Abstract
The objective of this study was to evaluate the potential antimicrobial and antibiofilm effect of ginger essential oil (GEO) and 6-gingerol on a multispecies biofilm formed by Listeria monocytogenes, Salmonella Typhimurium, and Pseudomonas aeruginosa on a polypropylene surface. The minimum inhibitory concentration concentrations obtained for GEO were 100 and 50 mg/mL and for 6-gingerol 1.25 mg/mL. Sessile cell counts ranged within 5.35–7.35 log CFU/cm2 in the control biofilm, with the highest sessile growth at 72 h. GEO treatments acted on the total population regardless of concentration at 1 and 48 h. L. monocytogenes behaved similarly to the total population, showing GEO action at 1 h and keeping the same pattern at 48, 72, and 96 h. Better action on S. Typhimurium was obtained at times of 1, 72, and 96 h. P. aeruginosa showed logarithmic reduction only when treated with GEO 50 mg at 24 h. As for 6-gingerol, in general, there was no significant action (p > 0.05) on the evaluated sessile cells. GEO showed antimicrobial activity against L. monocytogenes, S. Typhimurium, and P. aeruginosa, acting as an inhibitor of biofilm formation. As for 6-gingerol, it was considered a possible antimicrobial agent but without efficacy during biofilm formation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-023-01075-2.
Keywords: Antibiofilm, Antibacterial, Control methods, Essential oils
Introduction
Listeria monocytogenes, Salmonella Typhimurium, and Pseudomonas aeruginosa have the ability to adhere and form biofilm on the surfaces of equipment and utensils used in food processing [1–3]. Microorganisms secrete an extracellular matrix composed of polysaccharides, proteins, and nucleic acids during biofilm formation, which originates an ecosystem that provides cell protection and maintenance in hostile environments [4, 5]. This barrier help increase tolerance to biocides and antibiotics at usual concentrations, resulting in persistent bacterial contamination in the production environment, with a potential risk to public health [1].
The inappropriate use of commercial biocides has been responsible for negative effects on the environment, residual accumulation in the production system, reduced efficacy, and development of co-resistance in pathogenic microorganisms [6–8]. Thus, in recent years, the scientific community has shown greater interest in studying the use of natural compounds such as essential plant oils and their active principles in the industrial context [9, 10] owing to its bactericidal and biodegradable characteristics [11, 12], with great environmental gain and low toxicity.
Ginger essential oils (GEO) (Zingiber officinale) are liquid, oily, and aromatic substances extracted from roots that have anti-inflammatory [13], antioxidant [14], antimicrobial [10], and antifungal properties [15]. These natural compounds act on the cell membrane integrity of microorganisms, interfering in protein and microbiological marker synthesis as inhibitors of quorum sensing activity [16, 17]. The antimicrobial activity of non-volatile ginger compounds such as 6-gingerol has been described as effective against mycobacteria [18] and multi-resistant pathogens [19] and as a phytochemical with high capacity of inhibiting P. aeruginosa quorum sensing and virulence factors [20]. However, there is no report to date on its activity on sessile cells in multispecies biofilms. Thus, the objective of this study was to evaluate the antimicrobial and antibiofilm effect of GEO and 6-gingerol on a multispecies biofilm formed by L. monocytogenes, S. Typhimurium, and P. aeruginosa on a polypropylene surface.
Material and methods
Bacterial strains
The L. monocytogenes serotype IVb (LM) (n = 1) and S. Typhimurium serogroup O:4 (SAL) (n = 1) strains isolated from meat processing equipment and utensil surfaces [3, 21] were obtained from the culture bank of the Laboratory of Food and Water Quality Inspection and Control (LACOMA) at the Federal University of Paraná (UFPR). The P. aeruginosa ATCC 27853 strain was also used (PS) (n = 1). Isolates were stored at − 18 °C in tryptone soy broth supplemented with 0.6% yeast extract (TSB-YE; Oxoid; UK) containing 20% (v/v) glycerol.
Preparation of GEO and 6-gingerol
GEO (BATCH: 283, CAS: 8007–08-7—Table 1) and 6-gingerol active ingredient (BATCH: 59,126, CAS: 23,513–14-6) were respectively purchased from FERQUIMA—Indústria e Comércio Ltd. and Start Bioscience Materiais para Laboratórios Ltd. The GEO composition was provided by FERQUIMA—Indústria e Comércio Ltd. through a technical report. Working solutions were prepared to each experiment repetition and stored at room temperature, protected from light. To avoid changing component concentrations, the same batches were used throughout the experiment. The solutions were solubilized in dimethylsulfoxide (DMSO) 0.5% (Sigma-Aldrich, St. Louis, MO, USA) added to TSB-YE containing 0.5% polysorbate 80 (Tween 80®; Sigma-Aldrich).
Table 1.
GEO composition containing the main major compounds
| Main components of ginger essential oil (Zingiber officinale)* | |
|---|---|
| Chemical compost | Composition (%) |
| α-Zingiberene | 33 |
| β-Sesquifelandrene | 13 |
| α-Curcumene | 8 |
| β-Bisabolene | 6 |
| Camphene | 6 |
| α-Pinene | 2 |
*Extraction carried out through steam distillation of the root
Determination of minimum inhibitory concentration (MIC) in L. monocytogenes, S. Typhimurium, and P. aeruginosa
The MIC was determined using the broth microdilution method based on the CLSI protocol [22], with modifications. Pure (SAL, LM, and PS) and combined microorganism inoculums (LM + SAL, PS + LM, PS + SAL, SAL + LM + PS) were prepared from a 24 h microorganism culture until reaching the 0.5 McFarland scale. Each well of a 96-well plate received 100 µL of Mueller Hinton (MH) broth supplemented with 0.5% polysorbate 80 (Tween 80—Vetec®). Next, 200 μL of GEO and 6-gingerol active ingredient were added separately in the first line of wells at serial dilutions (1:2) of 200, 100, 50, 25, 12.5, 6.25, 3.12, 1.56, and 0.78 mg/mL for GEO, and of 10, 5, 2.5, 1.25, 0.62, 0.31, and 0.15 mg/mL for 6-gingerol. Then, 20 μL of inoculum and 80 μL of MH broth to complement the dilution were added, with a final concentration of 1.0 × 105 CFU/mL. The plates were incubated at 37 °C for 24 h under 120 rpm orbital shaking. Plates were macroscopically read by the visible presence or absence of bacterial growth, and for confirmation, 1 μL of the test solution was inoculated with a pin replicator into Petri plates containing MH agar with subsequent reading after incubation at 37 °C for 24 h. Standard antibiotics were used as positive controls of the antimicrobial action, with gentamicin for L. monocytogenes and S. Typhimurium and ampicillin for P. aeruginosa. The positive inoculum control was MH broth containing DMSO supplemented with 0.5% polysorbate 80 without test solution and 1.0 × 105 CFU/mL of inoculum. Negative process control included wells incubated with MH broth containing DMSO supplemented with 0.5% polysorbate 80 without inoculum. To carry out the experiment, the lowest concentration of oil capable of completely inhibiting microbial growth was chosen.
Multispecies biofilm formation on polypropylene surface with GEO interference
L. monocytogenes, S. Typhimurium, and P. aeruginosa isolates were subjected to multispecies biofilm formation on polypropylene coupons for up to 96 h at 10 °C, under the following conditions: multispecies biofilm formation (C +), multispecies biofilm formation with ½ GEO MIC, and multispecies biofilm formation with GEO MIC.
Prior to the bacterial adhesion tests, the polypropylene coupons (1.0 × 1.0 × 0.1 cm) (n = 90) were washed with 70% w/w alcohol (70°INPM), rinsed three times in distilled water, and sterilized in an autoclave at 121 °C for 15 min. The conditions for preparing and confirming the inoculum were performed as described by Santos et al. [23]. Three flasks containing the mixed culture were prepared: one was used as an untreated positive control, one was treated with ½ MIC of GEO, and the third was treated with the MIC of GEO. The vials were kept at 10 °C under 120 rpm orbital shaking, and planktonic and sessile growth was evaluated at 1, 12, 24, 48, 72, and 96 h of incubation. Duplicate 1-mL TSB-YE aliquots were collected from each treatment at different incubation times for planktonic microorganism counting, and two coupons were used for sessile microorganism counting.
Sessile cells were quantified using the methodology by [24], with modifications. Coupons were washed with 10 mL of phosphate buffered saline (PBS), immersed in 1.0 mL of PBS solution supplemented with 1% polysorbate 80, and sessile cells were extracted through ultrasonic (40 kHz for 1.5 min, twice) and vortex ultrasonic baths (1.5 min, twice). After this procedure, appropriate serial dilutions were performed, and 10 µL aliquots were collected for sessile cell quantification using the drop plate method [25] and inoculated onto tryptone soy agar (KASVI, Brazil) for total biofilm population count, Oxford listeria agar (Oxoid) for L. monocytogenes, cetrimide agar (KASVI, Brazil) for P. aeruginosa, and xylose lysine deoxycholate agar (KASVI, Brazil) for S. Typhimurium. Plates were incubated at 37 °C for 24–48 h, as recommended by the manufacturer. The study included two repetitions for each condition, and the results were expressed in log CFU/mL and log CFU/cm2 for planktonic and sessile cells, respectively.
Multispecies biofilm formation on polypropylene surface with 6-gingerol interference
The dynamics of L. monocytogenes, S. Typhimurium, and P. aeruginosa multispecies biofilm formation was evaluated on polypropylene coupons with 6-gingerol at 1, 12, 24, 48, 72, and 96 h at 10 °C. Growth conditions, biofilm extraction, counting, and results were analyzed as previously described.
Relative distribution (RD) of the three-species biofilm
The calculation for evaluating the relative distribution followed the method described by Macho et al. [26] with adaptations. The total number of cultivable cells was enumerated by plating serial dilutions onto non-specific and specific agar plates. The RD of each of the three species in the multispecies biofilm was reported as a percentage (single species A + single species B + single species C) and was calculated before (control biofilm) and after treatment with the evaluated compounds (GEO and 6-gingerol), as follows:
Statistical analysis
The results were expressed as mean ± standard deviation. The Shapiro–Wilk and Kolmogorov–Smirnov tests for normality were used to evaluate. Subsequently, the non-parametric Mann–Whitney test was used to statistically compare count means with different times. The IBM® SPSS® statistics software version 2.0 was used for all analyses at a 0.05 significance level.
Results
GEO and 6-gingerol antimicrobial activity
The compounds were evaluated for their in vitro antimicrobial activity before being evaluated for their antibiofilm efficacy. GEO and 6-gingerol susceptibility evaluation results are described in Table 2. Lower 6-gingerol active ingredient concentrations inhibited microbial growth, compared to GEO (with L. monocytogenes being the most sensitive). The MIC values were the same in the evaluation of the action of 6-gingerol on S. Typhimurium and P. aeruginosa and on associated microorganisms (1.25 mg/mL).
Table 2.
GEO and 6-gingerol antimicrobial activity against L. monocytogenes, S. Typhimurium, and P. aeruginosa in individual and associated cultures
| Microorganisms | GEO (mg/mL) | 6-gingerol (mg/mL) |
|---|---|---|
| LM | 25 | 0.62 |
| SAL | 200 | 1.25 |
| PS | 100 | 1.25 |
|
LM + SAL LM + PS PS + SAL |
50 100 200 |
1.25 1.25 1.25 |
| LM + SAL + PS | 100 | 1.25 |
Results obtained in two individual experiments in triplicates
*LM: Listeria monocytogenes, SAL: Salmonella Typhimurium, PS: Pseudomonas aeruginosa
With GEO, microbial interaction led to different MIC responses compared to the values obtained for individual cultures. Like 6-gingerol, GEO showed better action on L. monocytogenes than on other microorganisms. The MIC varied between compounds, with the need for higher GEO concentrations to inhibit bacterial growth.
GEO and 6-gingerol effect on multispecies biofilm formation on polypropylene surface
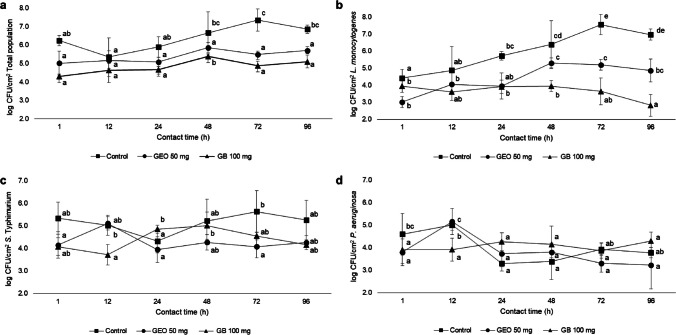
The effects of GEO and 6-gingerol on multispecies biofilm formation were evaluated using two GEO concentrations and one 6-gingerol concentration. These concentrations did not decrease the planktonic cell counts of the evaluated strains (data not shown). Figure 1 shows the behavior of the population of sessile cells without interference (C +), added with a GEO concentration of 50 mg/mL as ½ MIC and of 100 mg/mL as MIC during the incubation times. Sessile cells were individually counted for each microorganism within the multispecies biofilm, and biofilm adhesion and maintenance processes depend on the evaluated conditions (species/time) (Fig. 1). The evaluation of total population behavior (Fig. 1a) showed different counts, with results ranging from 5.35 to 7.35 log CFU/cm2 between the control biofilm and the treatments used (Table S1). GEO showed its best effect during the adhesion process (1 h) and on the formed biofilm (48 h) at a concentration of 100 mg (p < 0.05).
Fig. 1.
Interference of adding 50 mg (1/2 MIC) and 100 mg (MIC) of GEO in sessile growth dynamics of multispecies biofilms with individual evaluation of the total population (a), L. monocytogenes (b), S. Typhimurium (c), and P. aeruginosa (d) at 10 °C over time. The results represent two individual experiments in triplicate. Different lowercase letters between the evaluated times (1, 12, 24, 48, 72, and 96 h) indicate statistical difference (p < 0.05)
There was greater sessile growth in the control biofilm at 72 h. L. monocytogenes counts (Fig. 1b) behaved similarly to the total population, decreasing cell counts when concentrations of 100 and 50 mg were applied in the adhesion process in 1 h. It maintained the same behavior pattern at 48, 72, and 96 h (p < 0.05). For S. Typhimurium (Fig. 1c) the times we obtained the lowest counts in the evaluated concentrations (50 and 100 mg) were 1, 72, and 96 h (Fig. 1c) in comparison with the control. P. aeruginosa sessile cell count (Fig. 1d) showed logarithmic reduction in 24 h (p < 0.05) compared to control, maintaining similar counts until the last evaluation time point at 72 h.
There was no significant action (p > 0.05) of the 6-gingerol compound on sessile cells under the influence of biofilm formation time (Table 3). The evaluation of total biofilm population compared to the logarithmic decrease between treatment and control showed lower counts with 1.25 mg of 6-gingerol (MIC) at 12 h (p < 0.05). Individual sessile cell counts showed action on L. monocytogenes during the adhesion process at 1 and 48 h (p < 0.05), and on S. Typhimurium and P. aeruginosa at 1 h (p < 0.05).
Table 3.
6-gingerol (1.25 mg/mL) activity against L. monocytogenes, S. Typhimurium, and P. aeruginosa in multispecies biofilm
| Microorganisms | Treatments | Contact time | |||||
|---|---|---|---|---|---|---|---|
| 1 h | 12 h | 24 h | 48 h | 72 h | 96 h | ||
| Total population | Control | 4.89A1;a2 | 5.07A;b | 4.85A;a | 6.71B;a | 5.54A;a | 5.56A;a |
| 6-gingerol | 5.10B;a | 3.86A;a | 5.51BC;a | 6.40C;a | 4.72AB;a | 5.58BC;a | |
| L. monocytogenes | Control | 5.61B;b | 4.14A;a | 3.76A;a | 7.01C;b | 4.60A;a | 5.57B;a |
| 6-gingerol | 3.75A;a | 4.38AB;a | 4.58AB;a | 5.83C;a | 5.22BC;a | 5.36BC;a | |
| S. Typhimurium | Control | 4.60A;a | 5.38A;a | 5.07A;a | 4.76A;a | 6.54B;a | 5.39A;a |
| 6-gingerol | 5.15A;a | 6.36B;b | 4.68A;a | 6.17B;b | 6.72B;a | 4.93A;a | |
| P. aeruginosa | Control | 4.10A;a | 6.19C;b | 4.29A;a | 5.34BC;a | 5.97C;a | 4.38AB;a |
| 6-gingerol | 4.24A;a | 4.35AB;a | 4.03A;a | 5.35BC;a | 5.72C;a | 5.22BC;a | |
*Results are expressed in log CFU/cm2
1Different capital letters on the same line indicate statistically significant difference (p < 0.05) for the same microorganism
2Different lowercase letters in the same column indicate statistically significant difference (p < 0.05) for the same microorganism
The comparison between 6-gingerol and GEO actions shows that GEO was more effective, with results varying by observation time (Fig. 2). Although the application of GEO showed lower counts in the total population at a concentration of 100 mg at all times evaluated, this action was not observed statistically (p > 0.05).
Fig. 2.
Interference of 6-gingerol concentration of 1.25 mg (MIC), and GEO concentration of 50 mg (1/2 MIC) and 100 mg (MIC) in multispecies biofilm formation. Asterisks between treatments (6-gingerol × GEO) demonstrate statistical difference (p < 0.05) in incubation time
The proportion of L. monocytogenes gradually increased over time on the control multispecies biofilm (Fig. 3), with a 2.5 log CFU/cm2 increase in sessile cells ranging between 4.42 and 6.97 log CFU/cm2 (30 to 40% of the biofilm) at 96 h of incubation. The proportions in microbial consortium exposed to 100 mg/mL of GEO (MIC) showed the opposite behavior, with P. aeruginosa and S. Typhimurium growth instead of L. monocytogenes, with the highest population increase at 96 h, reaching 4.3, 4.16, and 2.82 CFU/cm2, respectively. In general, the percentage composition of the multispecies biofilm was altered because of exposure to the evaluated compound, according to the applied concentration (Fig. 3).
Fig. 3.
L. monocytogenes, S. Typhimurium, and P. aeruginosa relative distribution (RD) within the multispecies biofilm as a function of time and treatment
Discussion
The use of natural compounds has been evaluated as an alternative and complement to the use of biocides [10, 17]. The results of this study indicate that GEO and 6-gingerol have different antimicrobial actions. Previous studies reported that 6-gingerol showed considerable inhibitory and bactericidal activity against planktonic Staphylococcus aureus and Escherichia coli cells at a concentration of 0.4 mg/mL [27] and against mycobacteria at a concentration of 0.05 mg/mL [18]. However, this is the first study on the antimicrobial activity of 6-gingerol against L. monocytogenes, S. Typhimurium, and P. aeruginosa. This study demonstrated the antibacterial effect of 6-gingerol active ingredient against microorganisms evaluated both individually and in combination, at concentrations similar to other microorganism groups.
GEO showed antimicrobial activity and a different behavior according to the evaluated microorganism [28, 29). Owing to GEO hydrophobia, insolubility, and instability, [28] evaluated its antimicrobial activity in an encapsulated form against L. monocytogenes, S. Typhimurium, and P. aeruginosa isolates. The present study showed different antimicrobial actions among the microorganisms tested, with evidence of better action of GEO on L. monocytogenes, a Gram-positive microorganism, than on S. Typhimurium and P. aeruginosa (Gram-negative), requiring a lower concentration to inhibit its growth.
Thus, owing to the difficult standardization of these compounds, the concentration to classify the antimicrobial activity of natural compounds has not been predefined [29, 30]. According to [31], natural compounds could be classified as inactive, active, moderately active, and extremely active. In view of this classification, the action of 6-gingerol active principle was moderately active in this study, whereas GEO would be considered inactive.
Many Zingiber species were identified and had their bioactive compounds described, with the presence of gingerol and shogaol, which possess antimicrobial activity [17, 32]. Responsible for the fresh ginger characteristic pungent aroma and flavor, 6-gingerol is one of the main non-volatile constituents of its rhizome [33]. Although GEO contains a large number of chemical components with antimicrobial activity [30], some authors attribute the efficacy of GEO to the synergistic activity of these compounds [29, 34]. The lipophilic structure is another characteristic that can be related to an effective antimicrobial activity, resulting in the call membrane destabilization and interfering with the transport of ions and solutes, leading to cell death [35, 36]. In this study, the two bioactive compounds evaluated demonstrated antimicrobial action on the analyzed microorganisms, thus being proposed as compounds with antimicrobial properties.
Several conditions favor cell adhesion and biofilm formation, such as the presence of residues that provide substrate, associated with the characteristics of certain surfaces such as hydrophobicity, and the presence of roughness, which can increase bacterial attachment [5]. In this study, the evaluated isolates formed a biofilm on the polypropylene surface within up to 96 h of incubation and at 10 °C. The evaluation of the antibiofilm action of GEO and 6-gingerol showed different action behaviors in the exposed cells.
6-Gingerol demonstrated antimicrobial action in planktonic cells, requiring low concentrations to inhibit bacterial growth. However, when evaluating the interference of MIC in cell adhesion, the same result was not possible, reinforcing the need for a higher concentration when working with sessile cells, as differences in metabolic behavior within the multispecies biofilm may impede the action of the evaluated compound [37]. This behavior is confirmed by L. monocytogenes cell count, which had a lower tolerated MIC (0.62 mg/mL) than the MIC exposed in the multispecies biofilm. L. monocytogenes adhered to the polypropylene surface (3.75 CFU/cm2) and survived for 96 h at 10 °C, demonstrating a possible cooperative interaction between the species, with S. Typhimurium and P. aeruginosa demonstrating a protective behavior that allowed L. monocytogenes survival under hostile conditions. Different results were found by [20], who reported that 6-gingerol inhibited P. aeruginosa adhesion in 19–53% (10 µM treatment) on glass surfaces. However, no previous studies were found on the efficacy of 6-gingerol against P. aeruginosa, L. monocytogenes, and S. Typhimurium sessile cells.
GEO had a better antibiofilm efficacy against sessile cells both at MIC concentrations and ½ MIC than 6-gingerol (p < 0.05). The best GEO activity can be attributed to its lipophilic characteristic, resulting in physicochemical changes on the cell surface and in antioxidant activity that interfere with cell-surface interaction, preventing cell adhesion [14, 38], a behavior demonstrated in our study by a cell biomass reduction compared to the control. However, there was no total elimination of sessile and planktonic cells in 96 h of incubation. An important fact to be discussed is the increased efficacy of GEO in logarithmic reductions at the first hours of incubation (1–48 h) for the three microorganisms evaluated. A fact that must be considered in the evaluation of different action between longer incubation times is the possible GEO volatility even at a temperature of 10 °C, resulting in less effective action at 72 and 96 h. Our study corroborates the study by [24], who reported that GEO decreased sessile counts in L. monocytogenes biofilm.
This result should be considered in the use of natural compounds as adjuvants in the sanitization process, as this stage is interspersed every 24 h in the industrial work process. Similarly, [39] evaluated the biofilm formation capacity of P. aeruginosa, demonstrating biofilm reduction of 68.13–88.63% using 0.05–0.2 mg/mL of GEO. In a different way, [40] did not report GEO antimicrobial activity on L. monocytogenes and S. Typhimurium cells.
GEO action was evaluated for the first time on a multispecies biofilm on a polypropylene surface. This study considered GEO as a possible antimicrobial agent and found potential antibiofilm activity against multispecies biofilm formed by L. monocytogenes, S. Typhimurium, and P. aeruginosa, depending on the concentration used. We proved that 6-gingerol has antibacterial effect on planktonic cells, but not showing the same potential for sessile cells in multispecies biofilms, whereas GEO has antimicrobial and antibiofilm efficacy against pathogenic microorganisms, representing a promising strategy in multispecies biofilm control. Therefore, GEO can be used as an adjunct to clean surfaces such as polypropylene, being considered a promising alternative in the control of pathogenic and deteriorating microorganisms in the food industry.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq, Brasília, DF, Brazil), the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES, Brasília, DF, Brazil—Code 001 and CAPES PRINT Project, 88881310254/2018-01), and the Federal University of Paraná (Universidade Federal do Paraná—UFPR; nº 02/2020—RESEARCH/PRPPG/UFPR—Araucária Foundation).
Author contribution
EARS: conceptualization, methodology, data curation, writing—original draft, writing—preparation of the original draft. LET: methodology, visualization, investigation. JAS: methodology, visualization, investigation. FSB: formal analysis, writing—review and editing, writing—revision and editing. MOP: formal analysis, writing—review and editing, writing—revision and editing. JGP: formal analysis, writing—review and editing, writing—revision and editing. LSB: supervision, project administration, writing—review and editing, writing, revision and editing.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Responsible Editor: Luis Augusto Nero
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ziech RE, Perin AP, Lampugnani C, Sereno MJ, Viana C, Soares VM, Pereira JG, Pinto JPAN, Bersot LS. Biofilm-producing ability and tolerance to industrial sanitizers in Salmonella spp. isolated from Brazilian poultry processing plants. LWT-Food Sci Technol. 2016;68:85–90. doi: 10.1016/j.lwt.2015.12.021. [DOI] [Google Scholar]
- 2.Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7(39):1–29. doi: 10.3389/fcimb.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sereno MJ, Viana C, Pegoraro K, Da Silva DAL, Yamatogi RS, Nero LA, Bersot LS. Distribution, adhesion, virulence and antibiotic resistance of persistence Listeria monocytogenes in a pig slaughterhouse in Brazil. Food Microbiol. 2019;84:103234. doi: 10.1016/j.fm.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Costerton JW. Introduction to biofilm. Int J Antimicrob Agents. 1999;11(3–4):217–221. doi: 10.1016/S0924-8579(99)00018-7. [DOI] [PubMed] [Google Scholar]
- 5.Fagerlund A, Langsrud S, Møretrø T. Microbial diversity and ecology of biofilms in food industry environments associated with Listeria monocytogenes persistence. Curr Opin Food Sci. 2020;37:171–178. doi: 10.1016/j.cofs.2020.10.015. [DOI] [Google Scholar]
- 6.Canton R, Ruiz-Garbajosa P. Co-resistance: an opportunity for the bacteria and resistance genes. Curr Opin Pharmacol. 2011;11(5):477–485. doi: 10.1016/j.coph.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Alonso-Calleja C, Guerrero-Ramos E, Alonso-Hernando A, Capita R. Adaptation and cross-adaptation of Escherichia coli ATCC 12806 to several food-grade biocides. Food Control. 2015;56:86–94. doi: 10.1016/j.foodcont.2015.03.012. [DOI] [Google Scholar]
- 8.Paul D, Chakraborty R, Mandal S. M. Biocides and health-care agents are more than just antibiotics: inducing cross to co-resistance in microbes. Ecotoxicol Environ Saf. 2019;174:601–610. doi: 10.1016/j.ecoenv.2019.02.083. [DOI] [PubMed] [Google Scholar]
- 9.Brusotti G, Cesari I, Dentamaro A, Caccialanza G, Massolini G. Isolation and characterization of bioactive compounds from plant resources: the role of analysis in the ethnopharmacological approach. J Pharm Biomed Anal. 2014;87:218–228. doi: 10.1016/j.jpba.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Imane NI, Fouzia H, Azzahra LF, Ahmed E, Ismail G, Idrissa D, Mohamed HH, Sirine F, Houcine O, Noureddine B. Chemical composition, antibacterial and antioxidant activities of some essential oils against multidrug resistant bacteria. Eur J Integr Med. 2020;35:101074. doi: 10.1016/j.eujim.2020.101074. [DOI] [Google Scholar]
- 11.Noori S, Zeynali F, Almasi H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control. 2018;84:312–320. doi: 10.1016/j.foodcont.2017.08.015. [DOI] [Google Scholar]
- 12.Mane MB, Bhandari VM, Ranade VV. Safe water and technology initiative for water disinfection: application of natural plant derived materials. J Water Process Eng. 2021;43:102280. doi: 10.1016/j.jwpe.2021.102280. [DOI] [Google Scholar]
- 13.Ezzat SM, Ezzat MI, Okba MM, Menze ET, Abdel-Naim AB. The hidden mechanism beyond ginger (Zingiber officinale Roscue) potent in vivo and in vitro anti-inflammatory activity. J Ethnopharmacol. 2018;214:113–123. doi: 10.1016/j.jep.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Yousfi F, Abrigach F, Petrovic JD, Sokovic M, Ramdani M. Phytochemical screening and evaluation of the antioxidant and antibacterial potential of Zingiber officinale extracts. S Afr J Bot. 2021;142:433–440. doi: 10.1016/j.sajb.2021.07.010. [DOI] [Google Scholar]
- 15.Yamamoto-Ribeiro MMG, Grespan R, Kohiyama CY, Ferreira FD, Mossini SAG, Silva EL, De Abreu-Filho BA, Mikcha JAM, Junior MM. Effect of Zingiberofficinale essential oil on Fusarium verticillioides and fumonisin production. Food Chem. 2013;141:3147–3152. doi: 10.1016/j.foodchem.2013.05.144. [DOI] [PubMed] [Google Scholar]
- 16.Kumar NV, Murthy PS, Manjunatha JR, Bettadaiah BK. Synthesis and quorum sensing inhibitory activity of key phenolic compounds of ginger and their derivatives. Food Chem. 2014;159:451–457. doi: 10.1016/j.foodchem.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 17.Mickymaray S. Efficacy and mechanism of traditional medicinal plants and bioactive compounds against clinically important pathogens. Antibiotics. 2019;8(4):257. doi: 10.3390/antibiotics8040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhaskar A, Kumari A, Singh M, Kumar S, Kumar S, Dabla A, Chaturvedi S, Yadav VC, Dwivedi VP. [6]-Gingerol exhibits potent anti-mycobacterial and immunomodulatory activity against tuberculosis. Int Immunopharmacol. 2020;87:106809. doi: 10.1016/j.intimp.2020.106809. [DOI] [PubMed] [Google Scholar]
- 19.Oyedemi BO, Kotsia EM, Stapleton PD, Gibbons S. Capsaicin and gingerol analogues inhibit the growth of efflux-multidrug resistant bacteria and R- plasmids conjugal transfer. J Ethnopharmacol. 2019;245:111871. doi: 10.1016/j.jep.2019.111871. [DOI] [PubMed] [Google Scholar]
- 20.Kim HS, Lee SH, Byun Y, Park HD. 6-Gingerol reduces Pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Sci Rep. 2015;1:1–11. doi: 10.1038/srep08656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viana C, Sereno MJ, Pegoraro K, Yamatogi RS, Call DR, Bersot LS, Nero LA. Distribution, diversity, virulence genotypes and antibiotic resistance for Salmonella isolated from a Brazilian pork production chain. Int J Food Microbiol. 2019;310:108310. doi: 10.1016/j.ijfoodmicro.2019.108310. [DOI] [PubMed] [Google Scholar]
- 22.CLSI, Clinical and Laboratory Standards Institute (2003) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—sixth edition. CLSI document M07-A6. NCCLS document M7-A6 (ISBN 1–56238–486–4). NCCLS, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087–1898 USA.
- 23.dos Santos EAR, Tadielo LE, Schmiedt JA, Orisio PHS, Brugeff EDCL, Possebon FS, dos Santos Bersot L. Inhibitory effects of piperine and black pepper essential oil on multispecies biofilm formation by Listeria monocytogenes, Salmonella Typhimurium, and Pseudomonas aeruginosa. LWT. 2023;182:114851. doi: 10.1016/j.lwt.2023.114851. [DOI] [Google Scholar]
- 24.Reis-Teixeira FB, Sousa IP, Alves VF, Furtado NAJC, De Martinis ECP. Evaluation of lemongrass and ginger essential oils to inhibit Listeria monocytogenes in biofilms. J Food Saf. 2019;39(4):e12627. doi: 10.1111/jfs.12627. [DOI] [Google Scholar]
- 25.Herigstad B, Hamilton M, Heersink J. How to optimize the drop plate method for enumerating bacteria. J Microbiol Methods. 2001;44(2):121–129. doi: 10.1016/S0167-7012(00)00241-4. [DOI] [PubMed] [Google Scholar]
- 26.Macho AP, Zumaquero A, Ortiz-Martín I, Beuzon CR. Competitive index in mixed infections: a sensitive and accurate assay for the genetic analysis of Pseudomonas syringae–plant interactions. Mol Plant Pathol. 2007;8(4):437–450. doi: 10.1111/j.1364-3703.2007.00404.x. [DOI] [PubMed] [Google Scholar]
- 27.Singh RP, Gangadharappa HV, Mruthunjaya K. Phytosome complexed with chitosan for gingerol delivery in the treatment of respiratory infection: vitro and in vivo evaluation. Eur J Pharm Sci. 2018;122:214–229. doi: 10.1016/j.ejps.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 28.Da Silva FT, Da Cunha KF, Fonseca LM, Antunes MD, El Halal SLM, Fiorentini ÂM, Zavareze ER, Dias ARG. Action of ginger essential oil (Zingiber officinale) encapsulated in proteins ultrafine fiber son the antimicrobial control in situ. Int J Biol Macromol. 2018;118(Pt A):107–115. doi: 10.1016/j.ijbiomac.2018.06.079. [DOI] [PubMed] [Google Scholar]
- 29.Dos Santos RN, De Santana NB, De Carvalho Tavares IM, Lessa OA, Dos Santos LR, Pereira NE, Soares GA, Oliveira RA, Oliveira JR, Franco M. Enzyme extraction by lab-scale hydrodistillation of ginger essential oil (Zingiber officinale Roscoe): chromatographic and micromorphological analyses. Ind Crops Prod. 2020;146:112210. doi: 10.1016/j.indcrop.2020.112210. [DOI] [Google Scholar]
- 30.Jayasundara NDB, Arampath P. Effect of variety, location & maturity stage at harvesting, on essential oil chemical composition, and weight yield of Zingiber officinale roscoe grown in Sri Lanka. Heliyon. 2021;7(3):06560. doi: 10.1016/j.heliyon.2021.e06560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Djabou N, Lorenzi V, Guinoiseau E, Andreani S, Giuliani MC, Desjobert JM, Muselli A. Phytochemical composition of Corsican Teucrium essential oils and antibacterial activity against foodborne or toxi-infectious pathogens. Food Control. 2013;30:354–363. doi: 10.1016/j.foodcont.2012.06.025. [DOI] [Google Scholar]
- 32.Sivasothy Y, Chong WK, Hamid A, Eldeen IM, Sulaiman SF, Awang K. Essential oils of Zingiber officinale var. rubrum Theilade and their antibacterial activities. Food Chem. 2011;124(2):514–517. doi: 10.1016/j.foodchem.2010.06.062. [DOI] [Google Scholar]
- 33.Jolad SD, Lantz RC, Solyom AM, Chen GJ, Bates RB, Timmermann BN. Fresh organically grown ginger (Zingiber officinale): composition and effects on LPS-induced PGE2 production. Phytochem. 2004;65(13):1937–1954. doi: 10.1016/j.phytochem.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Chan EWC, Lim YY, Wong SK, Lim KK, Tan SP, Lianto FS, Yong MY. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009;113(1):166–172. doi: 10.1016/j.foodchem.2008.07.090. [DOI] [Google Scholar]
- 35.Turina ADV, Nolan MV, Zygadlo JA, Perillo MA. Natural terpenes: self-assembly and membrane partitioning. Biophys Chem. 2006;122(2):101–113. doi: 10.1016/j.bpc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils—a review. Food Chem Toxicol. 2008;46(2):446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 37.Boucher C, Waite-Cusic J, Stone D, Kovacevic J. Relative performance of commercial citric acid and quaternary ammonium sanitizers against Listeria monocytogenes under conditions relevant to food industry. Food Microbiol. 2021;97:103752. doi: 10.1016/j.fm.2021.103752. [DOI] [PubMed] [Google Scholar]
- 38.Bellik Y. Total antioxidant activity and antimicrobial potency of the essential oil and oleoresin of Zingiber officinale roscoe. Asian Pac J Trop Dis. 2014;4(1):40–44. doi: 10.1016/S2222-1808(14)60311-X. [DOI] [Google Scholar]
- 39.Chakotiya AS, Tanwar A, Narula A, Sharma RK. Zingiber officinale: its antibacterial activity on Pseudomonas aeruginosa and mode of action evaluated by flow cytometry. Microb Pathog. 2017;107:254–260. doi: 10.1016/j.micpath.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 40.Mazzarrino G, Paparella A, Chaves-López C, Faberi A, Sergi M, Sigismondi C, Compagnone D, Serio A. Salmonella enterica and Listeria monocytogenes inactivation dynamics after treatment with selected essential oils. Food Control. 2015;50:794–803. doi: 10.1016/j.foodcont.2014.10.029. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.