Abstract
For decades, topical corticosteroids have been the mainstay of treatment for mild-to-moderate inflammatory skin diseases, even though only short-term use is approved for these agents and systemic inflammation is not addressed. Increased understanding of the immunopathogenesis of these conditions, especially for psoriasis and atopic dermatitis, has facilitated the development of antibody-based drugs that neutralize single key cytokines or their associated receptors, such as interleukin (IL)-17A/F, IL-23, and IL-17RA in psoriasis and IL-13 and IL-4Rα in atopic dermatitis. However, oral therapy is still preferred by many patients owing to the ease of use and needle-free administration. Phosphodiesterase 4 (PDE4) inhibitors have been approved for both oral and topical use for inflammatory skin diseases. In this review, we present a summary of an emerging class of selective PDE4B/D inhibitors under clinical development and compare the differences in selectivity of this new generation of PDE4 inhibitors with the less selective currently approved PDE4 inhibitors.
Keywords: PDE4 inhibitors, PDE4B, PDE4D, Nerandomilast, Orismilast, PF-07038124, Zatolmilast
Key Summary Points
| Chronic inflammatory skin diseases are estimated to affect 20–25% of the world’s population and the medical need persists for new safe and effective oral drugs for long-term treatment of chronic inflammatory skin diseases. |
| A new class of selective phosphodiesterase 4 subtype B and subtype D (PDE4B/D) inhibitors is emerging as four selective PDE4B/D inhibitor drug candidates (nerandomilast, zatolmilast, orismilast, and PF-07038124) are currently in late-stage clinical trials for diseases of the lung, brain, and skin. |
| Short isoforms of PDE4B/D—in particular PDE4B2 and PDE4D1/D2—are critical isoforms to block to achieve anti-inflammatory effects, and selective PDE4B/D inhibitors may drive higher efficacy than previously approved pan-phosphodiesterase 4 (pan-PDE4) inhibitors. |
| Next generation PDE4B/D inhibitors also have potential to affect comorbidities that are associated with chronic inflammatory skin diseases, including cardiometabolic disease. |
Introduction
Chronic inflammatory skin diseases, including atopic dermatitis (AD), psoriasis, and hidradenitis suppurativa, are estimated to affect 20–25% of the world’s population [1]. Beyond the burden of living with a life-long chronic skin disease, these patients also often have increased risk for comorbidities such as cardiovascular risk, and increased mortality [2]. Although biologic therapy for chronic inflammatory skin diseases has revolutionized the field of dermatology, from a patient perspective, safe and effective oral drugs are preferred by most [3]. Several orally available immunosuppressive drugs are available today for psoriasis and atopic dermatitis, including methotrexate, cyclosporine, and Janus kinase (JAK) inhibitors (e.g., JAK1 and TYK2 inhibitors such as upadacitinib, abrocitinib, and deucravacitinib); these medications, however, require monitoring for hepatotoxicity (methotrexate) [4, 5], nephrotoxicity (cyclosporine) [6], and/or serious adverse events such as infections, tuberculosis, thrombosis, cancer, and major adverse cardiovascular events (e.g., JAK1 and TYK2 inhibitors) [7]. While the development of apremilast, a pan-phosphodiesterase 4 (PDE4) inhibitor, offered patients with psoriasis a safer option than previous oral options, efficacy of this drug is limited. Accordingly, the medical need persists for new safe and effective oral drugs for long-term treatment of chronic inflammatory skin diseases. This review describes a new generation of oral PDE4B/D selective inhibitors under development and compares their PDE4 subtype profile with the approved PDE4 inhibitors currently used for treatment of psoriasis and atopic dermatitis. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
PDE4: An Immunomodulatory Target Linked to cAMP
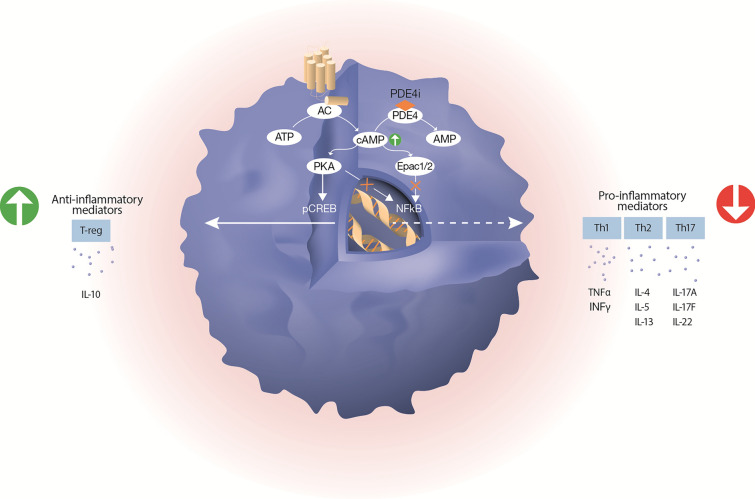
Cyclic adenosine monophosphate (cAMP) is a pivotal second messenger that regulates various cellular functions, including cell trafficking, release of inflammatory mediators, and immune cell proliferation. Drugs that elevate intracellular cAMP levels suppress immune functions of T cells, monocytes, macrophages, and neutrophils, by reducing the production of pro-inflammatory cytokines and by increasing the production of anti-inflammatory mediators (Fig. 1).
Fig. 1.
Schematic illustration of how PDE4 inhibitors and cAMP are involved in resolving inflammation. Increased level of cAMP inhibits the production of pro-inflammatory cytokines through simultaneous inhibition of PKA-NFkB and Epac1/2-NFkB pathways; and promotes the production of anti-inflammatory mediators by activation of the PKA-CREB pathway. The intracellular level of cAMP is mainly controlled by the activity of adenylyl cyclase (AC) and phosphodiesterase 4 (PDE4). Upon stimulation, AC increases cAMP levels by converting ATP to cAMP. PDE4 controls the amplitude and duration of the cAMP signal by catalyzing the degradation of cAMP to AMP. Inhibition of PDE4 increases the intracellular levels of cAMP. Adenylyl cyclase (AC), phosphodiesterase 4 (PDE4), protein kinase A (PKA), exchange protein 1/2 activated by cAMP (Epac1/2), phosphorylated cAMP-responsive element binding protein (pCREB), nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), inhibitor of PDE4 (PDE4i)
The figure was created with assistance from Erik Nylund, VisualizeThat AB
Intracellular levels of cAMP are tightly controlled on a subcellular level by phosphodiesterases (PDEs), which are a superfamily of enzymes that inactivate cAMP and cyclic guanosine monophosphate (cGMP) [8]. PDEs are grouped into 11 distinct gene members (PDE1–PDE11), which each demonstrate different selectivity for cAMP and cGMP. PDE4 selectively degrades cAMP and accounts for most of the cAMP-hydrolyzing capacity within cells. Four subtypes exist (PDE4A/4B/4C/4D), which are expressed as approximately 20 PDE4 isoforms (splice variants) [9]. These isoforms are grouped into long isoforms (acting as homo- and heterodimers) and short/super-short isoforms (acting as monomers). Importantly, all PDE4 isoforms are unique proteins and can differ in their intracellular localization, three-dimensional structure, and cell type expression. Consequently, when profiling the affinity of PDE4 inhibitors against the various PDE4 isoforms, a PDE4 isoform fingerprint is displayed that dictates the effect of the inhibitor on different cell types and potential efficacy in different diseases.
The clinical importance of different PDE4 isoforms is not fully understood and only sparse information is available regarding the function of the specific isoforms in human tissues. Inhibition of PDE4B and PDE4D subtypes is considered the main driver of the anti-inflammatory effects of PDE4 inhibitors, as these two subtypes are the only subtypes expressed in high levels in immune cells, e.g., human CD4+ T cells and peripheral blood mononuclear cells (PBMCs). Conversely, PDE4C is largely absent in immune and blood cells (Table 1) [10–12].
Table 1.
Expression of PDE4 subtypes in primary human CD4+ T cells and psoriatic PBMCs
| Gene expression in primary human CD4+ T cellsa | Protein expression in primary human CD4+ T cellsa | Gene expression in psoriatic PBMCsb | |
|---|---|---|---|
| PDE4A | 1 | 1 | 1 |
| PDE4B | Eight-fold higher levels | Two-fold higher levels | Five-fold higher levels |
| PDE4C | Not detected | Not detected | Not detected |
| PDE4D | 12-Fold higher levels | Three-fold higher levels | Two-fold higher levels |
aExpression levels of each PDE4 subtype are shown relative to PDE4A
bExpression levels of each PDE4 subtype are shown relative to healthy donors
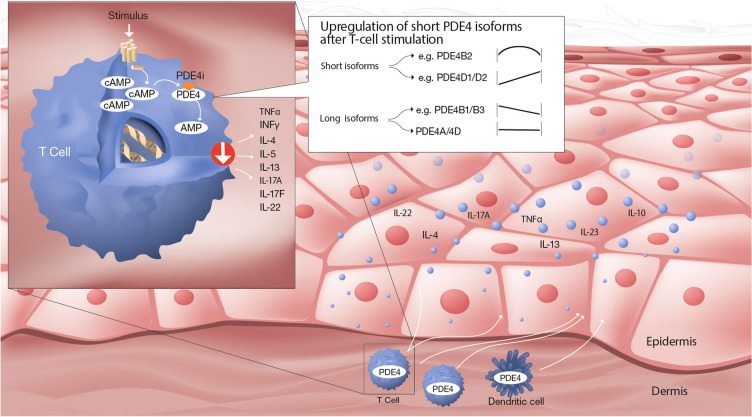
Upon CD3/CD28 stimulation of primary human CD4+ T cells, the PDE4B2 short isoform is transiently upregulated, whereas PDE4D1/D2 short isoforms are increasingly upregulated over time. By contrast, PDE4B long isoforms (PDE4B1/B3) are downregulated and PDE4A/PDE4D long forms (PDE4A4/A10 and PDED3/D4/D5/D7/D8/D9) are unaffected. The upregulation of short PDE4 splice variants was reported to account for the induction of PDE4 activity in stimulated CD4+ T cells [10]. In human neutrophils and monocytes, the short PDE4B2 isoform is the predominant PDE4 isoform [13]. On the basis of these data, we propose that the short isoforms of PDE4B/D—in particular PDE4B2, PDE4D1, and PDE4D2—are critical isoforms to block to achieve anti-inflammatory effects (Fig. 2). Additional studies, however, are needed to fully establish the functional roles of the various PDE4 isoforms across different immune and tissue cell types.
Fig. 2.
Schematic illustration of the regulation of PDE4 isoforms in stimulated T cells and the importance of inhibiting short isoforms to prevent production of inflammatory cytokines in skin. The figure is based on PDE4 isoform data obtained using anti-CD3/CD28 stimulation of CD4+ T cells [10]. The key findings were as follows: (i) The upregulation of short PDE4 splice variants was reported to account for the induction of PDE4 activity in stimulated CD4+ T cells; (ii) PDE4B2 was transiently upregulated; (iii) PDE4D1/D2 were upregulated in a time-dependent manner; (iv) PDE4B1/B3 were downregulated over time; (v) Long PDE4A/4D isoforms were unchanged; and (vi) Short isoforms of PDE4A/PDE4C and long isoforms of PDE4C were not detected. Inhibition of PDE4B2 and PDE4D1/D2 leads to increased levels of cAMP and reduced levels of disease driving cytokines in the skin
The figure was created with assistance from Erik Nylund, VisualizeThat AB
Of note, PDE4D protein expression was reported as significantly increased in both the epidermis and dermis of patients with psoriasis and atopic dermatitis compared to healthy controls, whereas a more complex expression pattern was reported for the other subtypes [14]. Interestingly, recent evidence suggests that inhibition of PDE4D5 improves diabetes-associated cardiac dysfunction [15]. PDE4 inhibition has also been shown to reduce inflammation in human vascular endothelial cells [16]. These findings highlight an important opportunity for next generation PDE4B/D inhibitors to not only positively impact chronic inflammatory skin disease but to also affect comorbidities that are associated with these diseases including cardiovascular diseases.
Approved PDE4 Inhibitors
Roflumilast was the first PDE4 inhibitor to be approved in 2010 for oral treatment of severe chronic obstructive pulmonary disease. This drug is rapidly metabolized to an active metabolite (roflumilast-N-oxide), which drives 90% of the efficacy and was reported to be a PDE4 inhibitor, without any particular selectivity for the various PDE4 isoforms (i.e., a pan-PDE4 inhibitor) [17]. Apremilast was the second PDE4 inhibitor to enter the market. Apremilast was initially approved for oral treatment of psoriasis in 2014, and later for psoriatic arthritis (2014) and Behcet’s disease (2019). Apremilast is also reported to be a pan-PDE4 inhibitor without any PDE4 isoform selectivity [18].
Apremilast is widely used and it is relatively easy to manage this drug for both patients and prescribers, largely owing to its oral dosing and benign safety profile. However, at times, adverse reactions, in particular diarrhea, nausea, emesis, and headache, can be challenging. In addition, efficacy is limited [19, 20]. A recent link between cAMP and cystic fibrosis transmembrane conductance regulator (CFTR) has been reported which may, in part, explain the diarrhea [21]. CFTR is a chloride ion channel at the apical membrane of epithelial cells, including the intestine, and has a critical role in transepithelial chloride transport and intestinal fluid secretion/homeostasis. Upregulation of intracellular cAMP levels activate the CFTR channel, causing excessive fluid secretion and secretory diarrhea. To circumvent this potentially limiting adverse reaction, topically applied PDE4 inhibitors have been developed and approved. Topical roflumilast was approved in 2022 for treatment of psoriasis, and topical crisaborole and topical difamilast for treatment of atopic dermatitis in 2017 and 2022, respectively.
Next Generation PDE4 Inhibitors Based on Selective Inhibition of the PDE4B/D Subtypes
Following the approval of pan-PDE4 inhibitors, development of compounds that selectively inhibit PDE4B/D subtypes has received renewed attention. Indeed, four selective PDE4B/D inhibitor drug candidates are currently in late-stage clinical trials for diseases of the lung, brain, and skin (Table 2). Clinical data across several indications support the hypothesis that PDE4B/D inhibitors can achieve high clinical efficacy, opening these drugs for new clinical applications.
Table 2.
Overview of approved PDE4 inhibitors and next generation PDE4B/D selective inhibitors in late-stage clinical development
| Compound | Indication | Phase | Isoform data | Selectivity ratioa | PDE4 profileb |
|---|---|---|---|---|---|
| Roflumilast (oral and topical) | COPD | Approved |
PDE4A1: 0.7 nM PDE4A4: 0.9 nM PDE4B1: 0.7 nM PDE4B2: 0.2 nM cPDE4C1: 3 nM cPDE4C2: 4.3 nM PDE4D2: 0.3 nM PDE4D3: 0.4 nM PDE4D4: 0.2 nM PDE4D5: 0.4 nM |
PDE4 A/B = 1.8 PDE4 A/D = 2.5 PDE4 B/D = 1.4 Unselective |
High-potency, unselective PDE4 inhibitor |
| Apremilast (oral) | Psoriasis, psoriatic arthritis, and Behcet’s disease | Approved |
PDE4A1: 78 nM PDE4A4: 42 nM PDE4A10: 140 nM PDE4B1: 61 nM PDE4B2: 97 nM PDE4B3: 117 nM cPDE4C2: 244 nM PDE4D1: 44 nM PDE4D2: 54 nM PDE4D3: 54 nM PDE4D4: 41 nM PDE4D5: 61 nM PDE4D7: 50 nM |
PDE4 A/B = 1.0 PDE4 A/D = 1.7 PDE4 B/D = 1.8 Unselective |
Medium-potency, unselective PDE4 inhibitor |
| Nerandomilast (oral) | Idiopathic pulmonary fibrosis and progressive fibrosing interstitial lung diseases | 3 |
PDE4A: 248 nM PDE4B2: 10 nM cPDE4C2: 8700 nM PDE4D2: 91 nM |
PDE4 A/B = 25 PDE4 A/D = 2.7 PDE4 D/B = 9.1 Selective for B |
Potent, selective PDE4B inhibitor |
| Zatolmilast (oral) | Fragile X syndrome | 2b/3 |
PDE4D2: 127 nM dPDE4D7: 1018 nM ePDE4D7: 8 nM PDE4D3: 7 nM |
ND | Potent allosteric, PDE4D modulator |
| Orismilast (oral) | Psoriasis and atopic dermatitis | 2b |
PDE4A1: 16 nM PDE4A4: 11 nM PDE4A10: 52 nM PDE4B1: 16 nM PDE4B2: 6 nM PDE4B3: 3 nM cPDE4C2: 104 nM PDE4D1: 9 nM PDE4D2: 2 nM PDE4D3: 2 nM PDE4D4: 3 nM PDE4D5: 2 nM PDE4D7: 3 nM |
PDE4 A/B = 3.2 PDE4 A/D = 7.5 PDE4 B/D = 2.5 Selective for B/D |
Potent, selective PDE4B/D inhibitor |
| Crisaborole [30] (topical) | Atopic dermatitis | Approved |
PDE4A1: 52 nM PDE4B1: 61 nM PDE4B2: 75 nM cPDE4C1: 340 nM PDE4D7: 170 nM |
PDE4 B/D = 1.3 PDE4 D/A = 3.3 PDE4 B/D = 1.4 Selective for A |
Medium-potency, selective PDE4A inhibitor |
| PF-07038124 (topical) | Psoriasis and atopic dermatitis | 2b | PDE4B2: 0.5 nM | ND | High-potency PDE4B2 inhibitor |
ND not determined
aUnselective was defined as a potency ratio < three-fold when comparing the isoform with highest potency to the isoform with lowest potency. Selective was defined as a potency ratio ≥ three-fold when comparing the isoform with highest potency to the isoform with lowest potency. When more isoforms were reported within a PDE4 subtype, the average of the isoform data of a given subtype was used
bHigh potency was defined as a potency range of 0.1–0.9 nM. Potent was defined as a potency range of 1–10 nM. Medium potency was defined as a potency range of 11–1000 nM
cPDE4C isoform data are listed in italics and not considered when describing the PDE4 profile, since PDE4C isoforms were reported to be absent in immune and blood cells (see Table 1)
dThis isoform was the basal dimer of PDE4D7
eThis isoform was the activated dimer of PDE4D7
Apremilast data were generated head-to-head with orismilast [26]
Nerandomilast is a selective PDE4B2 inhibitor, although rather limited PDE4 isoform data have been disclosed [22]. In addition to anti-inflammatory effects, nerandomilast also demonstrated an anti-fibrotic effect in preclinical models, as nerandomilast inhibited the transforming growth factor beta (TGFβ)-stimulated transformation of fibroblasts into myofibroblasts. In a phase 2 trial, nerandomilast prevented a decrease in lung function in patients with idiopathic pulmonary fibrosis over a period of 12 weeks [23]. Nerandomilast is currently undergoing phase 3 clinical testing in idiopathic pulmonary fibrosis and progressive fibrosing interstitial lung diseases.
Zatolmilast is a brain-penetrating allosteric modulator of PDE4D. The compound selectively and partially inhibits activated dimeric isoforms of PDE4D when compared to monomeric and basal forms [24]. In theory, modulation of PDE4D (rather than complete inhibition) is predicted to improve cognitive function by prolonging cAMP activity. In a phase 2 trial, zatolmilast met key secondary efficacy measures of cognition and daily function in patients with fragile X syndrome [25]. This compound is currently being studied in a phase 2b/3 trial in the same indication.
Orismilast is a PDE4B/D selective inhibitor that has been profiled against 13 PDE4 isoforms and shown to selectively inhibit PDE4B/D isoforms [26]. In addition, orismilast potently inhibits the secretion of several key disease-driving cytokines (e.g., tumor necrosis factor (TNF)α, IL-17A, and IL-13) in preclinical models. In the clinic, orismilast has shown promising efficacy data in a placebo-controlled, phase 2b study in patients with moderate-to-severe psoriasis [27]. Furthermore, orismilast has shown encouraging data in hidradenitis suppurativa [28] and is currently being studied in a phase 2b trial in patients with atopic dermatitis.
PF-07038124 is a potent PDE4B2 inhibitor designed to be applied to the skin, using a soft-drug approach, whereby the drug is rapidly inactivated when it reaches the systemic circulation. No additional PDE isoform data has been disclosed for PF-07038124. PF-07038124 is currently being studied for topical treatment of both psoriasis and atopic dermatitis [29].
Conclusion
A new class of selective PDE4B/D inhibitors is emerging. Based on clinical data across psoriasis, atopic dermatitis, idiopathic pulmonary fibrosis, and fragile X syndrome, the combination of potent yet selective mechanism of action offers new opportunities for clinical applications. Combined with the proven safety of the pan-PDE4 inhibitors, novel selective PDE4B/D inhibitors offer a potential new treatment approach for the long-term management of chronic inflammatory and fibrotic diseases.
Acknowledgments
Medical Writing/Editorial Assistance
The figures were created with assistance from Erik Nylund, VisualizeThat AB, with funding from UNION therapeutics
Author Contributions
All authors contributed to the interpretation of data and conception of the manuscript and critically revised and approved the manuscript content. The authors meet the authorship criteria recommended by the International Committee of Medical Journal Editors (ICMJE) and did not receive payment related to the development of this article.
Funding
UNION therapeutics supported the publication of this article including rapid publication fee.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
Andrew Blauvelt has served as a speaker (received honoraria) for AbbVie, Bristol-Myers Squibb, Eli Lilly and Company, Pfizer, Regeneron, and Sanofi, served as a scientific adviser (received honoraria) for AbbVie, Abcentra, Aclaris, Affibody, Aligos, Almirall, Alumis, Amgen, Anaptysbio, Apogee, Arcutis, Arena, Aslan, Athenex, Bluefin Biomedicine, Boehringer Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, CTI BioPharma, Dermavant, EcoR1, Eli Lilly and Company, Escient, Evelo, Evommune, Forte, Galderma, HighlightII Pharma, Incyte, InnoventBio, Janssen, Landos, Leo, Lipidio, Merck, Monte Rosa Therapeutics, Nektar, Novartis, Overtone Therapeutics, Pfizer, Rani, Rapt, Regeneron, Sanofi Genzyme, Spherix Global Insights, Sun Pharma, Takeda, TLL Pharmaceutical, TrialSpark, UCB Pharma, Union, Ventyx, Vibliome, and Xencor, and has acted as a clinical study investigator (institution has received clinical study funds) for AbbVie, Acelyrin, Allakos, Almirall, Alumis, Amgen, Arcutis, Athenex, Boehringer Ingelheim, Bristol-Myers Squibb, Concert, Dermavant, Eli Lilly and Company, Evelo, Evommune, Galderma, Incyte, Janssen, Leo, Merck, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, UCB Pharma, and Ventyx. Richard G. Langley has received honoraria from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Dermira, Eli Lilly, GlaxoSmithKline, Janssen, Leo Pharma, Novartis, Ortho Dermatologics, Pfizer, Sanofi Genzyme, Sun Pharma, and UCB. Kenneth B. Gordon has received grant support and consulting fees from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, and UCB, and has received consulting fees from Amgen, Almirall, Dermira, Leo Pharma, Pfizer, and Sun Pharma. Jonathan Silverberg is a consultant and/or advisory board member for Abbvie, Afyx, Aobiome, Arena, Asana, Aslan, BioMX, Bluefin, Bodewell, Boehringer-Ingelheim, Celgene, Connect Biopharma, Dermavant, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Kiniksa, Leo Pharma, Luna, Menlo, Novartis, Pfizer, RAPT, Regeneron, Sanofi-Genzyme; speaker for Abbvie, Eli Lilly, Leo Pharma, Pfizer, Regeneron, Sanofi-Genzyme; institution received grants from Galderma, Pfizer. LEF: Consultant and/or advisory board member Galderma, Janssen, Leo Pharma, Eli Lilly, Almirall, UNION therapeutics, Regeneron, Novartis, Amgen, Abbvie, UCB, Biotest, AC Immune and InflaRx. Kilian Eyerich has received speakers fees and/or advisory board member for Abbvie, Almirall, Boehringer Ingelheim, BMS, Incyte, Janssen, Leo, Lilly, Pfizer, Novartis, UCB; shares and co-founder of Dermagnostix and Dermagnostix R&D. Richard B. Warren has received research grants from AbbVie, Almirall, Amgen, Celgene, Janssen, Lilly, Leo Pharma, Novartis, Pfizer, and UCB; consulting fees from AbbVie, Almirall, Amgen, Arena, Astellas, Avillion, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, DiCE, GlaxoSmithKline, Janssen, Lilly, Leo Pharma, Novartis, Pfizer, Sanofi, Sun Pharma, UCB, and UNION Therapeutics.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Ujiie H, Rosmarin D, Schön MP, et al. Unmet medical needs in chronic, non-communicable inflammatory skin diseases. Front Med (Lausanne) 2022;9:875492. doi: 10.3389/fmed.2022.875492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hojman L, Karsulovic C. Cardiovascular disease-associated skin conditions. Vasc Health Risk Manag. 2022;18:43–53. doi: 10.2147/VHRM.S343319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeri M, Sutphin J, Hauber B, Cappelleri JC, Romero W, Di Bonaventura M. Quantifying patient preferences for systemic atopic dermatitis treatments using a discrete-choice experiment. J Dermatol Treat. 2022;33(3):1449–1458. doi: 10.1080/09546634.2020.1832185. [DOI] [PubMed] [Google Scholar]
- 4.West SG. Methotrexate hepatotoxicity. Rheum Dis Clin N Am. 1997;23(4):883–915. doi: 10.1016/S0889-857X(05)70365-3. [DOI] [PubMed] [Google Scholar]
- 5.Kreher MA, Konda S, Noland MMB, Longo MI, Valdes-Rodriguez R. Risk of melanoma and nonmelanoma skin cancer with immunosuppressants, part II: methotrexate, alkylating agents, biologics, and small molecule inhibitors. J Am Acad Dermatol. 2023;88(3):534–542. doi: 10.1016/j.jaad.2022.11.043. [DOI] [PubMed] [Google Scholar]
- 6.Burdmann EA, Andoh TF, Yu L, Bennett WM. Cyclosporine nephrotoxicity. Semin Nephrol. 2003;23(5):465–476. doi: 10.1016/S0270-9295(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 7.Samuel C, Cornman H, Kambala A, Kwatra SG. A review on the safety of using JAK inhibitors in dermatology: clinical and laboratory monitoring. Dermatol Ther (Heidelb) 2023;13(3):729–749. doi: 10.1007/s13555-023-00892-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conti M, Mika D, Richter W. Cyclic AMP compartments and signaling specificity: role of cyclic nucleotide phosphodiesterases. J Gen Physiol. 2014;143(1):29–38. doi: 10.1085/jgp.201311083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paes D, Schepers M, Rombaut B, van den Hove D, Vanmierlo T, Prickaerts J. The molecular biology of phosphodiesterase 4 enzymes as pharmacological targets: an interplay of isoforms, conformational states, and inhibitors. Pharmacol Rev. 2021;73(3):1016–1049. doi: 10.1124/pharmrev.120.000273. [DOI] [PubMed] [Google Scholar]
- 10.Peter D, Jin SL, Conti M, Hatzelmann A, Zitt C. Differential expression and function of phosphodiesterase 4 (PDE4) subtypes in human primary CD4+ T cells: predominant role of PDE4D. J Immunol. 2007;178(8):4820–4831. doi: 10.4049/jimmunol.178.8.4820. [DOI] [PubMed] [Google Scholar]
- 11.Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28(7):753–763. doi: 10.1016/j.cellsig.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Kolb M, Crestani B, Maher TM. Phosphodiesterase 4B inhibition: a potential novel strategy for treating pulmonary fibrosis. Eur Respir Rev. 2023;32(167):220206. doi: 10.1183/16000617.0206-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang P, Wu P, Ohleth KM, Egan RW, Billah MM. Phosphodiesterase 4B2 is the predominant phosphodiesterase species and undergoes differential regulation of gene expression in human monocytes and neutrophils. Mol Pharmacol. 1999;56(1):170. doi: 10.1124/mol.56.1.170. [DOI] [PubMed] [Google Scholar]
- 14.Schafer PH, Adams M, Horan G, Truzzi F, Marconi A, Pincelli C. Apremilast normalizes gene expression of inflammatory mediators in human keratinocytes and reduces antigen-induced atopic dermatitis in mice. Drugs R D. 2019;19(4):329–338. doi: 10.1007/s40268-019-00284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu R, Fu J, Hu Y, et al. Roflumilast-mediated phosphodiesterase 4D inhibition reverses diabetes-associated cardiac dysfunction and remodeling: effects beyond glucose lowering. Diabetes. 2022;71(8):1660–1678. doi: 10.2337/db21-0898. [DOI] [PubMed] [Google Scholar]
- 16.Otto M, Dorn B, Grasmik T, et al. Apremilast effectively inhibits TNFα-induced vascular inflammation in human endothelial cells. J Eur Acad Dermatol Venereol. 2022;36(2):237–246. doi: 10.1111/jdv.17769. [DOI] [PubMed] [Google Scholar]
- 17.Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163(1):53–67. doi: 10.1111/j.1476-5381.2011.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schafer PH, Parton A, Capone L, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26(9):2016–2029. doi: 10.1016/j.cellsig.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1) J Am Acad Dermatol. 2015;73(1):37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 20.Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2) Br J Dermatol. 2015;173(6):1387–1399. doi: 10.1111/bjd.14164. [DOI] [PubMed] [Google Scholar]
- 21.Moon C, Zhang W, Sundaram N, et al. Drug-induced secretory diarrhea: a role for CFTR. Pharmacol Res. 2015;102:107–112. doi: 10.1016/j.phrs.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrmann FE, Hesslinger C, Wollin L, Nickolaus P. BI 1015550 is a PDE4B inhibitor and a clinical drug candidate for the oral treatment of idiopathic pulmonary fibrosis. Front Pharmacol. 2022;13:838449. doi: 10.3389/fphar.2022.838449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richeldi L, Azuma A, Cottin V, et al. Trial of a preferential phosphodiesterase 4B inhibitor for idiopathic pulmonary fibrosis. N Engl J Med. 2022;386(23):2178–2187. doi: 10.1056/NEJMoa2201737. [DOI] [PubMed] [Google Scholar]
- 24.Gurney ME, Nugent RA, Mo X, et al. Design and synthesis of selective phosphodiesterase 4D (PDE4D) allosteric inhibitors for the treatment of fragile X syndrome and other brain disorders. J Med Chem. 2019;62(10):4884–4901. doi: 10.1021/acs.jmedchem.9b00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry-Kravis EM, Harnett MD, Reines SA, et al. Inhibition of phosphodiesterase-4D in adults with fragile X syndrome: a randomized, placebo-controlled, phase 2 clinical trial. Nat Med. 2021;27(5):862–870. doi: 10.1038/s41591-021-01321-w. [DOI] [PubMed] [Google Scholar]
- 26.Silverberg JI, French LE, Warren RB, et al. Pharmacology of orismilast, a potent and selective PDE4 inhibitor. J Eur Acad Dermatol Venereol. 2023;37(4):721–729. doi: 10.1111/jdv.18818. [DOI] [PubMed] [Google Scholar]
- 27.Warren RB, French LE, Blauvelt A, et al. Orismilast in moderate-to-severe psoriasis: efficacy and safety from a 16-week, randomized, double-blinded, placebo-controlled, dose-finding, phase 2b trial (IASOS). 2023. J Am Acad Dermatol. (In press). [DOI] [PubMed]
- 28.Frederiksen CG, Sedeh FB, Taudorf EH, Saunte DM, Jemec GBE. Orismilast for mild to severe hidradenitis suppurativa: preliminary data from OSIRIS, a phase 2a, open-label, single-center, single-arm clinical trial. Poster at the 12th European Hidradenitis Suppurativa Foundation (EHSF) conference. February 8–10, 2023, in Florence, Italy.
- 29.Strobach JW, Blakemore DC, Jones P, et al. Boron containing PDE4 inhibitors. 2023. WO2020070651.
- 30.Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358(3):413–422. doi: 10.1124/jpet.116.232819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.