium chloride in PBS for 30 min to neutralize free aldehyde groups, and transferred to blocking buffer (PBS supplemented with 0.01% Tween 20, 1.5% BSA, 0.5% fish gelatin, pH 7.4) for 1 h at room temperature. The grids were incubated with fibronectin at 10 µg/mL in PBS for 1 h, washed in PBS-BSA, subsequently incubated for 1 h with anti-fibronectin antibody (1:100 dilution). The samples were then washed twice with PBS-BSA and incubated for 1 h with gold-labeled (10 nm particle size) goat anti-rabbit IgG (1:100 dilution) as the secondary antibody. Controls were included in which incubation with the primary antibody was omitted (data not shown). Finally, the sections were then successively washed in PBS and water, stained with aqueous uranyl acetate and alkaline lead citrate, and observed in a ZEISS 900 transmission electron microscope.
Role of fibronectin-binding molecules on the interaction with lung cells
The human adenocarcinoma alveolar basal epithelial cell line A549 (ATCC CCL-185) and the human fetal lung fibroblast cell line MRC-5 (ATCC CCL-171) were cultured in sterile 75-cm2 culture flasks containing Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 2 mM L-glutamine at 37 °C in a 5% CO2 atmosphere. To verify the importance of fibronectin-binding proteins in the interaction between S. apiospermum and lung cells, 1 × 106 conidia/mL in PBS was incubated at room temperature for 1 h in the absence (control) or presence of soluble fibronectin at 100, 200 or 400 μg/mL [9, 16, 17]. Alternatively, lung cells were incubated with anti-fibronectin antibodies (Sigma) at 1:100, 1:200, or 1:400 dilutions. As controls, conidia were treated with BSA 400 μg/mL and lung cells were treated with irrelevant IgG. The systems were washed three times with PBS and allowed to interact in a proportion of 10:1 (fungi:lung cell) for 2 h at 37 °C and at 5% CO2. Then, the systems were washed with PBS and the coverslips containing the interactions were fixed with Bouin’s solution for 10 min, washed with alcohol to remove excess Bouin’s solution, and then washed exhaustively with distilled water. The coverslips were stained with Giemsa for 90 min, and then subjected to a battery of acetone/xylene. The percentage of infected lung cells was determined by randomly counting 200 lung cells on each of triplicate coverslips from three different experimental sets. The association index was obtained by multiplying the percentage of infected lung cells by the number of fungi per infected lung cell.
Statistical analyses
All experiments were performed in triplicate, in three independent experimental sets. The results were analyzed statistically by Student’s t-test (in the comparisons between two groups). In all analyses, P values of 0.05 or less were considered statistically significant. All analyses were performed using the program GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA, USA).
Results
S. apiospermum conidia bind to immobilized and soluble fibronectin
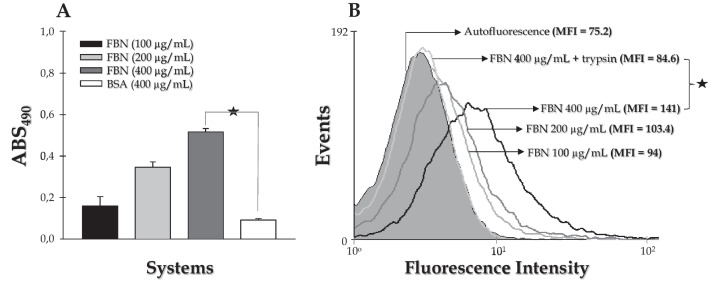
The ability of S. apiospermum conidial cells to bind to immobilized fibronectin adsorbed on a polystyrene surface was evaluated by ELISA method. The results showed a typical concentration-dependent effect of fibronectin on the number of adhered conidia (Fig. 1A). In contrast, the adhesion of S. apiospermum conidial cells to BSA-coated polystyrene, used as protein control, was significantly reduced (about 20-fold) compared to the surface coated with fibronectin, using both proteins at a concentration of 400 μg/mL (Fig. 1A).
Fig. 1.
Binding of S. apiospermum conidia to soluble and immobilized human fibronectin. A Analysis of conidia binding to immobilized fibronectin by ELISA method. A solution of conidia (1 × 106 cells) was added to fibronectin immobilized in 96-well polystyrene plates for 1 h at 37 °C. The reaction was developed using 3,3′,5,5′-tetramethylbenzidine and the color intensity was determined at 490 nm. Negative control wells were incubated only with BSA (400 µg/mL). B Analysis of conidia binding to soluble fibronectin by flow cytometry. Paraformaldehyde-fixed conidia (1 × 106 cells) were sequentially incubated with soluble fibronectin at different concentrations, followed with anti-fibronectin antibody and FITC-labeled anti-IgG. Conidia treated only with the secondary antibody were used as control (autofluorescence). Trypsin treated conidial cells were also used before adding fibronectin in order to check the proteinaceous nature of the potential ligand. The results were expressed as mean of fluorescence intensity (MFI). The symbols indicate the experimental systems considered statistically significant from the control (P < 0.05; Student’s t-test)
The characteristics of fibronectin binding to S. apiospermum conidia were further investigated by flow cytometry assay. Analysis of conidia incubated with soluble fibronectin at various concentrations showed a dose-dependent binding of the ligand to the fungal cells (Fig. 1B). To characterize the biochemical nature of the binding sites, conidia were treated with trypsin and then their ability to bind fibronectin was analyzed by flow cytometry (Fig. 1B). Proteolytic treatment of the conidial cells greatly reduced the binding of the ligand to conidia to levels similar to those detected in conidia incubated only with PBS (Fig. 1B).
Identification of S. apiospermum components that bind fibronectin
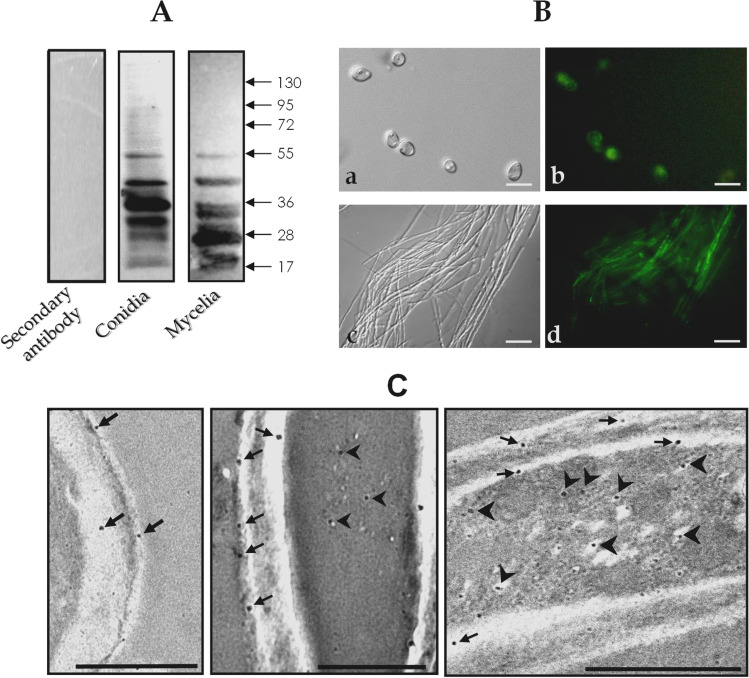
The statement describes a set of experiments in which potential fungal ligands to fibronectin were investigated. Firstly, whole extracts from both conidial and mycelial cells were transferred to a nitrocellulose membrane and sequentially incubated with fibronectin and anti-fibronectin antibodies. The results showed that fibronectin bound to at least seven polypeptides with molecular masses ranging from 17 to 55 kDa in both conidial and mycelial homogenates (Fig. 2A). The absence of polypeptide bands when the nitrocellulose membrane was neither incubated with fibronectin solution nor anti-fibronectin antibodies indicates that the observed binding between fibronectin and the fungal extracts is specific (Fig. 2A). Secondly, the fungal cells were observed using fluorescence microscopy in order to detect the fibronectin-binding molecules. The fluorescence observed in both conidia and mycelia of S. apiospermum indicated their interaction with fibronectin (Fig. 2B). It is worth noting that variations in fibronectin binding were detected within the cell population (Fig. 2B). Thirdly, the cellular distribution of the fibronectin-binding molecules in S. apiospermum conidial cells was investigated through immunocytochemistry analysis (Fig. 2C). Gold particles were clearly detected in fungal cell wall and in intracellular compartments (cytoplasm) (Fig. 2C).
Fig. 2.
Localization of fibronectin-binding sites in S. apiospermum cells. A Detection of fibronectin-binding proteins in S. apiospermum conidial and mycelial whole extracts was carried out using a Western blotting assay. A control was included by incubating the membrane only with the secondary antibody, omitting the previous incubation with fibronectin. The numbers on the right refer to the molecular masses of standard proteins, expressed in kilodalton (kDa). B Fluorescence microscopy showing binding of soluble fibronectin to conidia (a phase-contrast microscopy; b fluorescence microscopy) and mycelia (c phase-contrast microscopy; d fluorescence microscopy) of S. apiospermum. Bars: 4 µm. C Immunocytochemical localization of fibronectin-binding sites in S. apiospermum conidia. Labeling is evident in intracellular compartments (arrowhead) and fungal cell wall (arrow). Bars: 4 µm (left image), 0.5 µm (center image), and 1 µm (right image)
Participation of fibronectin-binding molecules on the interaction of S. apiospermum with lung cells
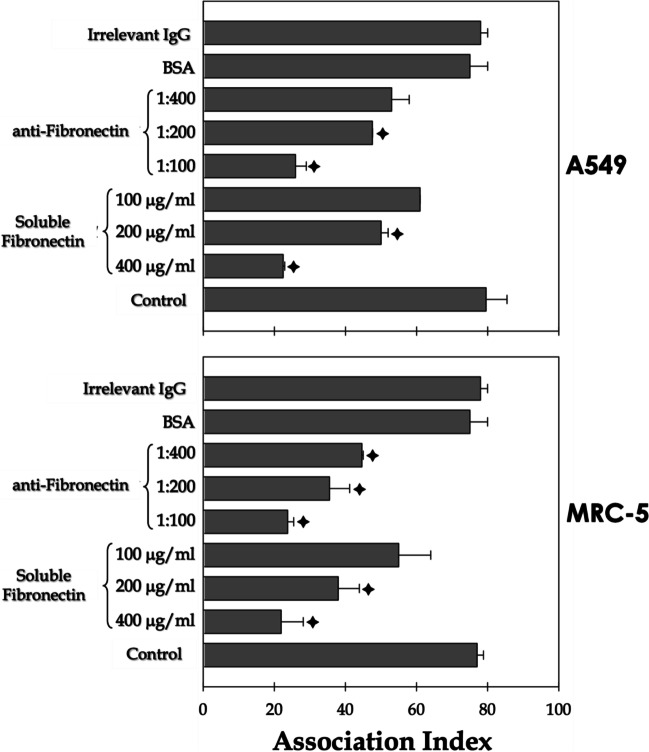
Recognition and attachment are primary and essential steps in the interaction process of live cells. Therefore, surface fungal molecules must be able to bind to their correspondent receptors in host cells. Based on these premises, herein, experiments were conducted to investigate the role of surface-located fibronectin-binding molecules of S. apiospermum in the adhesion to mammalian cells. Specifically, interaction assays were performed between S. apiospermum conidia and two lung cell lines (A549 and MRC-5) in the presence of soluble fibronectin and anti-fibronectin antibodies (Fig. 3). Our results evidenced that the pre-treatment of S. apiospermum conidial cells with soluble fibronectin inhibited the association indexes with both epithelial (A549) and fibroblast (MRC-5) cells in a classical dose-dependent fashion (Fig. 3). A similar inhibition profile was obtained when lung cells were pre-incubated with the anti-fibronectin antibodies (Fig. 3). Conversely, conidia pre-treated with soluble BSA as well as lung cells pre-incubated with irrelevant antibodies showed association indexes similar to those of the control systems (Fig. 3).
Fig. 3.
Participation of the fibronectin-binding molecules of S. apiospermum in the interaction with target host cells (epithelial and fibroblast lung cells). Conidia were incubated in the presence or absence of soluble human fibronectin at different concentrations (100–400 μg/ml) or with soluble BSA (400 μg/ml) at room temperature for 1 h. Alternatively, lung epithelial and fibroblast cells (A549 and MRC-5 lineages, respectively) were incubated with anti-fibronectin antibody at 1:100, 1:200, and 1:400 dilutions or with irrelevant IgG (1:400) for 1 h at 37 °C in an atmosphere with 5% CO2. Posteriorly, fungi and lung cells were placed to interact in a proportion of 10:1, respectively, for 2 h at 37 °C at 5% CO2. After the interaction period, the systems were washed and stained with Giemsa for subsequent determination of association indexes. The symbols indicate the experimental systems considered statistically significant from the control (P < 0.05; Student’s t-test)
Discussion
Fibronectin is a large glycoprotein present in the extracellular matrix and coating epithelial cells. It is also found circulating in plasma, and its soluble form can be found in inflammatory secretions [11, 22]. Numerous studies have reported the crucial role of binding to extracellular matrix proteins in microbial pathogenesis, as it enables tissue damage and invasion [10]. Is it well known that Candida species bind to soluble fibronectin via protein surface receptors. For instance, C. tropicalis has a 105-kDa fibronectin-binding protein on its surface [11, 23, 24]. The interaction of human fibronectin with C. glabrata is mediated by the epithelial adhesin 6 (Epa6), which is expressed on the surface of yeast cells [25]. In C. albicans, the cytosolic glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) has also been detected on the outer surface of the yeast cell wall and it has been implicated in mediating adhesion of fungal cells to fibronectin [26]. The interaction between soluble fibronectin and Candida could be an essential event in the dissemination process in vivo, where the microorganism would gain access to the interstitial space [23]. After binding to fibronectin, C. albicans would be able to secrete proteolytic enzymes, especially aspartic peptidase 2 (Sap2), capable of degrading this protein constituent [27]. In a previous study developed by our group, we found that the metallopeptidases secreted by S. apiospermum were capable of hydrolyzing human fibronectin [19]. However, nothing is known about the ability of this fungus to bind to this multifunctional protein. In the present study, we demonstrated that S. apiospermum conidia and mycelia can bind to human fibronectin, and this process is relevant for the interaction between conidia and lung cells to occur.
The binding of S. apiospermum conidial cells to immobilized fibronectin was both specific and a dose-dependent event. The presence of fibronectin-binding molecules appears to be uniformly distributed on the surface of either conidial or mycelial cells, indicating that the expression of these molecules is not suppressed during cell differentiation. Similar findings were reported for Sporothrix schenckii, in which the presence of binding molecules for fibronectin and laminin was detected in conidia, hyphae, and yeasts [28]. However, contrasting data were obtained in studies conducted with Penicillium marneffei, in which the presence of fibronectin-binding molecules was reported in the conidia and phialides but not in the hyphae [17, 29].
The polypeptide profile corresponding to the fibronectin-binding molecules from the whole extract of conidia and mycelia of S. apiospermum revealed a very similar labeling pattern, indicating seven polypeptide bands ranging from 17 to 55 kDa. These data support the absence of prominent modulation in the expression of fibronectin-binding molecules based on the morphotype of S. apiospermum. In Aspergillus fumigatus, the binding to fibronectin is mediated by two polypeptides with apparent molecular masses of 23 and 30 kDa, as well as the cell wall adhesin galactosaminogalactan [9, 30, 31]. Additionally, the enzymatic removal of sialic acid from the surface of A. fumigatus decreased the adherence of conidia to fibronectin by more than 65% [32], demonstrating the role of surface negatively charged carbohydrates of A. fumigatus in the adhesion to extracellular matrix components. Furthermore, the pretreatment of A. fumigatus with trypsin significantly reduced fibronectin binding, suggesting the presence of protein-binding sites on the surface of conidia [9]. A similar approach was used in our present study, which demonstrated that fibronectin-binding molecules of S. apiospermum conidia were removed from the fungal surface after trypsin treatment, as shown by flow cytometry assays.
It is known that S. apiospermum is capable of adhering to and invading epithelial, fibroblast, and macrophage cells, which are essential processes for the course of infection [4, 5]. For instance, our research group demonstrated that germinated conidia of S. apiospermum can penetrate the plasma membrane of mammalian cells, leading to irreversible damage that culminates in host cell death [4, 5]. However, further studies are necessary to clarify the adhesins present in the cell wall of S. apiospermum as well as the receptors present in the host cells. In this context, the interaction process between the conidia of S. apiospermum and larynx carcinoma cells (HEp2) occurs partially via peptidorhamnomannan (PRM) molecules, which are peptidopolysaccharides located in the fungal cell wall, and a 25-kDa surface polypeptide on the HEp2 plasma membrane [33]. The PRM molecules also mediated the in vitro interaction between Lomentospora prolificans (formerly S. prolificans) conidia and mouse peritoneal macrophages [34]. Moreover, the α-glucan extracted from the cell wall of S. apiospermum partially mediated the phagocytosis of conidia by both macrophage and dendritic cells [35].
Due to the scarcity of data on S. apiospermum molecules involved in adhesion events, we decided to explore the role of fibronectin and fibronectin-binding molecules in the interaction between S. apiospermum and target host cells. We found that pre-treating lung cells (epithelial and fibroblast cells) with anti-fibronectin antibodies, as well as pre-incubating conidia with soluble fibronectin, significantly decreased adhesion events in a dose-dependent manner. Taken together, these results demonstrate that fibronectin plays a role in the S. apiospermum-host interaction. Similarly, the interaction between Paracoccidioides brasiliensis conidia and A549 cells involves either fibronectin or fibrinogen located at the surface of epithelial lung cells [36]. The ability to adhere to epithelial cells may represent a mechanism by which the infective conidial cells avoid entrapment within the mucus that covers the respiratory ducts and physical removal by the movement of ciliary cells, thereby playing a role in pathogenesis. Interestingly, dexamethasone can increase invasiveness of A. fumigatus conidia by promoting fibronectin expression, which may partially explain why patients who are given large doses of glucocorticoids for extended periods are more susceptible to develop aspergillosis [37].
Conclusions
The results exposed in the present work demonstrated that fibronectin binding is an important event in the interaction of S. apiospermum conidia with target host cells, expanding the current knowledge on fungal molecules with adhesive properties. This fact leads us to assume that, as with other pathogens, the ability to adhere to fibronectin may be an essential step in the pathogenesis of S. apiospermum. However, the purification of the probable(s) adhesin(s) and the identification of receptors present in host cells are necessary to confirm this assumption.
Acknowledgements
We would like to thank Denise Rocha de Souza, who is supported by FAPERJ scholarship, for her technical assistance.
Funding
This work was supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES – financial support 001), and Fundação Oswaldo Cruz (FIOCRUZ).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Responsible Editor: Marcio Lourenço Rodrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cortez KJ, Roilides E, Quiroz-Telles F, Meletiadis J, Antachopoulos C, Knudsen T, Buchanan W, Milanovich J, Sutton DA, Fothergill A, Rinaldi MG, Shea YR, Zaoutis T, Kottilil S, Walsh TJ. Infections caused by Scedosporium spp. Clin Microbiol Rev. 2017;21(1):157–197. doi: 10.1128/CMR.00039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramirez-Garcia A, Pellon A, Rementeria A, Buldain I, Barreto-Bergter E, Rollin-Pinheiro R, de Meirelles JV, Xisto MIDS, Ranque S, Havlicek V, Vandeputte P, Govic YL, Bouchara JP, Giraud S, Chen S, Rainer J, Alastruey-Izquierdo A, Martin-Gomez MT, López-Soria LM, Peman J, Schwarz C, Bernhardt A, Tintelnot K, Capilla J, Martin-Vicente A, Cano-Lira J, Nagl M, Lackner M, Irinyi L, Meyer W, de Hoog S, Hernando FL. Scedosporium and Lomentospora: an updated overview of underrated opportunists. Med Mycol. 2018;56(suppl_1):102–125. doi: 10.1093/mmy/myx113. [DOI] [PubMed] [Google Scholar]
- 3.Mello TP, Bittencourt VCB, Liporagi-Lopes LC, Aor AC, Branquinha MH, Santos ALS. Insights into the social life and obscure side of Scedosporium/Lomentospora species: ubiquitous, emerging and multidrug-resistant opportunistic pathogens. Fungal Biol Rev. 2018;33:16–46. doi: 10.1016/j.fbr.2018.07.002. [DOI] [Google Scholar]
- 4.Aor AC, Mello TP, Sangenito LS, Fonseca BB, Rozental S, Lione VF, Veiga VF, Branquinha MH, Santos ALS. Ultrastructural viewpoints on the interaction events of Scedosporium apiospermum conidia with lung and macrophage cells. Mem Inst Oswaldo Cruz. 2018;113(10):e180311. doi: 10.1590/0074-02760180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mello TP, Aor AC, Branquinha MH, Santos ALS. Insights into the interaction ofScedosporium apiospermum,Scedosporium aurantiacum,Scedosporium minutisporum, andLomentospora prolificanswith lung epithelial cells. Braz J Microbiol. 2019;51(2):427–436. doi: 10.1007/s42770-019-00183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mello TP, Aor AC, Gonçalves DDS, Seabra SH, Branquinha MH, Santos ALS (2018) Scedosporium apiospermum, Scedosporium aurantiacum, Scedosporium minutisporum and Lomentospora prolificans: a comparative study of surface molecules produced by conidial and germinated conidial cells. 113:1–8. 10.1590/0074-02760180102 [DOI] [PMC free article] [PubMed]
- 7.Mello TP, Aor AC, Gonçalves DS, Seabra SH, Branquinha MH, Santos ALS. Assessment of biofilm formation by Scedosporium apiospermum, S. aurantiacum, S. minutisporum and Lomentospora prolificans. Biofouling. 2016;32:737–749. doi: 10.1080/08927014.2016.1192610. [DOI] [PubMed] [Google Scholar]
- 8.Mello TP, Oliveira SSC, Branquinha MH, Santos ALS. Decoding the antifungal resistance mechanisms in biofilms of emerging, ubiquitous and multidrug-resistant species belonging to the Scedosporium/Lomentospora genera. Med Mycol. 2022;60(6):myac036. doi: 10.1093/mmy/myac036. [DOI] [PubMed] [Google Scholar]
- 9.Peñalver M, O’Connor JE, Martinez JP, Gil ML. Binding of human fibronectin to Aspergillus fumigatus conidia. Infect Immun. 1996;64(4):1146–1153. doi: 10.1128/iai.64.4.1146-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh B, Fleury C, Jalalvand F, Riesbeck K. Human pathogens utilize host extracellular matrix proteins laminin and collagen for adhesion and invasion of the host. FEMS Microbiol Rev. 2012;36(6):1122–1180. doi: 10.1111/j.1574-6976.2012.00340.x. [DOI] [PubMed] [Google Scholar]
- 11.Kozik A, Karkowska-Kuleta J, Zajac D, Bochenska O, Kedracka-Krok S, Jankowska U, Rapala-Kozik M. Fibronectin-, vitronectin- and laminin-binding proteins at the cell walls of Candida parapsilosis and Candida tropicalis pathogenic yeasts. BMC Microbiol. 2015;15:197. doi: 10.1186/s12866-015-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalton CJ, Lemmon CA. Fibronectin: molecular structure, fibrillar structure and mechanochemical signaling. Cells. 2021;10(9):2443. doi: 10.3390/cells10092443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klotz SA, Smith RL. A fibronectin receptor on Candida albicans mediates adherence of the fungus to extracellular matrix. J Infect Dis. 1991;163(3):604–610. doi: 10.1093/infdis/163.3.604. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues ML, dos Reis FC, Puccia R, Travassos LR, Alviano CS. Cleavage of human fibronectin and other basement membrane-associated proteins by a Cryptococcus neoformans serine proteinase. Microb Pathog. 2003;34(2):65–71. doi: 10.1016/s0882-4010(02)00195-x. [DOI] [PubMed] [Google Scholar]
- 15.Lima OC, Figueiredo CC, Previato JO, Mendonça-Previato L, Morandi V, Lopes Bezerra LM. Involvement of fungal cell wall components in adhesion of Sporothrix schenckii to human fibronectin. Infect Immun. 2001;69(11):6874–6880. doi: 10.1128/IAI.69.11.6874-6880.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton AJ, Jeavons L, Youngchim S, Vanittanakom N, Hay RJ. Sialic acid-dependent recognition of laminin by Penicillium marneffei conidia. Infect Immun. 1998;66(10):6024–6026. doi: 10.1128/IAI.66.12.6024-6026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton AJ, Jeavons L, Youngchim S, Vanittanakom N. Recognition of fibronectin by Penicillium marneffei conidia via a sialic acid-dependent process and its relationship to the interaction between conidia and laminin. Infect Immun. 1999;67(10):5200–5205. doi: 10.1128/IAI.67.10.5200-5205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumari A, Tripathi AH, Gautam P, Gahtori R, Pande A, Singh Y, Madan T, Upadhyay SK. Adhesins in the virulence of opportunistic fungal pathogens of human. Mycology. 2021;12(4):296–324. doi: 10.1080/21501203.2021.1934176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva BA, Pinto MR, Soares RMA, Barreto-Bergter E, Santos ALS. Pseudallescheria boydii releases metallopeptidases capable of cleaving several proteinaceous compounds. Res Microbiol. 2006;157(5):425–432. doi: 10.1016/j.resmic.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Lowry OH, Rebrough NJ, Fan AL, Randal RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- 21.Brittingham A, Morrison CJ, McMaster WR, McGwire BS, Chang KP, Mosser DM. Role of the Leishmania surface protease gp63 in complement fixation, cell adhesion, and resistance to complement-mediated lysis. J Immunol. 1995;155(6):3102–3111. doi: 10.4049/jimmunol.155.6.3102. [DOI] [PubMed] [Google Scholar]
- 22.Dubreuil JD, Giudice GD, Rappuoli R. Helicobacter pylori interactions with host serum and extracellular matrix proteins: potential role in the infectious process. Microbiol Mol Biol Rev. 2002;66(4):617–629. doi: 10.1128/MMBR.66.4.617-629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penn C, Klotz SA. Binding of plasma fibronectin to Candida albicans occurs through the cell binding domain. Microb Pathog. 1994;17(6):387–393. doi: 10.1006/mpat. [DOI] [PubMed] [Google Scholar]
- 24.DeMuri GP, Hostetter MK. Evidence for a beta 1 integrin fibronectin receptor in Candida tropicalis. J Infect Dis. 1996;174(1):127–132. doi: 10.1093/infdis/174.1.127. [DOI] [PubMed] [Google Scholar]
- 25.Zajac D, Karkowska-Kuleta J, Bochenska O, Rapala-Kozik M, Kozik A. Interaction of human fibronectin with Candida glabrata epithelial adhesin 6 (Epa6) Acta Biochim Pol. 2016;63(3):417–26. doi: 10.18388/abp.2016_1328. [DOI] [PubMed] [Google Scholar]
- 26.Gozalbo D, Gil-Navarro I, Azorín I, Renau-Piqueras J, Martínez JP, Gil ML. The cell wall-associated glyceraldehyde-3-phosphate dehydrogenase of Candida albicans is also a fibronectin and laminin binding protein. Infect Immun. 1998;66(5):2052–2059. doi: 10.1128/IAI.66.5.2052-2059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hube B. Extracellular proteinases of human pathogenic fungi. Contrib Microbiol. 2000;5:126–137. doi: 10.1159/000060350. [DOI] [PubMed] [Google Scholar]
- 28.Lima OC, Bouchara JP, Renier G, Marot-Leblond A, Chabasse D, Lopes-Bezerra LM. Immunofluorescence and flow cytometry analysis of fibronectin and laminin binding to Sporothrix schenckii yeast cells and conidia. Microb Pathog. 2004;37(3):131–140. doi: 10.1016/j.micpath.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Srinoulprasert Y, Kongtawelert P, Chaiyaroj SC. Chondroitin sulfate B and heparin mediate adhesion of Penicillium marneffei conidia to host extracellular matrices. Microb Pathog. 2006;40(3):126–132. doi: 10.1016/j.micpath.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Wasylnka JA, Moore MM. Adhesion of Aspergillus species to extracellular matrix proteins: evidence for involvement of negatively charged carbohydrates on the conidial surface. Infect Immun. 2000;68(6):3377–3384. doi: 10.1128/IAI.68.6.3377-3384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gravelat FN, Beauvais A, Liu H, Lee MJ, Snarr BD, Chen D, Xu W, Kravtsov I, Hoareau CM, Vanier G, Urb M, Campoli P, Al Abdallah Q, Lehoux M, Chabot JC, Ouimet MC, Baptista SD, Fritz JH, Nierman WC, Latgé JP, Mitchell AP, Filler SG, Fontaine T, Sheppard DC. Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal β-glucan from the immune system. PLoS Pathog. 2013;9(8):e1003575. doi: 10.1371/journal.ppat.1003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warwas ML, Watson JN, Bennet AJ, Moore MM. Structure and role of sialic acids on the surface of Aspergillus fumigatus conidiospores. Glycobiol. 2007;17(4):401–410. doi: 10.1093/glycob/cwl085. [DOI] [PubMed] [Google Scholar]
- 33.Pinto MR, Sá ACM, Limongi CL, Rozental S, Santos ALS, Barreto-Bergter E. Involvement of peptidorhamnomannan in the interaction of Pseudallescheria boydii and HEp2 cells. Microbes Infect. 2004;6:1259–1267. doi: 10.1016/j.micinf.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Xisto MI, Bittencourt VC, Liporagi-Lopes LC, Haido RMT, Mendonça MSA, Sassaki G, Figueiredo RT, Romanos MT, Barreto-Bergter E. O-glycosylation in cell wall proteins in Scedosporium prolificans is critical for phagocytosis and inflammatory cytokines production by macrophages. PLoS One. 2015;10(4):e0123189. doi: 10.1371/journal.pone.0123189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bittencourt VCB, Figueiredo RT, Silva RB, Mourão-Sá DS, Fernandez PL, Sassaki GL, Mulloy D, Bozza MT, Barreto-Bergter E. An alfa-glucan ofPseudallescheria boydiiis in- volved in fungal phagocytosis and Toll-like receptor activation. J Biol Chem. 2006;281:22614–22623. doi: 10.1074/jbc.M511417200. [DOI] [PubMed] [Google Scholar]
- 36.González A, Caro E, Muñoz C, Restrepo A, Hamilton AJ, Cano LE. Paracoccidioides brasiliensis conidia recognize fibronectin and fibrinogen which subsequently participate in adherence to human type II alveolar cells: involvement of a specific adhesin. Microb Pathog. 2008;44(5):389–401. doi: 10.1016/j.micpath.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Li T, Li JC, Qi Q, Li Y. Dexamethasone enhances invasiveness of Aspergillus fumigatus conidia and fibronectin expression in A549 cells. Chin Med J (Engl) 2013;126(17):3289–3294. [PubMed] [Google Scholar]